User login
LAAOS III: Surgical LAA closure cuts AFib stroke risk by one third
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
Fresh look at ISCHEMIA bolsters conservative message in stable CAD
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.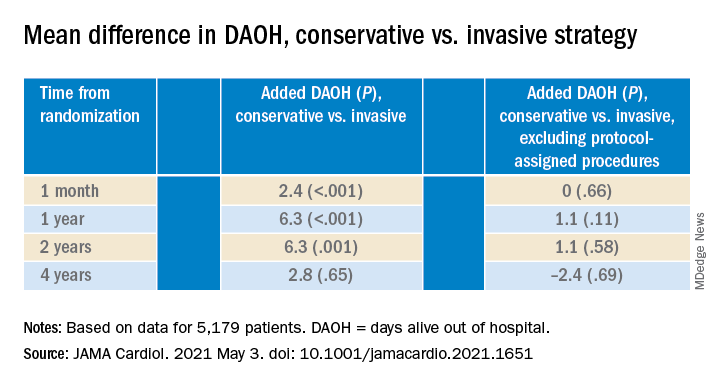
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
The more complicated a primary endpoint, the greater a puzzle it can be for clinicians to interpret the results. It’s likely even tougher for patients, who don’t help choose the events studied in clinical trials yet are increasingly sharing in the management decisions they influence.
That creates an opening for a more patient-centered take on one of cardiology’s most influential recent studies, ISCHEMIA, which bolsters the case for conservative, med-oriented management over a more invasive initial strategy for patients with stable coronary artery disease (CAD) and positive stress tests, researchers said.
The new, prespecified analysis replaced the trial’s conventional primary endpoint of major adverse cardiac events (MACE) with one based on “days alive out of hospital” (DAOH) and found an early advantage for the conservative approach, with caveats.
Those assigned to the conservative arm benefited with more out-of-hospital days throughout the next 2 years than those in the invasive-management group, owing to the latter’s protocol-mandated early cath-lab work-up with possible revascularization. The difference averaged more than 6 days for much of that time.
But DAOH evened out for the two groups by the fourth year in the analysis of more than 5,000 patients.
Protocol-determined cath procedures accounted for 61% of hospitalizations in the invasively managed group. A secondary DAOH analysis that excluded such required hospital days, also prespecified, showed no meaningful difference between the two strategies over the 4 years, noted the report published online May 3 in JAMA Cardiology.
DOAH is ‘very, very important’
The DAOH metric has been a far less common consideration in clinical trials, compared with clinical events, yet in some ways it is as “hard” a metric as mortality, encompasses a broader range of outcomes, and may matter more to patients, it’s been argued.
“The thing patients most value is time at home. So they don’t want to be in the hospital, they don’t want to be away from friends, they want to do recreation, or they may want to work,” lead author Harvey D. White, DSc, Green Lane Cardiovascular Services, Auckland (New Zealand) City Hospital, University of Auckland, told this news organization.
“When we need to talk to patients – and we do need to talk to patients – to have a days-out-of-hospital metric is very, very important,” he said. It is not only patient focused, it’s “meaningful in terms of the seriousness of events,” in that length of hospitalization tracks with clinical severity, observed Dr. White, who is slated to present the analysis May 17 during the virtual American College of Cardiology 2021 scientific sessions.
As previously reported, ISCHEMIA showed no significant effect on the primary endpoint of cardiovascular mortality, MI, or hospitalization for unstable angina, heart failure, or resuscitated cardiac arrest by assignment group over a median 3.2 years. Angina and quality of life measures were improved for patients in the invasive arm.
With an invasive initial strategy, “What we know now is that you get nothing of an advantage in terms of the composite endpoint, and you’re going to spend 6 days more in the hospital in the first 2 years, for largely no benefit,” Dr. White said.
That outlook may apply out to 4 years, the analysis suggests, but could conceivably change if DAOH is reassessed later as the ISCHEMIA follow-up continues for what is now a planned total of 10 years.
Meanwhile, the current findings could enhance doctor-patient discussions about the trade-offs between the two strategies for individuals whose considerations will vary.
“This is a very helpful measure to understand the burden of an approach to the patient,” observed E. Magnus Ohman, MD, an interventional cardiologist at Duke University, Durham, N.C., who was not involved in the trial.
With DAOH as an endpoint, “you as a clinician get another aspect of understanding of a treatment’s impact on a multitude of endpoints.” Days out of hospital, he noted, encompasses the effects of clinical events that often go into composite clinical endpoints – not death, but including nonfatal MI, stroke, need for revascularization, and cardiovascular hospitalization.
To patients with stable CAD who ask whether the invasive approach has merits in their case, the DAOH finding “helps you to say, well, at the end of the day, you will probably be spending an equal amount of time in the hospital. Your price up front is a little bit higher, but over time, the group who gets conservative treatment will catch up.”
The DAOH outcome also avoids the limitations of an endpoint based on time to first event, “not the least of which,” said Dr. White, is that it counts only the first of what might be multiple events of varying clinical impact. Misleadingly, “you can have an event that’s a small troponin rise, but that becomes more important in a person than dying the next day.”
The DAOH analysis was based on 5,179 patients from 37 countries who averaged 64 years of age and of whom 23% were women. The endpoint considered only overnight stays in hospitals, skilled nursing facilities, rehabilitation centers, and nursing homes.
There were many more hospital or extended care facility stays overall in the invasive-management group, 4,002 versus 1,897 for those following the conservative strategy (P < .001), but the numbers flipped after excluding protocol-assigned procedures: 1,568 stays in the invasive group, compared with 1,897 (P = .001)
There were no associations between DAOH and Seattle Angina Questionnaire 7–Angina Frequency scores or DAOH interactions by age, sex, geographic region, or whether the patient had diabetes, prior MI, or heart failure, the report notes.
The primary ISCHEMIA analysis hinted at a possible long-term advantage for the invasive initial strategy in that event curves for the two arms crossed after 2-3 years, Dr. Ohman observed.
Based on that, for younger patients with stable CAD and ischemia at stress testing, “an investment of more hospital days early on might be worth it in the long run.” But ISCHEMIA, he said, “only suggests it, it doesn’t confirm it.”
The study was supported in part by grants from Arbor Pharmaceuticals and AstraZeneca. Devices or medications were provided by Abbott Vascular, Amgen, Arbor, AstraZeneca, Esperion, Medtronic, Merck Sharp & Dohme, Phillips, Omron Healthcare, and Sunovion. Dr. White disclosed receiving grants paid to his institution and fees for serving on a steering committee from Sanofi-Aventis, Regeneron, Eli Lilly, Omthera, American Regent, Eisai, DalCor, CSL Behring, Sanofi-Aventis Australia, and Esperion Therapeutics, and personal fees from Genentech and AstraZeneca. Dr. Ohman reported receiving grants from Abiomed and Cheisi USA, and consulting for Abiomed, Cara Therapeutics, Chiesi USA, Cytokinetics, Imbria Pharmaceuticals, Otsuka Pharmaceuticals, Milestone Pharmaceuticals, and XyloCor Therapeutics.
A version of this article first appeared on Medscape.com.
Infective endocarditis with stroke after TAVR has ‘dismal’ prognosis
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who suffer a stroke during hospitalization for infective endocarditis (IE) after transcatheter aortic valve replacement (TAVR) have a dismal prognosis, with more than half dying during the index hospitalization and two-thirds within the first year, a new study shows.
The study – the first to evaluate stroke as an IE-related complication following TAVR in a large multicenter cohort – is published in the May 11 issue of the Journal of the American College of Cardiology.
The authors, led by David del Val, MD, Quebec Heart & Lung Institute, Quebec City, explain that IE after TAVR is a rare but serious complication associated with a high mortality rate. Neurologic events, especially stroke, remain one of the most common and potentially disabling IE-related complications, but until now, no study has attempted to evaluate the predictors of stroke and outcomes in patients with IE following TAVR.
For the current study, the authors analyzed data from the Infectious Endocarditis after TAVR International Registry, including 569 patients who developed definite IE following TAVR from 59 centers in 11 countries.
Patients who experienced a stroke during IE admission were compared with patients who did not have a stroke.
Results showed that 57 patients (10%) had a stroke during IE hospitalization, with no differences in the causative microorganism between groups. Stroke patients had higher rates of acute renal failure, systemic embolization, and persistent bacteremia.
Factors associated with a higher risk for stroke during the index IE hospitalization included stroke before IE, moderate or higher residual aortic regurgitation after TAVR, balloon-expandable valves, IE within 30 days after TAVR, and vegetation size greater than 8 mm.
The stroke rate was 3.1% in patients with none of these risk factors; 6.1% with one risk factor; 13.1% with two risk factors; 28.9% with three risk factors, and 60% with four risk factors.
“The presence of such factors (particularly in combination) may be considered for determining an earlier and more aggressive (medical or surgical) treatment in these patients,” the researchers say.
IE patients with stroke had higher rates of in-hospital mortality (54.4% vs. 28.7%) and overall mortality at 1 year (66.3% vs. 45.6%).
Surgery rates were low (25%) even in the presence of stroke and failed to improve outcomes in this population.
Noting that consensus guidelines for managing patients with IE recommend surgery along with antibiotic treatment for patients developing systemic embolism, particularly stroke, the researchers say their findings suggest that such surgery recommendations may not be extrapolated to TAVR-IE patients, and specific guidelines are warranted for this particular population.
Furthermore, the possibility of early surgery in those patients with factors increasing the risk for stroke should be evaluated in future studies.
The authors note that TAVR has revolutionized the treatment of aortic stenosis and is currently moving toward less complex and younger patients with lower surgical risk. Despite the relatively low incidence of IE after TAVR, the number of procedures is expected to grow exponentially, increasing the number of patients at risk of developing this life-threatening complication. Therefore, detailed knowledge of this disease and its complications is essential to improve outcomes.
They point out that the 10% rate of stroke found in this study is substantially lower, compared with the largest surgical prosthetic-valve infective endocarditis registries, but they suggest that the unique clinical profile of TAVR patients may lead to an underdiagnosis of stroke, with a high proportion of elderly patients who more frequently present with nonspecific symptoms.
They conclude that “IE post-TAVR is associated with a poor prognosis with high in-hospital and late mortality rates. Our study reveals that patients with IE after TAVR complicated by stroke showed an even worse prognosis.”
“The progressive implementation of advanced imaging modalities for early IE diagnosis, especially nuclear imaging, may translate into a better prognosis in coming years. Close attention should be paid to early recognition of stroke-associated factors to improve clinical outcomes,” they add.
In an accompanying editorial, Vuyisile Nkomo, MD, Daniel DeSimone, MD, and William Miranda, MD, Mayo Clinic, Rochester, Minn., say the current study “highlights the devastating consequences of IE after TAVR and the even worse consequences when IE was associated with stroke.”
This points to the critical importance of efforts to prevent IE with appropriate antibiotic prophylaxis and addressing potential sources of infection (for example, dental screening) before invasive cardiac procedures.
“Patient education is critical in regard to recognizing early signs and symptoms of IE. In particular, patients must be informed to obtain blood cultures with any episode of fever, as identification of bacteremia is critical in the diagnosis of IE,” the editorialists comment.
Endocarditis should also be suspected in afebrile patients with increasing transcatheter heart valve gradients or new or worsening regurgitation, they state.
Multimodality imaging is important for the early diagnosis of IE to facilitate prompt antibiotic treatment and potentially decrease the risk for IE complications, especially systemic embolization, they add.
“Despite the unequivocal advances in the safety and periprocedural complications of TAVR, IE with and without stroke in this TAVR population remains a dreadful complication,” they conclude.
Dr. Del Val was supported by a research grant from the Fundación Alfonso Martin Escudero. The editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
OCS heart system earns hard-won backing of FDA panel
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
After more than 10 hours of intense debate, a Food and Drug Administration advisory panel gave its support to a premarket approval application (PMA) for the TransMedics Organ Care System (OCS) Heart system.
The OCS Heart is a portable extracorporeal perfusion and monitoring system designed to keep a donor heart in a normothermic, beating state. The “heart in a box” technology allows donor hearts to be transported across longer distances than is possible with standard cold storage, which can safely preserve donor hearts for about 4 hours.
The Circulatory System Devices Panel of the Medical Devices Advisory Committee voted 12 to 5, with 1 abstention, that the benefits of the OCS Heart System outweigh its risks.
The panel voted in favor of the OCS Heart being effective (10 yes, 6 no, and 2 abstaining) and safe (9 yes, 7 no, 2 abstaining) but not without mixed feelings.
James Blankenship, MD, a cardiologist at the University of New Mexico, Albuquerque, voted yes to all three questions but said: “If it had been compared to standard of care, I would have voted no to all three. But if it’s compared to getting an [left ventricular assist device] LVAD or not getting a heart at all, I would say the benefits outweigh the risks.”
Marc R. Katz, MD, chief of cardiothoracic surgery, Medical University of South Carolina, Charleston, also gave universal support, noting that the rate of heart transplantations has been flat for years. “This is a big step forward toward being able to expand that number. Now all that said, it obviously was a less-than-perfect study and I do think there needs to be some constraints put on the utilization.”
The panel reviewed data from the single-arm OCS Heart EXPAND trial and associated EXPAND Continued Access Protocol (CAP), as well the sponsor’s first OCS Heart trial, PROCEED II.
EXPAND met its effectiveness endpoint, with 88% of donor hearts successfully transplanted, an 8% incidence of severe primary graft dysfunction (PGD) 24 hours after transplantation, and 94.6% survival at 30 days.
Data from 41 patients with 30-day follow-up in the ongoing EXPAND CAP show 91% of donor hearts were utilized, a 2.4% incidence of severe PGD, and 100% 30-day survival.
The sponsor and the FDA clashed over changes made to the trial after the PMA was submitted, the appropriateness of the effectiveness outcome, and claims by the FDA that there was substantial overlap in demographic characteristics between the extended criteria donor hearts in the EXPAND trials and the standard criteria donor hearts in PROCEED II.
TransMedics previously submitted a PMA based on PROCEED II but it noted in submitted documents that it was withdrawn because of “fundamental disagreements with FDA” on the interpretation of a post hoc analysis with United Network for Organ Sharing registry data that identified increased all-cause mortality risk but comparable cardiac-related mortality in patients with OCS hearts.
During the marathon hearing, FDA officials presented several post hoc analyses, including one stratified by donor inclusion criteria, in which 30-day survival estimates were worse in recipients of single-criterion organs than for those receiving donor organs with multiple inclusion criteria (85% vs. 91.4%). In a second analysis, 2-year point estimates of survival also trended lower with donor organs having only one extended criterion.
Reported EXPAND CAP 6- and 12-month survival estimates were 100% and 93%, respectively, which was higher than EXPAND (93% and 84%), but there was substantial censoring (>50%) at 6 months and beyond, FDA officials said.
When EXPAND and CAP data were pooled, modeled survival curves shifted upward but there was a substantial site effect, with a single site contributing 46% of data, which may affect generalizability of the results, they noted.
“I voted yes for safety, no for efficacy, and no for approval and I’d just like to say I found this to be the most difficult vote in my experience on this panel,” John Hirshfeld, MD, University of Pennsylvania, Philadelphia, said. “I was very concerned that the PROCEED data suggests a possible harm, and in the absence of an interpretable comparator for the EXPAND trial, it’s really not possible to decide if there’s efficacy.”
Keith B. Allen, MD, director of surgical research at Saint Luke’s Hospital of Kansas City (Mo.), said, “I voted no on safety; I’m not going to give the company a pass. I think their animal data was sorely lacking and a lot of issues over the last 10 years could have been addressed with some key animal studies.
“For efficacy and risk/benefit, I voted yes for both,” he said. “Had this been standard of care and only PROCEED II, I would have voted no, but I do think there are a lot of hearts that go in the bucket and this is a challenging population.”
More than a dozen physicians and patients spoke at the open public hearing about the potential for the device to expand donor heart utilization, including a recipient whose own father died while waiting on the transplant list. Only about 3 out of every 10 donated hearts are used for transplant. To ensure fair access, particularly for patients in rural areas, federal changes in 2020 mandate that organs be allocated to the sickest patients first.
Data showed that the OCS Heart System was associated with shorter waiting list times, compared with U.S. averages but longer preservation times than cold static preservation.
In all, 13% of accepted donor organs were subsequently turned down after OCS heart preservation. Lactate levels were cited as the principal reason for turn-down but, FDA officials said, the validity of using lactate as a marker for transplantability is unclear.
Pathologic analysis of OCS Heart turned-down donor hearts with stable antemortem hemodynamics, normal or near-normal anatomy and normal ventricular function by echocardiography, and autopsy findings of acute diffuse or multifocal myocardial damage “suggest that in an important proportion of cases the OCS Heart system did not provide effective organ preservation or its use caused severe myocardial damage to what might have been an acceptable graft for transplant,” said Andrew Farb, MD, chief medical officer of the FDA’s Office of Cardiovascular Devices.
Proposed indication
In the present PMA, the OCS Heart System is indicated for donor hearts with one or more of the following characteristics: an expected cross-clamp or ischemic time of at least 4 hours because of donor or recipient characteristics; or an expected total cross-clamp time of at least 2 hours plus one of the following risk factors: donor age 55 or older, history of cardiac arrest and downtime of at least 20 minutes, history of alcoholism, history of diabetes, donor ejection fraction of 40%-50%,history of left ventricular hypertrophy, and donor angiogram with luminal irregularities but no significant coronary artery disease
Several members voiced concern about “indication creep” should the device be approved by the FDA, and highlighted the 2-hour cross-clamp time plus wide-ranging risk factors.
“I’m a surgeon and I voted no on all three counts,” said Murray H. Kwon, MD, Ronald Reagan University of California, Los Angeles Medical Center. “As far as risk/benefit, if it was just limited to one group – the 4-hour plus – I would say yes, but if you’re going to tell me that there’s a risk/benefit for the 2-hour with the alcoholic, I don’t know how that was proved in anything.”
Dr. Kwon was also troubled by lack of proper controls and by the one quarter of patients who ended up on mechanical circulatory support in the first 30 days after transplant. “I find that highly aberrant.”
Joaquin E. Cigarroa, MD, head of cardiovascular medicine, Oregon Health & Science University, Portland, said the unmet need for patients with refractory, end-stage heart failure is challenging and quite emotional, but also voted no across the board, citing concerns about a lack of comparator in the EXPAND trials and overall out-of-body ischemic time.
“As it relates to risk/benefit, I thought long and hard about voting yes despite all the unknowns because of this emotion, but ultimately I voted no because of the secondary 2-hours plus alcoholism, diabetes, or minor coronary disease, in which the ischemic burden and ongoing lactate production concern me,” he said.
Although the panel decision is nonbinding, there was strong support from the committee members for a randomized, postapproval trial and more complete animal studies.
A version of this article first appeared on Medscape.com.
TAVR feasible, comparable with surgery in rheumatic heart disease
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
Patients with rheumatic heart disease (RHD) appear to have comparable outcomes, whether undergoing transcatheter or surgical aortic valve replacement (TAVR/SAVR), and when compared with TAVR in patients with nonrheumatic aortic stenosis, a new Medicare study finds.
An analysis of data from 1,159 Medicare beneficiaries with rheumatic aortic stenosis revealed that, over a median follow-up of 19 months, there was no difference in all-cause mortality with TAVR vs. SAVR (11.2 vs. 7.0 per 100 person-years; adjusted hazard ratio, 1.53; P = .2).
Mortality was also similar after a median follow-up of 17 months between TAVR in patients with rheumatic aortic stenosis and 88,554 additional beneficiaries with nonrheumatic aortic stenosis (15.2 vs. 17.7 deaths per 100 person-years; aHR, 0.87; P = .2).
“We need collaboration between industry and society leaders in developed countries to initiate a randomized, controlled trial to address the feasibility of TAVR in rheumatic heart disease in younger populations who aren’t surgical candidates or if there’s a lack of surgical capabilities in countries, but this is an encouraging first sign,” lead author Amgad Mentias, MD, MSc, Cleveland Clinic Foundation, said in an interview.
Although the prevalence of rheumatic heart disease (RHD) has fallen to less than 5% or so in the United States and Europe, it remains a significant problem in developing and low-income countries, with more than 1 million deaths per year, he noted. RHD patients typically present at younger ages, often with concomitant aortic regurgitation and mitral valve disease, but have less calcification than degenerative calcific aortic stenosis.
Commenting on the results, published in the Journal of the American College of Cardiology, David F. Williams, PhD, said in an interview that “it is only now becoming possible to entertain the use of TAVR in such patients, and this paper demonstrates the feasibility of doing so.
“Although the study is based on geriatric patients of an industrialized country, it opens the door to the massive unmet clinical needs in poorer regions as well as emerging economies,” said Dr. Williams, a professor at the Wake Forest Institute for Regenerative Medicine, Winston-Salem, N.C., and coauthor of an accompanying editorial.
The study included Medicare beneficiaries treated from October 2015 to December 2017 for rheumatic aortic stenosis (TAVR, n = 605; SAVR, n = 55) or nonrheumatic aortic stenosis (n = 88,554).
Among those with rheumatic disease, SAVR patients were younger than TAVR patients (73.4 vs. 79.4 years), had a lower prevalence of most comorbidities, and were less frail (median frailty score, 5.3 vs. 11.3).
SAVR was associated with significantly higher weighted risk for in-hospital acute kidney injury (22.3% vs. 11.9%), blood transfusion (19.8% vs. 7.6%), cardiogenic shock (5.7% vs. 1.5%), new-onset atrial fibrillation (21.1% vs. 2.2%), and had longer hospital stays (median, 8 vs. 3 days), whereas new permanent pacemaker implantations trended higher with TAVR (12.5% vs 7.2%).
The TAVR and SAVR groups had comparable rates of adjusted in-hospital mortality (2.4% vs. 3.5%), 30-day mortality (3.6% vs. 3.2%), 30-day stroke (2.4% vs. 2.8%), and 1-year mortality (13.1% vs. 8.9%).
Among the two TAVR cohorts, patients with rheumatic disease were younger than those with nonrheumatic aortic stenosis (79.4 vs. 81.2 years); had a higher prevalence of heart failure, ischemic stroke, atrial fibrillation, and lung disease; and were more frail (median score, 11.3 vs. 6.9).
Still, there was no difference in weighted risk of in-hospital mortality (2.2% vs. 2.6%), 30-day mortality (3.6% vs. 3.7%), 30-day stroke (2.0% vs. 3.3%), or 1-year mortality (16.0% vs. 17.1%) between TAVR patients with and without rheumatic stenosis.
“We didn’t have specific information on echo[cardiography], so we don’t know how that affected our results, but one of the encouraging points is that after a median follow-up of almost 2 years, none of the patients who had TAVR in the rheumatic valve and who survived required redo aortic valve replacement,” Dr. Mentias said. “It’s still short term but it shows that for the short to mid term, the valve is durable.”
Data were not available on paravalvular regurgitation, an Achilles heel for TAVR, but Dr. Mentias said rates of this complication have come down significantly in the past 2 years with modifications to newer-generation TAVR valves.
Dr. Williams and colleagues say one main limitation of the study also highlights the major shortcoming of contemporary TAVRs when treating patients with RHD: “namely, their inadequate suitability for AR [aortic regurgitation], the predominant rheumatic lesion of the aortic valve” in low- to middle-income countries.
They pointed out that patients needing an aortic valve where RHD is rampant are at least 30 years younger than the 79-year-old TAVR recipients in the study.
In a comment, Dr. Williams said there are several unanswered questions about the full impact TAVR could have in the treatment of young RHD patients in underprivileged regions. “These mainly concern the durability of the valves in individuals who could expect greater longevity than the typical heart valve patient in the USA, and the adaptation of transcatheter techniques to provide cost-effective treatment in regions that lack the usual sophisticated clinical infrastructure.”
Dr. Mentias received support from a National Research Service Award institutional grant to the Abboud Cardiovascular Research Center. Dr. Williams and coauthors are directors of Strait Access Technologies.
A version of this article first appeared on Medscape.com.
COVID-19 case fatality doubled in heart transplant patients
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart transplant recipients infected with SARS-CoV-2 are about twice as likely to die from COVID-19 and should be immediately referred to a transplant center for care, according to transplant experts from Northern Italy.
In a COVID Rapid Report published Dec. 9 in JACC Heart Failure, a group led by Tomaso Bottio, MD, PhD, from the University of Padua, Italy, presented findings on 47 heart transplant recipients who tested positive for SARS-Cov-2 between Feb. 21 and June 30.
The investigators found a case fatality rate of 29.7%, compared with 15.4% in the general population. Prevalence of infection was also much higher at 18 cases (vs. 7) per 1,000 population.
“In our opinion, prompt referral to a heart transplant center is crucial for immunosuppressive therapy optimization and cardiologic follow-up,” Dr. Bottio said in an interview.
Beyond the need for careful adjustment of immunosuppression, graft function should be assessed to “avoid acute rejection or decompensation,” he added.
Dr. Bottio and colleagues tracked COVID-19 cases from among the 2,676 heart transplant recipients alive before the onset of the pandemic at seven heart transplant centers in Northern Italy.
Of the 47 recipients who contracted SARS-CoV-2, 38 required hospitalization while 9 remained at home and 14 died. Mean length of stay in hospital was 17.8 days, much longer in survivors than nonsurvivors (23.2 days vs. 8.5 days; P < .001).
Nonsurvivors were significantly older than survivors (72 vs. 58 years; P = .002). Nonsurvivors were also more likely to present with diabetes (P = .04), extra-cardiac arteriopathy (P = .04), previous percutaneous coronary intervention (P = .04), more allograft vasculopathy (P = .04), and more symptoms of heart failure (P = .02).
Although the authors said the high case fatality rate was, unfortunately, expected, they did not expect so many patients to do well at home.
“What most surprised us was the proportion of a- or pauci-symptomatic heart transplanted patients who did well being treated at home without any therapy modifications,” Dr. Bottio shared. They were also surprised to see there were no cases of graft failure caused by infection-related myocarditis.
These findings from Northern Italy are not dissimilar from the 25% case fatality rate seen in a cohort of heart transplant recipients who caught COVID-19 in New York City early in the pandemic.
In another study, this time looking at a wider group of solid organ transplant recipients with SARS-CoV-2 infection at two centers during the first 3 weeks of the outbreak in New York City, 16 of 90 patients (18%) died.
Treatment recommendations?
Recognizing that there is no randomized trial data informing the treatment of this vulnerable patient population, Dr. Bottio and colleagues suggested that, based on their experience, no change in immunosuppression is needed in those who are “pauci-symptomatic” (mildly symptomatic).
“On the other hand, in hospitalized patients a partial reduction in immunosuppressive therapy avoiding full discontinuation and risk of graft rejection seems to be a common strategy in facing the viral infection,” he said. “In addition, the introduction of corticosteroids could help to suspend the onset of the inflammatory cascade responsible for severe forms of the disease.”
Antibiotic prophylaxis appears to be “fundamental,” he added, particularly in hospitalized patients, but “the role of specific antiviral therapies is still not fully understood in our population.”
Since July 1, they’ve seen an additional six patients with a positive test for SARS-CoV-2. Five were asymptomatic and quarantined at home without changing their immunosuppressive therapy. One patient was hospitalized for pneumonia and had immunosuppressive therapy reduced.
Dr. Bottio and the study coauthors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New-onset AFib common but unrecognized in the month after cardiac surgery
One in five patients at elevated stroke risk who underwent cardiac surgery with no history of atrial fibrillation preoperatively or at discharge developed postoperative AFib documented on a continuous cardiac rhythm monitoring device within the first 30 days after leaving the hospital in the randomized SEARCH-AF trial.
“Postoperative atrial fibrillation after cardiac surgery is not confined to the hospitalization period per se. We believe that these data should help inform on clinical practice guidelines on monitoring for postoperative atrial fibrillation in such patients,” said Subodh Verma, MD, PhD, reporting the results at the virtual American Heart Association scientific sessions.
“Guidelines provide little or no direction on optimal monitoring post cardiac surgery, particularly if patients are in sinus rhythm at discharge,” the surgeon noted.
SEARCH-AF was an open-label, multicenter study that included 336 patients at elevated stroke risk with an average CHA2DS2-VASc score of 4, no history of preoperative AFib, and none more than briefly with resolution during hospitalization. They were randomized to 30 days of postdischarge continuous cardiac rhythm monitoring with Medtronic’s SEEQ device, to Icentia’s CardioSTAT device, or to usual care, with Holter monitoring at the discretion of the treating physicians.
The primary result was a cumulative duration of AFib or atrial flutter of 6 minutes or longer during that 30-day period. This outcome occurred in 19.6% of the enhanced cardiac monitoring group and 1.7% of usual-care controls. Thus, there is an ongoing persistent occult risk of AFib that typically goes unrecognized. This 10-fold difference in the incidence of postoperative AFib translated into an absolute 17.9% between-group difference and a number-needed-to-treat of 6.
The secondary outcome of a cumulative atrial fib/flutter burden of 6 hours or more during 30 days occurred in 8.6% of the continuously monitored group and none of the controls. A cumulative AFib/flutter burden of 24 hours or greater occurred in 3.1% of the enhanced cardiac monitoring group and zero controls. These are AFib burdens that in other studies have been linked to increased risks of stroke and death, said Dr. Verma, professor of cardiovascular surgery at the University of Toronto.
“From a clinical standpoint, what this trial tells me is for my patients being discharged home tomorrow from the hospital, where they haven’t had AFib and I haven’t initiated anticoagulation, I have a low threshold to monitor these patients and to watch for periods of sustained unrecognized atrial fibrillation,” the surgeon added.
Experts: Results won’t change guidelines
Discussant Ben Freedman, MBBS, PhD, noted that the U.S. Preventive Services Task Force has stated that there are insufficient data available to recommend ECG screening for AFib to prevent stroke. Before the task force can be convinced to recommend it and for payers to cover it, a number of key questions need to be answered. And the SEARCH-AF trial doesn’t provide those answers, said Dr. Freedman, professor of cardiology and deputy director of the Heart Research Institute at the University of Sydney.
First off, it’ll be necessary to know if the risk posed by screen-detected AFib, including postoperative AFib, is similar to that of clinical AFib. Next, it must be shown that this screen-detected postoperative AFib is actionable; that is, that a screening strategy to detect postoperative AFib arising after discharge and then treat with oral anticoagulants will actually prevent more strokes than with usual care. There are large studies underway addressing that question, including HEARTLINE, STROKESTOP, and SAFERGUARD-AF, he observed.
In an interview, Rod S. Passman, MD, who gave a state-of-the-art talk on AFib detection at the meeting and wasn’t involved in SEARCH-AF, said he doesn’t consider the results practice-changing.
“It’s not guideline-changing because you’ve only shown that more intensive monitoring finds more AFib. Guideline-changing would be that finding that AFib and doing something about it impacts hard outcomes, and we don’t have that data yet,” said Dr. Passman, an electrophysiologist who is director of the Center for Arrhythmia Research and professor of medicine and preventive medicine at Northwestern University, Chicago.
The SEARCH-AF trial was funded by the Heart and Stroke Foundation of Canada, Bristol Myers Squibb, Pfizer, and Boehringer Ingelheim. Dr. Verma reported having received speaker’s fees and/or research support from those and other pharmaceutical companies. Dr. Freedman disclosed having no financial conflicts.
One in five patients at elevated stroke risk who underwent cardiac surgery with no history of atrial fibrillation preoperatively or at discharge developed postoperative AFib documented on a continuous cardiac rhythm monitoring device within the first 30 days after leaving the hospital in the randomized SEARCH-AF trial.
“Postoperative atrial fibrillation after cardiac surgery is not confined to the hospitalization period per se. We believe that these data should help inform on clinical practice guidelines on monitoring for postoperative atrial fibrillation in such patients,” said Subodh Verma, MD, PhD, reporting the results at the virtual American Heart Association scientific sessions.
“Guidelines provide little or no direction on optimal monitoring post cardiac surgery, particularly if patients are in sinus rhythm at discharge,” the surgeon noted.
SEARCH-AF was an open-label, multicenter study that included 336 patients at elevated stroke risk with an average CHA2DS2-VASc score of 4, no history of preoperative AFib, and none more than briefly with resolution during hospitalization. They were randomized to 30 days of postdischarge continuous cardiac rhythm monitoring with Medtronic’s SEEQ device, to Icentia’s CardioSTAT device, or to usual care, with Holter monitoring at the discretion of the treating physicians.
The primary result was a cumulative duration of AFib or atrial flutter of 6 minutes or longer during that 30-day period. This outcome occurred in 19.6% of the enhanced cardiac monitoring group and 1.7% of usual-care controls. Thus, there is an ongoing persistent occult risk of AFib that typically goes unrecognized. This 10-fold difference in the incidence of postoperative AFib translated into an absolute 17.9% between-group difference and a number-needed-to-treat of 6.
The secondary outcome of a cumulative atrial fib/flutter burden of 6 hours or more during 30 days occurred in 8.6% of the continuously monitored group and none of the controls. A cumulative AFib/flutter burden of 24 hours or greater occurred in 3.1% of the enhanced cardiac monitoring group and zero controls. These are AFib burdens that in other studies have been linked to increased risks of stroke and death, said Dr. Verma, professor of cardiovascular surgery at the University of Toronto.
“From a clinical standpoint, what this trial tells me is for my patients being discharged home tomorrow from the hospital, where they haven’t had AFib and I haven’t initiated anticoagulation, I have a low threshold to monitor these patients and to watch for periods of sustained unrecognized atrial fibrillation,” the surgeon added.
Experts: Results won’t change guidelines
Discussant Ben Freedman, MBBS, PhD, noted that the U.S. Preventive Services Task Force has stated that there are insufficient data available to recommend ECG screening for AFib to prevent stroke. Before the task force can be convinced to recommend it and for payers to cover it, a number of key questions need to be answered. And the SEARCH-AF trial doesn’t provide those answers, said Dr. Freedman, professor of cardiology and deputy director of the Heart Research Institute at the University of Sydney.
First off, it’ll be necessary to know if the risk posed by screen-detected AFib, including postoperative AFib, is similar to that of clinical AFib. Next, it must be shown that this screen-detected postoperative AFib is actionable; that is, that a screening strategy to detect postoperative AFib arising after discharge and then treat with oral anticoagulants will actually prevent more strokes than with usual care. There are large studies underway addressing that question, including HEARTLINE, STROKESTOP, and SAFERGUARD-AF, he observed.
In an interview, Rod S. Passman, MD, who gave a state-of-the-art talk on AFib detection at the meeting and wasn’t involved in SEARCH-AF, said he doesn’t consider the results practice-changing.
“It’s not guideline-changing because you’ve only shown that more intensive monitoring finds more AFib. Guideline-changing would be that finding that AFib and doing something about it impacts hard outcomes, and we don’t have that data yet,” said Dr. Passman, an electrophysiologist who is director of the Center for Arrhythmia Research and professor of medicine and preventive medicine at Northwestern University, Chicago.
The SEARCH-AF trial was funded by the Heart and Stroke Foundation of Canada, Bristol Myers Squibb, Pfizer, and Boehringer Ingelheim. Dr. Verma reported having received speaker’s fees and/or research support from those and other pharmaceutical companies. Dr. Freedman disclosed having no financial conflicts.
One in five patients at elevated stroke risk who underwent cardiac surgery with no history of atrial fibrillation preoperatively or at discharge developed postoperative AFib documented on a continuous cardiac rhythm monitoring device within the first 30 days after leaving the hospital in the randomized SEARCH-AF trial.
“Postoperative atrial fibrillation after cardiac surgery is not confined to the hospitalization period per se. We believe that these data should help inform on clinical practice guidelines on monitoring for postoperative atrial fibrillation in such patients,” said Subodh Verma, MD, PhD, reporting the results at the virtual American Heart Association scientific sessions.
“Guidelines provide little or no direction on optimal monitoring post cardiac surgery, particularly if patients are in sinus rhythm at discharge,” the surgeon noted.
SEARCH-AF was an open-label, multicenter study that included 336 patients at elevated stroke risk with an average CHA2DS2-VASc score of 4, no history of preoperative AFib, and none more than briefly with resolution during hospitalization. They were randomized to 30 days of postdischarge continuous cardiac rhythm monitoring with Medtronic’s SEEQ device, to Icentia’s CardioSTAT device, or to usual care, with Holter monitoring at the discretion of the treating physicians.
The primary result was a cumulative duration of AFib or atrial flutter of 6 minutes or longer during that 30-day period. This outcome occurred in 19.6% of the enhanced cardiac monitoring group and 1.7% of usual-care controls. Thus, there is an ongoing persistent occult risk of AFib that typically goes unrecognized. This 10-fold difference in the incidence of postoperative AFib translated into an absolute 17.9% between-group difference and a number-needed-to-treat of 6.
The secondary outcome of a cumulative atrial fib/flutter burden of 6 hours or more during 30 days occurred in 8.6% of the continuously monitored group and none of the controls. A cumulative AFib/flutter burden of 24 hours or greater occurred in 3.1% of the enhanced cardiac monitoring group and zero controls. These are AFib burdens that in other studies have been linked to increased risks of stroke and death, said Dr. Verma, professor of cardiovascular surgery at the University of Toronto.
“From a clinical standpoint, what this trial tells me is for my patients being discharged home tomorrow from the hospital, where they haven’t had AFib and I haven’t initiated anticoagulation, I have a low threshold to monitor these patients and to watch for periods of sustained unrecognized atrial fibrillation,” the surgeon added.
Experts: Results won’t change guidelines
Discussant Ben Freedman, MBBS, PhD, noted that the U.S. Preventive Services Task Force has stated that there are insufficient data available to recommend ECG screening for AFib to prevent stroke. Before the task force can be convinced to recommend it and for payers to cover it, a number of key questions need to be answered. And the SEARCH-AF trial doesn’t provide those answers, said Dr. Freedman, professor of cardiology and deputy director of the Heart Research Institute at the University of Sydney.
First off, it’ll be necessary to know if the risk posed by screen-detected AFib, including postoperative AFib, is similar to that of clinical AFib. Next, it must be shown that this screen-detected postoperative AFib is actionable; that is, that a screening strategy to detect postoperative AFib arising after discharge and then treat with oral anticoagulants will actually prevent more strokes than with usual care. There are large studies underway addressing that question, including HEARTLINE, STROKESTOP, and SAFERGUARD-AF, he observed.
In an interview, Rod S. Passman, MD, who gave a state-of-the-art talk on AFib detection at the meeting and wasn’t involved in SEARCH-AF, said he doesn’t consider the results practice-changing.
“It’s not guideline-changing because you’ve only shown that more intensive monitoring finds more AFib. Guideline-changing would be that finding that AFib and doing something about it impacts hard outcomes, and we don’t have that data yet,” said Dr. Passman, an electrophysiologist who is director of the Center for Arrhythmia Research and professor of medicine and preventive medicine at Northwestern University, Chicago.
The SEARCH-AF trial was funded by the Heart and Stroke Foundation of Canada, Bristol Myers Squibb, Pfizer, and Boehringer Ingelheim. Dr. Verma reported having received speaker’s fees and/or research support from those and other pharmaceutical companies. Dr. Freedman disclosed having no financial conflicts.
FROM AHA 2020
Who’ll get SAVR in 2020?
SNOWMASS, COLO. – The number of transcatheter aortic valve replacements (TAVRs) performed annually in the United States is forecast to rocket up from 75,000 in 2019 to 100,000 in 2020 in response to the procedure’s recent approval in low-surgical-risk patients with symptomatic aortic stenosis, Michael J. Mack, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“In 2020, TAVR seems like a tsunami that’s totally overwhelming SAVR [surgical aortic valve replacement]. And the question is, after the wave hits shore, is there going to be anything left in the surgical arena?” asked Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
He answered his own question with a quote from Mark Twain: “Reports of my death are greatly exaggerated.”
The trend is clear: TAVR will take over the market for isolated aortic valve replacement in much the same way that endovascular abdominal aortic aneurysm repair (EVAR) has come to dominate open surgical repair by an 80:20 margin. And By one estimate, it could include some 270,000 individuals per year in North America and the European Union (Eur Heart J. 2018 Jul 21;39[28]:2635-42).
But there’s no need to shed a tear at the prospect of SAVR surgeons standing in unemployment lines. They will continue to have their hands full performing combined SAVR plus coronary artery bypass graft (CABG) procedures, SAVR plus mitral or tricuspid valve operations, and Bentall procedures, Dr. Mack predicted.
Who should get SAVR for aortic stenosis in 2020? For starters, he said, the sorts of patients who were excluded from the major TAVR-versus-SAVR randomized trials. The low-surgical-risk trials were restricted to patients who had symptomatic aortic stenosis involving a tricuspid valve, no left ventricular outflow tract calcium, no or minimal coronary artery disease (CAD), a relatively normal left ventricular ejection fraction, and an aortic valve anatomy suitable for TAVR. And, 92% of study participants were over age 65 years.
Dr. Mack called the evidence for the safety and effectiveness of TAVR “the most robust evidence base in the history of medical devices,” backed by nine U.S. trials and 8,000 randomized patients during the last dozen years. He has played a major role in developing that evidence base, having served most recently as cochair of the landmark PARTNER 3 trial, which demonstrated superiority for TAVR over SAVR in low-surgical-risk patients. But the evidence base doesn’t apply to patients not enrolled in the trials. So for the foreseeable future, patients younger than age 65 years should probably stick with SAVR, mainly because of the still-open question of tissue valve durability and TAVR’s high rate of associated conduction system impairment and need for new pacemaker implantation. Younger patients find permanent pacemakers particularly problematic, he noted.
Others who should stick with surgery include patients with bicuspid valves, especially when aortopathy is present, individuals with low-lying coronary arteries, patients with heavy calcium deposits at the left ventricular outflow tract, those with infective endocarditis or rheumatic valve disease, and patients with structural valve deterioration after a valve-in-valve TAVR.
“Once you get beyond the first valve-in-valve, the outcomes are not going to be good. Those patients should preferentially be considered for surgery. The results for valve-in-valve have been very disappointing, with a 33% all-cause mortality at 3 years in the PARTNER Aortic Valve-in-Valve Registry,” according to the surgeon.
In patients with aortic stenosis and CAD, the clinical decision making should be based on the coronary disease. In a patient with triple-vessel disease, diabetes, and/or a high Syntax score for whom the collaborative multidisciplinary heart team would recommend surgical revascularization if aortic stenosis wasn’t present, the most appropriate option is SAVR plus CABG. On the other hand, if the CAD is amenable to percutaneous coronary intervention (PCI) and the Syntax score is low, TAVR plus PCI is a safe and solid strategy, he continued.
In addition to the unresolved issue of tissue valve durability, another unanswered question pushing against universal adoption of TAVR involves the clinical implications of bioprosthetic valve leaflet thrombosis and the optimal antithrombotic therapy, both early and late. Leaflet thrombosis post-TAVR is common – as well as post-SAVR with bioprosthetic valves, albeit less so – but the lesions often come and go. Although there is a theoretical concern that they might be a precursor to leaflet destruction, at this point, their clinical significance remains unclear. In the recent GALILEO trial, TAVR patients randomized to low-dose rivaroxaban (Xarelto) plus aspirin showed fewer leaflet motion abnormalities and less leaflet thickening than did those on dual-antiplatelet therapy, but a significantly higher all-cause mortality (N Engl J Med 2020 Jan 9;382:120-9).
“I know that nowhere else in the body is thrombus a good thing, so thrombus in the valve can’t be a good thing. The only question is, how bad is it? And right now all we know is, some of our treatments for it are worse than the disease,” the surgeon commented.
Dr. Mack indicated that, at this time, clinical decision making in aortic stenosis should begin on the basis of patient age, which influences the key decision of whether to opt for a mechanical versus tissue replacement valve. For patients aged 50-70 years, shared decision making between the heart team and patient is appropriate. The evidence suggests SAVR with a mechanical valve is the better option, but many patients in this intermediate age group loathe the ideal of lifelong oral anticoagulation and favor a tissue valve.
For patients under age 50 years, the best evidence indicates that SAVR with a mechanical valve is clearly the best option; however, most young patients are instead opting for a tissue valve, even after being cautioned about the lingering uncertainty surrounding tissue valve durability, be it SAVR or TAVR. For patients over age 70 years, a tissue valve is the best choice based on the outcomes in PARTNER 3 and other low-surgical-risk trials. If the patient is younger than 65 years and wants a tissue valve, Dr. Mack thinks the best evidence-based option is SAVR. Above age 80 years, TAVR is the clear choice. Age 65-80 years is shared–decision making territory regarding TAVR versus SAVR.
Dr. Mack reported serving as a consultant to Gore and receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
SNOWMASS, COLO. – The number of transcatheter aortic valve replacements (TAVRs) performed annually in the United States is forecast to rocket up from 75,000 in 2019 to 100,000 in 2020 in response to the procedure’s recent approval in low-surgical-risk patients with symptomatic aortic stenosis, Michael J. Mack, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“In 2020, TAVR seems like a tsunami that’s totally overwhelming SAVR [surgical aortic valve replacement]. And the question is, after the wave hits shore, is there going to be anything left in the surgical arena?” asked Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
He answered his own question with a quote from Mark Twain: “Reports of my death are greatly exaggerated.”
The trend is clear: TAVR will take over the market for isolated aortic valve replacement in much the same way that endovascular abdominal aortic aneurysm repair (EVAR) has come to dominate open surgical repair by an 80:20 margin. And By one estimate, it could include some 270,000 individuals per year in North America and the European Union (Eur Heart J. 2018 Jul 21;39[28]:2635-42).
But there’s no need to shed a tear at the prospect of SAVR surgeons standing in unemployment lines. They will continue to have their hands full performing combined SAVR plus coronary artery bypass graft (CABG) procedures, SAVR plus mitral or tricuspid valve operations, and Bentall procedures, Dr. Mack predicted.
Who should get SAVR for aortic stenosis in 2020? For starters, he said, the sorts of patients who were excluded from the major TAVR-versus-SAVR randomized trials. The low-surgical-risk trials were restricted to patients who had symptomatic aortic stenosis involving a tricuspid valve, no left ventricular outflow tract calcium, no or minimal coronary artery disease (CAD), a relatively normal left ventricular ejection fraction, and an aortic valve anatomy suitable for TAVR. And, 92% of study participants were over age 65 years.
Dr. Mack called the evidence for the safety and effectiveness of TAVR “the most robust evidence base in the history of medical devices,” backed by nine U.S. trials and 8,000 randomized patients during the last dozen years. He has played a major role in developing that evidence base, having served most recently as cochair of the landmark PARTNER 3 trial, which demonstrated superiority for TAVR over SAVR in low-surgical-risk patients. But the evidence base doesn’t apply to patients not enrolled in the trials. So for the foreseeable future, patients younger than age 65 years should probably stick with SAVR, mainly because of the still-open question of tissue valve durability and TAVR’s high rate of associated conduction system impairment and need for new pacemaker implantation. Younger patients find permanent pacemakers particularly problematic, he noted.
Others who should stick with surgery include patients with bicuspid valves, especially when aortopathy is present, individuals with low-lying coronary arteries, patients with heavy calcium deposits at the left ventricular outflow tract, those with infective endocarditis or rheumatic valve disease, and patients with structural valve deterioration after a valve-in-valve TAVR.
“Once you get beyond the first valve-in-valve, the outcomes are not going to be good. Those patients should preferentially be considered for surgery. The results for valve-in-valve have been very disappointing, with a 33% all-cause mortality at 3 years in the PARTNER Aortic Valve-in-Valve Registry,” according to the surgeon.
In patients with aortic stenosis and CAD, the clinical decision making should be based on the coronary disease. In a patient with triple-vessel disease, diabetes, and/or a high Syntax score for whom the collaborative multidisciplinary heart team would recommend surgical revascularization if aortic stenosis wasn’t present, the most appropriate option is SAVR plus CABG. On the other hand, if the CAD is amenable to percutaneous coronary intervention (PCI) and the Syntax score is low, TAVR plus PCI is a safe and solid strategy, he continued.
In addition to the unresolved issue of tissue valve durability, another unanswered question pushing against universal adoption of TAVR involves the clinical implications of bioprosthetic valve leaflet thrombosis and the optimal antithrombotic therapy, both early and late. Leaflet thrombosis post-TAVR is common – as well as post-SAVR with bioprosthetic valves, albeit less so – but the lesions often come and go. Although there is a theoretical concern that they might be a precursor to leaflet destruction, at this point, their clinical significance remains unclear. In the recent GALILEO trial, TAVR patients randomized to low-dose rivaroxaban (Xarelto) plus aspirin showed fewer leaflet motion abnormalities and less leaflet thickening than did those on dual-antiplatelet therapy, but a significantly higher all-cause mortality (N Engl J Med 2020 Jan 9;382:120-9).
“I know that nowhere else in the body is thrombus a good thing, so thrombus in the valve can’t be a good thing. The only question is, how bad is it? And right now all we know is, some of our treatments for it are worse than the disease,” the surgeon commented.
Dr. Mack indicated that, at this time, clinical decision making in aortic stenosis should begin on the basis of patient age, which influences the key decision of whether to opt for a mechanical versus tissue replacement valve. For patients aged 50-70 years, shared decision making between the heart team and patient is appropriate. The evidence suggests SAVR with a mechanical valve is the better option, but many patients in this intermediate age group loathe the ideal of lifelong oral anticoagulation and favor a tissue valve.
For patients under age 50 years, the best evidence indicates that SAVR with a mechanical valve is clearly the best option; however, most young patients are instead opting for a tissue valve, even after being cautioned about the lingering uncertainty surrounding tissue valve durability, be it SAVR or TAVR. For patients over age 70 years, a tissue valve is the best choice based on the outcomes in PARTNER 3 and other low-surgical-risk trials. If the patient is younger than 65 years and wants a tissue valve, Dr. Mack thinks the best evidence-based option is SAVR. Above age 80 years, TAVR is the clear choice. Age 65-80 years is shared–decision making territory regarding TAVR versus SAVR.
Dr. Mack reported serving as a consultant to Gore and receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
SNOWMASS, COLO. – The number of transcatheter aortic valve replacements (TAVRs) performed annually in the United States is forecast to rocket up from 75,000 in 2019 to 100,000 in 2020 in response to the procedure’s recent approval in low-surgical-risk patients with symptomatic aortic stenosis, Michael J. Mack, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“In 2020, TAVR seems like a tsunami that’s totally overwhelming SAVR [surgical aortic valve replacement]. And the question is, after the wave hits shore, is there going to be anything left in the surgical arena?” asked Dr. Mack, who is medical director of cardiothoracic surgery and chairman of the Baylor Scott & White The Heart Hospital – Plano (Tex.) Research Center.
He answered his own question with a quote from Mark Twain: “Reports of my death are greatly exaggerated.”
The trend is clear: TAVR will take over the market for isolated aortic valve replacement in much the same way that endovascular abdominal aortic aneurysm repair (EVAR) has come to dominate open surgical repair by an 80:20 margin. And By one estimate, it could include some 270,000 individuals per year in North America and the European Union (Eur Heart J. 2018 Jul 21;39[28]:2635-42).
But there’s no need to shed a tear at the prospect of SAVR surgeons standing in unemployment lines. They will continue to have their hands full performing combined SAVR plus coronary artery bypass graft (CABG) procedures, SAVR plus mitral or tricuspid valve operations, and Bentall procedures, Dr. Mack predicted.
Who should get SAVR for aortic stenosis in 2020? For starters, he said, the sorts of patients who were excluded from the major TAVR-versus-SAVR randomized trials. The low-surgical-risk trials were restricted to patients who had symptomatic aortic stenosis involving a tricuspid valve, no left ventricular outflow tract calcium, no or minimal coronary artery disease (CAD), a relatively normal left ventricular ejection fraction, and an aortic valve anatomy suitable for TAVR. And, 92% of study participants were over age 65 years.
Dr. Mack called the evidence for the safety and effectiveness of TAVR “the most robust evidence base in the history of medical devices,” backed by nine U.S. trials and 8,000 randomized patients during the last dozen years. He has played a major role in developing that evidence base, having served most recently as cochair of the landmark PARTNER 3 trial, which demonstrated superiority for TAVR over SAVR in low-surgical-risk patients. But the evidence base doesn’t apply to patients not enrolled in the trials. So for the foreseeable future, patients younger than age 65 years should probably stick with SAVR, mainly because of the still-open question of tissue valve durability and TAVR’s high rate of associated conduction system impairment and need for new pacemaker implantation. Younger patients find permanent pacemakers particularly problematic, he noted.
Others who should stick with surgery include patients with bicuspid valves, especially when aortopathy is present, individuals with low-lying coronary arteries, patients with heavy calcium deposits at the left ventricular outflow tract, those with infective endocarditis or rheumatic valve disease, and patients with structural valve deterioration after a valve-in-valve TAVR.
“Once you get beyond the first valve-in-valve, the outcomes are not going to be good. Those patients should preferentially be considered for surgery. The results for valve-in-valve have been very disappointing, with a 33% all-cause mortality at 3 years in the PARTNER Aortic Valve-in-Valve Registry,” according to the surgeon.
In patients with aortic stenosis and CAD, the clinical decision making should be based on the coronary disease. In a patient with triple-vessel disease, diabetes, and/or a high Syntax score for whom the collaborative multidisciplinary heart team would recommend surgical revascularization if aortic stenosis wasn’t present, the most appropriate option is SAVR plus CABG. On the other hand, if the CAD is amenable to percutaneous coronary intervention (PCI) and the Syntax score is low, TAVR plus PCI is a safe and solid strategy, he continued.
In addition to the unresolved issue of tissue valve durability, another unanswered question pushing against universal adoption of TAVR involves the clinical implications of bioprosthetic valve leaflet thrombosis and the optimal antithrombotic therapy, both early and late. Leaflet thrombosis post-TAVR is common – as well as post-SAVR with bioprosthetic valves, albeit less so – but the lesions often come and go. Although there is a theoretical concern that they might be a precursor to leaflet destruction, at this point, their clinical significance remains unclear. In the recent GALILEO trial, TAVR patients randomized to low-dose rivaroxaban (Xarelto) plus aspirin showed fewer leaflet motion abnormalities and less leaflet thickening than did those on dual-antiplatelet therapy, but a significantly higher all-cause mortality (N Engl J Med 2020 Jan 9;382:120-9).
“I know that nowhere else in the body is thrombus a good thing, so thrombus in the valve can’t be a good thing. The only question is, how bad is it? And right now all we know is, some of our treatments for it are worse than the disease,” the surgeon commented.
Dr. Mack indicated that, at this time, clinical decision making in aortic stenosis should begin on the basis of patient age, which influences the key decision of whether to opt for a mechanical versus tissue replacement valve. For patients aged 50-70 years, shared decision making between the heart team and patient is appropriate. The evidence suggests SAVR with a mechanical valve is the better option, but many patients in this intermediate age group loathe the ideal of lifelong oral anticoagulation and favor a tissue valve.
For patients under age 50 years, the best evidence indicates that SAVR with a mechanical valve is clearly the best option; however, most young patients are instead opting for a tissue valve, even after being cautioned about the lingering uncertainty surrounding tissue valve durability, be it SAVR or TAVR. For patients over age 70 years, a tissue valve is the best choice based on the outcomes in PARTNER 3 and other low-surgical-risk trials. If the patient is younger than 65 years and wants a tissue valve, Dr. Mack thinks the best evidence-based option is SAVR. Above age 80 years, TAVR is the clear choice. Age 65-80 years is shared–decision making territory regarding TAVR versus SAVR.
Dr. Mack reported serving as a consultant to Gore and receiving research grants from Abbott Vascular, Edwards Lifesciences, and Medtronic.
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Opioid deaths boost donor heart supply
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Why STEMI patients benefit from PCI of nonculprit lesions
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
PHILADELPHIA – Nearly half of patients with ST-elevation MI and multivessel coronary artery disease in the landmark COMPLETE trial had an obstructive coronary lesion with vulnerable plaque morphology in a segment far from the culprit lesion, Natalia Pinilla-Echeverri, MD, reported at the American Heart Association scientific sessions.
This novel finding from an optical coherence tomography (OCT) substudy of COMPLETE provides a likely mechanistic explanation for the major clinical benefits documented in the full COMPLETE trial, noted Dr. Pinilla-Echeverri, a cardiologist at the Population Health Research Institute at McMaster University, Hamilton, Ont.
COMPLETE was a multinational trial which randomized 4,041 ST-elevation MI (STEMI) patients with multivessel disease to culprit lesion–only percutaneous coronary intervention (PCI) or additional routine angiography–guided staged PCI of nonculprit obstructive lesions with at least 70% stenosis. As previously reported, the risk of the coprimary composite endpoint comprising cardiovascular death, new MI, or ischemia-driven revascularization was reduced by 49% over 3 years of follow-up in the group with staged PCI of nonculprit lesions, with an impressive number needed to treat of just 13 (N Engl J Med. 2019 Oct 10;381[15]:1411-21).
Dr. Pinella-Echeverri reported on the 93 patients who participated in the OCT substudy, the purpose of which was to determine the prevalence of high-risk, vulnerable plaque in obstructive and nonobstructive nonculprit lesions. For this purpose, vulnerable plaque was defined as thin-cap fibroatheroma (TCFA), a coronary lesion known to pose high risk of worsening stenosis, plaque rupture, and cardiovascular events.
Of note, these 93 patients had a total of 425 diseased segments: 150 obstructive and 275 nonobstructive.
“This is reassuring that the concept of acute coronary syndrome implies a diffuse pathophysiology of affecting not only the culprit segment but the coronary vasculature as a whole,” Dr. Pinella-Echeverri observed.
The main study finding, however, was that TCFA was significantly more prevalent in obstructive, compared with nonobstructive, nonculprit lesions by a margin of 35% to 23%. The obstructive and nonobstructive TCFA lesions had a similar lipid-rich composition; however, the obstructive ones were significantly longer and had a smaller mean lumen area.
When breaking down the prevalence of TCFA per patient, 47% of patients had a nonculprit obstructive lesion with vulnerable plaque morphology. Another 20% had nonobstructive TCFA lesions. And only 32% of the STEMI patients had no TCFA in their obstructive or nonobstructive segments.
Discussant Frans Van de Werf, MD, PhD, commented: “This [OCT substudy result] immediately explains the clinical benefit observed with preventive PCI in STEMI patients with obstructive multivessel disease.”
The finding that 20% of the STEMI patients had nonobstructive lesions with vulnerable plaque morphology by OCT provides powerful support for the current guideline-recommended strategy of immediately starting STEMI patients on intensive lipid-lowering therapy, added Dr. Van de Werf, professor of medicine at the Catholic University of Leuven (Belgium).
He argued that the decision to revascularize nonculprit lesions by means of PCI versus the more complete revascularization achieved via coronary artery bypass graft surgery shouldn’t be made during the initial primary PCI, citing evidence that when the decision gets made at that time, coronary artery bypass grafting (CABG) is less likely to be chosen.
“I believe that OCT and [fractional flow reserve] should not be performed during the index primary PCI, not only for the comfort of the patient, but also for the better selection of complete revascularization. Interventional cardiologists should not forget that CABG might be a better revascularization treatment in some cases, such as left main disease and diabetes mellitus,” the cardiologist cautioned.
The COMPLETE OCT Substudy was supported by Abbott Vascular, the Population Health Research Institute, Hamilton Health Sciences, and the Canadian Institutes of Health Research.
REPORTING FROM AHA 2019















