User login
Noteworthy advances in treatment and management of IBD
Although it had been thought that incidence rates of IBD were plateauing in high-incidence areas, a Danish study found a steady increase in incidence of Crohn’s disease and ulcerative colitis (UC).1 The highest increase in rates occurred in children and young adults, which will have repercussions as people get older and contribute to higher compounding prevalence. We need to get better at dealing with other health conditions as patients get older. A very large prospective Spanish study found that 42% of IBD patients scanned consecutively had MAFLD (metabolic-associated fatty liver disease) – even if they didn’t have high BMI and type 2 diabetes, suggesting that systemic inflammation in IBD contributes to the development of metabolic liver disease.2
The AGA has recently published guidelines for using biomarkers in the management of UC. Patients with very low fecal calprotectin (FCP) are unlikely to have active disease whereas FCP over 150 with significant symptoms may warrant empiric changes in treatment.3
Intestinal ultrasound is gaining wider acceptance as a noninvasive way to monitor IBD.4 In a UC study, improvement in bowel wall thickness following tofacitinib treatment correlated well with endoscopic activity.5
The majority of the presentation focused on the explosion of Food and Drug Administration–-approved medications for IBD in recent years. S1P receptor agonists, such as ozanimod and etrasimod, may work by trapping specific T-cell subsets in peripheral lymph nodes, preventing migration to intestinal tissues. Ozanimod is approved for UC. Etrasimod showed efficacy in UC with clinical remission rates of about 27% at week 12 and 32% at week 52.6,7
There has been a lot of excitement about JAK inhibitors for IBD. Upadacitinib has recently been approved for both UC and Crohn’s disease. Response rates of 73% and remission rates of 26% were seen in UC patients who had been largely biologic exposed.8 Similar results were seen in a biologic-exposed Crohn’s disease population treated with upadacitinib including in endoscopy.9 Upadacitinib was effective in maintaining remission at both 15-mg and 30-mg doses; but the higher dose had a greater effect on endoscopic endpoints.10
For Crohn’s disease, we now have risankizumab, an anti-p19/IL-23 inhibitor. Risankizumab was efficacious at inducing and maintain remission in the pivotal phase 3 studies, even with 75% of patients being biologic exposed. These studies used combined endpoints of clinical remission as well as endoscopic response.11 Guselkumab (anti-p19/IL-23) is also being studied for Crohn’s disease and early trials has appears to be efficacious.12
A head-to-head study of naive CD patients treated with ustekinumab or adalimumab (SEAVUE) showed comparable rates of clinical remission. At 52 weeks, the rates of clinical remission were quite high: >60% and endoscopic remission >30% with either therapy.13
We now have phase 3 data showing that a biologic is efficacious in patients with chronic pouchitis. The EARNEST trial demonstrated that vedolizumab has efficacy in treating pouchitis with improved clinical symptoms and endoscopy.14 Future treatment strategies may involve combinations of biologic therapies. The VEGA study showed that combining an anti-TNF, golimumab, with an anti-IL23, guselkumab, was superior than either alone with respect to clinical remission and endoscopic improvement in UC.15 We will see more studies combining therapies with diverse mechanisms of action.
In summary, there have been many noteworthy advances in treatment and management of IBD in the past year.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
Dr. Abreu is director of the Crohn’s and Colitis Center and professor of medicine, microbiology, and immunology at the University of Miami. She is president-elect of AGA. Dr. Allegretti is director of the Crohn’s and Colitis Center and director of the fecal microbiota transplant program at Brigham and Women’s Hospital, Boston. She is associate professor of medicine at Harvard Medical School, Boston. Dr. Loftus is the Maxine and Jack Zarrow Family Professor of Gastroenterology, codirector of the advanced IBD fellowship in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Ungaro is associate professor of medicine at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Agrawal M et al. Gastroenterology. 2022;163(6):1547-54.e5.
2. Rodriguez-Duque JC et al. Clin Gastroenterol Hepatol. 2023;21(2):406-14.e7.
3. Singh S, et al. Gastroenterology. 2023;164(3):344-72.
4. de Voogd F et al. Gastroenterology. 2022;163(6):1569-81.
5. Sandborn WJ et al. N Engl J Med. 2017;376(18):1723-36.
6. Sandborn WJ et al. N Engl J Med. 2021;385(14):1280-91.
7. Sandborn WJ et al. Lancet. 2023 Mar 25;401(10381):1000]. Lancet. 2023;401(10383):1159-71.
8. Danese S et al. Lancet. 2022 Sep 24;400(10357):996]. Lancet. 2022;399(10341):2113-28.
9. Loftus EV Jr et al. N Engl J Med. 2023 May 25;388(21):1966-80.
10. Panes J et al. Am J Gastroenterol 2022;117(S10). Abstract S37.
11. D’Haens G, et al. Lancet. 2022;399(10340):2015-30
12. Sandborn WJ et al. Gastroenterology. 2022;162(6):1650-64.e8.
13. Sands BE, et al. Lancet. 2022;399(10342):2200-11.
14. Travis S et al. N Engl J Med. 2023;388(13):1191-1200.
15. Feagan BG et al. Lancet Gastroenterol Hepatol. 2023;8(4):307-20.
Although it had been thought that incidence rates of IBD were plateauing in high-incidence areas, a Danish study found a steady increase in incidence of Crohn’s disease and ulcerative colitis (UC).1 The highest increase in rates occurred in children and young adults, which will have repercussions as people get older and contribute to higher compounding prevalence. We need to get better at dealing with other health conditions as patients get older. A very large prospective Spanish study found that 42% of IBD patients scanned consecutively had MAFLD (metabolic-associated fatty liver disease) – even if they didn’t have high BMI and type 2 diabetes, suggesting that systemic inflammation in IBD contributes to the development of metabolic liver disease.2
The AGA has recently published guidelines for using biomarkers in the management of UC. Patients with very low fecal calprotectin (FCP) are unlikely to have active disease whereas FCP over 150 with significant symptoms may warrant empiric changes in treatment.3
Intestinal ultrasound is gaining wider acceptance as a noninvasive way to monitor IBD.4 In a UC study, improvement in bowel wall thickness following tofacitinib treatment correlated well with endoscopic activity.5
The majority of the presentation focused on the explosion of Food and Drug Administration–-approved medications for IBD in recent years. S1P receptor agonists, such as ozanimod and etrasimod, may work by trapping specific T-cell subsets in peripheral lymph nodes, preventing migration to intestinal tissues. Ozanimod is approved for UC. Etrasimod showed efficacy in UC with clinical remission rates of about 27% at week 12 and 32% at week 52.6,7
There has been a lot of excitement about JAK inhibitors for IBD. Upadacitinib has recently been approved for both UC and Crohn’s disease. Response rates of 73% and remission rates of 26% were seen in UC patients who had been largely biologic exposed.8 Similar results were seen in a biologic-exposed Crohn’s disease population treated with upadacitinib including in endoscopy.9 Upadacitinib was effective in maintaining remission at both 15-mg and 30-mg doses; but the higher dose had a greater effect on endoscopic endpoints.10
For Crohn’s disease, we now have risankizumab, an anti-p19/IL-23 inhibitor. Risankizumab was efficacious at inducing and maintain remission in the pivotal phase 3 studies, even with 75% of patients being biologic exposed. These studies used combined endpoints of clinical remission as well as endoscopic response.11 Guselkumab (anti-p19/IL-23) is also being studied for Crohn’s disease and early trials has appears to be efficacious.12
A head-to-head study of naive CD patients treated with ustekinumab or adalimumab (SEAVUE) showed comparable rates of clinical remission. At 52 weeks, the rates of clinical remission were quite high: >60% and endoscopic remission >30% with either therapy.13
We now have phase 3 data showing that a biologic is efficacious in patients with chronic pouchitis. The EARNEST trial demonstrated that vedolizumab has efficacy in treating pouchitis with improved clinical symptoms and endoscopy.14 Future treatment strategies may involve combinations of biologic therapies. The VEGA study showed that combining an anti-TNF, golimumab, with an anti-IL23, guselkumab, was superior than either alone with respect to clinical remission and endoscopic improvement in UC.15 We will see more studies combining therapies with diverse mechanisms of action.
In summary, there have been many noteworthy advances in treatment and management of IBD in the past year.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
Dr. Abreu is director of the Crohn’s and Colitis Center and professor of medicine, microbiology, and immunology at the University of Miami. She is president-elect of AGA. Dr. Allegretti is director of the Crohn’s and Colitis Center and director of the fecal microbiota transplant program at Brigham and Women’s Hospital, Boston. She is associate professor of medicine at Harvard Medical School, Boston. Dr. Loftus is the Maxine and Jack Zarrow Family Professor of Gastroenterology, codirector of the advanced IBD fellowship in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Ungaro is associate professor of medicine at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Agrawal M et al. Gastroenterology. 2022;163(6):1547-54.e5.
2. Rodriguez-Duque JC et al. Clin Gastroenterol Hepatol. 2023;21(2):406-14.e7.
3. Singh S, et al. Gastroenterology. 2023;164(3):344-72.
4. de Voogd F et al. Gastroenterology. 2022;163(6):1569-81.
5. Sandborn WJ et al. N Engl J Med. 2017;376(18):1723-36.
6. Sandborn WJ et al. N Engl J Med. 2021;385(14):1280-91.
7. Sandborn WJ et al. Lancet. 2023 Mar 25;401(10381):1000]. Lancet. 2023;401(10383):1159-71.
8. Danese S et al. Lancet. 2022 Sep 24;400(10357):996]. Lancet. 2022;399(10341):2113-28.
9. Loftus EV Jr et al. N Engl J Med. 2023 May 25;388(21):1966-80.
10. Panes J et al. Am J Gastroenterol 2022;117(S10). Abstract S37.
11. D’Haens G, et al. Lancet. 2022;399(10340):2015-30
12. Sandborn WJ et al. Gastroenterology. 2022;162(6):1650-64.e8.
13. Sands BE, et al. Lancet. 2022;399(10342):2200-11.
14. Travis S et al. N Engl J Med. 2023;388(13):1191-1200.
15. Feagan BG et al. Lancet Gastroenterol Hepatol. 2023;8(4):307-20.
Although it had been thought that incidence rates of IBD were plateauing in high-incidence areas, a Danish study found a steady increase in incidence of Crohn’s disease and ulcerative colitis (UC).1 The highest increase in rates occurred in children and young adults, which will have repercussions as people get older and contribute to higher compounding prevalence. We need to get better at dealing with other health conditions as patients get older. A very large prospective Spanish study found that 42% of IBD patients scanned consecutively had MAFLD (metabolic-associated fatty liver disease) – even if they didn’t have high BMI and type 2 diabetes, suggesting that systemic inflammation in IBD contributes to the development of metabolic liver disease.2
The AGA has recently published guidelines for using biomarkers in the management of UC. Patients with very low fecal calprotectin (FCP) are unlikely to have active disease whereas FCP over 150 with significant symptoms may warrant empiric changes in treatment.3
Intestinal ultrasound is gaining wider acceptance as a noninvasive way to monitor IBD.4 In a UC study, improvement in bowel wall thickness following tofacitinib treatment correlated well with endoscopic activity.5
The majority of the presentation focused on the explosion of Food and Drug Administration–-approved medications for IBD in recent years. S1P receptor agonists, such as ozanimod and etrasimod, may work by trapping specific T-cell subsets in peripheral lymph nodes, preventing migration to intestinal tissues. Ozanimod is approved for UC. Etrasimod showed efficacy in UC with clinical remission rates of about 27% at week 12 and 32% at week 52.6,7
There has been a lot of excitement about JAK inhibitors for IBD. Upadacitinib has recently been approved for both UC and Crohn’s disease. Response rates of 73% and remission rates of 26% were seen in UC patients who had been largely biologic exposed.8 Similar results were seen in a biologic-exposed Crohn’s disease population treated with upadacitinib including in endoscopy.9 Upadacitinib was effective in maintaining remission at both 15-mg and 30-mg doses; but the higher dose had a greater effect on endoscopic endpoints.10
For Crohn’s disease, we now have risankizumab, an anti-p19/IL-23 inhibitor. Risankizumab was efficacious at inducing and maintain remission in the pivotal phase 3 studies, even with 75% of patients being biologic exposed. These studies used combined endpoints of clinical remission as well as endoscopic response.11 Guselkumab (anti-p19/IL-23) is also being studied for Crohn’s disease and early trials has appears to be efficacious.12
A head-to-head study of naive CD patients treated with ustekinumab or adalimumab (SEAVUE) showed comparable rates of clinical remission. At 52 weeks, the rates of clinical remission were quite high: >60% and endoscopic remission >30% with either therapy.13
We now have phase 3 data showing that a biologic is efficacious in patients with chronic pouchitis. The EARNEST trial demonstrated that vedolizumab has efficacy in treating pouchitis with improved clinical symptoms and endoscopy.14 Future treatment strategies may involve combinations of biologic therapies. The VEGA study showed that combining an anti-TNF, golimumab, with an anti-IL23, guselkumab, was superior than either alone with respect to clinical remission and endoscopic improvement in UC.15 We will see more studies combining therapies with diverse mechanisms of action.
In summary, there have been many noteworthy advances in treatment and management of IBD in the past year.
DDW is sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA), the American Society for Gastrointestinal Endoscopy (ASGE) and The Society for Surgery of the Alimentary Tract (SSAT).
Dr. Abreu is director of the Crohn’s and Colitis Center and professor of medicine, microbiology, and immunology at the University of Miami. She is president-elect of AGA. Dr. Allegretti is director of the Crohn’s and Colitis Center and director of the fecal microbiota transplant program at Brigham and Women’s Hospital, Boston. She is associate professor of medicine at Harvard Medical School, Boston. Dr. Loftus is the Maxine and Jack Zarrow Family Professor of Gastroenterology, codirector of the advanced IBD fellowship in the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn. Dr. Ungaro is associate professor of medicine at the Icahn School of Medicine at Mount Sinai, New York.
References
1. Agrawal M et al. Gastroenterology. 2022;163(6):1547-54.e5.
2. Rodriguez-Duque JC et al. Clin Gastroenterol Hepatol. 2023;21(2):406-14.e7.
3. Singh S, et al. Gastroenterology. 2023;164(3):344-72.
4. de Voogd F et al. Gastroenterology. 2022;163(6):1569-81.
5. Sandborn WJ et al. N Engl J Med. 2017;376(18):1723-36.
6. Sandborn WJ et al. N Engl J Med. 2021;385(14):1280-91.
7. Sandborn WJ et al. Lancet. 2023 Mar 25;401(10381):1000]. Lancet. 2023;401(10383):1159-71.
8. Danese S et al. Lancet. 2022 Sep 24;400(10357):996]. Lancet. 2022;399(10341):2113-28.
9. Loftus EV Jr et al. N Engl J Med. 2023 May 25;388(21):1966-80.
10. Panes J et al. Am J Gastroenterol 2022;117(S10). Abstract S37.
11. D’Haens G, et al. Lancet. 2022;399(10340):2015-30
12. Sandborn WJ et al. Gastroenterology. 2022;162(6):1650-64.e8.
13. Sands BE, et al. Lancet. 2022;399(10342):2200-11.
14. Travis S et al. N Engl J Med. 2023;388(13):1191-1200.
15. Feagan BG et al. Lancet Gastroenterol Hepatol. 2023;8(4):307-20.
AT DDW 2023
Defining difficult-to-treat inflammatory bowel disease
Up until now, one major obstacle has impeded our interpretation of studies focusing on patients suffering from this chronic condition: the lack of standard criteria and terminology among authors.
Under the guidance of the endpoints cluster of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD), a group of experts held a consensus meeting to propose a common operative definition for “difficult-to-treat IBD.” It’s the first step to better understanding this condition and designing targeted studies and interventions.
The definition
After the meeting, the experts agreed that “difficult-to-treat IBD” is defined by these characteristics:
- The failure of biologics and advanced small molecules with at least two different mechanisms of action.
- Postoperative recurrence of Crohn’s disease after two surgical resections in adults or one in children.
- Chronic antibiotic-refractory pouchitis (inflammation of the ileal pouch-anal anastomosis [J-pouch] created in patients with ulcerative colitis who have had total colectomy surgery).
- Complex perianal disease (difficult-to-treat Crohn’s disease).
- Comorbid psychosocial complications that impair disease management (for example, comorbid disorders that obstruct treatment compliance, participation in follow-up visits, or objective assessment of symptoms by clinicians).
The path here
The starting point was the IOIBD-sponsored 2022 global survey in which doctors treating patients with IBD were asked what they thought contributed to difficult-to-treat IBD. Using the responses from that survey, a series of statements were drawn up covering these three main areas: failure of medical and surgical treatments, disease phenotypes, and specific complaints from patients (not limited to bowel disease).
The statements were scrutinized by a 16-person task force made up of experts from eight European countries, Canada, Japan, Israel, and the United States. The project and its findings were published in the journal The Lancet Gastroenterology & Hepatology.
Using the modified Delphi technique, the experts argued for or against the 20 statements proposed. Consensus was achieved for five of these statements (meaning that at least 75% of voters were in agreement).
What does it mean?
“The scope of this consensus initiative was twofold,” explain the authors. “First, we wanted to help standardize study reporting and promote clinical study designs that include patients with difficult-to-treat IBD by proposing common terminology. Second, we hoped to identify, within clinical practice, a group of patients requiring specific treatment or referral to a specialist unit. For patients with conditions resistant to two or more advanced drug types (what is referred to as difficult-to-treat IBD), more aggressive treatment strategies, such as combined therapies or multidisciplinary approaches, should be taken into consideration.
“In the field of rheumatology, the creation of common criteria for difficult-to-treat rheumatoid arthritis has allowed researchers to concentrate their efforts on identifying progressive disease markers, assessing drug efficacy, mechanisms of inefficacy, personalized management strategies, and analyzing the use of health care resources and costs. Similar advances could be achieved in the area of inflammatory bowel disease.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
Up until now, one major obstacle has impeded our interpretation of studies focusing on patients suffering from this chronic condition: the lack of standard criteria and terminology among authors.
Under the guidance of the endpoints cluster of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD), a group of experts held a consensus meeting to propose a common operative definition for “difficult-to-treat IBD.” It’s the first step to better understanding this condition and designing targeted studies and interventions.
The definition
After the meeting, the experts agreed that “difficult-to-treat IBD” is defined by these characteristics:
- The failure of biologics and advanced small molecules with at least two different mechanisms of action.
- Postoperative recurrence of Crohn’s disease after two surgical resections in adults or one in children.
- Chronic antibiotic-refractory pouchitis (inflammation of the ileal pouch-anal anastomosis [J-pouch] created in patients with ulcerative colitis who have had total colectomy surgery).
- Complex perianal disease (difficult-to-treat Crohn’s disease).
- Comorbid psychosocial complications that impair disease management (for example, comorbid disorders that obstruct treatment compliance, participation in follow-up visits, or objective assessment of symptoms by clinicians).
The path here
The starting point was the IOIBD-sponsored 2022 global survey in which doctors treating patients with IBD were asked what they thought contributed to difficult-to-treat IBD. Using the responses from that survey, a series of statements were drawn up covering these three main areas: failure of medical and surgical treatments, disease phenotypes, and specific complaints from patients (not limited to bowel disease).
The statements were scrutinized by a 16-person task force made up of experts from eight European countries, Canada, Japan, Israel, and the United States. The project and its findings were published in the journal The Lancet Gastroenterology & Hepatology.
Using the modified Delphi technique, the experts argued for or against the 20 statements proposed. Consensus was achieved for five of these statements (meaning that at least 75% of voters were in agreement).
What does it mean?
“The scope of this consensus initiative was twofold,” explain the authors. “First, we wanted to help standardize study reporting and promote clinical study designs that include patients with difficult-to-treat IBD by proposing common terminology. Second, we hoped to identify, within clinical practice, a group of patients requiring specific treatment or referral to a specialist unit. For patients with conditions resistant to two or more advanced drug types (what is referred to as difficult-to-treat IBD), more aggressive treatment strategies, such as combined therapies or multidisciplinary approaches, should be taken into consideration.
“In the field of rheumatology, the creation of common criteria for difficult-to-treat rheumatoid arthritis has allowed researchers to concentrate their efforts on identifying progressive disease markers, assessing drug efficacy, mechanisms of inefficacy, personalized management strategies, and analyzing the use of health care resources and costs. Similar advances could be achieved in the area of inflammatory bowel disease.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
Up until now, one major obstacle has impeded our interpretation of studies focusing on patients suffering from this chronic condition: the lack of standard criteria and terminology among authors.
Under the guidance of the endpoints cluster of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD), a group of experts held a consensus meeting to propose a common operative definition for “difficult-to-treat IBD.” It’s the first step to better understanding this condition and designing targeted studies and interventions.
The definition
After the meeting, the experts agreed that “difficult-to-treat IBD” is defined by these characteristics:
- The failure of biologics and advanced small molecules with at least two different mechanisms of action.
- Postoperative recurrence of Crohn’s disease after two surgical resections in adults or one in children.
- Chronic antibiotic-refractory pouchitis (inflammation of the ileal pouch-anal anastomosis [J-pouch] created in patients with ulcerative colitis who have had total colectomy surgery).
- Complex perianal disease (difficult-to-treat Crohn’s disease).
- Comorbid psychosocial complications that impair disease management (for example, comorbid disorders that obstruct treatment compliance, participation in follow-up visits, or objective assessment of symptoms by clinicians).
The path here
The starting point was the IOIBD-sponsored 2022 global survey in which doctors treating patients with IBD were asked what they thought contributed to difficult-to-treat IBD. Using the responses from that survey, a series of statements were drawn up covering these three main areas: failure of medical and surgical treatments, disease phenotypes, and specific complaints from patients (not limited to bowel disease).
The statements were scrutinized by a 16-person task force made up of experts from eight European countries, Canada, Japan, Israel, and the United States. The project and its findings were published in the journal The Lancet Gastroenterology & Hepatology.
Using the modified Delphi technique, the experts argued for or against the 20 statements proposed. Consensus was achieved for five of these statements (meaning that at least 75% of voters were in agreement).
What does it mean?
“The scope of this consensus initiative was twofold,” explain the authors. “First, we wanted to help standardize study reporting and promote clinical study designs that include patients with difficult-to-treat IBD by proposing common terminology. Second, we hoped to identify, within clinical practice, a group of patients requiring specific treatment or referral to a specialist unit. For patients with conditions resistant to two or more advanced drug types (what is referred to as difficult-to-treat IBD), more aggressive treatment strategies, such as combined therapies or multidisciplinary approaches, should be taken into consideration.
“In the field of rheumatology, the creation of common criteria for difficult-to-treat rheumatoid arthritis has allowed researchers to concentrate their efforts on identifying progressive disease markers, assessing drug efficacy, mechanisms of inefficacy, personalized management strategies, and analyzing the use of health care resources and costs. Similar advances could be achieved in the area of inflammatory bowel disease.”
This article was translated from Univadis Italy. A version appeared on Medscape.com.
Nearly 1 in 100 people diagnosed with IBD in the U.S.
(IBD), and up to 56,000 new cases are diagnosed each year.
“The prevalence of IBD in the United States has been gradually increasing over the last decade, and thus the burden of caring for IBD is likely to increase as life expectancy increases,” said co-principal investigator Andrés Hurtado-Lorenzo, PhD, senior vice president, Translational Research and IBD Ventures, Crohn’s & Colitis Foundation.
These data provide “an initial step toward optimizing health care resources allocation and improving care of individuals with IBD,” said Manasi Agrawal, MD, a gastroenterologist at Mount Sinai Hospital, New York, who wasn’t involved in the study.
The study was published online in Gastroenterology.
For the federally funded study, researchers pooled data from commercial, Medicare, and Medicaid insurance plans to derive a population-based estimate of the incidence and prevalence of IBD throughout the United States.
“In essence, we consider this to be the most extensive study of the incidence and prevalence of IBD in the United States based on physician-diagnosed IBD, which is representative of nearly the entire U.S. population with health insurance,” Dr. Hurtado-Lorenzo said.
Trends identified
Key findings from the study include the following.
- The age- and sex-standardized incidence of IBD was 10.9 per 100,000 person years.
- The incidence of IBD peaks in the third decade of life, decreases to a relatively stable level across the fourth to eighth decades, and declines further beyond age 80.
- Ulcerative colitis is slightly more common than Crohn’s disease in most age groups, except in children, among whom this trend is reversed.
- The adjusted prevalence data show that IBD has been diagnosed in more than 0.7% of Americans, with 721 cases per 100,000, or nearly 1 in 100.
- Historically, IBD was slightly more common in men. Now it’s slightly more common in adult women and male children.
- IBD prevalence is highest in the Northeast and lowest in the western region of the United States.
- The overall prevalence of IBD increased gradually from 2011 to 2020.
“Environmental variables, such as ultra-processed foods, pollution, and urbanization, to name a few, are implicated in IBD risk. Shifts in our modern environment and improving diagnostics may be two reasons why we see rising trends in IBD,” said Dr. Agrawal, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai.
Prevalence highest among Whites
The data also point to significant differences in prevalence among different racial groups in the United States, with Whites having a rate of IBD that is seven times higher than Blacks, six times higher than Hispanics, and 21 times higher than Asians.
The prevalence of IBD per 100,000 population was 812 in Whites, 504 in Blacks, 403 in Asians, and 458 in Hispanics.
“It’s important to note that the reasons for ethnic disparities in IBD prevalence are complex and multifactorial, and further research is needed to better understand the specific mechanisms underlying these disparities,” said Dr. Hurtado-Lorenzo said.
Factors that could contribute to this disparity include genetic and environmental factors, socioeconomic factors, health care disparities, differences in disease awareness and reporting, and underdiagnosis in some populations.
The data suggest a lower prevalence of IBD among children with Medicaid insurance, “which underscores the need for further investigation into the influence of social determinants of health on IBD care,” Dr. Hurtado-Lorenzo said.
Insights important for planning
Because of the fragmented nature of the health care system, it’s been challenging to get an accurate estimate of how many patients in the United States have IBD, said Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, Boston.
“The authors and involved organizations are to be fully complemented on this really ambitious and important study. Having an idea of how common IBD is and how it is likely to increase in prevalence is important for resource planning for organizations and health care systems,” said Dr. Ananthakrishnan, who was not involved in the study.
Although IBD incidence and prevalence is lower in non-White populations, there is still a “sizeable burden of IBD in those groups, and it’s important to understand the implications of that in terms of disease biology, treatment availability, disparities, and access to care,” he added.
“With the aging of the population and increasing prevalence, it is also important to understand that the ‘face of IBD’ in the coming decades may be different than what we traditionally have estimated it to be. This is also important to incorporate in decision-making,” Dr. Ananthakrishnan said.
Funding for the study was provided by the Centers for Disease Control and Prevention. Dr. Hurtado-Lorenzo, Dr. Agrawal, and Dr. Ananthakrishnan have declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
(IBD), and up to 56,000 new cases are diagnosed each year.
“The prevalence of IBD in the United States has been gradually increasing over the last decade, and thus the burden of caring for IBD is likely to increase as life expectancy increases,” said co-principal investigator Andrés Hurtado-Lorenzo, PhD, senior vice president, Translational Research and IBD Ventures, Crohn’s & Colitis Foundation.
These data provide “an initial step toward optimizing health care resources allocation and improving care of individuals with IBD,” said Manasi Agrawal, MD, a gastroenterologist at Mount Sinai Hospital, New York, who wasn’t involved in the study.
The study was published online in Gastroenterology.
For the federally funded study, researchers pooled data from commercial, Medicare, and Medicaid insurance plans to derive a population-based estimate of the incidence and prevalence of IBD throughout the United States.
“In essence, we consider this to be the most extensive study of the incidence and prevalence of IBD in the United States based on physician-diagnosed IBD, which is representative of nearly the entire U.S. population with health insurance,” Dr. Hurtado-Lorenzo said.
Trends identified
Key findings from the study include the following.
- The age- and sex-standardized incidence of IBD was 10.9 per 100,000 person years.
- The incidence of IBD peaks in the third decade of life, decreases to a relatively stable level across the fourth to eighth decades, and declines further beyond age 80.
- Ulcerative colitis is slightly more common than Crohn’s disease in most age groups, except in children, among whom this trend is reversed.
- The adjusted prevalence data show that IBD has been diagnosed in more than 0.7% of Americans, with 721 cases per 100,000, or nearly 1 in 100.
- Historically, IBD was slightly more common in men. Now it’s slightly more common in adult women and male children.
- IBD prevalence is highest in the Northeast and lowest in the western region of the United States.
- The overall prevalence of IBD increased gradually from 2011 to 2020.
“Environmental variables, such as ultra-processed foods, pollution, and urbanization, to name a few, are implicated in IBD risk. Shifts in our modern environment and improving diagnostics may be two reasons why we see rising trends in IBD,” said Dr. Agrawal, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai.
Prevalence highest among Whites
The data also point to significant differences in prevalence among different racial groups in the United States, with Whites having a rate of IBD that is seven times higher than Blacks, six times higher than Hispanics, and 21 times higher than Asians.
The prevalence of IBD per 100,000 population was 812 in Whites, 504 in Blacks, 403 in Asians, and 458 in Hispanics.
“It’s important to note that the reasons for ethnic disparities in IBD prevalence are complex and multifactorial, and further research is needed to better understand the specific mechanisms underlying these disparities,” said Dr. Hurtado-Lorenzo said.
Factors that could contribute to this disparity include genetic and environmental factors, socioeconomic factors, health care disparities, differences in disease awareness and reporting, and underdiagnosis in some populations.
The data suggest a lower prevalence of IBD among children with Medicaid insurance, “which underscores the need for further investigation into the influence of social determinants of health on IBD care,” Dr. Hurtado-Lorenzo said.
Insights important for planning
Because of the fragmented nature of the health care system, it’s been challenging to get an accurate estimate of how many patients in the United States have IBD, said Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, Boston.
“The authors and involved organizations are to be fully complemented on this really ambitious and important study. Having an idea of how common IBD is and how it is likely to increase in prevalence is important for resource planning for organizations and health care systems,” said Dr. Ananthakrishnan, who was not involved in the study.
Although IBD incidence and prevalence is lower in non-White populations, there is still a “sizeable burden of IBD in those groups, and it’s important to understand the implications of that in terms of disease biology, treatment availability, disparities, and access to care,” he added.
“With the aging of the population and increasing prevalence, it is also important to understand that the ‘face of IBD’ in the coming decades may be different than what we traditionally have estimated it to be. This is also important to incorporate in decision-making,” Dr. Ananthakrishnan said.
Funding for the study was provided by the Centers for Disease Control and Prevention. Dr. Hurtado-Lorenzo, Dr. Agrawal, and Dr. Ananthakrishnan have declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
(IBD), and up to 56,000 new cases are diagnosed each year.
“The prevalence of IBD in the United States has been gradually increasing over the last decade, and thus the burden of caring for IBD is likely to increase as life expectancy increases,” said co-principal investigator Andrés Hurtado-Lorenzo, PhD, senior vice president, Translational Research and IBD Ventures, Crohn’s & Colitis Foundation.
These data provide “an initial step toward optimizing health care resources allocation and improving care of individuals with IBD,” said Manasi Agrawal, MD, a gastroenterologist at Mount Sinai Hospital, New York, who wasn’t involved in the study.
The study was published online in Gastroenterology.
For the federally funded study, researchers pooled data from commercial, Medicare, and Medicaid insurance plans to derive a population-based estimate of the incidence and prevalence of IBD throughout the United States.
“In essence, we consider this to be the most extensive study of the incidence and prevalence of IBD in the United States based on physician-diagnosed IBD, which is representative of nearly the entire U.S. population with health insurance,” Dr. Hurtado-Lorenzo said.
Trends identified
Key findings from the study include the following.
- The age- and sex-standardized incidence of IBD was 10.9 per 100,000 person years.
- The incidence of IBD peaks in the third decade of life, decreases to a relatively stable level across the fourth to eighth decades, and declines further beyond age 80.
- Ulcerative colitis is slightly more common than Crohn’s disease in most age groups, except in children, among whom this trend is reversed.
- The adjusted prevalence data show that IBD has been diagnosed in more than 0.7% of Americans, with 721 cases per 100,000, or nearly 1 in 100.
- Historically, IBD was slightly more common in men. Now it’s slightly more common in adult women and male children.
- IBD prevalence is highest in the Northeast and lowest in the western region of the United States.
- The overall prevalence of IBD increased gradually from 2011 to 2020.
“Environmental variables, such as ultra-processed foods, pollution, and urbanization, to name a few, are implicated in IBD risk. Shifts in our modern environment and improving diagnostics may be two reasons why we see rising trends in IBD,” said Dr. Agrawal, assistant professor of medicine at the Icahn School of Medicine at Mount Sinai.
Prevalence highest among Whites
The data also point to significant differences in prevalence among different racial groups in the United States, with Whites having a rate of IBD that is seven times higher than Blacks, six times higher than Hispanics, and 21 times higher than Asians.
The prevalence of IBD per 100,000 population was 812 in Whites, 504 in Blacks, 403 in Asians, and 458 in Hispanics.
“It’s important to note that the reasons for ethnic disparities in IBD prevalence are complex and multifactorial, and further research is needed to better understand the specific mechanisms underlying these disparities,” said Dr. Hurtado-Lorenzo said.
Factors that could contribute to this disparity include genetic and environmental factors, socioeconomic factors, health care disparities, differences in disease awareness and reporting, and underdiagnosis in some populations.
The data suggest a lower prevalence of IBD among children with Medicaid insurance, “which underscores the need for further investigation into the influence of social determinants of health on IBD care,” Dr. Hurtado-Lorenzo said.
Insights important for planning
Because of the fragmented nature of the health care system, it’s been challenging to get an accurate estimate of how many patients in the United States have IBD, said Ashwin Ananthakrishnan, MD, MPH, a gastroenterologist with Massachusetts General Hospital and Harvard Medical School, Boston.
“The authors and involved organizations are to be fully complemented on this really ambitious and important study. Having an idea of how common IBD is and how it is likely to increase in prevalence is important for resource planning for organizations and health care systems,” said Dr. Ananthakrishnan, who was not involved in the study.
Although IBD incidence and prevalence is lower in non-White populations, there is still a “sizeable burden of IBD in those groups, and it’s important to understand the implications of that in terms of disease biology, treatment availability, disparities, and access to care,” he added.
“With the aging of the population and increasing prevalence, it is also important to understand that the ‘face of IBD’ in the coming decades may be different than what we traditionally have estimated it to be. This is also important to incorporate in decision-making,” Dr. Ananthakrishnan said.
Funding for the study was provided by the Centers for Disease Control and Prevention. Dr. Hurtado-Lorenzo, Dr. Agrawal, and Dr. Ananthakrishnan have declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
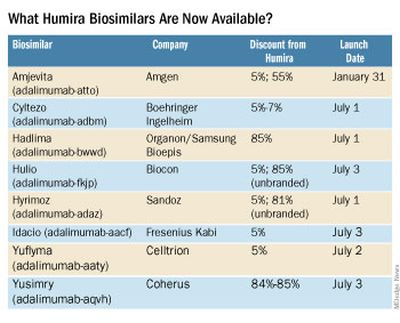
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Celiac disease: Update on diagnosis and monitoring
Celiac disease is a small bowel disorder. Specific antibodies along with a duodenal biopsy allow a secure diagnosis of celiac disease. Case detection rates have improved but many patients remain undiagnosed.
The only treatment available at present is a gluten-free diet (GFD). Most patients respond clinically to a GFD but histologic recovery is not always complete and may result in clinical consequences.
The anti-tissue transglutaminase IgA test (tTg-IgA) is the best initial serology test. A total IgA level appropriate for age is required to interpret a negative result. In patients with IgA deficiency, the deamidated gliadin peptide (DGP) antibodies, and/or tTg-IgA, may be helpful for diagnosis along with a duodenal biopsy.
First-degree female relatives with homozygous DQ2 positivity are at highest risk.
Both serology and duodenal biopsy have pitfalls in the diagnosis of celiac disease. In children, the diagnosis is secure with a tTg-IgA rate of at least 10 times the upper limit of normal (≥10×ULN) with positive endomysial antibodies (EMA).
There is less data on the correlation of tTg-IgA ≥10×ULN positive with villous atrophy in adults. All others require biopsy for diagnosis.
Considerations to forgo biopsy in adults include: tTg-IgA of ≥10×ULN positive, serology positive test in patients following GFD, or otherwise unable to undergo endoscopy with duodenal biopsy, or shared decision-making. Celiac disease recovery is assessed by clinical response to a GFD and antibody conversion to negative, which does not always correlate with histology.
Clinical consequences of persistent villous atrophy include increased risks for lymphoproliferative malignancy, hip fracture, and refractory celiac disease.
Dr. Semrad is director of the small bowel disease and nutrition program at the University of Chicago Medicine where she is a professor of medicine. She disclosed no conflicts of interest.
References
Rubio-Tapia et al. Am J Gastroenterol. 2023;118:59-76.
Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70:141-57.
Celiac disease is a small bowel disorder. Specific antibodies along with a duodenal biopsy allow a secure diagnosis of celiac disease. Case detection rates have improved but many patients remain undiagnosed.
The only treatment available at present is a gluten-free diet (GFD). Most patients respond clinically to a GFD but histologic recovery is not always complete and may result in clinical consequences.
The anti-tissue transglutaminase IgA test (tTg-IgA) is the best initial serology test. A total IgA level appropriate for age is required to interpret a negative result. In patients with IgA deficiency, the deamidated gliadin peptide (DGP) antibodies, and/or tTg-IgA, may be helpful for diagnosis along with a duodenal biopsy.
First-degree female relatives with homozygous DQ2 positivity are at highest risk.
Both serology and duodenal biopsy have pitfalls in the diagnosis of celiac disease. In children, the diagnosis is secure with a tTg-IgA rate of at least 10 times the upper limit of normal (≥10×ULN) with positive endomysial antibodies (EMA).
There is less data on the correlation of tTg-IgA ≥10×ULN positive with villous atrophy in adults. All others require biopsy for diagnosis.
Considerations to forgo biopsy in adults include: tTg-IgA of ≥10×ULN positive, serology positive test in patients following GFD, or otherwise unable to undergo endoscopy with duodenal biopsy, or shared decision-making. Celiac disease recovery is assessed by clinical response to a GFD and antibody conversion to negative, which does not always correlate with histology.
Clinical consequences of persistent villous atrophy include increased risks for lymphoproliferative malignancy, hip fracture, and refractory celiac disease.
Dr. Semrad is director of the small bowel disease and nutrition program at the University of Chicago Medicine where she is a professor of medicine. She disclosed no conflicts of interest.
References
Rubio-Tapia et al. Am J Gastroenterol. 2023;118:59-76.
Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70:141-57.
Celiac disease is a small bowel disorder. Specific antibodies along with a duodenal biopsy allow a secure diagnosis of celiac disease. Case detection rates have improved but many patients remain undiagnosed.
The only treatment available at present is a gluten-free diet (GFD). Most patients respond clinically to a GFD but histologic recovery is not always complete and may result in clinical consequences.
The anti-tissue transglutaminase IgA test (tTg-IgA) is the best initial serology test. A total IgA level appropriate for age is required to interpret a negative result. In patients with IgA deficiency, the deamidated gliadin peptide (DGP) antibodies, and/or tTg-IgA, may be helpful for diagnosis along with a duodenal biopsy.
First-degree female relatives with homozygous DQ2 positivity are at highest risk.
Both serology and duodenal biopsy have pitfalls in the diagnosis of celiac disease. In children, the diagnosis is secure with a tTg-IgA rate of at least 10 times the upper limit of normal (≥10×ULN) with positive endomysial antibodies (EMA).
There is less data on the correlation of tTg-IgA ≥10×ULN positive with villous atrophy in adults. All others require biopsy for diagnosis.
Considerations to forgo biopsy in adults include: tTg-IgA of ≥10×ULN positive, serology positive test in patients following GFD, or otherwise unable to undergo endoscopy with duodenal biopsy, or shared decision-making. Celiac disease recovery is assessed by clinical response to a GFD and antibody conversion to negative, which does not always correlate with histology.
Clinical consequences of persistent villous atrophy include increased risks for lymphoproliferative malignancy, hip fracture, and refractory celiac disease.
Dr. Semrad is director of the small bowel disease and nutrition program at the University of Chicago Medicine where she is a professor of medicine. She disclosed no conflicts of interest.
References
Rubio-Tapia et al. Am J Gastroenterol. 2023;118:59-76.
Husby S et al. J Pediatr Gastroenterol Nutr. 2020;70:141-57.
Biologics, thiopurines, or methotrexate doesn’t affect fertility or birth outcomes in men with IBD
published in Clinical Gastroenterology and Hepatology.
The effort is the first meta-analysis to assess semen parameters and the risk of adverse outcomes in pregnancy for male patients with IBD who have taken biologics, thiopurines or methotrexate for the condition, the researchers said.
“We provide encouraging evidence that biologic, thiopurine, and methotrexate therapy among male patients with IBD are not associated with impairments in male fertility or with increased risk of adverse pregnancy outcomes,” said the researchers, led in part by John Gubatan, MD, instructor in medicine at Stanford (Calif.) University, who worked with investigators in Copenhagen and Toronto. “Taken together, our data support the safety of continuing biologics, thiopurines, or methotrexate across the reproductive spectrum.”
Questions of fertility and pregnancy outcomes are of particular importance in IBD, since patients are often diagnosed around the time of their reproductive years – about 30 years old for Crohn’s disease and 35 years old for ulcerative colitis. There has been far more research attention paid to female than male reproductive considerations, mainly the health of the fetus when the mother takes biologic therapy for IBD during pregnancy, which has generally found to be safe.
Their search found 13 studies with male IBD patients exposed to biologics, 10 exposed to thiopurines and 6 to methotrexate. Researchers extracted data on sperm count, sperm motility, and abnormal sperm morphology – three metrics considered a proxy for male fertility – as well as early pregnancy loss, preterm birth and congenital malformations.
Researchers found no differences between sperm count, motility or morphology between those exposed and not exposed to biologics, thiopurines and methotrexate, with a couple of exceptions. They actually found that sperm count was higher for thiopurine users, compared with nonusers, and there was only one study on methotrexate and abnormal sperm morphology, so there was no data to pool together for that comparison.
In a subgroup analysis, there was a trend toward higher sperm count in thiopurine users, compared with biologic or methotrexate users, but no differences were seen in the other parameters.
Similarly, there were no significant differences for users and nonusers of these medications for early pregnancy loss, preterm births or congenital malformations, the researchers found.
A prior systematic review suggested that azathioprine might be associated with low sperm count, but this new analysis calls that into question.
“Our results, which demonstrated that thiopurine use among male patients with IBD is associated with increased sperm count, refute this prior finding,” the researchers said. The previous finding, they noted, was only qualitative because the authors didn’t do an analysis to calculate effect size or determine statistical significance.
“Furthermore,” the researchers said, “our study included more updated studies and a greater number of patients.”
The authors disclosed no conflicts of interest.
Understanding the impact of inflammatory bowel disease therapies on fertility and pregnancy outcomes is key toward managing patients with IBD. While there is substantial research on the implications of maternal exposure to IBD medications with reassuring safety data, research in the context of paternal exposure to IBD medications is limited.
This study represents the largest report summarizing data across diverse populations on the topic with reassuring results. It carries important implications in clinical practice and provides further evidence in support of continuing IBD therapy among male patients through pregnancy planning. Certainly, active IBD in male patients is associated with adverse effects on sperm quality and conception likelihood, and it is important to achieve remission prior to pregnancy planning.
Further research on the impact of paternal exposure to newer biologics, including small molecule drugs, and additional analyses after adjusting for potential confounders will advance the field and provide further guidance in clinical practice.
Manasi Agrawal, MD, MS, is an assistant professor of medicine in the Dr. Henry D. Janowitz Division of Gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. She is a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease. Aalborg University, Copenhagen. She reports no conflicts.
Understanding the impact of inflammatory bowel disease therapies on fertility and pregnancy outcomes is key toward managing patients with IBD. While there is substantial research on the implications of maternal exposure to IBD medications with reassuring safety data, research in the context of paternal exposure to IBD medications is limited.
This study represents the largest report summarizing data across diverse populations on the topic with reassuring results. It carries important implications in clinical practice and provides further evidence in support of continuing IBD therapy among male patients through pregnancy planning. Certainly, active IBD in male patients is associated with adverse effects on sperm quality and conception likelihood, and it is important to achieve remission prior to pregnancy planning.
Further research on the impact of paternal exposure to newer biologics, including small molecule drugs, and additional analyses after adjusting for potential confounders will advance the field and provide further guidance in clinical practice.
Manasi Agrawal, MD, MS, is an assistant professor of medicine in the Dr. Henry D. Janowitz Division of Gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. She is a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease. Aalborg University, Copenhagen. She reports no conflicts.
Understanding the impact of inflammatory bowel disease therapies on fertility and pregnancy outcomes is key toward managing patients with IBD. While there is substantial research on the implications of maternal exposure to IBD medications with reassuring safety data, research in the context of paternal exposure to IBD medications is limited.
This study represents the largest report summarizing data across diverse populations on the topic with reassuring results. It carries important implications in clinical practice and provides further evidence in support of continuing IBD therapy among male patients through pregnancy planning. Certainly, active IBD in male patients is associated with adverse effects on sperm quality and conception likelihood, and it is important to achieve remission prior to pregnancy planning.
Further research on the impact of paternal exposure to newer biologics, including small molecule drugs, and additional analyses after adjusting for potential confounders will advance the field and provide further guidance in clinical practice.
Manasi Agrawal, MD, MS, is an assistant professor of medicine in the Dr. Henry D. Janowitz Division of Gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. She is a research associate with the Center for Molecular Prediction of Inflammatory Bowel Disease. Aalborg University, Copenhagen. She reports no conflicts.
published in Clinical Gastroenterology and Hepatology.
The effort is the first meta-analysis to assess semen parameters and the risk of adverse outcomes in pregnancy for male patients with IBD who have taken biologics, thiopurines or methotrexate for the condition, the researchers said.
“We provide encouraging evidence that biologic, thiopurine, and methotrexate therapy among male patients with IBD are not associated with impairments in male fertility or with increased risk of adverse pregnancy outcomes,” said the researchers, led in part by John Gubatan, MD, instructor in medicine at Stanford (Calif.) University, who worked with investigators in Copenhagen and Toronto. “Taken together, our data support the safety of continuing biologics, thiopurines, or methotrexate across the reproductive spectrum.”
Questions of fertility and pregnancy outcomes are of particular importance in IBD, since patients are often diagnosed around the time of their reproductive years – about 30 years old for Crohn’s disease and 35 years old for ulcerative colitis. There has been far more research attention paid to female than male reproductive considerations, mainly the health of the fetus when the mother takes biologic therapy for IBD during pregnancy, which has generally found to be safe.
Their search found 13 studies with male IBD patients exposed to biologics, 10 exposed to thiopurines and 6 to methotrexate. Researchers extracted data on sperm count, sperm motility, and abnormal sperm morphology – three metrics considered a proxy for male fertility – as well as early pregnancy loss, preterm birth and congenital malformations.
Researchers found no differences between sperm count, motility or morphology between those exposed and not exposed to biologics, thiopurines and methotrexate, with a couple of exceptions. They actually found that sperm count was higher for thiopurine users, compared with nonusers, and there was only one study on methotrexate and abnormal sperm morphology, so there was no data to pool together for that comparison.
In a subgroup analysis, there was a trend toward higher sperm count in thiopurine users, compared with biologic or methotrexate users, but no differences were seen in the other parameters.
Similarly, there were no significant differences for users and nonusers of these medications for early pregnancy loss, preterm births or congenital malformations, the researchers found.
A prior systematic review suggested that azathioprine might be associated with low sperm count, but this new analysis calls that into question.
“Our results, which demonstrated that thiopurine use among male patients with IBD is associated with increased sperm count, refute this prior finding,” the researchers said. The previous finding, they noted, was only qualitative because the authors didn’t do an analysis to calculate effect size or determine statistical significance.
“Furthermore,” the researchers said, “our study included more updated studies and a greater number of patients.”
The authors disclosed no conflicts of interest.
published in Clinical Gastroenterology and Hepatology.
The effort is the first meta-analysis to assess semen parameters and the risk of adverse outcomes in pregnancy for male patients with IBD who have taken biologics, thiopurines or methotrexate for the condition, the researchers said.
“We provide encouraging evidence that biologic, thiopurine, and methotrexate therapy among male patients with IBD are not associated with impairments in male fertility or with increased risk of adverse pregnancy outcomes,” said the researchers, led in part by John Gubatan, MD, instructor in medicine at Stanford (Calif.) University, who worked with investigators in Copenhagen and Toronto. “Taken together, our data support the safety of continuing biologics, thiopurines, or methotrexate across the reproductive spectrum.”
Questions of fertility and pregnancy outcomes are of particular importance in IBD, since patients are often diagnosed around the time of their reproductive years – about 30 years old for Crohn’s disease and 35 years old for ulcerative colitis. There has been far more research attention paid to female than male reproductive considerations, mainly the health of the fetus when the mother takes biologic therapy for IBD during pregnancy, which has generally found to be safe.
Their search found 13 studies with male IBD patients exposed to biologics, 10 exposed to thiopurines and 6 to methotrexate. Researchers extracted data on sperm count, sperm motility, and abnormal sperm morphology – three metrics considered a proxy for male fertility – as well as early pregnancy loss, preterm birth and congenital malformations.
Researchers found no differences between sperm count, motility or morphology between those exposed and not exposed to biologics, thiopurines and methotrexate, with a couple of exceptions. They actually found that sperm count was higher for thiopurine users, compared with nonusers, and there was only one study on methotrexate and abnormal sperm morphology, so there was no data to pool together for that comparison.
In a subgroup analysis, there was a trend toward higher sperm count in thiopurine users, compared with biologic or methotrexate users, but no differences were seen in the other parameters.
Similarly, there were no significant differences for users and nonusers of these medications for early pregnancy loss, preterm births or congenital malformations, the researchers found.
A prior systematic review suggested that azathioprine might be associated with low sperm count, but this new analysis calls that into question.
“Our results, which demonstrated that thiopurine use among male patients with IBD is associated with increased sperm count, refute this prior finding,” the researchers said. The previous finding, they noted, was only qualitative because the authors didn’t do an analysis to calculate effect size or determine statistical significance.
“Furthermore,” the researchers said, “our study included more updated studies and a greater number of patients.”
The authors disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Mirikizumab performs well in UC, new data show
, according to new findings from the phase 3 LUCENT-1 induction and LUCENT-2 maintenance trials. The findings were reported in the New England Journal of Medicine.
Mirikizumab manufacturer Eli Lilly, which funded the study, is hoping the drug will become the first IL-23 inhibitor to be approved in the United States for ulcerative colitis. The drug targets the p19 subunit that is unique to IL-23. Ustekinumab, which targets the p40 subunit that is shared by IL-12 and IL-23, has been approved for UC and Crohn’s disease. Risankizumab, which targets the IL-23 p19 subunit, has been approved for Crohn’s treatment.
Earlier this year, the Food and Drug Administration rejected Lilly’s mirikizumab application over manufacturing issues, with no concerns about the clinical data, safety, or labelling. The company said it was working with the FDA to resolve the concerns, and hopes to “launch mirikizumab in the U.S. as soon as possible.” The drug has already been approved in Japan for moderately and severely active ulcerative colitis, and the drug was reviewed favorably by the European Medicines Agency.
Since 2014, the market size of interleukin inhibitors has grown fivefold with the greatest share belonging to IL-23 inhibitors.
The induction trial included 1,281 patients with moderately or severely active ulcerative colitis (UC), and 544 patients who had a response to mirikizumab were randomized again in the maintenance phase.
Significantly more patients in the mirikizumab arm – 24.1% (P < .001) – had clinical remission at week 12, although there was a high placebo remission rate, as is often seen in UC trials, at 13.3%. At week 40 of the maintenance trial, 49.9% of those on mirikizumab had clinical remission, compared to 25.1% for placebo (P < .001).
Mirikizumab also performed better than placebo on the trial’s five secondary endpoints: glucocorticoid-free clinical remission (44.9% to 21.8%), maintenance of clinical remission (63.6% to 36.9%), endoscopic remission (58.6% to 29.1%), histologic-endoscopic mucosal remission (43.3 %), and bowel-urgency remission (42.9% to 25.0%) (P < .001 for all).
Researchers led by Geert D’Haens, MD, PhD, professor of gastroenterology at Amsterdam University Medical Centers, emphasized the effects on acute inflammatory cell infiltration.
“Current recommendations for the treatment of ulcerative colitis include increasingly rigorous goals beyond symptomatic or endoscopic improvement,” the authors wrote. “Recent literature has recommended the absence of intraepithelial neutrophils as a minimal requirement for remission on the basis of histologic testing. After 1 year of mirikizumab treatment, more than 40% of the patients in the LUCENT-1 and LUCENT-2 trials had no mucosal neutrophils. “
Urgency NRS (Numeric Rating Scale) – a measure developed by Lilly in which patients report the urgency of bowel movements over the previous 24 hours – was used in the trial.
“Many patients with ulcerative colitis consider control of bowel movements to be more important than rectal bleeding or stool frequency,” the researchers said. “In the induction trial, patients reported reductions in bowel urgency with mirikizumab therapy, which were sustained during the maintenance trial.”
Of the 1,217 patients treated with mirikizumab during the placebo-controlled and non–placebo-controlled periods, opportunistic infections were seen in 15, with 6 herpes zoster infections. One case of an opportunistic infection was seen in a patient receiving placebo in the induction trial.
Cancer was seen in eight of the mirikizumab-treated patients, with adenocarcinoma of the colon in two patients in the induction trial. No cancers were seen in patients receiving placebo in induction.
“Additional and longer trials are ongoing,” the researchers said, “to further assess the efficacy and safety of mirikizumab therapy in patients with ulcerative colitis.”
The authors disclosed consultancies, or other relationships, with a number of pharmaceutical companies, including Eli Lilly, the maker of mirikizumab.
, according to new findings from the phase 3 LUCENT-1 induction and LUCENT-2 maintenance trials. The findings were reported in the New England Journal of Medicine.
Mirikizumab manufacturer Eli Lilly, which funded the study, is hoping the drug will become the first IL-23 inhibitor to be approved in the United States for ulcerative colitis. The drug targets the p19 subunit that is unique to IL-23. Ustekinumab, which targets the p40 subunit that is shared by IL-12 and IL-23, has been approved for UC and Crohn’s disease. Risankizumab, which targets the IL-23 p19 subunit, has been approved for Crohn’s treatment.
Earlier this year, the Food and Drug Administration rejected Lilly’s mirikizumab application over manufacturing issues, with no concerns about the clinical data, safety, or labelling. The company said it was working with the FDA to resolve the concerns, and hopes to “launch mirikizumab in the U.S. as soon as possible.” The drug has already been approved in Japan for moderately and severely active ulcerative colitis, and the drug was reviewed favorably by the European Medicines Agency.
Since 2014, the market size of interleukin inhibitors has grown fivefold with the greatest share belonging to IL-23 inhibitors.
The induction trial included 1,281 patients with moderately or severely active ulcerative colitis (UC), and 544 patients who had a response to mirikizumab were randomized again in the maintenance phase.
Significantly more patients in the mirikizumab arm – 24.1% (P < .001) – had clinical remission at week 12, although there was a high placebo remission rate, as is often seen in UC trials, at 13.3%. At week 40 of the maintenance trial, 49.9% of those on mirikizumab had clinical remission, compared to 25.1% for placebo (P < .001).
Mirikizumab also performed better than placebo on the trial’s five secondary endpoints: glucocorticoid-free clinical remission (44.9% to 21.8%), maintenance of clinical remission (63.6% to 36.9%), endoscopic remission (58.6% to 29.1%), histologic-endoscopic mucosal remission (43.3 %), and bowel-urgency remission (42.9% to 25.0%) (P < .001 for all).
Researchers led by Geert D’Haens, MD, PhD, professor of gastroenterology at Amsterdam University Medical Centers, emphasized the effects on acute inflammatory cell infiltration.
“Current recommendations for the treatment of ulcerative colitis include increasingly rigorous goals beyond symptomatic or endoscopic improvement,” the authors wrote. “Recent literature has recommended the absence of intraepithelial neutrophils as a minimal requirement for remission on the basis of histologic testing. After 1 year of mirikizumab treatment, more than 40% of the patients in the LUCENT-1 and LUCENT-2 trials had no mucosal neutrophils. “
Urgency NRS (Numeric Rating Scale) – a measure developed by Lilly in which patients report the urgency of bowel movements over the previous 24 hours – was used in the trial.
“Many patients with ulcerative colitis consider control of bowel movements to be more important than rectal bleeding or stool frequency,” the researchers said. “In the induction trial, patients reported reductions in bowel urgency with mirikizumab therapy, which were sustained during the maintenance trial.”
Of the 1,217 patients treated with mirikizumab during the placebo-controlled and non–placebo-controlled periods, opportunistic infections were seen in 15, with 6 herpes zoster infections. One case of an opportunistic infection was seen in a patient receiving placebo in the induction trial.
Cancer was seen in eight of the mirikizumab-treated patients, with adenocarcinoma of the colon in two patients in the induction trial. No cancers were seen in patients receiving placebo in induction.
“Additional and longer trials are ongoing,” the researchers said, “to further assess the efficacy and safety of mirikizumab therapy in patients with ulcerative colitis.”
The authors disclosed consultancies, or other relationships, with a number of pharmaceutical companies, including Eli Lilly, the maker of mirikizumab.
, according to new findings from the phase 3 LUCENT-1 induction and LUCENT-2 maintenance trials. The findings were reported in the New England Journal of Medicine.
Mirikizumab manufacturer Eli Lilly, which funded the study, is hoping the drug will become the first IL-23 inhibitor to be approved in the United States for ulcerative colitis. The drug targets the p19 subunit that is unique to IL-23. Ustekinumab, which targets the p40 subunit that is shared by IL-12 and IL-23, has been approved for UC and Crohn’s disease. Risankizumab, which targets the IL-23 p19 subunit, has been approved for Crohn’s treatment.
Earlier this year, the Food and Drug Administration rejected Lilly’s mirikizumab application over manufacturing issues, with no concerns about the clinical data, safety, or labelling. The company said it was working with the FDA to resolve the concerns, and hopes to “launch mirikizumab in the U.S. as soon as possible.” The drug has already been approved in Japan for moderately and severely active ulcerative colitis, and the drug was reviewed favorably by the European Medicines Agency.
Since 2014, the market size of interleukin inhibitors has grown fivefold with the greatest share belonging to IL-23 inhibitors.
The induction trial included 1,281 patients with moderately or severely active ulcerative colitis (UC), and 544 patients who had a response to mirikizumab were randomized again in the maintenance phase.
Significantly more patients in the mirikizumab arm – 24.1% (P < .001) – had clinical remission at week 12, although there was a high placebo remission rate, as is often seen in UC trials, at 13.3%. At week 40 of the maintenance trial, 49.9% of those on mirikizumab had clinical remission, compared to 25.1% for placebo (P < .001).
Mirikizumab also performed better than placebo on the trial’s five secondary endpoints: glucocorticoid-free clinical remission (44.9% to 21.8%), maintenance of clinical remission (63.6% to 36.9%), endoscopic remission (58.6% to 29.1%), histologic-endoscopic mucosal remission (43.3 %), and bowel-urgency remission (42.9% to 25.0%) (P < .001 for all).
Researchers led by Geert D’Haens, MD, PhD, professor of gastroenterology at Amsterdam University Medical Centers, emphasized the effects on acute inflammatory cell infiltration.
“Current recommendations for the treatment of ulcerative colitis include increasingly rigorous goals beyond symptomatic or endoscopic improvement,” the authors wrote. “Recent literature has recommended the absence of intraepithelial neutrophils as a minimal requirement for remission on the basis of histologic testing. After 1 year of mirikizumab treatment, more than 40% of the patients in the LUCENT-1 and LUCENT-2 trials had no mucosal neutrophils. “
Urgency NRS (Numeric Rating Scale) – a measure developed by Lilly in which patients report the urgency of bowel movements over the previous 24 hours – was used in the trial.
“Many patients with ulcerative colitis consider control of bowel movements to be more important than rectal bleeding or stool frequency,” the researchers said. “In the induction trial, patients reported reductions in bowel urgency with mirikizumab therapy, which were sustained during the maintenance trial.”
Of the 1,217 patients treated with mirikizumab during the placebo-controlled and non–placebo-controlled periods, opportunistic infections were seen in 15, with 6 herpes zoster infections. One case of an opportunistic infection was seen in a patient receiving placebo in the induction trial.
Cancer was seen in eight of the mirikizumab-treated patients, with adenocarcinoma of the colon in two patients in the induction trial. No cancers were seen in patients receiving placebo in induction.
“Additional and longer trials are ongoing,” the researchers said, “to further assess the efficacy and safety of mirikizumab therapy in patients with ulcerative colitis.”
The authors disclosed consultancies, or other relationships, with a number of pharmaceutical companies, including Eli Lilly, the maker of mirikizumab.
Surveillance colonoscopy: When and how to stop
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
Dear colleagues,
Colonoscopy is the bread and butter of endoscopy. Multidisciplinary updates continue to support screening colonoscopy in reducing the risk of developing colorectal cancer. But there has been debate about the best uses of resources, especially with increased recognition of colorectal cancer (CRC) in younger patients, and successive guidelines lengthening the intervals for most surveillance colonoscopy.
In particular, when do we feel comfortable recommending cessation of surveillance colonoscopy especially in those who are 75-85 years old? Routine colonoscopy remains a very low-risk procedure even in older patients with multiple comorbidities.
In this issue of Perspectives, Dr. Petr Protiva and Dr. Mariam Naveed address this issue and provide differing perspectives on approaching surveillance colonoscopy in the elderly.
We welcome your thoughts on this issue on Twitter at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Striking a balance: Deciding the optimal age to cease surveillance for colorectal neoplasia
BY PETR PROTIVA, MD, MPH
The appropriate age to stop surveillance for colorectal neoplasia remains uncertain. Screening for average-risk individuals is typically stopped at age 75, but personalized screening with shared decision-making may continue until age 85.1 Evidence suggests that any survival benefit of screening past age 86 would be outweighed by the harm of screening and/or natural mortality. Nevertheless, determining the optimal age for surveillance in those with a history of neoplasia still poses some challenges. The issue is confounded as many clinicians use the terms “screening” and “surveillance” interchangeably. It should be noted that screening implies the individual is at average risk, while surveillance refers to those at elevated risk because of a personal history of colonic adenomas or cancer
Comorbidities and life expectancy
Despite recent staggering setbacks and a drop in the average life expectancy in the United States, the proportion of individuals older than 65 years old has been steadily increasing to a currently estimated 58.9 million – 16.8% of the U.S. population – and is projected to increase in the future.2 However, the prevalence of comorbidities also increases with age, and these are crucial factors to weigh in the decision-making process. Severe comorbid conditions, such as chronic obstructive pulmonary disease, cirrhosis, chronic hepatitis, chronic renal failure, dementia, and congestive heart failure can limit a patient’s ability to undergo surveillance and diminish or negate its benefits. There are online tools that help clinicians estimate life expectancy and time to benefit (that is, time between the intervention and its benefit), such as the Lee or Schonberg index.3 Consensus on time to colorectal screening benefit is about 9-10 years, but may be much shorter for surveillance. Striking a balance between the potential benefits of continued surveillance and the risks and burdens imposed on older adults with limited life expectancy is essential for making well-informed decisions.
Age-related risk increase and risk of neoplasia in the surveillance population:
The absolute risk of developing colorectal cancer is dependent on age. In adults aged 45-49, it is 33.4/100,000, rising to 135.6 in those 70-74 years old; in persons aged 85 and older, it is 234.7/100,000.4 A significant challenge in determining the appropriate age to stop surveillance is the additional individual risk based on baseline polyp characteristics. It seems reasonable to treat low-risk adenomas similarly to screening and stop surveillance by 85 or 86 years old in most cases. The decision process is less clear in individuals with advanced lesions or a personal history of colon cancer. The prevalence of advanced adenomas on the baseline exam is about 15% and greater than 20% on the follow-up exam if the index adenoma(s) were at least 20 mm in diameter. For five or more adenomas at baseline, the prevalence of advanced lesions on follow-up exam is about 20%. On the other hand, a negative surveillance colonoscopy (that is, no polyps found) is associated with far fewer advanced lesions on follow-up.
Colonoscopy
The safety of colonoscopy in older adults should be considered. The colonoscopy procedure is generally very safe, but is associated with a higher risk of post-procedure complications after outpatient colonoscopy in patients 75 years and older. In addition, comorbidities such as anemia, cardiac arrhythmia, heart failure, hypertension, chronic kidney disease, liver disease, smoking history, and obesity are also risk factors. It should be noted that high-quality colonoscopy is key to the detection and full removal of neoplastic lesions and risk reduction. The inability to achieve adequate colon preparation for any reason or undertaking colonoscopy in patients at high risk for complications reduces its benefit.
Who should oversee surveillance, the primary care physician or gastroenterologist
Should a gastroenterologist oversee the decision on surveillance in older adults based on a combination of age, estimated procedure risk and benefits, patient preferences, and current guidelines? Certainly, it seems appropriate and that this is what most specialists think, according to a recent survey of gastroenterologists and primary care physicians (PCPs) on this topic.5 Perhaps, not surprisingly, most PCPs disagreed – PCPs are thoroughly familiar with their patients’ up-to-date comorbidities, functional status, and preferences, and they have the benefit of knowing them for a long time. They also integrate diagnostic results from multiple subspecialists, sometimes from different states. The role of PCPs is critical in centers that offer open-access colonoscopy. Gastroenterologists may be the most appropriate authority to evaluate older individuals for continued surveillance, but in most busy practices these patients are seen by mid-level practitioners. Specialists play an important role if the PCP is uncertain whether surveillance is still indicated or in older patients with a history of advanced adenoma. Therefore, the colonoscopy report or subsequent communication after pathology results are returned should include a recommendation for future surveillance and a clear provision for discontinuing the surveillance in case of future health decline.
Conclusion
Determining the optimal age to discontinue surveillance for colorectal neoplasia involves evaluating multiple factors. Although the age limit is clearer for average-risk screening and low-risk lesion surveillance, uncertainty remains for individuals with advanced neoplasia history. Significant factors in this decision-making process include subsequent neoplasia risk, comorbidities, life expectancy, and age-related risks associated with colonoscopy. Cooperation between PCPs and subspecialty physicians is essential in making surveillance decisions for older adults. PCPs are well positioned to consider detailed patient comorbidities, functional status, and patient preferences, especially with the help of online life-expectancy estimators for most elderly or comorbid patients. To assist in this process, gastroenterologists should state clearly in their procedure report and subsequent pathology letters whether surveillance is recommended and that it is conditional on future comorbidities and should be discontinued if the patient’s health significantly declines.
Dr. Protiva is associate professor of medicine, Yale University, and director of the colon cancer screening program, VA Connecticut Healthcare System, West Haven. Dr. Protiva has no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022;162:285-99
2. U.S. Census Bureau. Measuring America’s People, Places, and Economy
3. University of California, San Francisco. ePrognosis. 4. Siegel RL et al. CA Cancer J Clin. 2023 May-Jun;73(3):233-54.
5. Schoenborn NL et al. Am J Gastroenterol. 2023; 118(3):523-30.
The gastroenterologist should guide the decision to maintain, or halt, surveillance in older adults
BY MARIAM NAVEED, MD
Endoscopic screening and surveillance for CRC in older adults (≥ 75 years old) is a medical “gray area” that needs more high-quality data to inform clinical decision making. In the most recent 2022 clinical guideline update from the U.S. Multisociety Task Force on Colorectal Cancer, the recommendation to stop CRC screening in average risk patients older than 86 years is well supported because of colonoscopy-associated mortality risk outweighing the benefits of adenoma detection and removal. By comparison, screening recommendations for average risk individuals between 76-85 years old are ambiguous and ultimately the decision to proceed with colonoscopy in this clinical population should be individualized based on shared decision making between the provider and patient. Of note, the same guideline provides no specific guidance for ongoing surveillance in the same age group and similarly suggests a shared decision-making approach.1
As a practicing gastroenterologist in the retirement capital of Florida, older adults comprise a large portion of my clinical practice. I have noticed several aspects unique to this demographic that merit special consideration. For example, a significant percentage of these patients are seasonal (that is, “snowbird”) patients that have multiple sets of doctors (set of physicians in their home state and another set in Florida). Consequently, fragmentation of clinical data enables opportunities for colonoscopies to be wrongly ordered (either in an inappropriate time frame and/or for inaccurate indications). In my own practice, when such a patient is referred for consideration of CRC surveillance, any/all external records must first be obtained and validated as a prerequisite for appropriate clinical counseling and informed decision-making. Additionally, consideration of periprocedural risks is particularly relevant in older adults, secondary to both the increased rate of direct complications and the likelihood of pre-existing comorbidities affecting completion of a safe colonoscopy. Factors that can be easily overlooked include higher rates of poor bowel preparation and corresponding decreased completion rates. Moreover, if the patient has a history of high-risk adenomas or worrisome family history warranting ongoing evaluation, but they also have high-risk comorbidities, I will frequently involve the patient’s cardiologist or pulmonologist to provide medical clearance prior to offering CRC screening/surveillance.
In addition to the clinical ambiguity of appropriateness of continued CRC screening/surveillance in the setting of advanced age, there is also the question of which provider is best positioned to counsel patients regarding this decision-making. Does the onus fall on the gastroenterologist (the proceduralist ultimately performing the procedure) or the PCP (who is likely more familiar with the patient’s overall health profile)? In a recent survey, more than 50% of PCPs reported feeling uncertain in their understanding of risk versus benefit stratification of continued CRC screening in older adults.2 While there may be justification for both classes of providers to be involved, in my opinion, the decision to maintain or halt surveillance in older adults should be primarily guided by the gastroenterologist who is better equipped to provide individualized guidance regarding the nuanced risks of disease progression in these patients with prior history of adenomas, and who is clinically responsible for any procedure-related complications.
In an era of cost containment, insurance companies are increasingly placing barriers for approving surveillance and diagnostic colonoscopies. Thus, we need to be ever mindful of appropriately allocating resources to best benefit patients. The data on incidence of polyps and CRC in older adults is inconsistent and even difficult at times for a gastroenterologist to interpret. Therefore, in my opinion, the onus should not fall solely on the PCPs who are not routinely familiar with this information. We as gastroenterologists typically have greater domain-specific knowledge regarding current data and updated guidelines.
Gastroenterologists should wield this expertise to regulate overly liberalized recommendations for continued surveillance in fragile patients, or conversely to intervene in settings of prematurely halting surveillance in high-risk populations with appropriate life expectancy to experience disease progression. It is critical to carefully consider patient-individualized life expectancies, avoid surveillance in patients without clinically significant polyps, avoid over-weighting previous abnormal prior colonoscopies without reviewing more current procedure results, and take time to discuss patient preferences. As proceduralists, we must also be mindful of intrinsic biases towards performing surveillance in patients who are not likely to benefit from this intervention, and several studies have reported on the overuse of surveillance colonoscopies in the form of repeating surveillance earlier than recommended or in the context of limited life expectancy.3
Finally, it is necessary to emphasize that PCPs are critical allies for promoting overall patient health, especially in scenarios where recommendations to discontinue surveillance may not coincide with patient preference. It has been reported that patients usually do not consider poor overall health relevant to decisions regarding CRC surveillance (which I have also experienced to be true).4 In these scenarios, partnering with the PCP can be strategic, as patients may be more inclined to trust the guidance of their more familiar physician. At the end of the day, regardless of which provider takes ownership of initiating the discussion surrounding surveillance colonoscopy in older adults, communication is key between all providers and the patient to ensure optimal outcomes.
As the U.S. population continues to age, the demographic of patients aged 65 and older is projected to nearly double by 2060.5 Decisions regarding ongoing surveillance for CRC will continue to be frequent and increasingly relevant. The importance of studies generating high-quality data to inform appropriate guidelines specific to this population cannot be understated.
Dr. Naveed is a gastroenterologist and director of the gastroenterology and hepatology fellowship program at AdventHealth Medical Group, Altamonte Springs, Fla. Dr. Naveed had no relevant disclosures.
References
1. Patel SG et al. Gastroenterology. 2022 Jan;162(1):285-99.
2 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
3 Calderwood AH et al. JAMA Intern Med. 2023 May 1;183(5):426-34.
4 Schoenborn NL et al. Am J Gastroenterol. 2023 Mar 1;118(3):523-30.
5 U.S. Census Bureau. Population Projections.
No link between PPIs and dementia in new study
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A new study provides reassurance about the long-term safety of proton pump inhibitors (PPIs) and histamine-2 receptor antagonist (H2RA) use in older adults, finding no increased risk for dementia or cognitive changes.
METHODOLOGY:
- Post hoc observational study within the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial.
- 18,934 adults aged 65+ from the United States and Australia without dementia at baseline.
- 4,667 (25%) PPI users and 368 (2%) H2RA users at baseline.
- PPI and H2RA use, dementia incidence, and cognitive changes were tracked.
TAKEAWAY:
- In multivariable analysis, baseline PPI use was not associated with incident dementia (hazard ratio, 0.88) or cognitive impairment (HR, 1.00).
- PPI use was not linked to changes in overall cognitive test scores over time (beta –0.002).
- No associations were found between H2RA use and cognitive endpoints.
IN PRACTICE:
“Long-term use of PPIs in older adults is unlikely to have negative effects on cognition,” the study team concludes.
STUDY DETAILS:
The study was led by Raaj Mehta, MD, PhD, with Massachusetts General Hospital and Harvard Medical School in Boston. The study was published online in Gastroenterology. Funding was provided by grants from the National Institute on Aging, the National Cancer Institute, and other institutions.
LIMITATIONS:
Potential for residual confounding and underestimation of PPI and H2RA use, lack of data on medication dose and duration, and the absence of ApoE4 allele status.
DISCLOSURES:
Dr. Mehta has disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
IBD study characterizes biologic adherence, confirms nonadherence risk factors
, based on a retrospective study.
Nonadherence became increasingly common in the presence of four previously reported risk factors, including smoking status, narcotic use, psychiatric history, and prior biologic use, reported lead author Lauren A. George, MD, of the University of Maryland, Baltimore, and colleagues.
“Identifying patients at risk for nonadherence is important to develop strategies to improve adherence,” the investigators wrote in Gastro Hep Advances. “The aim of this analysis was to evaluate adherence across several academic centers with integrated specialty pharmacies, to assess if previously identified risk factors for nonadherence remained significant across several diverse IBD centers, and to evaluate outcomes associated with nonadherence.”
Three tertiary care IBD clinics provided data from 608 patients with IBD. Inclusion required at least three consecutive prescription claims. All biologics were self-injectable, including adalimumab, certolizumab, golimumab, and ustekinumab.
Primary outcomes were medication possession ratio (MPR) and adherence, with nonadherence defined by an MPR lower than 0.86. Secondary outcomes included ED visits and hospitalizations.
After a median follow-up period of 903 days, the overall MPR was 0.95, with adherence of 68%-70%, which is considered “high,” according to Dr. George and colleagues, as it exceeds previously reported national adherence rates.
“[Findings were] similar across all centers, geographic regions, and patient demographics,” the investigators noted.
The four previously described risk factors did in fact predict nonadherence, with likelihood of nonadherence significantly increasing with each additional risk factor present. Patients with all four risk factors had less than 50% adherence.
Nonadherence was also significantly associated with more ED visits and hospitalizations, highlighting “the impact of biologic adherence on direct patient outcomes and healthcare costs,” the investigators wrote.
“All healthcare industry stakeholders including healthcare systems, manufacturers, and third-party benefit providers need to understand the importance of improving patient adherence,” Dr. George and colleagues concluded. “Decreasing barriers to self-injectable medication acquisition, increasing direct patient interaction with integrated pharmacy teams, and comprehensive patient education are a start to improving patient adherence. In addition, we propose that enhanced care pathways for patients with risk factors for nonadherence would improve adherence and outcomes.”
No funding was reported. The investigators disclosed relationships with AbbVie, Janssen, UCB, and others.
This article was updated 7/13/23.
An important adage in medicine is that medications only work if patients take them. Inflammatory bowel disease is a chronic illness that, if inadequately treated, can lead to emergency department visits, hospitalizations, and surgery.
This study by George et al. showed that patients receiving care at academic medical centers with integrated pharmacies had high adherence to subcutaneous therapies. Unsurprisingly, patients with high adherence had fewer emergency room visits and hospitalizations.
An important contribution is the authors identified risk factors for nonadherence. Among others, opioid use, psychiatric illness, and Medicaid insurance were associated with lower adherence. Identifying patients with these risk factors may allow more intensive outreach to improve adherence. IBD centers with integrated pharmacies, such as those in this study, are likely best equipped to do this. Alternatively, these patients may be best served with infusions that are less frequent than injections and be regularly scheduled with an appointment.
While this study did not directly compare other practice models, adherence was much higher than in other studies. This suggests the addition of an integrated pharmacy improves adherence and lowers costs. Other factors such as highly trained IBD gastroenterologists and skilled support staff may have also helped improve adherence, but in any case the multidisciplinary care, especially integrated pharmacies, should be emulated by other IBD centers.
Martin H. Gregory, MD, MSCI, assistant professor of medicine, Washington University School of Medicine, St. Louis. Dr. Gregory disclosed serving on an advisory board for Bristol Myers Squibb.
An important adage in medicine is that medications only work if patients take them. Inflammatory bowel disease is a chronic illness that, if inadequately treated, can lead to emergency department visits, hospitalizations, and surgery.
This study by George et al. showed that patients receiving care at academic medical centers with integrated pharmacies had high adherence to subcutaneous therapies. Unsurprisingly, patients with high adherence had fewer emergency room visits and hospitalizations.
An important contribution is the authors identified risk factors for nonadherence. Among others, opioid use, psychiatric illness, and Medicaid insurance were associated with lower adherence. Identifying patients with these risk factors may allow more intensive outreach to improve adherence. IBD centers with integrated pharmacies, such as those in this study, are likely best equipped to do this. Alternatively, these patients may be best served with infusions that are less frequent than injections and be regularly scheduled with an appointment.
While this study did not directly compare other practice models, adherence was much higher than in other studies. This suggests the addition of an integrated pharmacy improves adherence and lowers costs. Other factors such as highly trained IBD gastroenterologists and skilled support staff may have also helped improve adherence, but in any case the multidisciplinary care, especially integrated pharmacies, should be emulated by other IBD centers.
Martin H. Gregory, MD, MSCI, assistant professor of medicine, Washington University School of Medicine, St. Louis. Dr. Gregory disclosed serving on an advisory board for Bristol Myers Squibb.
An important adage in medicine is that medications only work if patients take them. Inflammatory bowel disease is a chronic illness that, if inadequately treated, can lead to emergency department visits, hospitalizations, and surgery.
This study by George et al. showed that patients receiving care at academic medical centers with integrated pharmacies had high adherence to subcutaneous therapies. Unsurprisingly, patients with high adherence had fewer emergency room visits and hospitalizations.
An important contribution is the authors identified risk factors for nonadherence. Among others, opioid use, psychiatric illness, and Medicaid insurance were associated with lower adherence. Identifying patients with these risk factors may allow more intensive outreach to improve adherence. IBD centers with integrated pharmacies, such as those in this study, are likely best equipped to do this. Alternatively, these patients may be best served with infusions that are less frequent than injections and be regularly scheduled with an appointment.
While this study did not directly compare other practice models, adherence was much higher than in other studies. This suggests the addition of an integrated pharmacy improves adherence and lowers costs. Other factors such as highly trained IBD gastroenterologists and skilled support staff may have also helped improve adherence, but in any case the multidisciplinary care, especially integrated pharmacies, should be emulated by other IBD centers.
Martin H. Gregory, MD, MSCI, assistant professor of medicine, Washington University School of Medicine, St. Louis. Dr. Gregory disclosed serving on an advisory board for Bristol Myers Squibb.
, based on a retrospective study.
Nonadherence became increasingly common in the presence of four previously reported risk factors, including smoking status, narcotic use, psychiatric history, and prior biologic use, reported lead author Lauren A. George, MD, of the University of Maryland, Baltimore, and colleagues.
“Identifying patients at risk for nonadherence is important to develop strategies to improve adherence,” the investigators wrote in Gastro Hep Advances. “The aim of this analysis was to evaluate adherence across several academic centers with integrated specialty pharmacies, to assess if previously identified risk factors for nonadherence remained significant across several diverse IBD centers, and to evaluate outcomes associated with nonadherence.”
Three tertiary care IBD clinics provided data from 608 patients with IBD. Inclusion required at least three consecutive prescription claims. All biologics were self-injectable, including adalimumab, certolizumab, golimumab, and ustekinumab.
Primary outcomes were medication possession ratio (MPR) and adherence, with nonadherence defined by an MPR lower than 0.86. Secondary outcomes included ED visits and hospitalizations.
After a median follow-up period of 903 days, the overall MPR was 0.95, with adherence of 68%-70%, which is considered “high,” according to Dr. George and colleagues, as it exceeds previously reported national adherence rates.
“[Findings were] similar across all centers, geographic regions, and patient demographics,” the investigators noted.
The four previously described risk factors did in fact predict nonadherence, with likelihood of nonadherence significantly increasing with each additional risk factor present. Patients with all four risk factors had less than 50% adherence.
Nonadherence was also significantly associated with more ED visits and hospitalizations, highlighting “the impact of biologic adherence on direct patient outcomes and healthcare costs,” the investigators wrote.
“All healthcare industry stakeholders including healthcare systems, manufacturers, and third-party benefit providers need to understand the importance of improving patient adherence,” Dr. George and colleagues concluded. “Decreasing barriers to self-injectable medication acquisition, increasing direct patient interaction with integrated pharmacy teams, and comprehensive patient education are a start to improving patient adherence. In addition, we propose that enhanced care pathways for patients with risk factors for nonadherence would improve adherence and outcomes.”
No funding was reported. The investigators disclosed relationships with AbbVie, Janssen, UCB, and others.
This article was updated 7/13/23.
, based on a retrospective study.
Nonadherence became increasingly common in the presence of four previously reported risk factors, including smoking status, narcotic use, psychiatric history, and prior biologic use, reported lead author Lauren A. George, MD, of the University of Maryland, Baltimore, and colleagues.
“Identifying patients at risk for nonadherence is important to develop strategies to improve adherence,” the investigators wrote in Gastro Hep Advances. “The aim of this analysis was to evaluate adherence across several academic centers with integrated specialty pharmacies, to assess if previously identified risk factors for nonadherence remained significant across several diverse IBD centers, and to evaluate outcomes associated with nonadherence.”
Three tertiary care IBD clinics provided data from 608 patients with IBD. Inclusion required at least three consecutive prescription claims. All biologics were self-injectable, including adalimumab, certolizumab, golimumab, and ustekinumab.
Primary outcomes were medication possession ratio (MPR) and adherence, with nonadherence defined by an MPR lower than 0.86. Secondary outcomes included ED visits and hospitalizations.
After a median follow-up period of 903 days, the overall MPR was 0.95, with adherence of 68%-70%, which is considered “high,” according to Dr. George and colleagues, as it exceeds previously reported national adherence rates.
“[Findings were] similar across all centers, geographic regions, and patient demographics,” the investigators noted.
The four previously described risk factors did in fact predict nonadherence, with likelihood of nonadherence significantly increasing with each additional risk factor present. Patients with all four risk factors had less than 50% adherence.
Nonadherence was also significantly associated with more ED visits and hospitalizations, highlighting “the impact of biologic adherence on direct patient outcomes and healthcare costs,” the investigators wrote.
“All healthcare industry stakeholders including healthcare systems, manufacturers, and third-party benefit providers need to understand the importance of improving patient adherence,” Dr. George and colleagues concluded. “Decreasing barriers to self-injectable medication acquisition, increasing direct patient interaction with integrated pharmacy teams, and comprehensive patient education are a start to improving patient adherence. In addition, we propose that enhanced care pathways for patients with risk factors for nonadherence would improve adherence and outcomes.”
No funding was reported. The investigators disclosed relationships with AbbVie, Janssen, UCB, and others.
This article was updated 7/13/23.
FROM GASTRO HEP ADVANCES















