User login
Mycobacteria subset plagues pulmonary patients
Nontuberculous mycobacteria accounts for an increasing percentage of pulmonary disease, and nonsurgical treatment alone has not shown effectiveness, according to data from a meta-analysis of 24 studies and 1,224 patients. The study results were published online in Chest.
Data on therapeutic successes in cases of nontuberculosis mycobacteria (NTM)–related pulmonary disease are limited, in particular for those species not related to the Mycobacterium avium complex (non-MAC), wrote Roland Diel, MD, of University Medical Hospital Schleswig-Holstein, Germany, and his colleagues.
In particular, non-MAC species Mycobacterium xenopi (MX), Mycobacterium abscessus, Mycobacterium malmoense, and Mycobacterium kansasii (MK) were addressed in the studies, which included 16 retrospective chart reviews, 5 randomized trials, and 3 prospective, nonrandomized studies (Chest 2017. doi: 10.1016/j.chest.2017.04.166).
Treatment success was measured by rates of sputum culture conversion (SCC).
Overall, the average proportion of SCC for patients with M. abscessus was 41% after subtraction for posttreatment relapses, but reached 70% for subspecies M. massiliense in macrolide-containing treatments. The average proportion of SCC was 80% for patients with M. kansasii, 32% for those with MX, and 54% for those with M. malmoense.
Treatment success ranged from 9% to 73% for M. xenopi patients, but all-cause mortality was 69%. Of note, a 100% success rate was noted in M. kansasii patients using a three-drug TB regimen of isoniazid, rifampicin, and ethambutol, or with a combination of ethambutol, rifampicin, and clarithromycin, the researchers noted.
The percentage of SCC in 55 patients with lung resection and either MX or M. abscessus was considered high at 76%.
The study findings were limited by the diverse definitions of treatment success and by the variety of treatments and “an optimal multidrug treatment cannot be derived from the few studies and has yet to be determined,” the researchers said. In the absence of optimal drug therapy, functional and quality of life elements deserve greater consideration when evaluating outcomes in patients with non-MAC NTM pulmonary disease, they added.
Dr. Diel reported receiving lecturing and/or consulting fees from Insmed and Riemser.
Nontuberculous mycobacteria accounts for an increasing percentage of pulmonary disease, and nonsurgical treatment alone has not shown effectiveness, according to data from a meta-analysis of 24 studies and 1,224 patients. The study results were published online in Chest.
Data on therapeutic successes in cases of nontuberculosis mycobacteria (NTM)–related pulmonary disease are limited, in particular for those species not related to the Mycobacterium avium complex (non-MAC), wrote Roland Diel, MD, of University Medical Hospital Schleswig-Holstein, Germany, and his colleagues.
In particular, non-MAC species Mycobacterium xenopi (MX), Mycobacterium abscessus, Mycobacterium malmoense, and Mycobacterium kansasii (MK) were addressed in the studies, which included 16 retrospective chart reviews, 5 randomized trials, and 3 prospective, nonrandomized studies (Chest 2017. doi: 10.1016/j.chest.2017.04.166).
Treatment success was measured by rates of sputum culture conversion (SCC).
Overall, the average proportion of SCC for patients with M. abscessus was 41% after subtraction for posttreatment relapses, but reached 70% for subspecies M. massiliense in macrolide-containing treatments. The average proportion of SCC was 80% for patients with M. kansasii, 32% for those with MX, and 54% for those with M. malmoense.
Treatment success ranged from 9% to 73% for M. xenopi patients, but all-cause mortality was 69%. Of note, a 100% success rate was noted in M. kansasii patients using a three-drug TB regimen of isoniazid, rifampicin, and ethambutol, or with a combination of ethambutol, rifampicin, and clarithromycin, the researchers noted.
The percentage of SCC in 55 patients with lung resection and either MX or M. abscessus was considered high at 76%.
The study findings were limited by the diverse definitions of treatment success and by the variety of treatments and “an optimal multidrug treatment cannot be derived from the few studies and has yet to be determined,” the researchers said. In the absence of optimal drug therapy, functional and quality of life elements deserve greater consideration when evaluating outcomes in patients with non-MAC NTM pulmonary disease, they added.
Dr. Diel reported receiving lecturing and/or consulting fees from Insmed and Riemser.
Nontuberculous mycobacteria accounts for an increasing percentage of pulmonary disease, and nonsurgical treatment alone has not shown effectiveness, according to data from a meta-analysis of 24 studies and 1,224 patients. The study results were published online in Chest.
Data on therapeutic successes in cases of nontuberculosis mycobacteria (NTM)–related pulmonary disease are limited, in particular for those species not related to the Mycobacterium avium complex (non-MAC), wrote Roland Diel, MD, of University Medical Hospital Schleswig-Holstein, Germany, and his colleagues.
In particular, non-MAC species Mycobacterium xenopi (MX), Mycobacterium abscessus, Mycobacterium malmoense, and Mycobacterium kansasii (MK) were addressed in the studies, which included 16 retrospective chart reviews, 5 randomized trials, and 3 prospective, nonrandomized studies (Chest 2017. doi: 10.1016/j.chest.2017.04.166).
Treatment success was measured by rates of sputum culture conversion (SCC).
Overall, the average proportion of SCC for patients with M. abscessus was 41% after subtraction for posttreatment relapses, but reached 70% for subspecies M. massiliense in macrolide-containing treatments. The average proportion of SCC was 80% for patients with M. kansasii, 32% for those with MX, and 54% for those with M. malmoense.
Treatment success ranged from 9% to 73% for M. xenopi patients, but all-cause mortality was 69%. Of note, a 100% success rate was noted in M. kansasii patients using a three-drug TB regimen of isoniazid, rifampicin, and ethambutol, or with a combination of ethambutol, rifampicin, and clarithromycin, the researchers noted.
The percentage of SCC in 55 patients with lung resection and either MX or M. abscessus was considered high at 76%.
The study findings were limited by the diverse definitions of treatment success and by the variety of treatments and “an optimal multidrug treatment cannot be derived from the few studies and has yet to be determined,” the researchers said. In the absence of optimal drug therapy, functional and quality of life elements deserve greater consideration when evaluating outcomes in patients with non-MAC NTM pulmonary disease, they added.
Dr. Diel reported receiving lecturing and/or consulting fees from Insmed and Riemser.
FROM CHEST
Key clinical point: An optimal multidrug treatment has not yet been found for patients with nontuberculosis mycobacteria (NTM)–related pulmonary disease.
Major finding: The average proportion of sputum culture conversion (SCC) for patients with M. abscessus was 42% after subtraction for posttreatment relapses, but reached 79% for subspecies M. massiliense in macrolide-containing treatments. The average proportion of SCC was 80% for patients with M. kansasii, 32% for those with M. xenopi, and 54% for those with M. malmoense.
Data source: A meta-analysis of 24 studies and 1,224 patients.
Disclosures: Dr. Roland Diel reported receiving lecturing and/or consulting fees from Insmed and Riemser.
Atezolizumab improves OS in NSCLC with brain metastases
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
GENEVA – Immune checkpoint inhibitors may improve survival in patients with non–small cell lung cancer (NSCLC) and brain metastases compared with chemotherapy, without adding unacceptable toxicities, pooled analyses of clinical trials suggest.
Among 1452 patients with NSCLC treated with the programmed death ligand-1 (PD-L1) inhibitor atezolizumab (Tecentriq), rates of treatment-related serious adverse events (SAEs) were similar between patients with brain metastases at baseline and those without brain metastases, reported Rimas V Lukas, MD, from the University of Chicago.
“Overall, I think that these results support the investigation of atezolizumab in non–small cell lung cancer patients with CNS metastases,” Dr. Lukas said at the European Lung Cancer Conference.
Lung cancer accounts for about 40%-50% of all cases of brain metastases, and approximately 20%-40% of patients with advanced NSCLC will develop metastases, which are associated with poor overall survival, according to Solange Peters, MD, from the Centre Hospitalier Universitaire Vaudois in Lausanne, Switzerland, the invited discussant.
To evaluate the safety and efficacy of the PD-L1 inhibitor in patients with brain metastases at baseline, Lukas et al. looked at pooled safety data on patients enrolled in one of five treatment studies with atezolizumab – PCD4989g; BIRCH, POPLAR, FIR, and OAK – and efficacy data from a subset of patients in OAK.
The pooled analysis included 1452 patients, 79 of whom (5%) had brain metastases at baseline. This analysis showed that, although there was a higher incidence of any neurological AE, treatment-related AE, or treatment–related neurologic AE among patients with brain metastases, treatment-related SAEs and treatment related neurological SAEs were similar between the two groups. The most common neurological AE was headache, reported by 8% of patients with metastases and 3% without.
The efficacy analysis, which included a cohort of the first 850 patients with or without brain metastases treated, showed a significant survival for atezolizumab in the OAK trial, with median overall survival of 20.1 months for patients with brain metastases assigned to the PD-L1 inhibitor, compared with 11.9 months for patients assigned to docetaxel. This translated into a hazard ratio for atezolizumab of 0.54 (P = .0279).
Among the 750 patients without baseline brain metastases in OAK, the respective OS rates were 13.0 months, vs. 9.4 months (HR, 0.75; P = .001).
Although it was not statistically significant, the risk for developing new central nervous system lesions also appeared to be lower with atezolizumab than with docetaxel, with a median time to new lesions not reached, vs. 9.5 months with docetaxel (HR, 0.42; 95% confidence interval, 0.15-1.18).
Among patients without baseline brain metastases, there was also a hint that atezolizumab could delay onset of CNS metastases, although the trend was not significant, the investigators found.
In her commentary on the study, Dr. Peters said that “checkpoint inhibitors demonstrate activity in the brain that remains to be quantified and prospectively compared to systemic activity in larger series, and these are very small series.”
“Limitations in duration and level of activity might exist, however, related to the blood brain barrier and the brain immune system characteristics,” she added.
The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
Key clinical point: The PD-L1 inhibitor atezolizumab improved overall survival, vs. docetaxel, in patients with non–small cell lung cancer and brain metastases.
Major finding: The hazard ratio for overall survival was 0.54 for patients assigned to atezolizumab, vs. docetaxel, in the OAK trial.
Data source: Pooled safety analysis of 1452 patients and efficacy analysis of 850 patients with NSCLC with or without brain metastases.
Disclosures: The study was supported by F. Hoffmann-La Roche/Genentech, a member of the Roche Group. Dr. Lukas disclosed serving on advisory boards for AstraZeneca and Novocure and receiving honoraria from AbbVie. Two coauthors are employees of Genentech, and two are employed by Roche. The remaining author had no disclosures. Dr. Peters reported relationships with Bristol-Myers Squibb, F. Hoffmann-La Roche, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, Morphotek, Merrimack, Merck Sharp and Dohme, and Merck Serono.
FDA approves pembrolizumab for first-line advanced NSCLC
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
The Food and Drug Administration has granted accelerated approval to checkpoint inhibitor pembrolizumab in combination with pemetrexed and carboplatin for the treatment of patients with previously untreated metastatic nonsquamous non–small cell lung cancer (NSCLC).
The immunotherapy pembrolizumab was approved as a second-line treatment for metastatic NSCLC in 2015.
First-line approval was based on an improved overall response rate (ORR) and progression-free survival (PFS) in a cohort of 123 patients within an open-label, multicohort study (KEYNOTE-21). Enrollees in cohort G1 had locally advanced or metastatic NSCLC and no prior systemic treatment for metastatic disease. They were randomized to receive either pembrolizumab, in combination with pemetrexed and carboplatin (PC) for four cycles followed by pembrolizumab for a maximum of 24 months (n = 60) or PC alone (n = 63). Randomization was stratified by PD-L1 tumor expression (tumor proportion score [TPS] less than 1% vs. TPS greater than or equal to 1%).
The hazard ratio for PFS was 0.53 (95% CI: 0.31, 0.91, P = .0205). The median PFS was 13.0 months for the pembrolizumab plus PC arm and 8.9 months for the PC-alone arm. In the TPS less than 1% subgroup, the ORR was 57% and 13% in the pembrolizumab-plus-PC and in the PC-alone arms, respectively. In the TPS greater-than-or-equal-to-1% subgroup, the ORR was 54% in the pembrolizumab-plus-PC arm and 38% in the pembrolizumab-plus-PC arm, the FDA said.
There were serious adverse events in 41% of the patients in the pembrolizumab-plus-PC arm compared with 28% in the PC-alone arm. Pembrolizumab was discontinued for adverse reactions in 10% of patients, most commonly due to acute kidney injury. The most common grade 3-4 adverse reactions were fatigue, dyspnea, nausea, vomiting, diarrhea, and rash.
The FDA cautioned that immune-mediated adverse reactions can occur with pembrolizumab including pneumonitis, colitis, hepatitis, endocrinopathies, and nephritis. Based on the severity of the adverse reaction, pembrolizumab should be withheld or discontinued and corticosteroids administered when appropriate. The recommended dose and schedule for NSCLC is 200 mg as an intravenous infusion every 3 weeks until disease progression, unacceptable toxicity, or up to 24 months in patients without disease progression.
Pembrolizumab is marketed as Keytruda by Merck.
FDA: Fluoroquinolone use not linked to retina detachment, aortic problems
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
The Food and Drug Administration has found no evidence of a link between fluoroquinolone usage and retinal detachment or aortic aneurysm and dissection, according to a new Drug Safety Communication update on potential serious, disabling side effects of oral and injectable fluoroquinolone antibiotics.
Fluoroquinolones are used to treat acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections.
However, after reviewing patient cases and study findings, the FDA said the evidence didn’t support an association between fluoroquinolone usage and potential retinal or aortic dangers, according to its May 10, 2017, Drug Safety Communication update.
Serious side effects associated with fluoroquinolone use include hallucination, depression, suicidal thoughts, tendinitis and tendon rupture, a “pins and needles” feeling in the arms and legs, joint pain and swelling, skin rash, and severe diarrhea.
“We will continue to assess safety issues with fluoroquinolones, and will update the public if additional actions are needed,” the FDA said in a statement.
Lung cancer metastatic sites differ by histology, tumor factors
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
GENEVA – A review of data on more than 75,000 patients with lung cancer has revealed distinct patterns of metastasis according to subtype, a finding that could help in surveillance, treatment planning, and prophylaxis, an investigator contends.
Patients with small cell lung cancer (SCLC) had significantly higher rates of liver metastases than patients with non–small cell lung cancer (NSCLC), while patients with NSCLC had significantly higher rates of metastases to bone, reported Mohamed Hendawi, MD, a visiting scholar at the Ohio State University Medical Center in Columbus.
“Predictors for liver metastasis were small cell and adenocarcinoma histology, lower and upper lobe locations, and high grade tumors. Predictors for metastasis to brain were advanced age at diagnosis, adenocarcinoma and small-cell histology, lower lobe [and] main bronchus locations, and high grade tumors,” he wrote in a scientific poster presented at the European Lung Cancer Conference.
Dr. Hendawi drew records on all patients with metastatic lung cancer included in the 2010-2013 Surveillance, Epidemiology, and End Results database. He used univariate and multivariate logistic regression models to evaluate predictors of metastasis.
The data set included a total of 76,254 patients with metastatic lung cancer, of which 17% were SCLC and 83% were NSCLC tumors. In 54% of patients, the primary tumor was in the right lung; in 38%, it was in the left lung; and, in 8% of patients, the primary tumor was bilateral.
The rates of metastases to bone were high in both major lung cancer types but, as noted before, were significantly higher in patients with NSCLC: 37% compared with 34% for patients with SCLC (P less than .001).
In contrast, the incidence of liver metastases in SCLC was more than double that of NSCLC: 46% vs. 20%, respectively (P less than .001). There were slightly, but significantly, fewer cases of brain metastases at the time of diagnosis among patients with SCLC: 25% vs. 26% (P = .003).
Histologic subtypes significantly associated with both brain and liver metastases were, in descending order, adenocarcinomas, small cell, and squamous cell cancers.
Although carcinoid lung cancers accounted for only 2.1% of all tumors, they were associated with a high rate of metastasis to brain at diagnosis (44.8%).
As noted, independent risk factors for liver metastasis were small cell and adenocarcinoma histologies (P less than .001), tumors in the upper lobe (P = .028), and high-grade tumor (P less than .001).
Independent predictors for brain metastases were advanced age at diagnosis (P less than .001), adenocarcinoma and small-cell histologies (P less than .001), lower lobe or main bronchus locations (P = .004), and higher-grade tumors (P less than .001).
In a poster discussion session, Paolo Boffetta, MD, MPH, from the Icahn School of Medicine at Mount Sinai in New York City, the invited discussant, commented that, while he thought that the data were interesting, “the main issue I had with this poster is that it’s limited to patients with metastasis, so we cannot really evaluate the risk of metastasis according to the different histological types and the absolute risk of developing metastases in one or the other organ but only the relative risk of developing metastasis in one organ versus the other having one or the other histology.”
“So, we really don’t know whether the risk is increased in one group or decreased in the other one that generates these differences,” he said.
The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
FROM ELCC
Key clinical point: Lung cancers tend to metastasize to different organs based on histology and other clinical factors.
Major finding: The incidence of metastasis to bone was higher in patients with NSCLC than SCLC, while liver metastases were more than twice as high among patients with SCLC.
Data source: Review of Surveillance, Epidemiology, and End Results data on 76,254 patients with metastatic lung cancer.
Disclosures: The study did not receive outside support. Dr. Hendawi declared no conflicts of interest.
G-CSF safe, but antibiotics are more concerning in SCLC
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
GENEVA – In patients with limited stage–small cell lung cancer (LS-SCLC) treated with concurrent chemotherapy and radiation, the use of antibiotics to prevent febrile neutropenia was associated with worse outcomes, but granulocyte-colony stimulating factor (G-CSF) prescribed for the same purposes appeared to be safe, reported investigators.
In a subanalysis of data on patients with early SCLC enrolled in the phase III CONVERT trial comparing chemotherapy with concurrent once-daily vs. twice-daily radiation, the use of antibiotic prophylaxis of neutropenia was associated with worse overall survival (OS) and progression-free survival, (PFS) compared with no antibiotics, reported Fabio Gomes, MD, from the Christie NHS Foundation Trust Hospital in Manchester, England.
The use of G-CSF was, however, associated with higher rates of grade 3 or 4 thrombocytopenia and anemia, requiring supportive measures, he acknowledged.
The role of G-CSF with concurrent thoracic radiotherapy is controversial because of safety concerns, but data are scarce, Dr. Gomes said. He noted that the American Society of Clinical Oncology guidelines on the use of white blood cell growth factors recommend against their routine use.
However, some of those concerns arose in the mid-1990s when granulocyt macrophage–stimulating colony factor (GM-CSF) was used, rather than G-CSF, which acts on only a single blood lineage, namely neutrophils. Additionally, modern radiology techniques are more precise than they were 20 years ago, reducing the risk of toxicity, he noted.
In the CONVERT trial, 547 patients with LS-SCLC were randomly assigned to receive four to six cycles of cisplatin and etoposide chemotherapy concurrently with either once daily thoracic radiation for a total dose of 66 Gy divided into 33 fractions delivered over 45 days or to twice-daily radiation at a total dose of 45 Gy divided into 30 fractions delivered over 19 days.
There was no difference between the groups in the primary endpoint of overall survival.
In the subanalysis reported here, Gomes et al. looked at the use of G-CSF, delivered at the investigator’s discretion, in 487 patients. Approximately 40% of patients in the subanalysis received G-CSF during at least one treatment cycle.
Prophylactic antibiotics were recommended by the investigators for use in association with at least one chemotherapy cycle, and 49% of patients in the subanalysis received them during at least one cycle.
Hematological toxicities included grade 3 or 4 thrombocytopenia occurring in 29.9% of patients who received G-CSF, vs. 13.3% of those who did not (P less than .001). The rates were similar between the once-daily and twice-daily radiation groups.
Grade 3 or 4 anemia occurred in 16.9% of patients who received G-CSF, vs. 10.7% of those who didn’t (P = .027). The difference was significant only among patients in the twice-daily radiation arm (20.9% vs. 8.3%, respectively; P = .004).
Patients in the twice-daily radiation arms who received G-CSF also required more platelet transfusion, compared with the once-daily arm (P less than .001), and, in both arms, G-CSF was associated with more red-cell transfusions (P = .007 for once-daily and .001 for twice daily).
G-CSF was not associated with either pneumonitis or esophagitis, and there were no differences in treatment-related deaths with either G-CSF or antibiotics.
Median OS by G-CSF use was 29 months with and 27 months without, a difference that was not significant (P = .08). Median PFS also did not differ by G-CSF use or nonuse.
When it came to antibiotic prophylaxis, however, both median OS and PFS were significant worse with antibiotic use (OS, 24 months with vs. 33 months without; P = .016; PFS, P = .03).
“We are very reassured that there are no significant additional toxicities [with G-CSF] from radiation in the acute setting,” commented Sanjay Popat, FRCP, PhD, from the Royal Marsden Hospital in London, the invited discussant.
“However, we have no data as yet on the impact of G-CSF usage on febrile neutropenia, which is of course the fundamental that we’re aiming to improve in the hope that this will contribute to [lowering] costs,” he added.
The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
FROM ELCC
Key clinical point: Febrile neutropenia prophylaxis with G-CSF was safe, but prophylactic antibiotics were associated with worse overall survival in patients with limited stage–small cell lung cancer.
Major finding: Both median overall and progression-free survival were lower among patients who received prophylactic antibiotics. There were no differences in survival by G-CSF use.
Data source: Subanalysis of data on 487 patients in the phase III CONVERT trial comparing once-daily and twice daily radiation concurrent with chemotherapy in LS-SCLC.
Disclosures: The study was sponsored by the Christie Hospital National Health Service Foundation Trust, Cancer Research UK, EORTC, GECP, GFPC, and IFCT. Dr. Gomes reported no relevant disclosures. Dr. Popat reported consultation, honoraria, travel expenses, and institutional research from multiple entities.
Chemo sequencing in elderly adults with NSCLC linked with survival
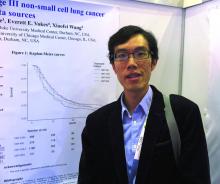
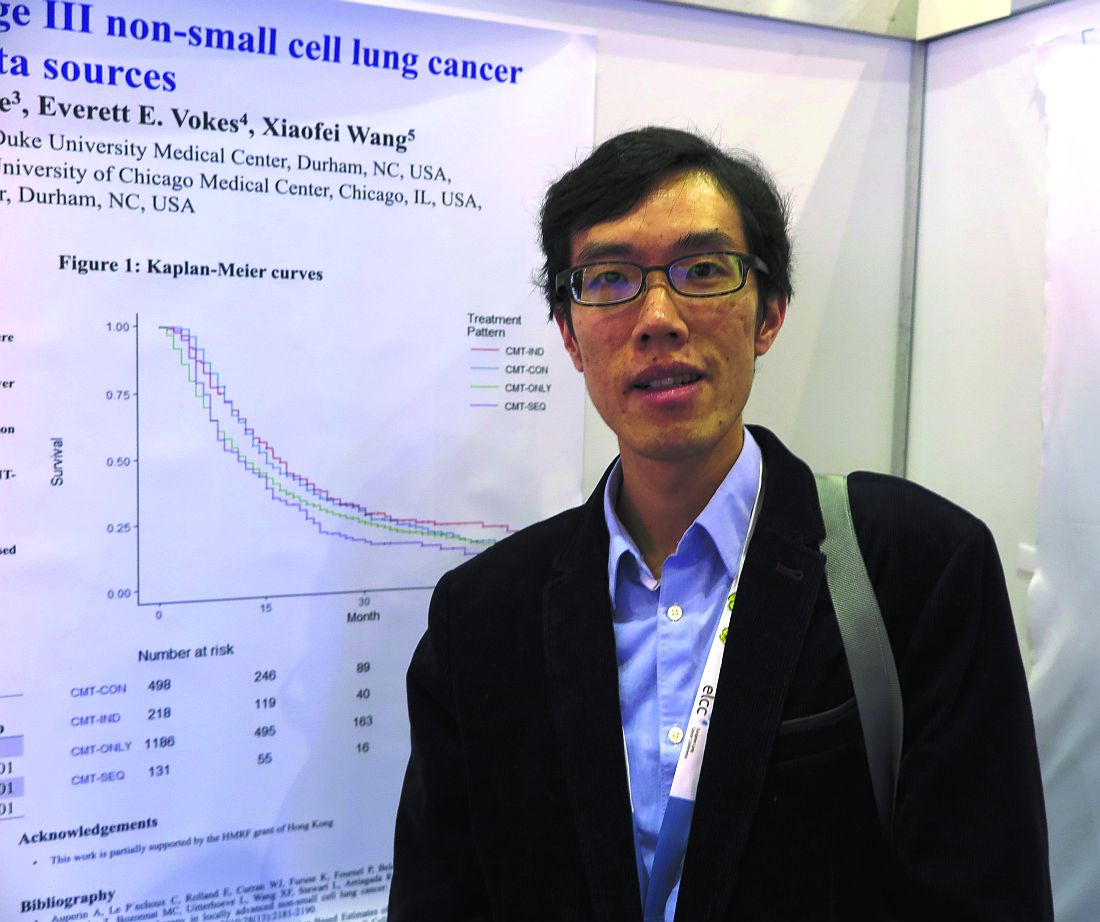
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
GENEVA – Among older adults with stage III non–small cell lung cancer (NSCLC), the sequencing of chemotherapy and radiation has a significant effect on overall survival, a team of investigators from Hong Kong and the United States reported.
Among 2,033 adults who were 65 years or older with locally advanced NSCLC and treated with one of four combined-modality therapy (CMT) schedules, both chemotherapy induction followed by concurrent therapy (CMT-IND) and concurrent therapy followed by consolidation chemoradiation (CMT-CON) were associated with an approximately 30% improvement in survival, compared with either sequential chemotherapy followed by radiation (CMT-SEQ) or concurrent therapy only (CMT-ONLY), reported Hei Man Herbert Pang, MD, of the University of Hong Kong and his colleagues.
The investigators used retrospective data from U.S. and Chinese sources to compare the relative survival benefits with various combined modality therapies. These included a Surveillance, Epidemiology, and End Results–Medicare cohort of patients 65 years and older with stage IIIA or IIIB NSCLC treated with CMT from 2006 through 2010 and a cohort of patients with the same age and NSCLC treated at Queen Mary Hospital in Hong Kong from 2007 through 2016.
They assessed neutropenia using inpatient claims data for episodes occurring within 130 days of the first chemotherapy cycle.
In an unadjusted analysis, they found that median overall survival, in descending order, was 16.1 months for CMT-SEQ,15.0 months for CMT-ONLY, 12.0 months for CMT-IND, and 11.0 months for CMT-CON.
When they controlled for variables, however, a different picture began to emerge.
For example, patients who were treated with CMT-SEQ had lower Charlson Comorbidity Index scores and, thus, were comparatively healthier than patients treated with other combined modalities.
Hospitalizations for neutropenia were most common with CMT-CON, occurring in 13.3% of patients, compared with 9.8% of patients treated with CMT-ONLY, 9.2% with CMT-IND, and 2.3% with CMT-SEQ.
In multivariable models controlling for sex, race, ethnicity, histology, and Charlson score, CMT-CON and CMT-IND were associated with significantly better overall, compared with CMT-SEQ, with respective hazard ratios for death of 0.68 (P less than .001) and 0.67 (P = .001). In this model, CMT-ONLY was not associated with significantly better survival.
In a propensity score model adjusted for the same factors, the respective HRs for CMT-CON, CMT-IND, and CMT-ONLY vs. CMT-SEQ were 0.69, 0.70, and 0.86 (P less than .001 for all three comparisons).
“The findings on efficacy and toxicity are quite consistent with previously reported studies based on clinical trials or observational databases,” the investigators said.
The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
FROM ELCC
Key clinical point: Some combined modality therapy options for older adults with NSCLC are associated with better overall survival.
Major finding: CMT-IND and CMT-CON were associated with a 30% improvement in overall survival, compared with CMT-SEQ.
Data source: Retrospective review of data on 2,033 adults 65 years and older with NSCLC in the United States and Hong Kong.
Disclosures: The study was supported by grants from the U.S. National Institutes of Health and the Hong Kong Health and Medical Research Fund. All authors have declared no conflicts of interest.
Sex-based differences in lung cancer screening intervals suggested
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
GENEVA – A lung-cancer screening CT interval of once-yearly for men and once every 3 years for women appears to be the optimum schedule for detecting most early-stage lung cancers while minimizing radiation exposure, results of a retrospective study suggest.
Among 96 patients (85 men and 11 women) with lung cancers detected on follow-up screening CT, the mean interval time between initial CT and diagnostic CT was significantly longer among women than among men, at 5.6 vs. 3.6 years (P = .02), reported Mi-Young Kim, MD, a radiologist at Asan Medical Center in Seoul, South Korea.
Men tended to have a higher stage at diagnosis, however. Stage I cancers were diagnosed in 82% of women, but only 49% of men. Tumor size was also larger among men at presentation at a mean of 29.5 mm vs. 15.5 mm, Dr. Kim and her colleagues found.
Current lung cancer screening guidelines vary somewhat, but most recommend annual screening for people aged 55-80 years who have a 30 pack-year or greater smoking history and are current smokers or have quit within the last 15 years.
Prior studies to see whether longer screening intervals were safe have yielded mixed results, possibly because of differences in clinical and radiologic presentation between men and women, Dr. Kim said.
To explore sex differences in lung cancer at the time of diagnosis, she and her colleagues retrospectively reviewed records for 46,766 patients who underwent screening at their center from January 2000 through February 2016, during which time, 282 patients were diagnosed with lung cancer. Of this group, 186 were diagnosed from the initial screening CT scan, and 96 – the cohort included in the study – were diagnosed from subsequent scans.
The authors found that the majority of men (72%) had solid nodules as the primary pathology. In contrast, ground-glass opacities were the most common nodular finding among women, occurring in 45% of the cases. The most common histology among men was adenocarcinoma (42%), followed by squamous-cell carcinoma (35%), small cell lung cancer (18%), and others (5%). All women presented with adenocarcinoma histology.
“Because ground-glass opacity nodule is the most common feature of lung cancer in women, and all cases are adenocarcinoma, the growth rate of cancers might be low,” Dr. Kim said in a statement.
Only 2 of the 11 women in the study were smokers, compared with 74 of the 85 men.
Looking at the operability of lung cancer according to screening intervals, the investigators found that 100% of tumors detected at 1 year in men were operable, compared with 94% of those detected at 2 years, and 55% for those detected at the 3-year interval. In contrast, among women, there were no tumors detected at 1 year, one operable tumor and no inoperable tumors at 2 years, and two operable and no inoperable tumors at 3 years. Beyond 3 years, however, the rate of inoperable tumors at the time of diagnosis was 32% in men and 25% in women.
“We included all patients screened for lung cancer in a 17-year period, but the number of women patients was low and further studies are needed to confirm the sex differences we found,” Dr. Kim acknowledged.
“Only when this is adjusted for – when we compare smokers of the same pack-year history – then we can look at whether women and men do have different rates of appearance of nodules,” he said.
“To me, this is a very interesting suggestion that indeed the biology of the growth of these nodules may be different in men and women, but only if we are sure that the main environmental factor is taken care of.”
The study funding source was not disclosed.
Dr. Kim and Dr. Boffetta reported no relevant disclosures.
FROM ELCC
Key clinical point:
Major finding: A repeated CT scan for lung cancer every year in men and every 3 years in women picked up most new, operable lesions.
Data source: Retrospective review of data on 96 patients with lung cancer diagnosed at a second or subsequent CT screening.
Disclosures: The study funding source was not disclosed. Dr. Kim and Dr. Boffetta reported no relevant disclosures.
VIDEO: Surgery use declines for non–small cell lung cancer
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
agallegos@frontlinemedcom.com
On Twitter @legal_med
AT THE AATS ANNUAL MEETING
FDA approves brigatinib for second-line advanced ALK-positive NSCLC
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.
The Food and Drug Administration has granted accelerated approval to brigatinib for the treatment of patients with metastatic anaplastic lymphoma kinase (ALK)–positive non–small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
Approval was based on a meaningful and durable overall response rate in a two-arm, open-label, multicenter trial of 222 patients with locally advanced or metastatic ALK-positive NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily (112 patients) or 180 mg once daily following a 7-day lead-in at 90 mg once daily (110 patients).
The median duration of response was 13.8 months in both arms.
The most common adverse reactions in 219 patients who received at least one dose were nausea, diarrhea, fatigue, cough, and headache. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. The rate of fatal adverse reactions was 3.7%; they were pneumonia in two patients and sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis, and urosepsis in one patient each. Visual disturbances also occurred in patients receiving brigatinib. The FDA cautions that patients receiving brigatinib should be monitored for new or worsening respiratory symptoms; hypertension; bradycardia; visual symptoms; and elevations in amylase, lipase, blood glucose, and creatine phosphokinase.
The recommended dosing of brigatinib, marketed as Alunbrig by Takeda Pharmaceutical, is 90 mg orally once daily for the first 7 days then, if tolerated, increase to 180 mg orally once daily.
Full prescribing information is available here.