User login
Guidelines for Treatment of Uncomplicated Cystitis and Pyelonephritis in Healthy, Community-Dwelling Women
Background
Uncomplicated cystitis is one of the most common indications for prescribing antimicrobial therapy to otherwise healthy women, but wide variation in prescribing practices has been described.1-2 This has prompted the need for guidelines to help providers in their selection of empiric antimicrobial regimens. Antibiotic selection should take into consideration the efficacy of individual agents, as well as their propensity for inducing resistance, altering gut flora, and increasing the risk of colonization or infection with multi-drug resistant organisms.
Guideline Update
In March 2010, the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESCMID) published new guidelines for the treatment of uncomplicated cystitis and pyelonephritis in healthy, community-dwelling women.3
First-line recommended agents for empiric treatment of uncomplicated cystitis are:
- nitrofurantoin for five days;
- trimethoprim-sulfamethoxazole for three days;
- fosfomycin in a single dose; or
- pivmecillinam (where available) for three to seven days.
Although highly efficacious, fluoroquinolones are not recommended as first-line treatment for acute cystitis because of their propensity for causing “collateral damage,” especially alteration of gut flora and increased risk of multi-drug resistant infection or colonization, including methicillin-resistant Staphylococcus aureus. Oral beta-lactams (other than pivmecillinam) have generally demonstrated inferior efficacy and more adverse effects when compared with the above agents, and should be used only if none of the preferred agents can be used. Specifically, amoxicillin and ampicillin are not recommended as empiric therapy due to their low efficacy in unselected patients, though may be appropriate when culture data is available to guide therapy. Narrow spectrum cephalosporins are also a potential agent for use in certain clinical situations, although the guidelines do not make any recommendation for or against their use, given a lack of studies.
For the treatment of acute pyelonephritis, the guidelines emphasize that all patients should have urine culture and susceptibility testing in order to tailor empiric therapy to the specific uropathogen. A 5-7 day course of an oral fluoroquinolone is appropriate when the prevalence of resistance in community uropathogens is ≤10%. Where resistance is more common, an initial intravenous dose of ceftriaxone or an aminoglycoside can be administered prior to starting oral therapy. Other alternatives include a 14-day course of trimethoprim-sulfamethoxazole or an oral beta-lactam.
Women requiring hospitalization for pyelonephritis should initially be treated with an intravenous antimicrobial regimen, the choice of which should be based on local resistance patterns. Recommended intravenous agents include fluoroquinolones, aminoglycosides (with or without ampicillin), extended-spectrum cephalosporins / penicillins, or carbapenems.
Analysis
Previous guidelines for the treatment of uncomplicated cystitis and pyelonephritis were published by the IDSA in 1999.4 The guidelines were updated based on the following factors:
- continued variability in prescribing practices;1-2
- increase in antimicrobial resistance among uropathogens;
- awareness of the unintended consequences of antimicrobial therapy, such as selection of drug-resistant organisms and colonization or infection with multi-drug resistant organisms; and
- study of newer agents and different durations of therapy.
Two important differences exist between the 1999 and 2010 guidelines:
- Nitrofurantoin has taken on more prominence in the 2010 guidelines for uncomplicated cystitis. The 1999 guidelines recommended trimethoprim-sulfamethoxazole as a first-line agent and mentioned nitrofurantoin and fosfomycin as potential alternative agents, but had few studies available to inform comparative efficacy or duration of therapy.
- For the outpatient treatment of mildly-ill patients with acute pyelonephritis, the 1999 guidelines recommended 14 days of therapy regardless of the agent used; in contrast, the 2010 guidelines recommend a five- to seven-day course for oral fluoroquinolones.
The American Congress of Obstetricians and Gynecologists, American Urological Association, Association of Medical Microbiology and Infectious Diseases-Canada, and the Society for Academic Emergency Medicine have endorsed the 2010 IDSA-ESCMID guidelines. The IDSA and ESCMID plan to evaluate the need for revisions to the 2010 guidelines based on an annual review of the current literature.
HM Takeaways
The 2010 IDSA-ESCMID guidelines are a resource available to hospitalists treating acute uncomplicated cystitis and pyelonephritis. As important differences exist between the target population and the hospitalist’s patient population, there are some key points to consider for clinicians treating cystitis or pyelonephritis in hospitalized patients.
Importantly, while nitrofurantoin is favored as a first-line antimicrobial agent for cystitis in the 2010 IDSA-ESCMID guidelines, it might be problematic in hospitalized patients for several reasons:
- it is not approved or recommended for the treatment of pyelonephritis;
- it is contraindicated in patients with creatinine clearance <60 ml/min; and
- it is generally not recommended for use in patients >65 years old because of the risk of renal impairment (Beers Criteria).5
Additionally, the treatment of acute cystitis in men requires special consideration. Notably, nitrofurantoin is not recommended in men because of poor prostatic tissue penetration, and although studies are limited, some sources recommend a longer treatment duration of at least 7 days.6 Finally, hospitalized patients commonly have other conditions, such as urological abnormalities, indwelling Foley catheters, recent urinary tract instrumentation, recent use of antibiotics, risk for multi-drug resistant organisms, potential interactions with other medications, and immunosuppression. The presence of any of these factors will influence the choice of empiric therapy and may warrant treatment for complicated cystitis or pyelonephritis, which are not addressed by these guidelines.
Drs. Tarvin and Sponsler are academic hospitalists at Vanderbilt University School of Medicine in Nashville, Tenn.
References
- Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2006;166:635-639.
- Kahan NR, Chinitz DP, Kahan E. Longer than recommended empiric antibiotic treatment of urinary tract infection in women: an avoidable waste of money. J Clin Pharm Therap. 2004;29:59-63.
- Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Inf Dis. 2011;52(5):e103-20.
- Warren JW, Abrutyn E, Hebel JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America. Clin Inf Dis. 1999;29(4):745-58.
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a U.S. consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
- Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. A Fam Phys. 2005;72(3):451-456.
Background
Uncomplicated cystitis is one of the most common indications for prescribing antimicrobial therapy to otherwise healthy women, but wide variation in prescribing practices has been described.1-2 This has prompted the need for guidelines to help providers in their selection of empiric antimicrobial regimens. Antibiotic selection should take into consideration the efficacy of individual agents, as well as their propensity for inducing resistance, altering gut flora, and increasing the risk of colonization or infection with multi-drug resistant organisms.
Guideline Update
In March 2010, the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESCMID) published new guidelines for the treatment of uncomplicated cystitis and pyelonephritis in healthy, community-dwelling women.3
First-line recommended agents for empiric treatment of uncomplicated cystitis are:
- nitrofurantoin for five days;
- trimethoprim-sulfamethoxazole for three days;
- fosfomycin in a single dose; or
- pivmecillinam (where available) for three to seven days.
Although highly efficacious, fluoroquinolones are not recommended as first-line treatment for acute cystitis because of their propensity for causing “collateral damage,” especially alteration of gut flora and increased risk of multi-drug resistant infection or colonization, including methicillin-resistant Staphylococcus aureus. Oral beta-lactams (other than pivmecillinam) have generally demonstrated inferior efficacy and more adverse effects when compared with the above agents, and should be used only if none of the preferred agents can be used. Specifically, amoxicillin and ampicillin are not recommended as empiric therapy due to their low efficacy in unselected patients, though may be appropriate when culture data is available to guide therapy. Narrow spectrum cephalosporins are also a potential agent for use in certain clinical situations, although the guidelines do not make any recommendation for or against their use, given a lack of studies.
For the treatment of acute pyelonephritis, the guidelines emphasize that all patients should have urine culture and susceptibility testing in order to tailor empiric therapy to the specific uropathogen. A 5-7 day course of an oral fluoroquinolone is appropriate when the prevalence of resistance in community uropathogens is ≤10%. Where resistance is more common, an initial intravenous dose of ceftriaxone or an aminoglycoside can be administered prior to starting oral therapy. Other alternatives include a 14-day course of trimethoprim-sulfamethoxazole or an oral beta-lactam.
Women requiring hospitalization for pyelonephritis should initially be treated with an intravenous antimicrobial regimen, the choice of which should be based on local resistance patterns. Recommended intravenous agents include fluoroquinolones, aminoglycosides (with or without ampicillin), extended-spectrum cephalosporins / penicillins, or carbapenems.
Analysis
Previous guidelines for the treatment of uncomplicated cystitis and pyelonephritis were published by the IDSA in 1999.4 The guidelines were updated based on the following factors:
- continued variability in prescribing practices;1-2
- increase in antimicrobial resistance among uropathogens;
- awareness of the unintended consequences of antimicrobial therapy, such as selection of drug-resistant organisms and colonization or infection with multi-drug resistant organisms; and
- study of newer agents and different durations of therapy.
Two important differences exist between the 1999 and 2010 guidelines:
- Nitrofurantoin has taken on more prominence in the 2010 guidelines for uncomplicated cystitis. The 1999 guidelines recommended trimethoprim-sulfamethoxazole as a first-line agent and mentioned nitrofurantoin and fosfomycin as potential alternative agents, but had few studies available to inform comparative efficacy or duration of therapy.
- For the outpatient treatment of mildly-ill patients with acute pyelonephritis, the 1999 guidelines recommended 14 days of therapy regardless of the agent used; in contrast, the 2010 guidelines recommend a five- to seven-day course for oral fluoroquinolones.
The American Congress of Obstetricians and Gynecologists, American Urological Association, Association of Medical Microbiology and Infectious Diseases-Canada, and the Society for Academic Emergency Medicine have endorsed the 2010 IDSA-ESCMID guidelines. The IDSA and ESCMID plan to evaluate the need for revisions to the 2010 guidelines based on an annual review of the current literature.
HM Takeaways
The 2010 IDSA-ESCMID guidelines are a resource available to hospitalists treating acute uncomplicated cystitis and pyelonephritis. As important differences exist between the target population and the hospitalist’s patient population, there are some key points to consider for clinicians treating cystitis or pyelonephritis in hospitalized patients.
Importantly, while nitrofurantoin is favored as a first-line antimicrobial agent for cystitis in the 2010 IDSA-ESCMID guidelines, it might be problematic in hospitalized patients for several reasons:
- it is not approved or recommended for the treatment of pyelonephritis;
- it is contraindicated in patients with creatinine clearance <60 ml/min; and
- it is generally not recommended for use in patients >65 years old because of the risk of renal impairment (Beers Criteria).5
Additionally, the treatment of acute cystitis in men requires special consideration. Notably, nitrofurantoin is not recommended in men because of poor prostatic tissue penetration, and although studies are limited, some sources recommend a longer treatment duration of at least 7 days.6 Finally, hospitalized patients commonly have other conditions, such as urological abnormalities, indwelling Foley catheters, recent urinary tract instrumentation, recent use of antibiotics, risk for multi-drug resistant organisms, potential interactions with other medications, and immunosuppression. The presence of any of these factors will influence the choice of empiric therapy and may warrant treatment for complicated cystitis or pyelonephritis, which are not addressed by these guidelines.
Drs. Tarvin and Sponsler are academic hospitalists at Vanderbilt University School of Medicine in Nashville, Tenn.
References
- Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2006;166:635-639.
- Kahan NR, Chinitz DP, Kahan E. Longer than recommended empiric antibiotic treatment of urinary tract infection in women: an avoidable waste of money. J Clin Pharm Therap. 2004;29:59-63.
- Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Inf Dis. 2011;52(5):e103-20.
- Warren JW, Abrutyn E, Hebel JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America. Clin Inf Dis. 1999;29(4):745-58.
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a U.S. consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
- Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. A Fam Phys. 2005;72(3):451-456.
Background
Uncomplicated cystitis is one of the most common indications for prescribing antimicrobial therapy to otherwise healthy women, but wide variation in prescribing practices has been described.1-2 This has prompted the need for guidelines to help providers in their selection of empiric antimicrobial regimens. Antibiotic selection should take into consideration the efficacy of individual agents, as well as their propensity for inducing resistance, altering gut flora, and increasing the risk of colonization or infection with multi-drug resistant organisms.
Guideline Update
In March 2010, the Infectious Diseases Society of America (IDSA) and the European Society for Microbiology and Infectious Diseases (ESCMID) published new guidelines for the treatment of uncomplicated cystitis and pyelonephritis in healthy, community-dwelling women.3
First-line recommended agents for empiric treatment of uncomplicated cystitis are:
- nitrofurantoin for five days;
- trimethoprim-sulfamethoxazole for three days;
- fosfomycin in a single dose; or
- pivmecillinam (where available) for three to seven days.
Although highly efficacious, fluoroquinolones are not recommended as first-line treatment for acute cystitis because of their propensity for causing “collateral damage,” especially alteration of gut flora and increased risk of multi-drug resistant infection or colonization, including methicillin-resistant Staphylococcus aureus. Oral beta-lactams (other than pivmecillinam) have generally demonstrated inferior efficacy and more adverse effects when compared with the above agents, and should be used only if none of the preferred agents can be used. Specifically, amoxicillin and ampicillin are not recommended as empiric therapy due to their low efficacy in unselected patients, though may be appropriate when culture data is available to guide therapy. Narrow spectrum cephalosporins are also a potential agent for use in certain clinical situations, although the guidelines do not make any recommendation for or against their use, given a lack of studies.
For the treatment of acute pyelonephritis, the guidelines emphasize that all patients should have urine culture and susceptibility testing in order to tailor empiric therapy to the specific uropathogen. A 5-7 day course of an oral fluoroquinolone is appropriate when the prevalence of resistance in community uropathogens is ≤10%. Where resistance is more common, an initial intravenous dose of ceftriaxone or an aminoglycoside can be administered prior to starting oral therapy. Other alternatives include a 14-day course of trimethoprim-sulfamethoxazole or an oral beta-lactam.
Women requiring hospitalization for pyelonephritis should initially be treated with an intravenous antimicrobial regimen, the choice of which should be based on local resistance patterns. Recommended intravenous agents include fluoroquinolones, aminoglycosides (with or without ampicillin), extended-spectrum cephalosporins / penicillins, or carbapenems.
Analysis
Previous guidelines for the treatment of uncomplicated cystitis and pyelonephritis were published by the IDSA in 1999.4 The guidelines were updated based on the following factors:
- continued variability in prescribing practices;1-2
- increase in antimicrobial resistance among uropathogens;
- awareness of the unintended consequences of antimicrobial therapy, such as selection of drug-resistant organisms and colonization or infection with multi-drug resistant organisms; and
- study of newer agents and different durations of therapy.
Two important differences exist between the 1999 and 2010 guidelines:
- Nitrofurantoin has taken on more prominence in the 2010 guidelines for uncomplicated cystitis. The 1999 guidelines recommended trimethoprim-sulfamethoxazole as a first-line agent and mentioned nitrofurantoin and fosfomycin as potential alternative agents, but had few studies available to inform comparative efficacy or duration of therapy.
- For the outpatient treatment of mildly-ill patients with acute pyelonephritis, the 1999 guidelines recommended 14 days of therapy regardless of the agent used; in contrast, the 2010 guidelines recommend a five- to seven-day course for oral fluoroquinolones.
The American Congress of Obstetricians and Gynecologists, American Urological Association, Association of Medical Microbiology and Infectious Diseases-Canada, and the Society for Academic Emergency Medicine have endorsed the 2010 IDSA-ESCMID guidelines. The IDSA and ESCMID plan to evaluate the need for revisions to the 2010 guidelines based on an annual review of the current literature.
HM Takeaways
The 2010 IDSA-ESCMID guidelines are a resource available to hospitalists treating acute uncomplicated cystitis and pyelonephritis. As important differences exist between the target population and the hospitalist’s patient population, there are some key points to consider for clinicians treating cystitis or pyelonephritis in hospitalized patients.
Importantly, while nitrofurantoin is favored as a first-line antimicrobial agent for cystitis in the 2010 IDSA-ESCMID guidelines, it might be problematic in hospitalized patients for several reasons:
- it is not approved or recommended for the treatment of pyelonephritis;
- it is contraindicated in patients with creatinine clearance <60 ml/min; and
- it is generally not recommended for use in patients >65 years old because of the risk of renal impairment (Beers Criteria).5
Additionally, the treatment of acute cystitis in men requires special consideration. Notably, nitrofurantoin is not recommended in men because of poor prostatic tissue penetration, and although studies are limited, some sources recommend a longer treatment duration of at least 7 days.6 Finally, hospitalized patients commonly have other conditions, such as urological abnormalities, indwelling Foley catheters, recent urinary tract instrumentation, recent use of antibiotics, risk for multi-drug resistant organisms, potential interactions with other medications, and immunosuppression. The presence of any of these factors will influence the choice of empiric therapy and may warrant treatment for complicated cystitis or pyelonephritis, which are not addressed by these guidelines.
Drs. Tarvin and Sponsler are academic hospitalists at Vanderbilt University School of Medicine in Nashville, Tenn.
References
- Huang ES, Stafford RS. National patterns in the treatment of urinary tract infections in women by ambulatory care physicians. Arch Intern Med. 2006;166:635-639.
- Kahan NR, Chinitz DP, Kahan E. Longer than recommended empiric antibiotic treatment of urinary tract infection in women: an avoidable waste of money. J Clin Pharm Therap. 2004;29:59-63.
- Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Inf Dis. 2011;52(5):e103-20.
- Warren JW, Abrutyn E, Hebel JR, Schaeffer AJ, Stamm WE. Guidelines for antimicrobial treatment of acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America. Clin Inf Dis. 1999;29(4):745-58.
- Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a U.S. consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724.
- Mehnert-Kay SA. Diagnosis and management of uncomplicated urinary tract infections. A Fam Phys. 2005;72(3):451-456.
In the Literature: Research You Need to Know
Clinical question: What is the relationship between well-being and demographic factors, educational debt, and medical knowledge in internal-medicine residents?
Background: Physician distress during training is common and can negatively impact patient care. There has never been a study of internal-medicine residents nationally that examined the patterns of distress across demographic factors or the association of these factors with medical knowledge.
Study design: Cross-sectional study.
Setting: U.S. internal-medicine residency programs.
Synopsis: Of the 21,208 U.S. internal-medicine residents who completed the 2008 in-training examination, 77.3% had both survey and demographic data available for analysis. Nearly 15% of these 16,394 residents rated quality of life “as bad as it can be” or “somewhat bad,” and 32.9% felt somewhat or very dissatisfied with work-life balance.
Overall burnout, high levels of weekly emotional exhaustion, and weekly depersonalization were reported by 51.5%, 45.8%, and 28.9% of residents, respectively. Symptoms of emotional exhaustion decreased as training increased, while depersonalization increased after the first postgraduate year. Residents reporting quality of life “as bad as it can be,” emotional exhaustion, or debt greater than $200,000 had mean exam scores 2.7, 4.2, and 5 points, respectively, lower than others surveyed.
Although unlikely given the study design, nonresponse bias could affect these results. Not all demographic variables or domains of well-being were studied, and self-reported educational debt could have been misclassified. Nonetheless, findings suggest that distress remains among residents despite the changes made to duty-hour regulations in 2003.
Bottom line: Suboptimal quality of life and burnout were common among internal-medicine residents nationally; symptoms of burnout were associated with higher debt and lower exam scores.
Citation: West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
Visit our website for more physician reviews of HM-related research.
Clinical question: What is the relationship between well-being and demographic factors, educational debt, and medical knowledge in internal-medicine residents?
Background: Physician distress during training is common and can negatively impact patient care. There has never been a study of internal-medicine residents nationally that examined the patterns of distress across demographic factors or the association of these factors with medical knowledge.
Study design: Cross-sectional study.
Setting: U.S. internal-medicine residency programs.
Synopsis: Of the 21,208 U.S. internal-medicine residents who completed the 2008 in-training examination, 77.3% had both survey and demographic data available for analysis. Nearly 15% of these 16,394 residents rated quality of life “as bad as it can be” or “somewhat bad,” and 32.9% felt somewhat or very dissatisfied with work-life balance.
Overall burnout, high levels of weekly emotional exhaustion, and weekly depersonalization were reported by 51.5%, 45.8%, and 28.9% of residents, respectively. Symptoms of emotional exhaustion decreased as training increased, while depersonalization increased after the first postgraduate year. Residents reporting quality of life “as bad as it can be,” emotional exhaustion, or debt greater than $200,000 had mean exam scores 2.7, 4.2, and 5 points, respectively, lower than others surveyed.
Although unlikely given the study design, nonresponse bias could affect these results. Not all demographic variables or domains of well-being were studied, and self-reported educational debt could have been misclassified. Nonetheless, findings suggest that distress remains among residents despite the changes made to duty-hour regulations in 2003.
Bottom line: Suboptimal quality of life and burnout were common among internal-medicine residents nationally; symptoms of burnout were associated with higher debt and lower exam scores.
Citation: West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
Visit our website for more physician reviews of HM-related research.
Clinical question: What is the relationship between well-being and demographic factors, educational debt, and medical knowledge in internal-medicine residents?
Background: Physician distress during training is common and can negatively impact patient care. There has never been a study of internal-medicine residents nationally that examined the patterns of distress across demographic factors or the association of these factors with medical knowledge.
Study design: Cross-sectional study.
Setting: U.S. internal-medicine residency programs.
Synopsis: Of the 21,208 U.S. internal-medicine residents who completed the 2008 in-training examination, 77.3% had both survey and demographic data available for analysis. Nearly 15% of these 16,394 residents rated quality of life “as bad as it can be” or “somewhat bad,” and 32.9% felt somewhat or very dissatisfied with work-life balance.
Overall burnout, high levels of weekly emotional exhaustion, and weekly depersonalization were reported by 51.5%, 45.8%, and 28.9% of residents, respectively. Symptoms of emotional exhaustion decreased as training increased, while depersonalization increased after the first postgraduate year. Residents reporting quality of life “as bad as it can be,” emotional exhaustion, or debt greater than $200,000 had mean exam scores 2.7, 4.2, and 5 points, respectively, lower than others surveyed.
Although unlikely given the study design, nonresponse bias could affect these results. Not all demographic variables or domains of well-being were studied, and self-reported educational debt could have been misclassified. Nonetheless, findings suggest that distress remains among residents despite the changes made to duty-hour regulations in 2003.
Bottom line: Suboptimal quality of life and burnout were common among internal-medicine residents nationally; symptoms of burnout were associated with higher debt and lower exam scores.
Citation: West CP, Shanafelt TD, Kolars JC. Quality of life, burnout, educational debt, and medical knowledge among internal medicine residents. JAMA. 2011;306:952-960.
Visit our website for more physician reviews of HM-related research.
What Is the Appropriate Use of Antibiotics In Acute Exacerbations of COPD?
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
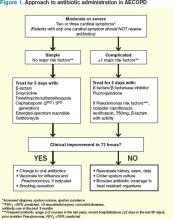
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
Case
A 58-year-old male smoker with moderate chronic obstructive pulmonary disease (COPD) (FEV1 56% predicted) is admitted with an acute exacerbation of COPD for the second time this year. He presented to the ED with increased productive cough and shortness of breath, similar to prior exacerbations. He denies fevers, myalgias, or upper-respiratory symptoms. Physical exam is notable for bilateral inspiratory and expiratory wheezing. His sputum is purulent. He is given continuous nebulizer therapy and one dose of oral prednisone, but his dyspnea and wheezing persist. Chest X-ray does not reveal an infiltrate.
Should this patient be treated with antibiotics and, if so, what regimen is most appropriate?
Overview
Acute exacerbations of COPD (AECOPD) present a major health burden, accounting for more than 2.4% of all hospital admissions and causing significant morbidity, mortality, and costs.1 During 2006 and 2007, COPD mortality in the United States topped 39 deaths per 100,000 people, and more recently, hospital costs related to COPD were expected to exceed $13 billion annually.2 Patients with AECOPD also experience decreased quality of life and faster decline in pulmonary function, further highlighting the need for timely and appropriate treatment.1
Several guidelines have proposed treatment strategies now considered standard of care in AECOPD management.3,4,5,6 These include the use of corticosteroids, bronchodilator agents, and, in select cases, antibiotics. While there is well-established evidence for the use of steroids and bronchodilators in AECOPD, the debate continues over the appropriate use of antibiotics in the treatment of acute exacerbations. There are multiple potential factors leading to AECOPD, including viruses, bacteria, and common pollutants; as such, antibiotic treatment may not be indicated for all patients presenting with exacerbations. Further, the risks of antibiotic treatment—including adverse drug events, selection for drug-resistant bacteria, and associated costs—are not insignificant.
However, bacterial infections do play a role in approximately 50% of patients with AECOPD and, for this population, use of antibiotics may confer important benefits.7
Interestingly, a retrospective cohort study of 84,621 patients admitted for AECOPD demonstrated that 85% of patients received antibiotics at some point during hospitalization.8
Support for Antibiotics
Several randomized trials have compared clinical outcomes in patients with AECOPD who have received antibiotics versus those who received placebos. Most of these had small sample sizes and studied only ββ-lactam and tetracycline antibiotics in an outpatient setting; there are limited data involving inpatients and newer drugs. Nevertheless, antibiotic treatment has been associated with decreased risk of adverse outcomes in AECOPD.
One meta-analysis demonstrated that antibiotics reduced treatment failures by 66% and in-hospital mortality by 78% in the subset of trials involving hospitalized patients.8 Similarly, analysis of a large retrospective cohort of patients hospitalized for AECOPD found a significantly lower risk of treatment failure in antibiotic-treated versus untreated patients.9 Specifically, treated patients had lower rates of in-hospital mortality and readmission for AECOPD and a lower likelihood of requiring subsequent mechanical ventilation during the index hospitalization.
Data also suggest that antibiotic treatment during exacerbations might favorably impact subsequent exacerbations.10 A retrospective study of 18,928 Dutch patients with AECOPD compared outcomes among patients who had received antibiotics (most frequently doxycycline or a penicillin) as part of their therapy to those who did not. The authors demonstrated that the median time to the next exacerbation was significantly longer in the patients receiving antibiotics.10 Further, both mortality and overall risk of developing a subsequent exacerbation were significantly decreased in the antibiotic group, with median follow-up of approximately two years.
Indications for Antibiotics
Clinical symptoms. A landmark study by Anthonisen and colleagues set forth three clinical criteria that have formed the basis for treating AECOPD with antibiotics in subsequent studies and in clinical practice.11 Often referred to as the “cardinal symptoms” of AECOPD, these include increased dyspnea, sputum volume, and sputum purulence. In this study, 173 outpatients with COPD were randomized to a 10-day course of antibiotics or placebo at onset of an exacerbation and followed clinically. The authors found that antibiotic-treated patients were significantly more likely than the placebo group to achieve treatment success, defined as resolution of all exacerbated symptoms within 21 days (68.1% vs. 55.0%, P<0.01).
Importantly, treated patients were also significantly less likely to experience clinical deterioration after 72 hours (9.9% vs. 18.9%, P<0.05). Patients with Type I exacerbations, characterized by all three cardinal symptoms, were most likely to benefit from antibiotic therapy, followed by patients with Type II exacerbations, in whom only two of the symptoms were present. Subsequent studies have suggested that sputum purulence correlates well with the presence of acute bacterial infection and therefore may be a reliable clinical indicator of patients who are likely to benefit from antibiotic therapy.12
Laboratory data. While sputum purulence is associated with bacterial infection, sputum culture is less reliable, as pathogenic bacteria are commonly isolated from patients with both AECOPD and stable COPD. In fact, the prevalence of bacterial colonization in moderate to severe COPD might be as high as 50%.13 Therefore, a positive bacterial sputum culture, in the absence of purulence or other signs of infection, is not recommended as the sole basis for which to prescribe antibiotics.
Serum biomarkers, most notably C-reactive protein (CRP) and procalcitonin, have been studied as a newer approach to identify patients who might benefit from antibiotic therapy for AECOPD. Studies have demonstrated increased CRP levels during AECOPD, particularly in patients with purulent sputum and positive bacterial sputum cultures.12 Procalcitonin is preferentially elevated in bacterial infections.
One randomized, placebo-controlled trial in hospitalized patients with AECOPD demonstrated a significant reduction in antibiotic usage based on low procalcitonin levels, without negatively impacting clinical success rate, hospital mortality, subsequent antibiotic needs, or time to next exacerbation.14 However, due to inconsistent evidence, use of these markers to guide antibiotic administration in AECOPD has not yet been definitively established.14,15 Additionally, these laboratory results are often not available at the point of care, potentially limiting their utility in the decision to initiate antibiotics.
Severity of illness. Severity of illness is an important factor in the decision to treat AECOPD with antibiotics. Patients with advanced, underlying airway obstruction, as measured by FEV1, are more likely to have a bacterial cause of AECOPD.16 Additionally, baseline clinical characteristics including advanced age and comorbid conditions, particularly cardiovascular disease and diabetes, increase the risk of severe exacerbations.17
One meta-analysis of placebo-controlled trials found that patients with severe exacerbations were likely to benefit from antibiotic therapy, while patients with mild or moderate exacerbations had no reduction in treatment failure or mortality rates.18 Patients presenting with acute respiratory failure necessitating intensive care and/or ventilator support (noninvasive or invasive) have also been shown to benefit from antibiotics.19
Current clinical guidelines vary slightly in their recommendations regarding when to give antibiotics in AECOPD (see Table 1). However, existing evidence favors antibiotic treatment for those patients presenting with two or three cardinal symptoms, specifically those with increased sputum purulence, and those with severe disease (i.e. pre-existing advanced airflow obstruction and/or exacerbations requiring mechanical ventilation). Conversely, studies have shown that many patients, particularly those with milder exacerbations, experience resolution of symptoms without antibiotic treatment.11,18
Antibiotic Choice in AECOPD
Risk stratification. In patients likely to benefit from antibiotic therapy, an understanding of the relationship between severity of COPD, host risk factors for poor outcomes, and microbiology is paramount to guide clinical decision-making. Historically, such bacteria as Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis have been implicated in the pathogenesis of AECOPD.3,7 In patients with simple exacerbations, antibiotics that target these pathogens should be used (see Table 2).
However, patients with more severe underlying airway obstruction (i.e. FEV1<50%) and risk factors for poor outcomes, specifically recent hospitalization (≥2 days during the previous 90 days), frequent antibiotics (>3 courses during the previous year), and severe exacerbations are more likely to be infected with resistant strains or gram-negative organisms.3,7 Pseudomonas aeruginosa, in particular, is of increasing concern in this population. In patients with complicated exacerbations, more broad-coverage, empiric antibiotics should be initiated (see Table 2).
With this in mind, patients meeting criteria for treatment must first be stratified according to the severity of COPD and risk factors for poor outcomes before a decision regarding a specific antibiotic is reached. Figure 1 outlines a recommended approach for antibiotic administration in AECOPD. The optimal choice of antibiotics must consider cost-effectiveness, local patterns of antibiotic resistance, tissue penetration, patient adherence, and risk of such adverse drug events as diarrhea.
Comparative effectiveness. Current treatment guidelines do not favor the use of any particular antibiotic in simple AECOPD.3,4,5,6 However, as selective pressure has led to in vitro resistance to antibiotics traditionally considered first-line (e.g. doxycycline, trimethoprim/sulfamethoxazole, amoxicillin), the use of second-line antibiotics (e.g. fluoroquinolones, macrolides, cephalosporins, β-lactam/ β-lactamase inhibitors) has increased. Consequently, several studies have compared the effectiveness of different antimicrobial regimens.
One meta-analysis found that second-line antibiotics, when compared with first-line agents, provided greater clinical improvement to patients with AECOPD, without significant differences in mortality, microbiologic eradication, or incidence of adverse drug events.20 Among the subgroup of trials enrolling hospitalized patients, the clinical effectiveness of second-line agents remained significantly greater than that of first-line agents.
Another meta-analysis compared trials that studied only macrolides, quinolones, and amoxicillin-clavulanate and found no difference in terms of short-term clinical effectiveness; however, there was weak evidence to suggest that quinolones were associated with better microbiological success and fewer recurrences of AECOPD.21 Fluoroquinolones are preferred in complicated cases of AECOPD in which there is a greater risk for enterobacteriaceae and Pseudomonas species.3,7
Antibiotic Duration
The duration of antibiotic therapy in AECOPD has been studied extensively, with randomized controlled trials consistently demonstrating no additional benefit to courses extending beyond five days. One meta-analysis of 21 studies found similar clinical and microbiologic cure rates among patients randomized to antibiotic treatment for ≤5 days versus >5 days.22 A subgroup analysis of the trials evaluating different durations of the same antibiotic also demonstrated no difference in clinical effectiveness, and this finding was confirmed in a separate meta-analysis.22,23
Advantages to shorter antibiotic courses include improved compliance and decreased rates of resistance. The usual duration of antibiotic therapy is three to seven days, depending upon the response to therapy.3
Back to the Case
As the patient has no significant comorbidities or risk factors, and meets criteria for a simple Anthonisen Type I exacerbation (increased dyspnea, sputum, and sputum purulence), antibiotic therapy with trimethoprim/sulfamethoxazole is initiated on admission, in addition to the previously started steroid and bronchodilator treatments. The patient’s clinical status improves, and he is discharged on hospital Day 3 with a prescription to complete a five-day course of antibiotics.
Bottom Line
Antibiotic therapy is effective in select AECOPD patients, with maximal benefits obtained when the decision to treat is based on careful consideration of characteristic clinical symptoms and severity of illness. Choice and duration of antibiotics should follow likely bacterial causes and current guidelines.
Dr. Cunningham is an assistant professor of internal medicine and academic hospitalist in the section of hospital medicine at Vanderbilt University School of Medicine in Nashville, Tenn. Dr. LaBrin is assistant professor of internal medicine and pediatrics and an academic hospitalist at Vanderbilt. Dr. Markley is a clinical instructor and academic hospitalist at Vanderbilt.
References
- Donaldson GC, Wedzicha JA. COPD exacerbations: 1. Epidemiology. Thorax. 2006;61:164-168.
- National Heart, Lung, and Blood Institute. 2009 NHLBI Morbidity and Mortality Chartbook. National Heart, Lung, and Blood Institute website. Available at: http://www.nhlbi.nih.gov/resources/docs/cht-book.htm Accessed Oct. 10, 2011.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) website. Available at: www.goldcopd.org/guidelines-resources.html Accessed Oct. 10, 2011.
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Resp J. 2004;23:932-946.
- National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. National Institute for Health and Clinical Excellence website. Available at: http://guidance.nice.org.uk/CG101/Guidance/pdf/English. Accessed Oct. 10, 2011.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J. 2007;14(Suppl B):5B-32B.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355-2565.
- Quon BS, Qi Gan W, Sin DD. Contemporary management of acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2008;133:756-766.
- Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035-2042.
- Roede BM, Bresser P, Bindels PJE, et al. Antibiotic treatment is associated with reduced risk of subsequent exacerbation in obstructive lung disease: a historical population based cohort study. Thorax. 2008;63:968-973.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GKM, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196-204.
- Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638-1645.
- Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005; 165:891-897.
- Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
- Daniels JMA, Schoorl M, Snijders D, et al. Procalcitonin vs C-reactive protein as predictive markers of response to antibiotic therapy in acute exacerbations of COPD. Chest. 2010;138:1108-1015.
- Miravitlles M, Espinosa C, Fernandez-Laso E, Martos JA, Maldonado JA, Gallego M. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest. 1999;116:40-46.
- Patil SP, Krishnan JA, Lechtzin N, Diette GB. In-hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003;163:1180-1186.
- Puhan MA, Vollenweider D, Latshang T, Steurer J, Steurer-Stey C. Exacerbations of chronic obstructive lung disease: when are antibiotics indicated? A systematic review. Resp Res. 2007;8:30-40.
- Nouira S, Marghli S, Belghith M, Besbes L, Elatrous S, Abroug F. Once daily ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomized placebo-controlled trial. Lancet. 2001;358:2020-2025.
- Dimopoulos G, Siempos II, Korbila IP, Manta KG, Falagas ME. Comparison of first-line with second-line antibiotics for acute exacerbations of chronic bronchitis: a metaanalysis of randomized controlled trials. Chest. 2007;132:447-455.
- Siempos II, Dimopoulos G, Korbila IP, Manta KG, Falagas ME. Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta-analysis. Eur Resp J. 2007;29:1127-1137.
- El-Moussaoui, Roede BM, Speelman P, Bresser P, Prins JM, Bossuyt PMM. Short-course antibiotic treatment in acute exacerbations of chronic bronchitis and COPD: a meta-analysis of double-blind studies. Thorax. 2008;63:415-422.
- Falagas ME, Avgeri SG, Matthaiou DK, Dimopoulos G, Siempos II. Short- versus long-duration antimicrobial treatment for exacerbations of chronic bronchitis: a meta-analysis. J Antimicrob Chemother. 2008;62:442-450.
In the Literature: Research You Need to Know
Clinical question: To what extent does diagnostic phlebotomy contribute to hospital-acquired anemia (HAA) during acute myocardial infarction (AMI)?
Background: During AMI, hospital-acquired HAA is associated with higher mortality and poorer health status. Moderate to severe HAA (nadir hemoglobin level <11 g/dL) has been shown to be prognostically important. The contribution of diagnostic phlebotomy blood loss on HAA is unknown and is a potentially modifiable factor.
Study design: Retrospective observational cohort study.
Setting: Fifty-seven U.S. hospitals.
Synopsis: Using Cerner Corp.'s Health Facts database, information was collected on 17,676 patients with AMI. Moderate to severe HAA developed in 3,551 (20%) patients who were not anemic upon admission. The diagnostic blood loss was estimated by assuming minimal blood volume per adult tube required to perform the lab work obtained. The mean phlebotomy volume was higher in patients with HAA compared with patients without HAA (173.8 mL vs. 83.5 mL; P<0.001). There was significant variation of diagnostic blood loss between hospitals. The risk of HAA increased by 18% (RR 1.18; 95% CI, 1.13-1.22) for every 50 mL of diagnostic blood loss.
Patients with HAA were noted to have greater disease severity and comorbidities. No causal inference can be made given the observational nature of the study. Randomized trials are needed to evaluate if strategies to reduce diagnostic blood loss can reduce HAA and improve clinical outcomes for patients with AMI.
Bottom line: Diagnostic blood loss is associated with development of hospital-acquired anemia in patients with acute myocardial infarction.
Citation: Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646-1653.
For more physician reviews of HM-relevant research, visit our website.
Clinical question: To what extent does diagnostic phlebotomy contribute to hospital-acquired anemia (HAA) during acute myocardial infarction (AMI)?
Background: During AMI, hospital-acquired HAA is associated with higher mortality and poorer health status. Moderate to severe HAA (nadir hemoglobin level <11 g/dL) has been shown to be prognostically important. The contribution of diagnostic phlebotomy blood loss on HAA is unknown and is a potentially modifiable factor.
Study design: Retrospective observational cohort study.
Setting: Fifty-seven U.S. hospitals.
Synopsis: Using Cerner Corp.'s Health Facts database, information was collected on 17,676 patients with AMI. Moderate to severe HAA developed in 3,551 (20%) patients who were not anemic upon admission. The diagnostic blood loss was estimated by assuming minimal blood volume per adult tube required to perform the lab work obtained. The mean phlebotomy volume was higher in patients with HAA compared with patients without HAA (173.8 mL vs. 83.5 mL; P<0.001). There was significant variation of diagnostic blood loss between hospitals. The risk of HAA increased by 18% (RR 1.18; 95% CI, 1.13-1.22) for every 50 mL of diagnostic blood loss.
Patients with HAA were noted to have greater disease severity and comorbidities. No causal inference can be made given the observational nature of the study. Randomized trials are needed to evaluate if strategies to reduce diagnostic blood loss can reduce HAA and improve clinical outcomes for patients with AMI.
Bottom line: Diagnostic blood loss is associated with development of hospital-acquired anemia in patients with acute myocardial infarction.
Citation: Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646-1653.
For more physician reviews of HM-relevant research, visit our website.
Clinical question: To what extent does diagnostic phlebotomy contribute to hospital-acquired anemia (HAA) during acute myocardial infarction (AMI)?
Background: During AMI, hospital-acquired HAA is associated with higher mortality and poorer health status. Moderate to severe HAA (nadir hemoglobin level <11 g/dL) has been shown to be prognostically important. The contribution of diagnostic phlebotomy blood loss on HAA is unknown and is a potentially modifiable factor.
Study design: Retrospective observational cohort study.
Setting: Fifty-seven U.S. hospitals.
Synopsis: Using Cerner Corp.'s Health Facts database, information was collected on 17,676 patients with AMI. Moderate to severe HAA developed in 3,551 (20%) patients who were not anemic upon admission. The diagnostic blood loss was estimated by assuming minimal blood volume per adult tube required to perform the lab work obtained. The mean phlebotomy volume was higher in patients with HAA compared with patients without HAA (173.8 mL vs. 83.5 mL; P<0.001). There was significant variation of diagnostic blood loss between hospitals. The risk of HAA increased by 18% (RR 1.18; 95% CI, 1.13-1.22) for every 50 mL of diagnostic blood loss.
Patients with HAA were noted to have greater disease severity and comorbidities. No causal inference can be made given the observational nature of the study. Randomized trials are needed to evaluate if strategies to reduce diagnostic blood loss can reduce HAA and improve clinical outcomes for patients with AMI.
Bottom line: Diagnostic blood loss is associated with development of hospital-acquired anemia in patients with acute myocardial infarction.
Citation: Salisbury AC, Reid KJ, Alexander KP, et al. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646-1653.
For more physician reviews of HM-relevant research, visit our website.
In the Literature: Research You Need to Know
Clinical question: What is the association between time to clinical stability (TCS) and post-discharge death or readmission in patients hospitalized with community-acquired pneumonia (CAP)?
Background: In patients with CAP, inflammatory response during hospitalization might be associated with adverse outcomes after discharge. Studies have not evaluated if time to clinical stability, a reflection of inflammatory response, can be used to identify patients at high risk of adverse outcomes after discharge.
Study design: Retrospective cohort study.
Setting: Veterans Hospital, Louisville, Ky.
Synopsis: Of 464 hospitalized patients with CAP, those with TCS >3 days had a higher rate of readmission or death within 30 days after discharge compared with those who had a TCS =3 days (26% versus 15%; OR 1.98; 95% CI, 1.19-3.3; P=0.008). Longer TCS during hospitalization was associated with a significantly increased risk of adverse outcomes (adjusted OR 1.06, 1.54, 2.40, 10.53 if TCS was reached at days 2, 3, 4, 5 versus Day 1, respectively). The authors proposed that patients with delays in reaching clinical stability should receive a special discharge management approach to decrease the risk of morbidity and mortality after discharge; this may include close observation, home visits, and a follow-up clinic appointment within 10 days.
As a retrospective cohort study, unaccounted-for confounders might exist between TCS and adverse outcomes. The small sample size precluded development of a fully predictive model. Additionally, the population studied was elderly men in a single hospital, which might limit generalizability.
Bottom line: Hospitalized patients with community-acquired pneumonia whose time to clinical stability was greater than three days had a higher risk of readmission or death within 30 days after discharge.
Citation: Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest. 2011;140:482-488.
For more physician reviews of HM-relevant research, visit our website.
Clinical question: What is the association between time to clinical stability (TCS) and post-discharge death or readmission in patients hospitalized with community-acquired pneumonia (CAP)?
Background: In patients with CAP, inflammatory response during hospitalization might be associated with adverse outcomes after discharge. Studies have not evaluated if time to clinical stability, a reflection of inflammatory response, can be used to identify patients at high risk of adverse outcomes after discharge.
Study design: Retrospective cohort study.
Setting: Veterans Hospital, Louisville, Ky.
Synopsis: Of 464 hospitalized patients with CAP, those with TCS >3 days had a higher rate of readmission or death within 30 days after discharge compared with those who had a TCS =3 days (26% versus 15%; OR 1.98; 95% CI, 1.19-3.3; P=0.008). Longer TCS during hospitalization was associated with a significantly increased risk of adverse outcomes (adjusted OR 1.06, 1.54, 2.40, 10.53 if TCS was reached at days 2, 3, 4, 5 versus Day 1, respectively). The authors proposed that patients with delays in reaching clinical stability should receive a special discharge management approach to decrease the risk of morbidity and mortality after discharge; this may include close observation, home visits, and a follow-up clinic appointment within 10 days.
As a retrospective cohort study, unaccounted-for confounders might exist between TCS and adverse outcomes. The small sample size precluded development of a fully predictive model. Additionally, the population studied was elderly men in a single hospital, which might limit generalizability.
Bottom line: Hospitalized patients with community-acquired pneumonia whose time to clinical stability was greater than three days had a higher risk of readmission or death within 30 days after discharge.
Citation: Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest. 2011;140:482-488.
For more physician reviews of HM-relevant research, visit our website.
Clinical question: What is the association between time to clinical stability (TCS) and post-discharge death or readmission in patients hospitalized with community-acquired pneumonia (CAP)?
Background: In patients with CAP, inflammatory response during hospitalization might be associated with adverse outcomes after discharge. Studies have not evaluated if time to clinical stability, a reflection of inflammatory response, can be used to identify patients at high risk of adverse outcomes after discharge.
Study design: Retrospective cohort study.
Setting: Veterans Hospital, Louisville, Ky.
Synopsis: Of 464 hospitalized patients with CAP, those with TCS >3 days had a higher rate of readmission or death within 30 days after discharge compared with those who had a TCS =3 days (26% versus 15%; OR 1.98; 95% CI, 1.19-3.3; P=0.008). Longer TCS during hospitalization was associated with a significantly increased risk of adverse outcomes (adjusted OR 1.06, 1.54, 2.40, 10.53 if TCS was reached at days 2, 3, 4, 5 versus Day 1, respectively). The authors proposed that patients with delays in reaching clinical stability should receive a special discharge management approach to decrease the risk of morbidity and mortality after discharge; this may include close observation, home visits, and a follow-up clinic appointment within 10 days.
As a retrospective cohort study, unaccounted-for confounders might exist between TCS and adverse outcomes. The small sample size precluded development of a fully predictive model. Additionally, the population studied was elderly men in a single hospital, which might limit generalizability.
Bottom line: Hospitalized patients with community-acquired pneumonia whose time to clinical stability was greater than three days had a higher risk of readmission or death within 30 days after discharge.
Citation: Aliberti S, Peyrani P, Filardo G, et al. Association between time to clinical stability and outcomes after discharge in hospitalized patients with community-acquired pneumonia. Chest. 2011;140:482-488.
For more physician reviews of HM-relevant research, visit our website.
In the Literature: The latest research you need to know
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Procalcitonin to guide antimicrobial use in ICU patients
- Platelet reactivity and event rates after PCI
- Conservative treatment of necrotizing pancreatitis
- Use of MRI to diagnose Takotsubo cardiomyopathy
- Rates of inadvertent medication discontinuation after hospitalization
- Role of history and physical exam in diagnosing medical illness
- Red-cell distribution width and rates of mortality
Procalcitonin-Guided Therapy Decreases Antimicrobial Duration in ICU Patients
Clinical question: Can utilization of serum procalcitonin (PCT) levels safely reduce antimicrobial exposure in ICU patients?
Background: Serum PCT levels are elevated in bacterial infections and sepsis and have been used in some settings to guide antimicrobial therapy. Randomized controlled trials have demonstrated reduction of antibiotic use with PCT measurement. This systematic review assessed the safety and effectiveness of PCT measurements in reducing antimicrobial exposure in ICU patients.
Study design: Systematic review.
Setting: Adult medical and surgical ICUs.
Synopsis: A search of MEDLINE and EMBASE yielded 1,018 publications related to PCT, critically ill patients, and antimicrobial therapy. Six randomized controlled trials involving 1,476 patients were reviewed. The duration of antimicrobial use was significantly decreased in all five studies that evaluated treatment duration. The remaining study only assessed the impact of PCT on initiation of antimicrobial therapy and did not demonstrate decreased antimicrobial exposure. Compared with the control group, patients randomized to PCT-guided therapy had relative reductions in duration of first antibiotic course by 21%, down to 38%, and decreases in days of antimicrobial therapy per 1,000 ICU patient days by 20%, down to 23%. PCT intervention also was associated with a 23% to 37% increase in days alive without antimicrobial therapy during the first 28 days. The length of ICU stay was significantly decreased in two studies but was not significantly different in the other studies. There were no significant differences in rates of mortality, infection relapse, or days free of mechanical ventilation.
Bottom line: PCT guidance reduced antimicrobial exposure of ICU patients without increasing rates of mortality or infection relapse.
Citation: Agarwal R, Schwartz DN. Procalcitonin to guide duration of antimicrobial therapy in intensive care units: a systematic review. Clin Infect Dis. 2011;53:379-387.
High Residual Platelet Reactivity Increases Risk of Cardiovascular Events among Patients with Acute Coronary Syndromes Undergoing PCI
Clinical question: Is high residual platelet reactivity (HRPR) in patients receiving clopidogrel associated with increased risk of ischemic events after percutaneous coronary intervention (PCI)?
Background: Studies have demonstrated an increased risk of cardiovascular events associated with HRPR in patients receiving clopidogrel while undergoing PCI. However, these studies have used a variety of platelet function tests, and thresholds for positive tests have not been established. In addition, these studies have enrolled heterogeneous populations with short-term follow-up and few have included patients with acute coronary syndromes (ACSs).
Study design: Prospective cohort study.
Setting: Cardiology service of a referral center in Italy.
Synopsis: This study included 1,789 consecutive patients with ACS undergoing PCI. Patients were given 325 mg aspirin and a 600-mg loading dose of clopidogrel on admission, followed by a daily maintenance dose of aspirin 325 mg and clopidogrel 75 mg for at least six months. Platelet reactivity was measured using adenosine diphosphate (ADP) testing, and those with HRPR (≥70% platelet aggregation) were given increased dosing of clopidogrel or switched to ticlopidine using ADP test guidance. At two-year follow-up, patients with HRPR experienced an increased composite endpoint of cardiac death, myocardial infarction, urgent coronary revascularization, and stroke with a combined event rate of 14.6% in patients with HRPR and 8.7% in patients with low residual platelet reactivity (95% CI, 1.6%-11.1%; P=0.003). Stent thrombosis was also higher in HRPR patients (absolute risk increase 3.2%; 95% CI, 0.4%-6.7%; P=0.01).
This study is nonrandomized, and residual unmeasured confounding cannot be excluded. In addition, use of non-antiplatelet drugs and adherence to recommended drugs might have influenced outcomes.
Bottom line: HRPR is associated with increased risk of ischemic events in patients with ACS receiving antiplatelet agents after PCI.
Citation: Parodi G, Marcucci R, Valenti R, et al. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215-1223.
Conservative Treatment of Necrotizing Pancreatitis Is Associated with Improved Outcomes
Clinical question: What are the outcomes of conservative and interventional management of necrotizing pancreatitis?
Background: Open necrosectomy was historically the treatment of choice for necrotizing pancreatitis, but, currently, pancreatic necrosis is only managed invasively when complicated by infection. Other changes in management over time have included a shift in the timing of intervention and the use of minimally invasive techniques. Existing studies do not reflect these changes in practice patterns and have been limited by small sample sizes or the exclusion of important subgroups of patients.
Study design: Prospective cohort study.
Setting: Twenty-one Dutch hospitals.
Synopsis: This study included 639 patients with acute necrotizing pancreatitis confirmed by imaging. Overall mortality was 15%. Conservative treatment was performed in 62% of the patients with a mortality of 7%; however, patients with organ failure (pulmonary, circulatory, and/or renal) who received conservative therapy had a mortality rate of 37%. Intervention (percutaneous drainage, video-assisted retroperitoneal debridement, endoscopic transluminal necrosectomy, laparotomy) in patients with suspected or confirmed infected pancreatic necrosis was performed on 38% of the patients with associated mortality of 27%. Interventions performed within the first 14 days of hospitalization resulted in a 56% mortality rate, whereas interventions performed after Day 29 resulted in a 15% mortality rate (P<0.001). Patients with organ failure experienced significantly greater mortality compared with patients with no organ failure (35% vs. 2%; P<0.001). Primary percutaneous drainage was associated with fewer complications than was primary necrosectomy (42% vs. 64%; P=0.003).
This study was nonrandomized, and final decisions regarding management were left to the treating physician. Notably, while there was no significant difference in APACHE II scores between the conservative and intervention groups, intervention patients had more severe pancreatic disease and scored higher on other measures of disease severity.
Bottom line: Patients with necrotizing pancreatitis can frequently be managed conservatively, though the presence of organ failure and parenchymal necrosis confer poorer prognosis. When intervention is indicated, postponing intervention and utilizing minimally invasive techniques decrease morbidity and mortality.
Citation: Van Santvoort HC, Bakker OJ, Bollen TL, et al. A conservative and minimally invasive approach to necrotizing pancreatitis improves outcome. Gastroenterology. 2011;141:1254-1263.
Cardiac MRI Complements Clinical Findings in Diagnosis of Stress Cardiomyopathy
Clinical question: What are the clinical features and cardiovascular MRI findings in patients with stress (Takotsubo) cardiomyopathy?
Background: Stress cardiomyopathy (SC) is characterized by acute and profound reversible left ventricular dysfunction that is thought to result from increased sympathetic activity related to emotional and/or physical stress. Prior studies evaluating the clinical features of SC were limited by small sample sizes and single-center enrollment, and cardiac MRI use in SC has not been well studied.
Study design: Prospective cohort study.
Setting: Seven North American and European tertiary-care centers.
Synopsis: This study enrolled 256 patients who met diagnostic criteria for SC according to Mayo criteria. Postmenopausal women were most commonly affected; only 11% of participants were men and 8% were women younger than 50 years old. An identifiable stressor was found in 71% of the patients. Clinical presentation was notable for symptoms of acute coronary syndrome (ACS) in 88% of patients, abnormal electrocardiogram in 87%, and elevated troponin T in 90%. Coronary angiography was normal in three-fourths of patients, and no patients had features of acute plaque rupture. Typical apical ballooning was seen on left ventriculography in 82% of patients.
Cardiac MRI findings included severe left ventricular dysfunction in a noncoronary distribution, myocardial edema in areas of regional wall abnormalities, absence of high signal areas in late gadolinium enhancement (e.g., absence of necrosis/fibrosis), and increased early gadolinium uptake (i.e. early inflammation). Repeat cardiac MRI four weeks after initial diagnosis showed near or complete resolution of imaging findings.
Bottom line: Stress cardiomyopathy typically presents like ACS, usually affects postmenopausal women, is often preceded by a stressful event, and is characterized by cardiac MRI findings of regional wall motion abnormalities, reversible myocardial injury, and the absence of fibrosis. Cardiac MRI may be valuable in diagnosing SC in patients who present without the classic clinical features.
Citation: Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277-286.
Increased Risk of Potentially Inadvertent Medication Discontinuation Following Acute-Care Hospitalization
Clinical question: Are medications for chronic diseases inadvertently discontinued after acute-care hospitalization, and is this risk increased in patients who had an ICU stay?
Background: Transitions of care are associated with medical errors. Two such transitions are a shift from the ICU to floor setting and from the inpatient to outpatient setting. Medications for chronic diseases might be held during hospitalization for a variety of reasons, and medication errors may occur if these drugs are not restarted when the acute problem resolves or the patient is discharged from the hospital.
Study design: Population-based cohort study.
Setting: Ontario, Canada.
Synopsis: Using four separate databases, administrative records were reviewed for 396,380 patients aged >65 years who were continuous users of at least one of five evidence-based medication groups for common chronic diseases. Medications included statins, antiplatelet/anticoagulant agents, levothyroxine, respiratory inhalers, and gastric acid suppressants. The primary outcome was potentially unintentional medication discontinuation (measured by failure to renew the prescription at 90 days) for hospitalized versus nonhospitalized patients. All medication groups had statistically significant adjusted odds ratios ranging from 1.18 (95% CI, 1.14-1.23) for discontinuation of levothyroxine to 1.86 (95% CI, 1.77-1.97) for discontinuation of antiplatelet/anticoagulant medications. Treatment in an ICU further increased this risk compared with nonhospitalized patients, and increased the risk for medication discontinuation in four of the five medication groups when compared with patients hospitalized without ICU treatment.
Important study limitations include the lack of appropriate clinical details to classify medication discontinuation as unintentional and the inability of administrative data to prove causality. This study highlights the importance of medication reconciliation and calls attention to inadvertent medication discontinuation during care transitions (see “Reconciliation Act,”).
Bottom line: Patients discharged from the hospital, particularly after ICU treatment, have a higher risk of discontinuation of long-term medications for chronic medical problems when compared with nonhospitalized patients.
Citation: Bell CM, Brener SS, Gunraj N, et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306:840-847.
Clinical Exam Remains Valuable in the Diagnosis of Patients Admitted to a Medicine Service
Clinical question: Is a clinical exam useful in the diagnosis of newly admitted patients to a general medicine service?
Background: The clinical exam, which comprises the history and physical examination, has long been described as essential to the diagnosis of illness. However, the literature supporting this claim is limited to the ambulatory setting. There has not been evaluation of the clinical exam as a diagnostic tool in the hospital setting, where more advanced testing is readily available.
Study design: Retrospective chart review.
Setting: Urban academic medical center.
Synopsis: The study included 442 adult patients consecutively admitted from the emergency department to the general medicine service who were separately assessed by one senior resident (SR) and one experienced hospital physician (HP) not involved with the case. The SR and HP each made an initial diagnosis and documented the most helpful component(s) in arriving at that diagnosis. Outcomes included comparison of the SR and HP’s admission diagnosis with the discharge diagnosis, and the diagnostic value of the various components of the clinical exam and initial studies.
Compared with the discharge diagnosis, the SR’s initial diagnosis was correct in 80.1% of cases, while the HP was correct in 84.4%. The patient’s history was the most important element in the initial assessment, independently influencing approximately 20% of the correct diagnoses for both physicians. Approximately 60% of correct diagnoses were established using the history and/or physical, and more than 90% were made using a combination of history, physical exam, and/or basic tests (admission labs, electrocardiogram, chest X-ray).
The generalizability of these results is limited by the retrospective, single-center study design, involvement of only one resident physician, and the lack of information regarding number of experienced clinicians and types of diagnoses.
Bottom line: Among patients admitted to a general medicine service, the most powerful tool in obtaining an accurate diagnosis was the combination of a patient’s history and a physical exam.
Citation: Paley L, Zornitzki T, Cohen J, et al. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171:1394-1396.
RDW Predicts All-Cause Mortality and Bloodstream Infection in ICU Patients
Clinical question: Among patients admitted to the ICU, is red blood cell distribution width (RDW) a reliable indicator of mortality?
Background: The RDW is an inexpensive test that is commonly included in routine laboratory studies. It has been associated with multiple disease processes and found to be a strong predictor of mortality in the general adult population. However, there has been limited study of the association between RDW and outcomes in critically ill patients.
Study design: Observational cohort study.
Setting: Urban tertiary-care academic medical center.
Synopsis: Data from 51,413 adult patients who received critical care between 1997 and 2007 were obtained from a computerized registry and evaluated for the primary outcome of 30-day mortality after critical-care initiation. Secondary outcomes included 90-day, 365-day, and in-hospital mortality, as well as bloodstream infection. Logistic regression examined both primary and secondary outcomes in association with pre-established RDW quintiles. After multivariable adjustment, RDW was found to be associated with mortality at 30, 90, and 365 days, in addition to in-hospital mortality. The highest RDW quintile (RDW >15.8%) had an adjusted OR of 2.61 (95% CI, 2.37-2.86; P<0.001) for the primary outcome, with similar risk for secondary outcomes of mortality. A subgroup of 18,525 patients with blood culture data was analyzed and an adjusted OR of 1.44 was found in the highest RDW quintile for the secondary outcome of bloodstream infection.
Bottom line: Red blood cell distribution width is a strong independent predictor of all-cause mortality and bloodstream infection in patients receiving intensive care.
Citation: Bazick HS, Chang D, Mahadevappa K, Gibbons FK, Christopher KB. Red cell distribution width and all-cause mortality in critically ill patients. Crit Care Med. 2011;39:1913-1921.