User login
Exploring options for POP treatment: Patient selection, surgical approaches, and ways to manage risks
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.
Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
A number of presentations at the 2019 Pelvic Anatomy and Gynecologic Surgery (PAGS) Symposium (Las Vegas, Nevada, December 12-14, 2019) focused on pelvic organ prolapse (POP) repair, including anatomic considerations, the evolution of surgical procedures, and transvaginal repair. OBG M
Nonsurgical approaches for POP: A good option for the right patient
John B. Gebhart, MD, MS: What are the nonsurgical options for POP?
Mark D. Walters, MD: Women who have prolapse could, of course, choose to continue to live with the prolapse. If they desire treatment, however, the main nonsurgical option is a combination of pessary use, possibly with some estrogen, and possibly with pelvic muscle exercises. Women who have a well-fitting pessary can be managed satisfactorily for years. If possible, women should be taught to take the pessary in and out on a regular basis to minimize their long-term complications.
Dr. Gebhart: How can nonsurgical treatment options be maximized?
Beri M. Ridgeway, MD: It depends on patient commitment. This is important to assess at the first visit when you are making management decisions, because if someone is not going to attend physical therapy or not going to continue to do the exercises, the expectation for the outcome is not going to be great.
Also, if a patient feels very uncomfortable using a pessary and really does not want it, I am fine proceeding with surgery as a first-line treatment. If the patient is committed, the ideal is to educate her and connect her with the right people, either a pelvic floor physical therapist or someone in your office who will encourage her and manage pessary use.
Dr. Gebhart: It goes back to assessing patient goals and expectations.
Mickey M. Karram, MD: If you have a patient who is a good candidate for a pessary—say she has a well-supported distal vagina and maybe a cervical prolapse or an apical prolapse—and you can fit a small pessary that will sit in the upper vagina in a comfortable fashion, it is worthwhile to explain to the patient that she is a really good candidate for this option. By contrast, someone who has a wide genital hiatus and a large rectocele will not have good success with a pessary.
Dr. Gebhart: That is important: Choose your nonsurgical patients well, those who will respond to therapy and maybe not get frustrated with it.
Dr. Walters: A problem I see is that some people are good at fitting a pessary, but they do not teach how to use it very well. When I see the patient back, she says, “What’s my long term on the pessary?” I say, “If we teach you to take it in and out, you are less likely to have any problems with it, and then you can manage it for years that way. Otherwise, you have to keep visiting a practitioner to change it and that is not necessarily a good long-term option.” At the very first visit, I teach them what a pessary is, its purpose, and how to maintain it themselves. I think that gives patients the best chance for long-term satisfaction.
Dr. Gebhart: Surgery is always an option if pessary management is not satisfactory.
Dr. Ridgeway: I also tell patients, especially those uncertain about using a pessary, “Worst case, you spend a little time to figure this out, but if it works, you can avoid surgery. If it doesn’t—the risks are very low and you perhaps wasted some time—but at least you’ll know you tried the conservative management.”
Dr. Gebhart: Mickey made an excellent point earlier that it can be a diagnostic treatment strategy as well.
Dr. Karram: If you are concerned about the prolapse worsening or negatively impacting a functional problem related to the bladder or bowel, it is good to place a pessary for a short period of time. This can potentially give you an idea of how your surgery will impact a patient’s bladder or bowel function.
Continue to: Decisions to make before choosing a surgical approach...
Decisions to make before choosing a surgical approach
Dr. Gebhart: Would you elaborate on the surgical options for managing POP?
Dr. Walters: For women with prolapse who decide they want to have surgery, the woman and the surgeon need to make a number of decisions. Some of these include whether the uterus, if present, needs to be removed; whether the woman would like to maintain sexual function or not; whether the repair would best be done vaginally only with native tissue suturing, vaginally with some augmentation (although that is not likely in the United States at this time), or through the abdomen, usually laparoscopically or robotically with a mesh-augmented sacrocolpopexy repair.
Also, we must decide whether to do additional cystocele and rectocele repairs and whether to add slings for stress incontinence, which can coexist or could develop after the prolapse repair. A lot of different decisions need to be made when choosing a prolapse repair for different women.
Dr. Ridgeway: It is shared decision-making with the patient. You need to understand her goals, the degree of prolapse, whether she has contraindications to uterine preservation, and how much risk she is willing to take.

Fundamentals of the clinical evaluation
Dr. Gebhart: For a woman who wants to manage her prolapse surgically, let us consider some fundamentals of clinical diagnosis. Take me through your office evaluation of the patient reporting prolapse symptoms—her history, yes, but from a physical exam standpoint, what is important?
Dr. Karram: You want to know if this is a primary prolapse or recurrent prolapse. You want to distinguish the various segments of the pelvic floor that are prolapsing and try to quantitate that in whatever way you would like. A standardized quantification system is useful, but you should have a system within your practice that you can standardize. Then, determine if there are coexisting functional derangements and how those are being impacted by the prolapse, because that is very important.
Take a good history, and identify how badly the prolapse bothers the patient and affects her quality of life. Understand how much she is willing to do about it. Does she just want to know what it is and has no interest in a surgical intervention, versus something she definitely wants to get corrected? Then do whatever potential testing around the bladder, and bowel, based on any functional derangements and finally determine interest in maintaining sexual function. Once all this information is obtained, a detailed discussion of surgical options can be undertaken.
Dr. Gebhart: What are your clinical pearls for a patient who has prolapse and does not describe any incontinence, voiding dysfunction, or defecatory symptoms? Do we need imaging testing of any sort or is the physical exam adequate for assessing prolapse?
Dr. Walters: When you do the standardized examination of the prolapse, it is important to measure how much prolapse affects the anterior wall of the apex and/or cervix and the posterior wall. Then note that in your notes and plan your surgery accordingly.
It is useful to have the patient fully bear down and then make your measurements; then, especially if she has a full bladder, have her cough while you hold up the prolapse with a speculum or your hand to see if she has stress urinary incontinence.
Continue to: I agree that to diagnose prolapse...
Dr. Ridgeway: I agree that to diagnose prolapse, it is physical exam alone. I would not recommend any significant testing other than testing for the potential for stress incontinence.
Dr. Gebhart: Is it necessary to use the POP-Q (Pelvic Organ Prolapse Quantification system) in a nonacademic private practice setting? Or are other systems, like a Baden-Walker scoring system, adequate in the everyday practice of the experienced generalist?
Dr. Walters: The Baden-Walker system actually is adequate for use in everyday practice. However, Baden-Walker is an outdated measurement system that really is not taught anymore. I think that as older physicians finish and newer doctors come in, no one will even know what Baden-Walker is.
It is better to go ahead and start learning the POP-Q system. Everyone has electronic charts now and if you learn to use the POP-Q, you can do it very quickly and get a grading system for your chart that is reproducible for everyone.
Dr. Ridgeway: The most important thing is to assess all 3 compartments and document the amount of prolapse of each compartment. A modified POP-Q is often adequate. To do this, perform a split speculum exam and use the hymen as the reference. Zero is at the hymen, +1 is 1 cm beyond the hyman. Covering the rectum, how much does the anterior compartment prolapse in reference to the hymen? Covering the anterior compartment, get an idea of what is happening posteriorly. And the crux of any decision in my mind is what is happening at the apex or to the uterus/cervix if it is still present. It is really important to document at least those 3 compartments.
Dr. Karram: I agree. The POP-Q is the ideal, but I don’t think generalists are motivated to use it. It is very important, though, to have some anatomic landmarks, as already mentioned by Dr. Ridgeway.
Choose a surgical approach based on the clinical situation
Dr. Gebhart: How do you choose the surgical approach for someone with prolapse?
Dr. Karram: Most surgeons do what they think they do best. I have spent the majority of my career operating through the vagina, and most of that involves native tissue repairs. I almost always will do a primary prolapse through the vagina and not consider augmentation except in rare circumstances. A recurrent prolapse, a prolapsed shortened vagina, scarring, or a situation that is not straightforward has to be individualized. My basic intervention initially is almost always vaginally with native tissue.
Dr. Ridgeway: For a primary prolapse repair, I also will almost always use native tissue repair as firstline. Whether that is with hysterectomy or without, most people in the long term do very well with that. At least 70% of my repairs are done with a native tissue approach.
For a woman who has a significant prolapse posthysterectomy, especially of the anterior wall or with recurrent prolapse, I offer a laparoscopic sacrocolpopexy. The only other time I offer that as a primary approach would be for a younger woman with very significant prolapse. In that case, I will review risks and benefits with the patient and, using shared decision-making, offer either a native tissue repair or a sacrocolpopexy. For that patient, no matter what you do, given that she has many years to live, the chances are that she will likely need a second intervention.
Dr. Gebhart: Mark, how do you choose an approach for prolapse?
Dr. Walters: I do things pretty much the way Dr. Karram and Dr. Ridgeway do. For women who have a primary prolapse, I usually take a vaginal approach, and for recurrences I frequently do sacrocolpopexy with mesh or I refer to one of my partners who does more laparoscopic or robotic sacrocolpopexy.
Whether the patient needs a hysterectomy or not is evolving. Traditionally, hysterectomy is almost always done at the first prolapse repair. That is being reassessed in the United States to match what is happening in some other countries. It is possible to do nice primary prolapse repair vaginally or laparoscopically and leave the uterus in, in selected women who desire that.
Continue to: Transvaginal prolapse repair: Mesh is no longer an option...
Transvaginal prolapse repair: Mesh is no longer an option
Dr. Gebhart: What led up to the US Food and Drug Administration’s (FDA) market removal of mesh for transvaginal repair of POP?
Dr. Ridgeway: To clarify, it was not a recall—a word that many people use—it was an order to stop producing and distributing surgical mesh intended for transvaginal repair of POP.1 There is a very long history. Transvaginal mesh was introduced with the goal of improving prolapse anatomic and subjective outcomes. Over the last 13 years or so, there were adverse events that led to FDA public health notifications. Consequently, these devices were reclassified, and now require additional testing prior to approval. The newest transvaginal mesh kits were studied.
These 522 studies were completed recently and needed to show superior outcomes because, historically, the risks associated with transvaginal mesh compared to those associated with native tissue repairs are higher: higher reoperation rates, higher rates of other complications, and very minimal improvements in subjective and objective outcomes. Data were presented to the FDA, and it was deemed that these mesh kits did not improve outcomes significantly compared with native tissue repairs.
Dr. Karram: Beri, you stated that very accurately. The pro-mesh advocates were taken back by the idea that the FDA made this recommendation without allowing the outcomes to be followed longer.
Dr. Gebhart: My understanding is that the FDA had a timeline where they had to do a report and the studies had not matured to that end point; thus, they had to go with the data they had even though the studies were not completed. I think they are requesting that they be completed.
Dr. Ridgeway: Additional data will be available, some through the 522 studies, others through randomized controlled trials in which patients were already enrolled and had surgery. As far as I know, I do not think that the decision will be reversed.
Continue to: Native tissue repair and failure risk...
Native tissue repair and failure risk
Dr. Gebhart: I hear a lot that native tissue repairs fail. Mickey, as you do a lot of vaginal surgery, what are your thoughts? Should you use augmentation of some sort because native tissue fails?
Dr. Karram: There is going to be a failure rate with whatever surgery you do. I think that the failure rate with native tissue is somewhat overstated. I think a lot of that dates back to some of the things that were being promoted by mesh advocates. Initially, there was a lot of cherry-picking of native tissue data in some of those studies to promote the idea that the recurrent prolapse rates were 40% to 80%. We certainly do not see that in our patient population.
Based on our 5-year data, we have a recurrence rate of about 15% and a reoperation rate of less than 10%. That is the best I can quote based on our data. We have not followed patients longer than 5 years.
I can’t do much better than that with an augmentation; even if I get another 5% or 10% better anatomic outcome, that will be at the expense of some erosions and other complications specific to the mesh. I do think that the native tissue failure rate being promoted by a lot of individuals is a higher failure rate than what we are seeing.
Dr. Gebhart: What do you think, Mark?
Dr. Walters: Large cohort studies both at your institution, Mayo Clinic, and ours at the Cleveland Clinic mirror what Dr. Karram said, in that we have a reoperation rate somewhere between 8% and 15%. Of course, we have some failures that are stage 2 failures where patients choose not to have another operation. In general, a 10% or 12% reoperation rate at 5 to 7 years is acceptable.
Native tissue repairs probably fail at the apex a little more than mesh sacrocolpopexy. Mesh sacrocolpopexy, depending on what else you do with that operation, may have more distal vaginal failures, rates like distal rectoceles and more de novo stress urinary incontinence than we probably get with native tissue. I get some failures of the apex with native tissue repairs, but I am okay with using sacrocolpopexy as the second-line therapy in those patients.
Hysteropexy technique and pros and cons
Dr. Gebhart: Is hysteropexy a fad, or is there something to this?
Dr. Ridgeway: I do not think it is a fad. Women do feel strongly about this, and we now have data supporting this choice: randomized controlled trials of hysterectomy and prolapse repair versus hysteropexy with comparable outcomes at the short and medium term.2
The outcomes are similar, but as we said, outcomes for all prolapse repair types are not perfect. We have recurrences with sacrocolpopexy, native tissue repair, and hysteropexy. We need more data on types of hysteropexy and long-term outcomes for uterine preservation.
Dr. Walters: We have been discussing what patients think of their uterus, and some patients have very strong opinions. Some prefer to have a hysterectomy because then they don’t need to worry about cancer or do screening for cancer, and they are very happy with that. Other women with the same kind of prolapse prefer not to have a hysterectomy because philosophically they think they are better off keeping their organs. Since satisfaction is an outcome, it is useful to know what the patient wants and what she thinks about the surgical procedure.
Dr. Gebhart: For hysteropexy, do the data show that suture or a mesh augment provide an advantage one way or the other? Do we know that yet?
Dr. Walters: No, there are not enough studies with suture. There are only a few very good studies with suture hysteropexy, and they are mostly sacrospinous suture hysteropexies. Only a few studies look at mesh hysteropexy (with the Uphold device that was put on hold), or with variations of uterosacral support using strips of mesh, mostly done in other countries.
A point I want to add, if native tissue repairs fail at the apex more, why don’t you just always do sacrocolpopexy? One reason is because it might have a little higher complication rate due to the abdominal access and the fact that you are putting mesh in. If you have, for example, a 4% complication rate with the mesh but you get a better cure rate, those things balance out, and the woman may not be that much better off because of the extra complications. You have to assess the pro and con with each patient to pick what is best for her—either a more durable repair with a mesh or a little safer repair with native tissue.
Continue to: Women feel very strongly about risk...
Dr. Ridgeway: Women feel very strongly about risk. Within the same clinic I will have similar patients, and I say, “Probably in the long term this one may last a little longer but the surgery takes longer and it has a little higher complication rate.” One patient will say, “I’m not worried about the risk, I want what’s going to last the longest,” whereas a very similar patient will say, “Why would anyone pick the higher-risk operation? I want the lower risk that probably will last a long time.”
Dr. Gebhart: Beri, who should not have a hysteropexy?
Dr. Ridgeway: The biggest factor would be someone who has ever had postmenopausal bleeding. From our data, we know that if they have even had a work-up with benign results, the risk of unanticipated pathology is high. I do not recommend hysteropexy for anyone who has had postmenopausal bleeding.
For a premenopausal woman who has irregular bleeding, I also do not recommend it, because you just do not know what that future will hold. If a patient has anatomic abnormalities like large fibroids, I would not recommend it either. I would like patients to have had standard cervical cancer screening without any abnormalities for about 10 years or so.
Dr. Gebhart: What about prior cervical dysplasia?
Dr. Ridgeway: If a patient had ASCUS or low-grade dysplasia decades ago, has been normal for at least 10 years, and is currently negative for human papillomavirus, I have no problem.
Dr. Gebhart: How about women at high genetic risk for cancer?
Dr. Ridgeway: If they are at high risk for endometrial cancer, I would not recommend hysteropexy. If they are going to need an oophorectomy and/or salpingectomy for risk reduction during prolapse treatment, I usually perform a hysterectomy.
Plan surgical steps and prepare for “what if’s”
Dr. Gebhart: What tips can you provide, either regarding the evaluation or something you do surgically, that are important in a transvaginal native tissue repair?
Dr. Karram: If you have a case of posthysterectomy apical prolapse, that you think is an indication for sacrocolpopexy, in reality these are very good candidates for either sacrospinous or uterosacral suspensions. I prefer a uterosacral suspension as I feel there is less distortion of the vaginal apex compared to a sacrospinous suspension.
Dr. Ridgeway: The most critical step is setting up the OR and positioning the patient. That sets up the case for success, preventing struggles during the case. I use a high lithotomy, with careful positioning of course, but I use candy cane stirrups so that I can have an instrument stand in front of me and not struggle during the case.
Dr. Walters: My tip for everyone who is doing native tissue surgery, whether it is high McCall colpopexy or uterosacral ligament suspension or sacrocolpopexy, would be to really learn well the anatomy of each operation, including how close the ureter is, where the risk for bleeding is, and where the risk for nerve damage is.
The complications for each of these surgeries are slightly different, but there is a small risk of kinking the ureter with both uterosacral ligament suspension and the McCall, so you should do a cystoscopy as part of that operation. If you do a sacrospinous ligament suspension, use an instrument that can get a stitch into a ligament—not too close to the ischial spine and not too close to the sacrum—to avoid the risk of damage to major nerves and blood vessels and to minimize buttock and leg pain.
Continue to: Another tip is to understand...
Dr. Karram: Another tip is to understand that you are going to have potential complications intraoperatively. Think through those presurgically. You do not want to start thinking about these things and making decisions as they are happening. For example, what if I do a uterosacral suspension and I don’t see efflux of urine from the ureter? What am I going to do, and how long am I going to wait before I intervene? If I do a sacrospinous and I start to see a lot of bleeding from that area, what am I going to do? My plan would be, “I will pack the area, get extra suction, etc.” Thinking these ideas through before they occur is very helpful.
Dr. Gebhart: That is critical, to have an algorithm or a scheme in your mind. You want to think through it before it occurs because you are not always thinking as clearly when things are not going well.
I would say get good at physical examination skills in the office, then have a plan for the OR based on what you see in the office. If what is going on with the prolapse is not completely investigated and other issues are not addressed, then failure results because you did not make the diagnosis. Certainly, modify the procedure according to what you find intraoperatively, but follow through.
Indications and tips for sacrocolpopexy
Dr. Gebhart: What are the indications for sacrocolpopexy?
Dr. Ridgeway: Indications include recurrent apical prolapse, posthysterectomy prolapse, or severe prolapse in someone quite young. It is a fantastic operation with overall low risks, but this needs to be discussed with the patient.
Dr. Walters: There are some unusual circumstances—for example, the woman has a short prolapsed vagina, usually after a prior surgery—in which the best repair is a bridging piece of mesh, usually done laparoscopically, because those operations cannot be done very well vaginally to obtain a durable result.
Dr. Karram: I agree. I do not think that all recurrent prolapses mandate a sacrocolpopexy. You need to individualize, but in general the short prolapsed vagina and patients who are very young are at high risk for a recurrence.
Dr. Gebhart: An older patient might be a very good candidate, even if she had recurrence from another vaginal repair.
Beri, does the patient with a high body mass index need augmentation?
Dr. Ridgeway: That is a great question, and this has to be individualized because, while heavier patients can benefit from augmentation, in a very heavy patient, getting into that abdomen has its own set of challenges. Anatomically they get a better repair with a mesh-augmented repair like a sacrocolpopexy, but they do have increased risks. That is important to acknowledge and clarify with the patient.
Dr. Gebhart: Any surgical tip you might offer on sacrocolpopexy?
Dr. Ridgeway: Perform the operation in the same way you would an open procedure. Meaning, use the same materials, the same sutures, the same placement, and the same type of dissection in order to obtain results similar to those with an open operation. Using your assistants to manipulate the vagina and rectum is important, as well as exposure and typical careful surgical technique.
Dr. Gebhart: What is important about the placement of sutures on the anterior longitudinal ligament, and what do you need to be cognizant of?
Dr. Ridgeway: Be careful of that left common iliac vein that is a little more medial than you would expect and of the middle sacral artery, and try to differentiate between L5 and S1. In an ideal circumstance, place the suture at S1 or L5 but not the inner disc space, which is the area to avoid placement.
Historically, the recommendation is S1. Some people do L5 because of some pull out strength studies, but also because it is easier, and sometimes in that area of the anterior longitudinal ligament is much better. The key is to do enough dissection and use haptic feedback, especially with conventional laparoscopy or an open approach, to avoid placing sutures through the disc space, as there is some concern that it increases the risk for discitis or osteomyelitis in that area.
Continue to: We also have found...
Dr. Gebhart: We also have found that if you have a combined surgery with colorectal colleagues, like a rectal prolapse repair, there is a little higher risk of discitis.
Dr. Ridgeway: In my own practice I saw a combined case with a rectopexy in someone who had a biologic mesh erosion. When we reviewed the literature, a number of reported cases of discitis had either an early post-op or concurrent urinary tract infection or vaginal infection that likely predisposed them to an infection that traveled up the material.
Dr. Karram: My final comment is that a sacrocolpopexy is not a few stitches or a little mesh right at the apex. If the patient has an isolated enterocele, okay, but it is a wide mesh for a reason and it should connect to the endopelvic fascia anteriorly, posteriorly. It is a mistake to suture just a little bit of the cuff and grab it and think, “I’ve done a colpopexy” when the procedure has not been executed as it should be.
Dr. Gebhart: I want to thank our expert panel and OBG M
Continue to: Some procedures call for cystoscopy...
Dr. Gebhart: Is cystoscopy necessary in patients undergoing native tissue repair or abdominal approaches to prolapse, and should the experienced generalist have this skill?
Dr. Walters: If you are going to do prolapse surgery or surgery for stress urinary incontinence, you need to learn to do cystoscopy. Almost all specialists in urogynecology and urology would do a cystoscopy at the time of a native tissue prolapse repair, a mesh-augmented prolapse repair, or a sling procedure. Whether a generalist doing simple hysterectomies needs to do cystoscopy is controversial, and it is probably based on risk assessment of the kind of hysterectomy being done. Definitely, if you are doing prolapse repair, you probably should be doing cystoscopy at the same time.
Dr. Karram: I would take it further. For certain procedures, cystoscopy is standard of care. For example, if you are doing anything around the uterosacral ligaments, whether a McCall culdoplasty or uterosacral suspension, it is standard of care. It would be a difficult medical-legal defense issue if it was not done in those cases.
To Mark’s point, it is controversial whether universal cystoscopy should be performed on every hysterectomy or every anterior to posterior repair. We are not there yet, but certainly it is in your best interest to have a very low threshold, so if you think about doing cystoscopy, you should probably do it.
Dr. Gebhart: Is cystoscopy needed in sacrocolpopexy?
Dr. Ridgeway: We know from our own data that the risk of lower urinary tract injury is very low with sacrocolpopexy. Having said that, I agree with the position statement of the American Urogynecologic Society that says, “Universal cystoscopy should be performed at the time of all pelvic reconstruction surgeries, with the exception of operations solely for posterior compartment defects.”1
Dr. Gebhart: The reality is that we just want to identify if there is a problem or not at the time of the surgery. It does not mean you have to manage it. You could get your partner, your urologist, or another person with expertise to come in to help you.
Dr. Ridgeway: Absolutely, because intraoperative identification and treatment will prevent many unfavorable outcomes in the postoperative period.
Reference
1. Cohen SA, Carberry CL, Smilen SW. American Urogynecologic Society Consensus Statement: cystoscopy at the time of prolapse repair. Female Pelvic Med Reconstr Surg. 2018;24:258-259.
Dr. Gebhart: If a patient is a smoker and/or utilizes tobacco and you think she is a candidate for a sacrocolpopexy, are there any special considerations? How would you counsel that patient?
Dr. Walters: The risk of mesh erosion is high enough that I would try to not do any mesh prolapse repair in a woman who was a smoker, especially a heavy smoker. A more common situation is, would I put a polypropylene midurethral sling in that patient? I usually am willing to do that because it is still the best option compared with the no-mesh options. In a patient who would be a good candidate for sacrocolpopexy, I can usually do a no-mesh surgery and keep the risk low. I could always give the woman an option to quit smoking, but that tends not to be successful.
Dr. Gebhart: What is the risk of using mesh in a smoker?
Dr. Walters: An increased risk of erosion through the vaginal walls. I am not sure of the magnitude of risk, maybe 2 or 3 times higher. That is high enough that I probably would not take the risk except in unusual circumstances.
Dr. Ridgeway: A good amount of data show increased risk of mesh exposure for smokers. Those patients also tend to have a higher risk of prolapse recurrence because of coughing. Sacrocolpopexy is not my favorite operation to do in a smoker. I will work with the patient to quit, but often if it is the right operation, I will do it, with preoperative estrogen and appropriate conseling.
Dr. Gebhart: Is there still a role for vaginal mesh? While it is no longer being sold in the United States, could you fashion your own mesh for a prolapse procedure?
Dr. Walters: I can do pretty much everything I need to do without adding transvaginal mesh, and if I need a meshaugmented repair, then I would go with the sacrocolpopexy route. Having said that, data for hysteropexy do show that a mesh-augmented hysteropexy could have some advantages, whether you do it with a kit or some fashioned pieces of mesh. Most of the experiences with this are outside of the United States, so we need much more standardization of technique and tracking to answer that question.
Dr. Gebhart: Mickey, what are your thoughts regarding someone who thinks, “Mesh has been good for me, I want to stay with that. I’m going to cut my own mesh”? Are they assuming some liability now that companies are no longer marketing mesh for vaginal repair?
Dr. Karram: Unfortunately, I really think they are. It would be easy to be put in a legal corner and asked, the FDA felt that this should be pulled off the market, why are you still utilizing it? At the end of the day, what the FDA said was not inaccurate.
The studies have not shown a significant better outcome with mesh, and it is an extra intervention that, again, in the best of hands is going to have some issues. That is a dilemma many surgeons faced because they felt that that was their main way of treating prolapse—”they took away my way of successfully treating patients for years.” I do think it increases their medical-legal liability.
Dr. Ridgeway: I agree that it does increase medical-legal liability, and I can’t imagine a situation in which I would offer that. Dr. Gebhart: There are risks with all procedures, including slings for stress incontinence, but sling use is appropriate in appropriately counseled patients.
Dr. Ridgeway: Correct. I feel very strongly that the risk profile for the midurethral sling is very different from that for transvaginal mesh. Very large data sets in large groups of people support that the outcomes are favorable and the risk profile is low. Having said that, slings are not risk free, but living with severe incontinence is not risk free either.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
- US Food and Drug Administration. FDA takes action to protect women's health, orders manufacturers of surgical mesh intended for transvaginal repair of pelvic organ prolapse to stop selling all devices. https://www.fda.gov/news-events/press-announcements/fda-takes-action-protect-womens-health-orders-manufacturers-surgical-mesh-intended-transvaginal. April 16, 2019. Accessed January 14, 2020.
- Detollenaere RJ, den Boon J, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with suspension of the uterosacral ligaments in women with uterine prolapse stage 2 or higher: multicentre randomised non-inferiority trial. BMJ. 2015;351:h3717.
Hysterectomy in patients with history of prior cesarean delivery: A reverse dissection technique for vesicouterine adhesions
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).
Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).
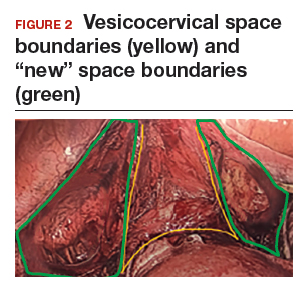
Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).
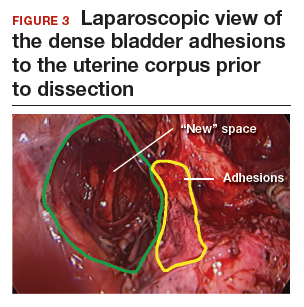
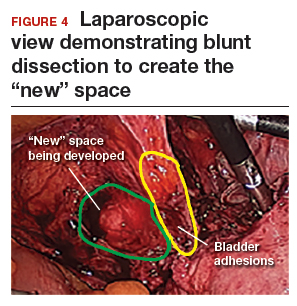
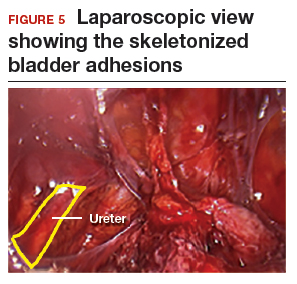
Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
Minimally invasive surgical techniques, which have revolutionized modern-day surgery, are the current standard of care for benign hysterectomies.1-4 Many surgeons use a video-laparoscopic approach, with or without robotic assistance, to perform a hysterectomy. The development of a bladder flap or vesicovaginal surgical space is a critical step for mobilizing the bladder. When properly performed, it allows for appropriate closure of the vaginal cuff while mitigating the risk of urinary bladder damage.
In patients with no prior pelvic surgeries, this vesicovaginal anatomic space is typically developed with ease. However, in patients who have had prior cesarean deliveries (CDs), the presence of vesicouterine adhesions could make this step significantly more challenging. As a result, the risk of bladder injury is higher.5-8
With the current tide of cesarean birth rates approaching 33% on a national scale, the presence of vesicouterine adhesions is commonly encountered.9 These adhesions can distort the anatomy and thereby create more difficult dissections and increase operative time, conversion to laparotomy, and inadvertent cystotomy. Such a challenge also presents an increased risk of injuring adjacent structures.
In this article, we describe an effective method of dissection that is especially useful in the setting of prior CDs. This method involves developing a "new" surgical space lateral and caudal to the vesicocervical space.
Steps in operative planning
Preoperative evaluation. A thorough preoperative evaluation should be performed for patients planning to undergo a laparoscopic hysterectomy. This includes obtaining details of their medical and surgical history. Access to prior surgical records may help to facilitate planning of the surgical approach. Previous pelvic surgery, such as CD, anterior myomectomy, cesarean scar defect repair, endometriosis treatment, or exploratory laparotomy, may predispose these patients to develop adhesions in the anterior cul-de-sac. Our method of reverse vesicouterine fold dissection can be particularly efficacious in these settings.
Surgical preparation and laparoscopic port placement. In the operative suite, the patient is placed under general anesthesia and positioned in the dorsal lithotomy position.10 Sterile prep and drapes are used in the standard fashion. A urinary catheter is inserted to maintain a decompressed bladder. A uterine manipulator is inserted with good placement ensured.
Per our practice, we introduce laparoscopic ports in 4 locations. The first incision is made in the umbilicus for the introduction of a 10-mm laparoscope. Three subsequent 5-mm incisions are made in the left and right lower lateral quadrants and medially at the level of the suprapubic region.10 Upon laparoscopic entry, we perform a comprehensive survey of the abdominopelvic cavity. Adequate mobility of the uterus is confirmed.11 Any posterior uterine adhesions or endometriosis are treated appropriately.12
First step in the surgical technique: Lateral dissection
We proceed by first desiccating and cutting the round ligament laterally near the inguinal canal. This technique is carried forward in a caudal direction as the areolar tissue near the obliterated umbilical artery is expanded by the pneumoperitoneum. With a vessel sealing-cutting device, we address the attachments to the adnexa. If the ovaries are to be retained, the utero-ovarian ligament is dessicated and cut. If an oophorectomy is indicated, the infundibulopelvic ligament is dessicated and cut.
Continue to: Using the tip of the vessel sealing...
Using the tip of the vessel sealing-cutting device, the space between the anterior and posterior leaves of the broad ligament is developed and opened. A grasping forceps is then used to elevate the anterior leaf of the broad ligament and maintain medial traction. A space parallel and lateral to the cervix and bladder is then created with blunt dissection.
The inferior and medial direction of this dissection is paramount to avoid injury to nearby structures in the pelvic sidewall. Gradually, this will lead to the identification of the vesciovaginal ligament and then the vesicocervical ligament. The development of these spaces allows for the lateral and inferior displacement of the ureter. These maneuvers can mitigate ureter injury by pushing it away from the planes of dissection during the hysterectomy.
Continued traction is maintained by keeping the medial aspect of the anterior leaf of the broad ligament intact. However, the posterior leaf is dissected next, which further lateralizes the ureter. Now, with the uterine vessels fully exposed, they are thoroughly dessicated and ligated. The same procedure is then performed on the contralateral side.11 (See the box below for links to videos that demonstrate the techniques described here.)
Creating the “new” space
In the “new” space that was partially developed during the lateral dissection, blunt dissection is continued, using a sweeping motion from an inferior-to-superior direction, to extend this avascular space. This is performed bilaterally until both sides are connected from the inferior aspect of the vesicouterine adhesions, if present. This thorough dissection creates what we refer to as a “new” space11 (FIGURE 1).

Medially, the new space is bordered by the vesicocervical-vaginal ligament, also known as the bladder pillar. Its distal landmark is the bladder. The remaining intact anterior leaf of the broad ligament lies adjacent to the space anteriorly. The inner aspect of the obliterated umbilical artery neighbors it laterally. Lastly, the vesicovaginal plane’s posterior margin is the parametrium, which is the region where the ureter courses into the bladder. The paravesical space lies lateral to the obliterated umbilical ligament.
Visualization of this new space is made possible in the laparoscopic setting. The pneumoperitoneum allows for better demarcation of the space. Additionally, laparoscopic views of the anatomic spaces differ from those of the laparotomy view because of the magnification and the insufflation of carbon dioxide gas in the spaces.13,14 In our experience, approaching the surgery from the “new” space could significantly decrease the risk of genitourinary injuries in patients with anterior cul-de-sac adhesions (FIGURE 2).

Using the reverse vesicouterine fold dissection technique
Among patients with prior CDs, adhesions often are at the level of or superior to the prior CD scar. By creating the new space, safe dissection from a previously untouched area can be accomplished and injury to the urinary bladder can be avoided.
The reverse vesicouterine fold dissection can be performed from this space. Using the previously described blunt sweeping motion from an inferior-to-superior direction, the vesicovaginal and vesicocervical space is further developed from an unscarred plane. This will separate the lowest portion of the bladder from the vagina, cervix, and uterus in a safe manner. Similar to the technique performed during a vaginal hysterectomy, this reverse motion of developing the bladder flap avoids erroneous and blind dissection through the vesicouterine adhesions (FIGURES 3–5).



Once the bladder adhesions are well delineated and separated from the uterus by the reverse vesicouterine fold dissection technique, it is safe to proceed with complete bladder mobilization. Sharp dissection can be used to dissect the remaining scarred bladder at its most superior attachments. Avoid the use of thermal energy to prevent heat injury to the bladder. Carefully dissect the bladder adhesions from the cervicouterine junction. Additional inferior bladder mobilization should be performed up to 3 cm past the leading edge of the cervicovaginal junction to ensure sufficient vaginal tissue for cuff closure. Note that the bladder pillars occasionally may be trapped inside a CD scar. This surgical technique could make it easier to release the pillars from inside the adhesions and penetrating into the scar.15
Continue to: Completing the surgery...
Completing the surgery
Once the bladder is freely mobilized and all adhesions have been dissected, the cervix is circumferentially amputated using monopolar cautery. The vaginal cuff can then be closed from either a laparoscopic or vaginal approach using polyglactin 910 (0-Vicryl) or barbed (V-Loc) suture in a running or interrupted fashion. Our practice uses a 1.5-cm margin depth with each suture. At the end of the surgery, routine cystoscopy is performed to verify distal ureteral patency.16 Postoperatively, we manage these patients using a fast-track, or enhanced recovery, model.17
From the Center for Special Minimally Invasive and Robotic Surgery
https://youtu.be/wgGssnd1JAo
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy
- Case 1: TLH with development of the "new space": The technique with prior C-section
- Case 2: A straightforward case: Dysmenorrhea and menorrhagia
- Case 3: History of multiple C-sections with adhesions and fibroids
https://youtu.be/6vHamfPZhdY
Reverse vesicouterine fold dissection for total laparoscopic hysterectomy after prior cesarean delivery
An effective technique in challenging situations
Genitourinary injury is a common complication of hysterectomy.18 The proximity of the bladder and ureters to the field of dissection during a hysterectomy can be especially challenging when the anatomy is distorted by adhesion formation from prior surgeries. One study demonstrated a 1.3% incidence of urinary tract injuries during laparoscopic hysterectomy.6 This included 0.54% ureteral injuries, 0.71% urinary bladder injuries, and 0.06% combined bladder and ureteral injuries.6 Particularly among patients with a prior CD, the risk of bladder injury can be significantly heightened.18
The reverse vesicouterine fold dissection technique that we described offers multiple benefits. By starting the procedure from an untouched and avascular plane, dissection into the plane of the prior adhesions can be circumvented; thus, bleeding is limited and injury to the bladder and ureters is avoided or minimized. By using blunt and sharp dissection, thermal injury and delayed necrosis can be mitigated. Finally, with bladder mobilization well below the colpotomy site, more adequate vaginal tissue is free to be incorporated into the vaginal cuff closure, thereby limiting the risk of cuff dehiscence.16
While we have found this technique effective for patients with prior cesarean deliveries, it also may be applied to any patient who has a scarred anterior cul-de-sac. This could include patients with prior myomectomy, cesarean scar defect, or endometriosis. Despite the technique being a safeguard against bladder injury, surgeons must still use care in developing the spaces to avoid ureteral injury, especially in a setting of distorted anatomy.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
- Page B. Nezhat & the advent of advanced operative video-laparoscopy. In: Nezhat C. Nezhat's History of Endoscopy. Tuttlingen, Germany: Endo Press; 2011:159-179. https://laparoscopy.blogs.com/endoscopyhistory/chapter_22. Accessed October 23, 2019.
- Podratz KC. Degrees of freedom: advances in gynecological and obstetric surgery. In: American College of Surgeons. Remembering Milestones and Achievements in Surgery: Inspiring Quality for a Hundred Years, 1913-2012. Tampa, FL: Faircount Media Group; 2013:113-119. http://endometriosisspecialists.com/wp-content/uploads/pdfs/Degrees-of-Freedom-Advances-in-Gynecological-and-Obstetrical-Surgery.pdf. Accessed October 31, 2019.
- Kelley WE Jr. The evolution of laparoscopy and the revolution in surgery in the decade of the 1990s. JSLS. 2008;12:351-357.
- Tokunaga T. Video surgery expands its scope. Stanford Med. 1993/1994;11(2)12-16.
- Rooney CM, Crawford AT, Vassallo BJ, et al. Is previous cesarean section a risk for incidental cystotomy at the time of hysterectomy? A case-controlled study. Am J Obstet Gynecol. 2005;193:2041-2044.
- Tan-Kim J, Menefee SA, Reinsch CS, et al. Laparoscopic hysterectomy and urinary tract injury: experience in a health maintenance organization. J Minim Invasive Gynecol. 2015;22:1278-1286.
- Sinha R, Sundaram M, Lakhotia S, et al. Total laparoscopic hysterectomy in women with previous cesarean sections. J Minim Invasive Gynecol. 2010;17:513-517.
- O'Hanlan KA. Cystosufflation to prevent bladder injury. J Minim Invasive Gynecol. 2009;16:195-197.
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1-65.
- Nezhat C, Nezhat F, Nezhat C, eds. Nezhat's Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy with DVD, 4th ed. New York, NY: Cambridge University Press; 2013.
- Nezhat C, Grace LA, Razavi GM, et al. Reverse vesicouterine fold dissection for laparoscopic hysterectomy after prior cesarean deliveries. Obstet Gynecol. 2016;128:629-633.
- Nezhat C, Xie J, Aldape D, et al. Use of laparoscopic modified nerve-sparing radical hysterectomy for the treatment of extensive endometriosis. Cureus. 2014;6:e159.
- Yabuki Y, Sasaki H, Hatakeyama N, et al. Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol. 2005;193:7-15.
- Uhlenhuth E. Problems in the Anatomy of the Pelvis: An Atlas. Philadelphia, PA: JB Lippincott Co; 1953.
- Nezhat C, Grace, L, Soliemannjad, et al. Cesarean scar defect: what is it and how should it be treated? OBG Manag. 2016;28(4):32,34,36,38-39,53.
- Nezhat C, Kennedy Burns M, Wood M, et al. Vaginal cuff dehiscence and evisceration: a review. Obstet Gynecol. 2018;132:972-985.
- Nezhat C, Main J, Paka C, et al. Advanced gynecologic laparoscopy in a fast-track ambulatory surgery center. JSLS. 2014;18:pii:e2014.00291.
- Nezhat C, Falik R, McKinney S, et al. Pathophysiology and management of urinary tract endometriosis. Nat Rev Urol. 2017;14:359-372.
Using slings for the surgical management of urinary incontinence: A safe, effective, evidence-based approach
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6
Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.
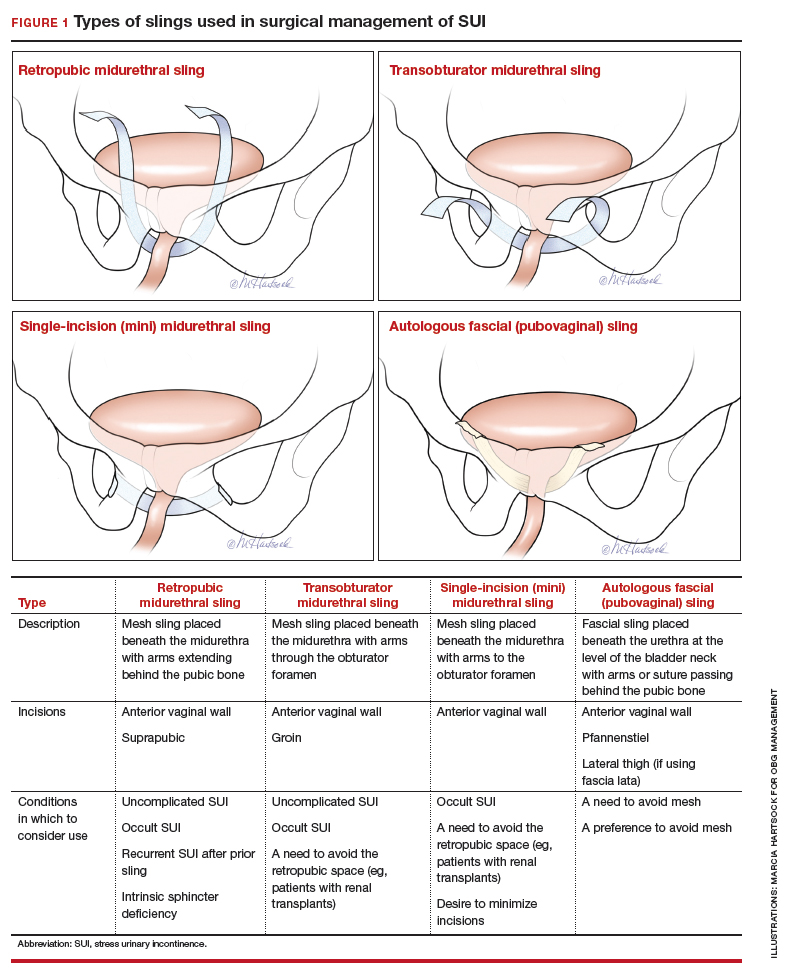
Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)
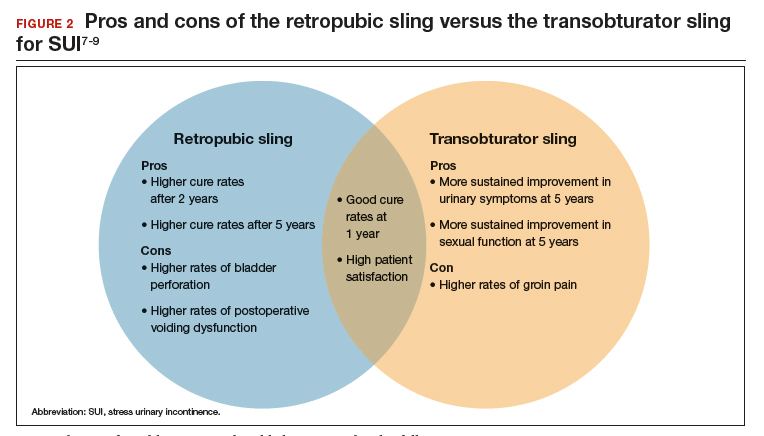
Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)
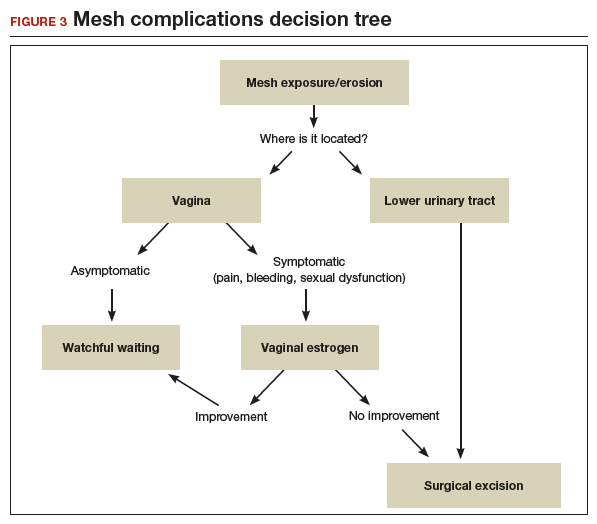
A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6

Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.

Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)

Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)

A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
Urinary incontinence affects approximately 50% of women, with up to 80% of these women experiencing stress urinary incontinence (SUI) at some point in their lives.1-3 While conservative measures can offer some improvement in symptoms, the mainstay of treatment for SUI is surgical intervention.4,5 The lifetime risk of undergoing surgery for SUI is 13.6%, and surgery leads to a major improvement in quality of life and productivity.1,6

Types of slings used for SUI
Sling procedures are the most commonly used surgical approach for the treatment of SUI. Two types of urethral slings are used: the midurethral sling and the autologous fascial (pubovaginal) sling. The midurethral sling, which is the most frequently used sling today, can be further characterized as the retropubic sling, the transobturator sling, and the mini sling (FIGURE 1).
Retropubic sling
A retropubic sling is a midurethral mesh sling that is placed beneath the urethra at the midpoint between the urethral meatus and the bladder neck. The arms of the sling extend behind the pubic symphysis, providing a hammock-like support that helps prevent leakage with increased abdominal pressures. The retropubic sling is the most commonly used type of sling. For women presenting with uncomplicated SUI who desire surgical correction, it often is the best choice for providing long-term treatment success.7
Transobturator sling
A transobturator sling is a midurethral mesh sling that is placed beneath the urethra as described above, but the arms of the sling extend outward through the obturator foramen and into the groin. This enables support of the midurethra, but this sling is less likely to result in such complications as bladder perforation or postoperative urinary retention. Transobturator slings also are associated with lower rates of voiding dysfunction and urinary urgency than retropubic slings.7-9 However, transobturator slings have higher rates of groin pain, and they are less effective in maintaining long-term cure of SUI.7
First introduced in 1996, the midurethral sling quickly grew in popularity for the treatment of SUI because of its high success rates and its minimally invasive approach.10 Both retropubic and transobturator slings are safe, extensively researched surgical approaches for the management of SUI.3 Midurethral slings have a very high rate of incontinence cure (80%–90%) and extremely high patient satisfaction rates (85%–90%), as even patients without complete cure report meaningful symptomatic improvement.7,8,11
Single-incision (mini) sling
A single-incision sling is a midurethral mesh sling that is designed to be shorter in length than standard midurethral slings. The placed sling lies under the midurethra and extends toward the superior edge of the obturator foramen but does not penetrate it. The sling is held in place by small pledgets on either side of the mesh hammock that anchor it in place to the obturator internus muscular fascia. Because this “mini” sling was introduced in 2006, fewer long-term data are available for this sling than for standard midurethral slings.
Continue to: Autologous (fascial) sling...
Autologous (fascial) sling
An autologous sling is a retropubic sling made from the patient’s own fascia; it is harvested from either the fascia lata of the lateral thigh or the rectus fascia of the abdomen. The sling is placed beneath the urethra in the bladder neck region, and sutures affixed to the sling edges pass behind the pubic symphysis and through the abdominal fascia to anchor it in place.

Choose a sling based on the clinical situation and patient goals
Consider the unique features of each sling when selecting the proper sling; this should be a shared decision with the patient after thorough counseling. Below, we present 4 clinical cases to exemplify scenarios in which different slings are appropriate, and we review the rationale for each selection.
CASE 1 SUI that interferes with exercise routine
Ms. P. is a 46-year-old (G3P3) active mother. She loves to exercise, but she has been working out less frequently because of embarrassing urinary leakage that occurs with activity. She has tried pelvic floor exercises and changing her fluid intake habits, but improvements have been minimal with these interventions. On evaluation, she has a positive cough stress test with a recently emptied bladder and a normal postvoid residual volume.
What type of sling would be best?
Because this patient is young, active, and has significant leakage with an empty bladder, a sling with good long-term treatment success is likely to provide her with the best results (Figure 1). We therefore offered her a retropubic midurethral sling. The retropubic approach is preferred here as it is less likely than the transobturator sling to cause groin/thigh pain, which is an important consideration in this young, active patient.
Further testing is not needed
For women with uncomplicated SUI who demonstrate leakage with stress (coughing, Valsalva stress test) and who have a normal postvoid residual volume, additional testing, such as urodynamic evaluation, is not necessary.12 These patients can be counseled on the range of conservative management options and as well as surgical inventions.
CASE 2 Return of SUI symptoms after transobturator sling placement
Ms. E. is a 70-year-old woman who had a transobturator sling placed 5 years ago. Initially, her SUI symptoms improved after surgery. Recently, however, she noticed a return of her SUI, which she finds bothersome and limiting to her quality of life.
How would you manage this patient?
While midurethral slings are highly effective, there are instances in which patients will have symptom recurrence. For women who already have a midurethral sling, consider the following important questions.
Is this truly recurrent SUI, or is it a new process?
Like any reconstructive procedure, midurethral sling success rates decline over time and recurrent SUI can develop.7 However, it also is possible for urge urinary incontinence to develop as a new process, and it is important to distinguish which type of urinary incontinence your patient has prior to counseling about treatment options.
To further evaluate patients with recurrent incontinence and a prior sling, we recommend urodynamic studies with cystoscopy (in addition to a detailed history and physical exam). This not only helps rule out other forms of incontinence, such as overactive bladder, but also evaluates for possible mesh erosion into the urethra or bladder, which can cause irritative voiding symptoms and incontinence.
Continue to: What type of sling did the patient have initially...
What type of sling did the patient have initially, and how does this impact a repeat procedure?
Regardless of the initial sling type used, repeat midurethral sling procedures have a significantly lower cure rate than primary midurethral sling procedures.13 Retropubic slings are more effective than transobturator slings for patients with recurrent SUI who have failed a prior sling. When a patient presents with recurrent SUI after a prior transobturator sling, the best option for a repeat procedure is usually a retropubic sling, as it achieves higher objective and subjective cure rates.13,14 (See FIGURE 2 for a comparison of retropubic and transobturator slings.)

Should I remove the old sling prior to placing a new one?
While it is recommended to remove the vaginal portion of the sling if the patient has a mesh exposure or is experiencing other symptoms, such as pain or bleeding, removal of the old sling is not necessarily indicated prior to (or during) a repeat incontinence procedure.15,16 Removing the sling, removing a portion of the sling, or leaving the sling in situ are all reasonable options.
CASE 3 Treated SUI has mesh exposure
Ms. R. is a 60-year-old woman with a history of SUI that was previously managed with a retropubic midurethral sling placed at an outside hospital. She is a smoker and has developed a vaginal mesh exposure. Although she would like the mesh removed, she does not want her incontinence to come back. She tells you that she does not think she would be able to quit smoking.
What would be a reasonable next option for Ms. R.?
While complications from a midurethral sling are rare, mesh exposures occur in approximately 2% of patients, and urinary retention requiring release of the sling occurs in about 1% of patients.3,6 It often helps to clarify for patients that the US Food and Drug Administration public health advisories on the use of transvaginal mesh have been directed specifically toward the use of transvaginal mesh for the treatment of pelvic organ prolapse (POP), not the use of mesh for midurethral slings for SUI or transabdominal mesh for POP.10,17
When considering use of a mesh sling, a thorough discussion of the potential risks, as well as the benefits and alternatives, is imperative. Patients must personally balance the probability of benefit with the potential risk of complications, and while physicians can help outline the benefits and risks through shared decision-making, ultimately it is the patient who should make this decision.
Certain patient populations may be at higher risk for mesh complications18 (See "Risk factors for mesh-related complications," below). These complications are managed in various ways (FIGURE 3). Patients who have experienced mesh complications previously are typically not good candidates for a repeat mesh sling, particularly when the risk factor for complications cannot be modified.
• Smoking
• Poorly controlled diabetes
• Decreased estrogen status
• Chronic steroid use
• Prior urethral surgery (urethral diverticulum, urethroplasty)

A mesh sling alternative
The most effective way to manage SUI in patients who are not good candidates for a mesh sling is to consider employing a sling that uses the patient’s own tissue.19-21 Common approaches include harvesting a graft of rectus fascia through a Pfannenstiel skin incision or using fascia lata from the patient’s iliotibial band in the lateral thigh. Autologous slings are safe and effective, and even after a mesh sling has failed, autologous slings have an almost 70% cure rate for SUI.20,21
Continue to: Timing of mesh removal and placement of an autologous fascial sling...
Timing of mesh removal and placement of an autologous fascial sling
Either concomitant or delayed placement of a pubovaginal sling is acceptable when removing mesh, though this should be a joint decision with the patient after counseling. If the risk for surgical complications is modifiable (for example, poorly controlled diabetes that could be improved with blood glucose control), it may be advisable to delay the fascial sling until the risk factors have been addressed. Similarly, if the reason for mesh removal is pain, it may be advisable to remove the mesh prior to placing a new sling to ensure that the pain resolves completely. Otherwise, if pain persists, it can be unclear whether the new sling is contributing to the pain, and this may lead to difficulties treating pain or incontinence in the future.
In this patient, who was an active smoker, we excised the exposed mesh and concomitantly placed an autologous fascial sling utilizing rectus fascia. This maintained continence without introducing mesh in a high-risk patient.
CASE 4 POP and occult SUI
Ms. B. is a 79-year-old woman with stage 3 POP planned for surgical repair. While she does not report urinary leakage, preoperative urodynamic testing revealed occult SUI with reduction of her prolapse. Her priorities are to avoid needing another surgery and to limit the chances of postoperative leakage, but she is nervous about her postoperative recovery and wants to avoid pain.
What approach would be appropriate?
Consider a mini sling for this patient
The single-incision (mini) sling is an option to consider for patients with mild incontinence or for those without evidence of intrinsic sphincter deficiency. It is also a good option for those who want to avoid the additional incisions required for full-length slings.
While currently there is not sufficient evidence to clearly state if single-incision slings are equivalent to other slings, recent studies show that single-incision slings appear to be safe and effective in the short term, with possibly fewer complications than traditional transobturator slings.22-24 As patients are often concerned about the potential for groin pain with a transobturator sling, a single-incision sling is an acceptable alternative that avoids groin incisions and also avoids the retropubic space.
Patient counseling is crucial
Regardless of the route, sling procedures are highly effective and safe for treating women with SUI.3 Understanding the characteristics of each type of sling and the distinct surgical approaches enables informed counseling for patients who are navigating the treatment options for SUI.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
- Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol. 2014;123:1201-1206.
- Jonsson Funk M, Levin PJ, Wu JM. Trends in the surgical management of stress urinary incontinence. Obstet Gynecol. 2012;119:845-851.
- Ford AA, Rogerson L, Cody JD, et al. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017;7:CD006375.
- Dumoulin C, Hay-Smith J, Habee-Seguin GM, et al. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women: a short version Cochrane systematic review with meta-analysis. Neurourol Urodyn. 2015;34:300-308.
- Cox A, Herschorn S, Lee L. Surgical management of female SUI: is there a gold standard? Nat Rev Urol. 2013;10:78-89.
- Schimpf MO, Rahn DD, Wheeler TL, et al; Society of Gynecologic Surgeons Systematic Review Group. Sling surgery for stress urinary incontinence in women: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:71.e1-71.e27.
- Kenton K, Stoddard AM, Zyczynski H, et al. 5-year longitudinal followup after retropubic and transobturator mid urethral slings. J Urol. 2015;193:203-210.
- Richter HE, Albo ME, Zyczynski HM, et al; Urinary Incontinence Treatment Network. Retropubic versus transobturator midurethral slings for stress incontinence. N Engl J Med. 2010;362:2066-2076.
- Albo ME, Litman HJ, Richter HE, et al; Urinary Incontinence Treatment Network. Treatment success of retropubic and transobturator midurethral slings at 24-months. J Urol. 2012;188:2281-2287.
- US Food and Drug Administration. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse. July 2011;1-15. https://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf. Accessed September 16, 2019.
- Nilsson CG, Palva K, Aarnio R, et al. Seventeen years’ follow up of the tension-free vaginal tape procedure for female stress urinary incontinence. Int Urogynecol J. 2013;24:1265-1269.
- Nager CW, Brubaker L, Litman HJ, et al; Urinary Incontinence Treatment Network. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med. 2012;366:1987-1997.
- Stav K, Dwyer PL, Rosamilia A, et al. Repeat synthetic mid urethral sling procedure for women with recurrent stress urinary incontinence. J Urol. 2010;183:241-246.
- Kim A, Kim MS, Park YJ, et al. Retropubic versus transobturator mid urethral slings in patients at high risk for recurrent stress incontinence: a systematic review and meta-analysis. J Urol. 2019;202:132-142.
- Kavanagh A, Sanaee M, Carison KV, et al. Management of patients with stress urinary incontinence after failed midurethral sling. Can Urol Assoc J. 2017;11(6 suppl 2):S143-S146.
- Steele SE, Hill AJ, Unger CA. Concurrent midurethral sling excision or lysis at the time of repeat sling for treatment of recurrent or persistent stress urinary incontinence. Int Urogynecol J. 2018;29:285-290.
- US Food and Drug Administration. Urogynecologic surgical mesh implants. https://www.fda.gov/medicaldevices/productsandmedicalprocedures/implantsandprosthetics/urogynsurgicalmesh/. Content current as of July 10, 2019. Accessed September 16, 2019.
- Kokanali MK, Doganay M, Aksakal O, et al. Risk factors for mesh erosion after vaginal sling procedures for urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 2014;177:146-150.
- Nikolopoulos KI, Betschart C, Doumouchtsis SK. The surgical management of recurrent stress urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2015;94:568-576.
- Milose JC, Sharp KM, He C, et al. Success of autologous pubovaginal sling after failed synthetic mid urethral sling. J Urol. 2015;193:916-920.
- Albo ME, Richter HE, Brubaker L, et al; Urinary Incontinence Treatment Network. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143-2155.
- Imamura M, Hudson J, Wallace SA, et al. Surgical interventions for women with stress urinary incontinence: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2019;365:I1842.
- Jiao B, Lai S, Xu X, et al. A systematic review and meta-analysis of single-incision mini-slings (MiniArc) versus transobturator mid-urethral slings in surgical management of female stress urinary incontinence. Medicine (Baltimore). 2018;97:e0283.
- Sun Z, Wang X, Lang J, et al. Comparison of outcomes between single-incision sling and transobturator sling for treating stress urinary incontinence: a 10-year prospective study. Neurourol Urodyn. 2019;38:1852-1858.
Native tissue repair of POP: Apical suspension, anterior repair, and posterior repair



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes



Videos courtesy of Mayo Clinic
Read the related article: Native tissue repair of POP: Surgical techniques to improve outcomes