User login
Digital Tools in the Management of IBS/ Functional GI Disorders
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
- Hasan SS et al. Neurogastroenterol Motil. 2023;35(4):e14554. doi:10.1111/nmo.14554
- Peters SL et al. Neurogastroenterol Motil. 2023;35(4):e14533. doi:10.1111/nmo.14533
- Zhou C et al. Neurogastroenterol Motil. 2019;31(2):e13461. doi:10.1111/nmo.13461
- Staudacher HM et al. Nat Rev Gastroenterol Hepatol. 2023;1-15. doi:10.1038/s41575-023-00794-z
- Qin HY et al. World J Gastroenterol. 2014;20(39):14126-14131. doi:10.3748/wjg.v20.i39.14126
- Varjú P et al. PLoS One. 2017;12(8):e0182942. doi:10.1371/journal.pone.0182942
- Saleh ZM et al. Am J Gastroenterol. 2023. doi:10.14309/ajg.0000000000002220
- Yu C et al. Clin Transl Gastroenterol. 2022;13(9):e00515. doi:10.14309/ctg.0000000000000515
- Jagannath B et al. Inflamm Bowel Dis. 2020;26(10):1533-1542. doi:10.1093/ibd/izaa191
- Zhang H et al. J Nutr. 2023;153(4):924-939. doi:10.1016/j.tjnut.2023.01.026
- Karakan T et al. Gut Microbes. 2022;14(1):2138672. doi:10.1080/19490976.2022.2138672
- Kordi M et al. Inform Med Unlocked. 2022;29:100891. doi:10.1016/j.imu.2022.100891
- Gubatan J et al. World J Gastroenterol. 2021;27(17):1920-1935. doi:10.3748/wjg.v27.i17.1920
- Boucher EM et al. Expert Rev Med Devices. 2021;18(suppl 1):37-49. doi:10.1080/17434440.2021.2013200
- Babel A et al. Front Digit Health. 2021;3:669869. doi:10.3389/fdgth.2021.669869
The Evolving Role of Surgery for IBD
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
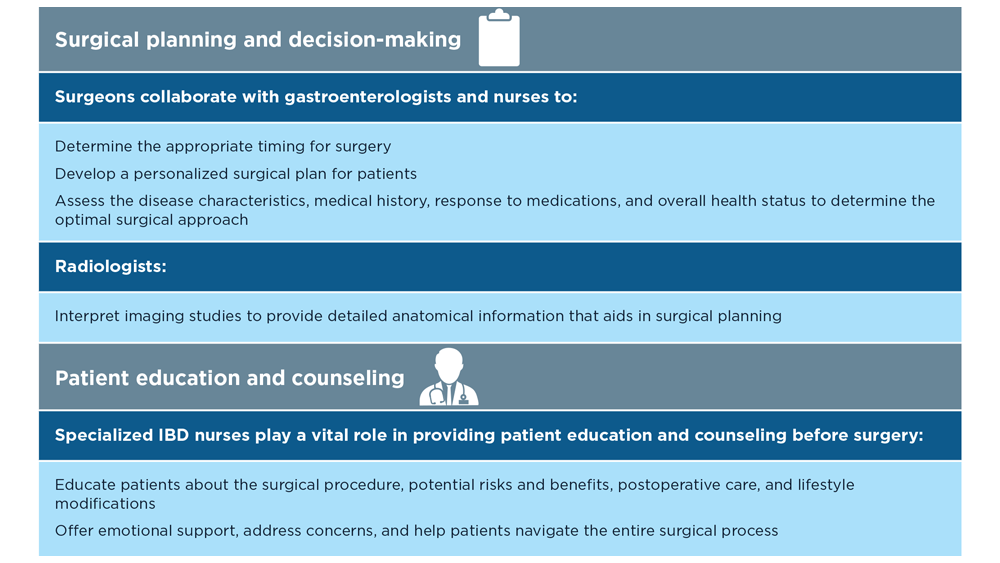
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
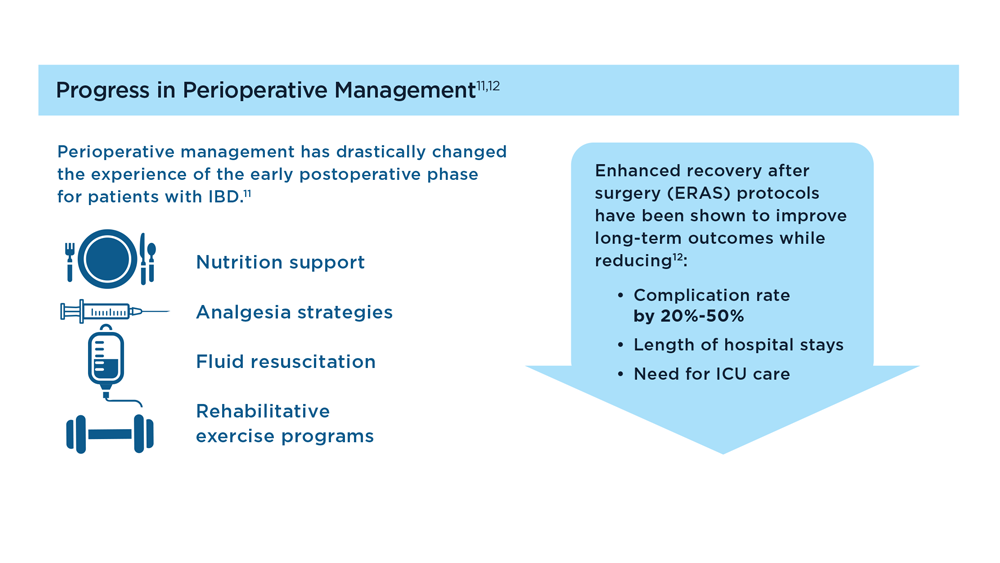
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
- Gul F et al. Ann Med Surg (Lond). 2022;81:104476. doi:10.1016/j.amsu.2022.104476
- Kotze PG et al. Clin Colon Rectal Surg. 2021;34(3):172-180. doi:10.1055/s-0040-1718685
- Bemelman WA; S-ECCO collaborators. J Crohns Colitis. 2018;12(8):1005-1007. doi:10.1093/ecco-jcc/jjy056
- Ricci C et al. Dig Liver Dis. 2008;40(suppl 2):S285-S288. doi:10.1016/S1590-8658(08)60539-3
- Lin X et al. Therap Adv Gastroenterol. 2022;15:17562848221104951. doi:10.1177/17562848221104951
- Parigi TL et al. Dis Colon Rectum. 2022;65(suppl 1):S119-S128. doi:10.1097/DCR.0000000000002548
- Pilonis ND et al. Transl Gastroenterol Hepatol. 2022;7:7. doi:10.21037/tgh.2020.04.02
- Misawa M et al. Clin Endosc. 2021;54(4):455-463. doi:10.5946/ce.2021.165
- de Sousa HT et al. Curr Opin Gastroenterol. 2018;34(4):194-207. doi:10.1097/MOG.0000000000000440
- Whitehead A, Cataldo PA. Clin Colon Rectal Surg. 2017;30(3):162-171. doi:10.1055/s-0037-1598156
- Cannon LM. The use of enhanced recovery pathways in patients undergoing surgery for inflammatory bowel disease. In: Hyman N, Fleshner P, Strong S, eds Mastery of IBD Surgery. Chicago, IL: University of Chicago Press; 2019:29-38. doi:10.1007/978-3-030-16755-4_4
- Ljungqvist O et al. World J Surg. 2020;44(10):3197–3198. doi:10.1007/s00268-020-05734-5
Gastroenterology Data Trends 2023
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
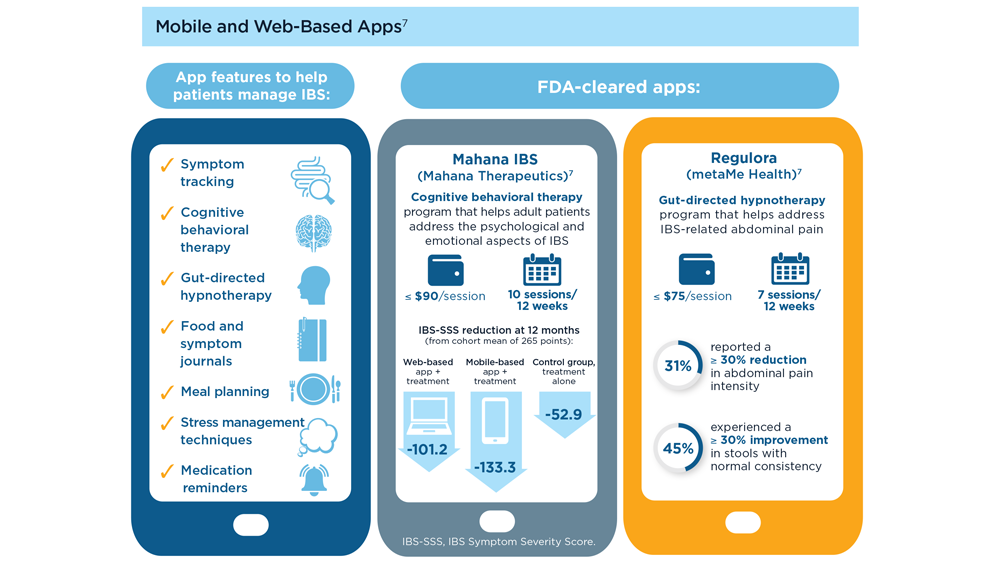
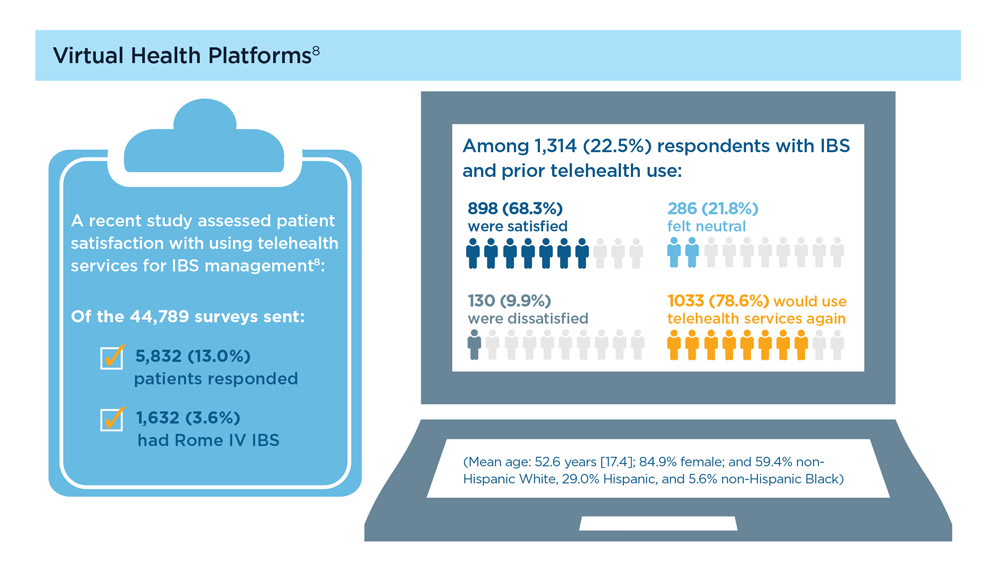
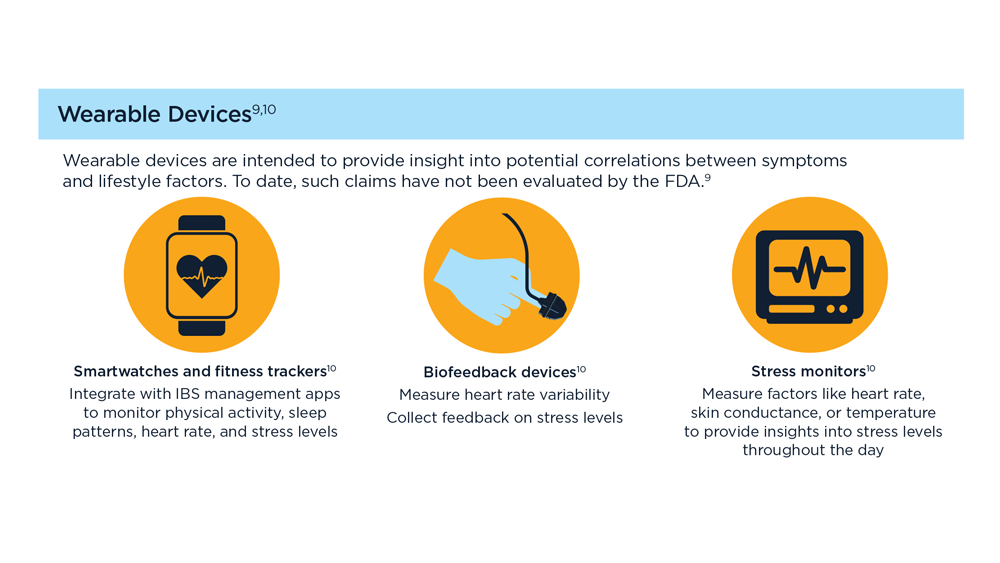
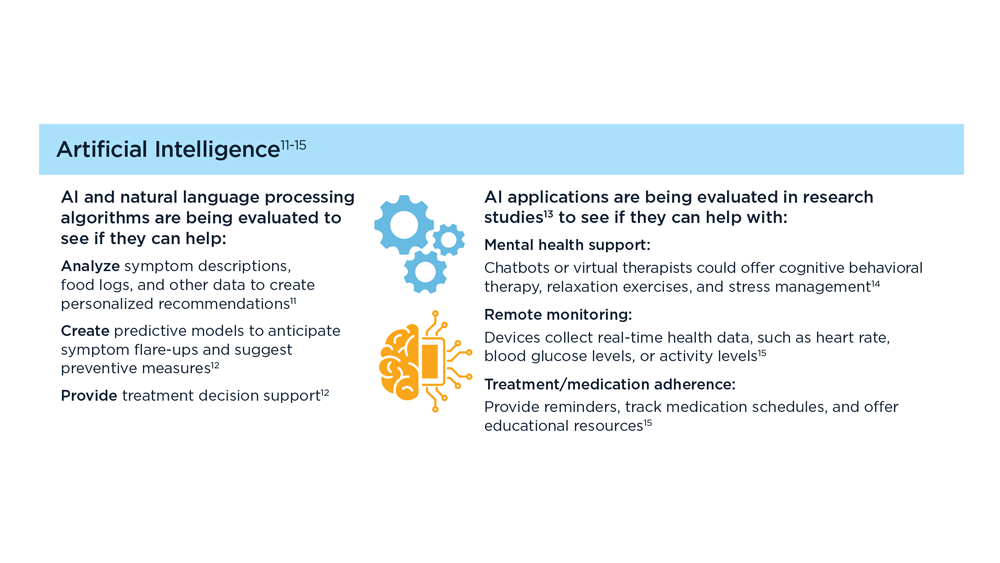
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
In this issue:
- Gastroenterology and Climate Change: Assessing and Mitigating Impacts
Swapna Gayam, MD, FACG - MASLD/MASH and Weight Loss
Arpan Mohanty, MD, MSc - Digital Tools in the Management of IBS/Functional GI Disorders
Eric D. Shah, MD, MBA, FACG - Long COVID and the Gastrointestinal System: Emerging Evidence
Daniel E. Freedberg, MD, MS, and Lin Chang, MD, AGAF - Germline Genetic Testing in CRC: Implications for Familial and Population-Based Testing
Fay Kastrinos, MD, MPH - Evolution of Targeted Therapies for C difficile
Sahil Khanna, MBBS, MS, FACG, AGAF - Harnessing the Power of AI to Enhance Endoscopy: Promises and Pitfalls
Eugenia Uche-Anya, MD, MPH - The Evolving Role of Surgery for IBD
Julie K.M. Thacker, MD, FACS, FASCRS
Rectal cancer risk is markedly higher at 10 years post colectomy
, shows a Danish population-based cohort study.
These findings suggest that more intensive long-term surveillance is needed for colectomized patients with IBD, wrote researchers who were led by Tine Jess, MD, DMSc, of the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen.
“Our nationwide population-based cohort study covering 4 decades shows that despite a relatively low absolute number of RC cases following colectomy for IBD, the risk of RC is markedly increased 10 years after the surgery. This calls for better long-term surveillance of colectomized IBD patients,” the authors wrote in Gastro Hep Advances.
Previous studies have suggested that patients with IBD have an increased risk of rectal cancer after colectomy, but these data “cannot stand alone,” and “need to be confirmed in other unselected patient cohorts,” investigators wrote.
The new study was based on an analysis of data from more than 9 million individuals in the Danish Civil Registration System between 1978 and 2018. The analyses were restricted to risk of rectal cancer in the population with diverted rectum.
The final dataset included 4,931 patients with IBD who had subtotal colectomy and diverted rectum, 49,251 matched patients with IBD who did not undergo colectomy, and 246,550 matched individuals without IBD to serve as a background population. Within these groups, rectal cancer occurred at a rate of 0.9%, 0.4%, and 0.4%, respectively, hinting at an increased risk of rectal cancer after colectomy among patients with IBD.
This signal was clarified by comparing rates of rectal cancer 10 years before and after colectomy. Rates 10 years before colectomy were not significantly different between groups.
Comparing colectomized IBD patients with the noncolectomized IBD patients at the 10-year postcolectomy mark revealed an eightfold increased risk of rectal cancer (hazard ratio, 7.56; 95% confidence interval, 5.21-10.86). Risk was slightly lower for patients with Crohn’s disease (HR, 5.10; 95% CI, 2.41-10.81) than for those with ulcerative colitis (HR, 9.42; 95% CI, 6.18-14.36).
A comparison at the same time point for colectomized IBD patients versus the background population showed an even higher relative risk for rectal cancer, up 10-fold (HR, 10.01; 95% CI, 7.20-13.94).
Despite variations in surgical methods, researchers concluded that the long-term risk of rectal cancer post colectomy increased among patients with IBD.
The findings should inform surveillance guidelines, they wrote.
“To reduce the risk of CRC in IBD, endoscopic surveillance guidelines have been developed both nationally and internationally. However, guidelines do not include clear recommendations for patients with a residual rectum, ileo-rectal anastomosis, or ileal pouch-anal anastomosis. The Danish guidelines, the Danish Society of Gastroenterology and Hepatology, mention a potential increased risk of rectal cancer post colectomy ... The European Crohn’s and Colitis Organization guideline consensus paper ‘European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies’ mentions that ‘the risk of rectal cancer is relatively high in IBD patients after subtotal colectomy’ [but] without further recommendation,” study authors wrote.
The study was supported by Laege Carl Emil Friis, Hustru Olga Doris Friis, and the Danish National Research Foundation. The investigators disclosed no conflicts of interest.
Rectal cancer risk in colectomized IBD patients is poorly understood, and most guidelines do not specify unique surveillance protocols for this subset of patients. As such, gastroenterologists are often left using their best judgement to decide surveillance intervals in this group.
In a Danish population-based cohort study, Akimenko and colleagues identify a markedly increased risk of rectal cancer 10 years after colectomy in patients with a diverted rectum. This risk is 8-fold compared to a matched IBD cohort without colectomy, 10-fold compared to the background population, and is slightly higher in ulcerative colitis than Crohn’s disease. The relative risk is similar to that identified in a Swedish nationwide study.
The study benefits from a large, unselected cohort and its use of a matched IBD population without colectomy. However, it is not sufficiently powered to assess cancer risk in patients with IRA or IPAA, thus limiting its generalizability. The lengthy 40-year inclusion period, while providing strength in numbers, may also impact the study findings, as significant changes have occurred in IBD management during this timeframe.
The authors herald an important reminder that post-colectomy IBD patients are not ‘out of the woods’ with regards to rectal cancer risk. Inconsistency exists amongst providers when it comes to surveillance intervals in these patients.
The study highlights the need for specific surveillance guidelines for this group, particularly in patients with a diverted rectum. Additional studies are needed to assess risk in patients with IRA or IPAA.
Dr. Maté Gergely is an assistant professor of medicine within the division of gastroenterology at Washington University School of Medicine, St. Louis. He has no relevant disclosures.
Rectal cancer risk in colectomized IBD patients is poorly understood, and most guidelines do not specify unique surveillance protocols for this subset of patients. As such, gastroenterologists are often left using their best judgement to decide surveillance intervals in this group.
In a Danish population-based cohort study, Akimenko and colleagues identify a markedly increased risk of rectal cancer 10 years after colectomy in patients with a diverted rectum. This risk is 8-fold compared to a matched IBD cohort without colectomy, 10-fold compared to the background population, and is slightly higher in ulcerative colitis than Crohn’s disease. The relative risk is similar to that identified in a Swedish nationwide study.
The study benefits from a large, unselected cohort and its use of a matched IBD population without colectomy. However, it is not sufficiently powered to assess cancer risk in patients with IRA or IPAA, thus limiting its generalizability. The lengthy 40-year inclusion period, while providing strength in numbers, may also impact the study findings, as significant changes have occurred in IBD management during this timeframe.
The authors herald an important reminder that post-colectomy IBD patients are not ‘out of the woods’ with regards to rectal cancer risk. Inconsistency exists amongst providers when it comes to surveillance intervals in these patients.
The study highlights the need for specific surveillance guidelines for this group, particularly in patients with a diverted rectum. Additional studies are needed to assess risk in patients with IRA or IPAA.
Dr. Maté Gergely is an assistant professor of medicine within the division of gastroenterology at Washington University School of Medicine, St. Louis. He has no relevant disclosures.
Rectal cancer risk in colectomized IBD patients is poorly understood, and most guidelines do not specify unique surveillance protocols for this subset of patients. As such, gastroenterologists are often left using their best judgement to decide surveillance intervals in this group.
In a Danish population-based cohort study, Akimenko and colleagues identify a markedly increased risk of rectal cancer 10 years after colectomy in patients with a diverted rectum. This risk is 8-fold compared to a matched IBD cohort without colectomy, 10-fold compared to the background population, and is slightly higher in ulcerative colitis than Crohn’s disease. The relative risk is similar to that identified in a Swedish nationwide study.
The study benefits from a large, unselected cohort and its use of a matched IBD population without colectomy. However, it is not sufficiently powered to assess cancer risk in patients with IRA or IPAA, thus limiting its generalizability. The lengthy 40-year inclusion period, while providing strength in numbers, may also impact the study findings, as significant changes have occurred in IBD management during this timeframe.
The authors herald an important reminder that post-colectomy IBD patients are not ‘out of the woods’ with regards to rectal cancer risk. Inconsistency exists amongst providers when it comes to surveillance intervals in these patients.
The study highlights the need for specific surveillance guidelines for this group, particularly in patients with a diverted rectum. Additional studies are needed to assess risk in patients with IRA or IPAA.
Dr. Maté Gergely is an assistant professor of medicine within the division of gastroenterology at Washington University School of Medicine, St. Louis. He has no relevant disclosures.
, shows a Danish population-based cohort study.
These findings suggest that more intensive long-term surveillance is needed for colectomized patients with IBD, wrote researchers who were led by Tine Jess, MD, DMSc, of the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen.
“Our nationwide population-based cohort study covering 4 decades shows that despite a relatively low absolute number of RC cases following colectomy for IBD, the risk of RC is markedly increased 10 years after the surgery. This calls for better long-term surveillance of colectomized IBD patients,” the authors wrote in Gastro Hep Advances.
Previous studies have suggested that patients with IBD have an increased risk of rectal cancer after colectomy, but these data “cannot stand alone,” and “need to be confirmed in other unselected patient cohorts,” investigators wrote.
The new study was based on an analysis of data from more than 9 million individuals in the Danish Civil Registration System between 1978 and 2018. The analyses were restricted to risk of rectal cancer in the population with diverted rectum.
The final dataset included 4,931 patients with IBD who had subtotal colectomy and diverted rectum, 49,251 matched patients with IBD who did not undergo colectomy, and 246,550 matched individuals without IBD to serve as a background population. Within these groups, rectal cancer occurred at a rate of 0.9%, 0.4%, and 0.4%, respectively, hinting at an increased risk of rectal cancer after colectomy among patients with IBD.
This signal was clarified by comparing rates of rectal cancer 10 years before and after colectomy. Rates 10 years before colectomy were not significantly different between groups.
Comparing colectomized IBD patients with the noncolectomized IBD patients at the 10-year postcolectomy mark revealed an eightfold increased risk of rectal cancer (hazard ratio, 7.56; 95% confidence interval, 5.21-10.86). Risk was slightly lower for patients with Crohn’s disease (HR, 5.10; 95% CI, 2.41-10.81) than for those with ulcerative colitis (HR, 9.42; 95% CI, 6.18-14.36).
A comparison at the same time point for colectomized IBD patients versus the background population showed an even higher relative risk for rectal cancer, up 10-fold (HR, 10.01; 95% CI, 7.20-13.94).
Despite variations in surgical methods, researchers concluded that the long-term risk of rectal cancer post colectomy increased among patients with IBD.
The findings should inform surveillance guidelines, they wrote.
“To reduce the risk of CRC in IBD, endoscopic surveillance guidelines have been developed both nationally and internationally. However, guidelines do not include clear recommendations for patients with a residual rectum, ileo-rectal anastomosis, or ileal pouch-anal anastomosis. The Danish guidelines, the Danish Society of Gastroenterology and Hepatology, mention a potential increased risk of rectal cancer post colectomy ... The European Crohn’s and Colitis Organization guideline consensus paper ‘European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies’ mentions that ‘the risk of rectal cancer is relatively high in IBD patients after subtotal colectomy’ [but] without further recommendation,” study authors wrote.
The study was supported by Laege Carl Emil Friis, Hustru Olga Doris Friis, and the Danish National Research Foundation. The investigators disclosed no conflicts of interest.
, shows a Danish population-based cohort study.
These findings suggest that more intensive long-term surveillance is needed for colectomized patients with IBD, wrote researchers who were led by Tine Jess, MD, DMSc, of the Center for Molecular Prediction of Inflammatory Bowel Disease, Aalborg University, Copenhagen.
“Our nationwide population-based cohort study covering 4 decades shows that despite a relatively low absolute number of RC cases following colectomy for IBD, the risk of RC is markedly increased 10 years after the surgery. This calls for better long-term surveillance of colectomized IBD patients,” the authors wrote in Gastro Hep Advances.
Previous studies have suggested that patients with IBD have an increased risk of rectal cancer after colectomy, but these data “cannot stand alone,” and “need to be confirmed in other unselected patient cohorts,” investigators wrote.
The new study was based on an analysis of data from more than 9 million individuals in the Danish Civil Registration System between 1978 and 2018. The analyses were restricted to risk of rectal cancer in the population with diverted rectum.
The final dataset included 4,931 patients with IBD who had subtotal colectomy and diverted rectum, 49,251 matched patients with IBD who did not undergo colectomy, and 246,550 matched individuals without IBD to serve as a background population. Within these groups, rectal cancer occurred at a rate of 0.9%, 0.4%, and 0.4%, respectively, hinting at an increased risk of rectal cancer after colectomy among patients with IBD.
This signal was clarified by comparing rates of rectal cancer 10 years before and after colectomy. Rates 10 years before colectomy were not significantly different between groups.
Comparing colectomized IBD patients with the noncolectomized IBD patients at the 10-year postcolectomy mark revealed an eightfold increased risk of rectal cancer (hazard ratio, 7.56; 95% confidence interval, 5.21-10.86). Risk was slightly lower for patients with Crohn’s disease (HR, 5.10; 95% CI, 2.41-10.81) than for those with ulcerative colitis (HR, 9.42; 95% CI, 6.18-14.36).
A comparison at the same time point for colectomized IBD patients versus the background population showed an even higher relative risk for rectal cancer, up 10-fold (HR, 10.01; 95% CI, 7.20-13.94).
Despite variations in surgical methods, researchers concluded that the long-term risk of rectal cancer post colectomy increased among patients with IBD.
The findings should inform surveillance guidelines, they wrote.
“To reduce the risk of CRC in IBD, endoscopic surveillance guidelines have been developed both nationally and internationally. However, guidelines do not include clear recommendations for patients with a residual rectum, ileo-rectal anastomosis, or ileal pouch-anal anastomosis. The Danish guidelines, the Danish Society of Gastroenterology and Hepatology, mention a potential increased risk of rectal cancer post colectomy ... The European Crohn’s and Colitis Organization guideline consensus paper ‘European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies’ mentions that ‘the risk of rectal cancer is relatively high in IBD patients after subtotal colectomy’ [but] without further recommendation,” study authors wrote.
The study was supported by Laege Carl Emil Friis, Hustru Olga Doris Friis, and the Danish National Research Foundation. The investigators disclosed no conflicts of interest.
FROM GASTRO HEP ADVANCES
AGA CPU: Essentials to prevent complications with ostomies
The American Gastroenterological Association has published a new Clinical Practice Update for the management of enteral ostomies, which are common in the management of patients with colorectal cancer, inflammatory bowel disease, diverticular disease, intestinal trauma, and intestinal perforation.
Approximately 750,000 people in the United States live with an ostomy, including colostomy, ileostomy, and continent ileostomy. Complications and challenges with self-care are common among patients with an enteral stoma, but most available guidance documents fail to offer management principles beyond the immediate perioperative period, wrote authors of the guidance which was led by Traci Hedrick, MD, of the University of Virginia Health, Charlottesville.
The update was published online in Clinical Gastroenterology and Hepatology. It includes best practice updates for managing short- and long-term complications, and perioperative considerations.
Early high ostomy output, defined by ostomy output greater than fluid intake that occurs within 3 weeks of stoma formation, causing dehydration, is a short-term complication associated with ostomies. It is more common among patients with an ileostomy than a colostomy, and requires rapid evaluation for infection and other associated complications. The cornerstone of treatment is rehydration, usually intravenously during hospital stay. Additional treatments may include bulking agents, antimotility agents, antisecretory agents, anti-inflammatory agents, adaptation-promoting agents, and surgery to reverse the ostomy.
Other short-term complications include ostomy leakage, stomal retraction, and mucocutaneous separation.
Dermatological problems are the most common of long-term complications. These typically involve skin irritation due to leakage. Other dermatological complaints include folliculitis, fungal rash, and allergic reaction to the appliance. Each of these must be addressed based on the nature and underlying cause of the complication.
Other long-term complications include chronic high ostomy output, parastomal hernia, and stomal prolapse.
Clinicians should also be aware of the psychological impact on patients. They may fear having a leakage or emitting an odor. They may also have anxiety in disclosing having an ostomy to partners and fear travel. “Difficulty with self-care should be addressed through preoperative and postoperative education. Preoperative education and stoma site marking has been shown to improve quality of life and decrease peristomal skin and pouching complications,” the authors wrote.
Health care providers should discuss and manage expectations for life with an ostomy, including managing ostomy output, maintaining pouching appliances, and the regular passage of mucus from the native rectum.
“High-quality ostomy care begins at the preoperative visit with wound ostomy and continence consultation. Stoma education and counseling are essential to prevent complications and manage patient expectations for living with a stoma. The early diagnosis and management of both early and late ostomy complications require ongoing communication between patients and care teams. Multidisciplinary coordination is imperative to prevent hospital readmissions and to improve the quality of life for our patients living with ostomies,” the authors wrote.
This Clinical Practice Update was commissioned by the AGA Institute. The investigators disclosed relationships with Johnson & Johnson, AbbVie, BMS, and others.
The American Gastroenterological Association has published a new Clinical Practice Update for the management of enteral ostomies, which are common in the management of patients with colorectal cancer, inflammatory bowel disease, diverticular disease, intestinal trauma, and intestinal perforation.
Approximately 750,000 people in the United States live with an ostomy, including colostomy, ileostomy, and continent ileostomy. Complications and challenges with self-care are common among patients with an enteral stoma, but most available guidance documents fail to offer management principles beyond the immediate perioperative period, wrote authors of the guidance which was led by Traci Hedrick, MD, of the University of Virginia Health, Charlottesville.
The update was published online in Clinical Gastroenterology and Hepatology. It includes best practice updates for managing short- and long-term complications, and perioperative considerations.
Early high ostomy output, defined by ostomy output greater than fluid intake that occurs within 3 weeks of stoma formation, causing dehydration, is a short-term complication associated with ostomies. It is more common among patients with an ileostomy than a colostomy, and requires rapid evaluation for infection and other associated complications. The cornerstone of treatment is rehydration, usually intravenously during hospital stay. Additional treatments may include bulking agents, antimotility agents, antisecretory agents, anti-inflammatory agents, adaptation-promoting agents, and surgery to reverse the ostomy.
Other short-term complications include ostomy leakage, stomal retraction, and mucocutaneous separation.
Dermatological problems are the most common of long-term complications. These typically involve skin irritation due to leakage. Other dermatological complaints include folliculitis, fungal rash, and allergic reaction to the appliance. Each of these must be addressed based on the nature and underlying cause of the complication.
Other long-term complications include chronic high ostomy output, parastomal hernia, and stomal prolapse.
Clinicians should also be aware of the psychological impact on patients. They may fear having a leakage or emitting an odor. They may also have anxiety in disclosing having an ostomy to partners and fear travel. “Difficulty with self-care should be addressed through preoperative and postoperative education. Preoperative education and stoma site marking has been shown to improve quality of life and decrease peristomal skin and pouching complications,” the authors wrote.
Health care providers should discuss and manage expectations for life with an ostomy, including managing ostomy output, maintaining pouching appliances, and the regular passage of mucus from the native rectum.
“High-quality ostomy care begins at the preoperative visit with wound ostomy and continence consultation. Stoma education and counseling are essential to prevent complications and manage patient expectations for living with a stoma. The early diagnosis and management of both early and late ostomy complications require ongoing communication between patients and care teams. Multidisciplinary coordination is imperative to prevent hospital readmissions and to improve the quality of life for our patients living with ostomies,” the authors wrote.
This Clinical Practice Update was commissioned by the AGA Institute. The investigators disclosed relationships with Johnson & Johnson, AbbVie, BMS, and others.
The American Gastroenterological Association has published a new Clinical Practice Update for the management of enteral ostomies, which are common in the management of patients with colorectal cancer, inflammatory bowel disease, diverticular disease, intestinal trauma, and intestinal perforation.
Approximately 750,000 people in the United States live with an ostomy, including colostomy, ileostomy, and continent ileostomy. Complications and challenges with self-care are common among patients with an enteral stoma, but most available guidance documents fail to offer management principles beyond the immediate perioperative period, wrote authors of the guidance which was led by Traci Hedrick, MD, of the University of Virginia Health, Charlottesville.
The update was published online in Clinical Gastroenterology and Hepatology. It includes best practice updates for managing short- and long-term complications, and perioperative considerations.
Early high ostomy output, defined by ostomy output greater than fluid intake that occurs within 3 weeks of stoma formation, causing dehydration, is a short-term complication associated with ostomies. It is more common among patients with an ileostomy than a colostomy, and requires rapid evaluation for infection and other associated complications. The cornerstone of treatment is rehydration, usually intravenously during hospital stay. Additional treatments may include bulking agents, antimotility agents, antisecretory agents, anti-inflammatory agents, adaptation-promoting agents, and surgery to reverse the ostomy.
Other short-term complications include ostomy leakage, stomal retraction, and mucocutaneous separation.
Dermatological problems are the most common of long-term complications. These typically involve skin irritation due to leakage. Other dermatological complaints include folliculitis, fungal rash, and allergic reaction to the appliance. Each of these must be addressed based on the nature and underlying cause of the complication.
Other long-term complications include chronic high ostomy output, parastomal hernia, and stomal prolapse.
Clinicians should also be aware of the psychological impact on patients. They may fear having a leakage or emitting an odor. They may also have anxiety in disclosing having an ostomy to partners and fear travel. “Difficulty with self-care should be addressed through preoperative and postoperative education. Preoperative education and stoma site marking has been shown to improve quality of life and decrease peristomal skin and pouching complications,” the authors wrote.
Health care providers should discuss and manage expectations for life with an ostomy, including managing ostomy output, maintaining pouching appliances, and the regular passage of mucus from the native rectum.
“High-quality ostomy care begins at the preoperative visit with wound ostomy and continence consultation. Stoma education and counseling are essential to prevent complications and manage patient expectations for living with a stoma. The early diagnosis and management of both early and late ostomy complications require ongoing communication between patients and care teams. Multidisciplinary coordination is imperative to prevent hospital readmissions and to improve the quality of life for our patients living with ostomies,” the authors wrote.
This Clinical Practice Update was commissioned by the AGA Institute. The investigators disclosed relationships with Johnson & Johnson, AbbVie, BMS, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Crohn’s disease indicators manifest years before diagnosis
Changes in proteins and antibodies suggesting immune dysregulation may be detectable in serum years before clinical manifestation of complicated Crohn’s disease, shows a study recently published in Clinical Gastroenterology and Hepatology.
These preclinical signatures could one day play a role in screening for complicated Crohn’s disease (CD) and in mapping underlying disease pathways, potentially opening doors to new preventive approaches, wrote study authors who were led by Joseph A. Murray, MD, of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“Mounting evidence suggests that the diagnosis of CD is preceded by a lengthy asymptomatic preclinical period,” investigators wrote. “Gaining insight into this phase may allow a better understanding of the primary events that lead to its development and offer potential strategies to predict and prevent the disease including its complications.”
The study, which was a nested case-control study based on the PREDICTS study, included 201 patients with CD who had serum samples archived 2, 4, and 6 years prior to diagnosis, as well as 201 healthy controls who provided serum samples for comparison. Serum samples were analyzed with a comprehensive panel of 1,129 proteomic markers and antimicrobial antibodies.
At time of diagnosis, 47 of the patients with CD (24%) had a complicated phenotype, including stricturing behavior, penetrating behavior, or need for early surgery.
“The unique availability of preclinical samples collected at multiple time points allowed us to examine the sequence of immunological changes and protein biomarkers that occurred before diagnosis,” the investigators wrote. “We also evaluated a wide array of protein biomarkers, utilizing a novel proteomic platform, and applied novel rigorous statistical approaches, which allowed us to discover the potential biomarkers and biologic pathways for the complicated phenotypes even before diagnosis.”
As early as 6 years before diagnosis, patients with complicated CD had significantly higher levels of antimicrobial antibodies than patients with noncomplicated CD, as well as elevations in 22 protein biomarkers indicating immune dysregulation.
Specifically, complicated CD was preceded by elevated anti–Saccharomyces cerevisiae antibodies (ASCA) IgA/IgG, anti-Flagellin antibodies, and other proteins linked with fibrosis, adaptive immunity, and innate immunity. Simultaneously, the same patients had reduced levels of protein biomarkers linked with protection against fibrosis and tissue damage. Network analysis added weight to these findings by demonstrating a significant correlation between ASCA IgA/IgG and protein biomarkers tied to innate immunity and lack of factors for tissue protection.
“Altogether, the hypothesis can be proposed that the combination of increasing levels of inflammatory cytokines, loss of anti-inflammatory proteins, and production of antimicrobial antibodies could accelerate and magnify tissue destruction and fibrosis driving complications at diagnosis in CD,” the investigators wrote. “These data support the concept that complicated CD may not always be the result of the progression of an uncontrolled inflammatory disease but may also be the consequence of a unique pathophysiological process.”
The findings deserve further investigation as they could lead to new clinical tools for screening and intervention. “The serological signature that we identified could help to further select subjects at risk of developing complicated CD who could be preferential candidates for preventative strategies,” investigators wrote.
The investigators disclosed relationships with Janssen, AbbVie, Galapagos, and others.
Certainly, insights into the preclinical phase of a disease state such as IBD may allow for potential preventive interventions and possible avoidance of disease-related complications. In this important study by Choung and colleagues, pre-diagnosis serum analyses of protein biomarkers and antimicrobial antibodies revealed that certain signatures are associated with a complicated phenotype of Crohn’s disease at time of diagnosis compared to patients with uncomplicated Crohn’s disease. Specifically, patients with complicated Crohn’s disease at diagnosis had higher levels of anti-microbial antibodies and altered protein biomarkers with overproduction of inflammatory proteins 6 years before their date of diagnosis, likely reflecting a dysregulated innate immune system, excessive adaptive response to microbial antigens, and fibrosis.
Although the results of this study are very exciting and may help support further research to risk stratify and possibly prevent progression of Crohn’s disease in the pre-clinical stage, additional confirmatory studies are needed before these observations are ready for real world application.
Jana G. Al Hashash, MD, MSc, associate professor of medicine, Mayo Clinic, Florida. She has no conflicts.
Certainly, insights into the preclinical phase of a disease state such as IBD may allow for potential preventive interventions and possible avoidance of disease-related complications. In this important study by Choung and colleagues, pre-diagnosis serum analyses of protein biomarkers and antimicrobial antibodies revealed that certain signatures are associated with a complicated phenotype of Crohn’s disease at time of diagnosis compared to patients with uncomplicated Crohn’s disease. Specifically, patients with complicated Crohn’s disease at diagnosis had higher levels of anti-microbial antibodies and altered protein biomarkers with overproduction of inflammatory proteins 6 years before their date of diagnosis, likely reflecting a dysregulated innate immune system, excessive adaptive response to microbial antigens, and fibrosis.
Although the results of this study are very exciting and may help support further research to risk stratify and possibly prevent progression of Crohn’s disease in the pre-clinical stage, additional confirmatory studies are needed before these observations are ready for real world application.
Jana G. Al Hashash, MD, MSc, associate professor of medicine, Mayo Clinic, Florida. She has no conflicts.
Certainly, insights into the preclinical phase of a disease state such as IBD may allow for potential preventive interventions and possible avoidance of disease-related complications. In this important study by Choung and colleagues, pre-diagnosis serum analyses of protein biomarkers and antimicrobial antibodies revealed that certain signatures are associated with a complicated phenotype of Crohn’s disease at time of diagnosis compared to patients with uncomplicated Crohn’s disease. Specifically, patients with complicated Crohn’s disease at diagnosis had higher levels of anti-microbial antibodies and altered protein biomarkers with overproduction of inflammatory proteins 6 years before their date of diagnosis, likely reflecting a dysregulated innate immune system, excessive adaptive response to microbial antigens, and fibrosis.
Although the results of this study are very exciting and may help support further research to risk stratify and possibly prevent progression of Crohn’s disease in the pre-clinical stage, additional confirmatory studies are needed before these observations are ready for real world application.
Jana G. Al Hashash, MD, MSc, associate professor of medicine, Mayo Clinic, Florida. She has no conflicts.
Changes in proteins and antibodies suggesting immune dysregulation may be detectable in serum years before clinical manifestation of complicated Crohn’s disease, shows a study recently published in Clinical Gastroenterology and Hepatology.
These preclinical signatures could one day play a role in screening for complicated Crohn’s disease (CD) and in mapping underlying disease pathways, potentially opening doors to new preventive approaches, wrote study authors who were led by Joseph A. Murray, MD, of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“Mounting evidence suggests that the diagnosis of CD is preceded by a lengthy asymptomatic preclinical period,” investigators wrote. “Gaining insight into this phase may allow a better understanding of the primary events that lead to its development and offer potential strategies to predict and prevent the disease including its complications.”
The study, which was a nested case-control study based on the PREDICTS study, included 201 patients with CD who had serum samples archived 2, 4, and 6 years prior to diagnosis, as well as 201 healthy controls who provided serum samples for comparison. Serum samples were analyzed with a comprehensive panel of 1,129 proteomic markers and antimicrobial antibodies.
At time of diagnosis, 47 of the patients with CD (24%) had a complicated phenotype, including stricturing behavior, penetrating behavior, or need for early surgery.
“The unique availability of preclinical samples collected at multiple time points allowed us to examine the sequence of immunological changes and protein biomarkers that occurred before diagnosis,” the investigators wrote. “We also evaluated a wide array of protein biomarkers, utilizing a novel proteomic platform, and applied novel rigorous statistical approaches, which allowed us to discover the potential biomarkers and biologic pathways for the complicated phenotypes even before diagnosis.”
As early as 6 years before diagnosis, patients with complicated CD had significantly higher levels of antimicrobial antibodies than patients with noncomplicated CD, as well as elevations in 22 protein biomarkers indicating immune dysregulation.
Specifically, complicated CD was preceded by elevated anti–Saccharomyces cerevisiae antibodies (ASCA) IgA/IgG, anti-Flagellin antibodies, and other proteins linked with fibrosis, adaptive immunity, and innate immunity. Simultaneously, the same patients had reduced levels of protein biomarkers linked with protection against fibrosis and tissue damage. Network analysis added weight to these findings by demonstrating a significant correlation between ASCA IgA/IgG and protein biomarkers tied to innate immunity and lack of factors for tissue protection.
“Altogether, the hypothesis can be proposed that the combination of increasing levels of inflammatory cytokines, loss of anti-inflammatory proteins, and production of antimicrobial antibodies could accelerate and magnify tissue destruction and fibrosis driving complications at diagnosis in CD,” the investigators wrote. “These data support the concept that complicated CD may not always be the result of the progression of an uncontrolled inflammatory disease but may also be the consequence of a unique pathophysiological process.”
The findings deserve further investigation as they could lead to new clinical tools for screening and intervention. “The serological signature that we identified could help to further select subjects at risk of developing complicated CD who could be preferential candidates for preventative strategies,” investigators wrote.
The investigators disclosed relationships with Janssen, AbbVie, Galapagos, and others.
Changes in proteins and antibodies suggesting immune dysregulation may be detectable in serum years before clinical manifestation of complicated Crohn’s disease, shows a study recently published in Clinical Gastroenterology and Hepatology.
These preclinical signatures could one day play a role in screening for complicated Crohn’s disease (CD) and in mapping underlying disease pathways, potentially opening doors to new preventive approaches, wrote study authors who were led by Joseph A. Murray, MD, of the division of gastroenterology and hepatology at Mayo Clinic, Rochester, Minn.
“Mounting evidence suggests that the diagnosis of CD is preceded by a lengthy asymptomatic preclinical period,” investigators wrote. “Gaining insight into this phase may allow a better understanding of the primary events that lead to its development and offer potential strategies to predict and prevent the disease including its complications.”
The study, which was a nested case-control study based on the PREDICTS study, included 201 patients with CD who had serum samples archived 2, 4, and 6 years prior to diagnosis, as well as 201 healthy controls who provided serum samples for comparison. Serum samples were analyzed with a comprehensive panel of 1,129 proteomic markers and antimicrobial antibodies.
At time of diagnosis, 47 of the patients with CD (24%) had a complicated phenotype, including stricturing behavior, penetrating behavior, or need for early surgery.
“The unique availability of preclinical samples collected at multiple time points allowed us to examine the sequence of immunological changes and protein biomarkers that occurred before diagnosis,” the investigators wrote. “We also evaluated a wide array of protein biomarkers, utilizing a novel proteomic platform, and applied novel rigorous statistical approaches, which allowed us to discover the potential biomarkers and biologic pathways for the complicated phenotypes even before diagnosis.”
As early as 6 years before diagnosis, patients with complicated CD had significantly higher levels of antimicrobial antibodies than patients with noncomplicated CD, as well as elevations in 22 protein biomarkers indicating immune dysregulation.
Specifically, complicated CD was preceded by elevated anti–Saccharomyces cerevisiae antibodies (ASCA) IgA/IgG, anti-Flagellin antibodies, and other proteins linked with fibrosis, adaptive immunity, and innate immunity. Simultaneously, the same patients had reduced levels of protein biomarkers linked with protection against fibrosis and tissue damage. Network analysis added weight to these findings by demonstrating a significant correlation between ASCA IgA/IgG and protein biomarkers tied to innate immunity and lack of factors for tissue protection.
“Altogether, the hypothesis can be proposed that the combination of increasing levels of inflammatory cytokines, loss of anti-inflammatory proteins, and production of antimicrobial antibodies could accelerate and magnify tissue destruction and fibrosis driving complications at diagnosis in CD,” the investigators wrote. “These data support the concept that complicated CD may not always be the result of the progression of an uncontrolled inflammatory disease but may also be the consequence of a unique pathophysiological process.”
The findings deserve further investigation as they could lead to new clinical tools for screening and intervention. “The serological signature that we identified could help to further select subjects at risk of developing complicated CD who could be preferential candidates for preventative strategies,” investigators wrote.
The investigators disclosed relationships with Janssen, AbbVie, Galapagos, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Bridging the gap between GI disorders and nutrition
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.
The gluten-free section in the grocery store didn’t exist when Renee Euler, MS, RD, LD, was diagnosed with celiac disease 30 years ago. A physician handed her a fax about the gluten-free diet from a national support group and said: “Here, read this.”
There was no Google to inform decisions. Patients had to rely on fact sheets or a book from the library.

“I didn’t realize how much nutrition was going to change my world,” said Ms. Euler, who worked as a landscape architect for 15 years before making a pivotal decision to go back to school and train as a dietitian.
Volunteering as a support group leader, and volunteering with the University of Chicago Celiac Disease Center guided this important career change. Ms. Euler discovered she enjoyed teaching people how to live a gluten-free life and that they could enjoy travel and social functions while adhering to dietary restrictions.
she emphasized.
Ms. Euler has made it her life’s work to navigate GI disorders with physicians and patients alike.
She runs her own business, Nutrition Redefined, in Albuquerque and is the chair of the National Celiac Association Celiac/Gluten Intolerance Support Group in Albuquerque. Previously, she chaired the Dietitians in Medical Nutrition Therapy Dietetic Practice Group, a part of the Academy of Nutrition and Dietetics.
In an interview, she talked about the unique dietary struggles people with celiac and other gastrointestinal conditions face, and the strategies she uses to help these patients overcome hurdles and live a more normal life.
Q: What fears did you have to push past to get to where you are in your career?
Ms. Euler: Leaving a successful career as a landscape architect and going back to school was definitely a huge hurdle. When I started my practice in 2017, in my area there were no outpatient GI dietitians providing specialized care for adults with conditions like celiac disease, irritable bowel syndrome (IBS), and inflammatory bowel disease (IBD). I was starting out with no real support.
Realizing that I was going to start a private practice of my own to help the people I wanted to help, was another big fear. “Am I going to succeed? Am I going to fail? What’s going to happen?” But over the years, my practice has grown as I learned to bill insurance and started receiving referrals from a large local GI practice, both of which have been the keys to my success. I have also limited my practice to GI clients so that I can focus my attention on this specialized area of nutrition and stay up to date on the latest developments.
Q: What interests you about the intersection between diet and GI disorders?
Ms. Euler: It’s not just about diet. We’re learning so much about how the gut microbiome can have a potential impact [on other parts of our health]. It’s interesting in terms of how we respond to certain foods, for instance, could affect our mental health. This especially applies to IBS and how the microbiome might be connected to these conditions.
It’s very challenging. There is so much information out there that is not super accurate, or it’s misleading.
Q: You serve as a liaison between the American Gastroenterological Association and the Academy of Nutrition and Dietetics. As a nutritionist with a focus on GI, how do you work with gastroenterologists to manage GI disorders?
Ms. Euler: Some of the dietary therapies that GI doctors recommend don’t provide sufficient guidance. They hand out that two-page fact sheet about diet and send the patient on their way. A lot of these diets have more nuance than what can be expressed in a two-page handout.
Many times, the physician doesn’t know the nuance, or they don’t have time to go over it. That’s where we can really help.
Patients often want diet to be the answer. They want to be told: “You need to eat this and only this, and everything will be fine, and diet’s going to change your world, and you won’t have to take medication.”
What they often don’t realize and understand, is a lot of these dietary therapies are not black and white. Celiac disease means a gluten-free diet for life. But a lot of these dietary therapies that get thrown out to patients as a possibility, like low FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols), are not lifetime diets. They’re tools for us to use to find out what the offending foods are for this person, and what can we do to get their symptoms under control.
Q: What is the biggest practice-related challenge in getting patients to alter their diet to improve their symptoms?
Ms. Euler: A lot of patients that come to me already have over restricted diets. They’re trying to solve things themselves. Rightfully so, a lot of them have a lot of food fears because they have been living with very uncomfortable symptoms for years, and they’re trying to find answers. Those food fears unfortunately are reinforced by social media and the news.
One of my biggest challenges with those clients is working through that process of building their confidence to broaden their diets and add foods back in, without causing their symptoms to flare up. The goal is to get them back on track to having a nutritious diet while trying to manage symptoms.
Q: Can you give me an anecdotal example of a case that wasn’t easy, and you ended up helping that person?
Ms. Euler: I had a patient who had been listening to all the wellness gurus. She was overrestricted to the point of eating just 10 different foods due to allergic and GI symptoms. Patients like this are definitely a challenge because you have to reorient them to the fact that what they’re doing isn’t necessarily working,
My initial assessments are 90 minutes long, so I have a lot of time to sit with a patient and hear their story and understand their background.
I suggested to the patient: “Why don’t we try adding these foods back in, but eliminating these other types of foods and see whether that would help?” 48 hours later, she sent me an email, telling me that she and her husband had talked this through, and they thought I hit the nail on the head: She was focusing on the wrong foods which were causing problems. Those are always great messages to get from patients, when they say: “Oh my gosh, I hadn’t even considered that.”
Q: Describe how you would spend a free Saturday afternoon.
Ms. Euler: They’re so rare – those free Saturday afternoons, but it would probably be a good book that would turn into a nap on the couch.
LIGHTNING ROUND
Do you prefer texting or talking?
Talking in person
What’s your favorite breakfast?
Greek yogurt with fiber, flax seeds, and berries
What’s your favorite junk food?
Ice cream
What’s your favorite fruit?
Garden grown strawberries
What’s your favorite holiday?
Thanksgiving
What’s your favorite type of music?
Jazz
If you weren’t a GI nutritionist, what would you be?
Probably a landscape architect.
CDC alerts clinicians to signs of alpha-gal syndrome
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.
AGS causes patients to become allergic to meat, and in some cases the reaction can be life-threatening. Symptoms typically start 2-6 hours after eating the meat.
The American Gastroenterological Association published a Clinical Practice Update in February notifying gastroenterologists that a subset of AGS patients are presenting with abdominal pain, nausea, diarrhea or vomiting, without skin changes or anaphylaxis. If alpha-gal is suspected, serum tests for immunoglobulin E (IgE) antibodies should be performed.
“It is important for gastroenterologists to be aware of this condition and to be capable of diagnosing and treating it in a timely manner,” wrote authors of the clinical practice update in Clinical Gastroenterology and Hepatology.
A Morbidity and Mortality Weekly Report demonstrates that health care provider knowledge is low surrounding AGS. Almost half of the 1,500 health care providers surveyed (42%) had never heard of the syndrome and another 35% were not confident in diagnosing or managing affected patients.
The low knowledge is concerning because the range of the lone star tick, which is the species primarily associated with this syndrome, is expanding. The knowledge gaps may lead to delayed or overlooked diagnoses.
“Improved health care provider education might facilitate a rapid diagnosis of AGS, improve patient care, and support public health understanding of this emerging condition,” write the report authors, led by Ann Carpenter, DVM, with the CDC.
Another Morbidity and Mortality Weekly Report, with lead author Johanna S. Salzer, DVM, PhD, of the CDC, also issued on July 28, notes that specific symptoms and severity of AGS vary and no cure or treatment is currently available. From 2010 to 2018, there were more than 34,000 suspected cases of AGS in the United States, but current knowledge of where the cases have occurred is limited, the study authors write.
According to the report, the suspected AGS cases were concentrated in areas where the lone star tick is known to be found, particularly throughout Arkansas, Kentucky, Missouri, and Suffolk County, N.Y.
The report also notes that, “during 2017-2021, there was an annual increase in positive test results for AGS in the United States. More than 90,000 suspected AGS cases were identified during the study period, and the number of new suspected cases increased by approximately 15,000 each year during the study.”
An AGS diagnosis “can be made with GI distress and increased serum alpha-gal IgE antibodies whose symptoms are relieved adequately on an alpha-gal avoidance diet that eliminates pork, beef, and mammalian-derived products,” the practice update says.
Patients whose symptoms also include facial swelling, urticaria, and trouble breathing should be referred to allergists, the AGA update states.
Patients should also be counseled to avoid further tick bites because additional bites can worsen the allergy.
The authors declare no relevant financial relationships.
Crohn’s link seen for ultraprocessed foods
Ultraprocessed foods contain large amounts of artificial flavors, stabilizers, emulsifiers, sweeteners, or preservatives. Studies have linked higher consumption of them to cardiovascular disease, diabetes, obesity, and cancers.
For their research, published in Clinical Gastroenterology and Hepatology, Neeraj Nerula, MD of McMaster University, Hamilton, Ont., and colleagues pooled data from five recent cohort studies to assess whether their consumption was also linked to inflammatory bowel disease.
The included cohort studies together enrolled more than 1 million participants (mean age, 43-56; 55%-85% female). Of these, 916 developed Crohn’s disease, and 1,934 developed ulcerative colitis, during follow-up. None of the participants had IBD at baseline, and all were followed up at least 1 year. All the studies used the same food classification system, called NOVA, to assess foods eaten, and all were conducted between 2020 and 2022.
People who consumed more ultraprocessed foods saw higher Crohn’s risk, compared with those classed as consuming lower amounts of these foods (hazard ratio, 1.71; 95% confidence interval, 1.37-2.14). Also, lower risk of Crohn’s was observed among participants who consumed more unprocessed or minimally processed foods, such as vegetables, chicken, milk, and eggs (HR, 0.71; 95% CI, 0.53-0.94). The same associations were not seen for ulcerative colitis.
“Our findings support the hypothesis that consumption of [ultraprocessed foods] and low consumption of unprocessed/minimally processed foods may increase the risk for CD,” Dr. Nerula and colleagues wrote. The lack of association seen with ulcerative colitis might be explained by differences in the pathogenesis of each disease.
Ultraprocessed foods might contribute to Crohn’s by disrupting gut microbiota, the authors wrote. “For instance, emulsifiers have been shown to increase epithelial permeability, disruption of the intestinal barrier, and gut dysbiosis in mice. Carboxymethyl cellulose has been shown to facilitate bacterial adherence to gut epithelium, possibly leading to bacterial overgrowth and invasion of bacteria in between the intestinal villi. Furthermore, additives such as carrageenan, titanium dioxide, and maltodextrin have been shown to promote intestinal inflammation.”
Dr. Nerula and colleagues described as strengths of their study its large size, the low heterogenicity of the included studies, and the use of validated, standardized questionnaires to measure dietary intake in each study. Nonetheless, they cautioned, the results might not apply to younger age groups, and the majority of participants were White North Americans and Europeans, making it difficult to generalize results.
“Advancements in food processing and associated changes in dietary patterns could explain the rise of IBD incidence during the 20th and 21st centuries,” Dr. Narula and colleagues concluded. “Further investigations are needed to identify the specific potential culprits among processed foods that could account for the increased risk of CD observed.”
The study authors did not report outside funding. Dr. Narula disclosed receiving fees from pharmaceutical manufacturers including Janssen, AbbVie, Takeda, Pfizer, Merck, and others. Two of coauthors also disclosed receiving funds from industry, and five additional coauthors had no conflicts.
The causes of inflammatory bowel disease (IBD) are thought to be multifactorial and include genetic predisposition, dysregulated immune responses, imbalances in the intestinal microbiota, and environmental exposures.
Incidence and prevalence of IBD has increased over time, including in developing countries, and appear to parallel industrialization and “Westernization” of societies. One of the potential contributors to IBD risk is diet. Dietary changes associated with more modern or “Westernized” diets, including increases in processed foods, are some of the factors hypothesized to contribute to rising rates of IBD.
In the meta-analysis by Narula and colleagues, the authors observed a significant increase in the risk of CD, but not UC, in individuals who consumed significantly higher amounts of ultraprocessed foods (that is, frozen or long-shelf-life foods, products with thickeners/emulsifiers, etc.). Although there are limitations to the studies included in the meta-analysis, the association is intriguing and could point to potential lifestyle modifications that could form the basis of preventative interventions for individuals at higher risk for IBD, such as first-degree relatives.
More immediately, prospective research is needed to understand if restricting ultraprocessed foods (or increasing less-processed foods) can decrease disease activity or prevent flares in patients with IBD.
Understanding factors that predispose to or trigger IBD, such as specific dietary components, will lead to improved management strategies and ultimately preventative interventions.
Ryan Ungaro, MD, MS, is an associate professor of medicine in the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. He is director of the Comprehensive Care for the Recently Diagnosed IBD Patient (COMPASS-IBD). He has served as an advisory board member or consultant for AbbVie, Bristol-Myers Squibb, Celltrion, Lilly, Janssen, Pfizer, Roivant, and Takeda, and has received research support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Lily, and Pfizer.
The causes of inflammatory bowel disease (IBD) are thought to be multifactorial and include genetic predisposition, dysregulated immune responses, imbalances in the intestinal microbiota, and environmental exposures.
Incidence and prevalence of IBD has increased over time, including in developing countries, and appear to parallel industrialization and “Westernization” of societies. One of the potential contributors to IBD risk is diet. Dietary changes associated with more modern or “Westernized” diets, including increases in processed foods, are some of the factors hypothesized to contribute to rising rates of IBD.
In the meta-analysis by Narula and colleagues, the authors observed a significant increase in the risk of CD, but not UC, in individuals who consumed significantly higher amounts of ultraprocessed foods (that is, frozen or long-shelf-life foods, products with thickeners/emulsifiers, etc.). Although there are limitations to the studies included in the meta-analysis, the association is intriguing and could point to potential lifestyle modifications that could form the basis of preventative interventions for individuals at higher risk for IBD, such as first-degree relatives.
More immediately, prospective research is needed to understand if restricting ultraprocessed foods (or increasing less-processed foods) can decrease disease activity or prevent flares in patients with IBD.
Understanding factors that predispose to or trigger IBD, such as specific dietary components, will lead to improved management strategies and ultimately preventative interventions.
Ryan Ungaro, MD, MS, is an associate professor of medicine in the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. He is director of the Comprehensive Care for the Recently Diagnosed IBD Patient (COMPASS-IBD). He has served as an advisory board member or consultant for AbbVie, Bristol-Myers Squibb, Celltrion, Lilly, Janssen, Pfizer, Roivant, and Takeda, and has received research support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Lily, and Pfizer.
The causes of inflammatory bowel disease (IBD) are thought to be multifactorial and include genetic predisposition, dysregulated immune responses, imbalances in the intestinal microbiota, and environmental exposures.
Incidence and prevalence of IBD has increased over time, including in developing countries, and appear to parallel industrialization and “Westernization” of societies. One of the potential contributors to IBD risk is diet. Dietary changes associated with more modern or “Westernized” diets, including increases in processed foods, are some of the factors hypothesized to contribute to rising rates of IBD.
In the meta-analysis by Narula and colleagues, the authors observed a significant increase in the risk of CD, but not UC, in individuals who consumed significantly higher amounts of ultraprocessed foods (that is, frozen or long-shelf-life foods, products with thickeners/emulsifiers, etc.). Although there are limitations to the studies included in the meta-analysis, the association is intriguing and could point to potential lifestyle modifications that could form the basis of preventative interventions for individuals at higher risk for IBD, such as first-degree relatives.
More immediately, prospective research is needed to understand if restricting ultraprocessed foods (or increasing less-processed foods) can decrease disease activity or prevent flares in patients with IBD.
Understanding factors that predispose to or trigger IBD, such as specific dietary components, will lead to improved management strategies and ultimately preventative interventions.
Ryan Ungaro, MD, MS, is an associate professor of medicine in the division of gastroenterology at the Icahn School of Medicine at Mount Sinai, New York. He is director of the Comprehensive Care for the Recently Diagnosed IBD Patient (COMPASS-IBD). He has served as an advisory board member or consultant for AbbVie, Bristol-Myers Squibb, Celltrion, Lilly, Janssen, Pfizer, Roivant, and Takeda, and has received research support from AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Lily, and Pfizer.
Ultraprocessed foods contain large amounts of artificial flavors, stabilizers, emulsifiers, sweeteners, or preservatives. Studies have linked higher consumption of them to cardiovascular disease, diabetes, obesity, and cancers.
For their research, published in Clinical Gastroenterology and Hepatology, Neeraj Nerula, MD of McMaster University, Hamilton, Ont., and colleagues pooled data from five recent cohort studies to assess whether their consumption was also linked to inflammatory bowel disease.
The included cohort studies together enrolled more than 1 million participants (mean age, 43-56; 55%-85% female). Of these, 916 developed Crohn’s disease, and 1,934 developed ulcerative colitis, during follow-up. None of the participants had IBD at baseline, and all were followed up at least 1 year. All the studies used the same food classification system, called NOVA, to assess foods eaten, and all were conducted between 2020 and 2022.
People who consumed more ultraprocessed foods saw higher Crohn’s risk, compared with those classed as consuming lower amounts of these foods (hazard ratio, 1.71; 95% confidence interval, 1.37-2.14). Also, lower risk of Crohn’s was observed among participants who consumed more unprocessed or minimally processed foods, such as vegetables, chicken, milk, and eggs (HR, 0.71; 95% CI, 0.53-0.94). The same associations were not seen for ulcerative colitis.
“Our findings support the hypothesis that consumption of [ultraprocessed foods] and low consumption of unprocessed/minimally processed foods may increase the risk for CD,” Dr. Nerula and colleagues wrote. The lack of association seen with ulcerative colitis might be explained by differences in the pathogenesis of each disease.
Ultraprocessed foods might contribute to Crohn’s by disrupting gut microbiota, the authors wrote. “For instance, emulsifiers have been shown to increase epithelial permeability, disruption of the intestinal barrier, and gut dysbiosis in mice. Carboxymethyl cellulose has been shown to facilitate bacterial adherence to gut epithelium, possibly leading to bacterial overgrowth and invasion of bacteria in between the intestinal villi. Furthermore, additives such as carrageenan, titanium dioxide, and maltodextrin have been shown to promote intestinal inflammation.”
Dr. Nerula and colleagues described as strengths of their study its large size, the low heterogenicity of the included studies, and the use of validated, standardized questionnaires to measure dietary intake in each study. Nonetheless, they cautioned, the results might not apply to younger age groups, and the majority of participants were White North Americans and Europeans, making it difficult to generalize results.
“Advancements in food processing and associated changes in dietary patterns could explain the rise of IBD incidence during the 20th and 21st centuries,” Dr. Narula and colleagues concluded. “Further investigations are needed to identify the specific potential culprits among processed foods that could account for the increased risk of CD observed.”
The study authors did not report outside funding. Dr. Narula disclosed receiving fees from pharmaceutical manufacturers including Janssen, AbbVie, Takeda, Pfizer, Merck, and others. Two of coauthors also disclosed receiving funds from industry, and five additional coauthors had no conflicts.
Ultraprocessed foods contain large amounts of artificial flavors, stabilizers, emulsifiers, sweeteners, or preservatives. Studies have linked higher consumption of them to cardiovascular disease, diabetes, obesity, and cancers.
For their research, published in Clinical Gastroenterology and Hepatology, Neeraj Nerula, MD of McMaster University, Hamilton, Ont., and colleagues pooled data from five recent cohort studies to assess whether their consumption was also linked to inflammatory bowel disease.
The included cohort studies together enrolled more than 1 million participants (mean age, 43-56; 55%-85% female). Of these, 916 developed Crohn’s disease, and 1,934 developed ulcerative colitis, during follow-up. None of the participants had IBD at baseline, and all were followed up at least 1 year. All the studies used the same food classification system, called NOVA, to assess foods eaten, and all were conducted between 2020 and 2022.
People who consumed more ultraprocessed foods saw higher Crohn’s risk, compared with those classed as consuming lower amounts of these foods (hazard ratio, 1.71; 95% confidence interval, 1.37-2.14). Also, lower risk of Crohn’s was observed among participants who consumed more unprocessed or minimally processed foods, such as vegetables, chicken, milk, and eggs (HR, 0.71; 95% CI, 0.53-0.94). The same associations were not seen for ulcerative colitis.
“Our findings support the hypothesis that consumption of [ultraprocessed foods] and low consumption of unprocessed/minimally processed foods may increase the risk for CD,” Dr. Nerula and colleagues wrote. The lack of association seen with ulcerative colitis might be explained by differences in the pathogenesis of each disease.
Ultraprocessed foods might contribute to Crohn’s by disrupting gut microbiota, the authors wrote. “For instance, emulsifiers have been shown to increase epithelial permeability, disruption of the intestinal barrier, and gut dysbiosis in mice. Carboxymethyl cellulose has been shown to facilitate bacterial adherence to gut epithelium, possibly leading to bacterial overgrowth and invasion of bacteria in between the intestinal villi. Furthermore, additives such as carrageenan, titanium dioxide, and maltodextrin have been shown to promote intestinal inflammation.”
Dr. Nerula and colleagues described as strengths of their study its large size, the low heterogenicity of the included studies, and the use of validated, standardized questionnaires to measure dietary intake in each study. Nonetheless, they cautioned, the results might not apply to younger age groups, and the majority of participants were White North Americans and Europeans, making it difficult to generalize results.
“Advancements in food processing and associated changes in dietary patterns could explain the rise of IBD incidence during the 20th and 21st centuries,” Dr. Narula and colleagues concluded. “Further investigations are needed to identify the specific potential culprits among processed foods that could account for the increased risk of CD observed.”
The study authors did not report outside funding. Dr. Narula disclosed receiving fees from pharmaceutical manufacturers including Janssen, AbbVie, Takeda, Pfizer, Merck, and others. Two of coauthors also disclosed receiving funds from industry, and five additional coauthors had no conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Study documents obesity-related defecation disorders
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
, as well as clinically significant rectocele and increased anal resting and rectal pressures.
The study, which was published in the American Journal of Gastroenterology and led by Pam Chaichanavichkij, MBChB, MRCS, of Queen Mary University, London, included 1,155 patients (84% female, median age 52) who were obese (31.7%), overweight (34.8%), or of normal weight 33.5%).
“These results support the notion that rectal evacuation disorder/incomplete evacuation may be an important underlying mechanism for fecal incontinence in obese patients,” the authors wrote.
Obese patients had higher odds of fecal incontinence to liquid stools (69.9 vs. 47.8%; odds ratio, 1.96 [confidence interval, 1.43-2.70]), use of containment products (54.6% vs. 32.6%; OR, 1.81 [CI, 1.31-2.51]), fecal urgency (74.6% vs. 60.7%; OR, 1.54 [CI, 1.11-2.14]), urge fecal incontinence (63.4% vs. 47.3%, OR, 1.68 [CI, 1.23-2.29]), and vaginal digitation (18.0% vs. 9.7%; OR, 2.18 [CI, 1.26-3.86]).
Obese patients were also more likely to have functional constipation (50.3%), compared with overweight (44.8%) and normal weight patients (41.1%).
There was a positive linear association between body mass index (BMI) and anal resting pressure (beta 0.45; R2, 0.25, P = 0.0003), though the odds of anal hypertension were not significantly higher after Benjamini-Hochberg correction. Obese patients more often had a large clinically significant rectocele (34.4% vs. 20.6%; OR, 2.62 [CI, 1.51-4.55]), compared with normal BMI patients.
The data showed higher rates of gynecological surgery, cholecystectomy, diabetes, and self-reported use of opioids, antidepressants, and anticholinergic medications in the obese group, compared with the others.
In morphological differences measured by x-ray defecography, obese patients had more than two-fold higher odds of having a rectocele and even greater odds of the rectocele being large and clinically significant. Anal and rectal resting pressures were linearly related to increasing BMI, the authors report.
Because most patients in the study were female, the findings may not be generalizable to the general population or male patients. Also, diet and exercise, two factors that may affect defecation disorders, were not accounted for in this study.
Dr. Chaichanavichkij reported no disclosures. Two other authors reported financial relationships with Medtronic Inc. and MMS/Laborie.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY