User login
CDC Report Confirms Hospitalists’ Role in Fight against Antibiotic-Resistant Pathogens
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Describing the enormity of the problem of antibiotic resistance and warning of the “potentially catastrophic consequences of inaction,” the Centers for Disease Control and Prevention (CDC) announced in September that more than 2 million people a year are sickened by infections that are resistant to treatment with antibiotics.
Moreover, the CDC says 23,000 people die as a result.
The report is a call to action for hospitalists, who are in a position to participate in efforts to prevent infections and control their spread once they’re discovered, says Jean Patel, PhD, deputy director of the office of antimicrobial resistance at the CDC. She also says the medical community cannot expect that new treatments will become available to fight all of these new infections.

—Robert Orenstein, DO, infectious disease expert, Mayo Clinic, Rochester, Minn.
“All of the drugs also are going to have some gaps in their range of activity” Dr. Patel explains. “For that reason, we’re sounding the alarm that it’s important to pay attention to infection control and antibiotic stewardship practices.”
The report, “Antibiotic Resistance Threats to the United States, 2013” creates three categories of antibiotic-resistant pathogens. In the “urgent” tier are Clostridium difficile, which the CDC estimates is responsible for 250,000 infections a year and 14,000 deaths; carbapenem-resistant Enterobacteriaceae, estimated to be responsible for 9,000 infections a year and 600 deaths; and Neisseria gonorrhoeae, at 246,000 infections.
These bacteria are considered an “immediate public health threat that requires urgent and aggressive action.”
Twelve pathogens in the second category, described as “a serious concern,” require “prompt and sustained action to ensure the problem does not grow.” Of particular interest to hospitalists in this group, Dr. Patel says, is methicillin-resistant Staphylococcus aureus (MRSA). The CDC estimates that more than 80,000 severe MRSA infections and more than 11,000 deaths occur in the U.S. every year.
MRSA was not ranked as an “urgent” threat only because the number of infections is decreasing, and because there are antibiotics that still work on MRSA.
Another infection that should be on hospitalists’ radar is Streptococcus pneumoniae. A new vaccine is helping to decrease the number of these infections, but hospitalists should be vigilant about infections that could escape the vaccine and become resistant, Dr. Patel says.
Ketino Kobaidze, MD, assistant professor at the Emory University School of Medicine in Atlanta and a member of the antimicrobial stewardship and infectious disease control committees at Emory University Hospital Midtown, says the most important thing for hospitalists “is to follow up with whatever you’re ordering and notice right away what happens with these tests. If it’s positive or negative, redirect your care.”
Robert Orenstein, DO, an infectious disease expert at Mayo Clinic, praises the report and says hospitalists have a key role to play. “We need to educate them and build systems that target antimicrobials to the infecting agents and limit their use,” he says. TH
Tom Collins is a freelance writer in South Florida.
Universal MRSA Decolonization in ICU Leads to Fewer Bloodstream Infections
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Clinical question
Does universal decolonization for methicillin-resistant Staphylococcus aureus (MRSA) in patients in the intensive care unit decrease the rate of MRSA-positive clinical cultures?
Bottom line
As compared with no decolonization or a targeted decolonization, a universal decolonization strategy for MRSA using intranasal mupirocin and chlorhexidine bathing cloths for all patients admitted to the intensive care unit (ICU) is most effective at decreasing MRSA-positive clinical cultures and ICU-acquired bloodstream infections. Overall, you would need to treat 54 patients with universal decolonization to prevent one bloodstream infection. The cost effectiveness of this strategy as well as the concern of emerging resistance was not addressed in this study. (LOE = 1b-)
Reference
Study design
Randomized controlled trial (nonblinded)
Funding source
Government
Allocation
Uncertain
Setting
Inpatient (ICU only)
Synopsis
Prior research has shown that daily bathing with chlorhexidine lowers the rate of MRSA acquisition and decreases the overall number of hospital-acquired bloodstream infections in the ICU (Daily POEM 4/26/13). The current study's goal was to identify whether targeted or universal MRSA decolonization is the most effective at reducing MRSA infections in the ICU. Investigators randomized 43 hospitals to use 1 of 3 strategies within all their adult ICUs: (1) MRSA screening and contact isolation only; (2) screening, isolation, and decolonization of MRSA carriers; (3) decolonization of all patients without any screening procedures. Screening for MRSA was performed via swabs of bilateral nares upon ICU admission in the first 2 groups. Contact precautions were implemented for those with a positive MRSA screening result in groups 1 and 2 and for those with history of MRSA colonization or infection in all groups. Decolonization in groups 2 and 3 consisted of 5 days of twice-daily intranasal mupirocin, as well as daily bathing with chlorhexidine cloths during the entire ICU stay. Baseline characteristics of the patient populations in each group were similar. Patients in all adult ICUs of a participating hospital were assigned to the same study group. Although both universal and targeted decolonization resulted in a significant reduction in the primary outcome of MRSA-positive clinical cultures, the universal strategy was found to be most effective (hazard ratio [HR] = 0.63 for the universal strategy; HR = 0.75 for the targeted strategy; and HR = 0.92 for screening and isolation; P = .01). Additionally, universal decolonization led to the greatest reduction of overall bloodstream infections (HR = 0.56 for universal; HR = 0.78 for targeted; HR = 0.99 for screening and isolation; P < .001). Of note, the universal decolonization group contained 3 of the 4 hospitals that performed bone marrow and solid-organ transplantations, resulting in a higher baseline risk of infection than the other groups, but this difference was not statistically significant. Overall, only severe adverse events were noted in this study and all were classified as mild pruritus or rash due to chlorhexidine bathing. Investigators did not evaluate the cost-effectiveness of the different strategies nor did they examine the emergence of resistance with widespread use of chlorhexidine and mupirocin.
Dr. Kulkarni is an assistant professor of hospital medicine at Northwestern University in Chicago.
Surgical-Site-Infection Risk Not Associated with Prophylactic Antibiotic Timing
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005 to 2009 were reviewed. A post-operative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated.
In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Visit our website for more physician reviews of recent HM-relevant literature.
Surgical-Site Infection Risk Not Associated with Prophylactic Antibiotic Timing
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Clinical question: How does timing of surgical antibiotic prophylaxis affect risk of postoperative surgical-site infections (SSIs)?
Background: Antibiotic prophylaxis for major surgical procedures has been proven in clinical trials to reduce rates of SSI. The Centers for Medicare & Medicaid Services’ (CMS) Surgical Care Improvement Project (SCIP) has implemented quality metrics to ensure antibiotics are administered within 60 minutes of incision; however, studies have failed to show that a 60-minute pre-incision window is advantageous.
Study design: Retrospective cohort.
Setting: Veterans Affairs hospitals.
Synopsis: Using SCIP and VA Surgical Quality Improvement Program data from 112 VA hospitals, 32,459 cases of hip or knee arthroplasty, colorectal surgery, arterial vascular surgery, and hysterectomy from 2005-2009 were reviewed. A postoperative SSI occurred in 1,497 cases (4.6%). Using several statistical methods, the relationship between timing of prophylactic antibiotic administration and postoperative SSI within 30 days was evaluated. In unadjusted models, higher SSI rates were observed with antibiotic administration more than 60 minutes prior to incision (OR 1.34, 95% CI 1.08-1.66) but not after incision (OR 1.26, 95% CI 0.92-1.72), compared with procedures with antibiotics administered within 60 minutes pre-incision. However, after adjustment for patient, procedure, and antibiotic variables, no significant relationship between timing and SSI was observed (P=0.50 for all specialties).
The study sample was comprised primarily of older men and did not include patients who underwent cardiac procedures, limiting the generalizability of the findings. Nonetheless, the study is the largest of its kind and confirms previous studies that suggest there is no significant relationship between timing of antibiotics and SSI. Prophylactic antibiotics should still be used when indicated; however, using timing of prophylactic antibiotics as a quality measure is unlikely to improve outcomes.
Bottom line: Adherence to the empiric 60-minute window metric for timing of prophylactic antibiotics is not significantly associated with risk of SSI.
Citation: Hawn MT, Richman JS, Vick CC, et al. Timing of surgical antibiotic prophylaxis and the risk of surgical site infection. JAMA Surg. 2013 March 20:1-8. doi: 10.1001/jamasurg.2013.134 [Epub ahead of print].
Probiotics prevent C. diff-associated diarrhea in patients taking antibiotics
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.
Clinical question
Does the use of probiotics prevent Clostridium difficile-associated diarrhea in patients taking antibiotics?
Bottom line
Moderate-quality evidence suggests that probiotic administration reduces the incidence of C. difficile-associated diarrhea (CDAD) in patients who are taking antibiotics. LOE = 1a-
Reference
Study Design
Meta-analysis (other)
Funding Source
None
Setting
Various (meta-analysis)
Synopsis
These investigators searched multiple databases, including the Cochrane Register, MEDLINE, EMBASE, as well as reviewed bibliographies of relevant articles and spoke to experts in the field, to find randomized controlled trials that compared probiotics with placebo in reducing the incidence of CDAD in patients taking antibiotics. Two reviewers independently selected the articles, extracted data, and assessed study quality. Half of the 20 studies selected had either an unclear or high risk of bias; 7 studies had an overall low risk of bias. Patients included in the individual studies (N = 3818) varied in age and baseline risk of CDAD. Meta-analysis of the data showed that probiotics, as compared with placebo, reduced the incidence of CDAD in patients taking antibiotics (relative risk = 0.34; 95% CI, 0.24-0.49). Subgroup analyses showed similar results in adults and children, with lower and higher doses of probiotics, and with different probiotic species. There was no evidence of an increased risk of adverse events in the probiotics group. The majority of the studies excluded immunocompromised patients, thus limiting the generalizability of the results. Addtionally, the authors downrated the level of evidence to moderate quality because the overall sample size was smaller than what would be required for an optimally powered single study, which decreases the precision of the results.
Discordant Antibiotic Use in Pediatric UTIs Associated with Higher LOS
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Discordant antibiotic therapy for urinary tract infections (UTIs) is common and associated with higher length of stay (LOS) in hospitalized children, according to a study published online last month in the Journal of Hospital Medicine. But lead author Karen Jerardi, MD, division of hospital medicine at Cincinnati Children's Hospital Medical Center says the reason might be related to physicians, not their patients.
"First, use our knowledge of local resistance patterns and patient factors to select an antibiotic likely to be concordant," she says. "The second thing is [that] we probably need to analyze our practice a little bit more and try to figure out if we are just keeping patients in the hospital because we want to see them be on the concordant antibiotic for X number of hours before we send them home. Does that benefit the patient more, or are we keeping them in the hospital longer for our own peace of mind?"
The report, "Discordant Antibiotic Therapy and Length of Stay in Children Hospitalized for Urinary Tract Infection," found that discordant therapy occurred in 10% of cases in which patients had laboratory-confirmed UTIs and, in adjusted analyses, was associated with a 1.8-day increase in LOS.
Dr. Jerardi says that future studies are needed to determine whether pediatric hospitalists are extending LOS by keeping patients longer than absolutely necessary. She cautions, though, that how long a child is kept in the hospital should be determined by case-specific circumstances.
"Hopefully, this will make people analyze how they do things," she adds, "and think to themselves, 'Would I keep that patient an extra day longer because I had to switch their antibiotic—even if their fever went away, they were drinking great, and Mom and Dad were ready to go home—just for my peace of mind?'"
Guidelines for Pneumonia Call for Decreased Use of Broad-Spectrum Antibiotics
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
Clinical question: What is the impact of a clinical practice guideline for hospitalized children with community-acquired pneumonia (CAP) on antibiotic selection?
Background: CAP is one of the most common reasons for hospitalizations in children. Broad-spectrum antibiotics frequently are prescribed for presumed bacterial pneumonia in children. Recent guidelines for CAP in children have emphasized that ampicillin is an appropriate empiric inpatient treatment option.
Study design: Retrospective review.
Setting: Tertiary referral children’s hospital.
Synopsis: Patients older than two months old with acute, uncomplicated CAP and without significant secondary illness were identified in the 12-month periods preceding and following the implementation of a clinical practice guideline (CPG) that recommended empiric treatment with ampicillin upon admission, and amoxicillin upon discharge.
A total of 1,033 patients were identified, 530 pre-CPG and 503 post-CPG, and the groups were similar. After the CPG, there was a significant increase in empiric ampicillin use (13% to 63%) and concomitant decrease in ceftriaxone use (72% to 21%). Rates of outpatient narrow-spectrum antibiotic prescribing increased as well, and the rate of treatment failure was similar between the groups.
Complex regression analysis was used to analyze the impact of a concomitant antibiotic stewardship program (ASP), implemented three months prior to the initiation of the CPG and demonstrating a separate and additive effect of both initiatives. Thus, changes in antibiotic prescribing were multifactorial over this time period.
The outcomes remain impressive in the context of two increasingly popular QI efforts—CPGs and ASPs. This study represents a meaningful contribution toward demonstration of outcomes-based quality improvement (QI).
Bottom line: In the context of a CPG, antibiotic spectrum may be safely narrowed in pediatric CAP.
Citation: Newman RE, Hedican EB, Herigon JC, Williams DD, Williams AR, Newland JG. Impact of a guideline on management of children hospitalized with community-acquired pneumonia. Pediatrics. 2012;129(3):e597-604.
Reviewed by Pediatric Editor Mark Shen, MD, FHM, medical director of hospital medicine at Dell Children’s Medical Center, Austin, Texas.
ONLINE EXCLUSIVE: Listen to an ID specialist explains why de-escalation of antibiotics isn't a simple proposition
Click here to listen to Dr. Allen
Click here to listen to Dr. Allen
Click here to listen to Dr. Allen
What Is the Appropriate Use of Antibiotics In Acute Exacerbations of COPD?
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
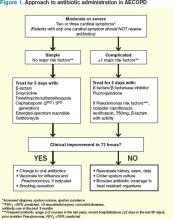
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
New Mindset on Antibiotics
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."
Hospitalists should consider the arena of antimicrobial stewardship one of the newest frontiers of clinical efficiency and cost savings, according to the author of a study in a supplement to this month's Journal of Hospital Medicine.
David Rosenberg, MD, MPH, FACP, SFHM, of the Department of Medicine, Section of Hospital Medicine at North Shore University Hospital in Manhasset, N.Y., says that changing the mindset on antibiotic resistance might seem like a daunting task, but it dovetails neatly with HM's current focus on quality and safety, particularly when it can help reduce length of stay (LOS).
"Think different about antibiotics and build that into your practice," he says.
The supplement highlights four related papers tackling the issues of appropriate initiation and selection of antibiotics, antimicrobial de-escalation strategies, duration and cessation of treatment, and Dr. Rosenberg's paper, "The Emerging Role of Hospitalists." The research includes an online CME component.
Dr. Rosenberg writes that hospitalists "are positioned as excellent champions of the principles and practices of antimicrobial stewardship." That means revamping the use of antibiotics both for individual patients and on an institutional level. That leadership means accepting that "culture change is slow" and physicians often feel "trapped" in letting an antibiotic treatment run its course rather than reassessing midstream.
Still, Dr. Rosenberg says, national guidelines on antibiotic overuse are likely to be developed in the coming years, and hospitalists would do well to get ahead of that curve.
"We're talking about the optimal treatment of patients we are already taking care of," he says. "Stewardship is a natural step forward."