User login
Novel inhibitor proves ‘potent’ in hematologic malignancies
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
MRD better measure of ALL remission than morphology
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
MONTREAL – In children with acute lymphoblastic leukemia, minimal residual disease findings appear to be better at defining remission than morphology, Children’s Oncology Group investigators reported.
A study of outcomes of more than 9,000 children and young adults with B-lineage or T-lineage acute lymphoblastic leukemia (ALL) showed that patients who would be defined as being in remission by morphology but have minimal residual disease (MRD) of 5% or greater have survival outcomes similar to those of patients who do get a morphologic remission. Additionally, patients with discordant morphologic and MRD findings have significantly worse outcomes than do patients who were in morphologic remission and had concordant MRD findings, said Sumit Gupta, MD, PhD, from the Hospital for Sick Children in Toronto.
“Given that, however, although MRD is used to measure the depth of remission either using flow cytometry or PCR [polymerase chain reaction]-based methods, remission itself continues to be defined by basic morphological assessment, whether that’s in clinical practice or clinical trials,” he added.
To see whether the practice of declaring remissions by morphology still makes sense, Dr. Gupta and his colleagues in the Children’s Oncology Group looked at outcomes for children and young adults with discordant ALL remissions as assessed by morphology, compared with MRD.
They looked at data on 9,350 patients from the ages of 1 to 31 years who were enrolled in one of three Children’s Oncology Group trials for patients with newly diagnosed ALL. Two of the trials (AALL0331 and AALL0232) were for patients with B-lineage ALL, and one (AALL0434) was for patients with T-lineage ALL.
They looked at morphologic responses as assessed by local centers, with M1 responses defined as less than 5% leukemic blasts (remission), M2 defined as 5% to less than 25% blasts, and M3 as 25% or more blasts. MRD was measured by flow cytometry at one of two central labs.
They found that discordant results (M1 morphology but MRD of 5% or greater) occurred in only 0.9% of patients with B-ALL, but in 6.9% of patients with T-ALL (P less than .0001).
In multivariate analysis, significant predictors of discordance in patients with B-ALL were patients age 10 years or older (P = .03), white blood cell counts of 50,000/mcL or greater (P = .005), and neutral or unfavorable cytogenetics vs. favorable (P less than .0001 for each).
Among patients with T-ALL, the only significant predictor of discordant results was the early T-precursor phenotype, with an odds ratio of 4.7 (P less than .0001).
Comparing event-free survival (EFS) between patients with concordant remission findings (M1/MRD less than 5%), they investigators saw that for patients with B-ALL, the 5-year EFS was 87%, compared with 59% for patients with discordant findings (M1/MRD 5% or greater, P less than .0001 vs. concordant remissions), and 39% for patients with concordant results showing a lack of remission (P = .009 vs. discordant findings).
Similarly, respective EFS rates for patients with T-ALL were 88%, 80% (P = .011) and 63% (not significant).
In a subanalysis of EFS by risk category, they found no differences according to concordance/discordance among patients with standard-risk B-ALL but a significant difference among patients with high-risk disease.
Attempting to determine what was driving the intermediate outcomes of patients with discordant findings, “we hypothesized that maybe it’s a difference in their actual MRD levels.” Specifically, they found that while both discordant and concordant not-in-remission patients had MRD levels of 5% or higher, the MRD levels were higher among those patients who were conclusively not in remission, Dr. Gupta said.
Finally, they found that for those patients with known overall survival data, concordant in remission patients with B-ALL had a 94% rate out to 12 years, compared with 73% for those with discordant results (P less than .0001). There was no significant difference in OS among patients with T-ALL, however.
“Should MRD assessment actually replace morphology in defining remission in subjects with ALL? I think these data strongly support that,” Dr. Gupta said.
The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
FROM ASPHO 2017
Key clinical point: Patients with ALL determined to be in remission by both morphology and minimal residual disease had better outcomes than did those with discordant results.
Major finding: Event-free survival of B-ALL was 87% for patients with concordant remission findings vs. 59% for patients with discordant findings and 39% for concordant not-in-remission findings.
Data source: Retrospective review of data on 9,350 children and young adults with ALL.
Disclosures: The study was supported by the National Institutes of Health. Dr. Gupta reported having no conflicts of interest.
Could refractory T-ALL be daratumumab’s next frontier?
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
MONTREAL – Daratumumab may do for patients with T-cell acute lymphoblastic leukemia (T-ALL) what it has done for those with multiple myeloma. That, at least, is the hope of a team of investigators who are conducting preclinical studies and planning human trials of the CD38 inhibitor in leukemia.
“We believe daratumumab significantly inhibits disease progression as shown in our different [patient-derived xenograft] models,” said Karen L. Bride, MD, of Children’s Hospital of Philadelphia.
The Food and Drug Administration approved daratumumab (Darzalex) in November 2015 for the treatment of patients with multiple myeloma who had received at least three prior lines of therapy. They then amended the approval last fall to “at least one prior medicine.”
When added to a standard regimen of bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma in the phase III CASTOR trial, daratumumab reduced the risk of disease progression or death by 61% with little increase in toxicity.
The drug is believed to work against multiple myeloma through both an on-target (anti-CD38) mechanism, and through off-target promotion of increases in T-helper cells, cytotoxic T-lymphocytes, T-cell function response, and T-cell receptor clonality (Blood. 2016 Jan. doi: 10.1182/blood-2015-12-687749).
CD38 in T-ALL
Dr. Bride and her colleagues hope to bring daratumumab’s anti-CD38 action to bear on relapsed or refractory T-ALL.
“One of the reasons this is particularly challenging is that we find T-ALL is clinically and genetically heterogeneous,” she said. “With a number of different genetic mutations that have been identified, there are certainly some potentially targetable pathways. However, finding an appropriate target that can be broadly applicable is still needed.”
CD38 may be one such target. It is expressed at relatively high levels on both T-ALL and B-precursor ALL blasts but at only low levels on normal immune cells.
The investigators first used flow cytometry to measure CD38 levels in samples from 10 patients with early T-cell precursor (ETP) T-ALL and 11 with non-ETP disease, both at diagnosis and after 1 month of induction chemotherapy. CD38 expression was detectable in all of the samples and did not change significantly after chemotherapy, suggesting that CD38 was indeed a valid target in T-ALL.
They then grafted primary ALL blasts from patients with ETP-ALL and non-ETP-ALL into mice and randomly assigned them to be treated for 3 to 5 weeks with daratumumab or to serve as controls. The mice were initially treated after they developed more than 1% of peripheral blood blasts.
Daratumumab-treated models had significant reductions in disease burden as measured by blasts in both peripheral blood (P = .0112) and spleen (P = .0003).
There were six responses to daratumumab in the seven treated mice grafted with ETP-ALL and no cases of toxicity. Among the eight mice with non-ETP ALL, however, there was only one response, and five animals became moribund roughly 1 hour after injection.
The investigators could not find an explanation for these reactions either on necropsy or pathology studies.
“We hypothesized that there was potentially massive tumor lysis syndrome being experienced by the mice, and, as a consequence, they were becoming moribund,” Dr. Bride said.
In subsequent experiments, they have begun introducing the drug within 5 days of adoptive transfer, prior to full engraftment. This is akin to treating during a minimal residual disease phase, she said.
Despite the observed but unexplained toxicities in some animals, “our data are promising enough that we’re hopeful that we will open a phase I/II trial of daratumumab starting next year,” Dr. Bride said.
Not so fast
However, a pediatric hematologist/oncologist who was not involved in the study said in an interview that Dr. Bride and her colleagues would be wise not to proceed too quickly into human trials, at least until the potential toxicities of daratumumab in T-ALL have been more fully elucidated.
“I found it very striking that the mice responded the way they did, and that was just from receiving the drug. So, there is something else that’s going on, and I think it behooves them to investigate further. It’s not that I’m skeptical about the activity of the drug; I just don’t want studies to be shut down because the investigators didn’t have the best trial design,” said Valerie I. Brown, MD.
Dr. Brown, director of experimental therapeutics at Penn State Health Milton S. Hershey (Penn.) Medical Center, was a comoderator of the session where Dr. Bride presented the study findings.
Asked about her response to Dr. Brown’s comments in an interview, Dr. Bride said that “because of the success of daratumumab in humans already, I think I’m a bit less worried about this agent. You can’t necessarily translate exactly across diseases, but I do think it’s very promising, and I don’t think [the toxicity] is a reason to pull back.”
The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported no conflicts of interest to disclose.
Key clinical point: CD38 may be a valid target for therapy against relapsed/refractory T-cell acute lymphoblastic leukemia.
Major finding: Six of seven models of early T-precursor T-ALL responded to daratumumab injections.
Data source: In vitro and in vivo studies evaluating the potential of daratumumab for treatment of T-ALL.
Disclosures: The study was supported by grants from the Leukemia and Lymphoma Society and the National Institutes of Health. Janssen donated the daratumumab. Dr. Bride and Dr. Brown reported having no conflicts of interest.
Cord blood/placental cell combo induces rapid immune recovery
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
MONTREAL – A combination of placenta-derived stem cells and umbilical cord blood was associated with early engraftment and high degrees of cord blood donor chimerism in the treatment of children with both malignant and nonmalignant hematologic conditions requiring stem cell transplantation, updated results of a pilot study show.
Among 16 children treated with the combination, the probability of neutrophil engraftment was 87.5%, and all patients who had neutrophil engraftment went on to have platelet engraftment. The probability of 12-month overall survival was 81.2%, reported Allyson Flower, MD, from Boston Children’s Health Physicians in Hawthorne, N.Y. “The probability of grade II-IV acute graft vs. host disease was 12.5%, compared with 32.5% seen with unrelated cord blood in our group’s previous studies. Cellular immune reconstitution was robust,” she said at the annual meeting of the American Society of Pediatric Hematology/Oncology.
Augmenting cord blood
Although unrelated donor cord blood transplantation expands the donor pool, is rapidly available, and is associated with decreases in both severe acute graft vs. host disease (GVHD) and chronic GVHD, compared with other stem cell sources, the technique is hampered by limited cell doses, prolonged immune reconstitution time, delays in hematopoietic recovery, and a higher incidence of graft failure.
Early studies of myeloablative conditioning followed by unrelated umbilical or placental blood transplantation showed a median of 22-24 days to neutrophil engraftment (Blood 1996 88:795-802; N Engl J Med. 1996;335:157-66), Dr. Flower noted.
More recently, a multivariate analysis of patients who underwent reduced-intensity conditioning followed by hematopoietic stem cell transplant with unrelated cord blood showed that graft failure was an independent risk factor for worse overall survival (Biol Blood Marrow Transplant. 2013 Apr;19:4;552-61).
Multiple groups have shown that adding human placenta–derived stem cells (HPDSC) to cord blood transplantation can facilitate more rapid hematopoietic engraftment by increasing the number of stem cells, increasing the proportion of hematopoietic progenitor cells, and providing additional, immature CD34+/CD45– progenitor cells.
In a single-arm, nonrandomized study, the investigators enrolled 16 patients ranging in age from 0.3 to 15.7 years with inborn errors of metabolism, marrow failure syndromes, severe immunodeficiency states, or hematologic malignancies.
Malignant conditions included B-cell precursor acute lymphoblastic leukemia (B-ALL; four patients), acute myeloid leukemia (AML; two), and T-cell ALL (one) in first complete remission, and T-cell lymphoblastic lymphoma following induction failure (one). Nonmalignant conditions included adrenoleukodystrophy (two patients), amegakaryotic thrombocytopenia (one), severe combined immunodeficiency (SCID; two), dyskeratosis congenita (one), chronic granulomatous disease (one), and severe congenital neutropenia (one).
The patients first underwent either myeloablative or reduced-intensity conditioning, followed 10 days later by infusion of unrelated cord blood and HPDSCs. Prior to HPDSC infusion, patients were medicated with diphenhydramine and hydrocortisone to prevent or reduce potential sensitivity reactions. HPDSCs were infused no sooner than 4 hours after the end of the cord blood infusion.
Patients received GVHD prophylaxis with either tacrolimus or cyclosporine, plus mycophenolate mofetil.
The combination appeared to be safe, with no cases of grade 3 or 4 toxicity secondary to HPDSC infusion.
The probability of neutrophil engraftment was 87.5%, with engraftment occurring at a median of 23 days (range 13-53). As noted before, all patients who had neutrophil engraftment had platelet engraftment, which was achieved at a median of 47 days (range, 20-98). In the group’s previous studies, median time to platelet engraftment was 53 days for patients who had undergone reduced-intensity conditioning, and 118 days for patients who had undergone myeloablation.
The probability of grade 2-4 acute GVHD within 100 days was 12.5%, and there were no cases of chronic GVHD.
Respective percentages of cord blood donor chimerism at days 30, 60, 100, and 180 were 88%, 98%, 99%, and 99%.
Immune reconstitution was strong, with normalization of mean CD3+, CD19+, and CD56+ cells occurring by day 100, CD8+ cells by day 180, and CD4+ cells by day 270.
There were three patient deaths: one from adenoviremia in a patient with B-ALL and CNS relapse, who had neutrophil engraftment at day 21; one in a patient with SCID, from adenoviremia and multiple system organ failure, who did not have engraftment before death; and one in a patient with severe congenital neutrophilia, who also did not have neutrophil engraftment.
None of the eight patients with malignant disease have experienced relapse to date, Dr. Flower noted.
The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
Key clinical point: A combination of donor cord blood and human placenta–derived stem cells induced more rapid engraftment than cord blood alone.
Major finding: The probability of 12-month overall survival was 81%.
Data source: Open-label single-arm study in 16 children with severe malignant and nonmalignant diseases requiring hematopoietic stem cell transplants.
Disclosures: The study was funded by a grant from Celgene Cellular Therapeutics. Dr. Flower reported having no conflicts of interest.
FDA approves first ready-to-use oral solution of methotrexate
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
The US Food and Drug Administration (FDA) has approved a ready-to-use oral solution of methotrexate (Xatmep) for use in certain pediatric patients.
The drug is approved as part of a multi-phase, combination chemotherapy maintenance regimen to treat pediatric patients with acute lymphoblastic leukemia.
Xatmep is also approved for use in pediatric patients with active polyarticular juvenile idiopathic arthritis who have had an insufficient response to, or cannot tolerate, an adequate trial of first-line therapy, including full-dose non-steroidal anti-inflammatory agents.
Xatmep is the first ready-to-use oral solution of methotrexate to be approved by the FDA.
There was previously no such formulation of the drug approved for use in pediatric patients requiring body surface area dosing (mg/m2), patients who have difficulty swallowing or cannot consume tablets, or those with needle-phobia.
Xatmep (methotrexate) Oral Solution, 2.5 mg/mL, requires no preparation. It eliminates the need for needles, crushing or splitting tablets, or for compounding tablets into a liquid formulation.
Xatmep requires refrigeration but may be stored at room temperature for 60 days after dispensing.
For more on Xatmep, see the prescribing information, which includes a boxed warning detailing the risk of severe toxic reactions, including embryo-fetal toxicity.
Xatmep is available through pharmacies and a qualified mail-order service. For information on how to obtain the drug, call 1-855-379-0382.
Xatmep is a product of Silvergate Pharmaceuticals, Inc. ![]()
Treatment-related hypertension, kidney injury are undertreated in kids with leukemia
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
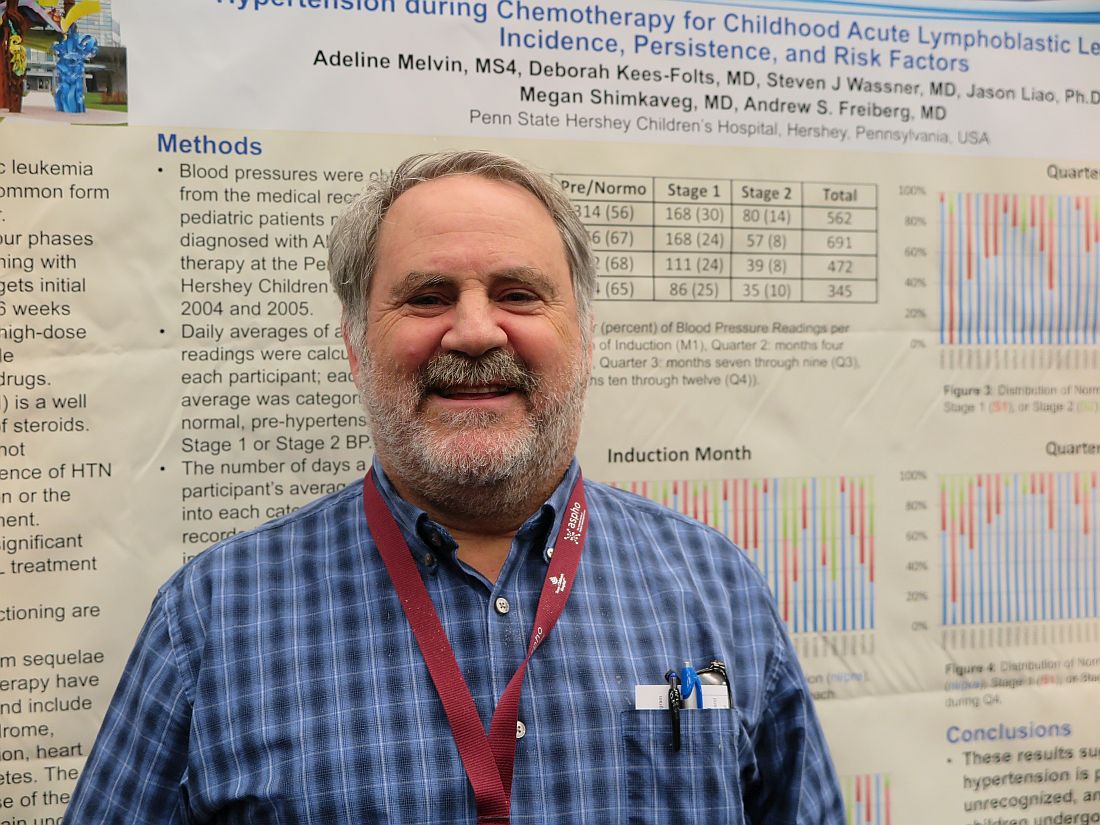
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
At ASPHO 2017
Key clinical point:
Major finding: The combined incidence of stage 1 or 2 hypertension in children during induction therapy for acute lymphoblastic leukemia was 44%.
Data source: Two retrospective institutional studies.
Disclosures: The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
CHMP recommends inotuzumab ozogamicin for adult ALL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion of inotuzumab ozogamicin (Besponsa®).
The CHMP is recommending approval of inotuzumab ozogamicin for the treatment of adults with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia (ALL), including patients with Philadelphia chromosome-positive ALL who have failed treatment with at least one tyrosine kinase inhibitor.
The CHMP’s opinion will be reviewed by the European Commission, which is expected to issue a decision on approval within 67 days from adoption of the opinion.
Inotuzumab ozogamicin is an antibody-drug conjugate that consists of a monoclonal antibody targeting CD22 and a cytotoxic agent known as calicheamicin.
The product originates from a collaboration between Pfizer and Celltech (now UCB), but Pfizer has sole responsibility for all manufacturing and clinical development activities.
The application for inotuzumab ozogamicin is supported by results from a phase 3 trial, which were published in NEJM in June 2016.
The trial enrolled 326 adult patients with relapsed or refractory B-cell ALL and compared inotuzumab ozogamicin to standard of care chemotherapy.
The rate of complete remission, including incomplete hematologic recovery, was 80.7% in the inotuzumab ozogamicin arm and 29.4% in the chemotherapy arm (P<0.001). The median duration of remission was 4.6 months and 3.1 months, respectively (P=0.03).
Forty-one percent of patients treated with inotuzumab ozogamicin and 11% of those who received chemotherapy proceeded to stem cell transplant directly after treatment (P<0.001).
The median progression-free survival was 5.0 months in the inotuzumab ozogamicin arm and 1.8 months in the chemotherapy arm (P<0.001).
The median overall survival was 7.7 months and 6.7 months, respectively (P=0.04). This did not meet the prespecified boundary of significance (P=0.0208).
Liver-related adverse events were more common in the inotuzumab ozogamicin arm than the chemotherapy arm. The most frequent of these were increased aspartate aminotransferase level (20% vs 10%), hyperbilirubinemia (15% vs 10%), and increased alanine aminotransferase level (14% vs 11%).
Veno-occlusive liver disease occurred in 11% of patients in the inotuzumab ozogamicin arm and 1% in the chemotherapy arm.
There were 17 deaths during treatment in the inotuzumab ozogamicin arm and 11 in the chemotherapy arm. Four deaths were considered related to inotuzumab ozogamicin, and 2 were thought to be related to chemotherapy. ![]()
ALL, HL guidelines added to radiation therapy resource
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
The National Comprehensive Cancer Network® (NCCN) has added 9 disease sites to its NCCN Radiation Therapy Compendium™, a resource that provides a single access point for NCCN recommendations pertaining to radiation therapy (RT).
The compendium provides guidance on all RT modalities recommended within NCCN guidelines, including intensity modulated RT, intra-operative RT, stereotactic radiosurgery/stereotactic body RT/stereotactic ablative RT, image-guided RT, low dose-rate brachytherapy/high dose-rate brachytherapy, radioisotope, and particle therapy.
The NCCN Radiation Therapy Compendium was launched with recommendations pertaining to 24 cancer types.
Now, NCCN has added RT recommendations from an additional 9 NCCN Clinical Practice Guidelines in Oncology:
Acute lymphoblastic leukemia (ALL), Version 2.2016
Basal cell skin cancer, Version 1.2017
Dermatofibrosarcoma protuberans, Version 1.2017
Gastric cancer, Version 1.2017
Hodgkin lymphoma (HL), Version 1.2017
Merkel cell carcinoma, Version 1.2017
Ovarian cancer, Version 1.2017
Squamous cell skin cancer, Version 1.2017
Thymomas and thymic carcinomas, Version 1.2017
The first 24 disease sites included in the NCCN Radiation Therapy Compendium were:
Acute myeloid leukemia
Anal cancer
B-cell lymphomas
Bladder cancer
Breast cancer
Chronic lymphocytic leukemia/small lymphoblastic lymphoma
Colon cancer
Hepatobiliary cancers
Kidney cancer
Malignant pleural mesothelioma
Melanoma
Multiple myeloma
Neuroendocrine tumors
Non-small cell lung cancer
Occult primary cancer
Pancreatic adenocarcinoma
Penile cancer
Primary cutaneous B-cell lymphomas
Prostate cancer
Rectal cancer
Small cell lung cancer
Soft tissue sarcoma
T-cell lymphomas
Testicular cancer
The NCCN said additional cancer types will be added to the NCCN Radiation Therapy Compendium on a rolling basis over the coming months.
For more information and to access the NCCN Radiation Therapy Compendium, visit NCCN.org/RTCompendium. ![]()
Second cancers take greater toll on younger patients
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Second cancers take a greater toll on patients under the age of 40, according to research published in JAMA Oncology.
Researchers studied 14 types of cancer occurring in more than 1 million patients.
For nearly all of the cancers studied, 5-year survival rates were much higher if the cancer occurred as a first malignancy rather than a second cancer.
These survival differences were more pronounced in pediatric patients and adolescents and young adults (AYAs) than they were in patients age 40 and older.
Researchers hope these findings will help guide clinicians in providing age-specific recommendations on cancer prevention, screening, treatment, and survivorship, especially among the AYA population.
“Although the increased incidence of second cancers is well known among cancer survivors, less is known about outcomes of these cancers or the influence of age,” said Theresa Keegan, PhD, of the UC Davis Comprehensive Cancer Center in Sacramento, California.
With this in mind, Dr Keegan and her colleagues analyzed data on patients diagnosed with either a single cancer or a first and second malignancy during 1992 through 2008. The researchers used Surveillance, Epidemiology and End Results program data collected from 13 cancer registries.
The team collected data on the 14 most common cancer types that affect AYAs: acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), soft tissue sarcoma, and bone sarcoma, as well as female breast, thyroid, testicular, colorectal, central nervous system, cervical, and ovarian cancers.
There were a total of 15,954 pediatric patients (younger than 15 years at diagnosis), 125,750 AYAs (ages 15 to 39), and 878,370 older adult patients (age 40 and older).
Survival rates
For pediatric patients, the 5-year relative survival was 80% for a first cancer and 47% for a second primary malignancy.
For AYAs, the 5-year relative survival was 81% for a first cancer and 60% for a second primary malignancy.
For older adults, the 5-year relative survival was 70% for a first cancer and 61% for a second primary malignancy.
When the researchers looked at 5-year survival by age and individual cancer types, they found striking differences depending on whether it was a first or second malignancy in all but 2 of the 14 cancer types, testicular cancer and melanoma.
“For almost every type of cancer, the AYA population did worse with a secondary cancer,” said study author Melanie Goldfarb, MD, of John Wayne Cancer Institute at Providence Saint John’s Health Center in Santa Monica, California.
“What struck us was that the second cancer caused such an increased risk of death.”
Lymphomas
For pediatric patients with HL, the 5-year relative survival was 95% when patients had HL as a first cancer. There were no data on HL as a second primary malignancy.
For AYAs, the 5-year relative survival was 93% when patients had HL as a first cancer and 72% when they had HL as a second primary malignancy.
For older adults, the 5-year relative survival was 69% when patients had HL as a first cancer and 54% when they had HL as a second primary malignancy.
For pediatric patients with NHL, the 5-year relative survival was 84% when patients had NHL as a first cancer and 63% when they had NHL as a second primary malignancy.
For AYAs, the 5-year relative survival was 64% when patients had NHL as a first cancer and 22% when they had NHL as a second primary malignancy.
For older adults, the 5-year relative survival was 57% when patients had NHL as a first cancer and 54% when they had NHL as a second primary malignancy.
Leukemias
For pediatric patients with ALL, the 5-year relative survival was 87% when patients had ALL as a first cancer and 63% when they had ALL as a second primary malignancy.
For AYAs, the 5-year relative survival was 48% when patients had ALL as a first cancer and 26% when they had ALL as a second primary malignancy.
For older adults, the 5-year relative survival was 17% when patients had ALL as a first cancer and 11% when they had ALL as a second primary malignancy.
For pediatric patients with AML, the 5-year relative survival was 57% when patients had AML as a first cancer and 29% when they had AML as a second primary malignancy.
For AYAs, the 5-year relative survival was 46% when patients had AML as a first cancer and 23% when they had AML as a second primary malignancy.
For older adults, the 5-year relative survival was 12% when patients had AML as a first cancer and 10% when they had AML as a second primary malignancy.
Why younger patients tend to fare worse after a second cancer than older patients is not fully understood or specifically addressed in the current study, the researchers noted.
Now, the team plans to examine how the time between getting a first and second cancer affects survival and whether the type of treatment for the first cancer influences the outcome of a second cancer. ![]()
Nanoparticles allow for creation of CAR T cells in vivo
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()
Researchers say they have developed biodegradable nanoparticles that can be used to genetically reprogram T cells while they are still in the body.
The nanoparticles were able to program T cells with genes encoding leukemia-specific chimeric antigen receptors (CARs).
The resulting CAR T cells were able to eliminate leukemia or slow the progression of disease in a mouse model of B-cell acute lymphoblastic leukemia (B-ALL).
Researchers reported these results in Nature Nanotechnology.
“Our technology is the first that we know of to quickly program tumor-recognizing capabilities into T cells without extracting them for laboratory manipulation,” said Matthias Stephan, MD, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“The reprogrammed cells begin to work within 24 to 48 hours and continue to produce these receptors for weeks. This suggests that our technology has the potential to allow the immune system to quickly mount a strong enough response to destroy cancerous cells before the disease becomes fatal.”
Dr Stephan and his colleagues designed their nanoparticles to carry genes that encode for CARs intended to target and eliminate B-ALL. The nanoparticles are coated with ligands that make them seek out and bind to T cells.
When a nanoparticle binds to a T cell, the cell engulfs the particle. The nanoparticle then travels to the cell’s nucleus and dissolves.
The CAR genes integrate into chromosomes housed in the nucleus, making it possible for the T cells to begin decoding the new genes and producing CARs within 1 or 2 days.
Once they determined their CAR-carrying nanoparticles reprogrammed a noticeable percentage of T cells, the researchers tested the T cells’ efficacy in a mouse model of B-ALL.
The team infused the nanoparticles into 10 mice and found the treatment eradicated tumors in 7 of the animals. The other 3 mice “showed substantial regression” of leukemia, the researchers said.
On average, mice that received CAR-carrying nanoparticles had a 58-day improvement in survival compared to control mice.
Mice that received the nanoparticles also had “dramatically reduced” B-cell numbers in their spleens. The researchers noted that this is consistent with the reversible B-cell aplasia observed in patients who receive conventional CD19 CAR T-cell therapy.
Dr Stephan and his colleagues also tested conventional CAR T-cell therapy in the B-ALL mouse model. The mice received cyclophosphamide followed by CAR T cells created ex vivo.
These mice had significantly better survival than controls, but their survival was comparable to that of the mice that received the CAR-carrying nanoparticles.
Although these nanoparticles are several steps away from the clinic, Dr Stephan said he imagines a future in which nanoparticles transform cell-based immunotherapies into easily administered, off-the-shelf treatments that are available anywhere.
“I’ve never had cancer, but if I did get a cancer diagnosis, I would want to start treatment right away,” Dr Stephan said. “I want to make cellular immunotherapy a treatment option the day of diagnosis and have it able to be done in an outpatient setting near where people live.” ![]()