User login
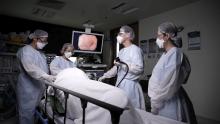
Ergonomic ‘Timeouts’ Make Endoscopy Easier For GIs
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights
Amandeep Shergill, MD, MS, AGAF, always thought she had good hand-eye coordination until she entered her gastroenterology fellowship.
“You’re learning how to scope and the endoscope just feels so awkward in the hands. It can be such a difficult instrument to both learn and to use,” said Dr. Shergill, professor of clinical medicine at University of California, San Francisco.
Her attendings and mentors couldn’t give her the feedback she needed.
“I was told that I wasn’t holding it right. But every time I tried to do something that someone was trying to tell me, it seemed like my hands were too small. I couldn’t hold it the way that they were teaching me to hold it.” She began to wonder: Was this about her or the tool itself?
A deep dive into hand tool interactions and medical device designs led her to human factors and ergonomics. Her fellowship mentor, Ken McQuaid, MD, AGAF, had gone to medical school with David Rempel, MD, MPH who was one of the top-funded ergonomists in the country. “He emailed David and wrote: I have a fellow who’s interested in learning more about ergonomics and applying it to endoscopy,” said Dr. Shergill.
Through her work with Dr. Rempel, she was able to uncover the mechanisms that lead to musculoskeletal disorders in endoscopists.
Over time, she has become a trailblazer in this field, helming the UC Berkeley Center for Ergonomic Endoscopy with Carisa Harris-Adamson PhD, CPE, her ergonomics collaborator. In an interview, she described the unique “timeout” algorithm she created to ease the process of endoscopy for GI physicians.
What is your favorite aspect of being a GI physician?
I really love the diversity of patients and cases. You’re always learning something new. It’s an internal medicine subspecialty and a cognitive field, so we must think about differential diagnoses, risks and benefits of procedures for patients. But as a procedural field, we get to diagnose and immediately treat certain disorders. What’s exciting about GI right now is there’s still so much to learn. I think that we’re still discovering more about how the brain-gut interaction works every day. There’s been additional research about the microbiome and the immense influence it has on both health and disease. The field is continuing to evolve rapidly. There’s always something new to learn, and I think it keeps us fresh.
Tell me about your work in ergonomics and endoscopy.
Ken McQuaid connected me with David Rempel. I worked with David to approach this problem of endoscopy ergonomics from a very rigorous ergonomics perspective. Early in my fellowship, endoscopy ergonomics wasn’t well known. There were few survey-based studies, including one from the American Society for Gastrointestinal Endoscopy (ASGE) that documented a high prevalence of endoscopist injury. But not a lot was known about what was causing injury in endoscopists.
What were the risk factors for endoscopist injury? Instead of just doing another survey, I wanted to show that there was this potential for causation given the design of the endoscopes. I worked with David to do a pilot study where we collected some pinch forces and forearm muscle loads. I was able to collect some pilot data that I used to apply for the ASGE Endoscopic Research Award. And luckily, ASGE supported that work.
Another award I received, the ASGE Career Development Award, was instrumental in allowing me to become more proficient in the science of ergonomics. I was able to leverage that career development award to go back to school. I went to UC Berkeley and got a master’s in environmental health sciences with a focus on ergonomics. It really helped me to lay the foundation and understanding for ergonomics and then apply that to endoscopy to generate a more rigorous scientific background for endoscopy ergonomics and start that conversation within the field of GI.
What leads to musculoskeletal disorders in endoscopists and how can it be prevented?
Musculoskeletal disorders are associated with the repetitive procedures that we’re performing, often utilizing high forces and in non-neutral postures. This is because of how we’re interacting with our tools and how we’re interacting with our environments. The studies I have done with Carisa Harris-Adamson have been able to demonstrate and document the high forces that are required to interact with the endoscope. To turn the control section dials and to torque and manipulate the insertion tube, there are really high distal upper extremity muscle loads that are being applied.
We were able to compare the loads and the forces we were seeing to established risk thresholds from the ergonomics literature and demonstrate that performing endoscopy was associated with moderate to high risk of development of distal upper extremity disorders.
What research are you doing now?
We’re trying to focus more on interventions. We’ve done some studies on engineering controls we can utilize to decrease the loads of holding the scope. First, it was an anti-gravity support arm. More recently we’re hoping to publish data on whether a scope stand can alleviate some of those left distal upper extremity loads because the stand is holding the scope instead of the hand holding the scope. Can we decrease injury risk by decreasing static loading?
Neck and back injuries, which have a high prevalence in endoscopists, are usually associated with how the room is set up. One of the things that I’ve tried to help promote is a pre-procedure ergonomic “timeout.” Before an endoscopist does a procedure, we’re supposed to perform a timeout focused on the patient’s safety. We should also try to advocate for physician safety and an ergonomic timeout. I developed a mnemonic device utilizing the word “MYSELF” to help endoscopists remember the ergonomic timeout checklist: M = monitor, Y = upside-down Y stance, S = scope, E = elbow/ bed position, L = lower extremities, F = free movement of endoscope/ processor placement.
First, thinking about the monitor, “M”, and fixing the monitor height so that the neck is in neutral position. Then, thinking of an upside down “Y” standing straight with the feet either hip width or shoulder width apart, so that the physician has a stable, neutral standing posture. Then “S” is for checking the scope to ensure you have a scope with optimal angulation that’s working properly.
“E” is for elbows — adjusting the bed to an optimal position so that elbows and shoulders are in neutral position. “L” is for lower extremities — are the foot pedals within an easy reach? Do you have comfortable shoes on, an anti-fatigue floor mat if you need it? And then the “F” in “MYSELF” is for the processor placement, to ensure “free movement” of the scope. By placing the processor directly behind you and lining up the processor with the orifice to be scoped, you can ensure free movement of the scope so that you can leverage large movements of the control section to result in tip deflection.
We studied the MYSELF mnemonic device for a pre-procedure ergonomic timeout in a simulated setting and presented our results at Digestive Disease Week (DDW) 2024, where we showed a reduction in ergonomic risk scores based on the Rapid Entire Body Assessment tool.
We presented the results of the scope stand study at DDW 2025 in San Diego this May.
What has been the feedback from physicians who use these supportive tools?
While physicians are very grateful for bringing attention to this issue, and many have found utility in some of the tools that I proposed, I think we still have so much work to do. We’re just all hoping to continue to move this field forward for better tools that are designed more with the breadth of endoscopists in mind.
How do you handle stress and maintain work-life balance?
A few years ago, during DDW I gave a talk entitled “Achieving Work-Life Harmony.” I disclosed at the beginning of the talk that I had not achieved work-life harmony. It’s definitely a difficult thing to do, especially in our field as GI proceduralists, where we’re frequently on call and there are potentially on-call emergencies.
One of the key things that I’ve tried to do is create boundaries to prioritize both things in my personal life and my professional life and really try to stay true to the things that are important to me. For instance, things like family time and mealtimes, I think that’s so critical. Trying to be home on evenings for dinnertime is so important.
One of my GI colleagues, Raj Keswani, MD, MS gave a talk about burnout and described imagining life as juggling balls; trying to figure out which balls are glass balls and need to be handled with care, and which balls are rubber balls.
More often, work is the rubber ball. If you drop it, it’ll bounce back and the work that you have will still be there the next day. Family, friends, our health, those are the glass balls that if they fall, they can get scuffed or shatter sometimes. That image helps me think in the moment. If I need to decide between two competing priorities, which one will still be here tomorrow? Which is the one that’s going to be more resilient, and which is the one that I need to focus on? That’s been a helpful image for me.
I also want to give a shout out to my amazing colleagues. We all pitch in with the ‘juggling’ and help to keep everyone’s ‘balls’ in the air, and cover for each other. Whether it’s a sick patient or whatever’s going on in our personal lives, we always take care of each other.
What advice would you give to aspiring GI fellows or graduating fellows?
GI is such an amazing field and many people end up focusing on the procedural aspect of it. What I think defines an exceptional gastroenterologist and physician in general is adopting both a “growth mindset” and a “mastery mindset.” And really, it starts out with when you’re exploring an area of focus, listening to what consistently draws your attention, what you’re excited about learning more about.
Finding mentors, getting involved in projects, doing deep learning, and really trying to develop an expertise in that area through additional training, coursework, and education. I think that idea of a mastery mindset will really help set you up for becoming deeply knowledgeable about a field.
Lightning Round
Coffee or tea?
Coffee
What’s your favorite book?
Project Hail Mary (audiobook)
Beach vacation or mountain retreat?
Mountain retreat
Early bird or night owl?
Night owl
What’s your go-to comfort food?
Chaat (Indian street food)
Do you prefer dogs or cats?
Dogs
What’s one hobby you’d like to pick up?
Sewing
If you could have dinner with any historical figure, who would it be?
Ruth Bader Ginsburg
What’s your go-to karaoke song?
I Wanna Dance with Somebody
What’s one thing on your bucket list?
To see the Northern Lights

Endoscopist Brings Cutting-Edge Tech to Asia-Pacific Region
As the COVID-19 crisis unfolded in early 2020, Tossapol Kerdsirichairat, MD, faced another challenge: his mother’s ovarian cancer diagnosis.
“She chose to remain in Thailand, so I decided to relocate to care for her,” said Dr. Kerdsirichairat, an interventional endoscopist who completed fellowships at the University of Michigan, Ann Arbor, and Johns Hopkins University in Baltimore. The move to Bangkok turned out to be one of the best decisions of his life, he said, as he could support his mother while introducing advanced endoscopic techniques and devices to the region.
“Bangkok is a hub for medical innovation in Asia, offering opportunities to work with a diverse patient population and access to cutting-edge technology,” said Dr. Kerdsirichairat, who works at Bumrungrad International Hospital as a clinical associate professor.
The program is the first of its kind in Thailand and one of the few in the Asia-Pacific region.
“I guide patients and families through understanding their risks and implementing preventive strategies, collaborating with multidisciplinary teams to ensure comprehensive care. It’s incredibly rewarding to see the impact of early tumor detection,” said Dr. Kerdsirichairat, an international member of AGA who was a participant in the AGA Young Delegates Program.
He has set several records in Thailand for the smallest tumor detected, including a 0.3-millimeter (mm) esophageal tumor, a 0.8-mm tumor for stomach cancer, a 5-mm pancreatic tumor, and a 1-mm tumor for colon cancer.
“These were detected through high-standard screening programs, as patients often do not develop symptoms from these subtle lesions,” said Dr. Kerdsirichairat, who discussed in an interview the unique challenges of practicing overseas.
Why did you choose GI?
Gastroenterology is a specialty that uniquely integrates procedural skill, clinical decision making, and a deep understanding of complex biological systems. I was drawn especially to the ability to make a direct and meaningful impact in patients’ lives through advanced endoscopic procedures, while also addressing both acute and chronic diseases, and focusing on cancer prevention. It is incredibly rewarding to perform an endoscopic retrograde cholangiopancreatography (ERCP) for cholangitis and see a patient return to normal the very next day, or to perform an endoscopic ultrasound (EUS) for pancreatic cancer screening in high-risk individuals and detect a sub-centimeter pancreatic tumor.
Realizing that early detection can improve survival by threefold after surgery is a powerful reminder of the difference we can make in patients’ lives. This specialty requires a delicate balance of precision and empathy, which perfectly aligns with my strengths and values as a physician.
You have a wide variety of clinical interests, from endoscopic procedures to cancer research to GERD. What’s your key subspecialty and why?
My primary specialty is advanced endoscopy, which includes techniques such as EUS, ERCP, and endoscopic resection of precancerous and early cancerous lesions. I also focus on cutting-edge, evidence-based techniques recently included in clinical guidelines, such as Transoral Incisionless Fundoplication (TIF). These minimally invasive options allow me to diagnose and treat conditions that once required surgery. The precision and innovation involved in advanced endoscopy enable me to effectively manage complex cases—from diagnosing early cancers to managing bile duct obstructions and resecting precancerous lesions.
Can you describe your work in cancer genetics and screening?
I am deeply committed to the early detection of gastrointestinal cancers, particularly through screening for precancerous conditions and hereditary syndromes. During my general GI training at the University of Michigan, I had the privilege of working with Grace Elta, MD, AGAF, and Michelle Anderson, MD, MSc, renowned experts in pancreatic cancer management. I was later trained by Anne Marie Lennon, PhD, AGAF, who pioneered the liquid biopsy technique for cancer screening through the CancerSEEK project, and Marcia (Mimi) Canto, MD, MHS, who initiated the Cancer of the Pancreas Screening project for high-risk individuals of pancreatic cancer.
I also had the distinction of being the first at Bumrungrad International Hospital to perform endoscopic drainage for pancreatic fluid collections in the setting of multi-organ failure. This endoscopic approach has been extensively validated in the medical literature as significantly improving survival rates compared to surgical drainage. My training in this specialized procedure was conducted under the guidance of the premier group for necrotizing pancreatitis, led by Martin Freeman, MD, at the University of Minnesota.
Later, I contributed to overseeing the Inherited Gastrointestinal Malignancy Clinic of MyCode, a large-scale population-based cohort program focused on cancer screening in Pennsylvania. By December 2024, MyCode had collected blood samples from over 258,000 individuals, analyzed DNA sequences from over 184,000, and provided clinical data that benefits over 142,000 patients. It’s not uncommon for healthy 25-year-old patients to come to our clinic for colon cancer screening after learning from the program that they carry a cancer syndrome, and early screening can potentially save their lives.
What are the key differences between training and practicing medicine in the United States and in an Asian country?
The U.S. healthcare system is deeply rooted in evidence-based protocols and multidisciplinary care, driven by an insurance-based model. In contrast, many Asian countries face challenges such as the dependency on government approval for certain treatments and insurance limitations. Practicing in Asia requires navigating unique cultural, economic, and systemic differences, including varying resource availability and disease prevalence.
What specific challenges have you faced as a GI in Thailand?
As an advanced endoscopist, one of the biggest challenges I faced initially was the difficulty in obtaining the same devices I used in the U.S. for use in Thailand. With support from device companies and mentors in the U.S., I was able to perform groundbreaking procedures, such as the TIF in Southeast Asia and the first use of a full-thickness resection device in Thailand. I am also proud to be part of one of the first few centers worldwide performing the combination of injectable semaglutide and endoscopic sleeve gastroplasty, resulting in a remarkable weight reduction of 44%, comparable to surgical gastric bypass.
In addition, Bumrungrad International Hospital, where I practice, sees over 1.1 million visits annually from patients from more than 190 countries. This offers a unique opportunity to engage with a global patient base and learn from diverse cultures. Over time, although the hospital has professional interpreters for all languages, I have become able to communicate basic sentences with international patients in their preferred languages, including Chinese, Japanese, and Arabic, which has enriched my practice.
What’s your favorite thing to do when you’re not practicing GI?
I enjoy traveling, exploring new cuisines, and spending quality time with family and friends. These activities help me recharge and offer fresh perspectives on life.
Lightning Round
Texting or talking?
Talking. It’s more personal and meaningful.
Favorite city in the U.S.?
Ann Arbor, Michigan
Cat or dog person?
Dog person
Favorite junk food?
Pizza
How many cups of coffee do you drink per day?
Two – just enough to stay sharp, but not jittery.
If you weren’t a GI, what would you be?
Architect
Best place you went on vacation?
Kyoto, Japan
Favorite sport?
Skiing
Favorite ice cream?
Matcha green tea
What song do you have to sing along with when you hear it?
“Everybody” by Backstreet Boys
Favorite movie or TV show?
Forrest Gump and Friends
Optimist or pessimist?
Optimist. I believe in focusing on solutions and possibilities.
As the COVID-19 crisis unfolded in early 2020, Tossapol Kerdsirichairat, MD, faced another challenge: his mother’s ovarian cancer diagnosis.
“She chose to remain in Thailand, so I decided to relocate to care for her,” said Dr. Kerdsirichairat, an interventional endoscopist who completed fellowships at the University of Michigan, Ann Arbor, and Johns Hopkins University in Baltimore. The move to Bangkok turned out to be one of the best decisions of his life, he said, as he could support his mother while introducing advanced endoscopic techniques and devices to the region.
“Bangkok is a hub for medical innovation in Asia, offering opportunities to work with a diverse patient population and access to cutting-edge technology,” said Dr. Kerdsirichairat, who works at Bumrungrad International Hospital as a clinical associate professor.
The program is the first of its kind in Thailand and one of the few in the Asia-Pacific region.
“I guide patients and families through understanding their risks and implementing preventive strategies, collaborating with multidisciplinary teams to ensure comprehensive care. It’s incredibly rewarding to see the impact of early tumor detection,” said Dr. Kerdsirichairat, an international member of AGA who was a participant in the AGA Young Delegates Program.
He has set several records in Thailand for the smallest tumor detected, including a 0.3-millimeter (mm) esophageal tumor, a 0.8-mm tumor for stomach cancer, a 5-mm pancreatic tumor, and a 1-mm tumor for colon cancer.
“These were detected through high-standard screening programs, as patients often do not develop symptoms from these subtle lesions,” said Dr. Kerdsirichairat, who discussed in an interview the unique challenges of practicing overseas.
Why did you choose GI?
Gastroenterology is a specialty that uniquely integrates procedural skill, clinical decision making, and a deep understanding of complex biological systems. I was drawn especially to the ability to make a direct and meaningful impact in patients’ lives through advanced endoscopic procedures, while also addressing both acute and chronic diseases, and focusing on cancer prevention. It is incredibly rewarding to perform an endoscopic retrograde cholangiopancreatography (ERCP) for cholangitis and see a patient return to normal the very next day, or to perform an endoscopic ultrasound (EUS) for pancreatic cancer screening in high-risk individuals and detect a sub-centimeter pancreatic tumor.
Realizing that early detection can improve survival by threefold after surgery is a powerful reminder of the difference we can make in patients’ lives. This specialty requires a delicate balance of precision and empathy, which perfectly aligns with my strengths and values as a physician.
You have a wide variety of clinical interests, from endoscopic procedures to cancer research to GERD. What’s your key subspecialty and why?
My primary specialty is advanced endoscopy, which includes techniques such as EUS, ERCP, and endoscopic resection of precancerous and early cancerous lesions. I also focus on cutting-edge, evidence-based techniques recently included in clinical guidelines, such as Transoral Incisionless Fundoplication (TIF). These minimally invasive options allow me to diagnose and treat conditions that once required surgery. The precision and innovation involved in advanced endoscopy enable me to effectively manage complex cases—from diagnosing early cancers to managing bile duct obstructions and resecting precancerous lesions.
Can you describe your work in cancer genetics and screening?
I am deeply committed to the early detection of gastrointestinal cancers, particularly through screening for precancerous conditions and hereditary syndromes. During my general GI training at the University of Michigan, I had the privilege of working with Grace Elta, MD, AGAF, and Michelle Anderson, MD, MSc, renowned experts in pancreatic cancer management. I was later trained by Anne Marie Lennon, PhD, AGAF, who pioneered the liquid biopsy technique for cancer screening through the CancerSEEK project, and Marcia (Mimi) Canto, MD, MHS, who initiated the Cancer of the Pancreas Screening project for high-risk individuals of pancreatic cancer.
I also had the distinction of being the first at Bumrungrad International Hospital to perform endoscopic drainage for pancreatic fluid collections in the setting of multi-organ failure. This endoscopic approach has been extensively validated in the medical literature as significantly improving survival rates compared to surgical drainage. My training in this specialized procedure was conducted under the guidance of the premier group for necrotizing pancreatitis, led by Martin Freeman, MD, at the University of Minnesota.
Later, I contributed to overseeing the Inherited Gastrointestinal Malignancy Clinic of MyCode, a large-scale population-based cohort program focused on cancer screening in Pennsylvania. By December 2024, MyCode had collected blood samples from over 258,000 individuals, analyzed DNA sequences from over 184,000, and provided clinical data that benefits over 142,000 patients. It’s not uncommon for healthy 25-year-old patients to come to our clinic for colon cancer screening after learning from the program that they carry a cancer syndrome, and early screening can potentially save their lives.
What are the key differences between training and practicing medicine in the United States and in an Asian country?
The U.S. healthcare system is deeply rooted in evidence-based protocols and multidisciplinary care, driven by an insurance-based model. In contrast, many Asian countries face challenges such as the dependency on government approval for certain treatments and insurance limitations. Practicing in Asia requires navigating unique cultural, economic, and systemic differences, including varying resource availability and disease prevalence.
What specific challenges have you faced as a GI in Thailand?
As an advanced endoscopist, one of the biggest challenges I faced initially was the difficulty in obtaining the same devices I used in the U.S. for use in Thailand. With support from device companies and mentors in the U.S., I was able to perform groundbreaking procedures, such as the TIF in Southeast Asia and the first use of a full-thickness resection device in Thailand. I am also proud to be part of one of the first few centers worldwide performing the combination of injectable semaglutide and endoscopic sleeve gastroplasty, resulting in a remarkable weight reduction of 44%, comparable to surgical gastric bypass.
In addition, Bumrungrad International Hospital, where I practice, sees over 1.1 million visits annually from patients from more than 190 countries. This offers a unique opportunity to engage with a global patient base and learn from diverse cultures. Over time, although the hospital has professional interpreters for all languages, I have become able to communicate basic sentences with international patients in their preferred languages, including Chinese, Japanese, and Arabic, which has enriched my practice.
What’s your favorite thing to do when you’re not practicing GI?
I enjoy traveling, exploring new cuisines, and spending quality time with family and friends. These activities help me recharge and offer fresh perspectives on life.
Lightning Round
Texting or talking?
Talking. It’s more personal and meaningful.
Favorite city in the U.S.?
Ann Arbor, Michigan
Cat or dog person?
Dog person
Favorite junk food?
Pizza
How many cups of coffee do you drink per day?
Two – just enough to stay sharp, but not jittery.
If you weren’t a GI, what would you be?
Architect
Best place you went on vacation?
Kyoto, Japan
Favorite sport?
Skiing
Favorite ice cream?
Matcha green tea
What song do you have to sing along with when you hear it?
“Everybody” by Backstreet Boys
Favorite movie or TV show?
Forrest Gump and Friends
Optimist or pessimist?
Optimist. I believe in focusing on solutions and possibilities.
As the COVID-19 crisis unfolded in early 2020, Tossapol Kerdsirichairat, MD, faced another challenge: his mother’s ovarian cancer diagnosis.
“She chose to remain in Thailand, so I decided to relocate to care for her,” said Dr. Kerdsirichairat, an interventional endoscopist who completed fellowships at the University of Michigan, Ann Arbor, and Johns Hopkins University in Baltimore. The move to Bangkok turned out to be one of the best decisions of his life, he said, as he could support his mother while introducing advanced endoscopic techniques and devices to the region.
“Bangkok is a hub for medical innovation in Asia, offering opportunities to work with a diverse patient population and access to cutting-edge technology,” said Dr. Kerdsirichairat, who works at Bumrungrad International Hospital as a clinical associate professor.
The program is the first of its kind in Thailand and one of the few in the Asia-Pacific region.
“I guide patients and families through understanding their risks and implementing preventive strategies, collaborating with multidisciplinary teams to ensure comprehensive care. It’s incredibly rewarding to see the impact of early tumor detection,” said Dr. Kerdsirichairat, an international member of AGA who was a participant in the AGA Young Delegates Program.
He has set several records in Thailand for the smallest tumor detected, including a 0.3-millimeter (mm) esophageal tumor, a 0.8-mm tumor for stomach cancer, a 5-mm pancreatic tumor, and a 1-mm tumor for colon cancer.
“These were detected through high-standard screening programs, as patients often do not develop symptoms from these subtle lesions,” said Dr. Kerdsirichairat, who discussed in an interview the unique challenges of practicing overseas.
Why did you choose GI?
Gastroenterology is a specialty that uniquely integrates procedural skill, clinical decision making, and a deep understanding of complex biological systems. I was drawn especially to the ability to make a direct and meaningful impact in patients’ lives through advanced endoscopic procedures, while also addressing both acute and chronic diseases, and focusing on cancer prevention. It is incredibly rewarding to perform an endoscopic retrograde cholangiopancreatography (ERCP) for cholangitis and see a patient return to normal the very next day, or to perform an endoscopic ultrasound (EUS) for pancreatic cancer screening in high-risk individuals and detect a sub-centimeter pancreatic tumor.
Realizing that early detection can improve survival by threefold after surgery is a powerful reminder of the difference we can make in patients’ lives. This specialty requires a delicate balance of precision and empathy, which perfectly aligns with my strengths and values as a physician.
You have a wide variety of clinical interests, from endoscopic procedures to cancer research to GERD. What’s your key subspecialty and why?
My primary specialty is advanced endoscopy, which includes techniques such as EUS, ERCP, and endoscopic resection of precancerous and early cancerous lesions. I also focus on cutting-edge, evidence-based techniques recently included in clinical guidelines, such as Transoral Incisionless Fundoplication (TIF). These minimally invasive options allow me to diagnose and treat conditions that once required surgery. The precision and innovation involved in advanced endoscopy enable me to effectively manage complex cases—from diagnosing early cancers to managing bile duct obstructions and resecting precancerous lesions.
Can you describe your work in cancer genetics and screening?
I am deeply committed to the early detection of gastrointestinal cancers, particularly through screening for precancerous conditions and hereditary syndromes. During my general GI training at the University of Michigan, I had the privilege of working with Grace Elta, MD, AGAF, and Michelle Anderson, MD, MSc, renowned experts in pancreatic cancer management. I was later trained by Anne Marie Lennon, PhD, AGAF, who pioneered the liquid biopsy technique for cancer screening through the CancerSEEK project, and Marcia (Mimi) Canto, MD, MHS, who initiated the Cancer of the Pancreas Screening project for high-risk individuals of pancreatic cancer.
I also had the distinction of being the first at Bumrungrad International Hospital to perform endoscopic drainage for pancreatic fluid collections in the setting of multi-organ failure. This endoscopic approach has been extensively validated in the medical literature as significantly improving survival rates compared to surgical drainage. My training in this specialized procedure was conducted under the guidance of the premier group for necrotizing pancreatitis, led by Martin Freeman, MD, at the University of Minnesota.
Later, I contributed to overseeing the Inherited Gastrointestinal Malignancy Clinic of MyCode, a large-scale population-based cohort program focused on cancer screening in Pennsylvania. By December 2024, MyCode had collected blood samples from over 258,000 individuals, analyzed DNA sequences from over 184,000, and provided clinical data that benefits over 142,000 patients. It’s not uncommon for healthy 25-year-old patients to come to our clinic for colon cancer screening after learning from the program that they carry a cancer syndrome, and early screening can potentially save their lives.
What are the key differences between training and practicing medicine in the United States and in an Asian country?
The U.S. healthcare system is deeply rooted in evidence-based protocols and multidisciplinary care, driven by an insurance-based model. In contrast, many Asian countries face challenges such as the dependency on government approval for certain treatments and insurance limitations. Practicing in Asia requires navigating unique cultural, economic, and systemic differences, including varying resource availability and disease prevalence.
What specific challenges have you faced as a GI in Thailand?
As an advanced endoscopist, one of the biggest challenges I faced initially was the difficulty in obtaining the same devices I used in the U.S. for use in Thailand. With support from device companies and mentors in the U.S., I was able to perform groundbreaking procedures, such as the TIF in Southeast Asia and the first use of a full-thickness resection device in Thailand. I am also proud to be part of one of the first few centers worldwide performing the combination of injectable semaglutide and endoscopic sleeve gastroplasty, resulting in a remarkable weight reduction of 44%, comparable to surgical gastric bypass.
In addition, Bumrungrad International Hospital, where I practice, sees over 1.1 million visits annually from patients from more than 190 countries. This offers a unique opportunity to engage with a global patient base and learn from diverse cultures. Over time, although the hospital has professional interpreters for all languages, I have become able to communicate basic sentences with international patients in their preferred languages, including Chinese, Japanese, and Arabic, which has enriched my practice.
What’s your favorite thing to do when you’re not practicing GI?
I enjoy traveling, exploring new cuisines, and spending quality time with family and friends. These activities help me recharge and offer fresh perspectives on life.
Lightning Round
Texting or talking?
Talking. It’s more personal and meaningful.
Favorite city in the U.S.?
Ann Arbor, Michigan
Cat or dog person?
Dog person
Favorite junk food?
Pizza
How many cups of coffee do you drink per day?
Two – just enough to stay sharp, but not jittery.
If you weren’t a GI, what would you be?
Architect
Best place you went on vacation?
Kyoto, Japan
Favorite sport?
Skiing
Favorite ice cream?
Matcha green tea
What song do you have to sing along with when you hear it?
“Everybody” by Backstreet Boys
Favorite movie or TV show?
Forrest Gump and Friends
Optimist or pessimist?
Optimist. I believe in focusing on solutions and possibilities.

Is AI Use Causing Endoscopists to Lose Their Skills?
, a large observational study suggested.
“The extent and consistency of the adenoma detection rate (ADR) drop after long-term AI use were not expected,” study authors Krzysztof Budzyń, MD, and Marcin Romańczyk, MD, of the Academy of Silesia, Katowice, Poland, told GI & Hepatology News. “We thought there might be a small effect, but the 6% absolute decrease — observed in several centers and among most endoscopists — points to a genuine change in behavior. This was especially notable because all participants were very experienced, with more than 2000 colonoscopies each.”
Another unexpected result, they said, “was that the decrease was stronger in centers with higher starting ADRs and in certain patient groups, such as women under 60. We had assumed experienced clinicians would be less affected, but our results show that even highly skilled practitioners can be influenced.”
The study was published online in The Lancet Gastroenterology & Hepatology.
ADR Reduced After AI Use
To assess how endoscopists who used AI regularly performed colonoscopy when AI was not in use, researchers conducted a retrospective, observational study at four endoscopy centers in Poland taking part in the ACCEPT trial.
These centers introduced AI tools for polyp detection at the end of 2021, after which colonoscopies were randomly assigned to be done with or without AI assistance.
The researchers assessed colonoscopy quality by comparing two different phases: 3 months before and 3 months after AI implementation. All diagnostic colonoscopies were included, except for those involving intensive anticoagulant use, pregnancy, or a history of colorectal resection or inflammatory bowel disease.
The primary outcome was the change in the ADR of standard, non-AI-assisted colonoscopy before and after AI exposure.
Between September 2021 and March 2022, a total of 2177 colonoscopies were conducted, including 1443 without AI use and 734 with AI. The current analysis focused on the 795 patients who underwent non-AI-assisted colonoscopy before the introduction of AI and the 648 who underwent non-AI-assisted colonoscopy after.
Participants’ median age was 61 years, and 59% were women. The colonoscopies were performed by 19 experienced endoscopists who had conducted over 2000 colonoscopies each.
The ADR of standard colonoscopy decreased significantly from 28.4% (226 of 795) before the introduction of AI to 22.4% (145 of 648) after, corresponding to a 20% relative and 6% absolute reduction in the ADR.
The ADR for AI-assisted colonoscopies was 25.3% (186 of 734).
The number of adenomas per colonoscopy (APC) in patients with at least one adenoma detected did not change significantly between the groups before and after AI exposure, with a mean of 1.91 before vs 1.92 after. Similarly, the number of mean advanced APC was comparable between the two periods (0.062 vs 0.063).
The mean advanced APC detection on standard colonoscopy in patients with at least one adenoma detected was 0.22 before AI exposure and 0.28 after AI exposure.
Colorectal cancers were detected in 6 (0.8%) of 795 colonoscopies before AI exposure and in 8 (1.2%) of 648 after AI exposure.
In multivariable logistic regression analysis, exposure to AI (odds ratio [OR], 0.69), patient’s male sex (OR, 1.78), and patient age at least 60 years (OR, 3.60) were independent factors significantly associated with ADR.
In all centers, the ADR for standard, non-AI-assisted colonoscopy was reduced after AI exposure, although the magnitude of ADR reduction varied greatly between centers, according to the authors.
“Clinicians should be aware that while AI can boost detection rates, prolonged reliance may subtly affect their performance when the technology is not available,” Budzyń and Romańczyk said. “This does not mean AI should be avoided — rather, it highlights the need for conscious engagement with the task, even when AI is assisting. Monitoring one’s own detection rates in both AI-assisted and non-AI-assisted procedures can help identify changes early.”
“Endoscopists should view AI as a collaborative partner, not a replacement for their vigilance and judgment,” they concluded. “Integrating AI effectively means using it to complement, not substitute, core observational and diagnostic skills. In short, enjoy the benefits of AI, but keep your skills sharp — your patients depend on both.”
Omer Ahmed, MD, of University College London, London, England, gives a similar message in a related editorial. The study “compels us to carefully consider the effect of AI integration into routine endoscopic practice,” he wrote. “Although AI continues to offer great promise to enhance clinical outcomes, we must also safeguard against the quiet erosion of fundamental skills required for high-quality endoscopy.”
‘Certainly a Signal’
Commenting on the study for GI & Hepatology News, Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, said, “On the face of it, these findings would seem to correlate with all our lived experiences as humans. Any skill or task that we give to a machine will inherently ‘de-skill’ or weaken our ability to perform it.”
“The only way to miss a polyp is either due to lack of attention/recognition of a polyp in the field of view or a lack of fold exposure and cleansing,” said Bhuta, who was not involved in the study. “For AI to specifically de-skill polyp detection, it would mean the AI is conditioning physicians to pay less active attention during the procedure, similar to the way a driver may pay less attention in a car that has self-driving capabilities.”
That said, he noted that this is a small retrospective observational study with a short timeframe and an average of fewer than 100 colonoscopies per physician.
“My own ADR may vary by 8% or more by random chance in such a small dataset,” he said. “It’s hard to draw any real conclusions, but it is certainly a signal.”
The issue of de-skilling goes beyond gastroenterology and medicine, Bhuta noted. “We have invented millions of machines that have ‘de-skilled’ us in thousands of small ways, and mostly, we have benefited as a society. However, we’ve never had a machine that can de-skill our attention, our creativity, and our reason.”
“The question is not whether AI will de-skill us but when, where, and how do we set the boundaries of what we want a machine to do for us,” he said. “What is lost and what is gained by AI taking over these roles, and is that an acceptable trade-off?”
The study was funded by the European Commission and the Japan Society for the Promotion of Science. Budzyń, Romańczyk, and Bhuta declared having no competing interests. Ahmed declared receiving medical consultancy fees from Olympus, Odin Vision, Medtronic, and Norgine.
A version of this article appeared on Medscape.com.
, a large observational study suggested.
“The extent and consistency of the adenoma detection rate (ADR) drop after long-term AI use were not expected,” study authors Krzysztof Budzyń, MD, and Marcin Romańczyk, MD, of the Academy of Silesia, Katowice, Poland, told GI & Hepatology News. “We thought there might be a small effect, but the 6% absolute decrease — observed in several centers and among most endoscopists — points to a genuine change in behavior. This was especially notable because all participants were very experienced, with more than 2000 colonoscopies each.”
Another unexpected result, they said, “was that the decrease was stronger in centers with higher starting ADRs and in certain patient groups, such as women under 60. We had assumed experienced clinicians would be less affected, but our results show that even highly skilled practitioners can be influenced.”
The study was published online in The Lancet Gastroenterology & Hepatology.
ADR Reduced After AI Use
To assess how endoscopists who used AI regularly performed colonoscopy when AI was not in use, researchers conducted a retrospective, observational study at four endoscopy centers in Poland taking part in the ACCEPT trial.
These centers introduced AI tools for polyp detection at the end of 2021, after which colonoscopies were randomly assigned to be done with or without AI assistance.
The researchers assessed colonoscopy quality by comparing two different phases: 3 months before and 3 months after AI implementation. All diagnostic colonoscopies were included, except for those involving intensive anticoagulant use, pregnancy, or a history of colorectal resection or inflammatory bowel disease.
The primary outcome was the change in the ADR of standard, non-AI-assisted colonoscopy before and after AI exposure.
Between September 2021 and March 2022, a total of 2177 colonoscopies were conducted, including 1443 without AI use and 734 with AI. The current analysis focused on the 795 patients who underwent non-AI-assisted colonoscopy before the introduction of AI and the 648 who underwent non-AI-assisted colonoscopy after.
Participants’ median age was 61 years, and 59% were women. The colonoscopies were performed by 19 experienced endoscopists who had conducted over 2000 colonoscopies each.
The ADR of standard colonoscopy decreased significantly from 28.4% (226 of 795) before the introduction of AI to 22.4% (145 of 648) after, corresponding to a 20% relative and 6% absolute reduction in the ADR.
The ADR for AI-assisted colonoscopies was 25.3% (186 of 734).
The number of adenomas per colonoscopy (APC) in patients with at least one adenoma detected did not change significantly between the groups before and after AI exposure, with a mean of 1.91 before vs 1.92 after. Similarly, the number of mean advanced APC was comparable between the two periods (0.062 vs 0.063).
The mean advanced APC detection on standard colonoscopy in patients with at least one adenoma detected was 0.22 before AI exposure and 0.28 after AI exposure.
Colorectal cancers were detected in 6 (0.8%) of 795 colonoscopies before AI exposure and in 8 (1.2%) of 648 after AI exposure.
In multivariable logistic regression analysis, exposure to AI (odds ratio [OR], 0.69), patient’s male sex (OR, 1.78), and patient age at least 60 years (OR, 3.60) were independent factors significantly associated with ADR.
In all centers, the ADR for standard, non-AI-assisted colonoscopy was reduced after AI exposure, although the magnitude of ADR reduction varied greatly between centers, according to the authors.
“Clinicians should be aware that while AI can boost detection rates, prolonged reliance may subtly affect their performance when the technology is not available,” Budzyń and Romańczyk said. “This does not mean AI should be avoided — rather, it highlights the need for conscious engagement with the task, even when AI is assisting. Monitoring one’s own detection rates in both AI-assisted and non-AI-assisted procedures can help identify changes early.”
“Endoscopists should view AI as a collaborative partner, not a replacement for their vigilance and judgment,” they concluded. “Integrating AI effectively means using it to complement, not substitute, core observational and diagnostic skills. In short, enjoy the benefits of AI, but keep your skills sharp — your patients depend on both.”
Omer Ahmed, MD, of University College London, London, England, gives a similar message in a related editorial. The study “compels us to carefully consider the effect of AI integration into routine endoscopic practice,” he wrote. “Although AI continues to offer great promise to enhance clinical outcomes, we must also safeguard against the quiet erosion of fundamental skills required for high-quality endoscopy.”
‘Certainly a Signal’
Commenting on the study for GI & Hepatology News, Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, said, “On the face of it, these findings would seem to correlate with all our lived experiences as humans. Any skill or task that we give to a machine will inherently ‘de-skill’ or weaken our ability to perform it.”
“The only way to miss a polyp is either due to lack of attention/recognition of a polyp in the field of view or a lack of fold exposure and cleansing,” said Bhuta, who was not involved in the study. “For AI to specifically de-skill polyp detection, it would mean the AI is conditioning physicians to pay less active attention during the procedure, similar to the way a driver may pay less attention in a car that has self-driving capabilities.”
That said, he noted that this is a small retrospective observational study with a short timeframe and an average of fewer than 100 colonoscopies per physician.
“My own ADR may vary by 8% or more by random chance in such a small dataset,” he said. “It’s hard to draw any real conclusions, but it is certainly a signal.”
The issue of de-skilling goes beyond gastroenterology and medicine, Bhuta noted. “We have invented millions of machines that have ‘de-skilled’ us in thousands of small ways, and mostly, we have benefited as a society. However, we’ve never had a machine that can de-skill our attention, our creativity, and our reason.”
“The question is not whether AI will de-skill us but when, where, and how do we set the boundaries of what we want a machine to do for us,” he said. “What is lost and what is gained by AI taking over these roles, and is that an acceptable trade-off?”
The study was funded by the European Commission and the Japan Society for the Promotion of Science. Budzyń, Romańczyk, and Bhuta declared having no competing interests. Ahmed declared receiving medical consultancy fees from Olympus, Odin Vision, Medtronic, and Norgine.
A version of this article appeared on Medscape.com.
, a large observational study suggested.
“The extent and consistency of the adenoma detection rate (ADR) drop after long-term AI use were not expected,” study authors Krzysztof Budzyń, MD, and Marcin Romańczyk, MD, of the Academy of Silesia, Katowice, Poland, told GI & Hepatology News. “We thought there might be a small effect, but the 6% absolute decrease — observed in several centers and among most endoscopists — points to a genuine change in behavior. This was especially notable because all participants were very experienced, with more than 2000 colonoscopies each.”
Another unexpected result, they said, “was that the decrease was stronger in centers with higher starting ADRs and in certain patient groups, such as women under 60. We had assumed experienced clinicians would be less affected, but our results show that even highly skilled practitioners can be influenced.”
The study was published online in The Lancet Gastroenterology & Hepatology.
ADR Reduced After AI Use
To assess how endoscopists who used AI regularly performed colonoscopy when AI was not in use, researchers conducted a retrospective, observational study at four endoscopy centers in Poland taking part in the ACCEPT trial.
These centers introduced AI tools for polyp detection at the end of 2021, after which colonoscopies were randomly assigned to be done with or without AI assistance.
The researchers assessed colonoscopy quality by comparing two different phases: 3 months before and 3 months after AI implementation. All diagnostic colonoscopies were included, except for those involving intensive anticoagulant use, pregnancy, or a history of colorectal resection or inflammatory bowel disease.
The primary outcome was the change in the ADR of standard, non-AI-assisted colonoscopy before and after AI exposure.
Between September 2021 and March 2022, a total of 2177 colonoscopies were conducted, including 1443 without AI use and 734 with AI. The current analysis focused on the 795 patients who underwent non-AI-assisted colonoscopy before the introduction of AI and the 648 who underwent non-AI-assisted colonoscopy after.
Participants’ median age was 61 years, and 59% were women. The colonoscopies were performed by 19 experienced endoscopists who had conducted over 2000 colonoscopies each.
The ADR of standard colonoscopy decreased significantly from 28.4% (226 of 795) before the introduction of AI to 22.4% (145 of 648) after, corresponding to a 20% relative and 6% absolute reduction in the ADR.
The ADR for AI-assisted colonoscopies was 25.3% (186 of 734).
The number of adenomas per colonoscopy (APC) in patients with at least one adenoma detected did not change significantly between the groups before and after AI exposure, with a mean of 1.91 before vs 1.92 after. Similarly, the number of mean advanced APC was comparable between the two periods (0.062 vs 0.063).
The mean advanced APC detection on standard colonoscopy in patients with at least one adenoma detected was 0.22 before AI exposure and 0.28 after AI exposure.
Colorectal cancers were detected in 6 (0.8%) of 795 colonoscopies before AI exposure and in 8 (1.2%) of 648 after AI exposure.
In multivariable logistic regression analysis, exposure to AI (odds ratio [OR], 0.69), patient’s male sex (OR, 1.78), and patient age at least 60 years (OR, 3.60) were independent factors significantly associated with ADR.
In all centers, the ADR for standard, non-AI-assisted colonoscopy was reduced after AI exposure, although the magnitude of ADR reduction varied greatly between centers, according to the authors.
“Clinicians should be aware that while AI can boost detection rates, prolonged reliance may subtly affect their performance when the technology is not available,” Budzyń and Romańczyk said. “This does not mean AI should be avoided — rather, it highlights the need for conscious engagement with the task, even when AI is assisting. Monitoring one’s own detection rates in both AI-assisted and non-AI-assisted procedures can help identify changes early.”
“Endoscopists should view AI as a collaborative partner, not a replacement for their vigilance and judgment,” they concluded. “Integrating AI effectively means using it to complement, not substitute, core observational and diagnostic skills. In short, enjoy the benefits of AI, but keep your skills sharp — your patients depend on both.”
Omer Ahmed, MD, of University College London, London, England, gives a similar message in a related editorial. The study “compels us to carefully consider the effect of AI integration into routine endoscopic practice,” he wrote. “Although AI continues to offer great promise to enhance clinical outcomes, we must also safeguard against the quiet erosion of fundamental skills required for high-quality endoscopy.”
‘Certainly a Signal’
Commenting on the study for GI & Hepatology News, Rajiv Bhuta, MD, assistant professor of clinical gastroenterology and hepatology at Temple University and a gastroenterologist at Temple University Hospital, both in Philadelphia, said, “On the face of it, these findings would seem to correlate with all our lived experiences as humans. Any skill or task that we give to a machine will inherently ‘de-skill’ or weaken our ability to perform it.”
“The only way to miss a polyp is either due to lack of attention/recognition of a polyp in the field of view or a lack of fold exposure and cleansing,” said Bhuta, who was not involved in the study. “For AI to specifically de-skill polyp detection, it would mean the AI is conditioning physicians to pay less active attention during the procedure, similar to the way a driver may pay less attention in a car that has self-driving capabilities.”
That said, he noted that this is a small retrospective observational study with a short timeframe and an average of fewer than 100 colonoscopies per physician.
“My own ADR may vary by 8% or more by random chance in such a small dataset,” he said. “It’s hard to draw any real conclusions, but it is certainly a signal.”
The issue of de-skilling goes beyond gastroenterology and medicine, Bhuta noted. “We have invented millions of machines that have ‘de-skilled’ us in thousands of small ways, and mostly, we have benefited as a society. However, we’ve never had a machine that can de-skill our attention, our creativity, and our reason.”
“The question is not whether AI will de-skill us but when, where, and how do we set the boundaries of what we want a machine to do for us,” he said. “What is lost and what is gained by AI taking over these roles, and is that an acceptable trade-off?”
The study was funded by the European Commission and the Japan Society for the Promotion of Science. Budzyń, Romańczyk, and Bhuta declared having no competing interests. Ahmed declared receiving medical consultancy fees from Olympus, Odin Vision, Medtronic, and Norgine.
A version of this article appeared on Medscape.com.
Repeat Intubation of the Sigmoid Colon Improves Adenoma Detection
, new research showed.
“After eliminating the impact of time, the adenoma-detection rate [with a second intubation vs standard withdrawal] was still significantly increased, indicating that the second intubation technique could enhance the visualization of the sigmoid colon mucosa and reduce the rate of missed lesions,” reported the authors of the study, published in The American Journal of Gastroenterology.
When precancerous polyps are removed during standard colonoscopies, as many as 70%-90% of colorectal cancers can be prevented; however, rates of missed polyps during colonoscopy are notoriously high.
Recent studies have shown improved adenoma-detection rates with the use of Endocuff, water-assisted colonoscopy, full-spectrum endoscopy, and repeat withdrawal examinations, which include retroflexion and forward-viewing methods.
The repeat colonoscopy examinations may represent “the easiest and most practical option for endoscopists as they do not require additional tools, staff, or funding,” the authors explained.
However, most studies on the issue have focused mainly on the right colon and forward-viewing examinations, whereas the sigmoid colon, which has the most turns and is the most easily compressed, can be easily missed during withdrawal observation.
To investigate if use of a second colon intubation of the sigmoid colon could improve detection rates, senior author Jianning Yao, MD, of the Department of Gastroenterology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, conducted a randomized trial, enrolling 650 patients between December 2023 and April 2024 who were aged 45 or older and had overweight or obesity (BMI ≥ 24).
At the time of the first withdrawal during the colonoscopy, the patients were randomized 1:1 to groups of 325 each to either receive standard withdrawal, with withdrawal to the anus, or to receive a second intubation, with reinsertion into the sigmoid colon.
In the second intubation, the colonoscope was pushed forward without straightening, “allowing for slight looping that could be used to flatten the colonic folds as the tip of the instrument was advanced,” they explained.
The patients had a mean age of 55; about 25% had a smoking habit, and the mean BMI was about 28. There were no significant differences in other baseline characteristics.
The results showed that patients in the second-intubation group vs standard-withdrawal group had a substantially higher adenoma-detection rate (24.3% vs 14.5%) and polyp-detection rate (29.2% vs 17.8%, P = .001 for both) in the sigmoid colon.
In the second-intubation group, 85% of the adenomas discovered throughout the second inspection in the sigmoid colon were 5 mm or smaller in size. In addition, 90% of the 40 adenomas were somewhat raised or pedunculated, and all were tubular adenomas.
No high-grade dysplasia adenomas were discovered.
Of note, the colonoscopy in the second-intubation group’s colonoscopic examinations took just 1.47 minute longer overall than the standard-withdrawal group’s examinations.
Factors that were determined in a multivariate analysis to be independent predictors of higher adenoma detection in the second-intubation group included older age, smoking habit, longer duration of the second inspection, and the identification of lesions during the initial withdrawal from the sigmoid colon.
Patients’ vital signs were monitored at intervals of 3 minutes throughout the colonoscopy procedure, and patients were followed up to monitor for any adverse events occurring within 2 weeks after the examination, with no notable disparities observed between the two groups.
Alternative to AKS Approach in Second Intubation
The authors explained that, in their approach in the second intubation, the common axis-keeping shortening (AKS) was not utilized, and instead they pushed the colonoscope forward without straightening it, which offers important advantages.
“In this way, slight looping of the colonoscope can be used to flatten the colonic folds as the tip of the instrument is advanced, thereby achieving an observation effect that cannot be reached by any number of withdrawal examinations.”
In general, the stimulation of peristalsis during a second examination allows for the observation of the colonic mucosa from different angles, thereby reducing the rate of missed lesions, the authors added.
“Although the detection of these lesions may not significantly affect clinical outcomes, it serves as a reminder for patients regarding regular follow-ups and lifestyle adjustments,” they explained. “Additionally, it may reduce the likelihood of missing some smaller lesions that progress rapidly, such as de novo cancer.”
Based on the results, the authors concluded that older patients, patients who smoke, or those with lesions found on the first sigmoid inspection have a higher chance of having missed adenomas discovered in the sigmoid colon during the second intubation examination.
“If one of these risk factors is present, a second examination of the sigmoid colon may be considered to detect missed lesions,” they said.
The added time commitment of just 1.47 minutes can be a worthwhile tradeoff, they added.
“Considering the improvements in the adenoma-detection rate provided by the second intubation, this modest time increase may be acceptable.”
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
, new research showed.
“After eliminating the impact of time, the adenoma-detection rate [with a second intubation vs standard withdrawal] was still significantly increased, indicating that the second intubation technique could enhance the visualization of the sigmoid colon mucosa and reduce the rate of missed lesions,” reported the authors of the study, published in The American Journal of Gastroenterology.
When precancerous polyps are removed during standard colonoscopies, as many as 70%-90% of colorectal cancers can be prevented; however, rates of missed polyps during colonoscopy are notoriously high.
Recent studies have shown improved adenoma-detection rates with the use of Endocuff, water-assisted colonoscopy, full-spectrum endoscopy, and repeat withdrawal examinations, which include retroflexion and forward-viewing methods.
The repeat colonoscopy examinations may represent “the easiest and most practical option for endoscopists as they do not require additional tools, staff, or funding,” the authors explained.
However, most studies on the issue have focused mainly on the right colon and forward-viewing examinations, whereas the sigmoid colon, which has the most turns and is the most easily compressed, can be easily missed during withdrawal observation.
To investigate if use of a second colon intubation of the sigmoid colon could improve detection rates, senior author Jianning Yao, MD, of the Department of Gastroenterology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, conducted a randomized trial, enrolling 650 patients between December 2023 and April 2024 who were aged 45 or older and had overweight or obesity (BMI ≥ 24).
At the time of the first withdrawal during the colonoscopy, the patients were randomized 1:1 to groups of 325 each to either receive standard withdrawal, with withdrawal to the anus, or to receive a second intubation, with reinsertion into the sigmoid colon.
In the second intubation, the colonoscope was pushed forward without straightening, “allowing for slight looping that could be used to flatten the colonic folds as the tip of the instrument was advanced,” they explained.
The patients had a mean age of 55; about 25% had a smoking habit, and the mean BMI was about 28. There were no significant differences in other baseline characteristics.
The results showed that patients in the second-intubation group vs standard-withdrawal group had a substantially higher adenoma-detection rate (24.3% vs 14.5%) and polyp-detection rate (29.2% vs 17.8%, P = .001 for both) in the sigmoid colon.
In the second-intubation group, 85% of the adenomas discovered throughout the second inspection in the sigmoid colon were 5 mm or smaller in size. In addition, 90% of the 40 adenomas were somewhat raised or pedunculated, and all were tubular adenomas.
No high-grade dysplasia adenomas were discovered.
Of note, the colonoscopy in the second-intubation group’s colonoscopic examinations took just 1.47 minute longer overall than the standard-withdrawal group’s examinations.
Factors that were determined in a multivariate analysis to be independent predictors of higher adenoma detection in the second-intubation group included older age, smoking habit, longer duration of the second inspection, and the identification of lesions during the initial withdrawal from the sigmoid colon.
Patients’ vital signs were monitored at intervals of 3 minutes throughout the colonoscopy procedure, and patients were followed up to monitor for any adverse events occurring within 2 weeks after the examination, with no notable disparities observed between the two groups.
Alternative to AKS Approach in Second Intubation
The authors explained that, in their approach in the second intubation, the common axis-keeping shortening (AKS) was not utilized, and instead they pushed the colonoscope forward without straightening it, which offers important advantages.
“In this way, slight looping of the colonoscope can be used to flatten the colonic folds as the tip of the instrument is advanced, thereby achieving an observation effect that cannot be reached by any number of withdrawal examinations.”
In general, the stimulation of peristalsis during a second examination allows for the observation of the colonic mucosa from different angles, thereby reducing the rate of missed lesions, the authors added.
“Although the detection of these lesions may not significantly affect clinical outcomes, it serves as a reminder for patients regarding regular follow-ups and lifestyle adjustments,” they explained. “Additionally, it may reduce the likelihood of missing some smaller lesions that progress rapidly, such as de novo cancer.”
Based on the results, the authors concluded that older patients, patients who smoke, or those with lesions found on the first sigmoid inspection have a higher chance of having missed adenomas discovered in the sigmoid colon during the second intubation examination.
“If one of these risk factors is present, a second examination of the sigmoid colon may be considered to detect missed lesions,” they said.
The added time commitment of just 1.47 minutes can be a worthwhile tradeoff, they added.
“Considering the improvements in the adenoma-detection rate provided by the second intubation, this modest time increase may be acceptable.”
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
, new research showed.
“After eliminating the impact of time, the adenoma-detection rate [with a second intubation vs standard withdrawal] was still significantly increased, indicating that the second intubation technique could enhance the visualization of the sigmoid colon mucosa and reduce the rate of missed lesions,” reported the authors of the study, published in The American Journal of Gastroenterology.
When precancerous polyps are removed during standard colonoscopies, as many as 70%-90% of colorectal cancers can be prevented; however, rates of missed polyps during colonoscopy are notoriously high.
Recent studies have shown improved adenoma-detection rates with the use of Endocuff, water-assisted colonoscopy, full-spectrum endoscopy, and repeat withdrawal examinations, which include retroflexion and forward-viewing methods.
The repeat colonoscopy examinations may represent “the easiest and most practical option for endoscopists as they do not require additional tools, staff, or funding,” the authors explained.
However, most studies on the issue have focused mainly on the right colon and forward-viewing examinations, whereas the sigmoid colon, which has the most turns and is the most easily compressed, can be easily missed during withdrawal observation.
To investigate if use of a second colon intubation of the sigmoid colon could improve detection rates, senior author Jianning Yao, MD, of the Department of Gastroenterology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, conducted a randomized trial, enrolling 650 patients between December 2023 and April 2024 who were aged 45 or older and had overweight or obesity (BMI ≥ 24).
At the time of the first withdrawal during the colonoscopy, the patients were randomized 1:1 to groups of 325 each to either receive standard withdrawal, with withdrawal to the anus, or to receive a second intubation, with reinsertion into the sigmoid colon.
In the second intubation, the colonoscope was pushed forward without straightening, “allowing for slight looping that could be used to flatten the colonic folds as the tip of the instrument was advanced,” they explained.
The patients had a mean age of 55; about 25% had a smoking habit, and the mean BMI was about 28. There were no significant differences in other baseline characteristics.
The results showed that patients in the second-intubation group vs standard-withdrawal group had a substantially higher adenoma-detection rate (24.3% vs 14.5%) and polyp-detection rate (29.2% vs 17.8%, P = .001 for both) in the sigmoid colon.
In the second-intubation group, 85% of the adenomas discovered throughout the second inspection in the sigmoid colon were 5 mm or smaller in size. In addition, 90% of the 40 adenomas were somewhat raised or pedunculated, and all were tubular adenomas.
No high-grade dysplasia adenomas were discovered.
Of note, the colonoscopy in the second-intubation group’s colonoscopic examinations took just 1.47 minute longer overall than the standard-withdrawal group’s examinations.
Factors that were determined in a multivariate analysis to be independent predictors of higher adenoma detection in the second-intubation group included older age, smoking habit, longer duration of the second inspection, and the identification of lesions during the initial withdrawal from the sigmoid colon.
Patients’ vital signs were monitored at intervals of 3 minutes throughout the colonoscopy procedure, and patients were followed up to monitor for any adverse events occurring within 2 weeks after the examination, with no notable disparities observed between the two groups.
Alternative to AKS Approach in Second Intubation
The authors explained that, in their approach in the second intubation, the common axis-keeping shortening (AKS) was not utilized, and instead they pushed the colonoscope forward without straightening it, which offers important advantages.
“In this way, slight looping of the colonoscope can be used to flatten the colonic folds as the tip of the instrument is advanced, thereby achieving an observation effect that cannot be reached by any number of withdrawal examinations.”
In general, the stimulation of peristalsis during a second examination allows for the observation of the colonic mucosa from different angles, thereby reducing the rate of missed lesions, the authors added.
“Although the detection of these lesions may not significantly affect clinical outcomes, it serves as a reminder for patients regarding regular follow-ups and lifestyle adjustments,” they explained. “Additionally, it may reduce the likelihood of missing some smaller lesions that progress rapidly, such as de novo cancer.”
Based on the results, the authors concluded that older patients, patients who smoke, or those with lesions found on the first sigmoid inspection have a higher chance of having missed adenomas discovered in the sigmoid colon during the second intubation examination.
“If one of these risk factors is present, a second examination of the sigmoid colon may be considered to detect missed lesions,” they said.
The added time commitment of just 1.47 minutes can be a worthwhile tradeoff, they added.
“Considering the improvements in the adenoma-detection rate provided by the second intubation, this modest time increase may be acceptable.”
The authors had no disclosures to report.
A version of this article appeared on Medscape.com.
Large Language Models Cut Time, Cost of Guideline Development
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
Ethan Goh, MD, executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, described the AGA pilot as both timely and promising.
“I’m certainly bullish about the use case,” he said in an interview. “Their study design and application is also robust, so I would congratulate them.”
Goh, a general editor for BMJ Digital Health & AI, predicted “huge potential” in the strategy for both clinicians and the general population, who benefit from the most up-to-date guidelines possible.
“I believe that using AI can represent a much faster, more cost effective, efficient way of gathering all these information sources,” he said.
Still, humans will need to be involved in the process.
“[This AI-driven approach] will always need some degree of expert oversight and judgement,” Goh said.
Speaking more broadly about automating study aggregation, Goh said AI may still struggle to determine which studies are most clinically relevant.
“When we use [AI models] to pull out medical references, anecdotally, I don’t think they’re always getting the best ones all the time, or even necessarily the right ones,” he said.
And as AI models grow more impressive, these shortcomings become less apparent, potentially lulling humans into overconfidence.
“Humans are humans,” Goh said. “We get lazy over time. That will be one of the challenges. As the systems get increasingly good, humans start to defer more and more of their judgment to them and say, ‘All right, AI, you’re doing good. Just do 100% automation.’ And then [people] start fact checking or reviewing even less.”
AI could also undermine automated reviews in another way: AI-generated publications that appear genuine, but aren’t, may creep into the dataset.
Despite these concerns, Goh concluded on an optimistic note.
“I think that there are huge ways to use AI, tools, not to replace, but to augment and support human judgment,” he said.
Ethan Goh, MD, is senior research engineer and executive director of the Stanford AI Research and Science Evaluation (ARISE) Network, at Stanford (Calif.) University. He declared no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
, according to a pilot study from the American Gastroenterological Association (AGA).
Faster, cheaper study screening could allow societies to update clinical recommendations more frequently, improving alignment with the latest evidence, lead author Sunny Chung, MD, of Yale School of Medicine, New Haven, Connecticut, and colleagues, reported.
“Each guideline typically requires 5 to 15 systematic reviews, making the process time-consuming (averaging more than 60 weeks) and costly (more than $140,000),” the investigators wrote in Gastroenterology . “One of the most critical yet time-consuming steps in systematic reviews is title and abstract screening. LLMs have the potential to make this step more efficient.”
To test this approach, the investigators developed, validated, and applied a dual-model LLM screening pipeline with human-in-the-loop oversight, focusing on randomized controlled trials in AGA guidelines.
The system was built using the 2021 guideline on moderate-to-severe Crohn’s disease, targeting biologic therapies for induction and maintenance of remission.
Using chain-of-thought prompting and structured inclusion criteria based on the PICO framework, the investigators deployed GPT-4o (OpenAI) and Gemini-1.5-Pro (Google DeepMind) as independent screeners, each assessing titles and abstracts according to standardized logic encoded in JavaScript Object Notation. This approach mimicked a traditional double-reviewer system.
After initial testing, the pipeline was validated in a 2025 update of the same guideline, this time spanning 6 focused clinical questions on advanced therapies and immunomodulators. Results were compared against manual screening by 2 experienced human reviewers, with total screening time documented.
The system was then tested across 4 additional guideline topics: fecal microbiota transplantation (FMT) for irritable bowel syndrome and Clostridioides difficile, gastroparesis, and hepatocellular carcinoma. A final test applied the system to a forthcoming guideline on complications of acute pancreatitis.
Across all topics, the dual-LLM system achieved 100% sensitivity in identifying randomized controlled trials (RCTs). For the 2025 update of the AGA guideline on Crohn’s disease, the models flagged 418 of 4,377 abstracts for inclusion, captur-ing all 25 relevant RCTs in just 48 minutes. Manual screening of the same dataset previously took almost 13 hours.
Comparable accuracy and time savings were observed for the other topics.
The pipeline correctly flagged all 13 RCTs in 4,820 studies on FMT for irritable bowel syndrome, and all 16 RCTs in 5,587 studies on FMT for Clostridioides difficile, requiring 27 and 66 minutes, respectively. Similarly, the system captured all 11 RCTs in 3,919 hepatocellular carcinoma abstracts and all 18 RCTs in 1,578 studies on gastroparesis, completing each task in under 65 minutes. Early testing on the upcoming guideline for pancreatitis yielded similar results.
Cost analysis underscored the efficiency of this approach. At an estimated $175–200 per hour for expert screeners, traditional abstract screening would cost around $2,500 per review, versus approximately $100 for the LLM approach—a 96% reduction.
The investigators cautioned that human oversight remains necessary to verify the relevance of studies flagged by the models. While the system’s sensitivity was consistent, it also selected articles that were ultimately excluded by expert reviewers. Broader validation will be required to assess performance across non-RCT study designs, such as observational or case-control studies, they added.
“As medical literature continues to expand, the integration of artificial intelligence into evidence synthesis processes will become increasingly vital,” Dr. Chung and colleagues wrote. “With further refinement and broader validation, this LLM-based pipeline has the potential to revolutionize evidence synthesis and set a new standard for guideline development.”
This study was funded by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. The investigators reported no conflicts of interest.
FROM GASTROENTEROLOGY
Forceps Assistance Improves Outcomes in Difficult ERCP Cannulations
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
The results emerged from the small, single-center SOCCER trial of 152 patients recruited from March 2022 to October 2024 and are published in The American Journal of Gastroenterology.
Both groups had a slightly higher number of female participants, and the mean ages of the participants were 61.9 years in the forceps group and 68.3 years in the no forceps group.
First author Steven M. Hadley Jr, an MD candidate at Northwestern Feinberg School of Medicine in Chicago, and colleagues reported that forceps assistance in difficult cannulations yielded significantly higher success rates than no forceps assistance (100% vs 83.9%; P < .001).
The investigators noted that difficult cannulations during ERCP have a frequency of 42%. Cannulation failure is associated with increased morbidity — including longer hospitalization, increased ICU admissions, readmissions, and increased financial cost — as well as mortality rates of up to 10%.
SOCCER defined difficult cannulation as a papilla in or on the rim of a diverticulum, five or more attempts, attempts lasting 5 or more minutes, or two or more unintended pancreatic duct wire passages. Other features were redundant tissue overlaying the papilla or a type 2, 3, or 4 papilla.
The study found forceps assistance also had a nonstatistically significant lower rate of difficult cannulations than no forceps (57.1% vs 69.1%; P = .132). The rate of post-ERCP pancreatitis (PEP) was similarly low in both groups: 5.7% with forceps vs 3.7% without forceps (P = .705). The no forceps group had significantly more cannulation attempts after randomization than the forceps group (14 vs 8.3; P = .026).
Patients who crossed over to forceps assistance all had successful cannulations.
The technique has long been used to overcome cannulation difficulties, said Timothy B. Gardner, MD, MS, a gastroenterologist at the Dartmouth Hitchcock Medical Center in Lebanon, New Hampshire, and a coauthor of the study. “It was particularly effective for cannulations with redundant tissue limiting access to the papilla,” Gardner told GI & Hepatology News. “We decided to design a randomized trial to determine the extent to which this technique worked. We believed our study would answer an important question that would hopefully lead to an improvement in endoscopy practice.”
While a few case reports and video demos had described the technique, no trials had assessed its effectiveness, Hadley added. “We found the technique to be effective based on our experience, but it was exciting to see that a rigorously designed randomized trial proved that it is indeed a very effective technique to facilitate cannulation.”
Hadley noted the technique does not increase PEP incidence, unlike the commonly used precut sphincterotomy and the double-wire method for difficult cannulations. “As a result, the forceps-assisted technique may be an effective first-line option and may reduce the need for additional, more invasive procedures including surgery and repeat ERCP to obtain the therapeutic intent of the original ERCP.”
The paper outlines the technique’s methodology, he added, “so we believe endoscopists who read the manuscript will be able to start implementing the technique into their practice.”
Commenting on the paper but not involved in it, Christopher J. DiMaio, MD, regional director of Endoscopy for Northwell Health Physician Partners Gastroenterology and a gastroenterologist in Greenlawn, New York, called it potentially helpful but aimed at a niche group of expert practitioners. “The technique appears safe and very effective, which is the number one concern, and I would definitely keep it in my back pocket,” he said. “I expect it will be used more commonly now because of this study.”
He added that although expert endoscopists are familiar with the approach, they use more time-tested and sometimes more aggressive maneuvers to cope with difficult cannulations. “But this is a simple technique using a device that should be available to most high-volume endoscopists.”
DiMaio also noted that he would have liked to see an actual decrease in PEP incidence in the intervention group.
Looking ahead, Hadley said it would be interesting to compare the effectiveness of the double-wire technique against forceps-assisted cannulation in a randomized context. “A study we’re already looking into is seeing whether physician experience with the technique impacts outcomes.”
This study was supported by the American College of Gastroenterology. The authors and DiMaio reported having no relevant competing interests.
A version of this article first appeared on Medscape.com.
Out-of-Pocket Prep Costs Reduce Screening Colonoscopy Uptake, Especially in Vulnerable Populations
, a large insurance-claims analysis in Gastroenterology reported.
Moreover, this cost-sharing contravenes the preventive-care provisions for bowel preparation mandated by the Affordable Care Act (ACA).
Led by Gastroenterologist Eric D. Shah, MD, MBA, a clinical associate professor at the University of Michigan in Ann Arbor, Michigan, the study found a significant proportion of prescribed bowel preparation claims — 53% for commercial plans and 83% for Medicare — still involve patient cost-sharing, indicating noncompliance with ACA guidelines. Although expense-sharing was less prevalent among Medicaid claims (just 27%), it was not eliminated, suggesting room for improvement in coverage enforcement across the board.
“Colon cancer is unique in that it can be prevented with colonoscopy, but where are the patients? Bowel prep is a major reason that patients defer screening,” Shah told GI & Hepatology News. He said his group was quite surprised that the majority in the study cohort were paying something out of pocket when these costs should have been covered. “Primary care doctors may not think to ask about bowel prep costs when they order screening colonoscopies.”
The findings emerged from an analysis of 2,593,079 prescription drug claims: 52.9% from commercial plans, 35% from Medicare Part D plans, and 8.3% from Medicaid plans.
“These patient costs of $30 or $50 are a real not a theoretical deterrent,” said Whitney Jones, MD, a gastroenterologist, adjunct clinical professor at the University of Louisville in Louisville, Kentucky, and founder of the nonprofit Colon Cancer Prevention Project. Jones was not involved in the analysis. “Some insurers require prior patient authorization for the low-dose preps, but gastroenterologists are doing so many colonoscopies they don’t always have time to get a PA [prior authorization] on everyone.”
With the increasing use of blood and stool-based CRC testing, he added, “when you get a positive result, it’s really important to have the procedure quickly.” And appropriate bowel preparation is a small, cost-effective portion of the total costs of colonoscopy, a procedure that ultimately saves insurers significant money in treatment costs.
The authors noted that while CRC is the second-leading cause of cancer-related deaths in the US, screening rates remain low, with only 59% of adults aged 45 years or older up to date with screening. Screening rates are particularly low among racial and ethnic minority groups as compared with White individuals, a disparity that highlights the need to address existing barriers and enhance screening efforts.
In the current study, shared costs by bowel preparation volume also varied. Low-volume formulations had consistently higher out-of-pocket costs: a median of $60 for low-volume vs $10 for high-volume in commercial plans. In Medicare, 75% of high-volume claims had shared costs compared with 90% for their low-volume counterparts. The cost-sharing difference was slightly narrower with Medicaid: 27% of high-volume claims vs 30% of low-volume claims.
This is concerning, as low-volume options, which are preferred by patients for their better tolerability, can enhance uptake and adherence and improve colonoscopy outcomes. Shah advises physicians to consider prescribing low-volume preparations. “Let patients know about the potential out-of-pocket cost and about copay cards and assistance programs and use high-volume preps as an alternative rather than a go-to,” he said.
As to costs across insurance types, among commercial plans, the median nonzero out-of-pocket cost was $10 for high-volume and $60 for low-volume product claims. For Medicare, the median nonzero out-of-pocket cost was $8 for high-volume and $55.99 for low-volume products.
Under the ACA, CRC screening is classified as a recommended preventive service, requiring health plans to cover it without cost-sharing. Although the Centers for Medicare & Medicaid Services previously tried to enforce this mandate in 2015 and 2016, stating that colonoscopy preparation medications should be covered at no cost, many health plans are still not compliant.
At the nonfederal level, Jones noted, Kentucky, which has a significant high-risk population, recently became the first state to pass legislation requiring health benefit plans to cover all guideline-recommended CRC exams and lab tests.
For its part, AGA has also called on payers to eliminate all cost-sharing barriers across the CRC screening continuum.
Of note, the study authors said, the higher compliance with the ACA mandate in commercial and Medicaid plans than in Medicare highlights disparities that may disproportionately affect vulnerable older adults. While nearly half of commercial patients and nearly three quarters of Medicaid patients incurred zero out-of-pocket costs, fewer than 17% of Medicare beneficiaries, or 1 in 6, did so.
Although these costs may be low relative to the colonoscopy, they nevertheless can deter uptake of preventive screenings, potentially leading to higher CRC incidence and mortality. “While some patients may be willing to pay modest out-of-pocket costs, any required payment, however small, can serve as a barrier to preventative care, particularly in underserved populations,” they wrote. “These financial barriers will continue to contribute to widening disparities and hinder progress toward equitable screening outcomes.”
In the meantime, said Shah, “Physicians should advocate now to their representatives in Congress that bowel prep costs should already be covered as part of the ACA.”
This study was funded by Sebela Pharmaceuticals, maker of SUFLAVE preparation. The authors had no conflicts of interest to declare. Jones is a speaker and consultant for Grail LLC.
A version of this article appeared on Medscape.com.
, a large insurance-claims analysis in Gastroenterology reported.
Moreover, this cost-sharing contravenes the preventive-care provisions for bowel preparation mandated by the Affordable Care Act (ACA).
Led by Gastroenterologist Eric D. Shah, MD, MBA, a clinical associate professor at the University of Michigan in Ann Arbor, Michigan, the study found a significant proportion of prescribed bowel preparation claims — 53% for commercial plans and 83% for Medicare — still involve patient cost-sharing, indicating noncompliance with ACA guidelines. Although expense-sharing was less prevalent among Medicaid claims (just 27%), it was not eliminated, suggesting room for improvement in coverage enforcement across the board.
“Colon cancer is unique in that it can be prevented with colonoscopy, but where are the patients? Bowel prep is a major reason that patients defer screening,” Shah told GI & Hepatology News. He said his group was quite surprised that the majority in the study cohort were paying something out of pocket when these costs should have been covered. “Primary care doctors may not think to ask about bowel prep costs when they order screening colonoscopies.”
The findings emerged from an analysis of 2,593,079 prescription drug claims: 52.9% from commercial plans, 35% from Medicare Part D plans, and 8.3% from Medicaid plans.
“These patient costs of $30 or $50 are a real not a theoretical deterrent,” said Whitney Jones, MD, a gastroenterologist, adjunct clinical professor at the University of Louisville in Louisville, Kentucky, and founder of the nonprofit Colon Cancer Prevention Project. Jones was not involved in the analysis. “Some insurers require prior patient authorization for the low-dose preps, but gastroenterologists are doing so many colonoscopies they don’t always have time to get a PA [prior authorization] on everyone.”
With the increasing use of blood and stool-based CRC testing, he added, “when you get a positive result, it’s really important to have the procedure quickly.” And appropriate bowel preparation is a small, cost-effective portion of the total costs of colonoscopy, a procedure that ultimately saves insurers significant money in treatment costs.
The authors noted that while CRC is the second-leading cause of cancer-related deaths in the US, screening rates remain low, with only 59% of adults aged 45 years or older up to date with screening. Screening rates are particularly low among racial and ethnic minority groups as compared with White individuals, a disparity that highlights the need to address existing barriers and enhance screening efforts.
In the current study, shared costs by bowel preparation volume also varied. Low-volume formulations had consistently higher out-of-pocket costs: a median of $60 for low-volume vs $10 for high-volume in commercial plans. In Medicare, 75% of high-volume claims had shared costs compared with 90% for their low-volume counterparts. The cost-sharing difference was slightly narrower with Medicaid: 27% of high-volume claims vs 30% of low-volume claims.
This is concerning, as low-volume options, which are preferred by patients for their better tolerability, can enhance uptake and adherence and improve colonoscopy outcomes. Shah advises physicians to consider prescribing low-volume preparations. “Let patients know about the potential out-of-pocket cost and about copay cards and assistance programs and use high-volume preps as an alternative rather than a go-to,” he said.
As to costs across insurance types, among commercial plans, the median nonzero out-of-pocket cost was $10 for high-volume and $60 for low-volume product claims. For Medicare, the median nonzero out-of-pocket cost was $8 for high-volume and $55.99 for low-volume products.
Under the ACA, CRC screening is classified as a recommended preventive service, requiring health plans to cover it without cost-sharing. Although the Centers for Medicare & Medicaid Services previously tried to enforce this mandate in 2015 and 2016, stating that colonoscopy preparation medications should be covered at no cost, many health plans are still not compliant.
At the nonfederal level, Jones noted, Kentucky, which has a significant high-risk population, recently became the first state to pass legislation requiring health benefit plans to cover all guideline-recommended CRC exams and lab tests.
For its part, AGA has also called on payers to eliminate all cost-sharing barriers across the CRC screening continuum.
Of note, the study authors said, the higher compliance with the ACA mandate in commercial and Medicaid plans than in Medicare highlights disparities that may disproportionately affect vulnerable older adults. While nearly half of commercial patients and nearly three quarters of Medicaid patients incurred zero out-of-pocket costs, fewer than 17% of Medicare beneficiaries, or 1 in 6, did so.
Although these costs may be low relative to the colonoscopy, they nevertheless can deter uptake of preventive screenings, potentially leading to higher CRC incidence and mortality. “While some patients may be willing to pay modest out-of-pocket costs, any required payment, however small, can serve as a barrier to preventative care, particularly in underserved populations,” they wrote. “These financial barriers will continue to contribute to widening disparities and hinder progress toward equitable screening outcomes.”
In the meantime, said Shah, “Physicians should advocate now to their representatives in Congress that bowel prep costs should already be covered as part of the ACA.”
This study was funded by Sebela Pharmaceuticals, maker of SUFLAVE preparation. The authors had no conflicts of interest to declare. Jones is a speaker and consultant for Grail LLC.
A version of this article appeared on Medscape.com.
, a large insurance-claims analysis in Gastroenterology reported.
Moreover, this cost-sharing contravenes the preventive-care provisions for bowel preparation mandated by the Affordable Care Act (ACA).
Led by Gastroenterologist Eric D. Shah, MD, MBA, a clinical associate professor at the University of Michigan in Ann Arbor, Michigan, the study found a significant proportion of prescribed bowel preparation claims — 53% for commercial plans and 83% for Medicare — still involve patient cost-sharing, indicating noncompliance with ACA guidelines. Although expense-sharing was less prevalent among Medicaid claims (just 27%), it was not eliminated, suggesting room for improvement in coverage enforcement across the board.
“Colon cancer is unique in that it can be prevented with colonoscopy, but where are the patients? Bowel prep is a major reason that patients defer screening,” Shah told GI & Hepatology News. He said his group was quite surprised that the majority in the study cohort were paying something out of pocket when these costs should have been covered. “Primary care doctors may not think to ask about bowel prep costs when they order screening colonoscopies.”
The findings emerged from an analysis of 2,593,079 prescription drug claims: 52.9% from commercial plans, 35% from Medicare Part D plans, and 8.3% from Medicaid plans.
“These patient costs of $30 or $50 are a real not a theoretical deterrent,” said Whitney Jones, MD, a gastroenterologist, adjunct clinical professor at the University of Louisville in Louisville, Kentucky, and founder of the nonprofit Colon Cancer Prevention Project. Jones was not involved in the analysis. “Some insurers require prior patient authorization for the low-dose preps, but gastroenterologists are doing so many colonoscopies they don’t always have time to get a PA [prior authorization] on everyone.”
With the increasing use of blood and stool-based CRC testing, he added, “when you get a positive result, it’s really important to have the procedure quickly.” And appropriate bowel preparation is a small, cost-effective portion of the total costs of colonoscopy, a procedure that ultimately saves insurers significant money in treatment costs.
The authors noted that while CRC is the second-leading cause of cancer-related deaths in the US, screening rates remain low, with only 59% of adults aged 45 years or older up to date with screening. Screening rates are particularly low among racial and ethnic minority groups as compared with White individuals, a disparity that highlights the need to address existing barriers and enhance screening efforts.
In the current study, shared costs by bowel preparation volume also varied. Low-volume formulations had consistently higher out-of-pocket costs: a median of $60 for low-volume vs $10 for high-volume in commercial plans. In Medicare, 75% of high-volume claims had shared costs compared with 90% for their low-volume counterparts. The cost-sharing difference was slightly narrower with Medicaid: 27% of high-volume claims vs 30% of low-volume claims.
This is concerning, as low-volume options, which are preferred by patients for their better tolerability, can enhance uptake and adherence and improve colonoscopy outcomes. Shah advises physicians to consider prescribing low-volume preparations. “Let patients know about the potential out-of-pocket cost and about copay cards and assistance programs and use high-volume preps as an alternative rather than a go-to,” he said.
As to costs across insurance types, among commercial plans, the median nonzero out-of-pocket cost was $10 for high-volume and $60 for low-volume product claims. For Medicare, the median nonzero out-of-pocket cost was $8 for high-volume and $55.99 for low-volume products.
Under the ACA, CRC screening is classified as a recommended preventive service, requiring health plans to cover it without cost-sharing. Although the Centers for Medicare & Medicaid Services previously tried to enforce this mandate in 2015 and 2016, stating that colonoscopy preparation medications should be covered at no cost, many health plans are still not compliant.
At the nonfederal level, Jones noted, Kentucky, which has a significant high-risk population, recently became the first state to pass legislation requiring health benefit plans to cover all guideline-recommended CRC exams and lab tests.
For its part, AGA has also called on payers to eliminate all cost-sharing barriers across the CRC screening continuum.
Of note, the study authors said, the higher compliance with the ACA mandate in commercial and Medicaid plans than in Medicare highlights disparities that may disproportionately affect vulnerable older adults. While nearly half of commercial patients and nearly three quarters of Medicaid patients incurred zero out-of-pocket costs, fewer than 17% of Medicare beneficiaries, or 1 in 6, did so.
Although these costs may be low relative to the colonoscopy, they nevertheless can deter uptake of preventive screenings, potentially leading to higher CRC incidence and mortality. “While some patients may be willing to pay modest out-of-pocket costs, any required payment, however small, can serve as a barrier to preventative care, particularly in underserved populations,” they wrote. “These financial barriers will continue to contribute to widening disparities and hinder progress toward equitable screening outcomes.”
In the meantime, said Shah, “Physicians should advocate now to their representatives in Congress that bowel prep costs should already be covered as part of the ACA.”
This study was funded by Sebela Pharmaceuticals, maker of SUFLAVE preparation. The authors had no conflicts of interest to declare. Jones is a speaker and consultant for Grail LLC.
A version of this article appeared on Medscape.com.
FROM GASTROENTEROLOGY
Journal Highlights: May-July 2025
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Esophagus/Motility
Nguyen AD, et al. AGA Clinical Practice Update on Incorporating Functional Lumen Imaging Probe Into Esophageal Clinical Practice: Expert Review. Gastroenterology. 2025 Jul. doi: 10.1053/j.gastro.2025.05.011.
Hartnett DA, et al. Distribution of Esophageal Eosinophilia as a Predictor of Proton Pump Inhibitor Response in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.06.032.
Gyawali CP, et al. pH Impedance Monitoring on Proton Pump Inhibitor Therapy Impacts Management Decisions in Proven GERD but not in Unproven GERD. Clin Gastroenterol Hepatol. 2025 May. doi: 10.1016/j.cgh.2025.02.032.
Stomach
Wiklund AK, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Feb. doi: 10.1053/j.gastro.2025.01.239.
Sonaiya S, et al. Over-the-Scope Clip versus Standard Endoscopic Therapy as First-Line Intervention for Nonvariceal Upper Gastrointestinal Bleeding: A Cost-Effectiveness Analysis. Tech Innov Gastrointest. 2025 Jun. doi: 10.1016/j.tige.2025.250935.
Colon
Hassan C, et al. Colon Cancer Screening, Surveillance, and Treatment: Novel Artificial Intelligence Driving Strategies in the Management of Colon Lesions. Gastroenterology. 2025 Mar. doi: 10.1053/j.gastro.2025.02.021.
Pancreas
Wilcox CM, et al; US Pancreatic Disease Study Group. Management of the Disconnected Pancreatic Duct in Pancreatic Necrosis. Clin Gastroenterol Hepatol. 2025 Jul. doi: 10.1016/j.cgh.2025.05.024.
Ghimire C, et al. The effect of advances in pancreatic cancer treatment in population mortality: A SEER-based study. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100739.
Hepatology
Canivet CM, et al. Validation of the AASLD/EASL Multi-Step Screening Strategies for MASLD. Gastro Hep Adv. 2025 Jul. doi: 10.1016/j.gastha.2025.100747.
Miscellaneous
Chang L, et al. Gut Feelings: The Critical Role of Interoception in Obesity and Disorders of Gut-Brain Interaction. Gastroenterology. 2025 Aug. doi: 10.1053/j.gastro.2025.04.002.
Bashiri K, et al. Advancing Hemostatic Powder Technologies for Management of Gastrointestinal Bleeding: Challenges and Solutions. Tech Innov Gastrointest. 2025 Jul. doi: 10.1016/j.tige.2025.250940.
Dr. Trieu is assistant professor of medicine, interventional endoscopy, in the Division of Gastroenterology at Washington University in St. Louis School of Medicine, Missouri.
Follow-Up Colonoscopies Low After Blood-Based Screening
Evolving Standards of Practice: Esophageal Varices and Barrett’s Esophagus
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.