User login
Cardiologist sues hospital, claims he was fired in retaliation
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
FDA tweaks Impella indications on basis of postapproval study
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has updated the Abiomed Impella RP System’s approved indications in a way that “better reflects the characteristics of the patients who may benefit the most from treatment with the device,” the agency has announced.
The revised language reflects the final results of a postapproval study in which survival rates for patients who met the premarket-study entry criteria were comparable to rates seen in the premarket studies, the FDA observed.
The postapproval study “further confirms that the device is safe and effective when used for the currently approved indication.” The indication’s added words, however, tighten the description of eligible patients in a way that more precisely reflects the premarket-study population.
The update states that the Impella RP System is “indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥ 1.5 m2, who develop acute right heart failure or decompensation for less than 48 hours following left ventricular assist device implantation, myocardial infarction, heart transplant, or open heart surgery, without the presence of profound shock, end organ failure, or acute neurologic injury.”
The FDA “believes that when the device is used for the currently approved indication in appropriately selected patients, the benefits of the Impella RP System continue to outweigh the risks.”
The reworded indication is the latest among several updates to the agency’s February 2019 letter to clinicians noting a signal of increased mortality associated with the Impella RP device in an interim analysis of the same postapproval study. Ultimately, no such signal has emerged among the subset of postapproval patients who would have been eligible for the premarket study.
A version of this article first appeared on Medscape.com.
In CABG, radial artery works best for second key graft: RAPCO at 15 years
Lower risk of MACE shown
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
Lower risk of MACE shown
Lower risk of MACE shown
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
CHICAGO – With more than 15 years of follow-up from two related trials, the best conduit for the second most important target vessel in coronary artery bypass grafting (CABG) appears to be resolved.
The radial artery (RA) graft is linked with a lower risk of major adverse cardiac events (MACE) relative to a saphenous vein (SV) or the free right internal thoracic artery (FRITA).
On the basis of these findings, “a radial artery graft should be considered in all isolated CABG operations unless there are contraindications,” reported David L. Hare, MBBS, director of research in the department of cardiology, University of Melbourne.
For the primary graft, there is general agreement that the left internal thoracic artery (LITA) is the first choice for the left anterior descending vessel, but the optimal graft for the second most important target has never been established, according to Dr. Hare.
Almost 25 years ago, two randomized controlled trials called RAPCO-RITA and RAPCO-SV were initiated to address the question. There is now 15 years of follow-up for both of the RAPCO (Radial Artery Patency and Clinical Outcomes) trials, which were presented together at the American Heart Association scientific sessions.
Two trials conducted simultaneously
The RAPCO-RITA trial randomized CABG patients less than 70 years of age (less than 60 years in those with diabetes) to grafting of the second target vessel with an RA or FRITA graft. The RAPCO-SV trial randomized those 70 years or older (60 years or older with diabetes) to an RA or SV graft.
The two primary endpoints were graft patency at 10 years and a composite MACE at 10 years. The assessment of the MACE endpoint, which consisted of cardiovascular mortality, acute myocardial infarction, and coronary revascularization, was later amended to include a comparison at 15 years.
Ten-year patency results, favoring the RA in both studies, were previously published in Circulation. In the new data presented at the meeting, the RA was associated with a significant reduction in MACE relative to the comparator graft in both studies.
“The main driver was a reduction in all-cause mortality,” Dr. Hare reported.
In RAPCO-RITA, 394 patients were randomized with follow-up data available for all but 1 patient at 15 years. Similarly, only 1 patient was lost to follow-up among the 225 randomized in RAPCO-SV. In both studies, baseline characteristics were well balanced.
MACE curves separate at 5 years
In RAPCO-RITA, the MACE survival curves began to separate at about 5 years and then gradually widened. By 15 years, the lower rate of MACE in the RA group (38% vs. 48%) translated into a 26% relative reduction (hazard ratio, 0.74; P = .04).
In RAPCO-SV, the pattern was similar, by 15 years, the rates of MACE were 60% and 73% for the RA and SV groups, respectively, translating into a 29% relative reduction (HR, 0.71; P = .04).
There was no heterogeneity in benefit across prespecified subgroups such as presence or absence of diabetes, gender, or age. In RAPCO-RITA, there was 8% absolute and 31% relative reduction in all-cause mortality. In RAPCO-SV, the absolute and relative reductions were 11% and 26%.
When the trial was initiated, Dr. Hare hypothesized that RITA would prove more durable than RA, so the outcome was not anticipated.
“This is the first randomized controlled trial program to address the question,” said Dr. Hare, who noted that there have been numerous retrospective and case control analyses that have produced mixed results in the past.
Discussant praises trial quality
The AHA-invited discussant, Marc Ruel, MD, chair of cardiac surgery, University of Ottawa (Ont.) Heart Institute, called these data “important,” and he congratulated Dr. Hare for conducting the first randomized trial to address the question about second graft durability.
However, he noted that, although the study was randomized, it was not blinded, and he questioned whether postoperative care, in particular, was similar. He also pointed out that the MACE rate seemed high, particularly among the older patients randomized in RAPCO-SV.
“All of the patients were referred to an independently run CABG rehab program that was quite separate from the trial but that provided identical mandated care,” Dr. Hare responded, indicating that there was no opportunity for differences in postprocedural management.
In the United States, the SV graft is often preferred on the basis of easy harvesting and handling characteristics, according to Dr. Hare, who estimated that fewer than 10% of the 200,000 CABG procedures performed in the United States employ the RA conduit for second target vessels. He believes the RAPCO trials data support a change.
“My personal view is [that, on the basis of] this data, given that it is from a controlled trial rather than from patient-level meta-analyses, all isolated CABG operations should be using a radial graft if it is suitable,” Dr. Hare said.
Dr. Hare reports financial relationships with Abbott, Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, CSL-Biotherapies, Lundbeck, Menarini, Merck, Novartis, Pfizer, Regeneron, Sanofi, Servier, and Vifor. Dr. Ruel reports financial relationships with Cryolife, Edwards, and Medtronic.
AT AHA 2022
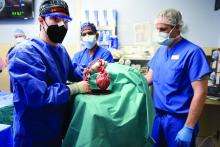
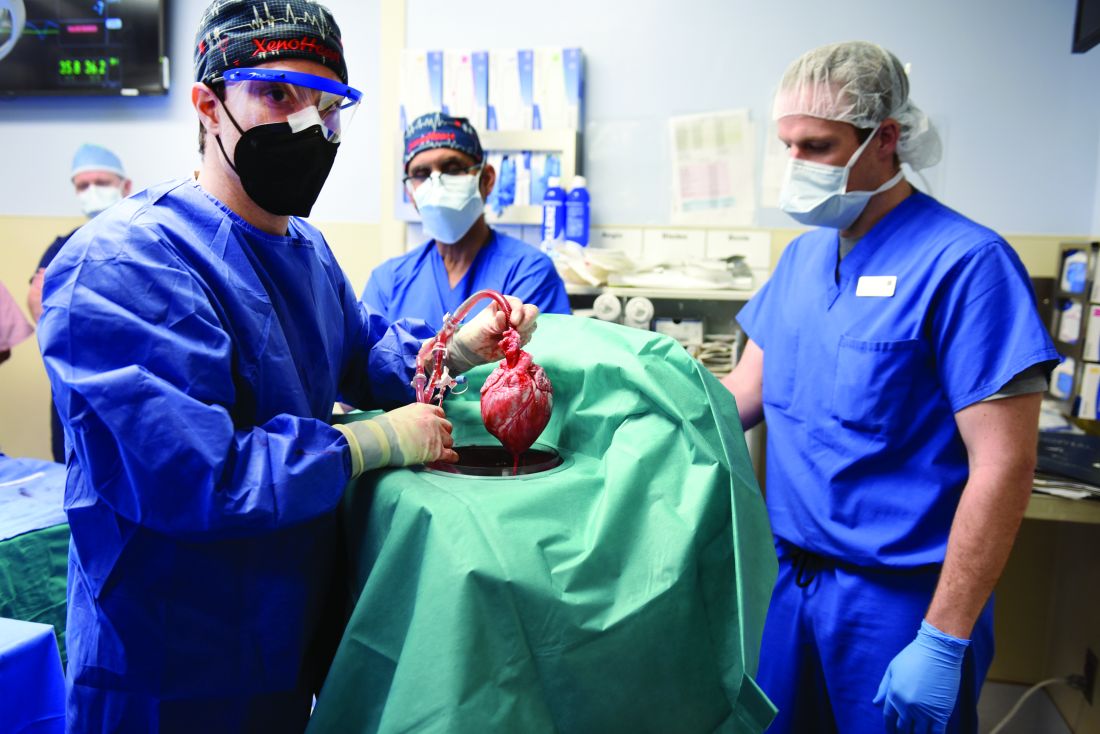
Puzzling, unique ECG from pig-to-human transplanted heart
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
In the first transplant of a genetically altered pig heart into a human in January, initial unexpected, prolonged ECG readings apparently did not affect the heart’s function, although the organ suddenly began to fail at day 50.
A study of these ECG changes, scheduled for presentation by Calvin Kagan, MD, and colleagues at the American Heart Association scientific sessions, offers insight into this novel operation.
As widely reported, the patient, 57-year-old David Bennett of Maryland, had end-stage heart disease and was a poor candidate for a ventricular assist device and was ineligible for a human heart, when he consented to be the first human to be transplanted with a pig heart that had a number of genes added or subtracted with the goal, in part, to prevent rejection.
The heart initially performed well after it was transplanted in an operation at the University of Maryland School of Medicine (UMSOM) in Baltimore on Jan. 7, but failed in the second month, and Mr. Bennett died on March 9.
The Food and Drug Administration had granted emergency authorization for the surgery through its expanded access (compassionate use) program, coauthor Muhammad Mohiuddin, MD, said in an interview.
“We have learned a lot and hope we can do more,” said Dr. Mohiuddin, scientific and program director of the cardiac xenotransplantation program at UMSOM.
“Suddenly on day 50, the heart started to get thicker and was not relaxing enough,” explained senior author Timm-Michael Dickfeld, MD, PhD, director of electrophysiology research at UMSOM. A biopsy revealed substantial buildup of interstitial fluid that restricted movement. The fluid was replaced by fibrous tissue, leading to irreversible damage.
Persistent, prolonged ECG parameters
In the heart from a genetically modified pig, three genes associated with antibody-mediated rejection and a gene associated with pig heart tissue growth had been inactivated and six human genes associated with immune acceptance had been added. The donor pig was supplied by Revivicor (Blacksburg, Va.).
The patient’s immunosuppressant therapy included an experimental antirejection medication (Kiniksa Pharmaceuticals; Lexington, Mass.).
The patient had daily 12-lead ECGs after the transplant.
In prior research using a pig heart transplanted into a pig body, ECG readings showed a short PR interval (50-120 ms), short QRS duration (70-90 ms) and short QT intervals (260-380 ms).
However, in the transplanted xenograft heart, the initial ECG readings showed a longer PR interval of 190 ms, QRS duration of 138 ms, and QT of 538 ms.
Prolonged intrinsic PR intervals remained stable during the postoperative course (210 ms, range 142-246 ms).
QRS duration also remained prolonged (145 ms, range 116-192 ms), but shortened during the postoperative course (days 21-40 vs. 41-60: 148 ms vs. 132 ms; P < .001).
Increased QT persisted (509 ms, range 384-650 ms) with dynamic fluctuations. The shortest QT duration was observed on day 14 (P < .001).
“In a human heart, when those parameters get longer, this can indicate signs of electrical or myocardial disease,” Dr. Dickfeld explained in a press release from the AHA.
“The QRS duration may prolong when, for example, the muscle and the electrical system itself is diseased, and that is why it takes a long time for electricity to travel from cell to cell and travel from one side of the heart to the other,” he said.
“In the human heart, the QT duration is correlated with an increased risk of abnormal heart rhythms,” he noted. “In our patient, it was concerning that the QT measure was prolonged. While we saw some fluctuations, the QT measure remained prolonged during the whole 61 days.”
‘Interesting study’
Two experts who were not involved with this research weighed in on the findings for this news organization.
“This very interesting study reinforces the difficulties in xenotransplantation, and the need for more research to be able to safely monitor recipients, as baseline values are unknown,” said Edward Vigmond, PhD.
Dr. Vigmond, from the Electrophysiology and Heart Modeling Institute at the University of Bordeaux in France, published a related study about a model of translation of pig to human electrophysiology.
The ECG is sensitive to the electrical activation pattern of the heart, along with the cellular and tissue electrical properties, he noted.
“Although pigs and humans may be similar in size, there are many differences between them,” Dr. Vigmond observed, including “the extent of the rapid conduction system of the heart, the number of nuclei in the muscle cells, the proteins in the cell membrane which control electrical activity, the orientation of the heart and thorax, and the handling of calcium inside the cell.”
“On top of this,” he continued, “donor hearts are denervated, so they no longer respond to nervous modulation, and circulating compounds in the blood which affect heart function vary between species.
“With all these differences, it is not surprising that the ECG of a pig heart transplanted into a human resembles neither that of a human nor that of a pig,” Dr. Vigmond said.
“It is interesting to note that the humanized-gene-edited porcine heart exhibited abnormal electrical conduction parameters from the outset,” said Mandeep R. Mehra, MD.
“Whether these changes were due to the gene modifications (i.e., already inherent in the pig ECG prior to transplant) or a result of the transplant operation challenges (such as the ischemia reperfusion injury and early immunological interactions) is uncertain and should be clarified,” said Dr. Mehra, of Harvard Medical School and Brigham and Women’s Medicine in Boston.
“Knowledge of these changes is important to determine whether a simple ECG parameter may be useful to identify changes that could indicate developing pathology,” Dr. Mehra added.
“In the older days of human transplantation, we often used ECG parameters such as a change in voltage amplitude to identify signals for rejection,” he continued. “Whether such changes occurred in this case could be another interesting aspect to explore as changes occurred in cardiac performance in response to the physiological and pathological challenges that were encountered in this sentinel case.”
The study authors reported having no outside sources of funding.
A version of this article first appeared on Medscape.com.
FROM AHA 2022
ACC/AHA issues updated guidance on aortic disease
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
focusing on surgical intervention considerations, consistent imaging practices, genetic and familial screenings, and the importance of multidisciplinary care.
“There has been a host of new evidence-based research available for clinicians in the past decade when it comes to aortic disease. It was time to reevaluate and update the previous, existing guidelines,” Eric M. Isselbacher, MD, MSc, chair of the writing committee, said in a statement.
“We hope this new guideline can inform clinical practices with up-to-date and synthesized recommendations, targeted toward a full multidisciplinary aortic team working to provide the best possible care for this vulnerable patient population,” added Dr. Isselbacher, codirector of the Thoracic Aortic Center at Massachusetts General Hospital, Boston.
The 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease was simultaneously published online in the Journal of the American College of Cardiology and Circulation.
The new guideline replaces the 2010 ACCF/AHA Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease and the 2015 Surgery for Aortic Dilation in Patients With Bicuspid Aortic Valves: A Statement of Clarification From the ACC/AHA Task Force on Clinical Practice Guidelines.
The new guideline is intended to be used with the 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease.
It brings together guidelines for both the thoracic and abdominal aorta and is targeted to cardiovascular clinicians involved in the care of people with aortic disease, including general cardiovascular care clinicians and emergency medicine clinicians, the writing group says.
Among the key recommendations in the new guideline are the following:
- Screen first-degree relatives of individuals diagnosed with aneurysms of the aortic root or ascending thoracic aorta, or those with aortic dissection to identify individuals most at risk for aortic disease. Screening would include genetic testing and imaging.
- Be consistent in the way CT or MRI are obtained and reported; in the measurement of aortic size and features; and in how often images are used for monitoring before and after repair surgery or other intervention. Ideally, all surveillance imaging for an individual should be done using the same modality and in the same lab, the guideline notes.
- For individuals who require aortic intervention, know that outcomes are optimized when surgery is performed by an experienced surgeon working in a multidisciplinary aortic team. The new guideline recommends “a specialized hospital team with expertise in the evaluation and management of aortic disease, in which care is delivered in a comprehensive, multidisciplinary manner.”
- At centers with multidisciplinary aortic teams and experienced surgeons, the threshold for surgical intervention for sporadic aortic root and ascending aortic aneurysms has been lowered from 5.5 cm to 5.0 cm in select individuals, and even lower in specific scenarios among patients with heritable thoracic aortic aneurysms.
- In patients who are significantly smaller or taller than average, surgical thresholds may incorporate indexing of the aortic root or ascending aortic diameter to either patient body surface area or height, or aortic cross-sectional area to patient height.
- Rapid aortic growth is a risk factor for rupture and the definition for rapid aneurysm growth rate has been updated. Surgery is now recommended for patients with aneurysms of aortic root and ascending thoracic aorta with a confirmed growth rate of ≥ 0.3 cm per year across 2 consecutive years or ≥ 0.5 cm in 1 year.
- In patients undergoing aortic root replacement surgery, valve-sparing aortic root replacement is reasonable if the valve is suitable for repair and when performed by experienced surgeons in a multidisciplinary aortic team.
- Patients with acute type A aortic dissection, if clinically stable, should be considered for transfer to a high-volume aortic center to improve survival. The operative repair of type A aortic dissection should entail at least an open distal anastomosis rather than just a simple supracoronary interposition graft.
- For management of uncomplicated type B aortic dissection, there is an increasing role for . Clinical trials of repair of thoracoabdominal aortic aneurysms with endografts are reporting results that suggest endovascular repair is an option for patients with suitable anatomy.
- Shared decision-making between the patient and multidisciplinary aortic team is highly encouraged, especially when the patient is on the borderline of thresholds for repair or eligible for different types of surgical repair.
- Shared decision-making should also be used with individuals who are pregnant or may become pregnant to consider the risks of pregnancy in individuals with aortic disease.
The guideline was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American College of Radiology, the Society of Cardiovascular Anesthesiologists, the Society for Cardiovascular Angiography and Interventions, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
It has been endorsed by the Society of Interventional Radiology and the Society for Vascular Surgery.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Similar transplant outcomes with hearts donated after circulatory death
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
Transplantation of hearts donated after circulatory death (DCD) is associated with short-term clinical outcomes similar to those of hearts donated after brain death (DBD), except for transient posttransplant right heart dysfunction, a single-center analysis suggests.
The right-heart dysfunction resolved by 3 weeks post transplant, and recipient mortality was similar for those receiving DCD and DBD, which is considered standard of care (SOC).
Furthermore, the median waiting list time was significantly shorter for DCD recipients than for SOC recipients (17 vs. 70 days).
The authors suggest that use of DCD hearts could expand the donor pool by as much as 30%.
“Now that we and others have demonstrated the safety of this technique, I believe it is our obligation as a transplant community to use these organs and not allow them to be wasted,” David A. D’Alessandro, MD, of Massachusetts General Hospital, Boston, told this news organization.
“I will caution that DCD heart transplantation is labor intensive, and there is a learning curve which can potentially put patients at risk,” he added. “It is vitally important, therefore, that we learn from each other’s experiences to flatten this curve.”
The study was published online in the Journal of the American College of Cardiology.
Similar outcomes
Dr. D’Alessandro and colleagues compared the hemodynamic and clinical profiles of 47 DCD hearts with 166 SOC hearts implanted at Massachusetts General Hospital between 2016 and 2022. DCD hearts were maintained with use of a proprietary warm perfusion circuit organ care system (OCS, TransMedics).
Baseline characteristics were similar between the groups, except the DCD heart recipients were younger (mean age, 55 vs. 59); they were less likely to be an inpatient at the time of transplant (26% vs. 49%); and they had lower pulmonary vascular resistance (1.73 WU vs. 2.26 WU).
The median time from DCD consent to transplant was significantly shorter than for SOC hearts (17 vs. 70 days). However, there was a higher, though not statistically significant, incidence of severe primary graft dysfunction at 24 hours post transplant with DCD (10.6% vs. SOC 3.6%), leading five DCD recipients (10.6%) and nine SOC recipients (5.4%) to receive venoarterial extracorporeal membrane oxygenation.
Right heart function was significantly impaired in DCD vs. SOC recipients 1 week post transplant, with higher median right atrial pressure (10 mm Hg vs. 7 mm Hg); higher right atrial pressure to pulmonary capillary wedge pressure ratio (0.64 vs. 0.57); and lower pulmonary arterial pulsatility index (1.66 vs. 2.52).
However, by 3 weeks post transplant, right heart function was similar between the groups, as was mortality at 30 days (0 vs. 2%) and 1 year (3% vs. 8%).
Furthermore, hospital length of stay following transplant, intensive care unit length of stay, ICU readmissions, and 30-day readmissions were similar between the groups.
“We and others will continue to push the boundaries of this technique to understand if we can safely extend the warm ischemic time, which could make additional organs available,” Dr. D’Alessandro said. “We will also be exploring additional ways to monitor and assess organ health and viability ex situ and potential avenues of treatment which could repair and optimize organ function.
“A successful DCD heart transplant program requires institutional and team commitment,” he added, “and there are clinical nuances which should be appreciated to minimize patient risks associated with the obligate learning curve.”
Ulrich P. Jorde, MD, of Montefiore Medical Center in New York, author of a related editorial, concluded that heart donation after circulatory death “promises significant expansion of the donor pool and will lead to many lives saved” and that “the current investigation is a timely and important contribution to this effort”.
However, he noted, “it must be acknowledged that donation after cardiac death has evoked significant controversy regarding the ethics of this approach,” particularly when using a technique called normothermic regional perfusion (NRP), in which, after declaration of death and ligation of cerebral vessels, the heart is resuscitated in situ using extracorporeal membrane oxygenation, as opposed to the proprietary warm perfusion OCS used in this study.
“Central to this discussion is the definition of death and its irreversibility,” Dr. Jorde noted. “In contrast to DBD, where brain death protocols are well established and accepted by societies across the globe, DCD protocol rules, e.g., standoff times after complete cessation of circulation, continue to vary even within national jurisdictions. Such variability and incomplete standardization of practice is particularly important when the organ is resuscitated in situ using normothermic regional perfusion.
“The International Society of Heart and Lung Transplantation has recently provided a framework within which donation after cardiac death, with or without the use of NRP, can be conducted to comply with ethical and legal norms and regulations, acknowledging that such norms and regulations may differ between societies,” he wrote. “To advance the field, and to ensure ongoing trust in the transplantation system, it is of critical importance that such discussions are held publicly and transparently.”
More ‘dry runs’
“Donor heart allographs are safe for our patients with heart failure if procured and transplanted in an organized and protocolized manner,” Philip J. Spencer, MD, a cardiovascular and transplant surgeon at Mayo Clinic in Rochester, Minn., told this news organization. “As the techniques are adopted globally, our patients will benefit.”
Nevertheless, like Dr. D’Alessandro, he noted that procurement of DCD hearts is more labor intensive. “A program and its patients must be willing to accept a higher number of ‘dry runs,’ which occurs when the team is sent for an organ and the donor does not progress to circulatory death in a time and manner appropriate for safe organ recovery.
“There is no doubt that being open to these organs will increase the patient’s chances of receiving a donor heart in a shorter period of time,” he said. “However, the experience of a dry run, or multiple, can be emotionally and financially stressful for the patient and the program.”
No commercial funding or relevant conflicts of interest were disclosed.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Angiography in patients with prior CABG does better when planned with CT
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
BOSTON – Coronary angiography in patients who have previously undergone cardiac artery bypass grafting (CABG) is challenging, but the procedure can be streamlined and made safer when preprocedural CT coronary angiography (CTCA) is performed to plan the intervention, according to a randomized controlled trial.
In this study, all three endpoints, including a reduction in the incidence of contrast-induced nephropathy (CIN) and duration of the procedure, were met, according to Daniel Jones, MBBS, PhD.
Preprocedural CTCA was also associated with about a 40% improvement in patient satisfaction.
“When logistically possible, CTCA should be considered for any stable postbypass patient undergoing coronary angiography,” said Dr. Jones, who supported this assertion with data presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
In this study, called BYPASS-CTCA, 688 patients with a prior CABG scheduled for invasive coronary angiography were randomized to a preprocedural CTCA or no preprocedural CTCA. Patients with stable angina and those with a non–ST elevated acute coronary syndrome were eligible. Those with ST-segment elevated MI or severe renal impairment (eGFR < 20 mL/min) were excluded.
All three co–primary endpoints favor CTCA
CTCA relative to no CTCA provided a significant advantage for all three of the coprimary endpoints, which were procedure duration, CIN as defined by KDIGO criteria, and patient satisfaction as measured by questionnaire.
The procedure duration was reduced by almost 21 minutes, cutting the time from nearly 39 minutes to less than 18 minutes (P < .001). This relative reduction was of similar magnitude across groups, such as those with or without acute coronary syndrome and procedures performed by a senior or a junior operator.
“Even when you include the preprocedural CTCA evaluation time, there was still a significant reduction [P < .001] in duration for those in the CTCA arm,” reported Dr. Jones, honorary consultant cardiologist, Barts Heart Centre, Queen Mary University, London.
The rates of CIN following the procedure in this study, which had a follow-up of 12 months, were 3.4% versus 27.9% (P < .0001) in the preprocedural CTCA and non-CTCA groups, respectively. Again, a sensitivity analysis showed a similar magnitude of risk reduction across all subgroups evaluated.
CTCA planning reduced contrast exposure
The reduced risk of CIN was consistent with a large reduction in contrast exposure for those in the CTCA group (77.4 vs. 173.0 mL; P < .001). The advantage narrowed substantially when adding in contrast exposure from CTCA, but still remained statistically significant (148.9 vs. 173.0 mL; P < .001).
Dr. Jones did not speculate about the specific reasons for the 40% improvement in patient satisfaction among those who underwent preprocedural CTCA relative to those who did not, but, again, a sensitivity analysis showed consistency across subgroups defined by race, operator experience, and underlying diagnosis.
Numerous secondary endpoints also favored CTCA over no CTCA. This included fewer catheters used to complete the procedure (three vs. four; P < .001), a greater likelihood that the procedure was performed with radial access (76.9% vs. 56.7%), and lower rates of procedural complications (2.3% vs. 10.8%; P < .001). This latter category included fewer vascular access complications such as bleeding (0.6 % vs. 4.4%; P = .007) and periprocedural MI (0.6% vs. 6.4%; P < 0.001).
In a graph of time to first major adverse cardiovascular event (MACE), the curves separated almost immediately with a consistently lower rate maintained in the CTCA arm over the 12 months of follow-up, but this is observational. Dr. Jones acknowledged that this trial was not powered to show a difference in MACE.
Study intriguing but not definitive
In a panel discussion that followed the presentation of these results at the meeting, sponsored by the Cardiovascular Research Foundation, some reservations with this study were expressed. In particular, several of the panelists, including Jeffrey W. Moses, MD, director of interventional cardiovascular therapeutics, Columbia University Medical Center, New York, expressed surprise at the 27% rate of CIN, which he considered uncommonly high even in a high-risk population.
The unusual rate of CIN was also considered problematic given that it was the most significant clinical outcome among the three co–primary endpoints. Procedural times and patient satisfaction, while valid endpoints, are important subjects of study, but Dr. Moses was not alone in suggesting this study deserves validation.
In particular, there appeared to be a consensus among panelists that a larger multicenter study looking at hard endpoints, such as MACE, would be more compelling. They indicated that even if CTCA poses a very low risk of meaningful complications, it does add expense and an extra step.
Dr. Jones reported no potential conflicts of interest. Dr. Moses reported financial relationships with Covanos, Orchestra Biomed, Ostial, and Xenter.
AT TCT 2022
PASCAL for MV repair noninferior to MitraClip in CLASP IID; FDA took notice
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
A newly available transcatheter device for edge-to-edge mitral valve (MV) repair, named for a famed scientist-inventor, is similar to the long-available MitraClip (Abbott) for short-term efficacy and safety, suggests an interim but prespecified analysis from a randomized trial.
In its comparison with MitraClip, the PASCAL transcatheter valve repair system (Edwards Lifesciences) was noninferior with respect to 30-day major adverse events and to success at achieving mitral regurgitation (MR) of no more than moderate severity within 6 months. The trial had entered patients with significant, symptomatic degenerative MR considered too high-risk for surgical repair or replacement.
The interim analysis covers 180 of the 300 patients followed in the study, of whom 117 received the PASCAL device and 63 were given MitraClip. Both groups showed significant gains in functional class, symptom status, and quality of life over 6 months, reported D. Scott Lim, MD, University of Virginia Health System Hospital, Charlottesville, and Konstantinos Koulogiannis, MD, Morristown Medical Center, N.J., jointly on Sept. 17 at the Transcatheter Cardiovascular Therapeutics (TCT) 2022 annual meeting in Boston.
Dr. Lim, one of the trial’s principal investigators, is also lead author on its same-day publication in JACC: Cardiovascular Interventions.
Based largely on those results from the CLASP IID pivotal trial, the U.S. Food and Drug Administration recently approved the PASCAL system for use in patients with degenerative MR, Edwards announced on Sept. 15. The device was approved in the European Union on Aug. 17.
MitraClip has been available in various iterations in the United States since 2013 and in Europe since 2008.
“It’s good for the field to be able to say we have two devices that are comparable,” giving clinicians more options, Vinod H. Thourani, MD, Piedmont Heart Institute, Atlanta, told this news organization.
The current analysis shows that “we’ve yet to figure out what patient pathologies will be beneficial” for each of the devices, Dr. Thourani said. “The goal will be to find out if there are certain anatomical considerations where one device is better than the other.”
It will be necessary to study “more patients, a larger cohort, with longer follow-up to allow us to see their true benefits,” he said, as well as to conduct more subgroup analyses. For now, the choice of device will probably be “operator-specific, which they feel comfortable with.”
Dr. Thourani, not an author on the current study, is the U.S. principal investigator for the CLASP IIF study looking at clinical outcomes with the two devices and says he consults for both Edwards and Abbott.
The findings are “preliminary for now,” said Michael Young, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., in part because, like most randomized trials, CLASP IID entered a select, not broadly representative population.
“They want to make, as best as they could, an apples-to-apples comparison, without confounding that might make it more difficult to interpret it afterwards,” Dr. Young, not associated with the trial, told this news organization.
But CLASP IID “did enroll patients that we do see and treat, so undoubtedly it’s a compelling study. We now have another device that is shown to be safe and effective. How we’re going to extrapolate it to all the patients that are being referred to our practices will, I think, be under debate and deliberation.”
The PASCAL and MitraClip devices each may be more suitable for different patients with varying mitral valve pathologies because of differences in their designs, Dr. Lim said. The PASCAL’s relative flexibility might make it preferable in patients with smaller mitral valves, and its ability to elongate during delivery could make it more suitable for patients with chordal-dense areas around the valve, he speculated.
MitraClip, Dr. Lim told this news organization, has a mechanical closure system for anchoring that may make it more appropriate for “more complicated, thicker leaflets with calcium.”
CLASP IID enrolled patients with grade 3+ or 4+ degenerative MR considered to be “at prohibitive surgical risk” at 43 sites in North America and Europe. It randomly assigned them 2-to-1 to receive the PASCAL device or MitraClip.
Either of two PASCAL versions were used, the original device or the “smaller, narrower” PASCAL Ace, Dr. Lim observed. Both versions are covered by the PASCAL Precision System FDA approval. About 40% of patients assigned to MitraClip received older versions of the device and about 60%, more recent versions, as they were entered into practice.
The mean procedure times were 88 minutes for PASCAL and 79 minutes for MitraClip (P = .023), with much of the difference attributable to the earliest PASCAL procedures. Procedure times for the device declined with greater operator experience, the published report states.
Rates of the primary safety endpoint of major adverse events at 30 days were 3.4% for PASCAL and 4.8% for MitraClip. The endpoint was a composite of cardiovascular mortality, stroke, myocardial infarction, new need for renal replacement therapy, severe bleeding, or nonelective MV reintervention.
The proportion of patients with MR grade 2+ or lower at 6 months, the primary effectiveness endpoint, assessed at a core laboratory, was 96.5% for the PASCAL group over a median follow-up of 179.5 days and 96.8% over a median of 184.5 days for those who received MitraClip.
Comparisons for both primary endpoints met the prespecified criteria for PASCAL noninferiority.
In a secondary analysis, the proportion of PASCAL patients with MR grade 1+ or less held about steady from postprocedure discharge out to 6 months, at 87.2% and 83.7%, respectively (P = .317).
But whereas 88.5% of MitraClip patients had MR grade 1+ or better at discharge, 71.2% were at that grade by 6 months (P = .003). That apparent hemodynamic deterioration raised some eyebrows at the TCT sessions as a potential sign that PASCAL functional results are more durable.
That sort of judgment is premature, offered Anita W. Asgar, MD, MSc, Montreal Heart Institute, Quebec City, as an invited discussant after the CLASP IID trial’s formal presentation at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The trial is notable in part for “showing how safe this procedure is and how successful it is for these patients – this is phenomenal,” she said, but “I would caution comparing one device being better than another with such a small number of patients.”
MitraClip, Dr. Young observed, “has been, up to this point, our only option for edge-to-edge repair of the mitral valve. And many of us have years of experience and a lot of patients that we treat with that device.” His center hasn’t yet used PASCAL, but that may change as the field gains more familiarity with the device. Operators may use either device in different cases, he said.
“Depending on the program, and depending on the volume of mitral patients that you see and edge-to-edge repair that you do, it could be that you stick with one, or switch to another, or you integrate both of them and try to decide which patients might be better suited for one or the other.”
CLASP IID was sponsored by Edwards Lifesciences. Dr. Lim discloses consulting for Philips, Venus, and Valgen and receiving research grants from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. Dr. Koulogiannis discloses consulting and serving on an advisory board for Edwards Lifesciences and as a speaker for Abbott and discloses holding equity, stocks, or stock options in 4C. Dr. Thourani discloses serving as a consultant to both Abbott and Edwards Lifesciences. Dr. Young discloses receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic. Dr. Asgar discloses receiving research support from or holding a research contract with Abbott Vascular and receiving consulting fees or honoraria or serving on a speaker’s bureau for Medtronic, Edwards Lifesciences, and W. Gore & Associates.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
TAVR now used in almost 50% of younger severe aortic stenosis patients
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
Among patients with severe isolated aortic stenosis younger than 65, the rate of transcatheter aortic valve replacement (TAVR) now almost matches that of surgical aortic valve replacement (SAVR), despite guideline recommendations to the contrary, a study in a national U.S. population shows.
The 2020 American Heart Association/American College of Cardiology (AHA/ACC) valve guideline recommends SAVR for patients younger than 65 with severe aortic stenosis, the researchers note, but their study showed “near equal utilization between TAVR and SAVR in these younger patients by 2021,” at 48% and 52% respectively.
Toishi Sharma, MD, and colleagues presented these findings in an oral poster session at Transcatheter Cardiovascular Therapeutics 2022, and the study was simultaneously published as a Research Letter in the Journal of the American College of Cardiology (JACC).
“To our knowledge, the current findings represent the first national temporal trends study stratifying [aortic stenosis] therapies according to guideline-recommended age groups: our observations demonstrate the dramatic growth of TAVR in all age groups, including young patients,” the researchers conclude.
They analyzed changes in rates of TAVR and SAVR in a U.S. sample stratified by age: younger than 65 years, 65-80, and older than 80 years.
These findings have implications for lifetime management of younger patients who undergo TAVR, they write, “including issues related to lifetime coronary access, valve durability, and the potential for subsequent TAVR procedures over time.”
Three age groups
In a study published in JACC, this group examined changes in uptake of TAVR versus SAVR in 4,161 patients with aortic stenosis in Vermont, New Hampshire, and Maine, senior author Harold L. Dauerman, MD, said in an interview.
The greatest rate of rise of TAVR was in the group younger than 65, but that study ended in 2019, said Dr. Dauerman, from the University of Vermont Health Network, Burlington.
The 2020 guideline stratifies TAVR and SAVR recommendations such that “less than 65 should primarily be a surgical approach and greater than 80 primarily a TAVR approach, while 65 to 80 is a gray zone, and shared decision-making becomes important,” he noted.
The group hypothesized that recent trials and technology have led to a national increase in TAVR in people younger than 65.
From the Vizient clinical database, including more than 250 U.S. academic centers that perform both TAVR and SAVR, the researchers identified 142,953 patients who underwent TAVR or SAVR for isolated aortic stenosis from Oct. 1, 2015, to Dec. 31, 2021. From 2015 to 2021, the valve replacement rates in the three age groups changed as follows:
- Age less than 65: TAVR rose from 17% to 48%; SAVR fell from 83% to 52%.
- Age 65-80: TAVR rose from 46% to 87%; SAVR fell from 54% to 12%.
- Age greater than 80: TAVR rose from 83% to 99%; SAVR fell from 16% to 1%.
“All ages have grown in the last 7 years in TAVR,” Dr. Dauerman summarized. “The one that’s surprising, and in contradiction to the guideline, is the growth of TAVR in young patients less than 65.”
Among patients younger than 65, prior bypass surgery and congestive heart failure predicted the use of TAVR instead of surgery, whereas bicuspid aortic valve disease was the biggest predictor of surgery instead of TAVR.
Most studies on TAVR valve durability are limited to patients in the randomized trials who were primarily in their mid-70s to mid-80s, some of whom died before a 10-year follow-up, Dr. Dauerman noted.
European guidelines recommend surgery for patients younger than 70, and it would be interesting to see if clinicians there follow this recommendation or if TAVR is now the preferred approach, he added.
There is a need for further, longer study of TAVR in younger patients, he said, to determine whether there are long-term clinical issues of concern.
Strategy depends on more than age
The “findings are not too surprising,” John Carroll, MD, who was not involved in this research, said in an email.
“Age is only one of multiple patient characteristics that enter into consideration of TAVR versus SAVR,” said Dr. Carroll, from Anschutz Medical Campus, University of Colorado, Aurora.
“As the article reports,” he noted, “those less than 65 having TAVR are more likely to have comorbid conditions that likely made the risk of SAVR higher.”
Dr. Carroll was lead author of a review article published in 2020 based on data from the ACC–Society of Thoracic Surgeons (STS)–Transcatheter Valve Therapy (TVT) registry on 276,316 patients who had TAVR in the United States from 2011-2019.
He pointed out that Figure 2 in that review shows that “SAVR is often performed in conjunction with other surgical procedures – another major reason why SAVR remains an important treatment for valvular heart disease.”
“We are awaiting long-term data comparing TAVR to SAVR durability,” Dr. Carroll added, echoing Dr. Dauerman. “So far [there are] no major differences, but it remains a key need to fully understand TAVR and the various models in commercial use.”
“Both TAVR and SAVR used in adults are tissue valves (SAVR with mechanical valves is used in younger patients),” Dr. Carroll noted, “and all tissue valves will eventually fail if the patient lives long enough.”
Patient management strategies need to consider what treatment options exist when the first valve fails. “If the first valve is SAVR, there is now extensive experience with placing a TAVR valve inside a failing SAVR valve, so called Valve-in-Valve or TAVR-in-SAVR. This is the preferred treatment in most patients with failing SAVR valves,” he said.
“On the other hand,” he continued, “we are just beginning to see more and more patients with failing TAVR valves, and the TAVR-in-TAVR procedure is less well understood.”
“Issues such as acute coronary occlusion and long-term difficulty in accessing coronary arteries are being encountered in some patients having TAVR-in-TAVR,” Dr. Carroll noted, which he discusses in a recent editorial he coauthored about the complexities of redo TAVR, published in JACC: Cardiovascular Interventions.
The study received no funding. Dr. Dauerman has research grants and is a consultant for Medtronic and Boston Scientific. Dr. Carroll is a local principal investigator in trials sponsored by Medtronic, Abbott, and Edwards Lifesciences.
A version of this article first appeared on Medscape.com.
FROM TCT 2022
In cardiogenic shock, edge-to-edge mitral valve repair improves outcome
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
In patients with severe mitral regurgitation (MR) and cardiogenic shock, successful transcatheter edge-to-edge repair (TEER) is associated with a substantial reduction in all-cause mortality and lower morbidity at 1 year, according to an analysis of registry data.
The data from this analysis also confirm that “successful reduction of MR is achievable with TEER in most patients with cardiogenic shock,” reported Mohamad A. Alkhouli, MD, an interventional cardiologist and professor of medicine at the Mayo Clinic, Rochester, Minn.
In those with device success, achieved in 85.6% of patients, all-cause mortality was about 21% lower (34.6% vs. 55.5%; P < .001) at 1 year than in those who were not successfully repaired, according to Dr. Alkhouli, who presented the findings at the Transcatheter Cardiovascular Therapeutics annual meeting in Boston. This translated into a reduction in the hazard ratio for death of nearly 50% (hazard ratio, 0.52; 95% confidence interval, 0.43-0.63).
A similar relative benefit was found for the composite endpoint of mortality and heart failure admissions at 1 year. Whether unadjusted (HR, 0.54; 95% CI, 0.45-0.66) or adjusted (HR, 0.51; 95% CI, 0.42-0.62), risk reductions with successful MR reduction, defined as greater than or equal to 1 grade improvement and a final MR grade of less than or equal to 2+, indicated that major adverse outcomes are reduced by about half.
STS/ACC TCT registry data queried
Drawn from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, 3,797 patients with cardiogenic shock underwent MR repair between November 2013 and December 2021. Outcomes at 1 year were evaluable in 2,773 of these patients. For inclusion, all had to meet at least one of the definitions of cardiogenic shock, such as inotrope use or mechanical circulatory support.
At baseline, 94.5% had a MR severity of at least 3+, and most of these had 4+. Thirty days after treatment, 88.8% had MR severity of 2+ or less, the majority of which had a severity of 1+.
These data address an important question not previously well studied, according to Dr. Alkhouli. In MR patients, cardiogenic shock is associated with a high risk of death, but there has been little evidence that valve repair does not exacerbate, let alone modify, this risk.
These data support the value of intervention, which was performed in almost all patients with MitraClipä (Abbott), the only device available for most of the period in which the registry was queried. However, Dr. Alkhouli cautioned that his data are best considered “hypothesis generating.”
“We need a randomized trial,” he said at the meeting sponsored by the Cardiovascular Research Foundation. He pointed out that this is a complex population for which multiple variables might have skewed results when data are analyzed retrospectively. Not least, those MR patients with cardiogenic shock in the database considered for TEER might well have been relatively healthy and not representative of an unselected population with both MR and cardiogenic shock.
The question might be better answered by the multicenter Canadian trial CAPITAL MINOS, which has just started. Described in an article in the American Heart Journal, it has a planned enrollment of about 150 MR patients with cardiogenic shock randomized to TEER or medical therapy. Results are expected in about 1 year, according to Dr. Alkhouli.
But regarding the present analysis, Dr. Alkhouli did note that sensitivity analyses conducted within his data across risk factors, such as degenerative versus nondegenerative MR, low (< 30%) versus higher left ventricular ejection fraction (LVEF), and presence or absence of an acute coronary syndrome (ACS), consistently supported a benefit from intervention.
Also, cardiogenic shock did not appear to be a factor in device failure, according to Dr. Alkhouli, addressing a potential criticism that cardiogenic shock was an underlying reason for device failure.
More than 90% in NYHA class III or IV heart failure
In this study, the mean age was 73 years. More than 90% were in class III or IV heart failure in the 2 weeks prior to TEER. More than half had established coronary artery disease. Other concomitant cardiovascular morbidities, including atrial fibrillation or flutter (65%), prior MI (39%), and prior stroke or transient ischemic attach (> 10%) were well represented.
When those with device success were compared with those with device failure, the risk profile was comparable. The predicted STS (Society of Thoracic Surgeons) mortality for mitral valve repair among these two groups was 14.8% versus 15% (P = 0.97), respectively.
However, those with device failure did have a lower baseline left ventricular ejection fraction (40.7% vs. 42.9%; P = .009) and a greater prevalence of moderate-to-severe or severe MR (96.1% vs. 84.9%; P < 0.001).
The growing experience with TEER means that benefit has now been shown in several complicated MR groups, such as those with severe ventricular dysfunction, renal insufficiency, and obstructive lung disease. This was a rationale for looking at the impact or repairing MR in patients with cardiogenic shock.
It is a pressing question, according to Dr. Alkhouli. He cited studies suggesting that up to 20% of patients hospitalized for cardiogenic shock have at least moderate-to-severe MR. Conversely, cardiogenic shock is not an uncommon finding in patients with MR.
While Dr. Alkhouli acknowledged that the many variables influencing outcome in patients with MR and cardiogenic shock will make a randomized trial “challenging,” many experts echoed this concern and even expressed some skepticism about the potential for an unbiased trial.
Data confirm MR repair is safe during shock
“These data do show that repair of MR is safe in patients safe in patients with cardiogenic shock,” said Anita W. Asgar, MD, an interventional cardiologist associated with the Montreal Heart Institute. She noted that there was a 5- to 6-day delay among the cardiogenic shock patients prior to undergoing MR repair in this analysis, potentially reflecting an elimination of those at very high risk. Similarly, she suggested that many interventionalists are likely to consider multiple variables before proceeding.
As a result, MR repair may not be amenable to randomization in a cardiogenic shock population, given that this decision is not typically undertaken out of the context of multiple variables.
“I am not sure that a clinical trial is ethical,” she said. She would expect that clinicians enrolling patients would only do so on a selective basis.
Alexandra J. Lansky, MD, Director of the Yale Heart and Vascular Research Program, Yale University, New Haven, Conn., also emphasized the difficulty of controlling for variables, such as the duration of cardiogenic shock, that influence decision-making.
Nevertheless, she called the data “very important” in that they at least lend some objective data for deciding whether to intervene a group of “challenging” patients not uncommonly faced in clinical practice.
Dr. Alkhouli reports financial relationships with Abbott Vascular, Boston Scientific, Johnson & Johnson, and Phillips. Dr. Asgar reports financial relationships with Abbott Vascular, Edwards Lifesciences, W.L. Gore & Associates, and Medtronic. Dr. Lasky reports no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM TCT 2022