User login
Blood test detects colon cancer in single-center study
Blood assay studied for colorectal cancer screening.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
Blood assay studied for colorectal cancer screening.
Blood assay studied for colorectal cancer screening.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
A blood test detected 11 of 11 cases of colorectal cancer in a study involving 354 patients, and also spotted a majority of cases – 40 out of 53 – in which participants had advanced adenomas, an investigator said.
Results from a single-center study of CellMax Life’s FirstSight blood test were released as a poster as part of the annual Digestive Disease Week®, which was canceled because of COVID-19.
For a study conducted at one site, the Veterans Affairs Palo Alto (Calif.) Healthcare System, Shai Friedland, MD, and colleagues recruited 354 patients between ages 45 and 80 who were scheduled for elective colonoscopy. The researchers excluded people with a personal history of cancer or inflammatory bowel disease. They used CellMax’s FirstSight test on blood samples from the study participants.
The FirstSight test result was positive for colorectal cancer in all 11 patients in the study who were found by colonoscopy to have this condition, said Dr. Friedland, who is a professor of medicine at Stanford (Calif.) University and chief of gastroenterology at the VA Palo Alto Healthcare System. Thus, the test showed a sensitivity of 100% in this instance.
Among the 53 study participants found by colonoscopy to have advanced adenoma, 40 were positive on FirstSight; thus, so the test has a sensitivity of 75.5% for this result.
Among 79 patients who had negative colonoscopy results, meaning they were judged free of cancer or polyps, the test showed 8 as having signs of disease or growths.
“If you had a large adenoma that was removed years ago and now you have a negative colonoscopy, your score might still be high,” Dr. Friedland said in a recorded presentation for DDW. “In other words, the changes that are detectable in your blood might persist even after the polypectomy.”
He said there are plans to soon start a large-scale multicenter study of the CellMax assay.
“The blood test has the potential to fill an unmet need by giving patients a highly sensitive convenient option for colorectal cancer screening,” he said.
CellMax already is seeking to position its test as a more convenient alternative to either colonoscopy or the Cologuard screening test. Many patients put off cancer screening because of the need to take time off from work and the invasive nature of colonoscopy. Exact Sciences has used direct-to-consumer advertising to promote its Cologuard home-based test as a more convenient alternative to colonoscopy, but its product requires patients to collect their own stool samples and mail them to a lab, a process many people find off-putting.
Public health advocates, including the U.S. Preventive Services Task Force (USPSTF), have for years been pressing for wider screening of American adults for colon cancer. USPSTF is in the midst of updating its recommendations on colon cancer. In announcing its latest update of these recommendations in 2016, USPSTF said “the best screening test is the one that gets done” (JAMA. 2016;315[23]:2564-75).
USPSTF pressed for maximizing the total proportion of the eligible population, a point Dr. Friedland echoed in a CellMax press release.
“For colon cancer screening to be most effective, it is essential to detect precancerous polyps and then perform a colonoscopy to remove the polyps,” said Dr. Friedland in the CellMax press release. “Giving patients the option of getting a blood test for screening would undoubtedly increase compliance and thereby reduce mortality from colorectal cancer.”
In the DDW presentation, Dr. Friedland and colleagues also said the CellMax test showed greater sensitivity (100%) for colorectal cancer and advanced precancerous lesions (75.5%) than did Cologuard (92.3% for colorectal cancer and 42.4% for advanced precancerous lesions).
Cara Connelly, Director of Public Relations and Corporate Communications for Exact Sciences said that the company “is dedicated to getting more people screened for colorectal cancer and applaud the researchers for their efforts. We look forward to hearing more about the performance of this test in a prospective multisite study with nonsymptomatic patients.”
Naresh T. Gunaratnam, MD, a gastroenterologist and research director at Huron Gastro in Ypsilanti, Mich., said he is concerned that aggressive promotion of alternative tests may obscure the benefits of colonoscopy. Dr. Gunaratnam, a 2019 winner of the American Gastroenterological Association (AGA) Distinguished Clinician Award, has been a public critic of the marketing of colon cancer tests, which emphasize the convenience of these products. When asked by MDedge to comment on the CellMax-funded study, Dr. Gunaratnam said alternative tests do have a place for the care of patients who cannot or will not have a colonoscopy.
“But if you convince a patient who would be willing to have a colonoscopy not to, that’s a disservice,” he said.
“If you want the best test, the one that is best at finding cancers and finding polyps and the only one that can remove the polyp, that’s colonoscopy,” Dr. Gunaratnam added. “One day there may be a pill you can swallow that blows up the polyps, but we’re not there yet. We have to mechanically remove them.”
SOURCE: Friedland S et al. DDW 2020, eposter 575.
FROM DDW 2020
Performance of the Veterans Choice Program for Improving Access to Colonoscopy at a Tertiary VA Facility
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
In April 2014, amid concerns for long wait times for care within the US Department of Veterans Affairs (VA) Veterans Health Administration (VHA), the Veterans Access, Choice, and Accountability Act was signed into law. This included the Veterans Choice Program (VCP), which included a provision for veterans to be referred outside of the VA to the community for care if their nearest VHA facility could not provide the requested care within 30 days of the clinically indicated date.1 Since implementation of the VCP, both media outlets and policy researchers have raised concerns about both the timeliness and quality of care provided through this program.2-4
Specifically for colonoscopy, referral outside of the VA in the pre-VCP era resulted in lower adenoma detection rate (ADR) and decreased adherence to surveillance guidelines when compared with matched VA control colonoscopies, raising concerns about quality assurance.5 Colorectal cancer (CRC) screening and timely colonoscopy is a VA priority; however, the performance of the VCP for colonoscopy timelines and quality has not been examined in detail.
Methods
We identified 3,855 veterans at the VA Pittsburgh Healthcare System (VAPHS) who were referred for colonoscopy in the community by using VCP from June 2015 through May 2017, using a query for colonoscopy procedure orders within the VA Corporate Data Warehouse. A total of 190 patients had a colonoscopy completed in the community by utilizing the VCP during this time frame.
At VAPHS, veterans who are referred for colonoscopy are contacted by a scheduler. The scheduler contacts the patient and offers the first available colonoscopy date at VAPHS and schedules the procedure for this date. However, if this date is > 30 days from the procedure order date, the scheduler gives the veteran the option of being contacted by VCP to schedule a colonoscopy within the community (Figure 1). We measured the time interval from the date of the initially scheduled first available colonoscopy at VAPHS to the date the colonoscopy was actually performed through VCP.
Quality assurance also was assessed by checking for the availability of records of colonoscopies performed through the VCP in the VA electronic health record (EHR) system. Colonoscopy procedure reports also were reviewed to assess for documentation of established colonoscopy quality metrics for examinations performed through the VCP. Additionally, we reviewed records scanned into the VA EHR pertaining to the VCP colonoscopy, including pathology information and pre- or postvisit records if available.
Data extraction was initiated in November 2017 to allow for at least 6 months of lead time for outside health records from the community to be received and scanned into the EHR for the veteran at VAPHS. For colonoscopy quality metrics, we chose 3 metrics that are universally documented for all colonoscopy procedures performed at VAPHS: quality of bowel preparation, cecal withdrawal time, and performance of retroflexion in the rectum. Documentation of these quality metrics is recommended in gastroenterology practice guidelinesand/or required by VA national policy.6,7
We separately reviewed a sample of 350 of the 3,855 patients referred for colonoscopy through VCP at VAPHS during the same time period to investigate overall VCP utilization. This sample was representative at a 95% CI with 5% margin of error of the total and sampled from 2 high-volume referral months (October and November 2015) and 3 low-volume months (January, February, and March 2017). Detailed data were collected regarding the colonoscopy scheduling and VCP referral process, including dates of colonoscopy procedure request; scheduling within the VAPHS; scheduling through the VCP; and ultimately if, when, and where (VAPHS vs community) a veteran had a colonoscopy performed. Wait times for colonoscopy procedures performed at the VAPHS and those performed through the VCP were compared.
The institutional review board at VAPHS reviewed and approved this quality improvement study.
Statistical Analysis
For the 190 veterans who had a colonoscopy performed through VCP, a 1-sample Wilcoxon signed rank test was used with a null hypothesis that the median difference in days between first available VAPHS colonoscopy and community colonoscopy dates was 0. For the utilization sample of 350 veterans, an independent samples median test was used to compare the median wait times for colonoscopy procedures performed at the VA and those performed through VCP. IBM SPSS Version 25 was used for all statistical analysis.
Results
Of the 190 identified colonoscopies completed in the community utilizing VCP, scanned records could not be found for 29 procedures (15.3%) (Table). VCP procedures were performed a median 2 days earlier than the first available VAPHS procedure, but this difference was not statistically significant (P = .62) (Figure 2). Although 52% of colonoscopies occurred sooner through VCP than the initially scheduled VAPHS date, 44% were performed later, and there was wide variability in the difference between these dates, ranging from 49 days sooner to 165 days later.
Pathology results from VCP procedures for which tissue samples were obtained were absent in 11.9% (14 of 118) of procedures. There were no clear follow-up recommendations to referring VA health care providers in the 18% (29 of 161) of available procedure reports. In VCP procedures, documentation of selected quality metrics: bowel preparation, cecal withdrawal time, and rectal retroflexion, were deficient in 27.3%, 70.2%, and 32.9%, respectively (Figure 3).
The utilization dataset sample included 350 veterans who were offered a VCP colonoscopy because the first available VAPHS procedure could not be scheduled for > 30 days. Of these patients, 231 (66%) ultimately had their colonoscopy performed at VAPHS. An additional 26.6% of the patients in the utilization sample were lost in the scheduling process (ie, could not be contacted, cancelled and could not be rescheduled, or were a “no show” their scheduled VAPHS procedure). An unknown number of these patients may have had a procedure outside of the VA, but there are no records to confirm or exclude this possibility. Ultimately, there were only 26 (7.4%) confirmed VCP colonoscopy procedures within the utilization sample (Figure 4). The median actual wait time for colonoscopy was 61 days for VA procedures and 66 days for procedures referred through the VCP, which was not statistically significant (P = .15).
Discussion
This is the first study to evaluate the performance of the VCP for colonoscopy referrals. Consistent with recently reported data in other specialties, colonoscopy referrals through VCP did not lead to more timely procedures overall, although there was wide variation.8 The use of VCP for veteran referral to the community for colonoscopy led to fragmentation of care—with 15% of records for VCP colonoscopies unavailable in the VA EHR 6 months after the procedure. In addition, there were 45 pre- or postprocedure visits in the community, which is not standard practice at VAPHS, and therefore may add to the cost of care for veterans.
Documentation of selected colonoscopy quality metrics were deficient in 27.3% to 70.2% of available VCP procedure reports. Although many veterans were eligible for VCP referral for colonoscopy, only 7.4% had a documented procedure through VCP, and two-thirds of veterans eligible for VCP participation had their colonoscopy performed at the VAPHS, reflecting overall low utilization of the program.
The national average wait time for VCP referrals for multiple specialties was estimated to be 51 days in a 2018 Government Accountability Office (GAO) report, which is similar to our findings.9 The GAO report also concluded that the VCP does not have timeliness standards and notes missed opportunities to develop a mechanism for record transfer between the community and the VA. Our finding of missing colonoscopy procedure and pathology reports within the VA EHR is consistent with this claim. Our analysis revealed that widely accepted quality standards for colonoscopy, those that are required at the VA and monitored for quality assurance at the VAPHS, are not being consistently reported for veterans who undergo procedures in the community. Last, the overall low utilization rate, combined with overall similar wait times for colonoscopies referred through the VCP vs those done at the VA, should lead to reconsideration of offering community care referral to all veterans based solely on static wait time cutoffs.
Limitations
There are several limitations to our analysis. First, all data were extracted via chart review by one author; therefore, some scanned procedure or pathology reports or pre- and postprocedure records may have been missed. Second, these data are representative of a single VA medical center and may not reflect trends nationwide. Third, there are many factors that can influence veteran decision making regarding when and where colonoscopy procedures are performed, which could be related to the VCP community care referral process or independent of this. Finally, colonoscopies performed through the VCP are grouped and may not reflect variability in the performance of community practices that veterans were referred to though the VCP.
Adenoma detection rates (ADR) were not included in the assessment for 2 reasons. First, there was an insufficient number of screening colonoscopies to use for the ADR calculation. Second, a composite non-VA ADR of multiple community endoscopists in different practices would likely be inaccurate and not clinically meaningful. Of note, the VAPHS does calculate and maintain ADR information as a practice for its endoscopists.
Conclusions
Our findings are particularly important as the VA expands access to care in the community through the VA Mission Act, which replaces the VCP but continues to include a static wait time threshold of 28 days for referral to community-based care.10 Especially for colonoscopies with the indication of screening or surveillance, wait times > 28 days are likely not clinically significant. Additionally, this study demonstrates that there also are delays in access to colonoscopy by community-based care providers, and potentially reflects widespread colonoscopy access issues that are not unique to the VA.
Our findings are similar to other published results and reports and raise similar concerns about the pitfalls of veteran referral into the community, including (1) similar wait times for the community and the VA; (2) the risk of fragmented care; (3) unevenquality of care; and (4) low overall utilization of VCP for colonoscopy.11 We agree with the GAO’s recommendations, which include establishing clinically meaningful wait time thresholds, systemic monitoring of the timeliness of care, and additional mechanisms for seamless transfer of complete records of care into the VA system. If a referral is placed for community-based care, this should come with an expectation that the care will be offered and can be delivered sooner than would be possible at the VA. We additionally recommend that standards for reporting quality metrics, including ADR, also be required of community colonoscopy providers contracted to provide care for veterans through the VA Mission Act. Importantly, we recommend that data for comparative wait times and quality metrics for VA and the community should be publicly available for veterans so that they may make more informed choices about where they receive health care.
Acknowledgments
The authors thank Kaneen Allen, PhD, for her administrative assistance and guidance.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
1. Veterans Access, Choice, and Accountability Act of 2014. 42 USC §1395 (2014).
2. Farmer CM, Hosek SD. Did we improve veterans health care? It’s unclear. https://www.rand.org/blog/2016/05/did-we-improve-veterans-health-care-its-unclear.html. Published May 24, 2016. Accessed April 20, 2020.
3. Farmer CM, Hosek SD, Adamson DM. balancing demand and supply for veterans’ health care: a summary of three RAND assessments conducted under the Veterans Choice Act. Rand Health Q. 2016;6(1):12.
4. Mattocks KM, Mengeling M, Sadler A, Baldor R, Bastian L. The Veterans Choice Act: a qualitative examination of rapid policy implementation in the Department of Veterans Affairs. Med Care. 2017;55(suppl 7)(suppl 1):S71-S75.
5. Bartel MJ, Robertson DJ, Pohl H. Colonoscopy practice for veterans within and outside the Veterans Affairs setting: a matched cohort study. Gastrointest Endosc. 2016;84(2):272-278.
6. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110(1):72-90.
7. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1015, colorectal cancer screening. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3068.Published December 30, 2014. Accessed April 12, 2020.
8. Penn M, Bhatnagar S, Kuy S, et al. Comparison of wait times for new patients between the private sector and United States Department of Veterans Affairs medical centers. JAMA Netw Open. 2019;2(1):e187096.
9. US Government Accountability Office. Veterans Choice Program: improvements needed to address access-related challenges as VA plans consolidation of its community care programs. https://www.gao.gov/assets/700/692271.pdf. Published June 4, 2018. Accessed April 12, 2020.
10. VA Maintaining Internal Systems and Strengthening Integrated Outside Networks Act of 2018. 38 USC §1703 (2018).
11. Barnett PG, Hong JS, Carey E, Grunwald GK, Joynt Maddox K, Maddox TM. Comparison of accessibility, cost, and quality of elective coronary revascularization between Veterans Affairs and community care hospitals. JAMA Cardiol. 2018;3(2):133-141.
Testicular Swelling as an Initial Presentation of a Patient With Metastatic Gastric Cancer (FULL)
Gastric cancer is one of the most common cancers and the second most common cause of cancer-related death worldwide.1 Patients with an early-stage gastric cancer are often asymptomatic, and about 30% to 40% of patients present with distant metastases.2 The most common sites of metastasis are the liver, peritoneum, and the lymph nodes. In more advanced stages, gastric cancers spread to the lungs, brain, bones, soft tissues, and other sites. Krukenberg tumors are classic but rare occurrences of gastric cancer metastasis to ovaries in females. In men, it is rare for gastric cancer to metastasize to the testes. Only a few cases of testicular metastasis from gastric cancer have been reported in the literature.3-5
Primary testicular neoplasms typically present with unilateral testicular swelling. In rare instances, testicular swelling can be an initial presentation of a metastatic cancer. In this article, we report a man who initially presented with right testicular swelling and was eventually diagnosed with a secondary testicular cancer from an aggressive metastatic gastric adenocarcinoma.
Case Presentation
A 43-year-old male presented to his primary care physician with a 6-month history of right testicular swelling and unintentional weight loss of 33 pounds. An ultrasound of the scrotum and the testes showed a 4.5-cm hypoechoic and predominantly cystic mass in the right testis. Serum levels of β-human chorionic gonadotropin, α-feto protein and lactate dehydrogenase were within normal limits.
A primary testicular neoplasm was suspected. The patient underwent a right-sided inguinal orchiectomy. The pathology of the resected testis revealed an intestinal type adenocarcinoma with mucinous and signet-ring cell features (Figure).
A staging Fluorine-18 fluorodeoxyglucose positron emission tomography scan revealed extensive hypermetabolic peritoneal nodules consistent with peritoneal carcinomatosis. The patient started palliative chemotherapy with 5-fluorouracil and oxaliplatin but rapidly declined with progressive abdominal symptoms after completion of 2 cycles. He discontinued chemotherapy and eventually opted for supportive care focusing on comfort.
Discussion
Testicular neoplasm is the most common solid tumor in men aged 20 to 34 years in the US.6 More than 90% of testicular neoplasms are germ-cell tumors and originate from the testes.6 Secondary neoplasms of the testes from solid tumors are rare. In an autopsy series, secondary neoplasms from solid tumors represented 4.6% of all testicular neoplasms.7 The reported frequencies of primary sites of origin vary based on the report. In a relatively large series, the most common solid tumors of origin were the prostate (35%) and lungs (20%).8 Among hematologic malignancies, lymphomas may originate from or secondarily involve the testes. Primary testicular lymphomas account for approximately 5% of testicular neoplasms and commonly occur in men aged > 60 years.9 Testicular involvement of acute myeloid or lymphoid leukemia may happen at diagnosis, but relapses of acute leukemia in a testicular site are more common and well recognized.10,11
Our report shows a rare occurrence of a testicular swelling as an initial presentation of an aggressive metastatic gastric adenocarcinoma. When a patient presents with a testicular swelling, a testicular neoplasm is suspected based on the clinical examination, serum tumor markers, and ultrasonographic examination of the testes. Inguinal orchiectomy is the initial standard of care, and pathologic examination of a resected testis renders the confirmatory diagnosis. The overwhelming majority of these patients have primary testicular neoplasms, predominantly testicular germ-cell tumors. A small group may have atypical and rare tumors requiring additional workup to establish a diagnosis.
Metastatic spread of a solid tumor to a testicular site is rare. A testis is somewhat protected by the distinct anatomy that is offered by the blood-testis barrier. The tunica vaginalis, an external fibrous covering sheath of the testis, separates the testis from the peritoneal cavity and provides additional protection. Nevertheless, possible routes of secondary metastatic spread may include hematogenous and lymphovascular spread, cavitary dissemination and peritoneal seeding. Among gastric cancers, diffuse gastric cancer subtype is commonly associated with signet-ring cells. These cells lack adhesion and invade as single cells or small groups, leading to scattered tumor cells and a higher probability of seeding.
Our patient exhibited extensive peritoneal carcinomatosis, so peritoneal seeding likely played a dominant role in disseminating the cancer to the testis. It should be noted that possible primary sites of signet-ring cell metastatic adenocarcinomas are broad and may include the stomach and other GI and pancreaticobiliary sites, as well as the lung, bladder, breast, and gynecologic tract. Immunohistochemistry is often of limited value in establishing the primary site. Diagnosis is best established on clinical grounds, as was done in our patient.
When signet-ring cells are encountered in an orchiectomy specimen and a primary extratesticular site of origin cannot be identified, it is important to consider rare variants of primary testicular tumors in the differential diagnosis. A number of primary testicular tumors have signetring morphology. These include primary signetring cell stromal tumor of the testis (PSRST),12 seminoma with signet-ring cell predominance,13 and a primary signet-ring cell germ-cell carcinoma of the testes (PSRGCT).14
Seminoma and PSRST variants generally are differentiated from adenocarcinoma by a lack of mucin production and the absence of keratin expression. PSRGCT is considered a
malignant somatic-type transformation of a testicular germ-cell tumor and germ-cell clonality is established by abnormal 12p chromosome through fluorescent in situ hybridization testing.13 PSRST is a benign tumor with excellent prognosis. Seminoma variants with signet-ring cell predominance and PSRGCT are managed as germ-cell tumors and are also considered to have good outcome. In contrast, testicular involvements from metastatic adenocarcinomas, including gastric cancers, are managed with multidisciplinary approach, including systemic chemotherapy. Despite this, patients have a poor outcome with a median survival of about 1 year.2
Conclusion
Advanced gastric adenocarcinoma can metastasize to the testes, and patients may present with a testicular swelling as an initial presentation. It is important to differentiate secondary testicular cancers from rare variants of primary testicular tumors because the prognosis and management vary substantially.
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-527.
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654-2664.
3. Qazi HA, Manikandan R, Foster CS, Fordham MV. Testicular metastasis from gastric carcinoma. Urology. 2006;68(4):890.e7-e8.
4. Civelek B, Aksoy S, Kos T, et al. Isolated testicular metastasis of gastric cancer. J Gastrointest Canc. 2012;43(suppl 1):S64-S66.
5. Li B, Cai H, Kang ZC, et al. Testicular metastasis from gastric carcinoma: a case report. World J of Gastroenterol. 2015;21(21):6764-6768.
6. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: testicular cancer. https://seer.cancer.gov/statfacts/html/testis.html. Updated September 10, 2018. Accessed April 10, 2019.
7. Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology. 2000;37(4):323-331.
8. Haupt HM, Mann RB, Trump DL, Abeloff MD. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer.1984;54 (4):709-714.
9. Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. 2014;123(4):486-493.
10. Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016;40(11):1473-1483.
11. Kulkarni KP, Marwaha RK, Trehan A, Bansal D. Testicular relapse in childhood acute lymphoblastic leukemia: the challenges and lessons. Indian J Cancer. 2010;47(2):134-138.
12. Kuo CY, Wen MC, Wang J, Jan YJ. Signet-ring stromal tumor of the testis: a case report and literature review. Hum Pathol. 2009;40(4):584-587.
13. Ulbright TM, Young RH. Seminoma with conspicuous signet ring cells: a rare, previously uncharacterized morphologic variant. Am J Surg Pathol. 2008;32(8):1175-1181.
14. Williamson SR, Kum JB, Shah SR, et al. Signet ring cell carcinoma of the testis: clinicopathologic and molecular evidence for germ cell tumor origin—a case report. Am J
Surg Pathol. 2012;36(2):311-315.
Gastric cancer is one of the most common cancers and the second most common cause of cancer-related death worldwide.1 Patients with an early-stage gastric cancer are often asymptomatic, and about 30% to 40% of patients present with distant metastases.2 The most common sites of metastasis are the liver, peritoneum, and the lymph nodes. In more advanced stages, gastric cancers spread to the lungs, brain, bones, soft tissues, and other sites. Krukenberg tumors are classic but rare occurrences of gastric cancer metastasis to ovaries in females. In men, it is rare for gastric cancer to metastasize to the testes. Only a few cases of testicular metastasis from gastric cancer have been reported in the literature.3-5
Primary testicular neoplasms typically present with unilateral testicular swelling. In rare instances, testicular swelling can be an initial presentation of a metastatic cancer. In this article, we report a man who initially presented with right testicular swelling and was eventually diagnosed with a secondary testicular cancer from an aggressive metastatic gastric adenocarcinoma.
Case Presentation
A 43-year-old male presented to his primary care physician with a 6-month history of right testicular swelling and unintentional weight loss of 33 pounds. An ultrasound of the scrotum and the testes showed a 4.5-cm hypoechoic and predominantly cystic mass in the right testis. Serum levels of β-human chorionic gonadotropin, α-feto protein and lactate dehydrogenase were within normal limits.
A primary testicular neoplasm was suspected. The patient underwent a right-sided inguinal orchiectomy. The pathology of the resected testis revealed an intestinal type adenocarcinoma with mucinous and signet-ring cell features (Figure).
A staging Fluorine-18 fluorodeoxyglucose positron emission tomography scan revealed extensive hypermetabolic peritoneal nodules consistent with peritoneal carcinomatosis. The patient started palliative chemotherapy with 5-fluorouracil and oxaliplatin but rapidly declined with progressive abdominal symptoms after completion of 2 cycles. He discontinued chemotherapy and eventually opted for supportive care focusing on comfort.
Discussion
Testicular neoplasm is the most common solid tumor in men aged 20 to 34 years in the US.6 More than 90% of testicular neoplasms are germ-cell tumors and originate from the testes.6 Secondary neoplasms of the testes from solid tumors are rare. In an autopsy series, secondary neoplasms from solid tumors represented 4.6% of all testicular neoplasms.7 The reported frequencies of primary sites of origin vary based on the report. In a relatively large series, the most common solid tumors of origin were the prostate (35%) and lungs (20%).8 Among hematologic malignancies, lymphomas may originate from or secondarily involve the testes. Primary testicular lymphomas account for approximately 5% of testicular neoplasms and commonly occur in men aged > 60 years.9 Testicular involvement of acute myeloid or lymphoid leukemia may happen at diagnosis, but relapses of acute leukemia in a testicular site are more common and well recognized.10,11
Our report shows a rare occurrence of a testicular swelling as an initial presentation of an aggressive metastatic gastric adenocarcinoma. When a patient presents with a testicular swelling, a testicular neoplasm is suspected based on the clinical examination, serum tumor markers, and ultrasonographic examination of the testes. Inguinal orchiectomy is the initial standard of care, and pathologic examination of a resected testis renders the confirmatory diagnosis. The overwhelming majority of these patients have primary testicular neoplasms, predominantly testicular germ-cell tumors. A small group may have atypical and rare tumors requiring additional workup to establish a diagnosis.
Metastatic spread of a solid tumor to a testicular site is rare. A testis is somewhat protected by the distinct anatomy that is offered by the blood-testis barrier. The tunica vaginalis, an external fibrous covering sheath of the testis, separates the testis from the peritoneal cavity and provides additional protection. Nevertheless, possible routes of secondary metastatic spread may include hematogenous and lymphovascular spread, cavitary dissemination and peritoneal seeding. Among gastric cancers, diffuse gastric cancer subtype is commonly associated with signet-ring cells. These cells lack adhesion and invade as single cells or small groups, leading to scattered tumor cells and a higher probability of seeding.
Our patient exhibited extensive peritoneal carcinomatosis, so peritoneal seeding likely played a dominant role in disseminating the cancer to the testis. It should be noted that possible primary sites of signet-ring cell metastatic adenocarcinomas are broad and may include the stomach and other GI and pancreaticobiliary sites, as well as the lung, bladder, breast, and gynecologic tract. Immunohistochemistry is often of limited value in establishing the primary site. Diagnosis is best established on clinical grounds, as was done in our patient.
When signet-ring cells are encountered in an orchiectomy specimen and a primary extratesticular site of origin cannot be identified, it is important to consider rare variants of primary testicular tumors in the differential diagnosis. A number of primary testicular tumors have signetring morphology. These include primary signetring cell stromal tumor of the testis (PSRST),12 seminoma with signet-ring cell predominance,13 and a primary signet-ring cell germ-cell carcinoma of the testes (PSRGCT).14
Seminoma and PSRST variants generally are differentiated from adenocarcinoma by a lack of mucin production and the absence of keratin expression. PSRGCT is considered a
malignant somatic-type transformation of a testicular germ-cell tumor and germ-cell clonality is established by abnormal 12p chromosome through fluorescent in situ hybridization testing.13 PSRST is a benign tumor with excellent prognosis. Seminoma variants with signet-ring cell predominance and PSRGCT are managed as germ-cell tumors and are also considered to have good outcome. In contrast, testicular involvements from metastatic adenocarcinomas, including gastric cancers, are managed with multidisciplinary approach, including systemic chemotherapy. Despite this, patients have a poor outcome with a median survival of about 1 year.2
Conclusion
Advanced gastric adenocarcinoma can metastasize to the testes, and patients may present with a testicular swelling as an initial presentation. It is important to differentiate secondary testicular cancers from rare variants of primary testicular tumors because the prognosis and management vary substantially.
Gastric cancer is one of the most common cancers and the second most common cause of cancer-related death worldwide.1 Patients with an early-stage gastric cancer are often asymptomatic, and about 30% to 40% of patients present with distant metastases.2 The most common sites of metastasis are the liver, peritoneum, and the lymph nodes. In more advanced stages, gastric cancers spread to the lungs, brain, bones, soft tissues, and other sites. Krukenberg tumors are classic but rare occurrences of gastric cancer metastasis to ovaries in females. In men, it is rare for gastric cancer to metastasize to the testes. Only a few cases of testicular metastasis from gastric cancer have been reported in the literature.3-5
Primary testicular neoplasms typically present with unilateral testicular swelling. In rare instances, testicular swelling can be an initial presentation of a metastatic cancer. In this article, we report a man who initially presented with right testicular swelling and was eventually diagnosed with a secondary testicular cancer from an aggressive metastatic gastric adenocarcinoma.
Case Presentation
A 43-year-old male presented to his primary care physician with a 6-month history of right testicular swelling and unintentional weight loss of 33 pounds. An ultrasound of the scrotum and the testes showed a 4.5-cm hypoechoic and predominantly cystic mass in the right testis. Serum levels of β-human chorionic gonadotropin, α-feto protein and lactate dehydrogenase were within normal limits.
A primary testicular neoplasm was suspected. The patient underwent a right-sided inguinal orchiectomy. The pathology of the resected testis revealed an intestinal type adenocarcinoma with mucinous and signet-ring cell features (Figure).
A staging Fluorine-18 fluorodeoxyglucose positron emission tomography scan revealed extensive hypermetabolic peritoneal nodules consistent with peritoneal carcinomatosis. The patient started palliative chemotherapy with 5-fluorouracil and oxaliplatin but rapidly declined with progressive abdominal symptoms after completion of 2 cycles. He discontinued chemotherapy and eventually opted for supportive care focusing on comfort.
Discussion
Testicular neoplasm is the most common solid tumor in men aged 20 to 34 years in the US.6 More than 90% of testicular neoplasms are germ-cell tumors and originate from the testes.6 Secondary neoplasms of the testes from solid tumors are rare. In an autopsy series, secondary neoplasms from solid tumors represented 4.6% of all testicular neoplasms.7 The reported frequencies of primary sites of origin vary based on the report. In a relatively large series, the most common solid tumors of origin were the prostate (35%) and lungs (20%).8 Among hematologic malignancies, lymphomas may originate from or secondarily involve the testes. Primary testicular lymphomas account for approximately 5% of testicular neoplasms and commonly occur in men aged > 60 years.9 Testicular involvement of acute myeloid or lymphoid leukemia may happen at diagnosis, but relapses of acute leukemia in a testicular site are more common and well recognized.10,11
Our report shows a rare occurrence of a testicular swelling as an initial presentation of an aggressive metastatic gastric adenocarcinoma. When a patient presents with a testicular swelling, a testicular neoplasm is suspected based on the clinical examination, serum tumor markers, and ultrasonographic examination of the testes. Inguinal orchiectomy is the initial standard of care, and pathologic examination of a resected testis renders the confirmatory diagnosis. The overwhelming majority of these patients have primary testicular neoplasms, predominantly testicular germ-cell tumors. A small group may have atypical and rare tumors requiring additional workup to establish a diagnosis.
Metastatic spread of a solid tumor to a testicular site is rare. A testis is somewhat protected by the distinct anatomy that is offered by the blood-testis barrier. The tunica vaginalis, an external fibrous covering sheath of the testis, separates the testis from the peritoneal cavity and provides additional protection. Nevertheless, possible routes of secondary metastatic spread may include hematogenous and lymphovascular spread, cavitary dissemination and peritoneal seeding. Among gastric cancers, diffuse gastric cancer subtype is commonly associated with signet-ring cells. These cells lack adhesion and invade as single cells or small groups, leading to scattered tumor cells and a higher probability of seeding.
Our patient exhibited extensive peritoneal carcinomatosis, so peritoneal seeding likely played a dominant role in disseminating the cancer to the testis. It should be noted that possible primary sites of signet-ring cell metastatic adenocarcinomas are broad and may include the stomach and other GI and pancreaticobiliary sites, as well as the lung, bladder, breast, and gynecologic tract. Immunohistochemistry is often of limited value in establishing the primary site. Diagnosis is best established on clinical grounds, as was done in our patient.
When signet-ring cells are encountered in an orchiectomy specimen and a primary extratesticular site of origin cannot be identified, it is important to consider rare variants of primary testicular tumors in the differential diagnosis. A number of primary testicular tumors have signetring morphology. These include primary signetring cell stromal tumor of the testis (PSRST),12 seminoma with signet-ring cell predominance,13 and a primary signet-ring cell germ-cell carcinoma of the testes (PSRGCT).14
Seminoma and PSRST variants generally are differentiated from adenocarcinoma by a lack of mucin production and the absence of keratin expression. PSRGCT is considered a
malignant somatic-type transformation of a testicular germ-cell tumor and germ-cell clonality is established by abnormal 12p chromosome through fluorescent in situ hybridization testing.13 PSRST is a benign tumor with excellent prognosis. Seminoma variants with signet-ring cell predominance and PSRGCT are managed as germ-cell tumors and are also considered to have good outcome. In contrast, testicular involvements from metastatic adenocarcinomas, including gastric cancers, are managed with multidisciplinary approach, including systemic chemotherapy. Despite this, patients have a poor outcome with a median survival of about 1 year.2
Conclusion
Advanced gastric adenocarcinoma can metastasize to the testes, and patients may present with a testicular swelling as an initial presentation. It is important to differentiate secondary testicular cancers from rare variants of primary testicular tumors because the prognosis and management vary substantially.
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-527.
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654-2664.
3. Qazi HA, Manikandan R, Foster CS, Fordham MV. Testicular metastasis from gastric carcinoma. Urology. 2006;68(4):890.e7-e8.
4. Civelek B, Aksoy S, Kos T, et al. Isolated testicular metastasis of gastric cancer. J Gastrointest Canc. 2012;43(suppl 1):S64-S66.
5. Li B, Cai H, Kang ZC, et al. Testicular metastasis from gastric carcinoma: a case report. World J of Gastroenterol. 2015;21(21):6764-6768.
6. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: testicular cancer. https://seer.cancer.gov/statfacts/html/testis.html. Updated September 10, 2018. Accessed April 10, 2019.
7. Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology. 2000;37(4):323-331.
8. Haupt HM, Mann RB, Trump DL, Abeloff MD. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer.1984;54 (4):709-714.
9. Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. 2014;123(4):486-493.
10. Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016;40(11):1473-1483.
11. Kulkarni KP, Marwaha RK, Trehan A, Bansal D. Testicular relapse in childhood acute lymphoblastic leukemia: the challenges and lessons. Indian J Cancer. 2010;47(2):134-138.
12. Kuo CY, Wen MC, Wang J, Jan YJ. Signet-ring stromal tumor of the testis: a case report and literature review. Hum Pathol. 2009;40(4):584-587.
13. Ulbright TM, Young RH. Seminoma with conspicuous signet ring cells: a rare, previously uncharacterized morphologic variant. Am J Surg Pathol. 2008;32(8):1175-1181.
14. Williamson SR, Kum JB, Shah SR, et al. Signet ring cell carcinoma of the testis: clinicopathologic and molecular evidence for germ cell tumor origin—a case report. Am J
Surg Pathol. 2012;36(2):311-315.
1. Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505-527.
2. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654-2664.
3. Qazi HA, Manikandan R, Foster CS, Fordham MV. Testicular metastasis from gastric carcinoma. Urology. 2006;68(4):890.e7-e8.
4. Civelek B, Aksoy S, Kos T, et al. Isolated testicular metastasis of gastric cancer. J Gastrointest Canc. 2012;43(suppl 1):S64-S66.
5. Li B, Cai H, Kang ZC, et al. Testicular metastasis from gastric carcinoma: a case report. World J of Gastroenterol. 2015;21(21):6764-6768.
6. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: testicular cancer. https://seer.cancer.gov/statfacts/html/testis.html. Updated September 10, 2018. Accessed April 10, 2019.
7. Dutt N, Bates AW, Baithun SI. Secondary neoplasms of the male genital tract with different patterns of involvement in adults and children. Histopathology. 2000;37(4):323-331.
8. Haupt HM, Mann RB, Trump DL, Abeloff MD. Metastatic carcinoma involving the testis. Clinical and pathologic distinction from primary testicular neoplasms. Cancer.1984;54 (4):709-714.
9. Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. 2014;123(4):486-493.
10. Kawamoto K, Miyoshi H, Yoshida N, Takizawa J, Sone H, Ohshima K. Clinicopathological, cytogenetic, and prognostic analysis of 131 myeloid sarcoma patients. Am J Surg Pathol. 2016;40(11):1473-1483.
11. Kulkarni KP, Marwaha RK, Trehan A, Bansal D. Testicular relapse in childhood acute lymphoblastic leukemia: the challenges and lessons. Indian J Cancer. 2010;47(2):134-138.
12. Kuo CY, Wen MC, Wang J, Jan YJ. Signet-ring stromal tumor of the testis: a case report and literature review. Hum Pathol. 2009;40(4):584-587.
13. Ulbright TM, Young RH. Seminoma with conspicuous signet ring cells: a rare, previously uncharacterized morphologic variant. Am J Surg Pathol. 2008;32(8):1175-1181.
14. Williamson SR, Kum JB, Shah SR, et al. Signet ring cell carcinoma of the testis: clinicopathologic and molecular evidence for germ cell tumor origin—a case report. Am J
Surg Pathol. 2012;36(2):311-315.
Cardiovascular Effects of Tyrosine Kinase Inhibitors in Patients With Advanced Renal Cell Carcinoma at the VA San Diego Healthcare System (FULL)
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
Treating rectal cancer in the COVID-19 era: Expert guidance
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
As the COVID-19 pandemic continues, minimizing risks of infection to patients with cancer while maintaining good outcomes remains a priority. An international panel of experts has now issued recommendations for treating patients with rectal cancer, which includes using a short pre-operative course of radiotherapy (SCRT) and then delaying surgery.
Using SCRT translates to fewer hospital appointments, which will keep patients safer and allow them to maintain social distancing. The panel also found that surgery can be safely delayed by up to 12 weeks, and thus will allow procedures to be rescheduled after the pandemic peaks.
“The COVID-19 pandemic is a global emergency and we needed to work very quickly to identify changes that would benefit patients,” said David Sebag-Montefiore, MD, a professor of clinical oncology at the University of Leeds and honorary clinical oncologist with the Leeds Teaching Hospitals NHS Trust, who led the 15 member panel. “Our recommendations were published 20 days after our first meeting.”
“This process normally takes many months, if not years,” he said in a statement.
The recommendations were published online April 2 in Radiotherapy and Oncology.
The panel used the European Society for Medical Oncology (ESMO) rectal cancer guidelines as a framework to describe these new recommendations.
Recommendations by Stage
The recommendations were categorized into four subgroups based on cancer stage.
Early stage
- The ESMO guidelines recommend total mesorectal excision (TME) surgery without pre-operative radiotherapy for most cases.
- Panel recommendation also strongly supports the use of TME without pre-operative radiotherapy.
Intermediate stage
- The ESMO guidelines recommend TME alone or combined with SCRT or conventional radiotherapy (CRT) if there is uncertainty that a good quality mesorectal excision can be achieved.
- The panel strongly recommends TME alone in regions where high quality surgery is performed. The use of radiotherapy in this subgroup requires careful discussion, as the benefits of preoperative radiotherapy are likely to be small. If radiotherapy is used, then the preferred option should be SCRT.
Locally advanced
- The ESMO guideline recommends either pre-operative SCRT or CRT.
- The panel strongly recommends the use of SCRT and notes two phase 3 trials have compared SCRT and CRT and showed comparable outcomes for local recurrence, disease-free survival, overall survival, and late toxicity. In the COVID-19 setting, the panel points out that SCRT has many advantages over CRT, namely that there is less acute toxicity, fewer treatments which translate to less travel and contact with other patients and staff, and a significantly reduced risk of COVID-19 infection during treatment.
Timing of surgery after SCRT
- The ESMO guideline does not have any recommendations as they were issued before the Stockholm III trial (Lancet Oncol. 2017;18:336-46).
- The panel notes that the use of SCRT and delaying surgery has advantages that can be beneficial in both routine clinical practice and the COVID-19 setting. Several clinical trials have recommended that surgery should be performed within 3-7 days of completing radiotherapy, but the Stockholm III trial reported no difference in outcomes when surgery was delayed. It compared surgery performed within 1 week versus 4-8 weeks following SCRT and there was no difference in any survival endpoints. In addition, a longer delay to surgery was associated with a reduction in post-operative and surgical morbidity although no differences in severe complications or re-operations.
Advanced subgroup
- The ESMO guidelines recommend the use of pre-operative CRT or SCRT followed by neoadjuvant chemotherapy. CRT should be given as a fluoropyrimidine (usually capecitabine) combined with radiotherapy of 45-50.4 Gy over 5-5.5 weeks. Adjuvant chemotherapy should be considered but there is wide international variation in its use.
- The panel recommends that two options be considered based on the current evidence. The first is pre-op CRT, which is the most established standard of care, with the duration of concurrent capecitabine chemotherapy limited to 5-5.5 weeks. The second option is SCRT with or without neoadjuvant chemotherapy. In this case, the duration of radiotherapy is substantially less and has advantages versus CRT. “We consider both options to be acceptable but note the advantages of using SCRT in the COVID-19 setting,” the authors write. “The decision to use neoadjuvant chemotherapy in option 2 will reflect the attitudes to neoadjuvant and adjuvant chemotherapy in each country, the assessment of the risk-benefit ratio, considering the risk factors for COVID-19 increased mortality, and the capacity and prioritization of chemotherapy delivery.”
Organ Preservation
Organ preservation is being increasingly considered when a complete clinical response is achieved after CRT or SCRT, the panel points out. “An organ preservation approach may be considered during the COVID-19 period providing that resources for an adequate surveillance including imaging and endoscopy are available to detect local failures that require salvage surgery,” they write.
This article first appeared on Medscape.com.
Colorectal cancer: Proposed treatment guidelines for the COVID-19 era
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer
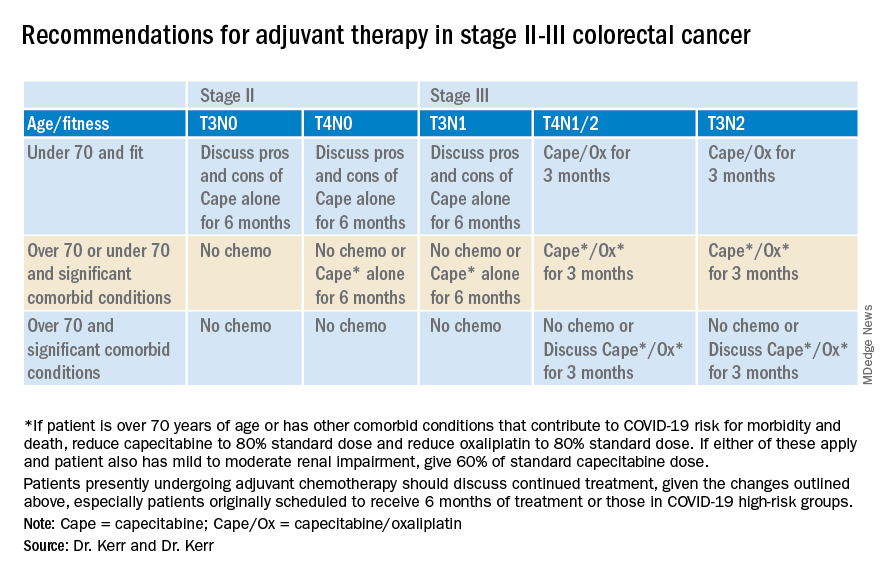
Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
In light of the rapid changes affecting cancer clinics due to the COVID-19 pandemic, Dr. David Kerr and Dr. Rachel Kerr, both specialists in gastrointestinal cancers at the University of Oxford in Oxford, United Kingdom, drafted these guidelines for the use of chemotherapy in colorectal cancer patients. Dr. Kerr and Dr. Kerr are putting forth this guidance as a topic for discussion and debate.
Our aim in developing these recommendations for the care of colorectal cancer patients in areas affected by the COVID-19 outbreak is to reduce the comorbidity of chemotherapy and decrease the risk of patients dying from COVID-19, weighed against the potential benefits of receiving chemotherapy. These recommendations are also designed to reduce the burden on chemotherapy units during a time of great pressure.
We have modified the guidelines in such a way that, we believe, will decrease the total number of patients receiving chemotherapy – particularly in the adjuvant setting – and reduce the overall immune impact of chemotherapy on these patients. Specifically, we suggest changing doublet chemotherapy to single-agent chemotherapy for some groups; changing to combinations involving capecitabine rather than bolus and infusional 5-FU for other patients; and, finally, making reasonable dose reductions upfront to reduce the risk for cycle 1 complications.
By changing from push-and-pump 5-FU to capecitabine for the vast majority of patients, we will both reduce the rates of neutropenia and decrease throughput in chemotherapy outpatient units, reducing requirements for weekly line flushing, pump disconnections, and other routine maintenance.
We continue to recommend the use of ToxNav germline genetic testing as a genetic screen for DPYD/ENOSF1 single-nucleotide polymorphisms (SNPs) to identify patients at high risk for fluoropyrimidine toxicity.
Use of biomarkers to sharpen prognosis should also be considered to refine therapeutic decisions.
Recommendations for stage II-III colorectal cancer

Recommendations for advanced colorectal cancer
Which regimen? Capecitabine/oxaliplatin should be the default backbone chemotherapy (rather than FOLFOX) in order to decrease the stress on infusion units.
Capecitabine plus irinotecan should be considered rather than FOLFIRI. However, in order to increase safety, reduce the dose of the capecitabine and the irinotecan, both to 80%, in all patient groups; and perhaps reduce the capecitabine dose further to 60% in those over the age of 70 or with significant comorbid conditions.
Treatment breaks. Full treatment breaks should be considered after 3 months of treatment in most patients with lower-volume, more indolent disease.
Treatment deintensification to capecitabine alone should be used in those with higher-volume disease (for example, more than 50% of liver replaced by tumor) at the beginning of treatment.
Deferring the start of any chemotherapy. Some older patients, or those with significant other comorbidities (that is, those who will be at increased risk for COVID-19 complications and death); who have low-volume disease, such as a couple of small lung metastases or a single liver metastasis; or who were diagnosed more than 12 months since adjuvant chemotherapy may decide to defer any chemotherapy for a period of time.
In these cases, we suggest rescanning at 3 months and discussing further treatment at that point. Some of these patients will be eligible for other interventions, such as resection, ablation, or stereotactic body radiation therapy. However, it will be important to consider the pressures on these other services during this unprecedented time.
Chemotherapy after resection of metastases. Given the lack of evidence and the present extenuating circumstances, we would not recommend any chemotherapy in this setting.
David J. Kerr, MD, CBE, MD, DSc, is a professor of cancer medicine at the University of Oxford. He is recognized internationally for his work in the research and treatment of colorectal cancer, and has founded three university spin-out companies: COBRA Therapeutics, Celleron Therapeutics, and Oxford Cancer Biomarkers. In 2002, he was appointed Commander of the British Empire by Queen Elizabeth. Rachel S. Kerr, MBChB, is a medical oncologist and associate professor of gastrointestinal oncology at the University of Oxford. She holds a UK Department of Health Fellowship, where she is clinical director of phase 3 trials in the oncology clinical trials office.
This article first appeared on Medscape.com.
ASCO guidelines take global view of late-stage colorectal cancer
Ideally, all cases of colorectal cancer would be detected at an early and curable stage, but, as new guidelines for late-stage colorectal cancer suggest, the world is far from perfect.
“Different regions of the world, both among and within countries, differ with respect to access to early detection,” the guideline authors wrote in JCO Global Oncology. “Many regions do not have mass or even opportunistic screening, and even within regions with mass screening, subpopulations may not have access to screening.”
The guidelines were developed by the American Society of Clinical Oncology’s Resource-Stratified Guidelines Advisory Group. Based on and adapted from existing guidelines developed by four international agencies, the ASCO guidelines take into account economic and social realities and offer recommendations for diagnosis, staging, and treatment by resource level: basic, limited, enhanced, or maximal.
“We made these guidelines to apply to countries or regions that have basic resources,” lead author E. Gabriela Chiorean, MD, of the University of Washington, Seattle, and the Seattle Cancer Care Alliance, said in an interview.
“We decided what should be the most basic resources – diagnostics, imaging, and treatment – that should be available to patients, and we make recommendations for the use of limited resources and supplies,” she added.
The guidelines pose and answer seven questions about optimal initial symptom management, diagnosis, and staging; optimal first and later lines of therapy; liver-directed therapy options for patients with late-stage colorectal cancer and liver metastases; and optimal on-treatment surveillance and follow-up strategies for patients treated for metastatic colorectal cancer.
For each question, the document offers guidance based on the availability of resources. As defined by the authors, the recommendations are stratified according to the following categories:
- Basic resources – “Core resources or fundamental services that are absolutely necessary for any cancer health care system to function.”
- Limited resources – “Second-tier resources or services that are intended to produce major improvements in outcome, such as increased survival and cost effectiveness, and are attainable with limited financial means and modest infrastructure.”
- Enhanced resources – “Third-tier resources or services that are optional but important; enhanced-level resources should produce further improvements in outcome and increase the number and quality of options and patient choice.”
- Maximal resources – “High-level/state-of-the art resources or services that may be used/available in some high-resource regions and/or may be recommended by high-resource setting guidelines that do not adapt to resource constraints but that nonetheless should be considered a lower priority than those resources or services listed in the other categories on the basis of extreme cost and/or impracticality for broad use in a resource-limited environment.”
The guidelines address common elements of symptom management for patients with acute disease, such as diagnosis involving the primary tumor, endoscopy when possible, and staging to include digital rectal exam and/or imaging when possible. The guidelines also include information tailored to resource level about chemotherapy and surgical resection.
“If, for example, a patient presents with bleeding and you suspect it to be of colorectal origin, we make recommendations that if the patient has symptoms of obstruction and bleeding and is resectable, they should undergo surgery, which should be available in countries of all resource levels,” Dr. Chiorean said.
The guidelines also recommend following the ASCO palliative care guidelines (J Clin Oncol. 2017 Jan;35[1]:96-112) for those patients who present with clinically unstable disease because of bowel obstruction, uncontrolled bleeding, or uncontrolled pain. Patients with clinically stable disease and ongoing bleeding from the primary tumor site are recommended to undergo transfusion and primary-site resection if only basic resources are available or transfusion plus multidisciplinary specialized evaluation when higher-level resources are available.
The ASCO guidelines are adapted from guidelines developed by Cancer Council Australia; the European Society for Medical Oncology; the National Institute for Health and Care Excellence, including separate recommendation for therapy combinations (https://www.nice.org.uk/guidance/ta212, https://www.nice.org.uk/guidance/ta439); and the National Comprehensive Cancer Network. Some of these guidelines have been updated since the creation of the ASCO guidelines.
ASCO funds the guideline development process. Dr. Chiorean and other authors disclosed relationships with multiple companies.
SOURCE: Chiorean EG et al. JCO Glob Oncol. 2020 Mar;6:414-38.
Ideally, all cases of colorectal cancer would be detected at an early and curable stage, but, as new guidelines for late-stage colorectal cancer suggest, the world is far from perfect.
“Different regions of the world, both among and within countries, differ with respect to access to early detection,” the guideline authors wrote in JCO Global Oncology. “Many regions do not have mass or even opportunistic screening, and even within regions with mass screening, subpopulations may not have access to screening.”
The guidelines were developed by the American Society of Clinical Oncology’s Resource-Stratified Guidelines Advisory Group. Based on and adapted from existing guidelines developed by four international agencies, the ASCO guidelines take into account economic and social realities and offer recommendations for diagnosis, staging, and treatment by resource level: basic, limited, enhanced, or maximal.
“We made these guidelines to apply to countries or regions that have basic resources,” lead author E. Gabriela Chiorean, MD, of the University of Washington, Seattle, and the Seattle Cancer Care Alliance, said in an interview.
“We decided what should be the most basic resources – diagnostics, imaging, and treatment – that should be available to patients, and we make recommendations for the use of limited resources and supplies,” she added.
The guidelines pose and answer seven questions about optimal initial symptom management, diagnosis, and staging; optimal first and later lines of therapy; liver-directed therapy options for patients with late-stage colorectal cancer and liver metastases; and optimal on-treatment surveillance and follow-up strategies for patients treated for metastatic colorectal cancer.
For each question, the document offers guidance based on the availability of resources. As defined by the authors, the recommendations are stratified according to the following categories:
- Basic resources – “Core resources or fundamental services that are absolutely necessary for any cancer health care system to function.”
- Limited resources – “Second-tier resources or services that are intended to produce major improvements in outcome, such as increased survival and cost effectiveness, and are attainable with limited financial means and modest infrastructure.”
- Enhanced resources – “Third-tier resources or services that are optional but important; enhanced-level resources should produce further improvements in outcome and increase the number and quality of options and patient choice.”
- Maximal resources – “High-level/state-of-the art resources or services that may be used/available in some high-resource regions and/or may be recommended by high-resource setting guidelines that do not adapt to resource constraints but that nonetheless should be considered a lower priority than those resources or services listed in the other categories on the basis of extreme cost and/or impracticality for broad use in a resource-limited environment.”
The guidelines address common elements of symptom management for patients with acute disease, such as diagnosis involving the primary tumor, endoscopy when possible, and staging to include digital rectal exam and/or imaging when possible. The guidelines also include information tailored to resource level about chemotherapy and surgical resection.
“If, for example, a patient presents with bleeding and you suspect it to be of colorectal origin, we make recommendations that if the patient has symptoms of obstruction and bleeding and is resectable, they should undergo surgery, which should be available in countries of all resource levels,” Dr. Chiorean said.
The guidelines also recommend following the ASCO palliative care guidelines (J Clin Oncol. 2017 Jan;35[1]:96-112) for those patients who present with clinically unstable disease because of bowel obstruction, uncontrolled bleeding, or uncontrolled pain. Patients with clinically stable disease and ongoing bleeding from the primary tumor site are recommended to undergo transfusion and primary-site resection if only basic resources are available or transfusion plus multidisciplinary specialized evaluation when higher-level resources are available.
The ASCO guidelines are adapted from guidelines developed by Cancer Council Australia; the European Society for Medical Oncology; the National Institute for Health and Care Excellence, including separate recommendation for therapy combinations (https://www.nice.org.uk/guidance/ta212, https://www.nice.org.uk/guidance/ta439); and the National Comprehensive Cancer Network. Some of these guidelines have been updated since the creation of the ASCO guidelines.
ASCO funds the guideline development process. Dr. Chiorean and other authors disclosed relationships with multiple companies.
SOURCE: Chiorean EG et al. JCO Glob Oncol. 2020 Mar;6:414-38.
Ideally, all cases of colorectal cancer would be detected at an early and curable stage, but, as new guidelines for late-stage colorectal cancer suggest, the world is far from perfect.
“Different regions of the world, both among and within countries, differ with respect to access to early detection,” the guideline authors wrote in JCO Global Oncology. “Many regions do not have mass or even opportunistic screening, and even within regions with mass screening, subpopulations may not have access to screening.”
The guidelines were developed by the American Society of Clinical Oncology’s Resource-Stratified Guidelines Advisory Group. Based on and adapted from existing guidelines developed by four international agencies, the ASCO guidelines take into account economic and social realities and offer recommendations for diagnosis, staging, and treatment by resource level: basic, limited, enhanced, or maximal.
“We made these guidelines to apply to countries or regions that have basic resources,” lead author E. Gabriela Chiorean, MD, of the University of Washington, Seattle, and the Seattle Cancer Care Alliance, said in an interview.
“We decided what should be the most basic resources – diagnostics, imaging, and treatment – that should be available to patients, and we make recommendations for the use of limited resources and supplies,” she added.
The guidelines pose and answer seven questions about optimal initial symptom management, diagnosis, and staging; optimal first and later lines of therapy; liver-directed therapy options for patients with late-stage colorectal cancer and liver metastases; and optimal on-treatment surveillance and follow-up strategies for patients treated for metastatic colorectal cancer.
For each question, the document offers guidance based on the availability of resources. As defined by the authors, the recommendations are stratified according to the following categories:
- Basic resources – “Core resources or fundamental services that are absolutely necessary for any cancer health care system to function.”
- Limited resources – “Second-tier resources or services that are intended to produce major improvements in outcome, such as increased survival and cost effectiveness, and are attainable with limited financial means and modest infrastructure.”
- Enhanced resources – “Third-tier resources or services that are optional but important; enhanced-level resources should produce further improvements in outcome and increase the number and quality of options and patient choice.”
- Maximal resources – “High-level/state-of-the art resources or services that may be used/available in some high-resource regions and/or may be recommended by high-resource setting guidelines that do not adapt to resource constraints but that nonetheless should be considered a lower priority than those resources or services listed in the other categories on the basis of extreme cost and/or impracticality for broad use in a resource-limited environment.”
The guidelines address common elements of symptom management for patients with acute disease, such as diagnosis involving the primary tumor, endoscopy when possible, and staging to include digital rectal exam and/or imaging when possible. The guidelines also include information tailored to resource level about chemotherapy and surgical resection.
“If, for example, a patient presents with bleeding and you suspect it to be of colorectal origin, we make recommendations that if the patient has symptoms of obstruction and bleeding and is resectable, they should undergo surgery, which should be available in countries of all resource levels,” Dr. Chiorean said.
The guidelines also recommend following the ASCO palliative care guidelines (J Clin Oncol. 2017 Jan;35[1]:96-112) for those patients who present with clinically unstable disease because of bowel obstruction, uncontrolled bleeding, or uncontrolled pain. Patients with clinically stable disease and ongoing bleeding from the primary tumor site are recommended to undergo transfusion and primary-site resection if only basic resources are available or transfusion plus multidisciplinary specialized evaluation when higher-level resources are available.
The ASCO guidelines are adapted from guidelines developed by Cancer Council Australia; the European Society for Medical Oncology; the National Institute for Health and Care Excellence, including separate recommendation for therapy combinations (https://www.nice.org.uk/guidance/ta212, https://www.nice.org.uk/guidance/ta439); and the National Comprehensive Cancer Network. Some of these guidelines have been updated since the creation of the ASCO guidelines.
ASCO funds the guideline development process. Dr. Chiorean and other authors disclosed relationships with multiple companies.
SOURCE: Chiorean EG et al. JCO Glob Oncol. 2020 Mar;6:414-38.
FROM JCO GLOBAL ONCOLOGY
Colorectal cancer burden rises in younger age groups
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
Current trends in the incidence and mortality of colorectal cancer (CRC) in the United States suggest CRC will become a disease that largely affects young and middle-aged adults, according to a report published in CA: A Cancer Journal for Clinicians.
As the second leading cause of cancer-related death in the United States, and with modifiable risk factors accounting for over 50% of cases and deaths, CRC is largely a preventable disease, explained study author Rebecca L. Siegel, of the American Cancer Society, and colleagues.
According to the investigators, CRC incidence dropped by 3.3% per year from 2011 through 2016 among individuals aged 65 years or older, but the opposite was observed for those aged 50-64 years, with rates increasing by 1% per year. The increase was even greater for those younger than 50 years, with an increase of 2.2% per year.
The CRC incidence from 2012 through 2016 was highest among Alaska Natives (89 cases per 100,000 persons) and lowest among Asian/Pacific Islanders (30 cases per 100,000 persons).
“CRC has been the most commonly diagnosed cancer in Alaska Natives since the early 1970s for reasons that are unknown but may include a higher prevalence of risk factors,” the investigators wrote.
The risk of developing CRC is related to several factors, including obesity, vitamin D deficiency, diabetes, smoking, and other dietary factors, the team further explained.
Among those aged 65 years or older, CRC death rates decreased by 3% per year from 2008 through 2017. For those aged 50-64 years, death rates dropped by 0.6% per year. In contrast, death rates rose by 1.3% per year for those younger than 50 years.
“The uptick in young adults, which is most rapid among non-Hispanic whites (2% per year), began around 2004 and was preceded by declines of 1% to 2% per year since at least 1975,” the investigators wrote.
The reduction in incidence and mortality among older adults is partially attributable to higher uptake of CRC screening. According to recent data, CRC screening rates were lower for those aged 50-64 years compared with individuals aged 65 years and older.
Based on current recommendations from the American Cancer Society, CRC screening should begin at age 45, with some higher-risk patients starting at age 40.
“Progress against CRC can be accelerated by increasing access to guideline-recommended screening and high quality treatment, particularly among Alaska Natives, and elucidating causes for rising incidence in young and middle-aged adults,” the investigators concluded.
The authors disclosed financial affiliations with the American Cancer Society, which funded the study, as well as Array Biopharma, Bayer, RGenix, Tesaro, and Seattle Genetics.
SOURCE: Siegel RL et al. CA Cancer J Clin. 2020 Mar 5. doi: 10.3322/caac.21601.
FROM CA: A CANCER JOURNAL FOR CLINICIANS
Sometimes medication is enough for a Crohn’s abscess
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
MAUI, HAWAII – If an intra-abdominal abscess in a recently diagnosed Crohn’s disease patient is less than 6 cm across with no downstream stenosis, involves only a short segment of bowel, and the patient has no perianal disease, then infliximab and azathioprine after drainage and antibiotics might be enough to heal it, according to Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at the Cleveland Clinic.
That will work in about 30% of patients who hit the mark; the rest will eventually need surgery, said Dr. Regueiro, a clinical researcher who has worked extensively with surgical GI patients and is also a coauthor on the American College of Gastroenterology 2018 Crohn’s disease guidelines (Am J Gastroenterol. 2018 Apr;113[4]:481-517).
Intra-abdominal abscesses are common in Crohn’s, usually from an inflammation-induced fistula or sinus in the small intestines that spills luminal contents into the abdominal cavity. Drainage and antibiotics are first line, but then there’s the question of who needs to go to the operating room and who doesn’t.
It has to do with “how much the hole in the intestines is actually reversible. Evidence of a stricture is of paramount importance. If you have a stricture below a fistula and prestenotic dilatation, that’s a high-pressure zone.” It’s a “fixed complication that, in my opinion, no medication is ever going to treat,” he said at the Gastroenterology Updates, IBD, Liver Disease Conference.
“Infliximab is still probably the best medicine for fistulizing disease,” so Dr. Regueiro opts for that if patients haven’t been on it before, in combination with an immunomodulator, generally azathioprine at half the standard dose, to prevent patients from forming antibodies to the infliximab.
When patients do go to the operating room, there is a good chance they will end up with a temporary ostomy, and definitely so if the abscess can’t be drained completely to prevent spillage. The risk of dehiscence and other complications is too great for primary anastomosis.
“I mentally prepare my patients for that; I tell them up front. I never guarantee that they are not going to have an ostomy bag,” Dr. Regueiro said.
He also said abscess formation isn’t necessarily a sign the biologic patients were on before has failed, especially if they were only on it for 6 months or so. More likely, “the disease was too far gone at that point” for short-term treatment to have much of an effect.
So he’s often likely to continue patients on the same biologic after surgery. “We’ve done a lot of study on” this and have “actually found that” patients do well with the approach. He will switch treatment, however, if they otherwise no longer seem to respond to a biologic they have been taking a while, despite adequate serum levels.
There’s no need to delay surgery for patients on biologics. “If they get a biologic the day before, they can still go to the [operating room]. We are not seeing increased postop complications, infections, or wound dehiscence,” he said.
Dr. Regueiro generally restarts biologics 2-4 weeks after surgery, which is enough time to know if there is going to be a surgical complication but not so long that patients will have a Crohn’s relapse. He restarts the maintenance dose, as “it’s not necessary to reinduct patients after such a short break,” he said.
He also noted that opioids and steroids should be avoided with Crohn’s abscesses. Opioids increase the risk of ileus, and steroids the risk of sepsis.
Dr. Regueiro reported no relevant disclosures.
EXPERT ANALYSIS FROM GUILD 2020
CRC task force updates colonoscopy follow-up guidance
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
The U.S. Multi-Society Task Force on Colorectal Cancer (CRC) recently updated recommendations for patient follow-up after colonoscopy and polypectomy.
The new guidance was based on advancements in both research and technology since the last recommendations were published in 2012, reported lead author Samir Gupta, MD, AGAF, of the University of California, San Diego, and colleagues.
“[Since 2012,] a number of articles have been published on risk of CRC based on colonoscopy findings and patient characteristics, as well as the potential impact of screening and surveillance colonoscopy on outcomes, such as incident CRC and polyps,” the investigators wrote in Gastroenterology. “Further, recent studies increasingly reflect the modern era of colonoscopy with more awareness of the importance of quality factors (e.g., adequate bowel preparation, cecal intubation, adequate adenoma detection, and complete polyp resection), and utilization of state of the art technologies (e.g., high-definition colonoscopes).”
The task force, which comprised the American College of Gastroenterology, the American Gastroenterological Association, and the American Society of Gastrointestinal Endoscopy, identified key topics using PICO (patient, intervention, comparison, and outcome) questions before conducting a comprehensive literature review that included 136 articles. Based on these findings, two task force members generated recommendations that were further refined through consensus discussion. The recommendations were copublished in the March issues of the American Journal of Gastroenterology, Gastroenterology, and Gastrointestinal Endoscopy.
According to Dr. Gupta and colleagues, some of the new recommendations, particularly those that advise less stringent follow-up, may encounter resistance from various stakeholders.
“Patients, primary care physicians, and colonoscopists may have concerns about lengthening a previously recommended interval, and will need to engage in shared decision making regarding whether to lengthen the follow-up interval based upon the guidance here or utilize the recommendation made at the time of the prior colonoscopy,” the task force wrote.
The most prominent recommendations of this kind concern patients who undergo removal of tubular adenomas less than 10 mm in size. For patients who have 1-2 of these adenomas removed, the task force now recommends follow-up after 7-10 years, instead of the previously recommended interval of 5-10 years.
“[This decision was] based on the growing body of evidence to support low risk for metachronous advanced neoplasia,” the task force wrote. “In this population, the risk for metachronous advanced neoplasia is similar to that for individuals with no adenoma. Importantly, the observed risk for fatal CRC among individuals with 1-10 adenomas less than 10 mm is lower than average for the general population.”
Along similar lines, patients who undergo removal of 3-4 small adenomas now have a recommended 3-5 year follow-up window, instead of the previously strict recommendation for follow-up at 3 years.
But not all of the new guidance is less stringent. While the task force previously recommended a follow-up period of less than 3 years after removal of more than 10 adenomas, they now recommend follow-up at 1 year. This change was made to simplify guidance, the investigators wrote, noting that the evidence base in this area “has not been markedly strengthened” since 2012.
Compared with the old guidance, the updated publication offers more detailed recommendations for follow-up after removal of serrated polyps. On this topic, 10 clinical scenarios are presented, with follow-up ranging from 6 months after piecemeal resection of a sessile serrated polyp greater than 20 mm to 10 years after removal of 20 or fewer hyperplastic polyps less than 10 mm that were located in the rectum or sigmoid colon. Incidentally, these two recommendations are strong and based on moderate evidence, whereas the remaining recommendations for serrated polyps are weak and based on very-low-quality evidence.
Because of such knowledge gaps, the investigators emphasized the need for more data. The publication includes extensive discussion of pressing research topics and appropriate methods of investigation.
“Our review highlights several opportunities for research to clarify risk stratification and management of patients post-polypectomy,” the task force wrote. “In order to optimize risk-reduction strategies, the mechanisms driving metachronous advanced neoplasia after baseline polypectomy and their relative frequency need to be better understood through studies that include large numbers of patients with interval cancers and/or advanced neoplasia after baseline polypectomy. Mechanisms may include new/incident growth, incomplete baseline resection, and missed neoplasia; each of these potential causes may require different interventions for improvement.”
The task force also suggested that some basic questions beyond risk stratification remain unanswered, such as the impact of surveillance on CRC incidence and mortality.
“Such evidence is needed given the increasing proportion of patients who are having adenomas detected as part of increased participation in CRC screening,” the task force wrote.
Other suggested topics of investigation include age-related analyses that incorporate procedural risk, cost-effectiveness studies, and comparisons of nonendoscopic methods of surveillance, such as fecal immunochemical testing.
The study was funded by the National Institutes of Health and the Department of Veterans Affairs. The investigators reported relationships with Covidien, Ironwood, Medtronic, and others.
SOURCE: Gupta S et al. Gastroenterology. 2020 Feb 7. doi: 10.1053/j.gastro.2019.10.026.
FROM GASTROENTEROLOGY














