User login
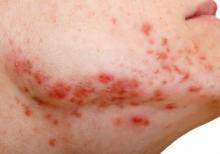
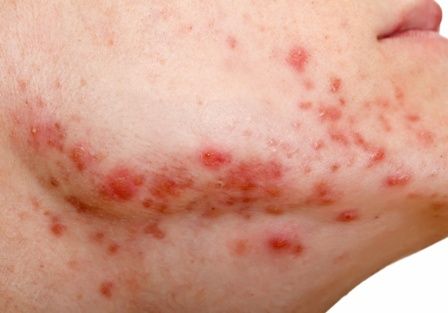
Celiac disease appears to double COVID-19 hospitalization risk
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a single-center U.S. study shows.
Vaccination against COVID-19 reduced the risk for hospitalization by almost half for both groups, however, the study finds.
“To our knowledge this is the first study that demonstrated a vaccination effect on mitigation of the risk of hospitalization in celiac disease patients with COVID-19 infection,” write Alberto Rubio-Tapia, MD, director, Celiac Disease Program, Digestive Disease and Surgery Institute, Cleveland Clinic Foundation, and colleagues.
Despite the increased risk for hospitalization among patients with celiac disease, there were no significant differences between those with and without the condition with respect to intensive care unit requirement, mortality, or thrombosis, the researchers found.
The findings suggest that celiac disease patients with COVID-19 are “not inherently at greater risk for more severe outcomes,” they wrote.
The study was published online in Clinical Gastroenterology and Hepatology.
Comparing outcomes
Although it has been shown that patients with celiac disease have increased susceptibility to viral illnesses, research to date has found similar COVID-19 incidence and outcomes, including hospitalization, between patients with celiac disease and the general population, the researchers wrote.
However, the impact of COVID-19 vaccination is less clear, so the researchers set out to compare the frequency of COVID-19–related outcomes between patients with and without celiac disease before and after vaccination.
Through an analysis of patient medical records, researchers found 171,763 patients diagnosed and treated for COVID-19 at their institution between March 1, 2020, and Jan 1, 2022. Of them, 110 adults had biopsy-proven celiac disease.
The median time from biopsy diagnosis of celiac disease to COVID-19 was 217 months, 66.3% of patients were documented to be following a gluten-free diet, and tissue transglutaminase IgA was positive in 46.2% at the time of COVID-19.
The celiac group was matched by age, ethnicity, sex, and date of COVID-19 diagnosis with a control group of 220 adults without a clinical diagnosis of celiac disease. The two cohorts had similar rates of comorbid obesity, type 2 diabetes, preexisting lung disease, and tobacco use.
Patients with celiac disease were significantly more likely to be hospitalized for COVID-19 than were the control participants, at 24% vs. 11% (hazard ratio, 2.1; P = .009), the researchers wrote.
However, hospitalized patients with celiac disease were less likely to require supplementary oxygen than were the control participants, at 63% vs. 84%.
Vaccination rates for COVID-19 were similar between the two groups, at 64.5% among patients with celiac disease and 70% in the control group. Vaccination was associated with a lower risk for hospitalization on multivariate analysis (HR, 0.53; P = .026).
There was no significant difference in hospitalization rates between vaccinated patients with celiac disease and vaccinated patients in the control group (odds ratio, 1.12; P = .79), the team reported.
The secondary outcomes of ICU requirement, mortality, and thrombosis were minimal in both groups, the researchers wrote.
Vaccination’s importance
The different findings regarding hospitalization risk among patients with celiac disease between this study and previous research are likely due to earlier studies not accounting for vaccination status, the researchers wrote.
“This study shows significantly different rates of hospitalization among patients with [celiac disease] depending on their vaccination status, with strong evidence for mitigation of hospitalization risk through vaccination,” they added.
“Vaccination against COVID-19 should be strongly recommended in patients with celiac disease,” the researchers concluded.
No funding was declared. Dr. Rubio-Tapia reported a relationship with Takeda. No other financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Integrating intestinal ultrasound into inflammatory bowel disease training and practice in the United States
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)
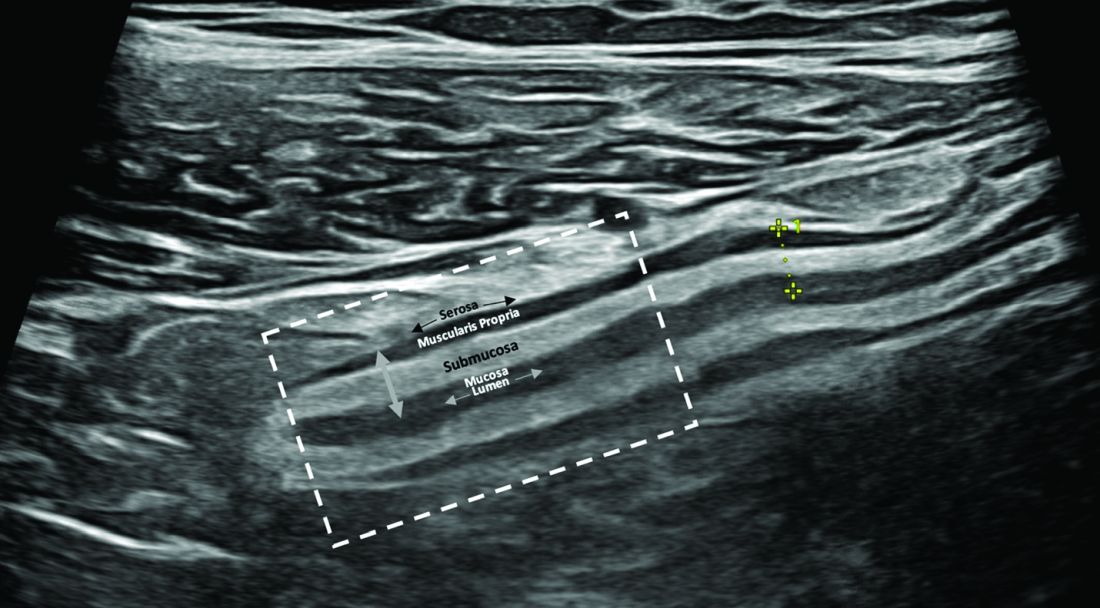
The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)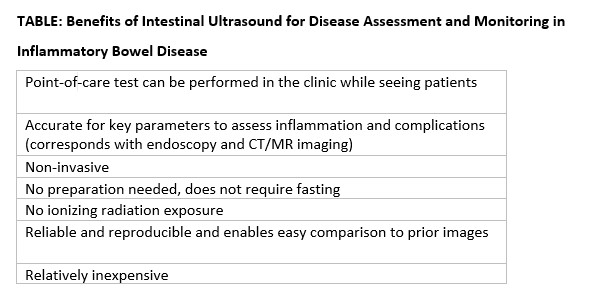
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Evolving endpoints and treat-to-target strategies in inflammatory bowel disease (IBD) incorporate a need for more frequent assessments of the disease, including objective measures of inflammation.1,2 Intestinal ultrasound (IUS) is a noninvasive, well-tolerated,3 repeatable, point-of-care (POC) test that is highly sensitive and specific in detection of bowel inflammation, transmural healing,4,5 and response to therapy in both Crohn’s disease (CD) and ulcerative colitis (UC).6-8 As IUS is taking hold in the United States, there is a great need to teach the next generation of gastroenterologists about its value, how to incorporate it into clinical practice, and how to become appropriately trained and maintain competency.
Why incorporate IUS in the United States now?
As IBD management has evolved, so has the appreciation for the value of bedside IUS as a tool that addresses very real needs for the field. Unlike other parts of the world in which ultrasound skills are part of the training curriculum, this has not been the case in internal medicine and gastroenterology training in the United States. In addition, there have been no specific billing codes or clear renumeration processes outlined for IUS,9 nor have there been any local training opportunities. Because of these challenges, it was not until recently that several leaders in IBD in the United States championed the potential of this technology and incorporated it into IBD management. Subsequently, a number of gastroenterologists have been trained and are now leading the effort to disseminate this tool throughout the United States. A consequence of these efforts resulted in support from the Helmsley Charitable Trust (Helmsley) and the creation of the Intestinal Ultrasound Group of the United States and Canada to address the gaps unique to North America as well as to strengthen the quality of IUS research through collaborations across the continent.
What is IUS, and when is it performed?
IUS is a sonographic exam performed by a gastroenterology-trained professional who scans the abdominal wall (and perineum when the rectum and perineal disease is evaluated), using both a convex low-frequency probe and linear high-frequency probe to evaluate the small intestine, colon, and rectum. The bowel is composed of five layers with alternating hyperechoic and hypoechoic layers: the mucosal-lumen interface (not a true part of the bowel wall), deep mucosa, submucosa, muscularis propria, and serosa. (Figure)

The most sensitive parameter for assessment of IBD activity is bowel wall thickness (≤ 3 mm in the small bowel and colon and ≤ 4 mm in the rectum are considered normal in adults).8,10 The second key parameter is the assessment of vascularization, in which presence of hyperemia suggests active disease.11 There are a number of indices to quantify hyperemia, with the most widely used being the Limberg score.12 Additional parameters include assessment of loss of the delineation of the bowel wall layers (loss of stratification signifies active inflammation), increased thickness of the submucosa,13 increased mesenteric fatty proliferation (with increased inflammation, mesenteric fat proliferation will appear as a hyperechoic area surrounding the bowel), lymphadenopathy, bowel strictures, and extramural complications such as fistulae and abscess. Shear wave elastography may be an effective way to differentiate severe fibrotic strictures, but this is an area that requires more investigation.14
IUS has been shown to be an excellent tool in not only assessing disease activity and disease complication (with higher sensitivity than the Harvey-Bradshaw Index, serum C-reactive protein),15 but, unique to IUS, can provide early prediction of response in moderate to severe active UC.6,7 This has also been shown with transperineal ultrasound in patients with UC, with the ability to predict response to therapy as early as 1 week from induction therapy.16 Furthermore, it can be used to assess transmural healing, which has been shown to be associated with improved outcomes in Crohn’s patient, such as lower rates of hospitalizations, surgery, medication escalation, and need for corticosteroids.17 IUS is associated with great patient satisfaction and greater understanding of disease-related symptoms when the patient sees the inflammation of the bowel. (Table)
How can you get trained in IUS?
Training in IUS varies across the globe, from incorporation of IUS into the standard training curriculum to available training programs that can be followed and attended outside of medical training. In the United States, interested gastroenterologists can now be trained by becoming a member of the International Bowel Ultrasound Group (IBUS Group) and applying to the workshops now available. The IBUS Group has developed an IUS-specific training curriculum over the last 16 years, which is comprised of three modules: a 2-day hands-on workshop (Module 1) with final examination of theoretical competency, a preceptorship at an “expert center” with an experienced sonographer for a total of 4 weeks to complete 40 supervised IUS examinations (Module 2), and didactics and a final examination (Module 3). Also with support from Helmsley, the first Module 1 to be offered in the United States was hosted at Mount Sinai Medical Center in New York in 2022, the second was hosted at the University of Chicago in March 2023, and the third is planned to take place at Cedars-Sinai Medical Center in Los Angeles in March 2024.18 With the growing interest and demand for IUS training in the United States, U.S. experts are working to develop new training options that will be less time consuming, scalable, and still provide appropriate training and competency assessment.
How do you integrate IUS into your practice?
The keys to integrating IUS are a section chief or practice manager’s support of a trainee or faculty member for both funding of equipment and protected time for training and building of the program, as well as a permissive environment and collegial relationship with radiology. An ultrasound machine and additional transducers may range in price from $50,000-$120,000. Funding may be a limiting step for many, however. A detailed business plan is imperative to the success and investment of funds in an IUS program. With current billing practices in place that include ”limited abdominal ultrasound” (76705) and “Doppler ultrasound of the abdomen” (93975),19 reimbursement should include a technical fee, professional fee, and if in a hospital-based clinic, a facility fee. IUS pro-fee combined with technical fee is reimbursed at approximately 0.80 relative value units. When possible, the facility fee is included for approximately $800 per IUS visit. For billing and compliance with HIPAA, all billed IUS images must be stored in a durable and accessible format. It is recommended that the images and cine loops be digitally stored to the same or similar platform used by radiologists at the same institution. This requires early communication with the local information technology department for the connection of an ultrasound machine to the storage platform and/or electronic health record. Reporting results should be standardized with unique or otherwise available IUS templates, which also satisfy all billing components.9 The flow for incorporation of IUS into practice can be at the same time patients are seen during their visit, or alternatively, in a dedicated IUS clinic in which patients are referred by other providers and scheduled back to back.
Conclusions
In summary, the confluence of treat-to-target strategies in IBD, new treatment options in IBD, and successful efforts to translate IUS training and billing practices to the United States portends a great future for the field and for our patients.
Dr. Cleveland and Dr. Rubin, of the University of Chicago’s Inflammatory Bowel Disease Center, are speakers for Samsung/Boston Imaging.
References
1. Turner D et al. Gastroenterology. Apr 2021;160(5):1570-83. doi: 10.1053/j.gastro.2020.12.031
2. Hart AL and Rubin DT. Gastroenterology. Apr 2022;162(5):1367-9. doi: 10.1053/j.gastro.2022.02.013
3. Rajagopalan A et al. JGH Open. Apr 2020;4(2):267-72. doi: 10.1002/jgh3.12268
4. Calabrese E et al. Clin Gastroenterol Hepatol. Apr 2022;20(4):e711-22. doi: 10.1016/j.cgh.2021.03.030
5. Ripolles T et al. Inflamm Bowel Dis. Oct 2016;22(10):2465-73. doi10.1097/MIB.0000000000000882
6. Maaser C et al. Gut. Sep 2020;69(9):1629-36. doi: 10.1136/gutjnl-2019-319451
7. Ilvemark J et al. J Crohns Colitis. Nov 23 2022;16(11):1725-34. doi: 10.1093/ecco-jcc/jjac083
8. Sagami S et al. Aliment Pharmacol Ther. Jun 2020;51(12):1373-83. doi: 10.1111/apt.15767
9. Dolinger MT et al. Guide to Intestinal Ultrasound Credentialing, Documentation, and Billing for Gastroenterologists in the United States. Am J Gastroenterol. 2023.
10. Maconi G et al. Ultraschall Med. Jun 2018;39(3):304-17. doi: 10.1055/s-0043-125329
11. Sasaki T et al. Scand J Gastroenterol. Mar 2014;49(3):295-301. doi: 10.3109/00365521.2013.871744
12. Limberg B. Z Gastroenterol. Jun 1999;37(6):495-508.
13. Miyoshi J et al. J Gastroenterol. Feb 2022;57(2):82-9. doi: 10.1007/s00535-021-01847-3
14. Chen YJ et al. Inflamm Bowel Dis. Sep 15 2018;24(10):2183-90. doi: 10.1093/ibd/izy115
15. Kucharzik T et al. Apr 2017;15(4):535-42e2. doi: 10.1016/j.cgh.2016.10.040
16. Sagami S et al. Aliment Pharmacol Ther. May 2022;55(10):1320-9. doi: 10.1111/apt.16817
17. Vaughan R et al. Aliment Pharmacol Ther. Jul 2022;56(1):84-94. doi: 10.1111/apt.16892
18. International Bowel Ultrasound Group. https://ibus-group.org/
19. American Medical Association. CPT (Current Procedural Terminology). https://www.ama-assn.org/amaone/cpt-current-procedural-terminology
Does CRC risk in IBD extend to close family members?
new research suggests.
In a large Swedish study, a history of IBD among first-degree relatives was not associated with an increased risk of CRC, even when considering various characteristics of IBD and CRC history.
The findings suggest that extra screening for CRC may not be needed for children, siblings, or parents of those with IBD, say the study authors, led by Kai Wang, MD, PhD, with Harvard School of Public Health, Boston. The findings strengthen the theory that it’s inflammation or atypism of the colon of people with IBD that confers the increased CRC risk.
“There is nothing in this study that changes our existing practice,” said Ashwin Ananthakrishnan, MD, MPH, with Massachusetts General Hospital and Harvard Medical School in Boston, who was not involved in the research. “It is already the thought that inflammation in IBD increases risk of cancer,” which would not increase CRC risk among family members.
The study appeared in the International Journal of Cancer.
Patients with IBD are known to be at increased risk for CRC. However, the association between family history of IBD and CRC risk remains less clear. Current CRC screening recommendations are the same for patients who have family members with IBD and for those who do not.
The Swedish nationwide case-control study included 69,659 individuals with CRC, of whom 1,599 (2.3%) had IBD, and 343,032 matched control persons who did not have CRC, of whom 1,477 (0.4%) had IBD.
Overall, 2.2% of CRC case patients and control patients had at least one first-degree relative who had a history of IBD.
After adjusting for family history of CRC, the authors did not find an increase in risk for CRC among first-degree relatives of people with IBD (odds ratio, 0.96; 95% confidence interval, 0.91-1.02).
The null association was consistently observed regardless of IBD subtype (Crohn’s disease or ulcerative colitis), the number of first-degree relatives with IBD, age at first IBD diagnosis, maximum location or extent of IBD, or type of relative (parent, sibling, or offspring). The null association remained for early-onset CRC diagnosed before age 50.
Overall, these findings suggest that IBD and CRC may not have substantial familial clustering or shared genetic susceptibility and provide “robust evidence that a family history of IBD did not increase the risk of CRC, supporting use of the same routine CRC screening strategy in offspring, siblings, and parents of IBD patients as in the general population,” Dr. Wang and colleagues conclude.
This “well-done” study is one of the largest to date to evaluate first-degree relatives of IBD patients and their risk of CRC, said Shannon Chang, MD, with NYU Langone Health Inflammatory Bowel Disease Center, who wasn’t involved in the research.
The findings are reassuring, as the authors assessed several factors and found that family members of patients with IBD are not at higher risk for CRC, compared with the general population, Dr. Chang added.
Support for the study was provided by the National Institutes of Health, the American Cancer Society, ALF funding, the Swedish Research Council, and the Swedish Cancer Foundation. Dr. Wang, Dr. Chang, and Dr. Ananthakrishnan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large Swedish study, a history of IBD among first-degree relatives was not associated with an increased risk of CRC, even when considering various characteristics of IBD and CRC history.
The findings suggest that extra screening for CRC may not be needed for children, siblings, or parents of those with IBD, say the study authors, led by Kai Wang, MD, PhD, with Harvard School of Public Health, Boston. The findings strengthen the theory that it’s inflammation or atypism of the colon of people with IBD that confers the increased CRC risk.
“There is nothing in this study that changes our existing practice,” said Ashwin Ananthakrishnan, MD, MPH, with Massachusetts General Hospital and Harvard Medical School in Boston, who was not involved in the research. “It is already the thought that inflammation in IBD increases risk of cancer,” which would not increase CRC risk among family members.
The study appeared in the International Journal of Cancer.
Patients with IBD are known to be at increased risk for CRC. However, the association between family history of IBD and CRC risk remains less clear. Current CRC screening recommendations are the same for patients who have family members with IBD and for those who do not.
The Swedish nationwide case-control study included 69,659 individuals with CRC, of whom 1,599 (2.3%) had IBD, and 343,032 matched control persons who did not have CRC, of whom 1,477 (0.4%) had IBD.
Overall, 2.2% of CRC case patients and control patients had at least one first-degree relative who had a history of IBD.
After adjusting for family history of CRC, the authors did not find an increase in risk for CRC among first-degree relatives of people with IBD (odds ratio, 0.96; 95% confidence interval, 0.91-1.02).
The null association was consistently observed regardless of IBD subtype (Crohn’s disease or ulcerative colitis), the number of first-degree relatives with IBD, age at first IBD diagnosis, maximum location or extent of IBD, or type of relative (parent, sibling, or offspring). The null association remained for early-onset CRC diagnosed before age 50.
Overall, these findings suggest that IBD and CRC may not have substantial familial clustering or shared genetic susceptibility and provide “robust evidence that a family history of IBD did not increase the risk of CRC, supporting use of the same routine CRC screening strategy in offspring, siblings, and parents of IBD patients as in the general population,” Dr. Wang and colleagues conclude.
This “well-done” study is one of the largest to date to evaluate first-degree relatives of IBD patients and their risk of CRC, said Shannon Chang, MD, with NYU Langone Health Inflammatory Bowel Disease Center, who wasn’t involved in the research.
The findings are reassuring, as the authors assessed several factors and found that family members of patients with IBD are not at higher risk for CRC, compared with the general population, Dr. Chang added.
Support for the study was provided by the National Institutes of Health, the American Cancer Society, ALF funding, the Swedish Research Council, and the Swedish Cancer Foundation. Dr. Wang, Dr. Chang, and Dr. Ananthakrishnan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In a large Swedish study, a history of IBD among first-degree relatives was not associated with an increased risk of CRC, even when considering various characteristics of IBD and CRC history.
The findings suggest that extra screening for CRC may not be needed for children, siblings, or parents of those with IBD, say the study authors, led by Kai Wang, MD, PhD, with Harvard School of Public Health, Boston. The findings strengthen the theory that it’s inflammation or atypism of the colon of people with IBD that confers the increased CRC risk.
“There is nothing in this study that changes our existing practice,” said Ashwin Ananthakrishnan, MD, MPH, with Massachusetts General Hospital and Harvard Medical School in Boston, who was not involved in the research. “It is already the thought that inflammation in IBD increases risk of cancer,” which would not increase CRC risk among family members.
The study appeared in the International Journal of Cancer.
Patients with IBD are known to be at increased risk for CRC. However, the association between family history of IBD and CRC risk remains less clear. Current CRC screening recommendations are the same for patients who have family members with IBD and for those who do not.
The Swedish nationwide case-control study included 69,659 individuals with CRC, of whom 1,599 (2.3%) had IBD, and 343,032 matched control persons who did not have CRC, of whom 1,477 (0.4%) had IBD.
Overall, 2.2% of CRC case patients and control patients had at least one first-degree relative who had a history of IBD.
After adjusting for family history of CRC, the authors did not find an increase in risk for CRC among first-degree relatives of people with IBD (odds ratio, 0.96; 95% confidence interval, 0.91-1.02).
The null association was consistently observed regardless of IBD subtype (Crohn’s disease or ulcerative colitis), the number of first-degree relatives with IBD, age at first IBD diagnosis, maximum location or extent of IBD, or type of relative (parent, sibling, or offspring). The null association remained for early-onset CRC diagnosed before age 50.
Overall, these findings suggest that IBD and CRC may not have substantial familial clustering or shared genetic susceptibility and provide “robust evidence that a family history of IBD did not increase the risk of CRC, supporting use of the same routine CRC screening strategy in offspring, siblings, and parents of IBD patients as in the general population,” Dr. Wang and colleagues conclude.
This “well-done” study is one of the largest to date to evaluate first-degree relatives of IBD patients and their risk of CRC, said Shannon Chang, MD, with NYU Langone Health Inflammatory Bowel Disease Center, who wasn’t involved in the research.
The findings are reassuring, as the authors assessed several factors and found that family members of patients with IBD are not at higher risk for CRC, compared with the general population, Dr. Chang added.
Support for the study was provided by the National Institutes of Health, the American Cancer Society, ALF funding, the Swedish Research Council, and the Swedish Cancer Foundation. Dr. Wang, Dr. Chang, and Dr. Ananthakrishnan have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF CANCER
AGA guideline defines role of biomarkers in ulcerative colitis
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
The American Gastroenterological Association (AGA) has released a new clinical practice guideline defining the role of biomarkers in monitoring and managing ulcerative colitis (UC).
, reported lead guideline panelist Siddharth Singh, MD, of University of California San Diego, La Jolla, Calif., and colleagues.
“[I]n routine clinical practice, repeated endoscopic assessment is invasive, expensive, and may be impractical,” the panelists wrote. Their report is in Gastroenterology. “There is an important need for understanding how noninvasive biomarkers may serve as accurate and reliable surrogates for endoscopic assessment of inflammation and whether they can be more readily implemented in a UC care pathway.”
After reviewing relevant randomized controlled trials and observational studies, Dr. Singh and colleagues issued seven conditional recommendations, three of which concern patients in symptomatic remission, and four of which apply to patients with symptomatically active UC.
“The key take-home message is that the routine measurement of noninvasive biomarkers in addition to assessment of patient reported symptoms is critical in evaluating the disease burden of UC,” said Jordan E. Axelrad, MD, MPH, director of clinical and translational research at NYU Langone Health’s Inflammatory Bowel Disease Center, New York. “Many of these recommendations regarding the assessment of disease activity beyond symptoms alone are widely accepted, particularity at tertiary IBD centers; however, this guideline serves to formalize and structure the recommendations, with appropriate test cutoff values, in a simple UC care pathway.”
Recommendations for patients in symptomatic remission
For patients in remission, the guideline advises monitoring both symptoms and biomarkers, with biomarkers measured every 6-12 months.
Asymptomatic patients with normal biomarkers can skip routine endoscopy to evaluate disease activity, according to the guideline, but those with abnormal fecal calprotectin, fecal lactoferrin, or serum C-reactive protein (CRP) are candidates for endoscopic assessment instead of empiric treatment adjustment. Patients may still need periodic colonoscopy for dysplasia surveillance.
“The most important pearl [from the guideline] is that fecal calprotectin less than 150 mcg/g, normal fecal lactoferrin, or normal CRP, can be used to rule out active inflammation in patients in symptomatic remission,” according to Dr. Axelrad.
The guideline suggests that the two fecal biomarkers “may be optimal for monitoring and may be particularly useful in patients where biomarkers have historically correlated with endoscopic disease activity.” In contrast, normal CRP may be insufficient to rule out moderate to severe endoscopic inflammation in patients who recently entered remission following treatment adjustment.
While abnormal biomarkers in asymptomatic patients are sufficient cause for endoscopy, the guideline also suggests that retesting in 3-6 months is a reasonable alternative. If biomarkers are again elevated, then endoscopic evaluation should be considered.
Recommendations for patients with symptomatically active disease
The recommendations for patients with symptomatically active UC follow a similar pathway. The guideline advises an evaluation strategy combining symptoms and biomarkers instead of symptoms alone.
For example, patients with moderate to severe symptoms suggestive of flare and elevated biomarkers are candidates for treatment adjustment without endoscopy.
Still, patient preferences should be considered, Dr. Singh and colleagues noted.
“Patients who place greater value in confirming inflammation, particularly when making significant treatment decisions (such as starting or switching immunosuppressive therapies), and lesser value on the inconvenience of endoscopy, may choose to pursue endoscopic evaluation before treatment adjustment,” they wrote.
For patients with mild symptoms, endoscopy is generally recommended, according to the guideline, unless the patient recently had moderate to severe symptoms and has improved after treatment adjustment; in that case, biomarkers can be used to fine-tune therapy without the need for endoscopy.
Again, providers should engage in shared-decision making, the guideline advises. Patients with mild symptoms but no biomarker results may reasonably elect to undergo endoscopy prior to testing biomarkers, while patients with mild symptoms and normal biomarkers may reasonably elect to retest biomarkers in 3-6 months.
Data remain insufficient to recommend biomarkers over endoscopy
Dr. Singh and colleagues concluded the guideline by highlighting an insufficient level of direct evidence necessary to recommend a biomarker-based treat-to-target strategy over endoscopy-based monitoring strategy, despite indirect evidence suggesting this may be the case.
“[T]here have not been any studies comparing a biomarker-based strategy with an endoscopy-based strategy for assessment and monitoring of endoscopic remission,” they wrote. “This was identified as a knowledge gap by the panel.”
The authors disclosed relationships with Pfizer, AbbVie, Lilly, and others. Dr. Axelrad disclosed relationships with Janssen, AbbVie, Pfizer, and others.
FROM GASTROENTEROLOGY
New influx of Humira biosimilars may not drive immediate change
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yardstick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars .
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yardstick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars .
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yardstick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Help your patients understand biologics and biosimilars by using AGA resources for providers and patients available at gastro.org/biosimilars .
Gastroparesis referrals often based on misdiagnosis
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
, a new retrospective review suggests.
The researchers analyzed the records of 339 patients referred for tertiary evaluation of GP at one center. Overall, 19.5% of patients were confirmed to have GP, whereas 80.5% were given an alternative diagnosis, with FD being the most common (44.5%).
Contributing to initial misdiagnosis are the similarity in presentation between patients with GP and FD and low rates of gastric emptying evaluation using the recommended test protocol, lead author David J. Cangemi, MD, division of gastroenterology, Mayo Clinic, Jacksonville, Fla., and colleagues write.
The findings “reaffirm guidelines noting that gastroparesis cannot be diagnosed based on symptoms alone,” they write.
Because FD is more prevalent than GP, FD “should be considered first in patients with characteristic upper GI symptoms,” they add.
The review was published online in Clinical Gastroenterology and Hepatology.
Similarities breed confusion
GP and FD are the two most common sensorimotor disorders of the stomach, and both are characterized by abdominal pain, nausea, early satiety, and vomiting, the researchers write.
While GP is defined by delayed gastric emptying, it is also seen in 20%-30% of patients with FD. This overlap and symptom commonality make “the diagnosis difficult for many health care providers,” they write.
The researchers hypothesized that GP is frequently incorrectly overdiagnosed in the community and that FD, along with other disorders that mimic GP, are underdiagnosed.
Their retrospective review involved adult patients referred to their institution for the evaluation of GP between January 2019 and July 2021.
The team gathered information on patient demographics, medical comorbidities, diagnostic tests, and laboratory results. Researchers determined a final diagnosis after reviewing clinical notes, communications, and the results of tests conducted by experts.
Of the 339 patients, 82.1% were female and 85.6% were White.
Diabetes was diagnosed in 21.7% of patients, of whom 59.7% had type 2 disease. Most patients (71.7%) had previously been diagnosed with gastroesophageal reflux disease, and 5.6% had been diagnosed with Helicobacter pylori. Anxiety and depression were also seen in 56.9% and 38.8% of patients, respectively.
The team found that 14.5% of patients were taking opioids, and 19.2% were using cannabis. Less than half (41.3%) had undergone cholecystectomy and 6.8% a fundoplication procedure.
The most common presenting symptom was nausea, in 89.1% of patients, followed by abdominal pain in 76.4%, constipation in 70.5%, and vomiting in 65.8%.
Related treatments included at least one pyloric injection of botulinum toxin in 13% of patients, whereas 2.4% had a gastric electrical stimulator implanted.
Importantly, only 57.8% of the patients had received a definitive evaluation with a gastric emptying study (GES), of whom 38.3% had undergone the recommended 4-hour study, and just 6.8% had ingested radiolabeled eggs as the test meal, the study notes.
Besides FD, alternative final diagnoses included rapid gastric emptying (12.1% of patients), pelvic floor dysfunction (9.9%), constipation (8.4%), and cannabinoid hyperemesis syndrome (7%).
Patient differences found
Compared with patients with a definitive GP diagnosis, patients with alternative diagnoses were younger (P = .001) and had a lower median body mass index (P = .017).
Patients who were correctly diagnosed with GP more often had diabetes (P < .001) and a history of Barrett’s esophagus (P = .042) and were less likely to have chronic kidney disease (P = .036) and rheumatoid arthritis (P = .035).
Patients with confirmed GP were also more likely to have undergone cholecystectomy (P = .008), fundoplication (P = .025), and botulinum toxin injection of the pylorus (P = .013) than those with an alternative diagnosis. They were also more likely to use a proton pump inhibitor (P < .001) and less likely to use less cannabis (P = .034).
After tertiary evaluation, patients with a definitive diagnosis of GP were more likely to be treated with metoclopramide (P < .001), prucalopride (P < .001), ondansetron (P = .005), promethazine (P = .05), and dietary interventions (P = .024) than those with alternative diagnoses.
On the other hand, patients with alternative diagnoses more often received a tricyclic antidepressant (P = .039) and were advised to discontinue cannabis (P = .05) than those confirmed as having GP.
‘Striking’ finding
Although researchers predicted that GP was overdiagnosed in the community, the finding that nearly 80% of people referred for tertiary evaluation did not have the condition was “quite striking,” Dr. Cangemi told this news organization.
The findings regarding gastric emptying evaluations highlight the result of a previous study “demonstrating low compliance with gastric emptying protocol guidelines among U.S. medical institutions,” the researchers write.
“Improperly performed GES appears to play a critical role in misdiagnosis of GP,” they add.
The study’s main message is the “importance of performing a proper gastric emptying study,” Dr. Cangemi said. If GES isn’t conducted according to the guidelines, the results may be “misleading,” he added.
Another key point is that FD is a much more prevalent disorder, affecting approximately 10% of the United States population, while GP is “much rarer,” Dr. Cangemi said.
“That might be another reason why patients are mislabeled with gastroparesis – the lack of recognition of functional dyspepsia as a common disorder of gut-brain interaction – and perhaps some hesitation of among some providers to make a confident clinical diagnosis of functional dyspepsia,” he said.
Moreover, Dr. Cangemi said, patients can “go back and forth” between the two disorders. A recent study demonstrated that roughly 40% of patients transition between the two over the course of a year, he noted.
“So being locked into one diagnosis is, I think, not appropriate anymore. Providers really need to keep an open mind and think critically about the results of a gastric emptying study, especially if it was not done recently and especially if the test did not adhere to standard protocol,” he said.
No funding was declared. Co-author Brian E. Lacy, MD, PhD, declared relationships with Ironwood, Urovant, Salix, Sanofi, and Viver. No other relevant financial relationships were declared.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Consider cultural differences in IBD diet planning
AURORA, COLO. – Inflammatory bowel disease doesn’t respect international borders, and
“Many patients are in an environment that they’re not used to, an environment where most people speak English and their customs and their language may differ from the individual providing care to them. They’re often told, in addition, to eat foods that they may not even have heard of. It can really be a scary situation for many of these patients,” said Neha D. Shah, MPH, RD, CNSC, a dietitian at University of California San Francisco Health.
“Put yourself in their shoes. [Consider] what would make you feel more comfortable in that environment, and then apply that perspective to the care of your patient,” she advised colleagues at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Ms. Shah explained that by incorporating understanding of cultural differences and food culture into the care of persons with IBD, clinicians can help patients from different ethnic backgrounds accept diets that both contain familiar foods and also help to ameliorate their gastrointestinal symptoms.
Food culture and acculturation
As of 2016, the estimated prevalence of IBD among pediatric patients in the United States was 77 per 100,000, and the prevalence in adults was estimated at 478.4 per 100,000. In a 2021 study of the effects of race and ethnicity on the diagnosis and management of IBD, the authors estimated that the prevalence of IBD in the United States was about 3.1 million persons, or 1.3% of the population, with an increase in prevalence in non-White persons and ethnicities, she noted.
Some of the increasing prevalence among minority populations may be attributable to diet acculturation, when members of a particular group partially or completely adopt the eating patterns and/or food choices of the host country.
Culturally appropriate foods
The term “food culture” refers to “the sociocultural aspect of eating, and include[s] the beliefs, values, and attitudes a community may accept around food,” she said.
Ms. Shah provided examples of culturally appropriate foods that may be tolerated by patients with IBD, such as beans, tortillas, chicken with rice, guacamole, mangos, and tomatoes in persons from South America, or lentils, breads, rice, oats, spinach, and tea among patients from the Indian subcontinent.
By understanding and respecting cultural differences, learning how to best communicate with persons of other cultures, and by being aware of one’s own biases, clinicians can better help patients create diet plans that fit within their expectations and lifestyles, she said.
For example, patients can be encouraged to incorporate more culturally familiar plant-based foods such as legumes to manage active disease and maintain remissions.
Patients with active disease should have at least one-half cup of one form of culturally appropriate fiber at each meal. The dietitian should consider recommending blending fiber into other foods or serving it cooked, mashed, or minced, depending upon the patient’s level of tolerance.
During the transition phase, patients can reintroduce an additional half cup of fiber at one meal, then at two meals, and finally at three daily meals. Patients can see whether they can tolerate more raw or whole high-fiber foods at this stage.
During remissions, patients should be advised to add two to three foods containing culturally appropriate fiber at each meal, she said.
‘Eye-opening’ realization
“I think it’s really eye-opening for us to think about how we have to have culturally sensitive discussions with our patients,” commented Sandra Kim, MD, from the University of Pittsburgh Medical Center, who moderated the session.
Dr. Kim asked Ms. Shah what advice she’d give to pediatric gastroenterologists about engaging patients and their families.
The clinician should ask both patients and parents about what the child eats and what the challenges of eating under certain circumstances are, and have culturally appropriate resources on hand.
Ms. Shah did not report a funding source for her work. She disclosed compensation as editor of the Journal of Practical Gastroeneterology and as GI on Demand–consultant for a joint virtual platform from the American College of Gastroenterology and Gastro Girl. She also serves as treasurer and director of operations for the South Asian IBD Alliance.
AURORA, COLO. – Inflammatory bowel disease doesn’t respect international borders, and
“Many patients are in an environment that they’re not used to, an environment where most people speak English and their customs and their language may differ from the individual providing care to them. They’re often told, in addition, to eat foods that they may not even have heard of. It can really be a scary situation for many of these patients,” said Neha D. Shah, MPH, RD, CNSC, a dietitian at University of California San Francisco Health.
“Put yourself in their shoes. [Consider] what would make you feel more comfortable in that environment, and then apply that perspective to the care of your patient,” she advised colleagues at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Ms. Shah explained that by incorporating understanding of cultural differences and food culture into the care of persons with IBD, clinicians can help patients from different ethnic backgrounds accept diets that both contain familiar foods and also help to ameliorate their gastrointestinal symptoms.
Food culture and acculturation
As of 2016, the estimated prevalence of IBD among pediatric patients in the United States was 77 per 100,000, and the prevalence in adults was estimated at 478.4 per 100,000. In a 2021 study of the effects of race and ethnicity on the diagnosis and management of IBD, the authors estimated that the prevalence of IBD in the United States was about 3.1 million persons, or 1.3% of the population, with an increase in prevalence in non-White persons and ethnicities, she noted.
Some of the increasing prevalence among minority populations may be attributable to diet acculturation, when members of a particular group partially or completely adopt the eating patterns and/or food choices of the host country.
Culturally appropriate foods
The term “food culture” refers to “the sociocultural aspect of eating, and include[s] the beliefs, values, and attitudes a community may accept around food,” she said.
Ms. Shah provided examples of culturally appropriate foods that may be tolerated by patients with IBD, such as beans, tortillas, chicken with rice, guacamole, mangos, and tomatoes in persons from South America, or lentils, breads, rice, oats, spinach, and tea among patients from the Indian subcontinent.
By understanding and respecting cultural differences, learning how to best communicate with persons of other cultures, and by being aware of one’s own biases, clinicians can better help patients create diet plans that fit within their expectations and lifestyles, she said.
For example, patients can be encouraged to incorporate more culturally familiar plant-based foods such as legumes to manage active disease and maintain remissions.
Patients with active disease should have at least one-half cup of one form of culturally appropriate fiber at each meal. The dietitian should consider recommending blending fiber into other foods or serving it cooked, mashed, or minced, depending upon the patient’s level of tolerance.
During the transition phase, patients can reintroduce an additional half cup of fiber at one meal, then at two meals, and finally at three daily meals. Patients can see whether they can tolerate more raw or whole high-fiber foods at this stage.
During remissions, patients should be advised to add two to three foods containing culturally appropriate fiber at each meal, she said.
‘Eye-opening’ realization
“I think it’s really eye-opening for us to think about how we have to have culturally sensitive discussions with our patients,” commented Sandra Kim, MD, from the University of Pittsburgh Medical Center, who moderated the session.
Dr. Kim asked Ms. Shah what advice she’d give to pediatric gastroenterologists about engaging patients and their families.
The clinician should ask both patients and parents about what the child eats and what the challenges of eating under certain circumstances are, and have culturally appropriate resources on hand.
Ms. Shah did not report a funding source for her work. She disclosed compensation as editor of the Journal of Practical Gastroeneterology and as GI on Demand–consultant for a joint virtual platform from the American College of Gastroenterology and Gastro Girl. She also serves as treasurer and director of operations for the South Asian IBD Alliance.
AURORA, COLO. – Inflammatory bowel disease doesn’t respect international borders, and
“Many patients are in an environment that they’re not used to, an environment where most people speak English and their customs and their language may differ from the individual providing care to them. They’re often told, in addition, to eat foods that they may not even have heard of. It can really be a scary situation for many of these patients,” said Neha D. Shah, MPH, RD, CNSC, a dietitian at University of California San Francisco Health.
“Put yourself in their shoes. [Consider] what would make you feel more comfortable in that environment, and then apply that perspective to the care of your patient,” she advised colleagues at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Ms. Shah explained that by incorporating understanding of cultural differences and food culture into the care of persons with IBD, clinicians can help patients from different ethnic backgrounds accept diets that both contain familiar foods and also help to ameliorate their gastrointestinal symptoms.
Food culture and acculturation
As of 2016, the estimated prevalence of IBD among pediatric patients in the United States was 77 per 100,000, and the prevalence in adults was estimated at 478.4 per 100,000. In a 2021 study of the effects of race and ethnicity on the diagnosis and management of IBD, the authors estimated that the prevalence of IBD in the United States was about 3.1 million persons, or 1.3% of the population, with an increase in prevalence in non-White persons and ethnicities, she noted.
Some of the increasing prevalence among minority populations may be attributable to diet acculturation, when members of a particular group partially or completely adopt the eating patterns and/or food choices of the host country.
Culturally appropriate foods
The term “food culture” refers to “the sociocultural aspect of eating, and include[s] the beliefs, values, and attitudes a community may accept around food,” she said.
Ms. Shah provided examples of culturally appropriate foods that may be tolerated by patients with IBD, such as beans, tortillas, chicken with rice, guacamole, mangos, and tomatoes in persons from South America, or lentils, breads, rice, oats, spinach, and tea among patients from the Indian subcontinent.
By understanding and respecting cultural differences, learning how to best communicate with persons of other cultures, and by being aware of one’s own biases, clinicians can better help patients create diet plans that fit within their expectations and lifestyles, she said.
For example, patients can be encouraged to incorporate more culturally familiar plant-based foods such as legumes to manage active disease and maintain remissions.
Patients with active disease should have at least one-half cup of one form of culturally appropriate fiber at each meal. The dietitian should consider recommending blending fiber into other foods or serving it cooked, mashed, or minced, depending upon the patient’s level of tolerance.
During the transition phase, patients can reintroduce an additional half cup of fiber at one meal, then at two meals, and finally at three daily meals. Patients can see whether they can tolerate more raw or whole high-fiber foods at this stage.
During remissions, patients should be advised to add two to three foods containing culturally appropriate fiber at each meal, she said.
‘Eye-opening’ realization
“I think it’s really eye-opening for us to think about how we have to have culturally sensitive discussions with our patients,” commented Sandra Kim, MD, from the University of Pittsburgh Medical Center, who moderated the session.
Dr. Kim asked Ms. Shah what advice she’d give to pediatric gastroenterologists about engaging patients and their families.
The clinician should ask both patients and parents about what the child eats and what the challenges of eating under certain circumstances are, and have culturally appropriate resources on hand.
Ms. Shah did not report a funding source for her work. She disclosed compensation as editor of the Journal of Practical Gastroeneterology and as GI on Demand–consultant for a joint virtual platform from the American College of Gastroenterology and Gastro Girl. She also serves as treasurer and director of operations for the South Asian IBD Alliance.
AT THE CROHN’S & COLITIS CONGRESS
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Herbal combination tames active UC in small study
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.
“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.

“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AURORA, COLO. –
Among 42 patients randomized on a 2:1 basis to receive either an enteric-coated pill containing 3 g of curcumin and qing-dai (CurQD) or placebo for 8 weeks, 43% of those assigned to receive the combination met the co-primary endpoint of a significant reduction in disease activity and objective evidence of response, compared with 8% of those assigned to placebo, reported Shomron Ben-Horin, MD, of Sheba Medical Center in Tel Aviv, Israel, and colleagues.
“In this randomized multicenter placebo-controlled trial, combination CurQD was found effective for inducing remission in active UC patients, including biologic-experienced patients,” they wrote in a scientific poster presented at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Nice spice
Curcumin is a polyphenolic compound derived from the spice turmeric that has been shown to have antioxidative and anti-inflammatory properties. Qing-dai (QD), also known as indigo naturalis, has been used in traditional Chinese medicine as an anti-inflammatory. Both agents are available over the counter in the United States, and have been on the market in Israel as a combination since 2016, said coauthor Nir Salomon, a certified herbalist at Sheba Medical Center.

“What we have here is a combination of these two compounds that are specifically sourced – the gut-directed curcumin, which we developed, and the specifically-sourced QD, and we use them in a specific protocol with a formulation suitable for moderate to severe disease,” he said in an interview.
Mr. Salomon and colleagues in Israel and in Athens, Greece, tested CurQD in a two-part trial. The first part was a 4-week open-label study of CurQD in 10 patients with active UC defined by a Simple Clinical Colitis Activity Index (SCCAI) score of 5 or greater and a modified Mayo endoscopic subscore of 2 or greater.
Part 2 was the placebo-controlled trial described before, with 42 patients with active UC. For 49% of these patients immunomodulatory and/or biologic therapies had failed to induce or maintain remissions.
A total of 43% of patients assigned to CurQD met the primary combined endpoint of a reduction in SCCAI of at least 3 points and objective evidence of response, consisting of either a Mayo endoscopic subscore improvement of 1 or greater, or at least 50% reduction in calprotectin.
In all, 85.7% of patients assigned to CurQD had a clinical response, compared with 30.7% of those assigned to placebo (P < .001).
In addition, 75% of patients on CurQD had endoscopic improvement, compared with 20% on placebo (P = .036), and more patients on the combined supplement had at least 50% reductions in calprotectin levels (46.4% vs. 15.4%, respectively), although the difference did not reach statistical significance.
Patients randomized to CurQD had significantly better resolution of rectal bleeding by day 12 (P value not shown).
Eight additional weeks of maintenance on curcumin alone resulted in 93% retention at week 16 of clinical response, 80% retention of remissions, and 40% maintenance of clinical biomarker responses.
CurQD, but not placebo, was associated with activation of the aryl-hydrocarbon receptor (AhR) pathway. AhR is a nuclear receptor that has been implicated as a mediator of inflammatory bowel disease.
“Induction of AhR merits further study as [a] potential treatment target in active UC,” the investigators wrote.
Small molecule
“This is a very promising and nicely conducted trial. Previously there are separate trials both determining potential mechanisms of action as well as efficacy of curcumin and Qing Dai separately in this population. This is a nice study that uses the combination in patients with mild to moderate UC,” said Ashwin N. Ananthakrishnan, MBBS, MPH, a gastroenterology physician and researcher at Massachusetts General Hospital in Boston.
“Immunosuppressive treatments are very effective in our patients with IBD but there remains concern (particularly for patients) about the consequences of immunosuppression including risk of treatment associated cancer. Thus, there is a lot of interest in rigorous studies of nonimmunosuppressive treatments that may still be effective in relieving objective inflammation (apart from just symptomatic improvement). This study provides a nice evidence base for that. There remain multiple limitations including small sample size, potential generalizability to other populations, and importantly whether the efficacy is driven by curcumin or Qing Dai,” he said in reply to a request for independent commentary.
Dr. Ananthakrishnan was not involved in the study.
“This is great work! We are also studying Qing Dai/indigo naturalis and have developed a single small molecule that works similarly to this therapy,” Matt Davidson, PhD, of Azora Therapeutics in Encino, Calif., said in an online chat section of the meeting website.
In a separate scientific poster presented at the meeting, Dr. Davidson and Julie Saiki, PhD, also from Azora, reported that their company is developing a novel synthetic small molecule prodrug of indirubin, an AhR agonist derived from indigo that is purported to maximize colonic exposure while minimizing systemic exposure.
In mouse models of colitis, oral administration of the prodrug significantly reduced Disease Activity Index and weight loss similar in magnitude to the active compound indirubin, they reported.
The study was supported by Sheba Medical Center. Mr. Salomon disclosed speaking fees from various companies and has received consulting fees and has an equity position in EvNature, the manufacturer of CurQD. Dr. Ananthakrishnan reported having no disclosures relative to the study. Dr. Davidson is CEO and cofounder of Avora Therapeutics.
AT THE CROHN’S & COLITIS CONGRESS
Adult stem cells can heal intractable perianal Crohn’s fistulae
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AURORA, COLO. – Perianal Crohn’s disease with fistula is notoriously difficult to treat and can make patients’ lives miserable, but a new, minimally invasive approach involving local injection of mesenchymal stem cells is both safe and, in a significant proportion of patients, highly effective, according to a colorectal surgeon.
“It’s a really debilitating phenotype, a spectrum of phenotypes,” Amy Lightner, MD, of the Cleveland Clinic said at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association.
Although some patients have minimal symptoms, others may require multiple setons to aid in drainage and healing, while others may require fistulotomy, endorectal advancement flap, intersphincteric fistula tract (LIFT) procedure, diversion, or proctectomy.
“Why is it so difficult to treat? Well, part of it is that this is an anatomic defect, and this is why 90% of patients will come to the operating room and will see their surgeons on a frequent basis. The other part of that is that we have medical therapies to treat these fistulas but they’re really largely ineffective, because there is that anatomical defect, the hole there that needs to be closed,” Dr. Lightner said.
Up to 20% of patients may require a permanent stoma, and an additional 20% may require temporary fecal diversion.
Mesenchymal stem cells (MSC) are derived from bone marrow, fat stores, or umbilical cord tissues. Unlike embryonic stem cells, which have the ability to metamorphose into a multitude of other cell types, mesenchymal stem cells are differentiated “adult” cells.
They work by secreting anti-inflammatory cytokines and recruiting immune cells to stimulate tissue repair and healing. The cells are delivered in a minimally invasive outpatient setting, and there is no risk of incontinence compared with more invasive procedures such as fistulotomy or advancement flaps.
Effective and safe
MSCs were first used in Spain in 2003 to successfully treat a young women with a complex fistula with five perianal tracts converging into a rectovaginal fistula. The investigators injected a single dose of 9 x 106 MSCs into the site, and the fistula healed within 3 months.
Since then in multiple clinical trials involving more than 400 patients, injection of MSCs has resulted in fistula closure and complete healing by 8-12 weeks in 50%-85% of patients, Dr. Lightner said.
The treatment effect is also durable, she said, pointing to data from the ADMIRE-CD study, in which 51.5% of Crohn’s disease patients with treatment-refractory complex perianal fistula were healed at 24 weeks following injection of adipose-derived stem cells, compared with 35.6% of controls. At 1 year of follow-up, respective rates of healing were 56.3% vs. 38.6%.
Dr. Lightner also cited a case report of a patient whose fistula remained healed 4 years after receiving MSCs for refractory perianal Crohn’s fistulas.
Although MSCs are derived from healthy donors, they do not bear cellular surface antigens that would instigate a destructive host immune response, and to date, there have been no reports from clinical trials of systemic infections or complications. The most frequently reported adverse events have been injection-site pain in about 12%-15% of patients, and perianal abscess in 5%-13%, with similar frequencies in treatment and control groups.
Dr. Lightner and colleagues are currently exploring additional indications for stem cell therapy with MSCs, including other complex fistula phenotypes, intestinal Crohn’s disease, and ulcerative colitis.
Other approaches
In a separate presentation, James D. Lewis, MD, MSCE, of the University of Pennsylvania in Philadelphia talked about what would be needed to achieve a “medical moonshot” with the goal of curing inflammatory bowel disease (IBD), and touched on hematopoietic stem cell transplants as a potential option for patients with chronic, severe, and intractable disease.
One of his patients was a woman in her 60s who was diagnosed with stricturing and penetrating Crohn’s disease in her 30s, with the disease involving the ileum and entire colon. She had previously undergone three small bowel resections and a partial colon resection, and had never experienced remission despite taking steroids, azathioprine, methotrexate, four anti-TNF drugs, ustekinumab (Stelara), and vedolizumab (Entyvio).
Following an autologous hematopoietic stem cell transplant, she had a Simple Endoscopic Score for Crohn’s Disease (SES-CD) of 0. Her course was complicated by demand ischemia and acute kidney injury.
An IBD specialist who was not involved in either study commented in an interview that both MSCs and stem cell transplants show promise for treatment-refractory IBD,
“Both approaches are very promising, but stem cell transplants for IBD haven’t been formally studied yet so the data aren’t as strong, but there is promise for the future,” said Berkeley N. Limketkai, MD, PhD, from the University of California, Los Angeles.
“The challenges, however, are also the morbidity associated with actually undergoing such procedures,” he continued. Short- and long-term morbidities associated with hematopoietic stem cell transplants may include mucositis; hemorrhagic cystitis; prolonged, severe pancytopenia; infection; graft-versus-host disease; graft failure; pulmonary complications, veno-occlusive disease of the liver; and thrombotic microangiopathy.
Dr. Limketkai said that over time as the protocols for stem cell transplants in IBD improve, the benefits for select patients may more clearly outweigh the risks.
Dr. Lightner’s work is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the American Society of Colon and Rectal Surgery. She disclosed consulting fees from Boomerang Medical, Mesoblast Limited, Ossium Health, and Takeda Pharmaceuticals USA. Dr. Lewis’ work is supported by grants from the National Institutes of Health, and from AbbVie, Takeda, Janssen, and Nestlé Health Science. He has also served as a consultant to and data safety monitoring board member for several entities. Dr. Limketkai disclosed consulting for Azora Therapeutics.
AT CROHN’S & COLITIS CONGRESS