User login
In a Parallel Universe, “I’d Be a Concert Pianist” Says Tennessee GI
She also relishes opportunities to think, to analyze, and solve problems for her patients.
One of her chief interests is inflammatory bowel disease (IBD). It’s reassuring to focus on a field of work “where I know exactly what’s causing the issue, and I can select a therapeutic approach (medication and lifestyle changes) that help a patient achieve remission,” said Dr. Pointer, co-owner and managing partner of Digestive and Liver Health Specialists in Hendersonville, Tenn. She’s also the medical director and a principal investigator of Quality Medical Research in Nashville, and currently serves as chair of the AGA Trainee and Early Career Committee.
Starting her own practice has been just as challenging and rewarding as going through medical school. Medical training does not prepare you for starting your own practice, Dr. Pointer said, so she and her business partner have had to learn as they go. “But I think we’ve done very well. We’ve taken the ups and downs in stride.”
In an interview, Dr. Pointer spoke more about her work in IBD and the ways in which she’s given back to the community through music and mentoring.
Q: Why did you choose GI?
I knew from a very young age that I was going to be a physician. I had always been interested in science. When I got into medical school and became exposed to the different areas, I really liked the cognitive skills where you had to think through a problem or an issue. But I also liked the procedural things as well.
During my internal medicine residency training, I felt that I had a knack for it. As I was looking at different options, I decided on gastroenterology because it combined both cognitive thinking through issues, but also taking it to the next step and intervening through procedures.
Q: During fellowship, your focus was inflammatory bowel disease. What drew your interest to this condition?
There are a lot of different areas within gastroenterology that one can subspecialize in, as we see the full gamut of gastrointestinal and hepatic disorders. But treating some conditions, like functional disorders, means taking more of a ‘trial and error’ approach, and you may not always get the patient a hundred percent better. That’s not to say that we can’t improve a patient’s quality of life, but it’s not always a guarantee.
But inflammatory bowel disease is a little bit different. Because I can point to an exact spot in the intestines that’s causing the problem, it’s very fulfilling for me as a physician to take a patient who is having 10-12 bloody bowel movements a day, to normal form stools and no abdominal pain. They’re able to gain weight and go on about their lives and about their day. So that was why I picked inflammatory bowel disease as my subspecialty.
Q: Tell me about the gastroenterology elective you developed for family medicine residents and undergraduate students. What’s the status of the program now?
I’ve always been interested in teaching and giving back to the next generations. I feel like I had great mentor opportunities and people who helped me along the way. In my previous hospital position, I was able to work with the family medicine department and create an elective through which residents and even undergraduate students could come and shadow and work with me in the clinic and see me performing procedures.
That elective ended once I left that position, at least as far as I’m aware. But in the private practice that I co-own now, we have numerous shadowing opportunities. I was able to give a lecture at Middle Tennessee State University for some students. And through that lecture, many students have reached out to me to shadow. I have allowed them to come shadow and do clinic work as a medical assistant and watch me perform procedures. I have multiple students working with me weekly.
Q: Years ago, you founded the non-profit Enchanted Fingers Piano Lessons, which gave free piano lessons to underserved youth. What was that experience like?
Piano was one of my first loves. In some parallel universe, there’s a Dr. Pointer who is a classical, concert pianist. I started taking piano lessons when I was in early middle school, and I took to it very quickly. I was able to excel. I just loved it. I enjoyed practicing and I still play.
The impetus for starting Enchanted Fingers Piano lessons was because I wanted to give back again to the community. I came from an underserved community. Oftentimes children and young adults in those communities don’t get exposed to extracurricular activities and they don’t even know what they could potentially have a passion for. And I definitely had a passion for piano. I partnered with a church organization and they allowed me to use their church to host these piano lessons, and it was a phenomenal and rewarding experience. I would definitely like to start it up again one day in the future. It was an amazing experience.
It’s actually how I met my husband. He was one of the young adult students who signed up to take lessons. We both still enjoy playing the piano together.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
I’m a creative at heart. I really enjoy sewing and I’m working on a few sewing projects. I just got a serger. It is a machine that helps you finish a seam. It can also be used to sew entire garments. That has been fun, learning how to thread that machine. When I’m not doing that or just relaxing with my family, I do enjoy curling up with a good book. Stephen King is one of my favorite authors.
Lightning Round
Texting or talking?
Talking
Favorite junk food?
Chocolate chip cookies
Cat or dog person?
Cat
Favorite vacation?
Hawaii
How many cups of coffee do you drink per day?
I don’t drink coffee
Favorite ice cream?
Butter pecan
Favorite sport?
I don’t watch sports
Optimist or pessimist?
Optimist
She also relishes opportunities to think, to analyze, and solve problems for her patients.
One of her chief interests is inflammatory bowel disease (IBD). It’s reassuring to focus on a field of work “where I know exactly what’s causing the issue, and I can select a therapeutic approach (medication and lifestyle changes) that help a patient achieve remission,” said Dr. Pointer, co-owner and managing partner of Digestive and Liver Health Specialists in Hendersonville, Tenn. She’s also the medical director and a principal investigator of Quality Medical Research in Nashville, and currently serves as chair of the AGA Trainee and Early Career Committee.
Starting her own practice has been just as challenging and rewarding as going through medical school. Medical training does not prepare you for starting your own practice, Dr. Pointer said, so she and her business partner have had to learn as they go. “But I think we’ve done very well. We’ve taken the ups and downs in stride.”
In an interview, Dr. Pointer spoke more about her work in IBD and the ways in which she’s given back to the community through music and mentoring.
Q: Why did you choose GI?
I knew from a very young age that I was going to be a physician. I had always been interested in science. When I got into medical school and became exposed to the different areas, I really liked the cognitive skills where you had to think through a problem or an issue. But I also liked the procedural things as well.
During my internal medicine residency training, I felt that I had a knack for it. As I was looking at different options, I decided on gastroenterology because it combined both cognitive thinking through issues, but also taking it to the next step and intervening through procedures.
Q: During fellowship, your focus was inflammatory bowel disease. What drew your interest to this condition?
There are a lot of different areas within gastroenterology that one can subspecialize in, as we see the full gamut of gastrointestinal and hepatic disorders. But treating some conditions, like functional disorders, means taking more of a ‘trial and error’ approach, and you may not always get the patient a hundred percent better. That’s not to say that we can’t improve a patient’s quality of life, but it’s not always a guarantee.
But inflammatory bowel disease is a little bit different. Because I can point to an exact spot in the intestines that’s causing the problem, it’s very fulfilling for me as a physician to take a patient who is having 10-12 bloody bowel movements a day, to normal form stools and no abdominal pain. They’re able to gain weight and go on about their lives and about their day. So that was why I picked inflammatory bowel disease as my subspecialty.
Q: Tell me about the gastroenterology elective you developed for family medicine residents and undergraduate students. What’s the status of the program now?
I’ve always been interested in teaching and giving back to the next generations. I feel like I had great mentor opportunities and people who helped me along the way. In my previous hospital position, I was able to work with the family medicine department and create an elective through which residents and even undergraduate students could come and shadow and work with me in the clinic and see me performing procedures.
That elective ended once I left that position, at least as far as I’m aware. But in the private practice that I co-own now, we have numerous shadowing opportunities. I was able to give a lecture at Middle Tennessee State University for some students. And through that lecture, many students have reached out to me to shadow. I have allowed them to come shadow and do clinic work as a medical assistant and watch me perform procedures. I have multiple students working with me weekly.
Q: Years ago, you founded the non-profit Enchanted Fingers Piano Lessons, which gave free piano lessons to underserved youth. What was that experience like?
Piano was one of my first loves. In some parallel universe, there’s a Dr. Pointer who is a classical, concert pianist. I started taking piano lessons when I was in early middle school, and I took to it very quickly. I was able to excel. I just loved it. I enjoyed practicing and I still play.
The impetus for starting Enchanted Fingers Piano lessons was because I wanted to give back again to the community. I came from an underserved community. Oftentimes children and young adults in those communities don’t get exposed to extracurricular activities and they don’t even know what they could potentially have a passion for. And I definitely had a passion for piano. I partnered with a church organization and they allowed me to use their church to host these piano lessons, and it was a phenomenal and rewarding experience. I would definitely like to start it up again one day in the future. It was an amazing experience.
It’s actually how I met my husband. He was one of the young adult students who signed up to take lessons. We both still enjoy playing the piano together.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
I’m a creative at heart. I really enjoy sewing and I’m working on a few sewing projects. I just got a serger. It is a machine that helps you finish a seam. It can also be used to sew entire garments. That has been fun, learning how to thread that machine. When I’m not doing that or just relaxing with my family, I do enjoy curling up with a good book. Stephen King is one of my favorite authors.
Lightning Round
Texting or talking?
Talking
Favorite junk food?
Chocolate chip cookies
Cat or dog person?
Cat
Favorite vacation?
Hawaii
How many cups of coffee do you drink per day?
I don’t drink coffee
Favorite ice cream?
Butter pecan
Favorite sport?
I don’t watch sports
Optimist or pessimist?
Optimist
She also relishes opportunities to think, to analyze, and solve problems for her patients.
One of her chief interests is inflammatory bowel disease (IBD). It’s reassuring to focus on a field of work “where I know exactly what’s causing the issue, and I can select a therapeutic approach (medication and lifestyle changes) that help a patient achieve remission,” said Dr. Pointer, co-owner and managing partner of Digestive and Liver Health Specialists in Hendersonville, Tenn. She’s also the medical director and a principal investigator of Quality Medical Research in Nashville, and currently serves as chair of the AGA Trainee and Early Career Committee.
Starting her own practice has been just as challenging and rewarding as going through medical school. Medical training does not prepare you for starting your own practice, Dr. Pointer said, so she and her business partner have had to learn as they go. “But I think we’ve done very well. We’ve taken the ups and downs in stride.”
In an interview, Dr. Pointer spoke more about her work in IBD and the ways in which she’s given back to the community through music and mentoring.
Q: Why did you choose GI?
I knew from a very young age that I was going to be a physician. I had always been interested in science. When I got into medical school and became exposed to the different areas, I really liked the cognitive skills where you had to think through a problem or an issue. But I also liked the procedural things as well.
During my internal medicine residency training, I felt that I had a knack for it. As I was looking at different options, I decided on gastroenterology because it combined both cognitive thinking through issues, but also taking it to the next step and intervening through procedures.
Q: During fellowship, your focus was inflammatory bowel disease. What drew your interest to this condition?
There are a lot of different areas within gastroenterology that one can subspecialize in, as we see the full gamut of gastrointestinal and hepatic disorders. But treating some conditions, like functional disorders, means taking more of a ‘trial and error’ approach, and you may not always get the patient a hundred percent better. That’s not to say that we can’t improve a patient’s quality of life, but it’s not always a guarantee.
But inflammatory bowel disease is a little bit different. Because I can point to an exact spot in the intestines that’s causing the problem, it’s very fulfilling for me as a physician to take a patient who is having 10-12 bloody bowel movements a day, to normal form stools and no abdominal pain. They’re able to gain weight and go on about their lives and about their day. So that was why I picked inflammatory bowel disease as my subspecialty.
Q: Tell me about the gastroenterology elective you developed for family medicine residents and undergraduate students. What’s the status of the program now?
I’ve always been interested in teaching and giving back to the next generations. I feel like I had great mentor opportunities and people who helped me along the way. In my previous hospital position, I was able to work with the family medicine department and create an elective through which residents and even undergraduate students could come and shadow and work with me in the clinic and see me performing procedures.
That elective ended once I left that position, at least as far as I’m aware. But in the private practice that I co-own now, we have numerous shadowing opportunities. I was able to give a lecture at Middle Tennessee State University for some students. And through that lecture, many students have reached out to me to shadow. I have allowed them to come shadow and do clinic work as a medical assistant and watch me perform procedures. I have multiple students working with me weekly.
Q: Years ago, you founded the non-profit Enchanted Fingers Piano Lessons, which gave free piano lessons to underserved youth. What was that experience like?
Piano was one of my first loves. In some parallel universe, there’s a Dr. Pointer who is a classical, concert pianist. I started taking piano lessons when I was in early middle school, and I took to it very quickly. I was able to excel. I just loved it. I enjoyed practicing and I still play.
The impetus for starting Enchanted Fingers Piano lessons was because I wanted to give back again to the community. I came from an underserved community. Oftentimes children and young adults in those communities don’t get exposed to extracurricular activities and they don’t even know what they could potentially have a passion for. And I definitely had a passion for piano. I partnered with a church organization and they allowed me to use their church to host these piano lessons, and it was a phenomenal and rewarding experience. I would definitely like to start it up again one day in the future. It was an amazing experience.
It’s actually how I met my husband. He was one of the young adult students who signed up to take lessons. We both still enjoy playing the piano together.
Q: When you’re not being a GI, how do you spend your free weekend afternoons?
I’m a creative at heart. I really enjoy sewing and I’m working on a few sewing projects. I just got a serger. It is a machine that helps you finish a seam. It can also be used to sew entire garments. That has been fun, learning how to thread that machine. When I’m not doing that or just relaxing with my family, I do enjoy curling up with a good book. Stephen King is one of my favorite authors.
Lightning Round
Texting or talking?
Talking
Favorite junk food?
Chocolate chip cookies
Cat or dog person?
Cat
Favorite vacation?
Hawaii
How many cups of coffee do you drink per day?
I don’t drink coffee
Favorite ice cream?
Butter pecan
Favorite sport?
I don’t watch sports
Optimist or pessimist?
Optimist

Does Bezlotoxumab Boost FMT Efficacy in IBD Patients With Recurrent CDI?
PHILADELPHIA – , according to a randomized controlled trial.
“Given the high efficacy of FMT, the addition of bezlotoxumab may not provide a further reduction in CDI recurrence,” said study author Jessica R. Allegretti, MD, MPH, AGAF, with Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts.
Allegretti presented the findings during a plenary session at the annual meeting of the American College of Gastroenterology (ACG).
Common and Deadly
CDI is the most common cause of healthcare-associated infection in the United States, leading to roughly 4.8 billion in excess healthcare costs. There are an estimated 500,000 cases each year in the United States, with roughly 30,000 of those cases leading to death.
Patients with IBD have a prevalence of CDI that is 2.5- to 8-fold higher than in peers without IBD, and they also have 4.5-fold higher risk of recurrence. Sequelae of CDI in IBD include exacerbations of IBD, increased hospitalizations, escalation of IBD therapy, and colectomy.
FMT has been shown to be safe and effective in patients with IBD and rCDI.
Bezlotoxumab — a fully human monoclonal antibody that binds to C difficile toxin B — was approved by the US Food and Drug Administration (FDA) in 2016 to reduce the recurrence of CDI in patients aged 18 years and older.
However, there is only limited data on the value of combining these two strategies.
Allegretti and colleagues conducted a multicenter randomized controlled trial to evaluate the impact of FMT in combination with bezlotoxumab in patients with IBD and rCDI.
They enrolled 61 patients (mean age, 38 years, 54% men) with two or more episodes of CDI who received a single colonoscopic FMT. Twenty patients had Crohn’s disease, and 41 had ulcerative colitis.
Thirty patients were randomly allocated to receive a single bezlotoxumab infusion and 31 to receive a placebo infusion prior to FMT.
A total of five participants (8%) experienced a CDI recurrence with confirmed EIA+ stool –4 in the treatment group and 1 in the placebo group (13% vs 3%, P = .15).
Participants in the treatment group had higher odds of CDI recurrence, though this was not statistically significant (odds ratio [OR], 4.6; 95% CI, 0.5-43.9), Allegretti reported.
With regards to C difficile colonization, more patients in the treatment group were decolonized compared with placebo at week 1 (82% vs 68%, P = .22) and at week 12 (83% vs 72%, P = .34).
Steroid use at the time of FMT was associated with a significant increased risk of ongoing colonization of C difficile at week 12 post-FMT (OR, 4.90; 95% CI, 1.18-20.37; P = .03).
While there were no significant differences in IBD outcomes between groups, there were numerically higher rates of IBD improvement in the treatment group compared to the placebo group 56% vs 46%.
Only one patient had IBD worsen, and this patient was in the placebo group. There were no de novo IBD flares.
FMT alone and with bezlotoxumab were both safe and well tolerated. Two serious adverse events were reported; neither were deemed to be treatment-related.
“This is the first clinical trial to assess the clinical effect of FMT in combination with bezlotoxumab in patients with IBD and rCDI. The data suggest no clear efficacy benefit to this combination compared to FMT alone,” Allegretti told attendees.
“This finding is not surprising given the high rate of efficacy of FMT,” said Ashwin N. Ananthakrishnan, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, Boston, who was not involved in the study.
“It would have been interesting to compare bezlotoxumab vs FMT as primary treatment for recurrent CDI in this population,” Ananthakrishnan added.
This was an investigator-initiated study funded by Merck. Allegretti disclosed various relationships with Abbvie, Artugen, Bristol Myers Squibb, Ferring, Finch Therapeutics, Janssen, Merck, Pfizer, and Seres. Ananthakrishnan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – , according to a randomized controlled trial.
“Given the high efficacy of FMT, the addition of bezlotoxumab may not provide a further reduction in CDI recurrence,” said study author Jessica R. Allegretti, MD, MPH, AGAF, with Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts.
Allegretti presented the findings during a plenary session at the annual meeting of the American College of Gastroenterology (ACG).
Common and Deadly
CDI is the most common cause of healthcare-associated infection in the United States, leading to roughly 4.8 billion in excess healthcare costs. There are an estimated 500,000 cases each year in the United States, with roughly 30,000 of those cases leading to death.
Patients with IBD have a prevalence of CDI that is 2.5- to 8-fold higher than in peers without IBD, and they also have 4.5-fold higher risk of recurrence. Sequelae of CDI in IBD include exacerbations of IBD, increased hospitalizations, escalation of IBD therapy, and colectomy.
FMT has been shown to be safe and effective in patients with IBD and rCDI.
Bezlotoxumab — a fully human monoclonal antibody that binds to C difficile toxin B — was approved by the US Food and Drug Administration (FDA) in 2016 to reduce the recurrence of CDI in patients aged 18 years and older.
However, there is only limited data on the value of combining these two strategies.
Allegretti and colleagues conducted a multicenter randomized controlled trial to evaluate the impact of FMT in combination with bezlotoxumab in patients with IBD and rCDI.
They enrolled 61 patients (mean age, 38 years, 54% men) with two or more episodes of CDI who received a single colonoscopic FMT. Twenty patients had Crohn’s disease, and 41 had ulcerative colitis.
Thirty patients were randomly allocated to receive a single bezlotoxumab infusion and 31 to receive a placebo infusion prior to FMT.
A total of five participants (8%) experienced a CDI recurrence with confirmed EIA+ stool –4 in the treatment group and 1 in the placebo group (13% vs 3%, P = .15).
Participants in the treatment group had higher odds of CDI recurrence, though this was not statistically significant (odds ratio [OR], 4.6; 95% CI, 0.5-43.9), Allegretti reported.
With regards to C difficile colonization, more patients in the treatment group were decolonized compared with placebo at week 1 (82% vs 68%, P = .22) and at week 12 (83% vs 72%, P = .34).
Steroid use at the time of FMT was associated with a significant increased risk of ongoing colonization of C difficile at week 12 post-FMT (OR, 4.90; 95% CI, 1.18-20.37; P = .03).
While there were no significant differences in IBD outcomes between groups, there were numerically higher rates of IBD improvement in the treatment group compared to the placebo group 56% vs 46%.
Only one patient had IBD worsen, and this patient was in the placebo group. There were no de novo IBD flares.
FMT alone and with bezlotoxumab were both safe and well tolerated. Two serious adverse events were reported; neither were deemed to be treatment-related.
“This is the first clinical trial to assess the clinical effect of FMT in combination with bezlotoxumab in patients with IBD and rCDI. The data suggest no clear efficacy benefit to this combination compared to FMT alone,” Allegretti told attendees.
“This finding is not surprising given the high rate of efficacy of FMT,” said Ashwin N. Ananthakrishnan, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, Boston, who was not involved in the study.
“It would have been interesting to compare bezlotoxumab vs FMT as primary treatment for recurrent CDI in this population,” Ananthakrishnan added.
This was an investigator-initiated study funded by Merck. Allegretti disclosed various relationships with Abbvie, Artugen, Bristol Myers Squibb, Ferring, Finch Therapeutics, Janssen, Merck, Pfizer, and Seres. Ananthakrishnan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA – , according to a randomized controlled trial.
“Given the high efficacy of FMT, the addition of bezlotoxumab may not provide a further reduction in CDI recurrence,” said study author Jessica R. Allegretti, MD, MPH, AGAF, with Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts.
Allegretti presented the findings during a plenary session at the annual meeting of the American College of Gastroenterology (ACG).
Common and Deadly
CDI is the most common cause of healthcare-associated infection in the United States, leading to roughly 4.8 billion in excess healthcare costs. There are an estimated 500,000 cases each year in the United States, with roughly 30,000 of those cases leading to death.
Patients with IBD have a prevalence of CDI that is 2.5- to 8-fold higher than in peers without IBD, and they also have 4.5-fold higher risk of recurrence. Sequelae of CDI in IBD include exacerbations of IBD, increased hospitalizations, escalation of IBD therapy, and colectomy.
FMT has been shown to be safe and effective in patients with IBD and rCDI.
Bezlotoxumab — a fully human monoclonal antibody that binds to C difficile toxin B — was approved by the US Food and Drug Administration (FDA) in 2016 to reduce the recurrence of CDI in patients aged 18 years and older.
However, there is only limited data on the value of combining these two strategies.
Allegretti and colleagues conducted a multicenter randomized controlled trial to evaluate the impact of FMT in combination with bezlotoxumab in patients with IBD and rCDI.
They enrolled 61 patients (mean age, 38 years, 54% men) with two or more episodes of CDI who received a single colonoscopic FMT. Twenty patients had Crohn’s disease, and 41 had ulcerative colitis.
Thirty patients were randomly allocated to receive a single bezlotoxumab infusion and 31 to receive a placebo infusion prior to FMT.
A total of five participants (8%) experienced a CDI recurrence with confirmed EIA+ stool –4 in the treatment group and 1 in the placebo group (13% vs 3%, P = .15).
Participants in the treatment group had higher odds of CDI recurrence, though this was not statistically significant (odds ratio [OR], 4.6; 95% CI, 0.5-43.9), Allegretti reported.
With regards to C difficile colonization, more patients in the treatment group were decolonized compared with placebo at week 1 (82% vs 68%, P = .22) and at week 12 (83% vs 72%, P = .34).
Steroid use at the time of FMT was associated with a significant increased risk of ongoing colonization of C difficile at week 12 post-FMT (OR, 4.90; 95% CI, 1.18-20.37; P = .03).
While there were no significant differences in IBD outcomes between groups, there were numerically higher rates of IBD improvement in the treatment group compared to the placebo group 56% vs 46%.
Only one patient had IBD worsen, and this patient was in the placebo group. There were no de novo IBD flares.
FMT alone and with bezlotoxumab were both safe and well tolerated. Two serious adverse events were reported; neither were deemed to be treatment-related.
“This is the first clinical trial to assess the clinical effect of FMT in combination with bezlotoxumab in patients with IBD and rCDI. The data suggest no clear efficacy benefit to this combination compared to FMT alone,” Allegretti told attendees.
“This finding is not surprising given the high rate of efficacy of FMT,” said Ashwin N. Ananthakrishnan, MD, MPH, AGAF, with Massachusetts General Hospital and Harvard Medical School, Boston, who was not involved in the study.
“It would have been interesting to compare bezlotoxumab vs FMT as primary treatment for recurrent CDI in this population,” Ananthakrishnan added.
This was an investigator-initiated study funded by Merck. Allegretti disclosed various relationships with Abbvie, Artugen, Bristol Myers Squibb, Ferring, Finch Therapeutics, Janssen, Merck, Pfizer, and Seres. Ananthakrishnan had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Angiotensin Receptor Blockers May Lead to Worse Outcomes in Celiac Disease
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a study presented at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
The association may be related to the similar pathophysiology between ARB-associated enteropathy and celiac disease, though additional research is needed.
“Based on our findings, people should take caution when prescribing angiotensin receptor blockers to people with celiac disease,” said lead author Isabel Hujoel, MD, clinical assistant professor of gastroenterology and clinic director of the Celiac Disease Center at the University of Washington, Seattle.
“When we see someone with nonresponsive celiac disease, meaning persistent symptoms despite a gluten-free diet, I do think we should review their medication list, and if they’re on an ARB, we should consider a trial off those medications to see if they respond,” she said. “A primary care provider may choose other hypertensives as well.”
Hujoel and co-author Margaux Hujoel, PhD, a postdoctoral research fellow at Brigham and Women’s Hospital, Boston; Broad Institute, Cambridge; and Harvard Medical School, Boston, analyzed data from the National Institutes of Health’s All of Us, a large publicly available US longitudinal dataset.
The researchers conducted a survival analysis of time-to-first event after celiac disease diagnosis, allowing patients to have a time-dependent covariate of ARB use. They looked at outcomes such as iron deficiency, diarrhea, abdominal pain, vitamin deficiency, vitamin D deficiency, malabsorption, low hemoglobin, and weight loss.
The analysis included 1849 patients with celiac disease, including 1460 women and 389 men, with a median age of nearly 50 years at diagnosis. While the vast majority of patients (nearly 1600) didn’t take an ARB, 120 started one before celiac disease diagnosis and 142 started one after diagnosis.
Overall, taking an ARB was associated with increased hazard ratios [HRs] for low hemoglobin, iron deficiency, diarrhea, and abdominal pain. There weren’t increased risks for weight loss, malabsorption, or vitamin deficiencies.
When excluding those who had an ARB prescription before diagnosis, the HRs remained significantly higher for low hemoglobin (HR, 1.98) and iron deficiency (HR, 1.72) for those who started an ARB after diagnosis.
“The use of angiotensin receptor blockers may be associated with worse outcomes in the setting of celiac disease, specifically persistent symptoms and possibly poor small bowel healing as evidenced by malabsorption,” Hujoel said.
Future studies could look specifically at losartan, which was the most common ARB prescribed in this analysis, she said. Other studies could also analyze different patient outcomes, whether patients were on a gluten-free diet, medication adherence, and recurrence or persistence of symptoms rather than initial occurrence. The associations between ARB use and celiac disease could shift among patients who are in remission, for instance.
“ARBs are some of the most widely used medications, so studies like these can help people to understand that they may have symptoms but not know it’s related to their medication. Public awareness of this fact is key,” said Patricia Jones, MD, a hepatologist and associate professor of clinical medicine at the University of Miami Miller School of Medicine, Miami. Jones co-moderated the plenary session on small intestine, functional, and liver research.
“There are many types of antihypertensives, so while ARBs are used often, other options are available if people have symptoms, especially if they have worsening symptoms with celiac disease,” she said. “It’s important to make changes in your practice.”
The study was named an ACG Newsworthy Abstract. Isabel Hujoel and Patricia Jones reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Common Crohn’s Immune Response to Gut Bacteria Suggests Therapeutic Target
Many patients with Crohn’s disease (CD) have a heightened immune response to flagellins expressed by commensal gut bacteria Lachnospiraceae, with seroreactivity appearing up to 5 years prior to development of Crohn’s complications, according to investigators.
These findings suggest that lead author Qing Zhao, MD, PhD, of the University of Alabama at Birmingham, and colleagues reported.
Previously, Zhao and colleagues found that about 30% of patients with CD had elevated IgG responses to multiple Lachnospiraceae flagellins, and stronger reactivity was associated with higher flagellin-specific CD4+ T cells in circulation.
“In this study, we aimed to identify immunodominant B cell peptide epitopes shared among Lachnospiraceae bacterial flagellins in patients with CD and to correlate this immune reactivity with the clinical disease course,” the investigators wrote in Gastroenterology.
To this end, the investigators analyzed serum samples from adult CD patients, pediatric CD patients, and healthy infants without inflammatory bowel disease, with data derived from multiple sources. Adult patients with CD were part of a regional cohort recruited at the University of Alabama at Birmingham, while pediatric patients with CD came from the RISK Stratification Study, a multisite cohort study across the United States and Canada. Samples from healthy infants were collected from three diverse geographic locations: Uganda, Sweden, and the United States, providing a broad comparison of immune responses to Lachnospiraceae flagellin across populations.
Samples were analyzed via two main methods: a flagellin peptide microarray and a cytometric bead array. The microarray, comprising sequential Lachnospiraceae-derived peptides, enabled identification of IgG responses specific to individual bacterial peptides. The cytometric bead array allowed for multiplexed detection of IgG, IgA, and IgM antibodies to these peptides, quantifying immune reactivity and enabling correlation with clinical disease data.
This approach revealed that nearly half of patients with CD — both adults and children — had a strong IgG immune response targeting a specific bacterial peptide in the Lachnospiraceae flagellin hinge region. This response was linked to an increased risk of disease complications over time, suggesting the peptide’s potential as a biomarker for CD severity and progression, according to the investigators.
Of note, healthy infants also exhibited an elevated IgG response to the same bacterial peptide at around 1 year of age, but this response declined as they grew older, in contrast to its persistence in CD patients. This difference points to a possible failure in immune tolerance in CD, where the natural immune response to gut bacteria in infancy may become dysregulated, Zhao and colleagues explained.
“The flagellin cytometric bead array used in this study holds potential for a simplified yet robust diagnostic and prognostic assay for Crohn’s disease,” they concluded. “Given that reactivity to the dominant flagellin epitope is strongly associated with the development of disease complications, this technique may also assist in identifying patients with Crohn’s disease who would benefit from early therapy.”
Zhao and colleagues also called for future studies to characterize the role of flagellin hinge peptide–specific IgG antibodies in CD pathogenesis, and to explore the hinge peptide as a potential therapeutic target.The study was supported by a Synergy Award from the Kenneth Rainin Foundation, a Career Development Award from the Crohn’s and Colitis Foundation, and grants from the Department of Veterans Affairs, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor and the University of Alabama at Birmingham hold a patent on Lachnospiraceae A4 Fla2, licensed for clinical application by Prometheus Laboratories. Four study coauthors have filed a patent for the flagellin peptide cytometric bead array. One coauthor serves as the founder and chief scientific officer of ImmPrev Bio, a company developing an antigen-directed immunotherapy for Crohn’s disease.
Many patients with Crohn’s disease (CD) have a heightened immune response to flagellins expressed by commensal gut bacteria Lachnospiraceae, with seroreactivity appearing up to 5 years prior to development of Crohn’s complications, according to investigators.
These findings suggest that lead author Qing Zhao, MD, PhD, of the University of Alabama at Birmingham, and colleagues reported.
Previously, Zhao and colleagues found that about 30% of patients with CD had elevated IgG responses to multiple Lachnospiraceae flagellins, and stronger reactivity was associated with higher flagellin-specific CD4+ T cells in circulation.
“In this study, we aimed to identify immunodominant B cell peptide epitopes shared among Lachnospiraceae bacterial flagellins in patients with CD and to correlate this immune reactivity with the clinical disease course,” the investigators wrote in Gastroenterology.
To this end, the investigators analyzed serum samples from adult CD patients, pediatric CD patients, and healthy infants without inflammatory bowel disease, with data derived from multiple sources. Adult patients with CD were part of a regional cohort recruited at the University of Alabama at Birmingham, while pediatric patients with CD came from the RISK Stratification Study, a multisite cohort study across the United States and Canada. Samples from healthy infants were collected from three diverse geographic locations: Uganda, Sweden, and the United States, providing a broad comparison of immune responses to Lachnospiraceae flagellin across populations.
Samples were analyzed via two main methods: a flagellin peptide microarray and a cytometric bead array. The microarray, comprising sequential Lachnospiraceae-derived peptides, enabled identification of IgG responses specific to individual bacterial peptides. The cytometric bead array allowed for multiplexed detection of IgG, IgA, and IgM antibodies to these peptides, quantifying immune reactivity and enabling correlation with clinical disease data.
This approach revealed that nearly half of patients with CD — both adults and children — had a strong IgG immune response targeting a specific bacterial peptide in the Lachnospiraceae flagellin hinge region. This response was linked to an increased risk of disease complications over time, suggesting the peptide’s potential as a biomarker for CD severity and progression, according to the investigators.
Of note, healthy infants also exhibited an elevated IgG response to the same bacterial peptide at around 1 year of age, but this response declined as they grew older, in contrast to its persistence in CD patients. This difference points to a possible failure in immune tolerance in CD, where the natural immune response to gut bacteria in infancy may become dysregulated, Zhao and colleagues explained.
“The flagellin cytometric bead array used in this study holds potential for a simplified yet robust diagnostic and prognostic assay for Crohn’s disease,” they concluded. “Given that reactivity to the dominant flagellin epitope is strongly associated with the development of disease complications, this technique may also assist in identifying patients with Crohn’s disease who would benefit from early therapy.”
Zhao and colleagues also called for future studies to characterize the role of flagellin hinge peptide–specific IgG antibodies in CD pathogenesis, and to explore the hinge peptide as a potential therapeutic target.The study was supported by a Synergy Award from the Kenneth Rainin Foundation, a Career Development Award from the Crohn’s and Colitis Foundation, and grants from the Department of Veterans Affairs, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor and the University of Alabama at Birmingham hold a patent on Lachnospiraceae A4 Fla2, licensed for clinical application by Prometheus Laboratories. Four study coauthors have filed a patent for the flagellin peptide cytometric bead array. One coauthor serves as the founder and chief scientific officer of ImmPrev Bio, a company developing an antigen-directed immunotherapy for Crohn’s disease.
Many patients with Crohn’s disease (CD) have a heightened immune response to flagellins expressed by commensal gut bacteria Lachnospiraceae, with seroreactivity appearing up to 5 years prior to development of Crohn’s complications, according to investigators.
These findings suggest that lead author Qing Zhao, MD, PhD, of the University of Alabama at Birmingham, and colleagues reported.
Previously, Zhao and colleagues found that about 30% of patients with CD had elevated IgG responses to multiple Lachnospiraceae flagellins, and stronger reactivity was associated with higher flagellin-specific CD4+ T cells in circulation.
“In this study, we aimed to identify immunodominant B cell peptide epitopes shared among Lachnospiraceae bacterial flagellins in patients with CD and to correlate this immune reactivity with the clinical disease course,” the investigators wrote in Gastroenterology.
To this end, the investigators analyzed serum samples from adult CD patients, pediatric CD patients, and healthy infants without inflammatory bowel disease, with data derived from multiple sources. Adult patients with CD were part of a regional cohort recruited at the University of Alabama at Birmingham, while pediatric patients with CD came from the RISK Stratification Study, a multisite cohort study across the United States and Canada. Samples from healthy infants were collected from three diverse geographic locations: Uganda, Sweden, and the United States, providing a broad comparison of immune responses to Lachnospiraceae flagellin across populations.
Samples were analyzed via two main methods: a flagellin peptide microarray and a cytometric bead array. The microarray, comprising sequential Lachnospiraceae-derived peptides, enabled identification of IgG responses specific to individual bacterial peptides. The cytometric bead array allowed for multiplexed detection of IgG, IgA, and IgM antibodies to these peptides, quantifying immune reactivity and enabling correlation with clinical disease data.
This approach revealed that nearly half of patients with CD — both adults and children — had a strong IgG immune response targeting a specific bacterial peptide in the Lachnospiraceae flagellin hinge region. This response was linked to an increased risk of disease complications over time, suggesting the peptide’s potential as a biomarker for CD severity and progression, according to the investigators.
Of note, healthy infants also exhibited an elevated IgG response to the same bacterial peptide at around 1 year of age, but this response declined as they grew older, in contrast to its persistence in CD patients. This difference points to a possible failure in immune tolerance in CD, where the natural immune response to gut bacteria in infancy may become dysregulated, Zhao and colleagues explained.
“The flagellin cytometric bead array used in this study holds potential for a simplified yet robust diagnostic and prognostic assay for Crohn’s disease,” they concluded. “Given that reactivity to the dominant flagellin epitope is strongly associated with the development of disease complications, this technique may also assist in identifying patients with Crohn’s disease who would benefit from early therapy.”
Zhao and colleagues also called for future studies to characterize the role of flagellin hinge peptide–specific IgG antibodies in CD pathogenesis, and to explore the hinge peptide as a potential therapeutic target.The study was supported by a Synergy Award from the Kenneth Rainin Foundation, a Career Development Award from the Crohn’s and Colitis Foundation, and grants from the Department of Veterans Affairs, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and National Institute of Diabetes and Digestive and Kidney Diseases. One coauthor and the University of Alabama at Birmingham hold a patent on Lachnospiraceae A4 Fla2, licensed for clinical application by Prometheus Laboratories. Four study coauthors have filed a patent for the flagellin peptide cytometric bead array. One coauthor serves as the founder and chief scientific officer of ImmPrev Bio, a company developing an antigen-directed immunotherapy for Crohn’s disease.
FROM GASTROENTEROLOGY
Guselkumab Efficacy in Crohn’s Disease Unaffected by Prior Biologic Use
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
FROM UEG 2024
IBS: Understanding a Common Yet Misunderstood Condition
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
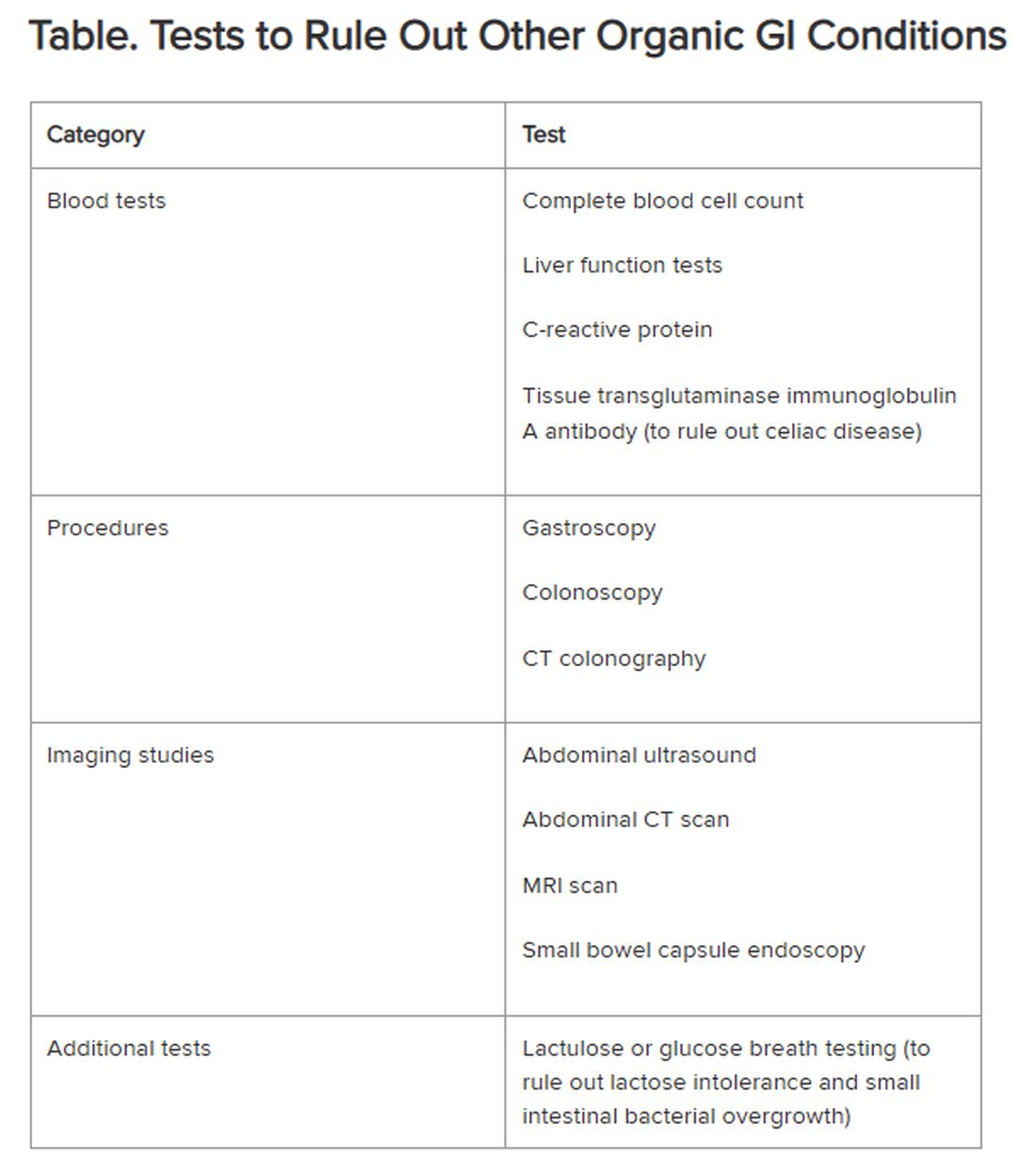
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Breath Gas Patterns Predict Response to Low FODMAP Diet
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Ultraprocessed Foods Associated With Relapse Risk in Crohn’s Disease
VIENNA —
Certain subgroups of UPFs, specifically bread, pastries, and starch as well as oil and spreads, exhibited the strongest association with relapse risks of approximately threefold.
“In addition to treating active inflammatory bowel disease (IBD), we want to maintain remission for the long term,” Chen Sarbagili Shabat, PhD, clinical dietitian from Tel Aviv Medical Center in Israel, said in an interview. “It’s highly important. We know environmental factors are associated with the disease, which is why we can treat active disease with diet. Likewise, we can manage CD in a remission state with diet.”
This is the first prospective study of this particular level of UPFs in people with Crohn’s disease who are in remission, noted Shabat, who presented the findings at United European Gastroenterology (UEG) Week 2024.
Previously, a meta-analysis of prospective cohort studies showed that a diet high in UPFs is associated with a 70% increased risk for development of CD, and a longitudinal study showed that “Western” dietary patterns were associated with relapse risk in patients with IBD, Shabat reported.
Effect of High vs Low Intake of UPFs
The current single-center, prospective cohort study, followed 111 patients with CD every 3 months until relapse for up to 1 year.
Participants were aged 18-75 years (mean age, 38 years), with a median disease duration of 8.7 years. They were required to have maintained steroid-free clinical remission (Harvey-Bradshaw Index (HBI), < 5) for 3 months or more. The median duration of clinical remission at recruitment was 3 years.
Data collection included HBI level, medication type and dosage to ensure constant therapy and full compliance, and a stool sample for fecal calprotectin measurement.
The primary outcome comprised a clinical relapse HBI ≥ 5 over the 12-month follow-up or a change in disease activity requiring a change in medication, hospitalization, or any IBD-related surgery.
Participants were asked to complete a processed food questionnaire to assess the intake of UPFs and a food frequency questionnaire to assess the total intake of energy, macronutrients, and micronutrients. UPFs were divided into high and low intakes using a median cutoff of 3.6 servings/day.
The low intake group included 57 participants, and the high intake group included 54.
A total of 24 patients (21.6%) experienced a clinical relapse event, 7 in the low intake group vs 17 in the high intake group (hazard ratio [HR], 3.86; 95% CI, 1.30-11.47; P = .015 after adjustments).
In a subset of 97 patients with baseline fecal calprotectin measurements, 6 (n = 50) in the low intake group experienced a clinical relapse vs 15 (n = 47) in the high intake group (HR, 4.32; 95% CI, 1.36-13.73; P = .013 after adjustments).
Fecal calprotectin results were also suggestive of an association between high intake of UPFs and gut inflammation, Shabat reported.
Food Groups and Emulsifiers
UPFs were divided into subgroups: Bread, pastries, and starch; oils and spreads; ultraprocessed meat; sweet products and desserts; and ultraprocessed beverages.
The highest associations with relapse were in the subgroup of bread, pastries, and starch (HR, 3.37; 95% CI, 1.26-8.25) and the subgroup of oils and spreads (HR, 2.76; 95% CI, 1.02-7.45).
“The selection of healthy food is highly important, especially since we know that certain food ingredients can contribute to the pathogenesis of CD,” Shabat said. Patients can use partial enteral nutrition to provide 40%-50% of daily caloric intake in order to maintain remission, but she acknowledged it can be really difficult to adhere to.
She concluded by asserting that the study results, along with future research, should contribute to establishing nutritional guidelines to reduce UPF consumption in patients with CD in order to maintain remission.
Commenting on the study, Kevin Whelan, PhD, professor of dietetics and head of the Department of Nutritional Sciences at King’s College London in England, said that he was intrigued by the subgroup analysis that showed breads, pastries, oils, and spreads as having the strongest association with relapse risk.
He also remarked that these foods almost ubiquitously contain emulsifiers, and so the association might have less to do with UPFs in general and more to do with emulsifiers.
Concurring, Shabat noted that, while emulsifiers can negatively influence the microbiota and the gut barrier function, as well as contribute to intestinal inflammation, further mechanistic studies are required to understand these effects.
We need to determine if all additives have the same effect on the inflammatory process and also need studies looking at UPFs alone, she added.
Shabat reported receiving personal fees from Nestle Health Science (Wolfson Medical Center IP) for consulting and speaking and from Takeda and Ferring for speaking. Whelan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
VIENNA —
Certain subgroups of UPFs, specifically bread, pastries, and starch as well as oil and spreads, exhibited the strongest association with relapse risks of approximately threefold.
“In addition to treating active inflammatory bowel disease (IBD), we want to maintain remission for the long term,” Chen Sarbagili Shabat, PhD, clinical dietitian from Tel Aviv Medical Center in Israel, said in an interview. “It’s highly important. We know environmental factors are associated with the disease, which is why we can treat active disease with diet. Likewise, we can manage CD in a remission state with diet.”
This is the first prospective study of this particular level of UPFs in people with Crohn’s disease who are in remission, noted Shabat, who presented the findings at United European Gastroenterology (UEG) Week 2024.
Previously, a meta-analysis of prospective cohort studies showed that a diet high in UPFs is associated with a 70% increased risk for development of CD, and a longitudinal study showed that “Western” dietary patterns were associated with relapse risk in patients with IBD, Shabat reported.
Effect of High vs Low Intake of UPFs
The current single-center, prospective cohort study, followed 111 patients with CD every 3 months until relapse for up to 1 year.
Participants were aged 18-75 years (mean age, 38 years), with a median disease duration of 8.7 years. They were required to have maintained steroid-free clinical remission (Harvey-Bradshaw Index (HBI), < 5) for 3 months or more. The median duration of clinical remission at recruitment was 3 years.
Data collection included HBI level, medication type and dosage to ensure constant therapy and full compliance, and a stool sample for fecal calprotectin measurement.
The primary outcome comprised a clinical relapse HBI ≥ 5 over the 12-month follow-up or a change in disease activity requiring a change in medication, hospitalization, or any IBD-related surgery.
Participants were asked to complete a processed food questionnaire to assess the intake of UPFs and a food frequency questionnaire to assess the total intake of energy, macronutrients, and micronutrients. UPFs were divided into high and low intakes using a median cutoff of 3.6 servings/day.
The low intake group included 57 participants, and the high intake group included 54.
A total of 24 patients (21.6%) experienced a clinical relapse event, 7 in the low intake group vs 17 in the high intake group (hazard ratio [HR], 3.86; 95% CI, 1.30-11.47; P = .015 after adjustments).
In a subset of 97 patients with baseline fecal calprotectin measurements, 6 (n = 50) in the low intake group experienced a clinical relapse vs 15 (n = 47) in the high intake group (HR, 4.32; 95% CI, 1.36-13.73; P = .013 after adjustments).
Fecal calprotectin results were also suggestive of an association between high intake of UPFs and gut inflammation, Shabat reported.
Food Groups and Emulsifiers
UPFs were divided into subgroups: Bread, pastries, and starch; oils and spreads; ultraprocessed meat; sweet products and desserts; and ultraprocessed beverages.
The highest associations with relapse were in the subgroup of bread, pastries, and starch (HR, 3.37; 95% CI, 1.26-8.25) and the subgroup of oils and spreads (HR, 2.76; 95% CI, 1.02-7.45).
“The selection of healthy food is highly important, especially since we know that certain food ingredients can contribute to the pathogenesis of CD,” Shabat said. Patients can use partial enteral nutrition to provide 40%-50% of daily caloric intake in order to maintain remission, but she acknowledged it can be really difficult to adhere to.
She concluded by asserting that the study results, along with future research, should contribute to establishing nutritional guidelines to reduce UPF consumption in patients with CD in order to maintain remission.
Commenting on the study, Kevin Whelan, PhD, professor of dietetics and head of the Department of Nutritional Sciences at King’s College London in England, said that he was intrigued by the subgroup analysis that showed breads, pastries, oils, and spreads as having the strongest association with relapse risk.
He also remarked that these foods almost ubiquitously contain emulsifiers, and so the association might have less to do with UPFs in general and more to do with emulsifiers.
Concurring, Shabat noted that, while emulsifiers can negatively influence the microbiota and the gut barrier function, as well as contribute to intestinal inflammation, further mechanistic studies are required to understand these effects.
We need to determine if all additives have the same effect on the inflammatory process and also need studies looking at UPFs alone, she added.
Shabat reported receiving personal fees from Nestle Health Science (Wolfson Medical Center IP) for consulting and speaking and from Takeda and Ferring for speaking. Whelan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
VIENNA —
Certain subgroups of UPFs, specifically bread, pastries, and starch as well as oil and spreads, exhibited the strongest association with relapse risks of approximately threefold.
“In addition to treating active inflammatory bowel disease (IBD), we want to maintain remission for the long term,” Chen Sarbagili Shabat, PhD, clinical dietitian from Tel Aviv Medical Center in Israel, said in an interview. “It’s highly important. We know environmental factors are associated with the disease, which is why we can treat active disease with diet. Likewise, we can manage CD in a remission state with diet.”
This is the first prospective study of this particular level of UPFs in people with Crohn’s disease who are in remission, noted Shabat, who presented the findings at United European Gastroenterology (UEG) Week 2024.
Previously, a meta-analysis of prospective cohort studies showed that a diet high in UPFs is associated with a 70% increased risk for development of CD, and a longitudinal study showed that “Western” dietary patterns were associated with relapse risk in patients with IBD, Shabat reported.
Effect of High vs Low Intake of UPFs
The current single-center, prospective cohort study, followed 111 patients with CD every 3 months until relapse for up to 1 year.
Participants were aged 18-75 years (mean age, 38 years), with a median disease duration of 8.7 years. They were required to have maintained steroid-free clinical remission (Harvey-Bradshaw Index (HBI), < 5) for 3 months or more. The median duration of clinical remission at recruitment was 3 years.
Data collection included HBI level, medication type and dosage to ensure constant therapy and full compliance, and a stool sample for fecal calprotectin measurement.
The primary outcome comprised a clinical relapse HBI ≥ 5 over the 12-month follow-up or a change in disease activity requiring a change in medication, hospitalization, or any IBD-related surgery.
Participants were asked to complete a processed food questionnaire to assess the intake of UPFs and a food frequency questionnaire to assess the total intake of energy, macronutrients, and micronutrients. UPFs were divided into high and low intakes using a median cutoff of 3.6 servings/day.
The low intake group included 57 participants, and the high intake group included 54.
A total of 24 patients (21.6%) experienced a clinical relapse event, 7 in the low intake group vs 17 in the high intake group (hazard ratio [HR], 3.86; 95% CI, 1.30-11.47; P = .015 after adjustments).
In a subset of 97 patients with baseline fecal calprotectin measurements, 6 (n = 50) in the low intake group experienced a clinical relapse vs 15 (n = 47) in the high intake group (HR, 4.32; 95% CI, 1.36-13.73; P = .013 after adjustments).
Fecal calprotectin results were also suggestive of an association between high intake of UPFs and gut inflammation, Shabat reported.
Food Groups and Emulsifiers
UPFs were divided into subgroups: Bread, pastries, and starch; oils and spreads; ultraprocessed meat; sweet products and desserts; and ultraprocessed beverages.
The highest associations with relapse were in the subgroup of bread, pastries, and starch (HR, 3.37; 95% CI, 1.26-8.25) and the subgroup of oils and spreads (HR, 2.76; 95% CI, 1.02-7.45).
“The selection of healthy food is highly important, especially since we know that certain food ingredients can contribute to the pathogenesis of CD,” Shabat said. Patients can use partial enteral nutrition to provide 40%-50% of daily caloric intake in order to maintain remission, but she acknowledged it can be really difficult to adhere to.
She concluded by asserting that the study results, along with future research, should contribute to establishing nutritional guidelines to reduce UPF consumption in patients with CD in order to maintain remission.
Commenting on the study, Kevin Whelan, PhD, professor of dietetics and head of the Department of Nutritional Sciences at King’s College London in England, said that he was intrigued by the subgroup analysis that showed breads, pastries, oils, and spreads as having the strongest association with relapse risk.
He also remarked that these foods almost ubiquitously contain emulsifiers, and so the association might have less to do with UPFs in general and more to do with emulsifiers.
Concurring, Shabat noted that, while emulsifiers can negatively influence the microbiota and the gut barrier function, as well as contribute to intestinal inflammation, further mechanistic studies are required to understand these effects.
We need to determine if all additives have the same effect on the inflammatory process and also need studies looking at UPFs alone, she added.
Shabat reported receiving personal fees from Nestle Health Science (Wolfson Medical Center IP) for consulting and speaking and from Takeda and Ferring for speaking. Whelan reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM UEG 2024
Live Rotavirus Vaccine Safe for Newborns of Biologic-Treated Moms With IBD
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
No adverse events or impairment of the immune system emerged in babies at 7 days, 1 month, and 9 months post vaccination, in findings from a small Canadian study published in Clinical Gastroenterology and Hepatology.
The study found normal extended immune function testing in infants despite third-trimester maternal biologic therapy and regardless of circulating drug levels. The data provide reassurance about live rotavirus vaccination in this population and may also offer insights into the safety of other live vaccines in biologic-exposed individuals, wrote investigators led by gastroenterologist Cynthia H. Seow, MD, a professor in the Cumming School of Medicine at the University of Calgary in Alberta, Canada.
“Despite the well-established safety and effectiveness of non–live vaccination in individuals with IBD, including those on immunomodulators and biologic therapy, vaccine uptake in pregnant women with IBD and their infants remains suboptimal,” Seow said in an interview. This largely arises from maternal and physician concerns regarding transplacental transfer of IBD therapies and their impact on the safety of vaccination.
“These concerns were heightened after reports emerged of five fatal outcomes following the administration of the live Bacille Calmette-Guérin [BCG] vaccine in biologic-exposed infants. However, it had already been reported that inadvertent administration of the live oral rotavirus vaccine, a very different vaccine in terms of target and mechanism of action, in biologic-exposed individuals had not been associated with significant adverse effects,” she said.
They undertook their analysis with the hypothesis that vaccination would carry low risk, although the live oral vaccine is not currently recommended in biologic-exposed infants. “Yet rotavirus is a leading cause of severe, dehydrating diarrhea in children under the age of 5 years globally, and vaccination has led to significant reductions in hospitalizations and mortality,” Seow added.
Provision of the vaccine to anti–tumor necrosis factor (TNF)–exposed infants has been incorporated into the Canadian Public Health and Immunization guidelines, as the majority of the biologic-exposed infants were exposed to anti-TNF agents. “And with collection of further data, we expect that this will be extended to other biologic agent exposure. These data are important to pregnant women with IBD as they help to normalize their care. Pregnancy is difficult enough without having to remember exceptions to care,” Seow said.
“Before some of the studies came out, broad guidelines recommended that live vaccines should not be used in biologic-exposed infants, but this had been thought to be overly zealous and too conservative, and the risk was thought to be low,” said Elizabeth Spencer, MD, an assistant professor of pediatrics in the Division of Pediatric Gastroenterology at the Icahn School of Medicine at Mount Sinai in New York City, in an interview. Spencer was not involved in the Canadian study.
“At our center, we had some moms on biologics during pregnancy who forgot and had their babies vaccinated for rotavirus, and the babies were all fine,” she said.
The safety of this vaccine has been confirmed by several small studies and recently the PIANO Helmsley Global Consensus on Pregnancy and Inflammatory Bowel Disease, which was presented at Digestive Disease Week 2024. The consensus encompasses preconception counseling and the safety of IBD medications during pregnancy and lactation.
“Another concern, however, was that giving a live GI bug like rotavirus to babies might overstimulate their immune systems and provoke IBD,” Spencer added. “While a number of population-based studies in the US and Europe showed that was not the case, at least in the general population, there was a suggestion that, down the road, vaccination might be mildly protective against IBD in some cases.”
She added the caveat that these studies were not done in mothers and their babies with IBD, who might be inherently at greater risk for IBD. “So, a question for future research would be, ‘Is immune stimulation of the gut in IBD moms and their babies a good or a bad thing for their gut?’ ”
Spencer conceded that “the data present a bit of a blurry picture, but I think it’s always better just to vaccinate according to the regular schedule. The current data say there is no added risk, but it would be nice to look specifically at risk in moms with IBD and their children.”
The Study
The prospective cohort study is a substudy of a larger 2023 one that included biologic use in a range of maternal illnesses, not just IBD.
For the current study, Seow and colleagues identified 57 infants born to 52 mothers with IBD attending a pregnancy clinic at the University of Calgary in the period 2019-2023. Almost 81% of the mothers had Crohn’s disease, and the median duration of IBD was 10 years. The median gestational age at delivery was 39 weeks, and almost 60% of deliveries were vaginal. The infants had been exposed in utero to infliximab (n = 21), adalimumab (n = 19), vedolizumab (n = 10), and ustekinumab (n = 7) in the third trimester.
The 57 biologic-exposed infants underwent standardized clinical assessments, drug concentration, and immune function testing. The live oral rotavirus vaccine series was provided to 50 infants, with the first dose at a median of 13 weeks of age. Immunologic assessments validated for age were normal in all infants despite median infliximab concentrations of 6.1 μg/mL (range, 0.4-28.8 μg/mL), adalimumab concentrations of 1.7 μg/mL (range, 0.7-7.9 μg/mL), ustekinumab concentrations of 0.6 μg/mL (range, 0-1.1), and undetectable for vedolizumab at 10.7 weeks of age.
As anticipated, infant immune function was normal regardless of circulating drug levels.
The overall message, said Seow, is “healthy mum equals healthy baby. Be more concerned regarding active inflammation than active medications. In almost all circumstances, treat to target in pregnancy as you would in the nonpregnant state.” She added, however, that further studies are needed to determine the safety and optimal timing of other live vaccines, such as the BCG, in the presence of biologic therapy.
This study was funded by the Alberta Children’s Hospital Research Institute. Seow reported advisory/speaker’s fees for Janssen, AbbVie, Takeda, Pfizer, Fresenius Kabi, Bristol-Myers Squibb, Pharmascience, and Lilly, as well as funding from Alberta Children’s Hospital Research Institute, Crohn’s and Colitis Canada, the Canadian Institutes of Health Research, and Calgary Health Trust, and data safety monitoring from New South Wales Government Health, Australia. Multiple coauthors disclosed similar consulting or speaker relationships with private industry. Spencer had no competing interests with regard to her comments.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
GI Docs Will Need to Forge a ‘Human-Computer Cooperative’
Several artificial intelligence (AI) technologies are emerging that will change the management of gastrointestinal (GI) diseases sooner rather than later. One of the leading researchers working toward that AI-driven future is Ryan W. Stidham, MD, MS, AGAF, associate professor of gastroenterology and computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
Stidham’s work focuses on leveraging AI to develop automated systems that better quantify disease activity and aid gastroenterologists in their decision-making. He also serves as a meber of AGA's AI Task Force. what the technology may do to improve physician efficiency, and why gastroenterologists shouldn’t be worried about being replaced by machines any time soon.
How did you first become involved in studying AI applications for GI conditions?
My medical training coincided with the emergence of electronic health records (EHRs) making enormous amounts of data, ranging from laboratory results to diagnostic codes and billing records, readily accessible.
I quickly contracted data analytics fever, but a major problem became apparent: EHRs and medical claims data alone only weakly describe a patient. Researchers in the field were excited to use machine learning for personalizing treatment decisions for GI conditions, including inflammatory bowel disease (IBD). But no matter how large the dataset, the EHRs lacked the most rudimentary descriptions: What was the patient’s IBD phenotype? Where exactly was the disease located?
I could see machine learning had the potential to learn and reproduce expert decision-making. Unfortunately, we were fueling this machine-learning rocket ship with crude data unlikely to take us very far. Gastroenterologists rely on data in progress notes, emails, interpretations of colonoscopies, and radiologists’ and pathologists’ reviews of imaging to make treatment decisions, but that information is not well organized in any dataset.
I wanted to use AI to retrieve that key information in text, images, and video that we use every day for IBD care, automatically interpreting the data like a seasoned gastroenterologist. Generating higher-quality data describing patients could take our AI models from interesting research to useful and reliable tools in clinical care.
How did your early research go about trying to solve that problem?
My GI career began amid the IBD field shifting from relying on symptoms alone to objective biomarkers for IBD assessment, particularly focusing on standardized scoring of endoscopic mucosal inflammation. However, these scores were challenged with interobserver variability, prompting the need for centralized reading. More importantly, these scores are qualitative and do not capture all the visual findings an experienced physician appreciates when assessing severity, phenotype, and therapeutic effect. As a result, even experts could disagree on the degree of endoscopic severity, and patients with obvious differences in the appearance of mucosa could have the same endoscopic score.
I asked myself: Are we really using these measures to make treatment decisions and determine the effectiveness of investigational therapies? I thought we could do better and aimed to improve endoscopic IBD assessments using then-emerging digital image analysis techniques.
Convolutional neural network (CNN) modeling was just becoming feasible as computing performance increased. CNNs are well suited for complex medical image interpretation, using an associated “label,” such as the presence or grade of disease, to decipher the complex set of image feature patterns characterizing an expert’s determination of disease severity.
How did you convert the promise of CNN into tangible results?
The plan was simple: Collect endoscopic images from patients with IBD, find some experts to grade IBD severity on the images, and train a CNN model using the images and expert labels.
In 2016, developing a CNN wasn’t easy. There was no database of endoscopic images or simple methods for image labeling. The CNN needed tens of thousands of images. How were we to collect enough images with a broad range of IBD severity? I also reached some technical limits and needed help solving computational challenges.
Designing our first IBD endoscopic CNN took years of reading, coursework, additional training, and a new host of collaborators.
Failure was frequent, and my colleagues and I spent a lot of nights and weekends looking at thousands of individual endoscopic images. But we eventually had a working model for grading endoscopic severity, and its performance exceeded our expectations.
To our surprise, the CNN model grading of ulcerative colitis severity almost perfectly matched the opinion of IBD experts. We introduced the proof of concept that AI could automate complex disease measurement for IBD.
What took us 3 years in 2016 would take about 3 weeks today.
You have said that AI could help reduce the substantial administrative burdens in medicine today. What might an AI-assisted future look like for time-strapped gastroenterologists?
We will be spending more time on complex decision-making and developing treatment plans, with less time needed to hunt for information in the chart and administrative tasks.
The practical applications of AI will chip away at tedious mechanical tasks, soon to be done by machines, reclaiming time for gastroenterologists.
For example, automated documentation is almost usable, and audio recordings in the clinic could be leveraged to generate office notes.
Computer vision analysis of endoscopic video is generating draft procedural notes and letters to patients in a shared language, as well as recommending surveillance intervals based on the findings.
Text processing is already being used to automate billing and manage health maintenance like vaccinations, laboratory screening, and therapeutic drug monitoring.
Unfortunately, I don’t think that AI will immediately help with burnout. These near-term AI administrative assistant advantages, however, will help us manage the increasing patient load, address physician shortages, and potentially improve access to care in underserved areas.
Were there any surprises in your work?
I must admit, I was certain AI would put us gastroenterologists to shame. Over time, I have reversed that view.
AI really struggles to understand the holistic patient context when interpreting disease and predicting what to do for an individual patient. Humans anticipate gaps in data and customize the weighting of information when making decisions for individuals. An experienced gastroenterologist can incorporate risks, harms, and costs in ways AI is several generations from achieving.
With certainty, AI will outperform gastroenterologists for tedious and repetitive tasks, and we should gladly expect AI to assume those responsibilities. However, many unknowns remain in the daily management of GI conditions. We will continue to rely on the clinical experience, creativity, and improvisation of gastroenterologists for years to come.
Has there been a turning-point moment when it felt like this technology moved from being more theoretical to something with real-world clinical applications?
Last spring, I saw a lecture by Peter Lee, who is president of Microsoft Research and a leader in developing AI-powered applications in medicine and scientific research, demonstrating how a large language model (LLM) could “understand” medical text and generate responses to questions. My jaw dropped.
We watched an LLM answer American Board of Internal Medicine questions with perfect explanations and rationale. He demonstrated how an audio recording of a clinic visit could be used to automatically generate a SOAP (subjective, objective assessment and plan) note. It was better than anything I would have drafted. He also showed how the LLM could directly ingest EHR data, without any modification, and provide a great diagnosis and treatment plan. Finally, LLM chatbots could carry on an interactive conversation with a patient that would be difficult to distinguish from a human physician.
The inevitability of AI-powered transformations in gastroenterology care became apparent.
Documentation, billing, and administrative work will be handled by AI. AI will collect and organize information for me. Chart reviews and even telephone/email checkups on patients will be a thing of the past. AI chatbots will be able to discuss an individual patient’s condition and test results. Our GI-AI assistants will proactively collect information from patients after hospitalization or react to a change in labs.
AI will soon be an amazing diagnostician and will know more than me. So do we need to polish our resumes for new careers? No, but we will need to adapt to changes, which I believe on the whole will be better for gastroenterologists and patients.
What does adaptation look like for gastroenterologists over the next handful of years?
Like any other tool, gastroenterologists will be figuring out how to use AI prediction models, chatbots, and imaging analytics. Value, ease of use, and information-gain will drive which AI tools are ultimately adopted.
Memory, information recall, calculations, and repetitive tasks where gastroenterologists occasionally error or find tiresome will become the job of machines. We will still be the magicians, now aided by machines, applying our human strengths of contextual awareness, judgment, and creativity to find customized solutions for more patients.
That, I think, is the future that we are reliably moving toward over the next decade — a human-computer cooperative throughout gastroenterology (including IBD) and, frankly, all of medicine.
A version of this article appeared on Medscape.com.
Several artificial intelligence (AI) technologies are emerging that will change the management of gastrointestinal (GI) diseases sooner rather than later. One of the leading researchers working toward that AI-driven future is Ryan W. Stidham, MD, MS, AGAF, associate professor of gastroenterology and computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
Stidham’s work focuses on leveraging AI to develop automated systems that better quantify disease activity and aid gastroenterologists in their decision-making. He also serves as a meber of AGA's AI Task Force. what the technology may do to improve physician efficiency, and why gastroenterologists shouldn’t be worried about being replaced by machines any time soon.
How did you first become involved in studying AI applications for GI conditions?
My medical training coincided with the emergence of electronic health records (EHRs) making enormous amounts of data, ranging from laboratory results to diagnostic codes and billing records, readily accessible.
I quickly contracted data analytics fever, but a major problem became apparent: EHRs and medical claims data alone only weakly describe a patient. Researchers in the field were excited to use machine learning for personalizing treatment decisions for GI conditions, including inflammatory bowel disease (IBD). But no matter how large the dataset, the EHRs lacked the most rudimentary descriptions: What was the patient’s IBD phenotype? Where exactly was the disease located?
I could see machine learning had the potential to learn and reproduce expert decision-making. Unfortunately, we were fueling this machine-learning rocket ship with crude data unlikely to take us very far. Gastroenterologists rely on data in progress notes, emails, interpretations of colonoscopies, and radiologists’ and pathologists’ reviews of imaging to make treatment decisions, but that information is not well organized in any dataset.
I wanted to use AI to retrieve that key information in text, images, and video that we use every day for IBD care, automatically interpreting the data like a seasoned gastroenterologist. Generating higher-quality data describing patients could take our AI models from interesting research to useful and reliable tools in clinical care.
How did your early research go about trying to solve that problem?
My GI career began amid the IBD field shifting from relying on symptoms alone to objective biomarkers for IBD assessment, particularly focusing on standardized scoring of endoscopic mucosal inflammation. However, these scores were challenged with interobserver variability, prompting the need for centralized reading. More importantly, these scores are qualitative and do not capture all the visual findings an experienced physician appreciates when assessing severity, phenotype, and therapeutic effect. As a result, even experts could disagree on the degree of endoscopic severity, and patients with obvious differences in the appearance of mucosa could have the same endoscopic score.
I asked myself: Are we really using these measures to make treatment decisions and determine the effectiveness of investigational therapies? I thought we could do better and aimed to improve endoscopic IBD assessments using then-emerging digital image analysis techniques.
Convolutional neural network (CNN) modeling was just becoming feasible as computing performance increased. CNNs are well suited for complex medical image interpretation, using an associated “label,” such as the presence or grade of disease, to decipher the complex set of image feature patterns characterizing an expert’s determination of disease severity.
How did you convert the promise of CNN into tangible results?
The plan was simple: Collect endoscopic images from patients with IBD, find some experts to grade IBD severity on the images, and train a CNN model using the images and expert labels.
In 2016, developing a CNN wasn’t easy. There was no database of endoscopic images or simple methods for image labeling. The CNN needed tens of thousands of images. How were we to collect enough images with a broad range of IBD severity? I also reached some technical limits and needed help solving computational challenges.
Designing our first IBD endoscopic CNN took years of reading, coursework, additional training, and a new host of collaborators.
Failure was frequent, and my colleagues and I spent a lot of nights and weekends looking at thousands of individual endoscopic images. But we eventually had a working model for grading endoscopic severity, and its performance exceeded our expectations.
To our surprise, the CNN model grading of ulcerative colitis severity almost perfectly matched the opinion of IBD experts. We introduced the proof of concept that AI could automate complex disease measurement for IBD.
What took us 3 years in 2016 would take about 3 weeks today.
You have said that AI could help reduce the substantial administrative burdens in medicine today. What might an AI-assisted future look like for time-strapped gastroenterologists?
We will be spending more time on complex decision-making and developing treatment plans, with less time needed to hunt for information in the chart and administrative tasks.
The practical applications of AI will chip away at tedious mechanical tasks, soon to be done by machines, reclaiming time for gastroenterologists.
For example, automated documentation is almost usable, and audio recordings in the clinic could be leveraged to generate office notes.
Computer vision analysis of endoscopic video is generating draft procedural notes and letters to patients in a shared language, as well as recommending surveillance intervals based on the findings.
Text processing is already being used to automate billing and manage health maintenance like vaccinations, laboratory screening, and therapeutic drug monitoring.
Unfortunately, I don’t think that AI will immediately help with burnout. These near-term AI administrative assistant advantages, however, will help us manage the increasing patient load, address physician shortages, and potentially improve access to care in underserved areas.
Were there any surprises in your work?
I must admit, I was certain AI would put us gastroenterologists to shame. Over time, I have reversed that view.
AI really struggles to understand the holistic patient context when interpreting disease and predicting what to do for an individual patient. Humans anticipate gaps in data and customize the weighting of information when making decisions for individuals. An experienced gastroenterologist can incorporate risks, harms, and costs in ways AI is several generations from achieving.
With certainty, AI will outperform gastroenterologists for tedious and repetitive tasks, and we should gladly expect AI to assume those responsibilities. However, many unknowns remain in the daily management of GI conditions. We will continue to rely on the clinical experience, creativity, and improvisation of gastroenterologists for years to come.
Has there been a turning-point moment when it felt like this technology moved from being more theoretical to something with real-world clinical applications?
Last spring, I saw a lecture by Peter Lee, who is president of Microsoft Research and a leader in developing AI-powered applications in medicine and scientific research, demonstrating how a large language model (LLM) could “understand” medical text and generate responses to questions. My jaw dropped.
We watched an LLM answer American Board of Internal Medicine questions with perfect explanations and rationale. He demonstrated how an audio recording of a clinic visit could be used to automatically generate a SOAP (subjective, objective assessment and plan) note. It was better than anything I would have drafted. He also showed how the LLM could directly ingest EHR data, without any modification, and provide a great diagnosis and treatment plan. Finally, LLM chatbots could carry on an interactive conversation with a patient that would be difficult to distinguish from a human physician.
The inevitability of AI-powered transformations in gastroenterology care became apparent.
Documentation, billing, and administrative work will be handled by AI. AI will collect and organize information for me. Chart reviews and even telephone/email checkups on patients will be a thing of the past. AI chatbots will be able to discuss an individual patient’s condition and test results. Our GI-AI assistants will proactively collect information from patients after hospitalization or react to a change in labs.
AI will soon be an amazing diagnostician and will know more than me. So do we need to polish our resumes for new careers? No, but we will need to adapt to changes, which I believe on the whole will be better for gastroenterologists and patients.
What does adaptation look like for gastroenterologists over the next handful of years?
Like any other tool, gastroenterologists will be figuring out how to use AI prediction models, chatbots, and imaging analytics. Value, ease of use, and information-gain will drive which AI tools are ultimately adopted.
Memory, information recall, calculations, and repetitive tasks where gastroenterologists occasionally error or find tiresome will become the job of machines. We will still be the magicians, now aided by machines, applying our human strengths of contextual awareness, judgment, and creativity to find customized solutions for more patients.
That, I think, is the future that we are reliably moving toward over the next decade — a human-computer cooperative throughout gastroenterology (including IBD) and, frankly, all of medicine.
A version of this article appeared on Medscape.com.
Several artificial intelligence (AI) technologies are emerging that will change the management of gastrointestinal (GI) diseases sooner rather than later. One of the leading researchers working toward that AI-driven future is Ryan W. Stidham, MD, MS, AGAF, associate professor of gastroenterology and computational medicine and bioinformatics at the University of Michigan, Ann Arbor.
Stidham’s work focuses on leveraging AI to develop automated systems that better quantify disease activity and aid gastroenterologists in their decision-making. He also serves as a meber of AGA's AI Task Force. what the technology may do to improve physician efficiency, and why gastroenterologists shouldn’t be worried about being replaced by machines any time soon.
How did you first become involved in studying AI applications for GI conditions?
My medical training coincided with the emergence of electronic health records (EHRs) making enormous amounts of data, ranging from laboratory results to diagnostic codes and billing records, readily accessible.
I quickly contracted data analytics fever, but a major problem became apparent: EHRs and medical claims data alone only weakly describe a patient. Researchers in the field were excited to use machine learning for personalizing treatment decisions for GI conditions, including inflammatory bowel disease (IBD). But no matter how large the dataset, the EHRs lacked the most rudimentary descriptions: What was the patient’s IBD phenotype? Where exactly was the disease located?
I could see machine learning had the potential to learn and reproduce expert decision-making. Unfortunately, we were fueling this machine-learning rocket ship with crude data unlikely to take us very far. Gastroenterologists rely on data in progress notes, emails, interpretations of colonoscopies, and radiologists’ and pathologists’ reviews of imaging to make treatment decisions, but that information is not well organized in any dataset.
I wanted to use AI to retrieve that key information in text, images, and video that we use every day for IBD care, automatically interpreting the data like a seasoned gastroenterologist. Generating higher-quality data describing patients could take our AI models from interesting research to useful and reliable tools in clinical care.
How did your early research go about trying to solve that problem?
My GI career began amid the IBD field shifting from relying on symptoms alone to objective biomarkers for IBD assessment, particularly focusing on standardized scoring of endoscopic mucosal inflammation. However, these scores were challenged with interobserver variability, prompting the need for centralized reading. More importantly, these scores are qualitative and do not capture all the visual findings an experienced physician appreciates when assessing severity, phenotype, and therapeutic effect. As a result, even experts could disagree on the degree of endoscopic severity, and patients with obvious differences in the appearance of mucosa could have the same endoscopic score.
I asked myself: Are we really using these measures to make treatment decisions and determine the effectiveness of investigational therapies? I thought we could do better and aimed to improve endoscopic IBD assessments using then-emerging digital image analysis techniques.
Convolutional neural network (CNN) modeling was just becoming feasible as computing performance increased. CNNs are well suited for complex medical image interpretation, using an associated “label,” such as the presence or grade of disease, to decipher the complex set of image feature patterns characterizing an expert’s determination of disease severity.
How did you convert the promise of CNN into tangible results?
The plan was simple: Collect endoscopic images from patients with IBD, find some experts to grade IBD severity on the images, and train a CNN model using the images and expert labels.
In 2016, developing a CNN wasn’t easy. There was no database of endoscopic images or simple methods for image labeling. The CNN needed tens of thousands of images. How were we to collect enough images with a broad range of IBD severity? I also reached some technical limits and needed help solving computational challenges.
Designing our first IBD endoscopic CNN took years of reading, coursework, additional training, and a new host of collaborators.
Failure was frequent, and my colleagues and I spent a lot of nights and weekends looking at thousands of individual endoscopic images. But we eventually had a working model for grading endoscopic severity, and its performance exceeded our expectations.
To our surprise, the CNN model grading of ulcerative colitis severity almost perfectly matched the opinion of IBD experts. We introduced the proof of concept that AI could automate complex disease measurement for IBD.
What took us 3 years in 2016 would take about 3 weeks today.
You have said that AI could help reduce the substantial administrative burdens in medicine today. What might an AI-assisted future look like for time-strapped gastroenterologists?
We will be spending more time on complex decision-making and developing treatment plans, with less time needed to hunt for information in the chart and administrative tasks.
The practical applications of AI will chip away at tedious mechanical tasks, soon to be done by machines, reclaiming time for gastroenterologists.
For example, automated documentation is almost usable, and audio recordings in the clinic could be leveraged to generate office notes.
Computer vision analysis of endoscopic video is generating draft procedural notes and letters to patients in a shared language, as well as recommending surveillance intervals based on the findings.
Text processing is already being used to automate billing and manage health maintenance like vaccinations, laboratory screening, and therapeutic drug monitoring.
Unfortunately, I don’t think that AI will immediately help with burnout. These near-term AI administrative assistant advantages, however, will help us manage the increasing patient load, address physician shortages, and potentially improve access to care in underserved areas.
Were there any surprises in your work?
I must admit, I was certain AI would put us gastroenterologists to shame. Over time, I have reversed that view.
AI really struggles to understand the holistic patient context when interpreting disease and predicting what to do for an individual patient. Humans anticipate gaps in data and customize the weighting of information when making decisions for individuals. An experienced gastroenterologist can incorporate risks, harms, and costs in ways AI is several generations from achieving.
With certainty, AI will outperform gastroenterologists for tedious and repetitive tasks, and we should gladly expect AI to assume those responsibilities. However, many unknowns remain in the daily management of GI conditions. We will continue to rely on the clinical experience, creativity, and improvisation of gastroenterologists for years to come.
Has there been a turning-point moment when it felt like this technology moved from being more theoretical to something with real-world clinical applications?
Last spring, I saw a lecture by Peter Lee, who is president of Microsoft Research and a leader in developing AI-powered applications in medicine and scientific research, demonstrating how a large language model (LLM) could “understand” medical text and generate responses to questions. My jaw dropped.
We watched an LLM answer American Board of Internal Medicine questions with perfect explanations and rationale. He demonstrated how an audio recording of a clinic visit could be used to automatically generate a SOAP (subjective, objective assessment and plan) note. It was better than anything I would have drafted. He also showed how the LLM could directly ingest EHR data, without any modification, and provide a great diagnosis and treatment plan. Finally, LLM chatbots could carry on an interactive conversation with a patient that would be difficult to distinguish from a human physician.
The inevitability of AI-powered transformations in gastroenterology care became apparent.
Documentation, billing, and administrative work will be handled by AI. AI will collect and organize information for me. Chart reviews and even telephone/email checkups on patients will be a thing of the past. AI chatbots will be able to discuss an individual patient’s condition and test results. Our GI-AI assistants will proactively collect information from patients after hospitalization or react to a change in labs.
AI will soon be an amazing diagnostician and will know more than me. So do we need to polish our resumes for new careers? No, but we will need to adapt to changes, which I believe on the whole will be better for gastroenterologists and patients.
What does adaptation look like for gastroenterologists over the next handful of years?
Like any other tool, gastroenterologists will be figuring out how to use AI prediction models, chatbots, and imaging analytics. Value, ease of use, and information-gain will drive which AI tools are ultimately adopted.
Memory, information recall, calculations, and repetitive tasks where gastroenterologists occasionally error or find tiresome will become the job of machines. We will still be the magicians, now aided by machines, applying our human strengths of contextual awareness, judgment, and creativity to find customized solutions for more patients.
That, I think, is the future that we are reliably moving toward over the next decade — a human-computer cooperative throughout gastroenterology (including IBD) and, frankly, all of medicine.
A version of this article appeared on Medscape.com.