User login
Cancer Data Trends 2024: B-Cell Lymphoma
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
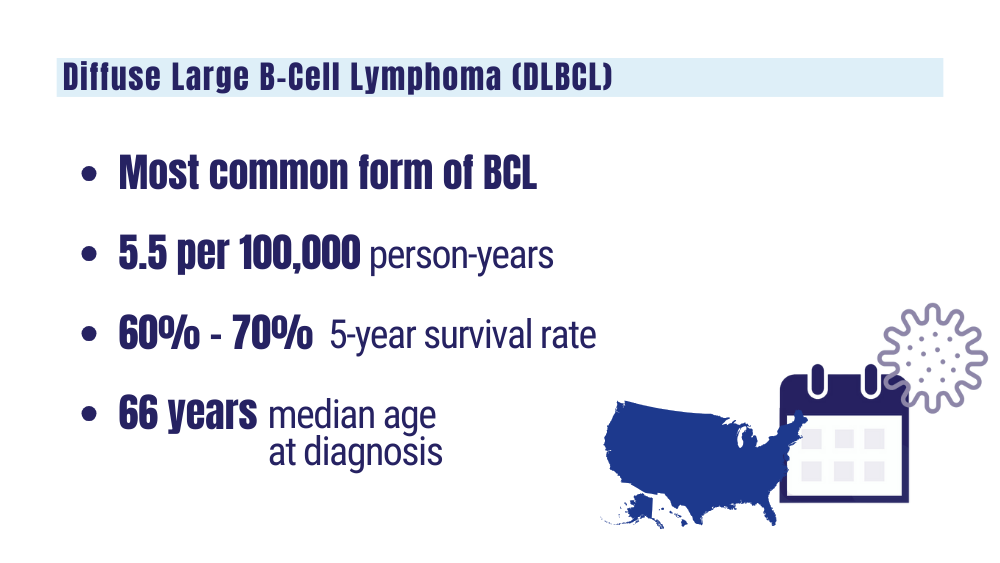
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
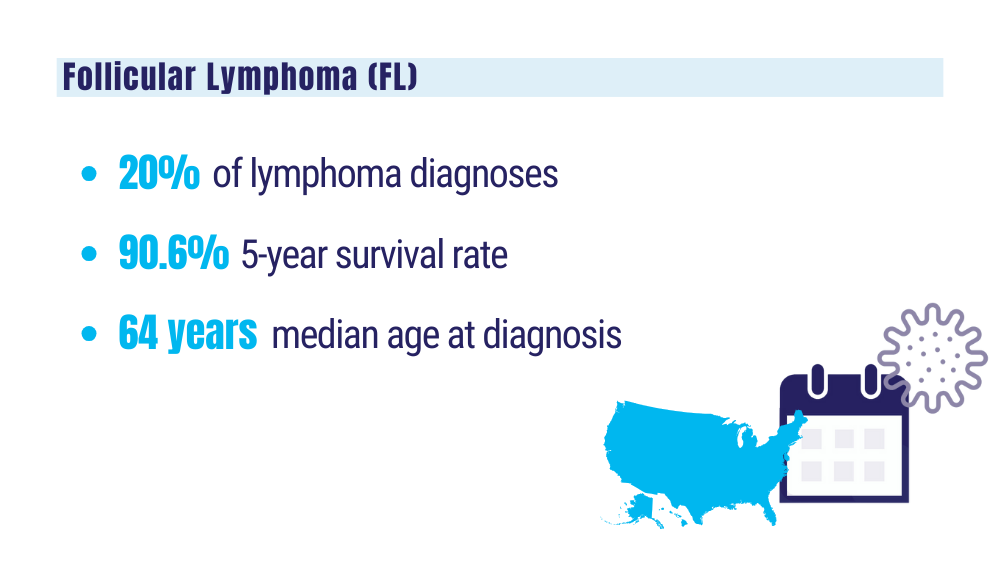
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
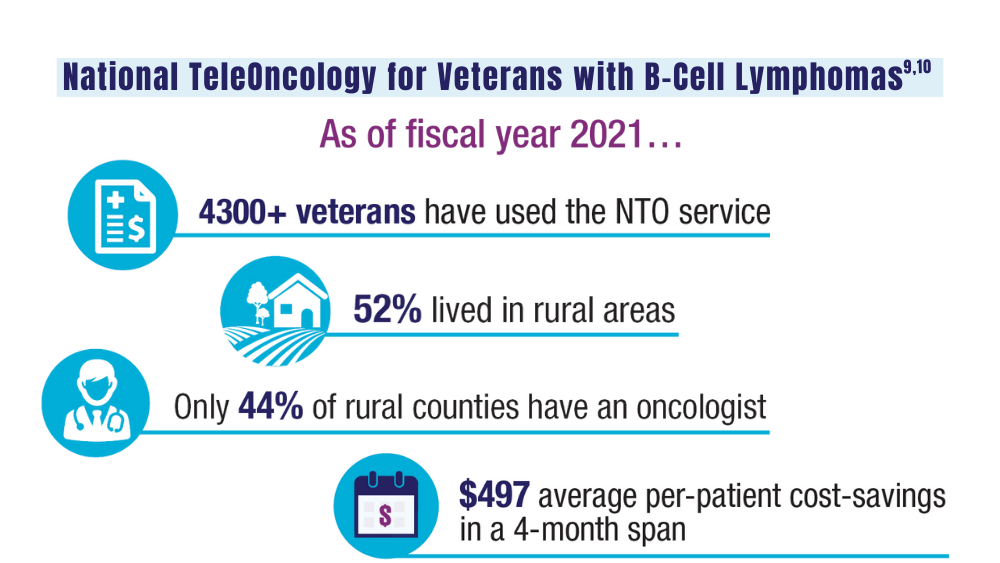
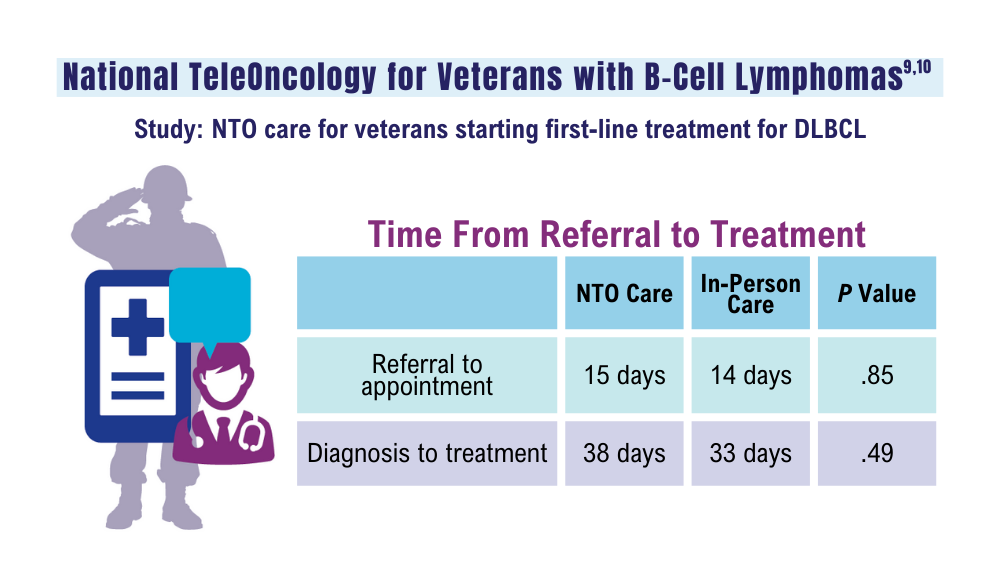
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
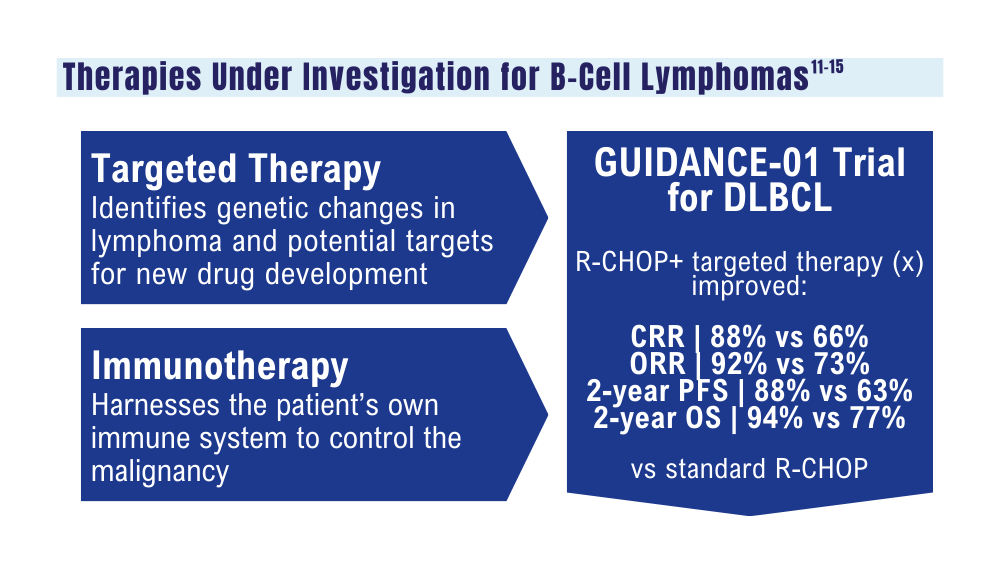
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
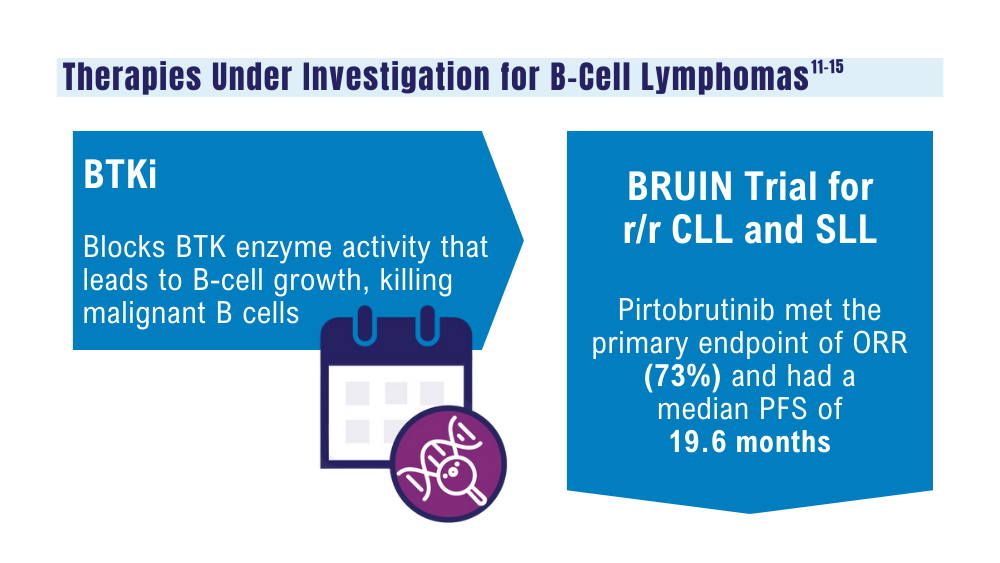
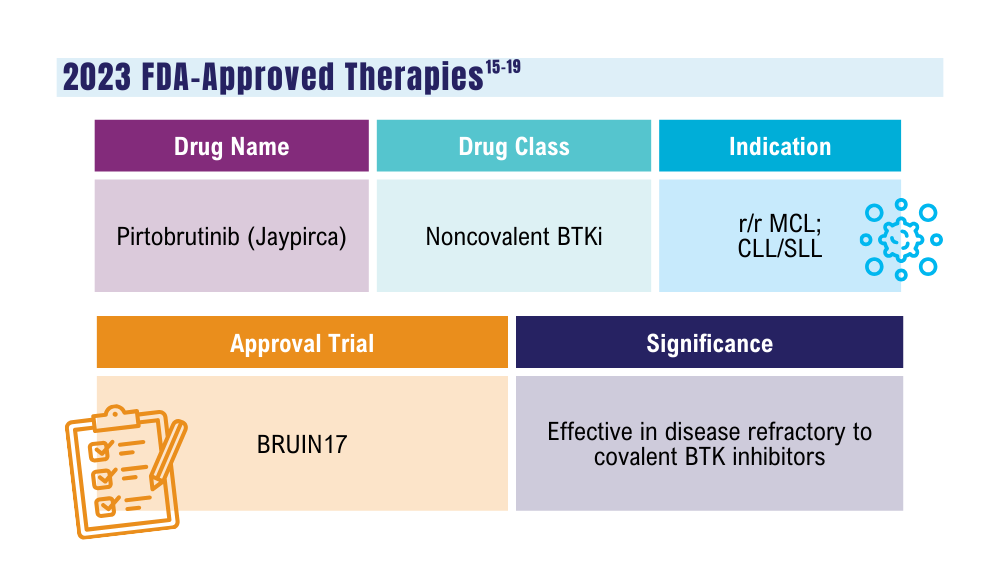
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
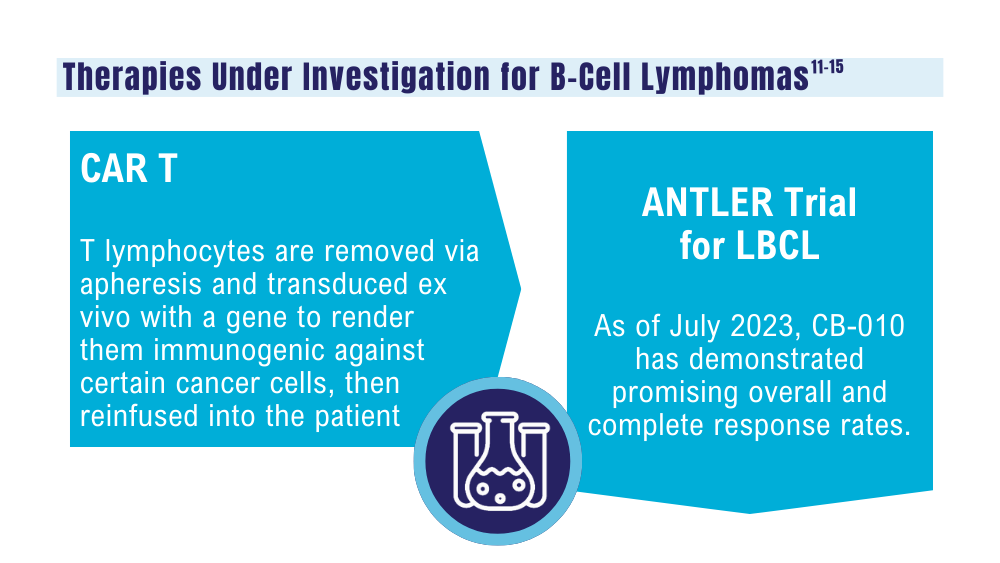
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
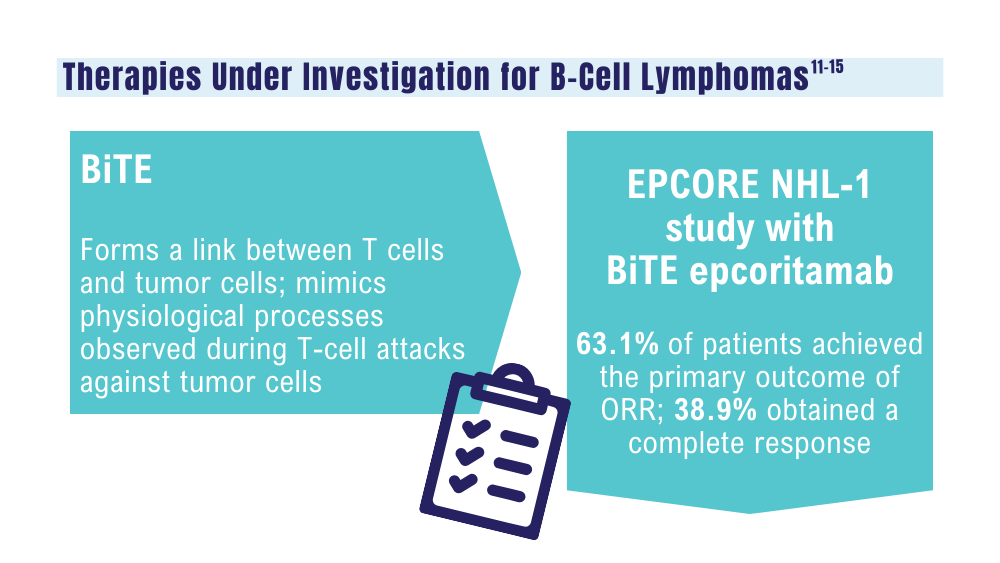
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
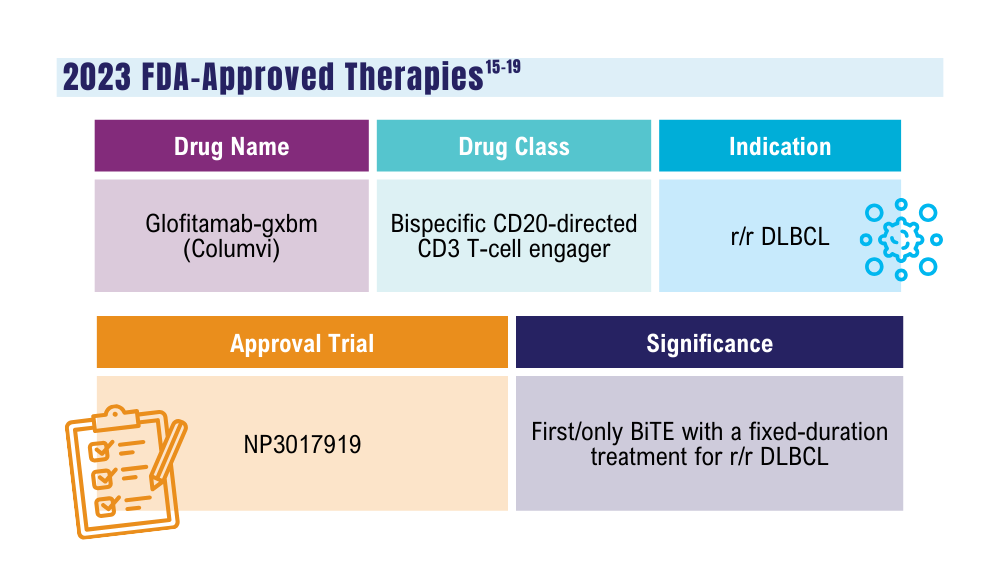
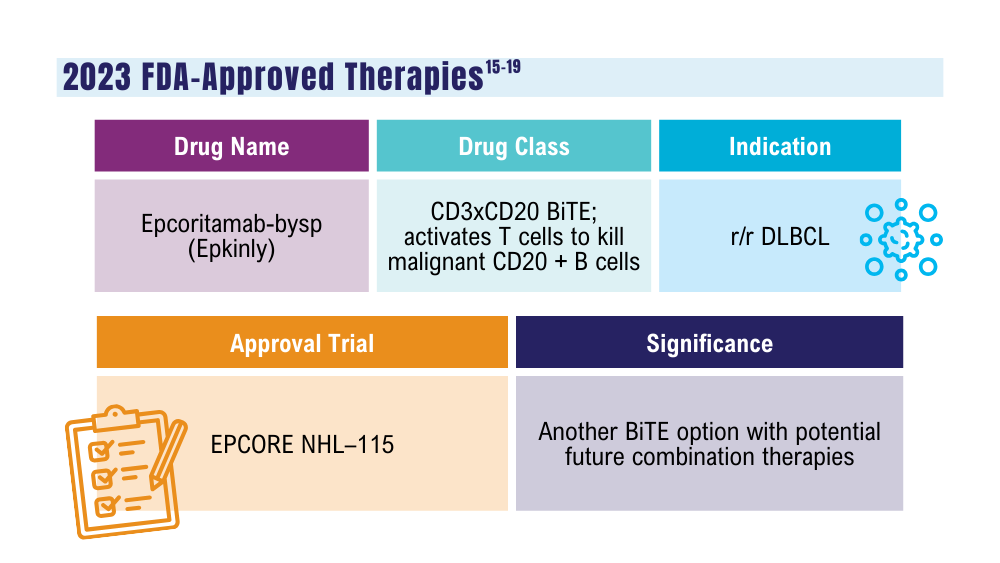
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
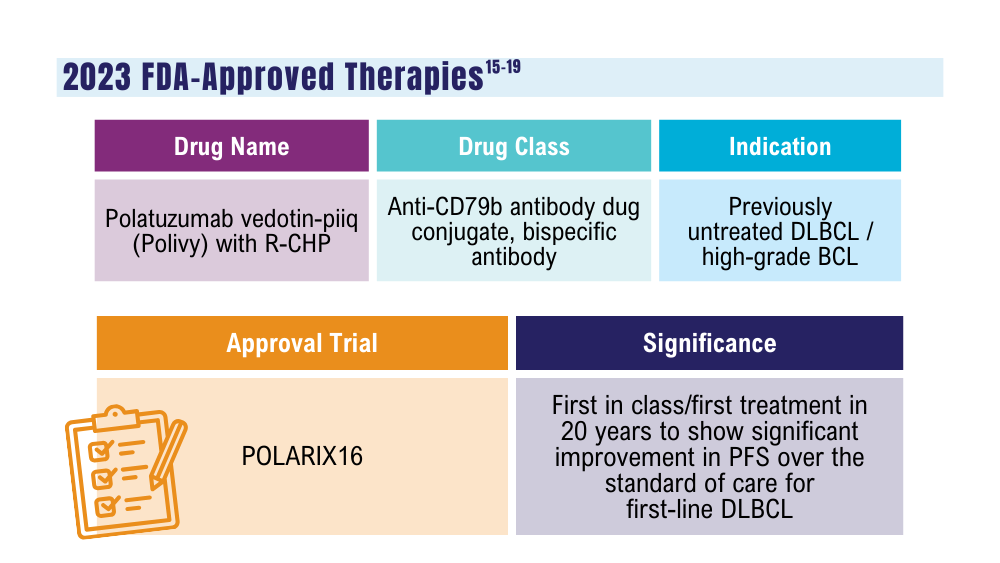
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
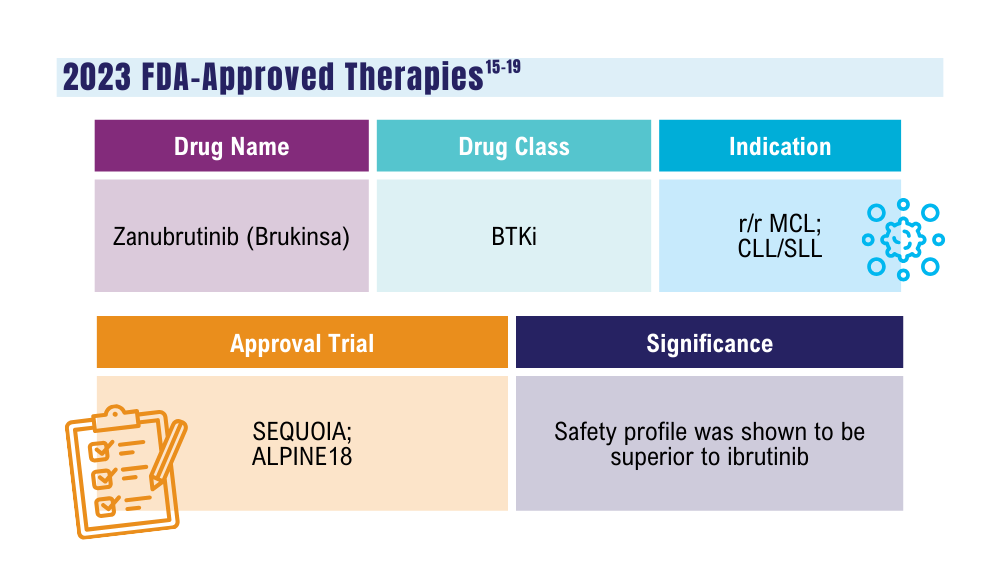
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
Lu W, Chen W, Zhou Y, et al. A model to predict the prognosis of diffuse large B-cell lymphoma based on ultrasound images. Sci Rep. 2023;13(1):3346. doi:10.1038/s41598-023-30533-y
Leukemia - Chronic lymphocytic - CLL: statistics. Cancer.net. Published February 2023. Accessed January 24, 2024. https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/statistics
Harmanen M, Hujo M, Sund R, et al. Survival of patients with mantle cell lymphoma in the rituximab era: retrospective binational analysis between 2000 and 2020. Br J Haematol. 2023;201(1):64-74. doi:10.1111/bjh.18597
Romancik JT, Cohen JB. Management of older adults with mantle cell lymphoma. Drugs Aging. 2020;37(7):469-481. doi:10.1007/s40266-020-00765-y
Marginal zone lymphoma (MZL). Leukemia and Lymphoma Society. Accessed January 24, 2024. https://www.lls.org/research/marginal-zone-lymphoma-mzl
Understanding lymphoma: diffuse large B-cell lymphoma. Lymphoma Research Foundation fact sheet. Updated 2023. Accessed January 24, 2024 https://lymphoma.org/wp-content/uploads/2023/10/LRF_Understanding_Lymphoma_Diffuse_Large_B_Cell_Lymphoma_Fact_Sheet.pdf
Key statistics for non-Hodgkin lymphoma. American Cancer Society. Updated January 17, 2024. Accessed January 24, 2024. https://www.cancer.org/cancer/types/non-hodgkin-lymphoma/about/key-statistics.html
Zullig LL, Raska W, McWhirter G, et al. Veterans Health Administration National TeleOncology Service. JCO Oncol Pract. 2023;19(4):e504-e510. doi:10.1200/OP.22.00455
Lin C, Zhou KI, Burningham ZR, et al. Telemedicine-supervised cancer therapy for patients with an aggressive lymphoma and metastatic lung cancer in the U.S. Veterans Affairs National TeleOncology Service. J Clin Oncol. 2023;41(16 suppl)16:Abstract 1602. doi:10.1200/JCO.2023.41.16_suppl.1602
Zhang MC, Tian S, Fu D, et al. Genetic subtype-guided immunochemotherapy in diffuse large B-cell lymphoma: the randomized GUIDANCE-01 trial. Cancer Cell. 2023;41(10):1705-1716.e5. doi:10.1016/j.ccell.2023.09.004
Mato AR, Woyach JA, Brown JR, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. N Engl J Med. 2023;389(1):33-44. doi:10.1056/NEJMoa2300696
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi:10.1038/s41408-021-00459-7
Caribou Biosciences reports positive clinical data from dose escalation of CB-010 ANTLER phase 1 trial in r/r B-NHL [news release]. Globalnewswire.com. Published July 13, 2023. Accessed January 24, 2024. https://www.globenewswire.com/news-release/2023/07/13/2704702/0/en/Caribou-Biosciences-Reports-Positive-Clinical-Data-from-Dose-Escalation-of-CB-010-ANTLER-Phase-1-Trial-in-r-r-B-NHL.html
Ma J, Mo Y, Tang M, et al. Bispecific antibodies: from research to clinical application. Front Immunol. 2021;12:626616. doi:10.3389/fimmu.2021.626616
Davis JA, Granger K, Sakowski A, et al. Dual target dilemma: navigating epcoritamab vs. glofitamab in relapsed refractory diffuse large B-cell lymphoma. Expert Rev Hematol. 2023;16(12):915-918. doi:10.1080/17474086.2023.2285978
Lynch RC, Poh C, Ujjani CS, et al. Polatuzumab vedotin with infusional chemotherapy for untreated aggressive B-cell non-Hodgkin lymphomas. Blood Adv. 2023;7(11):2449-2458. doi:10.1182/bloodadvances.2022009145
Thompson PA, Tam CS. Pirtobrutinib: a new hope for patients with BTK inhibitor-refractory lymphoproliferative disorders. Blood. 2023;141(26):3137-3142. doi:10.1182/blood.2023020240
US Food and Drug Administration. FDA approves zanubrutinib for chronic lymphocytic leukemia or small lymphocytic lymphoma. Published January 19, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-zanubrutinib-chronic-lymphocytic-leukemia-or-small-lymphocytic-lymphoma
US Food and Drug Administration. FDA grants accelerated approval to glofitamab-gxbm for selected relapsed or refractory large B-cell lymphomas. Published June 16, 2023. Accessed January 24, 2024. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-grants-accelerated-approval-glofitamab-gxbm-selected-relapsed-or-refractory-large-b-cell
How Does Military Service Impact Cancer Risk? It’s Complicated
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
CHICAGO—While it’s extremely difficult to link cancer rates to military service, researchers are starting to get some initial inklings of possible connections, a US Department of Veterans Affairs (VA) oncologist told an audience at the 2023 annual meeting of the Association of VA Hematology/Oncology.
One study found surprising levels of abnormal proteins and cancer in the blood of service members, said Christin DeStefano, MD, of David Grant US Air Force Medical Center at Travis Air Force Base in California. Another may have uncovered a link between military trauma and lymphoma. And an analysis of pilots found they have higher rates of certain kinds of cancer— but lower levels of other cancer types.
“It is hard to tell if service-related exposures heighten the risk of cancer. Some aspects of military service might increase cancer risk,” DeStefano said. “But other aspects of military service might decrease cancer risk.”
The VA has been especially focused on the possible link between military service and cancer since the passage of the PACT Act in 2022. The legislation prioritizes claims for cancer, terminal illnesses, and homelessness, and it’s sparked more than 4.1 million free toxic-exposure screenings for veterans.
VA Under Secretary for Health Shereef Elnahal, MD, MBA, noted in the keynote address at the 2023 AVAHO annual meeting that “Every type of solid tumor is now considered a presumptive condition associated with burden of exposure to veterans deployed anywhere in Central Command, either in the Persian Gulf War or the post-9/11 conflicts.”
DeStefano noted that there are a variety of challenges to analyzing data regarding connections between military exposure and cancer. For one, “it’s hard to include people from the time they enter the military to postmilitary service. Some are getting their health care in the civilian health care systems.” In addition, “There are a lot of problems with ICD-9 and ICD-10 codes, which can be very erroneous. Maybe somebody came in with a mass, the doctor or the nurse practitioner was busy and they put down ‘suspected cancer,’ and now they have that ICD code in their chart where they never actually had cancer.” This is why more reliable cancer registries are so important, she said.
Another challenge is figuring out when exposures occurred and whether they actually occurred in the military at all. “There are multiple studies suggesting that a first driver event is often acquired 20 to 40 years before a cancer diagnosis, often in one’s 20s and 30s,” she said. “It's hard to quantify an exposure, since there can be exposures before military service and exposures after military service. The amount of exposure and duration of exposure might differ, and individuals might metabolize the exposures differently from each other.”
To make matters more complicated, research is pointing in surprising directions. DeStefano highlighted her not-yet-published study of monoclonal gammopathy (MG), a condition in which abnormal proteins are found in the blood, in 534 service members. MG can be a cancer precursor. Those exposed to burn pits in Iraq had similar risks of MG (6.7%) vs an unexposed, matched control group (5.4%; P = .22), Dr. DeStefano said. Over a mean follow-up of 14 years and 10 years, respectively, 7% of participants in each group developed cancer.
“You might think, ‘this is a negative study. There's no difference.’ However, it is very notable that the prevalence of monoclonal gammopathy was 6.1%. That is 3 times as high as we would expect in somebody in their 40s,” she said. “Also, 7% having a cancer diagnosis is not insignificant.” She added: “It is very possible that many of these service members already have full-blown multiple myeloma or something associated with monoclonal gammopathy that just has not been diagnosed yet.”
In another study, this one published in 2021, Dr. DeStefano and colleagues tracked 8834 injured Iraq/Afghanistan veterans and compared them with matched controls to see if there was a link between severe trauma and cancer. There wasn’t except for lymphoma (22 vs 7 cases, respectively; odds ratio = 3.1; 95% CI, 1.34-7.37; P = .008). The connection remained after adjustment for confounders.
What’s going on? “It’s possible that blast injury might induce some alterations to the immune system that might set the stage for lymphoma genesis,” she said. “Or maybe that blast injury is a surrogate for a toxic exposure: Maybe carcinogens are released during a blast injury.”
Dr. DeStefano also highlighted a 2022 study that tracked 386,190 Air Force officers. The study found that combat pilots (9.1% of the total) had greater adjusted odds of testicular and prostate cancers and melanoma than the other officers. Why? “Military pilots have exposure to cosmic ionizing radiation as well as ultraviolet radiation,” she said.
But while “these are scary, sobering things,” she noted that combat pilots were less likely to develop several cancers than the general population, including kidney, testicular, colorectal, bladder and thyroid cancer, and they were less likely to die from colorectal cancer.
When to treat DLBCL with radiotherapy?
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
FROM ASTRO 2023
Metastatic Urothelial Carcinoma Presenting as Mediastinal Lymphadenopathy Without Appreciable Bladder Mass in a Patient With Chronic Lymphocytic Leukemia
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
INTRODUCTION
Lymphadenopathy in Chronic Lymphocytic Leukemia (CLL) is a very common feature. However, sudden increase in lymphadenopathy or other symptoms like weight loss should be evaluated for possible metastatic malignancy. We describe a CLL patient with diffuse mediastinal lymphadenopathy who was diagnosed with metastatic bladder cancer without a primary bladder tumor mass on imaging.
CASE DESCRIPTION
A 60-year-old man with a 60 pack-year smoking history, alcoholic cirrhosis, and a 5-year history of stage 1 CLL presented with 3 months of progressive shortness of breath; persistent cough; chills; hemoptysis; and a steady weight loss of 35 lbs. Notably, he had no bladder symptoms. Initial labs showed leukocytosis of 35.8k with a lymphocytic predominance. Screening low-dose chest CT was positive for diffuse mediastinal lymphadenopathy. Subsequent PET/CT revealed numerous hypermetabolic lymph nodes in the neck, mediastinum, left hilum, and right periaortic abdominal region. CT Chest, Abdomen, Pelvis revealed progressive lymphadenopathy as seen in prior imaging, stable pulmonary nodules up to 4 mm in size, and splenomegaly. No distant primary sites, including of the bladder, were identified. Mediastinal lymph node biopsy confirmed metastatic poorly differentiated carcinoma with immunohistochemical staining negative for p40, p63, CK20, TTF-1, Napsin A, CDX2, CA19- 9, Calretinin, and D2-40 and positive for CK7, GATA3, Ber-EP4, and Uroplakin, supporting bladder as primary origin. Urology deferred a cystoscopy given his lack of urinary symptoms and positive biopsy and was started on Carboplatin/Gemcitabine for his metastatic disease. He was ineligible for Cisplatin given his cirrhosis and hearing impairment.
DISCUSSION
In patients with CLL, new onset mediastinal lymphadenopathy is concerning for disease progression and possible transformation to a diffuse b-cell lymphoma. However, this symptom has a broad differential, including primary lung carcinomas, sarcomas, and metastatic disease. While our patient’s PET/CT and pan-CT failed to identify a distant primary site, maintaining a low clinical suspicion for metastatic disease and doing a thorough work-up was paramount. Only through immunohistochemical staining were we able to diagnosis this patient with urothelial carcinoma.
CONCLUSIONS
Biopsy with immunohistochemical staining and maintaining a low suspicion for worsening lymphadenopathy can identify unusually presenting urothelial carcinomas in CLL patients.
FDA approves motixafortide for stem cell mobilization in myeloma
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.
The success of autologous stem cell transplantation (ASCT) depends on adequate mobilization of stem cells during the treatment process. Collection of a sufficient number of stem cells to perform two transplantations is recommended. However, in up to 47% of patients, collecting target numbers of hematopoietic stem cells for ASCT after one apheresis session remains a challenge, BioLineRx explained in a press release today, announcing the approval.
The goal of combining motixafortide with filgrastim is to mobilize stem cells more reliably than filgrastim can alone, with fewer days of apheresis sessions and fewer doses of filgrastim.
“We believe [motixafortide] will play a critical role in addressing unmet needs and introduce a new treatment paradigm for” patients with multiple myeloma, CEO Philip Serlin said in the release.
The drug approval was based on the GENESIS trial, which randomized 122 patients to either motixafortide plus filgrastim or placebo plus filgrastim.
BioLineRx said the trial included patients considered representative of the typical multiple myeloma population undergoing ASCT, with a median age of 63 years and with about 70% of patients in both arms of the trial receiving lenalidomide-containing induction therapy.
Motixafortide plus filgrastim enabled 67.5% of patients to achieve the stem cell collection goal of 6 million or more CD34+ cells/kg within two apheresis sessions, versus 9.5% of patients receiving the placebo plus filgrastim regimen. Additionally, 92.5% of patients reached the stem cell collection goal in up to two apheresis sessions in the motixafortide arm and 21.4% in the placebo arm.
However, “the data are descriptive and were not statistically powered nor prespecified. The information should be cautiously interpreted,” the company said.
Serious adverse reactions occurred in 5.4% of patients in the motixafortide arm, including vomiting, injection-site reaction, hypersensitivity reaction, injection-site cellulitis, hypokalemia, and hypoxia. The most common adverse reactions, occurring in more than 20% of patients, were injection site reactions (pain, erythema, and pruritus), pruritus, flushing, and back pain.
Labeling for the subcutaneous injection is available online.
A version of this article first appeared on Medscape.com.
Chimeric Antigen Receptor T-Cell Therapy in the Veterans Affairs Network: the Tennessee Valley Healthcare System Experience
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
BACKGROUND
Chimeric antigen receptor T-cell (CAR-T) therapy is a novel treatment for hematologic malignancies, with 6 FDA agents approved for commercial use. The Veterans Affairs (VA) Tennessee Valley Healthcare System (TVHS) is currently the only VA facility accredited to administer these agents and we are reporting the TVHS experience thus far.
METHODS
TVHS became an authorized treatment center for CAR-T therapy in September 2019 and performed its first CAR-T infusion in December 2019. This is a retrospective electronic chart review of all CAR-T veterans referred to TVHS from the program’s inception, December 1, 2019 through July 31, 2022 to evaluate at least one year of post infusion data. The primary objective is to evaluate the outcomes of veterans who received CAR-T therapy at TVHS including overall response rates (ORR), progression free survival (PFS), and overall survival (OS). Secondary objectives include assessment of toxicities, including rates and maximum grades of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).
RESULTS
A total of 41 veterans have received CAR-T infusion at TVHS to date. Twenty-nine of these veterans have at least one year post-CAR-T infusion data and are included in this analysis. The majority of veterans were White (72%), male (93%), and were treated for diffuse large B-cell lymphoma (86%). Twenty-eight percent of veterans were under-represented minorities. Average age was 61 years with 62% being 65 years and older and five (17%) veterans being over the age of 74. Day 30 ORR was 90% (45% complete response [CR]). One-year PFS was 55.2% and 1-year OS was 65.5%. Of the 19 veterans who achieved CR by day 100, 79% remain in CR to date. CRS toxicity was observed in 66% of veterans (0% Grade 3 or higher). ICANS was observed in 27.5% of veterans (24% Grade 3 or higher). Only 5 (26%) veterans required transfer to the intensive care unit for additional monitoring.
CONCLUSIONS
CAR-T therapy has become a wellestablished practice at TVHS and is a safe and effective treatment option for veterans with aggressive lymphoid malignancies. Our outcomes are similar to that seen nationally with better access to under-represented minorities in an aging population.
Does Gemcitabine Have a Curative Role in Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia?
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
INTRODUCTION
Gemcitabine is a part of National Comprehensive Cancer Network (NCCN) guidelines as salvage therapy for relapsed/refractory B-cell lymphomas, but its role in chronic lymphocytic leukemia (CLL) remains unclear. We describe a case of relapsed CLL showing complete response while on gemcitabine for another primary malignancy, suggesting a potential curative role of gemcitabine for CLL.
CASE REPORT
A 78-year-old male with relapsed CD38+ CLL with del11q on ibrutinib with partial response, presented with gross hematuria for one week. Of note, he was diagnosed with BRCA-negative Stage Ib pancreatic adenocarcinoma within the previous year, treated with surgery and adjuvant capecitabine-gemcitabine. Physical examination was unremarkable and bloodwork showed a white cell count of 32,000 cells/ mm3 with 1.5% lymphocytes, hemoglobin 9.5 g/dL, and platelets 866,000 cells/mm3. Hematuria remained persistent despite frequent bladder irrigations but resolved within a week of stopping ibrutinib. Eight months later, his white cell count is 6,600 cells/mm3, with 16% lymphocytes, hemoglobin 10.2 g/dL, platelets 519,000/m3, and CT scans show no pathological lymphadenopathy. A recent flow cytometry done for academic purposes showed no clonal B cells.
DISCUSSION
Relapsed CLL has a poor prognosis with no curative treatment. Gemcitabine is a part of NCCN guidelines for relapse/refractory B-cell lymphomas but is not included in guidelines for CLL. A study by Jamie et al in 2001 suggested the pre-clinical effectiveness of gemcitabine for relapsed/refractory CLL and phase II trials conducted in 2005 and 2012 on combination chemotherapy including gemcitabine have shown overall CLL response rates of 50-65%. The resolution of B-cell clonality and improvement in biochemical markers after treatment with gemcitabine for an alternate primary malignancy suggested that gemcitabine played a potential curative role in our patient. Further prospective studies are needed to explore this avenue for the role of gemcitabine as a salvage as well as potentially curative therapy for relapsed CLL with variable cytogenetics and treatment histories.
CONCLUSIONS
Gemcitabine is not part of NCCN guidelines for CLL currently but it is a reasonable treatment option for relapsed/refractory CLL. Further studies are needed to explore its potential curative role for relapsed CLL, and update existing guidelines.
Asciminib Chronic Phase Chronic Myeloid Leukemia: A Real-World Single Institution Case Series
INTRODUCTION
The development of imatinib and now newer tyrosine kinase inhibitors (TKIs) has revolutionized the overall survival of patients with CML. However, toxicity and treatment-resistance can result in premature discontinuation of therapy. Asciminib, a novel TKI, may have fewer off-target effects. It also bypasses the mechanism of resistance to first-line TKIs by binding to a different site on the BCR-ABL fusion protein. In our institution, three patients have been initiated on asciminib thus far. We present their cases, with a focus on quality of life.
CASE PRESENTATIONS
(1) A 76-year-old male with a history of diffuse vascular disease experienced off-target effects on multiple TKIs (i.e. intolerable nausea on imatinib, pleural effusion on dasatinib, complete heart block on nilotinib), so he was switched to asciminib. He has been tolerating asciminib well over five months and continues to see significant log reduction in BCR-ABL transcripts. (2) A 71-year-old male with a history of multiple complicated gastrointestinal infections never achieved major molecular remission on imatinib and was unable to tolerate dasatinib or bosutinib due to severe nausea and vomiting. He was switched to asciminib, which he has been tolerating well for one year, and has achieved complete hematologic response. (3) A 73-year-old male with a history of chronic kidney disease experienced kidney injury thought to be due to imatinib and was switched to bosutinib. His BCRABL transcripts rose on bosutinib, so patient was started on asciminib, which he has been tolerating well.
DISCUSSION
In this series of patients in their 70s with multiple underlying comorbidities, the unifying theme is that of intolerance to first-line TKIs due to toxicity (cardiac, pulmonary, gastrointestinal, and renal). Existing data suggests that asciminib results in less toxicity than other first-line TKIs, and this is evident in our patients. More importantly, the combination of efficacy and tolerability gives these patients the opportunity to proceed with life-prolonging therapy, even for those who face treatment resistance with other agents.
CONCLUSIONS
For CML patients who have failed at least two lines of treatment, whether it is due to disease progression or intolerable toxicity, asciminib is an effective alternative. Further study may result in its promotion to first-line therapy for this disease.
INTRODUCTION
The development of imatinib and now newer tyrosine kinase inhibitors (TKIs) has revolutionized the overall survival of patients with CML. However, toxicity and treatment-resistance can result in premature discontinuation of therapy. Asciminib, a novel TKI, may have fewer off-target effects. It also bypasses the mechanism of resistance to first-line TKIs by binding to a different site on the BCR-ABL fusion protein. In our institution, three patients have been initiated on asciminib thus far. We present their cases, with a focus on quality of life.
CASE PRESENTATIONS
(1) A 76-year-old male with a history of diffuse vascular disease experienced off-target effects on multiple TKIs (i.e. intolerable nausea on imatinib, pleural effusion on dasatinib, complete heart block on nilotinib), so he was switched to asciminib. He has been tolerating asciminib well over five months and continues to see significant log reduction in BCR-ABL transcripts. (2) A 71-year-old male with a history of multiple complicated gastrointestinal infections never achieved major molecular remission on imatinib and was unable to tolerate dasatinib or bosutinib due to severe nausea and vomiting. He was switched to asciminib, which he has been tolerating well for one year, and has achieved complete hematologic response. (3) A 73-year-old male with a history of chronic kidney disease experienced kidney injury thought to be due to imatinib and was switched to bosutinib. His BCRABL transcripts rose on bosutinib, so patient was started on asciminib, which he has been tolerating well.
DISCUSSION
In this series of patients in their 70s with multiple underlying comorbidities, the unifying theme is that of intolerance to first-line TKIs due to toxicity (cardiac, pulmonary, gastrointestinal, and renal). Existing data suggests that asciminib results in less toxicity than other first-line TKIs, and this is evident in our patients. More importantly, the combination of efficacy and tolerability gives these patients the opportunity to proceed with life-prolonging therapy, even for those who face treatment resistance with other agents.
CONCLUSIONS
For CML patients who have failed at least two lines of treatment, whether it is due to disease progression or intolerable toxicity, asciminib is an effective alternative. Further study may result in its promotion to first-line therapy for this disease.
INTRODUCTION
The development of imatinib and now newer tyrosine kinase inhibitors (TKIs) has revolutionized the overall survival of patients with CML. However, toxicity and treatment-resistance can result in premature discontinuation of therapy. Asciminib, a novel TKI, may have fewer off-target effects. It also bypasses the mechanism of resistance to first-line TKIs by binding to a different site on the BCR-ABL fusion protein. In our institution, three patients have been initiated on asciminib thus far. We present their cases, with a focus on quality of life.
CASE PRESENTATIONS
(1) A 76-year-old male with a history of diffuse vascular disease experienced off-target effects on multiple TKIs (i.e. intolerable nausea on imatinib, pleural effusion on dasatinib, complete heart block on nilotinib), so he was switched to asciminib. He has been tolerating asciminib well over five months and continues to see significant log reduction in BCR-ABL transcripts. (2) A 71-year-old male with a history of multiple complicated gastrointestinal infections never achieved major molecular remission on imatinib and was unable to tolerate dasatinib or bosutinib due to severe nausea and vomiting. He was switched to asciminib, which he has been tolerating well for one year, and has achieved complete hematologic response. (3) A 73-year-old male with a history of chronic kidney disease experienced kidney injury thought to be due to imatinib and was switched to bosutinib. His BCRABL transcripts rose on bosutinib, so patient was started on asciminib, which he has been tolerating well.
DISCUSSION
In this series of patients in their 70s with multiple underlying comorbidities, the unifying theme is that of intolerance to first-line TKIs due to toxicity (cardiac, pulmonary, gastrointestinal, and renal). Existing data suggests that asciminib results in less toxicity than other first-line TKIs, and this is evident in our patients. More importantly, the combination of efficacy and tolerability gives these patients the opportunity to proceed with life-prolonging therapy, even for those who face treatment resistance with other agents.
CONCLUSIONS
For CML patients who have failed at least two lines of treatment, whether it is due to disease progression or intolerable toxicity, asciminib is an effective alternative. Further study may result in its promotion to first-line therapy for this disease.
Rasburicase Use and Glucose-6-Phosphate Dehydrogenase Testing
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
BACKGROUND/PURPOSE
Tumor lysis syndrome (TLS) occurs when malignant cells rapidly break down. This may lead to hyperuricemia, hyperkalemia, hyperphosphatemia, and/or hypocalcemia. Rasburicase reduces uric acid in cancer patients undergoing anti-cancer therapy. However, caution is required as rasburicase is contraindicated for patients with glucose- 6-phosphate dehydrogenase (G6PD) deficiency due to the increased risk of hemolysis. G6PD deficiency is more prevalent among African Americans (AA), affecting approximately 12% of this population. The FDA recommends testing for G6PD deficiency in higher risk groups before administering rasburicase.
METHODS
A retrospective analysis was conducted at the Louis Stokes Cleveland VAMC from February 1, 2018, to January 31, 2023 addressing appropriate use of rasburicase and incidence of G6PD deficiency and hemolysis. Appropriate use was defined by: TLS (2 or more: uric acid ≥ 8 or 25% increase; K+ ≥ 6.0 or 25% increase; Phos > 4.5mg/dL, or 25% increase; or calcium < 7, or 25% decrease, from baseline) or at high risk for TLS (CLL: venetoclax use w/lymph node > 10cm or WBC > 25k and elevated uric acid; AML: WBC > 100k; ALL: WBC > 100k and LDH 2x ULN; Burkitt lymphoma: LDH 2x ULN).
RESULTS
50 patients were identified who received rasburicase. 21/50 (42%) did not meet criteria for appropriate use. 44/50 (88%) underwent G6PD testing. The average time from G6PD testing order to obtaining the results was 3.4 days; 18/50 patients (36%) had G6PD resulted prior to rasburicase administration, and 26 patients (52%) received rasburicase prior to G6PD results. Overall, 13/50 (26%) were AA. Of the AA pts, 12/13 (92%) were tested for G6PD. Of these 12, 1/12 was found to be G6PD deficient and this patient experienced G6PD deficiency-induced hemolysis after rasburicase. None of the non-AA pts (0/31) tested were found to be G6PD deficient.
IMPLICATIONS
There was a high (42%) level of inappropriate use of rasburicase. G6PD deficiency was uncommon and only found in the AA population. To reduce inappropriate use, rasburicase orders will be restricted to medical oncology. G6PD testing will be limited to AA pts, with pathology to develop a rapid turnaround time for results prior to rasburicase administration to prevent hemolysis.
Comparing Outcomes and Toxicities With Standard and Reduced Dose Melphalan in Autologous Stem Cell Transplant Patients With Multiple Myeloma
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.
BACKGROUND
Multiple myeloma, an incurable plasma cell malignancy, has an average age of diagnosis over 65 years. For transplant-eligible patients, high-dose melphalan 200 mg/m2 (MEL200), followed by autologous stem cell rescue (ASCR) is the standard in consolidation therapy. Most clinical trials evaluating MEL200 with ASCR excluded patients over 65 due to concerns for toxicity and treatment-related mortality, leading to use of reduced dose melphalan 140 mg/m2 (MEL140) in clinical practice for older patients. As this dose has limited studies surrounding its reduction, the purpose of this study was to compare outcomes and toxicities of MEL140 in patients over the age of 65 to MEL200 in patients 65 and under.
METHODS
This single-center institutional review board approved retrospective study was conducted at VA Tennessee Valley Healthcare System. All multiple myeloma patients greater than 18 years of age who received melphalan with ASCR from January 1, 2018, to December 31, 2021, were included. Patients were divided into two arms: age < 65 treated with MEL200 and age >65 treated with MEL140. The primary endpoint was oneyear progression-free survival (PFS). The secondary endpoints were one-year overall survival (OS), treatment related mortality, time to neutrophil engraftment, and toxicities including febrile neutropenia, diarrhea, mucositis, infection, and intensive care unit transfers.
RESULTS
A total of 222 patients were included, 114 patients in the MEL200 arm and 108 patients in the MEL140 arm. The primary endpoint of one-year PFS had no significant difference, with 103 (90.4%) patients in the MEL200 group compared to 99 (91.7%) patients in the MEL140 group (p=0.732). Similarly, there was no statistically significant difference in the secondary endpoint of one-year OS with 112 (98.3%) patients in the MEL200 group compared to 106 (98.2%) in the MEL140 group (p=0.956). Toxicities were similar; however, grade 3 mucositis was higher in the MEL200 arm.
CONCLUSIONS
Our study found no difference in oneyear PFS or one-year OS when comparing MEL140 to MEL200 with minimal differences in regimen-related toxicities. Although not powered to detect statistical difference, results suggests that dose reduction with MEL140 in patients >65 years does not impact one-year PFS when compared to patients <65 receiving standard MEL200.