User login
Fewer than 20% of eligible children received the recommended two doses of flu vaccine
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
A second booster dose of the influenza vaccine in vaccine-naive children may significantly reduce their likelihood of getting the disease, new research suggests.
Writing for JAMA Pediatrics, researchers reported on a case-control study of 7,533 children presenting to outpatient clinics – all in the U.S. Influenza Vaccine Effectiveness Network – with acute respiratory tract illnesses from 2014 to 2018. The study looked at the effectiveness of vaccination against laboratory-confirmed influenza.
Current U.S. guidelines recommend that children aged 6 months to 8 years receive two doses of the influenza vaccine initially – a priming dose and a booster dose – while those aged 9 years or older are considered to be ‘immunologically primed’ and therefore only require one annual dose.
The study found that 60% of the children had received two doses of the influenza vaccine during their first vaccination season, and 68% were first vaccinated before the current influenza season. Of those who had been vaccinated, 89% had received their first influenza vaccine dose when they were younger than 2 years.
Among the 2,140 children who were unvaccinated before the current influenza season, the 436 children who received two doses of the influenza vaccine had 43% lower odds of influenza compared with the 466 children who received one dose. The overall vaccine effectiveness among this vaccine-naive group aged under 2 years was 38%; for those who received two doses it was 53%, and for those who received one dose it was 23%.
“The higher risk of infection resulting from underdeveloped immune and respiratory tract systems provides a reason to identify vaccination strategies focusing on this vulnerable population of younger children,” wrote Jessie R. Chung, MPH, of the Influenza Division of the Centers for Disease Control and Prevention, and coauthors. “Promoting efforts to improve influenza vaccine coverage—particularly with 2 doses in the first vaccination season – may reduce the burden of influenza illness among young children, who are particularly vulnerable to complications and death from influenza infection.”
Overall 52% of children were unvaccinated for the current influenza season and 9% were partially vaccinated. Of those who were fully vaccinated for the current season, 83% had received one dose in the current season, and 17% had received two doses.
The authors found that full vaccination against any influenza was associated with a 22% lower odds of influenza compared with partial vaccination (95% confidence interval, 0.61-1.01), with partial vaccination defined as anything less than two doses of vaccine in the current season – at least 4 weeks apart – or two or more doses before the current season and one or more doses in the current season. However, even children who were only partially vaccinated still showed statistically significant vaccine effectiveness, except for those who received one dose of vaccine in the current season and were aged under 2 years.
“Compared with older children, young children, even if healthy, are at an elevated risk of influenza infection and influenza-associated complications, such as hospitalization,” the authors wrote. “One recent simulation study reported that even small improvements in either vaccine coverage or VE [vaccine effectiveness], and ideally both, may avert substantial amounts of influenza-associated illnesses, medical visits, and hospitalizations.”
The study also noted that children who had received only a single previous vaccine dose rarely received two doses in the current season.
In an accompanying editorial, Claire Abraham, MD, and Melissa S. Stockwell, MD, of Columbia University Medical Center, New York, wrote that modeling suggested that in the 2017-2018 influenza season, vaccination prevented 1.3 million cases of infection, 895,000 medical visits, 10,500 hospitalizations and 111 deaths in children aged under 5 years.
“This study highlights the importance of administering 2 doses of the influenza vaccine to children younger than 9 years for whom 2 doses are needed, and especially to vaccine naive children younger than 2 years,” they wrote.
But despite many studies showing the impact and importance of influenza vaccination, uptake of this vaccine remained lower than for other pediatric vaccines.
“This present study reemphasizes the need for further research exploring why families who are seemingly willing to vaccinate their children against influenza, as indicated by their receiving the first needed dose of influenza vaccine, find barriers to receiving all of the needed doses, placing their children at higher risk for contracting a potentially devastating virus.”
The U.S. Influenza Vaccine Effectiveness Network is funded by the CDC, and this project also received support from the National Institutes of Health. Eight authors declared grants from the CDC during the conduct of the study, and five declared grants and other funding from private industry outside the study.
SOURCE: Chung J et al. JAMA Pediatrics 2020 May 4. doi: 10.1001/jamapediatrics.2020.0372.
FROM JAMA PEDIATRICS
2019-2020 flu season ends with ‘very high’ activity in New Jersey
The 2019-2020 flu season is ending, but not without a revised map to reflect the COVID-induced new world order.

For the week ending April 11, those additions encompass only New Jersey at level 13 and New York City at level 12, the CDC reported April 17.
Eight states, plus the District of Columbia and Puerto Rico, were in the “high” range of flu activity, which runs from level 8 to level 10, for the same week. Those eight states included Connecticut, Georgia, Louisiana, Maryland, Massachusetts, New York, South Carolina, and Wisconsin.
The CDC’s influenza division included this note with its latest FluView report: “The COVID-19 pandemic is affecting healthcare seeking behavior. The number of persons and their reasons for seeking care in the outpatient and ED settings is changing. These changes impact data from ILINet [Outpatient Influenza-like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels, therefore ILINet data should be interpreted with caution.”
Outpatient visits for influenza-like illness made up 2.9% of all visits to health care providers for the week ending April 11, which is the 23rd consecutive week that it’s been at or above the national baseline level of 2.4%. Twenty-three weeks is longer than this has occurred during any flu season since the CDC started setting a baseline in 2007, according to ILINet data.
Mortality from pneumonia and influenza, at 11.7%, was well above the epidemic threshold of 7.0%, although, again, pneumonia mortality “is being driven primarily by an increase in non-influenza pneumonia deaths due to COVID-19,” the CDC wrote.
The total number of influenza-related deaths in children, with reports of two more added this week, is 168 for the season – higher than two of the last three seasons: 144 in 2018-2019, 188 in 2017-2018, and 110 in 2016-2017, according to the CDC.
The 2019-2020 flu season is ending, but not without a revised map to reflect the COVID-induced new world order.

For the week ending April 11, those additions encompass only New Jersey at level 13 and New York City at level 12, the CDC reported April 17.
Eight states, plus the District of Columbia and Puerto Rico, were in the “high” range of flu activity, which runs from level 8 to level 10, for the same week. Those eight states included Connecticut, Georgia, Louisiana, Maryland, Massachusetts, New York, South Carolina, and Wisconsin.
The CDC’s influenza division included this note with its latest FluView report: “The COVID-19 pandemic is affecting healthcare seeking behavior. The number of persons and their reasons for seeking care in the outpatient and ED settings is changing. These changes impact data from ILINet [Outpatient Influenza-like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels, therefore ILINet data should be interpreted with caution.”
Outpatient visits for influenza-like illness made up 2.9% of all visits to health care providers for the week ending April 11, which is the 23rd consecutive week that it’s been at or above the national baseline level of 2.4%. Twenty-three weeks is longer than this has occurred during any flu season since the CDC started setting a baseline in 2007, according to ILINet data.
Mortality from pneumonia and influenza, at 11.7%, was well above the epidemic threshold of 7.0%, although, again, pneumonia mortality “is being driven primarily by an increase in non-influenza pneumonia deaths due to COVID-19,” the CDC wrote.
The total number of influenza-related deaths in children, with reports of two more added this week, is 168 for the season – higher than two of the last three seasons: 144 in 2018-2019, 188 in 2017-2018, and 110 in 2016-2017, according to the CDC.
The 2019-2020 flu season is ending, but not without a revised map to reflect the COVID-induced new world order.

For the week ending April 11, those additions encompass only New Jersey at level 13 and New York City at level 12, the CDC reported April 17.
Eight states, plus the District of Columbia and Puerto Rico, were in the “high” range of flu activity, which runs from level 8 to level 10, for the same week. Those eight states included Connecticut, Georgia, Louisiana, Maryland, Massachusetts, New York, South Carolina, and Wisconsin.
The CDC’s influenza division included this note with its latest FluView report: “The COVID-19 pandemic is affecting healthcare seeking behavior. The number of persons and their reasons for seeking care in the outpatient and ED settings is changing. These changes impact data from ILINet [Outpatient Influenza-like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels, therefore ILINet data should be interpreted with caution.”
Outpatient visits for influenza-like illness made up 2.9% of all visits to health care providers for the week ending April 11, which is the 23rd consecutive week that it’s been at or above the national baseline level of 2.4%. Twenty-three weeks is longer than this has occurred during any flu season since the CDC started setting a baseline in 2007, according to ILINet data.
Mortality from pneumonia and influenza, at 11.7%, was well above the epidemic threshold of 7.0%, although, again, pneumonia mortality “is being driven primarily by an increase in non-influenza pneumonia deaths due to COVID-19,” the CDC wrote.
The total number of influenza-related deaths in children, with reports of two more added this week, is 168 for the season – higher than two of the last three seasons: 144 in 2018-2019, 188 in 2017-2018, and 110 in 2016-2017, according to the CDC.
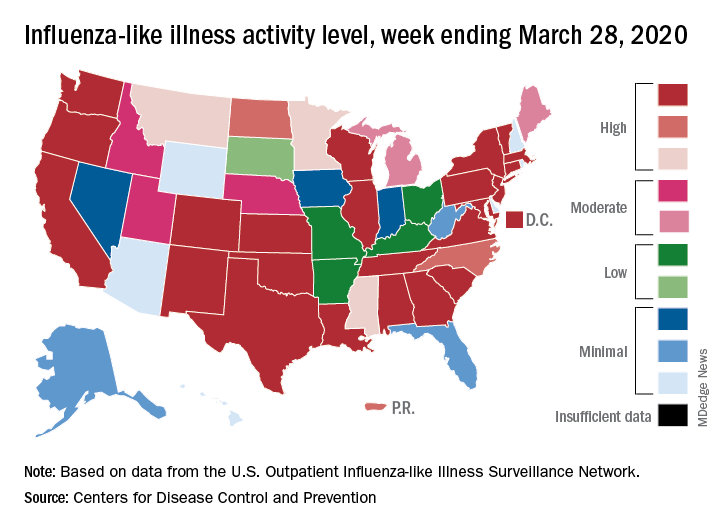
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
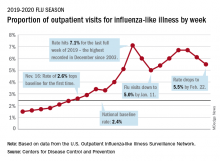
Flu activity measures continue COVID-19–related divergence
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
Flu now riding on COVID-19’s coattails
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
The viral tsunami that is COVID-19 has hit the United States, and influenza appears to be riding the crest of the wave.

according to the Centers for Disease Control. Flu-related visits went from 5.2% of all outpatient visits the week before to 5.8% during the week ending March 14.
“The COVID-19 outbreak unfolding in the United States may affect healthcare seeking behavior which in turn would impact data from” the U.S. Outpatient Influenza-like Illness Surveillance Network, the CDC explained.
Data from clinical laboratories show that, despite the increased activity, fewer respiratory specimens tested positive for influenza: 15.3% for the week of March 8-14, compared with 21.1% the week before, the CDC’s influenza division said in its latest FluView report.
Influenza activity also increased slightly among the states, with 35 states and Puerto Rico at the highest level on the CDC’s 1-10 scale, versus 34 states and Puerto Rico the previous week. The count was down to 33 for the last week of February, CDC data show.
Severity measures remain mixed as overall hospitalization continues to be moderate but rates for children aged 0-4 years and adults aged 18-49 years are the highest on record and rates for children aged 5-17 years are the highest since the 2009 pandemic, the influenza division said.
Mortality data present a similar picture: The overall death rate is low, but the 149 flu-related deaths reported among children is the most for this point of the season since 2009, the CDC said.
Inactivated flu vaccine succeeds among autoimmune rheumatic disease patients
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
Use of the inactivated influenza vaccine by adults with autoimmune rheumatic diseases significantly reduced their risk of influenza-like illness, hospitalization for pneumonia and chronic obstructive pulmonary disease, and death from pneumonia, according to findings from an observational study of more than 30,000 patients in the U.K. Clinical Practice Research Datalink.
Although the inactivated vaccine has been recommended for patients with autoimmune rheumatic diseases (AIRDs), including rheumatoid arthritis and spondyloarthritis, the vaccine’s impact on patient outcomes including pneumonia, hospitalization, and death has not been well studied, wrote Georgina Nakafero, PhD, of the University of Nottingham, England, and colleagues.
In a study published in Rheumatology, the researchers identified 30,788 adults with AIRDs from the longitudinal Clinical Practice Research Datalink database in the United Kingdom. Of these, 66% were women, 76% had rheumatoid arthritis, and 61% had been prescribed methotrexate. The study included a total of 125,034 flu cycles between 2006 and 2009 and between 2010 and 2015.
Overall, vaccination with the inactivated influenza vaccine (IIV) reduced the risk of primary care consultation for influenza-like illness (adjusted odds ratio, 0.70), hospitalization for pneumonia (aOR, 0.61), exacerbation of chronic obstructive pulmonary disease (aOR, 0.67), and death caused by pneumonia (aOR, 0.48) in the study population. In a propensity score–adjusted analysis, only protection from influenza-like illness lost statistical significance.
In addition, vaccination was associated with a reduction in all-cause mortality among AIRDs patients, but restricting the outcomes to the active influenza periods may have confounded this result, the researchers said.
The study findings were limited by several factors including observational design, the use of a single vaccine efficacy estimate for each outcome, potential missed vaccination cycles, and potential confounding by indication and healthy user bias that could inflate the vaccine effectiveness, the researchers noted. However, the results were strengthened by the large sample size, including a range of AIRDs, and the use of both diagnostic and prescription codes, they said.
“The findings of this study, together with the results of our previous study demonstrating the safety of IIV in people with AIRDs, provides evidence to promote seasonal flu vaccination in this population,” they concluded. They still emphasized that randomized, controlled trials are needed for an assessment of vaccine efficacy.
The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
SOURCE: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
FROM RHEUMATOLOGY
Key clinical point: Adults with autoimmune rheumatic diseases who received the inactivated flu vaccine had lower rates of flu-like illness, hospitalization, and death than did those not vaccinated.
Major finding: Vaccination significantly reduced the risk of flu-like illness, hospitalization for pneumonia or COPD exacerbation, and death from pneumonia by 30%, 39%, 33%, and 52%, respectively.
Study details: The data come from 30,788 adults with AIRD and included 125,034 influenza cycles.
Disclosures: The study was supported by Versus Arthritis and the National Institute of Health Research. Lead author Dr. Nakafero had no financial conflicts to disclose. Several coauthors disclosed relationships with companies, including AstraZeneca, Roche, and Pfizer.
Source: Nakafero G et al. Rheumatology. 2020 Mar 11. doi: 10.1093/rheumatology/keaa078.
After weeks of decline, influenza activity increases slightly
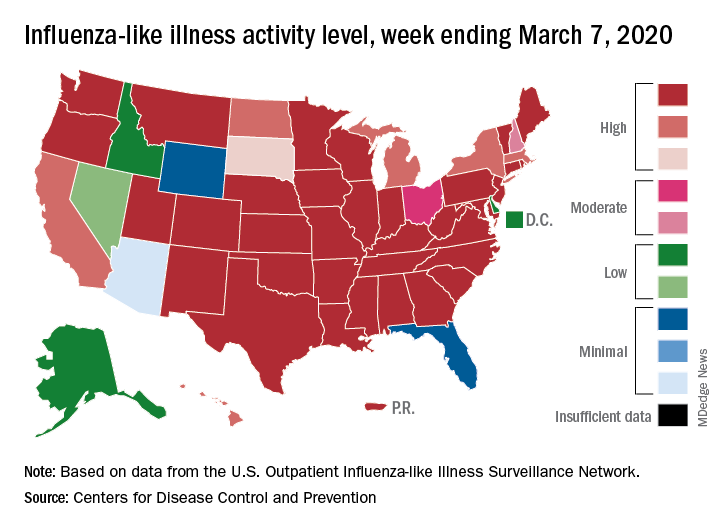
The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.

The two leading measures of influenza activity – the percentage of respiratory specimens testing positive for influenza and the proportion of visits to health care providers for influenza-like illness (ILI) – had been following a similar downward path since mid-February. But during the week ending March 7, their paths diverged, according to the Centers for Disease Control and Prevention.
The percentage of respiratory specimens testing positive for influenza dropped for the fourth consecutive week, falling from 26.1% to 21.5%, while the proportion of visits to health care providers for ILI increased from 5.1% to 5.2%, the CDC’s influenza division reported.
One possible explanation for that rise: “The largest increases in ILI activity occurred in areas of the country where COVID-19 is most prevalent. More people may be seeking care for respiratory illness than usual at this time,” the influenza division said March 13 in its weekly Fluview report.
This week’s map puts 34 states and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity, one more state than the week before, and 43 jurisdictions in the “high” range of 8-10, compared with 42 the previous week, the CDC said.
Rates of hospitalizations associated with influenza “remain moderate compared to recent seasons, but rates for children 0-4 years and adults 18-49 years are now the highest CDC has on record for these age groups, surpassing rates reported during the 2009 H1N1 pandemic,” the Fluview report said. Rates for children aged 5-17 years “are higher than any recent regular season but remain lower than rates experienced by this age group during the pandemic.”
The number of pediatric deaths this season is now up to 144, equaling the total for all of the 2018-2019 season. This year’s count led the CDC to invoke 2009 again, since it “is higher for the same time period than in every season since reporting began in 2004-2005, except for the 2009 pandemic.”
For the 2019-2020 season so far there have been 36 million flu illnesses, 370,000 hospitalizations, and 22,000 deaths from flu and pneumonia, the CDC estimated.
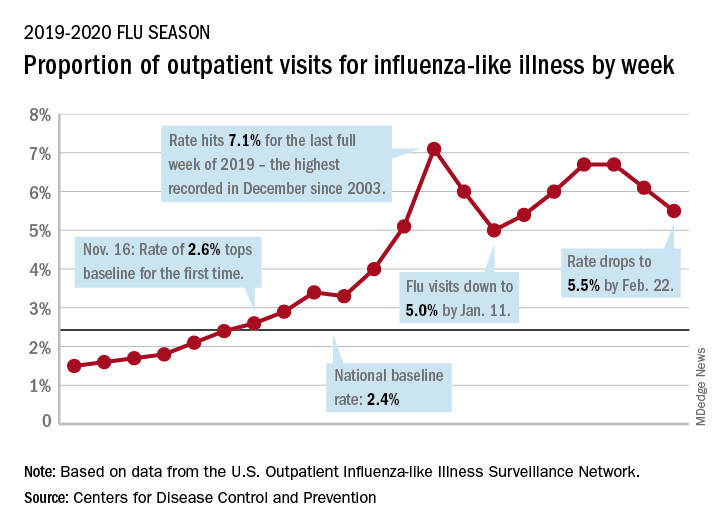
Flu activity declines again but remains high
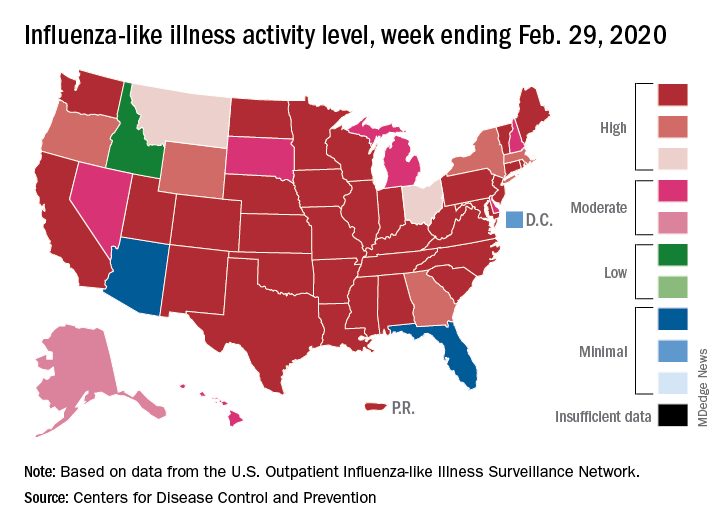
Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.

Outpatient visits to health care providers for influenza-like illness dropped from 5.5% the previous week to 5.3% of all visits for the week ending Feb. 29, the Centers for Disease Control and Prevention said on March 6.
The national baseline rate of 2.4% was first reached during the week of Nov. 9, 2019 – marking the start of flu season – and has remained at or above that level for 17 consecutive weeks. Last year’s season, which also was the longest in a decade, lasted 21 consecutive weeks but started 2 weeks later than the current season and had a lower outpatient-visit rate (4.5%) for the last week of February, CDC data show.
This season’s earlier start could mean that even a somewhat steep decline in visits to below the baseline rate – marking the end of the season – might take 5 or 6 weeks and would make 2019-2020 even longer than 2018-2019.
The activity situation on the state level reflects the small national decline. For the week ending Feb. 29, there were 33 states at level 10 on the CDC’s 1-10 activity scale, compared with 37 the week before, and a total of 40 in the “high” range of 8-10, compared with 43 the week before, the CDC’s influenza division reported.
The other main measure of influenza activity, percentage of respiratory specimens testing positive, also declined for the third week in a row and is now at 24.3% after reaching a high of 30.3% during the week of Feb. 2-8, the influenza division said.
The overall cumulative hospitalization rate continues to remain at a fairly typical 57.9 per 100,000 population, but rates for school-aged children (84.9 per 100,000) and young adults (31.2 per 100,000) are among the highest ever recorded at this point in the season. Mortality among children – now at 136 for 2019-2020 – is higher than for any season since reporting began in 2004, with the exception of the 2009 pandemic, the CDC said.
Children bearing the brunt of declining flu activity
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
National flu activity decreased for the second consecutive week, but pediatric mortality is heading in the opposite direction, according to the Centers for Disease Control and Prevention.
Influenza-like illness (ILI) represented 5.5% of all visits to outpatient health care providers during the week ending Feb. 22, compared with 6.1% the previous week, the CDC’s influenza division reported Feb. 28. The ILI visit rate had reached 6.6% in early February after dropping to 5.0% in mid-January, following a rise to a season-high 7.1% in the last week of December.
Another measure of ILI activity, the percentage of laboratory specimens testing positive, also declined for the second week in a row. The rate was 26.4% for the week ending Feb. 22, which is down from the season high of 30.3% reached 2 weeks before, the influenza division said.
ILI-related deaths among children, however, are not dropping. The total for 2019-2020 is now up to 125, and that “number is higher for the same time period than in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Hospitalization rates, which have been fairly typical in the general population, also are elevated for young adults and school-aged children, the agency said, and “rates among children 0-4 years old are now the highest CDC has on record at this point in the season, surpassing rates reported during the second wave of the 2009 H1N1 pandemic.”
ACIP: Flu vaccines for older adults show similar safety profiles
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) recommends that age-appropriate vaccines be used when possible, said Kenneth E. Schmader, MD, professor of medicine at Duke University, Durham, N.C. However, no study to date had directly compared the safety of the trivalent high dose (HD-IIV3) and adjuvanted (aIIV3) vaccines or their impact on health-related quality of life. Dr. Schmader presented findings from a randomized trial at the February ACIP meeting.
To compare the safety of the vaccines, the researchers recruited community-dwelling volunteers aged 65 years and older who were cognitively intact, not immunosuppressed, and had no contraindications for influenza vaccination. A total of 378 individuals were randomized to aIIV3 and 379 to HD-IIV3. The average age was 72 years; 80 individuals in the aIIV3 group and 83 in the HDIIV3 group were 80 years and older. The primary outcome was moderate or severe injection site pain.
Overall, the proportion of participants with moderate or severe injection site pain was not significantly different after aIIV3 vs. HD-IIV3 (3.2% vs. 5.8%).
Nine participants in the aIIV3 group and three participants in the HD-IIV3 group experienced at least one serious adverse event, but no serious adverse events were deemed vaccine related, and the occurrence of serious adverse events was not significantly different between groups.
In addition, measures of short-term, postvaccination health-related quality of life were not significantly different between the groups. Changes in scores from day 1 prevaccination to day 3 postvaccination on the EuroQOL-5 dimensions-5 levels (EQ-5D-5L) were –0.05 for both groups.
The findings were limited in part by the lack of inclusion of older adults in nursing homes or similar settings, Dr. Schmader noted. However, the results suggest that “from the standpoint of safety, either vaccine is an acceptable option for the prevention of influenza in older adults.”
Studies comparing the immunogenicity of the vaccines are ongoing, and the data should be available within the next few months, he noted.
Dr. Schmader had no financial conflicts to disclose.
FROM AN ACIP MEETING