User login
FDA approves Phexxi for use as an on-demand contraceptive
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.
Evofem Biosciences expects to release Phexxi – the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5 – in September 2020 alongside the Phexxi Concierge Experience, a comprehensive patient and health care provider telemedicine support system, according to the company’s press release. The service is designed to provide physicians with on-demand educational support, and to speed and simplify women’s access to Phexxi.
In an open-label multicenter trial, women aged 18-35 with regular menstrual cycles intravaginally administered a 5-gram dose of Phexxi vaginal gel up to 1 hour prior to intercourse; they did so for up to seven cycles. There were 101 pregnancies in 1,183 subjects during 4,769 cycles. The 7-cycle cumulative pregnancy rate was 14% (95% confidence interval: 10.0%, 17.5%).
The most common adverse events associated with Phexxi were vulvovaginal burning sensation, vulvovaginal pruritus, vulvovaginal mycotic infection, urinary tract infection, bacterial vaginosis, vaginal discharge, dysuria, and vulvovaginal pain.
Racial Limitations of Fitzpatrick Skin Type
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
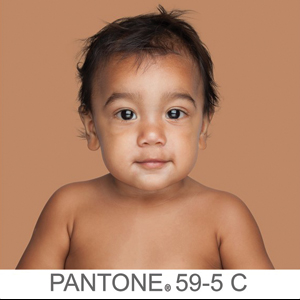
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
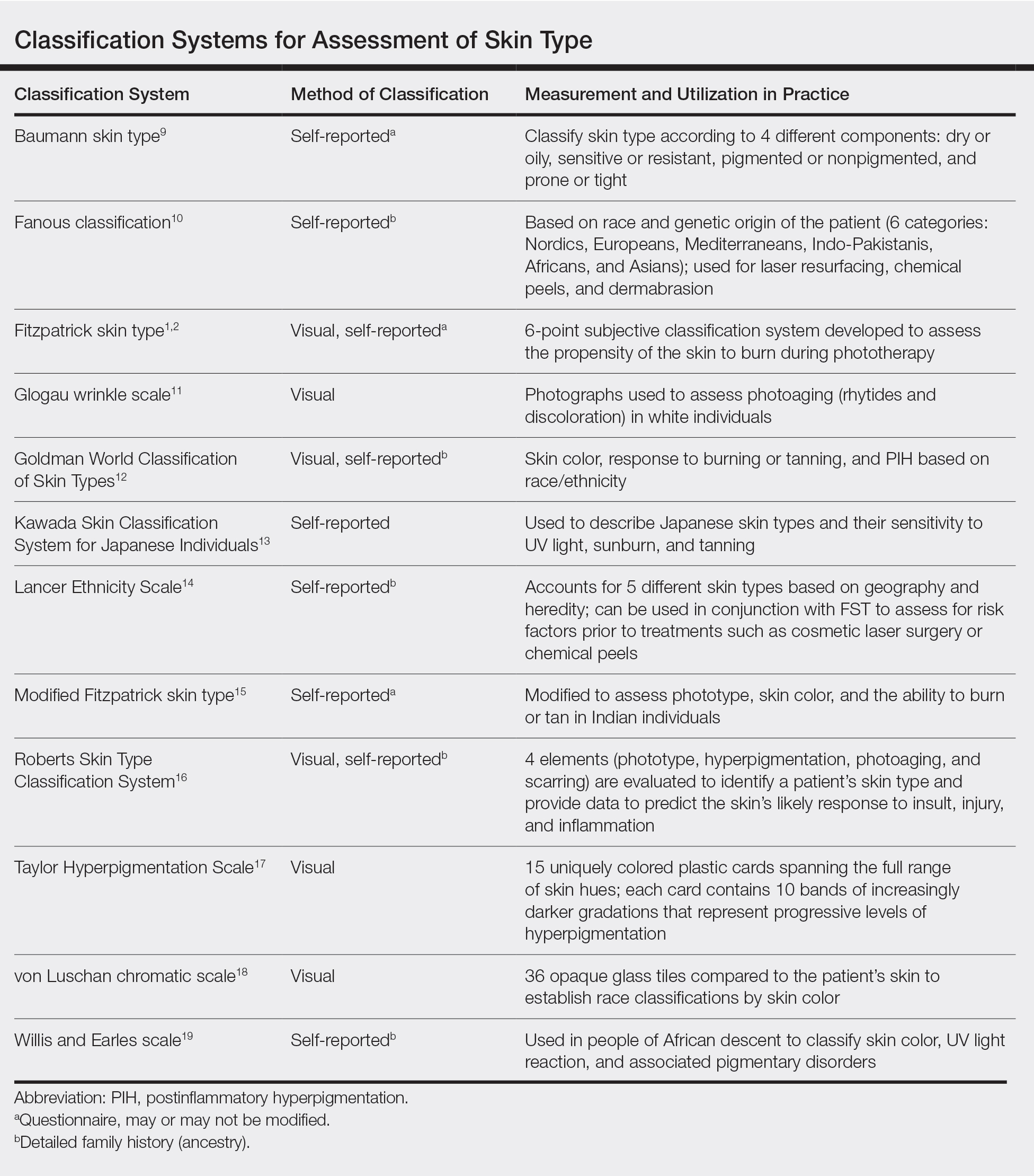
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20
Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
Fitzpatrick skin type (FST) is the most commonly used classification system in dermatologic practice. It was developed by Thomas B. Fitzpatrick, MD, PhD, in 1975 to assess the propensity of the skin to burn during phototherapy.1 Fitzpatrick skin type also can be used to assess the clinical benefits and efficacy of cosmetic procedures, including laser hair removal, chemical peel and dermabrasion, tattoo removal, spray tanning, and laser resurfacing for acne scarring.2 The original FST classifications included skin types I through IV; skin types V and VI were later added to include individuals of Asian, Indian, and African origin.1 As a result, FST often is used by providers as a means of describing constitutive skin color and ethnicity.3
How did FST transition from describing the propensity of the skin to burn from UV light exposure to categorizing skin color, thereby becoming a proxy for race? It most likely occurred because there has not been another widely adopted classification system for describing skin color that can be applied to all skin types. Even when the FST classification scale is used as intended, there are inconsistencies with its accuracy; for example, self-reported FSTs have correlated poorly with sunburn risk as well as physician-reported FSTs.4,5 Although physician-reported FSTs have been demonstrated to correlate with race, race does not consistently correlate with objective measures of pigmentation or self-reported FSTs.5 For example, Japanese women often self-identify as FST type II, but Asian skin generally is considered to be nonwhite.1 Fitzpatrick himself acknowledged that race and ethnicity are cultural and political terms with no scientific basis.6 Fitzpatrick skin type also has been demonstrated to correlate poorly with constitutive skin color and minimal erythema dose values.7
We conducted an anonymous survey of dermatologists and dermatology trainees to evaluate how providers use FST in their clinical practice as well as how it is used to describe race and ethnicity.
Methods
The survey was distributed electronically to dermatologists and dermatology trainees from March 13 to March 28, 2019, using the Association of Professors of Dermatology listserv, as well as in person at the annual Skin of Color Society meeting in Washington, DC, on February 28, 2019. The 8-item survey included questions about physician demographics (ie, primary practice setting, board certification, and geographic location); whether the respondent identified as an individual with skin of color; and how the respondent utilized FST in clinical notes (ie, describing race/ethnicity, skin cancer risk, and constitutive [baseline] skin color; determining initial phototherapy dosage and suitability for laser treatments, and likelihood of skin burning). A t test was used to determine whether dermatologists who identified as having skin of color utilized FST differently.
Results
A total of 141 surveys were returned, and 140 respondents were included in the final analysis. Given the methods used to distribute the survey, a response rate could not be calculated. The respondents included more board-certified dermatologists (70%) than dermatology trainees (30%). Ninety-three percent of respondents indicated an academic institution as their primary practice location. Notably, 26% of respondents self-identified as having skin of color.
Forty-one percent of all respondents agreed that FST should be included in their clinical documentation. In response to the question “In what scenarios would you refer to FST in a clinical note?” 31% said they used FST to describe patients’ race or ethnicity, 47% used it to describe patients’ constitutive skin color, and 22% utilized it in both scenarios. Respondents who did not identify as having skin of color were more likely to use FST to describe constitutive skin color, though this finding was not statistically significant (P=.063). Anecdotally, providers also included FST in clinical notes on postinflammatory hyperpigmentation, melasma, and treatment with cryotherapy.
Comment
The US Census Bureau has estimated that half of the US population will be of non-European descent by 2050.8 As racial and ethnic distinctions continue to be blurred, attempts to include all nonwhite skin types under the umbrella term skin of color becomes increasingly problematic. The true number of skin colors is unknown but likely is infinite, as Brazilian artist Angélica Dass has demonstrated with her photographic project “Humanae” (Figure). Given this shift in demographics and the limitations of the FST, alternative methods of describing skin color must be developed.

© Angélica Dass | Humanae Work in Progress (Courtesy of the artist).
The results of our survey suggest that approximately one-third to half of academic dermatologists/dermatology trainees use FST to describe race/ethnicity and/or constitutive skin color. This misuse of FST may occur more frequently among physicians who do not identify as having skin of color. Additionally, misuse of FST in academic settings may be problematic and confusing for medical students who may learn to use this common dermatologic tool outside of its original intent.
We acknowledge that the conundrum of how to classify individuals with nonwhite skin or skin of color is not simply answered. Several alternative skin classification models have been proposed to improve the sensitivity and specificity of identifying patients with skin of color (Table). Refining FST classification is one approach. Employing terms such as skin irritation, tenderness, itching, or skin becoming darker from sun exposure rather than painful burn or tanning may result in better identification.1,4 A study conducted in India modified the FST questionnaire to acknowledge cultural behaviors.15 Because lighter skin is culturally valued in this population, patient experience with purposeful sun exposure was limited; thus, the questionnaire was modified to remove questions on the use of tanning booths and/or creams as well as sun exposure and instead included more objective questions regarding dark brown eye color, black and dark brown hair color, and dark brown skin color.15 Other studies have suggested that patient-reported photosensitivity assessed via a questionnaire is a valid measure for assessing FST but is associated with an overestimation of skin color, known as “the dark shift.”20

Sharma et al15 utilized reflectance spectrophotometry as an objective measure of melanin and skin erythema. The melanin index consistently showed a positive correlation with FSTs as opposed to the erythema index, which correlated poorly.15 Although reflectance spectrometry accurately identifies skin color in patients with nonwhite skin,21,22 it is an impractical and cost-prohibitive tool for daily practice. A more practical tool for the clinical setting would be a visual color scale with skin hues spanning FST types I to VI, including bands of increasingly darker gradations that would be particularly useful in assessing skin of color. Once such tool is the Taylor Hyperpigmentation Scale.17 Although currently not widely available, this tool could be further refined with additional skin hues.
Conclusion
Other investigators have criticized the various limitations of FST, including physician vs patient assessment, interview vs questionnaire, and phrasing of questions on skin type.23 Our findings suggest that medical providers should be cognizant of conflating race and ethnicity with FST. Two authors of this report (O.R.W. and J.E.D.) are medical students with skin of color and frequently have observed the addition of FST to the medical records of patients who were not receiving phototherapy as a proxy for race. We believe that more culturally appropriate and clinically relevant methods for describing skin of color need to be developed and, in the interim, the original intent of FST should be emphasized and incorporated in medical school and resident education.
Acknowledgment
The authors thank Adewole Adamson, MD (Austin, Texas), for discussion and feedback.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: The McGraw-Hill Companies; 2012.
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian J Dermatol Venereol Leprol. 2009;75:93-96.
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clin Nurs Res. 2012;21:495-516.
- Eilers S, Bach DQ, Gaber R, et al. Accuracy of self-report in assessingFitzpatrick skin phototypes I through VI. JAMA Dermatol. 2013;149:1289-1294.
- He SY, McCulloch CE, Boscardin WJ, et al. Self-reported pigmentary phenotypes and race are significant but incomplete predictors of Fitzpatrick skin phototype in an ethnically diverse population. J Am Acad Dermatol. 2014;71:731-737.
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869-871.
- Leenutaphong V. Relationship between skin color and cutaneous response to ultraviolet radiation in Thai. Photodermatol Photoimmunol Photomed. 1996;11:198-203.
- Colby SL, Ortman JM. Projections of the Size and Composition of the US Population: 2014 to 2060. Washington, DC: US Census Bureau; 2015.
- Baumann L. Understanding and treating various skin types: the Baumann Skin Type Indicator. Dermatol Clin. 2008;26:359-373.
- Fanous N. A new patient classification for laser resurfacing and peels: predicting responses, risks, and results. Aesthetic Plast Surg. 2002;26:99-104.
- Glogau RG. Chemical peeling and aging skin. J Geriatric Dermatol. 1994;2:30-35.
- Goldman M. Universal classification of skin type. In: Shiffman M, Mirrafati S, Lam S, et al, eds. Simplified Facial Rejuvenation. Berlin, Heidelberg, Germany: Springer; 2008:47-50.
- Kawada A. UVB-induced erythema, delayed tanning, and UVA-induced immediate tanning in Japanese skin. Photodermatol. 1986;3:327-333.
- Lancer HA. Lancer Ethnicity Scale (LES). Lasers Surg Med. 1998;22:9.
- Sharma VK, Gupta V, Jangid BL, et al. Modification of the Fitzpatrick system of skin phototype classification for the Indian population, and its correlation with narrowband diffuse reflectance spectrophotometry. Clin Exp Dermatol. 2018;43:274-280.
- Roberts WE. The Roberts Skin Type Classification System. J Drugs Dermatol. 2008;7:452-456.
- Taylor SC, Arsonnaud S, Czernielewski J. The Taylor hyperpigmentation scale: a new visual assessment tool for the evaluation of skin color and pigmentation. Cutis. 2005;76:270-274.
- Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339-342.
- Willis I, Earles RM. A new classification system relevant to people of African descent. J Cosmet Dermatol. 2005;18:209-216.
- Reeder AI, Hammond VA, Gray AR. Questionnaire items to assess skin color and erythemal sensitivity: reliability, validity, and “the dark shift.” Cancer Epidemiol Biomarkers Prev. 2010;19:1167-1173.
- Dwyer T, Muller HK, Blizzard L, et al. The use of spectrophotometry to estimate melanin density in Caucasians. Cancer Epidemiol Biomarkers Prev. 1998;7:203-206.
- Pershing LK, Tirumala VP, Nelson JL, et al. Reflectance spectrophotometer: the dermatologists’ sphygmomanometer for skin phototyping? J Invest Dermatol. 2008;128:1633-1640.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
Practice Points
- Medical providers should be cognizant of conflating race and ethnicity with Fitzpatrick skin type (FST).
- Misuse of FST may occur more frequently among physicians who do not identify as having skin of color.
- Although alternative skin type classification systems have been proposed, more clinically relevant methods for describing skin of color need to be developed.
Calif. woman poisoned by methylmercury-containing skin cream
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
The first known case of contamination of skin-lightening cream with methylmercury was identified in July 2019 in a Mexican American woman in Sacramento, Calif., according to Anita Mudan, MD, of the department of emergency medicine at the University of California, San Francisco, and associates.
The woman, aged 47 years, sought medical care for dysesthesias and weakness in the upper extremities, the investigators wrote in a report published in Morbidity and Mortality Weekly Report.
This progressed to dysarthria, blurry vision, and unsteady gait over a 2-week period, leading to her hospitalization. Over the next 2 weeks, she declined into an agitated delirium; screening blood and urine tests detected levels of mercury exceeding the upper limit of quantification.
Oral dimercaptosuccinic acid at 10 mg/kg every 8 hours was administered via feeding tube. The California Department of Public Health (CDPH) conducted interviews with the patient’s family, discovering that the patient had used a skin-lightening cream obtained from Mexico for the past 7 years. The cream was analyzed and found to contain mercury at a concentration of 12,000 ppm. A Raman spectral analysis showed that the sample contained the organic compound methylmercury iodide.
Typically, contaminated skin-lightening creams contain inorganic mercury at levels up to 200,000 ppm; the significantly lower mercury content of the cream in this case “underscores the far higher toxicity of organic mercury compounds,” the investigators wrote.
The patient has undergone extensive chelation therapy, but remains unable to verbalize or care for herself, requiring continued tube feeding for nutritional support, Dr. Mudan and associates noted.
“CDPH is actively working to warn the public of this health risk, actively screening other skin lightening cream samples for mercury, and is investigating the case of a family member with likely exposure but less severe illness,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Mudan A et al. MMWR Morb Mortal Wkly Rep. 2019 Dec 20. doi: 10.15585/mmwr.mm6850a4.
FROM MMWR
The ABCs of COCs: A Guide for Dermatology Residents on Combined Oral Contraceptives
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4


Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
Resident Pearls
- Screen for contraindications to combined oral contraceptives (COCs) by taking a medical history, measuring a baseline blood pressure, and excluding pregnancy. A baseline pelvic examination is unnecessary.
- Characteristics to consider when selecting a COC include the formulation, hormone-free interval, estrogen dose, and progestin type.
- Combined oral contraceptives can be initiated on any day of the menstrual cycle, with the need for backup contraception based on the number of days since the first day of the patient’s most recent period.
- Management of risks and side effects includes simple lifestyle changes, skipping the hormone-free interval, switching the COC, and referring to an obstetrician/gynecologist.
Melatonin update, Part 2
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at dermnews@mdedge.com.
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.