User login
The DNA Mismatch Repair System in Sebaceous Tumors: An Update on the Genetics and Workup of Muir-Torre Syndrome
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
It is well known by now that tumor formation is driven by accumulation of numerous genetic and epigenetic mutations. Human cells are equipped with an apparatus called the DNA mismatch repair (MMR) system that corrects errors during replication.1 If these genes are themselves mutated, cells then start accumulating mutations in other genes, including oncogenes and tumor suppressor genes, which results in the development of sustained proliferative signaling pathways, evasion of growth suppression, resistance to cell death, and the potential for invasion and metastasis.2
Gene mutations in DNA MMR have been detected in several tumors, such as sebaceous tumors,3 colorectal adenocarcinomas,4 keratoacanthomas,5 and other visceral malignancies.6 Sebaceous tumors are rare in the general population; however, they are common in patients with inherited or acquired mutations in MMR genes.5 These patients also have been found to have other visceral malignancies such as colorectal adenocarcinomas and breast, lung, and central nervous system (CNS) tumors.7 This observation was made in the 1960s, and patients were referred to as having Muir-Torre syndrome (MTS).8 This article serves to briefly describe the DNA MMR system and its implication in sebaceous tumors as well as discuss the recent recommendations for screening for MTS in patients presenting with sebaceous tumors.
The DNA MMR System
Mismatch repair proteins are responsible for detecting and repairing errors during cell division, especially in microsatellite regions.9 Microsatellites are common and widely distributed DNA motifs consisting of repeated nucleotide sequences that normally account for 3% of the genome.10 Mutations in MMR result in insertion or deletion of nucleotides in these DNA motifs, making them either abnormally long or short, referred to as microsatellite instability (MSI), which results in downstream cumulative accumulation of mutations in oncogenes and tumor suppressor genes, and thus carcinogenesis.9
There are 7 human MMR proteins: MLH1, MLH3, MSH2, MSH3, MSH6, PMS1, and PMS2. These proteins are highly conserved across different living species.11 Loss of MMR proteins can be due to a mutation in the coding sequence of the gene or due to epigenetic hypermethylation of the gene promoter.12 These alterations can be inherited or acquired and in most cases result in MSI.
When assessing for MSI, tumor genomes can be divided into 3 subtypes: high-level and low-level MSI and stable microsatellites.13 Tumors with high-level MSI respond better to treatment and show a better prognosis than those with low-level MSI or stable microsatellites,14 which is thought to be due to tumor-induced immune activation. Microsatellite instability results in the generation of frameshift peptides that are immunogenic and induce tumor-specific immune responses.15 Several research laboratories have artificially synthesized frameshift peptides as vaccines and have successfully used them as targets for immune therapy as a way for preventing and treating malignancies.16
Sebaceous Tumors in MTS
A typical example of tumors that arise from mutations in the DNA MMR system is seen in MTS,a rare inherited genetic syndrome that predisposes patients to sebaceous neoplasms, keratoacanthomas, and visceral malignancies.17 It was first described as an autosomal-dominant condition in patients who have at least 1 sebaceous tumor and 1 visceral malignancy, with or without keratoacanthomas. It was then later characterized as a skin variant of Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer syndrome.18
Sebaceous tumors are the hallmark of MTS. Although sebaceous hyperplasia is common in the general population, sebaceous tumors are rare outside the context of MTS. There are 3 types of sebaceous tumors with distinct pathologic features: adenoma, epithelioma, and carcinoma.19 Sebaceous adenomas and epitheliomas are benign growths; however, sebaceous carcinomas can be aggressive and have metastatic potential.20 Because it is difficult to clinically distinguish carcinomas from the benign sebaceous growths, biopsy of a large, changing, or ulcerated lesion is important in these patients to rule out a sebaceous carcinoma. Other aggressive skin tumors can develop in MTS, such as rapidly growing keratoacanthomas and basal cell carcinomas with sebaceous differentiation.21
Types of MTS
For most cases, MTS is characterized by germline mutations in DNA MMR genes. The most common mutation involves MSH2 (MutS Homolog 2)—found in approximately 90% of patients—followed by MLH1 (MutL Homolog 1)—found in approximately 10% of patients.22 Other MMR genes such as MSH6 (MutS Homolog 6), PMS2 (PMS1 homolog 2, mismatch repair system component), and MLH3 (MutL Homolog 3) less commonly are reported in MTS. There is a subset of patients who lose MSH2 or MLH1 expression due to promoter hypermethylation rather than a germline mutation. Methylation results in biallelic inactivation of the gene and loss of expression.23
A new subtype of MTS has been identified that demonstrates an autosomal-recessive pattern of inheritance and is referred to as MTS type 2 (autosomal-recessive colorectal adenomatous polyposis).24 In contrast to the classic MTS type 1, MTS type 2 exhibits microsatellite stability. Recent molecular analyses revealed that type 2 is due to a mutation in a base excision repair gene called MUTYH (mutY DNA glycosylase).25 These patients are likely to develop hundreds of polyps at an early age.
Muir-Torre syndrome also can occur sporadically without inheriting a germline mutation, which has been reported in a transplant patient from de novo somatic mutations or promoter hypermethylation.26 A case report of a renal transplant patient showed that switching from tacrolimus to sirolimus halted the appearance of new sebaceous neoplasms, which suggests that patients with MTS who undergo organ transplantation should potentially avoid tacrolimus and be put on sirolimus instead.27
Visceral Malignancies in MTS
Apart from frequent skin examinations, MTS patients should have frequent and rigorous visceral malignancy screening. Patients most commonly develop colorectal adenocarcinoma, especially in the proximal parts of the colon.28 In addition, they can develop numerous premalignant tumors, especially in MTS type 2. Other common tumors include endometrial, ovarian, genitourinary, hepatobiliary, breast, lung, hematopoietic, and CNS malignancies.29
Studies showed that specific loss of certain MMR proteins predispose patients to different types of visceral malignancies.30-32 For example, loss of MSH2 predisposes patients to development of extracolonic tumors, while loss of MLH1 more strongly is associated with development of colorectal adenocarcinoma.30 Patients with MSH2 also are at risk for development of CNS tumors, while patients with MLH1 mutations have never been reported to develop CNS tumors.31 Patients with loss of PMS2 have the lowest risk for development of any visceral malignancy.32
Diagnosing MTS
Let us consider a scenario whereby a dermatologist biopsied a solitary lesion and it came back as a sebaceous tumor. What would be the next step to establish a diagnosis of MTS?
Sebaceous tumors are rare outside the context of MTS. Therefore, patients presenting with a solitary sebaceous tumor should be worked up for MTS, as there are implications for further cancer screening. One helpful clue that can affect the pretest probability for MTS diagnosis is location of the tumor. A sebaceous tumor inferior to the neck most likely is associated with MTS. On the other hand, tumors on the head and neck can be spontaneous or associated with MTS.33 Another helpful tool is the Mayo score, a risk score for MTS in patients with sebaceous tumors.34 The score is established by adding up points, with 1 point given to each of the following: age of onset of a sebaceous tumor less than 60 years, personal history of visceral malignancy, and family history of Lynch syndrome–related visceral malignancy. Two points are given if the patient has 2 or more sebaceous tumors. The score ranges from 0 to 5. A risk score of 2 or more has a sensitivity of 100% and specificity of 81% for predicting a germline mutation in MMR genes.34
Testing for loss of MMR proteins is performed using immunohistochemistry (IHC) as well as microsatellite gene analysis on the biopsied tumor. There is no need to perform another biopsy, as these tests can be performed on the paraffin-embedded formalin fixed tissue. Immunohistochemistry testing looks for loss of expression of one of the MMR proteins. Staining usually is performed for MSH2, MSH6, and MLH1, as the combination offers a sensitivity of 81% and a positive predictive value of 100%.23,35,36
If IHC shows loss of MMR proteins, then MSI gene analysis should be performed as a confirmatory test by using MSI gene locus assays, which utilize 5 markers of mononucleotide and dinucleotide repeats. If the genome is positive for 2 of 5 of these markers, then the patient most likely has MTS.13
One caveat for IHC analysis is that there is a subset of patients who develop a solitary sebaceous tumor due to a sporadic loss of MMR protein without having MTS. These tumors also exhibit BRAF (B-Raf proto-oncogene, serine/threonine kinase) mutations or loss of p16, features that distinguish these tumors from those developed in MTS.37 As such, in a patient with a low Mayo score who developed a solitary sebaceous tumor that showed loss of MMR protein on IHC without evidence of MSI, it is reasonable to perform IHC for BRAF and p16 to avoid inaccurate diagnosis of MTS.
Another caveat is that standard MSI analysis will not detect MSI in tumors with loss of MSH6 because the markers used in the MSI analysis do not detect MSI caused by MSH6 loss. For these patients, MSI analysis using a panel composed of mononucleotides alone (pentaplex assay) should be performed in lieu of the standard panel.38
It is important to note that these molecular tests are not helpful for patients with MTS type 2, as the sebaceous tumors maintain MMR proteins and have microsatellite stability. As such, if MTS is highly suspected based on the Mayo score (either personal history of malignancy or strong family history) but the IHC and MSI analysis are negative, then referral to a geneticist for identification for MUTYH gene mutation is a reasonable next step. These patients with high Mayo scores should still be managed as MTS patients and should be screened for visceral malignancies despite lack of confirmatory tests.
Final Thoughts
Dermatologists should be highly suspicious of MTS when they diagnose sebaceous tumors. Making a diagnosis of MTS notably affects patients’ primary care. Patients with MTS should have annual skin examinations, neurologic examinations, colonoscopies starting at the age of 18 years, and surveillance for breast and pelvic cancers in women (by annual transvaginal ultrasound and endometrial aspirations) or for prostate and testicular cancers in men.17,39,40 Other tests to be ordered annually include complete blood cell count with differential and urinalysis.19
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
- Yamamoto H, Imai K. An updated review of microsatellite instability in the era of next-generation sequencing and precision medicine. Semin Oncol. 2019;46:261-270.
- Tamura K, Kaneda M, Futagawa M, et al. Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome. Int J Clin Oncol. 2019;24:999-1011.
- Shiki M, Hida T, Sugano K, et al. Muir-Torre syndrome caused by exonic deletion of MLH1 due to homologous recombination. Eur J Dermatol. 2017;27:54-58.
- Büttner R, Friedrichs N. Hereditary colon cancer in Lynch syndrome/HNPCC syndrome in Germany. Pathologe. 2019;40:584-591.
- Kuwabara K, Suzuki O, Chika N, et al. Prevalence and molecular characteristics of DNA mismatch repair protein-deficient sebaceous neoplasms and keratoacanthomas in a Japanese hospital-based population. Jpn J Clin Oncol. 2018;48:514-521.
- Burris CKH, Rodriguez ME, Raven ML, et al. Muir-torre syndrome: the importance of a detailed family history. Case Rep Ophthalmol. 2019;10:180-185.
- Walsh MD, Jayasekara H, Huang A, et al. Clinico-pathological predictors of mismatch repair deficiency in sebaceous neoplasia: a large case series from a single Australian private pathology service. Australas J Dermatol. 2019;60:126-133.
- Georgeson P, Walsh MD, Clendenning M, et al. Tumor mutational signatures in sebaceous skin lesions from individuals with Lynch syndrome. Mol Genet Genomic Med. 2019;7:E00781.
- Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev. 2008;129:391-407.
- Li YC, Korol AB, Fahima T, et al. Microsatellites within genes: structure, function, and evolution [published online February 12, 2004]. Mol Biol Evol. 2004;21:991-1007.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet. 2004;5:435-445.
- Everett JN, Raymond VM, Dandapani M, et al. Screening for germline mismatch repair mutations following diagnosis of sebaceous neoplasm. JAMA Dermatol. 2014;150:1315-1321.
- Nojadeh JN, Sharif SB, Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159-168.
- Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. J Cancer Res Clin Oncol. 2019;145:2891-2899.
- Garbe Y, Maletzki C, Linnebacher M. An MSI tumor specific frameshift mutation in a coding microsatellite of MSH3 encodes for HLA-A0201-restricted CD8+ cytotoxic T cell epitopes. PLoS One. 2011;6:E26517.
- Peng M, Mo Y, Wang Y, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128.
- Rubay D, Ohanisian L, Bank MP, et al. Muir-Torre syndrome, a rare phenotype of hereditary nonpolyposis colorectal cancer with cutaneous manifestations. ACG Case Reports J. 2019;6:E00188.
- Velter C, Caussade P, Fricker JP, et al. Muir-Torre syndrome and Turcot syndrome [in French]. Ann Dermatol Venereol. 2017;144:525-529.
- John AM, Schwartz RA. Muir-Torre syndrome (MTS): an update and approach to diagnosis and management. J Am Acad Dermatol. 2016;74:558-566.
- Kibbi N, Worley B, Owen JL, et al. Sebaceous carcinoma: controversies and their evidence for clinical practice. Arch Dermatol Res. 2020;312:25-31.
- Marcoval J, Talavera-Belmonte A, Fornons-Servent R, et al. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44:506-511.
- Roth RM, Haraldsdottir S, Hampel H, et al. Discordant mismatch repair protein immunoreactivity in Lynch syndrome-associated neoplasms: a recommendation for screening synchronous/metachronous neoplasms. Am J Clin Pathol. 2016;146:50-56.
- Westwood A, Glover A, Hutchins G, et al. Additional loss of MSH2 and MSH6 expression in sporadic deficient mismatch repair colorectal cancer due to MLH1 promoter hypermethylation. J Clin Pathol. 2019;72:443-447.
- Claes K, Dahan K, Tejpar S, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP). Acta Gastroenterol Belg. 2011;74:421-426.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet. 2003;362:39-41.
- Tomonari M, Shimada M, Nakada Y, et al. Muir-Torre syndrome: sebaceous carcinoma concurrent with colon cancer in a kidney transplant recipient; a case report. BMC Nephrol. 2019;20:394
- Levi Z, Hazazi R, Kedar-Barnes I, et al. Switching from tacrolimus to sirolimus halts the appearance of new sebaceous neoplasms in Muir-Torre syndrome. Am J Transplant. 2007;7:476-479.
- Mork ME, Rodriguez A, Taggart MW, et al. Identification of MSH2 inversion of exons 1–7 in clinical evaluation of families with suspected Lynch syndrome. Fam Cancer. 2017;16:357-361.
- Schwartz RA, Torre DP. The Muir-Torre syndrome: a 25-year retrospect. J Am Acad Dermatol. 1995;33:90-104.
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24.
- Bansidhar BJ. Extracolonic manifestations of Lynch syndrome. Clin Colon Rectal Surg. 2012;25:103-110.
- Kato A, Sato N, Sugawara T, et al. Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol. 2016;40:770-776.
- Singh RS, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936-942.
- Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome [published online March 6, 2014]. Genet Med. 2014;16:711-716.
- Chhibber V, Dresser K, Mahalingam M. MSH-6: extending the reliability of immunohistochemistry as a screening tool in Muir-Torre syndrome. Mod Pathol. 2008;21:159-164.
- Orta L, Klimstra DS, Qin J, et al. Towards identification of hereditary DNA mismatch repair deficiency: sebaceous neoplasm warrants routine immunohistochemical screening regardless of patient’s age or other clinical characteristics. Am J Surg Pathol. 2009;33:934-944.
- Mathiak M, Rütten A, Mangold E, et al. Loss of DNA mismatch repair proteins in skin tumors from patients with Muir-Torre syndrome and MSH2 or MLH1 germline mutations: establishment of immunohistochemical analysis as a screening test. Am J Surg Pathol. 2002;26:338-343.
- Campanella NC, Berardinelli GN, Scapulatempo-Neto C, et al. Optimization of a pentaplex panel for MSI analysis without control DNA in a Brazilian population: correlation with ancestry markers. Eur J Hum Genet. 2014;22:875-880.
- Ponti G, Manfredini M, Tomasi A, et al. Muir-Torre Syndrome and founder mismatch repair gene mutations: a long gone historical genetic challenge. Gene. 2016;589:127-132.
- Ferreira I, Wiedemeyer K, Demetter P, et al. Update on the pathology, genetics and somatic landscape of sebaceous tumours [published online December 10, 2019]. Histopathology. doi:10.1111/his.14044
Resident Pearls
- When patients present with a solitary sebaceous tumor, there is a high likelihood they have Muir-Torre syndrome (MTS) and thus are at a high risk to develop visceral malignancies.
- It is important to perform further testing using immunohistochemistry for DNA mismatch repair proteins and microsatellite instability gene analysis in some cases to confirm the diagnosis of MTS and to perform the appropriate cancer screening tests.
Clinical Case-Viewing Sessions in Dermatology: The Patient Perspective
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
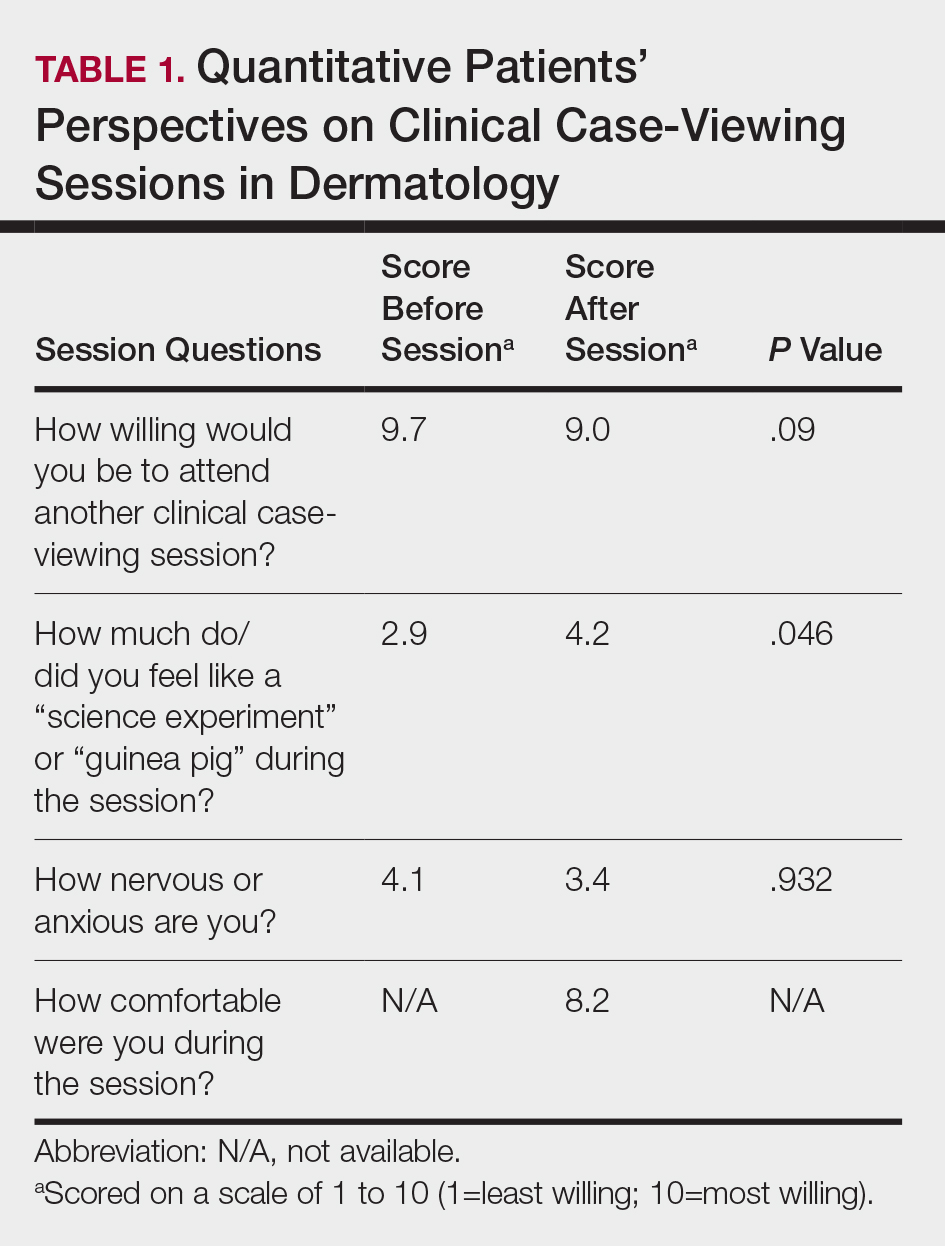
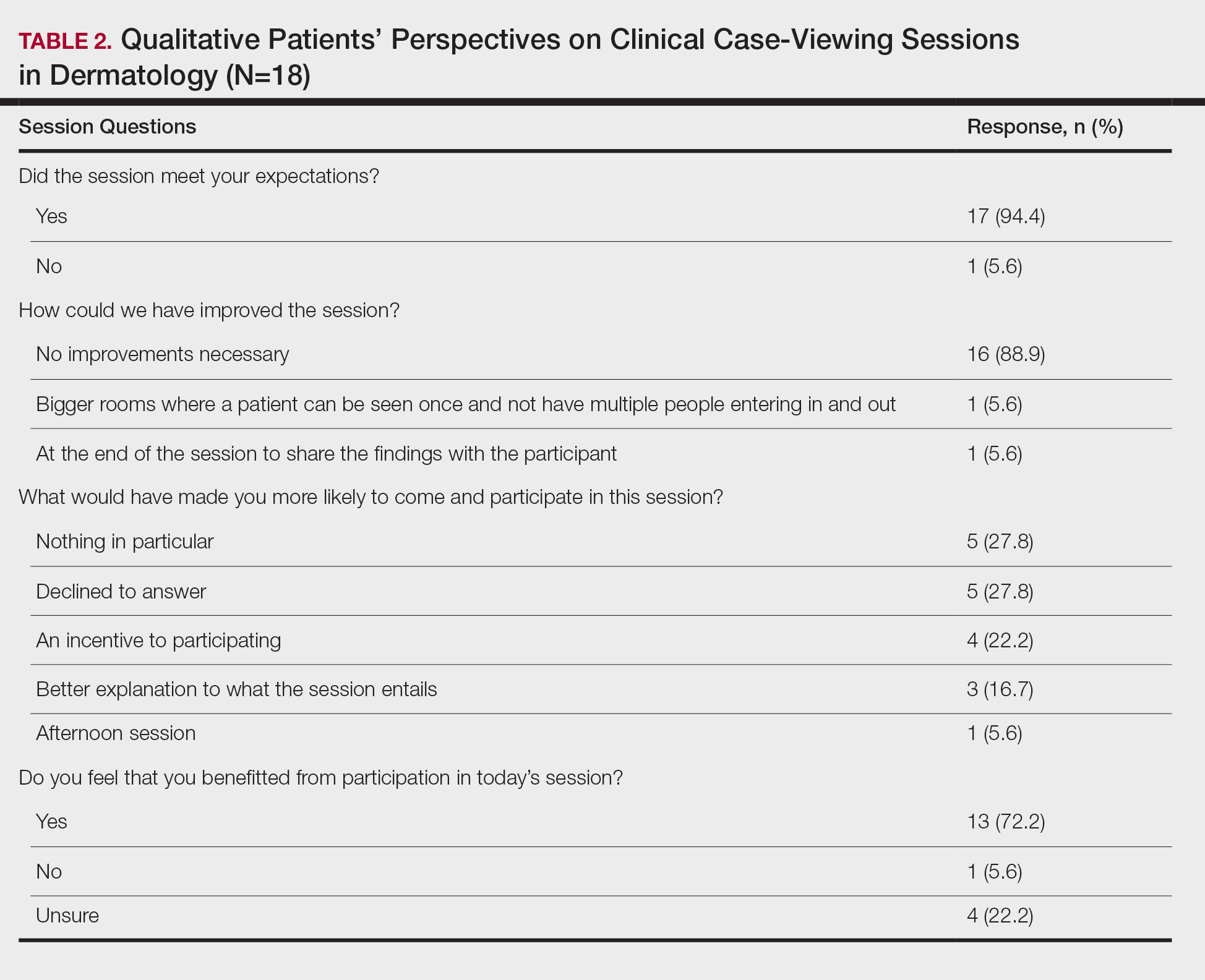
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
To the Editor:
Dermatology clinical case-viewing (CCV) sessions, commonly referred to as Grand Rounds, are of core educational importance in teaching residents, fellows, and medical students. The traditional format includes the viewing of patient cases followed by resident- and faculty-led group discussions. Clinical case-viewing sessions often involve several health professionals simultaneously observing and interacting with a patient. Although these sessions are highly academically enriching, they may be ill-perceived by patients. The objective of this study was to evaluate patients’ perception of CCV sessions.
This study was approved by the Wake Forest School of Medicine (Winston-Salem, North Carolina) institutional review board and was conducted from February 2017 to August 2017. Following informed consent, 18 patients older than 18 years who were present at the Wake Forest Department of Dermatology CCV sessions were recruited. Patients were each assigned to a private clinical examination room, and CCV attendees briefly visited each room to assess the pathologic findings of interest. Patients received written quantitative surveys before and after the CCV sessions assessing their perspectives on the session (Table 1). Quantitative surveys were assessed using a 10-point Likert scale (1=least willing; 10=most willing). Patients also received qualitative surveys following the session (Table 2). Scores on a 10-item Likert scale were evaluated using a 2-tailed t test.
The mean age of patients was 57.6 years, and women comprised 66.7% (12/18). Patient willingness to attend CCV sessions was relatively unchanged before and after the session, with a mean willingness of 9.7 before the session and 9.0 after the session (P=.09). There was a significant difference in the extent to which patients perceived themselves as experimental subjects prior to the session compared to after the session (2.9 vs 4.2)(P=.046). Following the session, 94.4% (17/18) of patients had the impression that the session met their expectations, and 72.2% (13/18) of patients felt they directly benefitted from the session.
Clinical case-viewing sessions are the foundation of any dermatology residency program1-3; however, characterizing the sessions’ psychosocial implications on patients is important too. Although some patients did feel part of a “science experiment,” this finding may be of less importance, as patients generally considered the sessions to be a positive experience and were willing to take part again.
Limitations of the study were typical of survey-based research. All participants were patients at a single center, which may limit the generalization of the results, in addition to the small sample size. Clinical case-viewing sessions also are conducted slightly differently between dermatology programs, which may further limit the generalization of the results. Future studies may aim to assess varying CCV formats to optimize both medical education as well as patient satisfaction.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
- Mehrabi D, Cruz PD Jr. Educational conferences in dermatology residency programs. J Am Acad Dermatol. 2006;55:523-524.
- Hull AL, Cullen RJ, Hekelman FP. A retrospective analysis of grand rounds in continuing medical education. J Contin Educ Health Prof. 1989;9:257-266.
- Cruz PD Jr, Chaker MB. Teaching conferences in dermatology residency programs revisited. J Am Acad of Dermatol. 1995;32:675-677.
Practice Points
- Patient willingness to attend dermatology clinical case-viewing (CCV) sessions is relatively unchanged before and after the session.
- Participants generally consider CCV sessions to be a positive experience.
Handoffs in Dermatology Residency
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
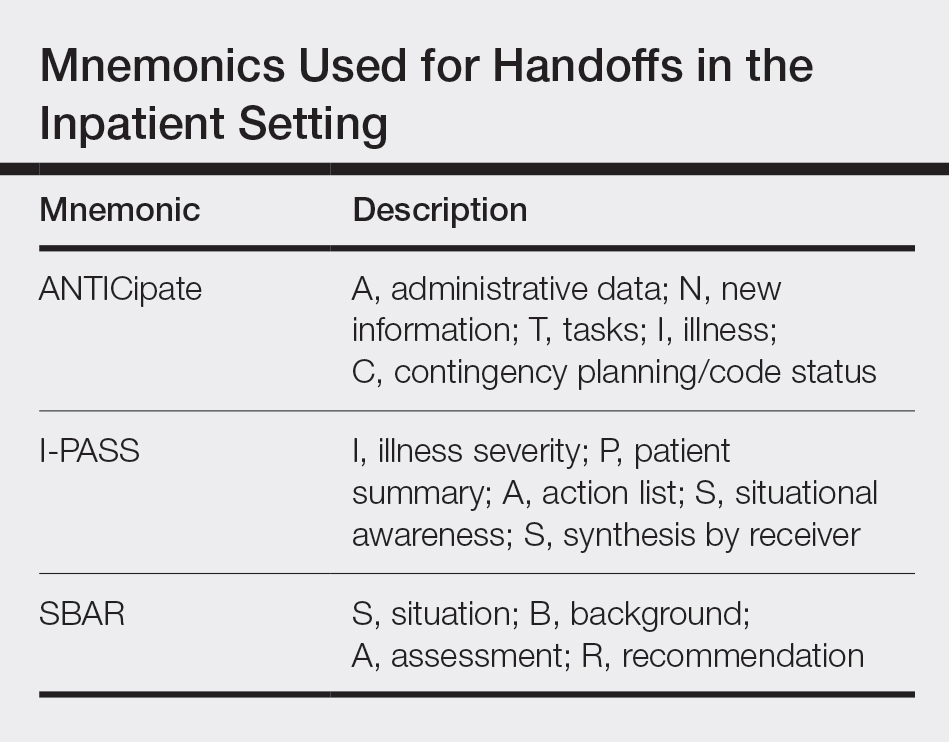
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
As a dermatologist, there are innumerable items to track after each patient encounter, such as results from biopsies, laboratory tests, cultures, and imaging, as well as ensuring follow-up with providers in other specialties. In residency, there is the complicating factor of switching rotations and therefore transitioning care to different providers (Figure). Ensuring organized handoff practices is especially important in residency. In a study of malpractice claims involving residents, handoff problems were a notable contributing factor in 19% of malpractice cases involving residents vs 13% of cases involving attending physicians.1 There still is a high percentage of malpractice cases involving handoff problems among attending physicians, highlighting the fact that these issues persist beyond residency.
This article will review a variety of handoff and organizational practices that dermatology residents currently use, discuss the evidence behind best practices, and highlight additional considerations relevant when selecting organizational tools.
Varied Practices
Based on personal discussions with residents from 7 dermatology residency programs across the country, there is marked variability in both the frequency of handoffs and organizational methods utilized. Two major factors that dictate these practices are the structure of the residency program and electronic health record (EHR) capacities.
Program structure and allocation of resident responsibilities affect the frequency of handoffs in the outpatient dermatology residency setting. In some programs, residents are responsible for all pending studies for patients they have seen, even after switching clinical sites. In other programs, residents sign out patients, including pending test results, when transitioning from one clinical rotation to another. The frequency of these handoffs varies, ranging from every few weeks to every 4 months.
Many dermatology residents report utilizing features in the EHR to organize outstanding tasks and results, obviating the need for additional documentation. Some EHRs have the capacity to assign proxies, which allows for a seamless transition to another provider. When the EHR lacks these capabilities, organization of outstanding tasks relies more heavily on supplemental documentation. Residents noted using spreadsheets, typed documents, electronic applications designed to organize handoffs outside of the EHR, and handwritten notes.
There is room for formal education on the best handoff and organizational practices in dermatology residency. A study of anesthesiology residents at a major academic institution suggested that education regarding sign-out practices is most effective when it is multimodal, using both formal and informal methods.2 Based on my discussions with other dermatology residents, these practices generally are informally learned; often, dermatology residents did not realize that organization practices varied so widely at other institutions.
Evidence Behind Handoff Practices
There are data in the dermatology literature to support utilizing electronic means for handoff practices. At a tertiary dermatology department in Melbourne, Australia, providers created a novel electronic handover system using Microsoft programs to be used alongside the main hospital EHR to help practitioners keep track of outpatient studies.3 An audit of this system demonstrated that its use provided a reliable system for follow-up on all outpatient results, with benefits in clinical, organizational, and health research domains.4 The investigators noted that residents, registrars, nurses, and consultants utilized the electronic handover system, with residents completing 90% of all tasks.3 Similarly, several residents I spoke with personally cited using Listrunner (www.listrunnerapp.com), a Health Insurance Portability and Accountability Act–compliant electronic tool outside of the EHR designed for collaborative management of patient lists.
Outside of the dermatology literature, resident handoff in the outpatient setting mainly has been studied in the primary care year-end transition of care, with findings that are certainly relevant to dermatology residency. Pincavage et al5 performed a targeted literature search on year-end handoff practices, and Donnelly et al6 studied internal medicine residents in an outpatient ambulatory clinic; both supported implementing a standardized process for sign-out. Pincavage et al5 also recommended focusing on high-risk patients, educating residents on handoff practices, preparing patients for the transition, and performing safety audits. Donnelly et al6 found that providing time dedicated to patient handoff and clear expectations improved handoff practices.
There is extensive literature on handoff practices in the inpatient setting sparked by an increasing number of handoffs after the implementation of Accreditation Council for Graduate Medical Education duty hour restrictions in 1989. Some of the guiding principles may be applied to the outpatient dermatology setting. Many residents may be familiar with mnemonics that have been developed to organize content during sign-out, which have been s
Other Considerations
An important consideration during patient handoffs is security, especially when implementing documentation and tools outside of the EHR. It is important for providers to be compliant with institutional policies as well as the Health Insurance Portability and Accountability Act and ensure protection against cyberattacks, which have been on the rise; 83% of 1300 physicians surveyed have been the victim of a cyberattack.9 Providers also should be mindful of redundancies in organizational and handoff practices. Multiple methods for keeping track of information helps ensure that important results do not fall through the cracks. However, too many redundancies may be wasteful of a practice’s resources and providers’ time.
Final Thoughts
There are varied practices regarding organization of handoff and follow-up. Residency should serve as an opportunity for physicians to become familiar with different practices. Becoming familiar with the varied options may be helpful to take forward in one’s career, especially given that dermatologists may enter a work setting postresidency with practices that are different from where they trained. Additionally, given rapid shifts in technologies, providers must change how they stay organized. This evolving landscape provides an opportunity for the next generation of dermatologists to take leadership to shape the future of organizational practices.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
- Singh H, Thomas EJ, Petersen LA, et al. Medical errors involving trainees: a study of closed malpractice claims from 5 insurers. Arch Intern Med. 2007;167:2030-2036.
- Muralidharan M, Clapp JT, Pulos BP, et al. How does training in anesthesia residency shape residents’ approaches to patient care handoffs? a single-center qualitative interview study. BMC Med Educ. 2018;18:271.
- Poon F, Martyres R, Denahy A, et al. Improving patient safety: the impact of an outpatients’ electronic handover system in a tertiary dermatology department. Australas J Dermatol. 2018;59:E183-E188.
- Listrunner website. https://www.listrunnerapp.com. Accessed January 30, 2020.
- Pincavage AT, Donnelly MJ, Young JQ, et al. Year-end resident clinic handoffs: narrative review and recommendations for improvement. Jt Comm J Qual Patient Saf. 2017;43:71-79.
- Donnelly MJ, Clauser JM, Weissman NJ. An intervention to improve ambulatory care handoffs at the end of residency. J Grad Med Educ. 2012;4:381-384.
- Vidyarthi AR, Arora V, Schnipper JL, et al. Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign-out. J Hosp Med. 2006;1:257-266.
- Breaux J, Mclendon R, Stedman RB, et al. Developing a standardized and sustainable resident sign-out process: an AIAMC National Initiative IV Project. Ochsner J. 2014;14:563-568.
- American Medical Association and Accenture. Taking the physician’s pulse: tackling cyber threats in healthcare. https://www.accenture.com/_acnmedia/accenture/conversion-assets/dotcom/documents/local/en/accenture-health-taking-the-physicians-pulse.pdf. Accessed January 30, 2020.
Resident Pearl
- For dermatology residents, ensuring organized handoff and follow-up practices is essential. Residency provides an opportunity to become familiar with different practices to take forward in one’s career.
Top Residents Selected for 2020 dermMentors™ Resident of Distinction Award™
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
The dermMentors™ Resident of Distinction Award™ was presented to 5 dermatology residents at the 19th Annual Caribbean Dermatology Symposium, January 21–25, 2020, in Paradise Island, Bahamas. Recipients of the award include Rachel Giesey, DO, Case Western Reserve University, Cleveland, Ohio, and University Hospitals Cleveland Medical Center, Cleveland, Ohio; Janice Tiao, MD, Boston University Medical Center, Boston, Massachusetts; Jordan V. Wang, MD, MBE, MBA, Thomas Jefferson University, Philadelphia, Pennsylvania; Jacqueline D. Watchmaker, MD, Boston University School of Medicine, Boston, Massachusetts; and Jennifer E. Yeh, MD, PhD, Brigham and Women’s Hospital, Boston, Massachusetts. The residents presented their research during the general sessions on January 25, 2020.
The overall grand prize was awarded to Dr. Yeh for her research entitled, “Topical Imiquimod in Combination With Brachytherapy for Unresectable Cutaneous Melanoma Metastases.” Dr. Yeh presented the utility of combining topical imiquimod with brachytherapy for locoregional control of cutaneous metastases through the presentation of 3 patients with scalp melanoma initially treated with wide local excision who developed numerous cutaneous metastases. “While surgical excision is the first-line treatment of single, discrete cutaneous metastases, it may not be practical in patients with multiple foci of disease distributed over large areas, as seen in the 3 patients presented here who achieved complete resolution of their cutaneous metastatic burden with concurrent topical imiquimod and brachytherapy,” Dr. Yeh reported.
Presentations by the other residents included a study of the burden of common skin diseases in the Caribbean and the potential correlation with a country’s socioeconomic status (Dr. Giesey), a study of the use of doxycycline in patients with lichen planopilaris and frontal fibrosing alopecia (Dr. Tiao), a discussion of counterfeit medical devices and injectables as well as medical spas in dermatology (Dr. Wang), and a study of the most common reasons patients are dissatisfied with minimally and noninvasive cosmetic procedures (Dr. Watchmaker). Access all of the abstracts presented by the top residents here.
The dermMentors™ Resident of Distinction Award™ recognizes top residents in dermatology. DermMentors.org and the dermMentors™ Resident of Distinction Award™ are sponsored by Beiersdorf Inc and administered by DermEd, Inc. The 2020 dermMentors™ Residents of Distinction™ presented new scientific research during the general sessions of the 19th Annual Caribbean Dermatology Symposium on January 25, 2020.
The Lowdown on Low-Dose Naltrexone
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
Low-dose naltrexone (LDN) has shown efficacy in off-label treatment of a variety of inflammatory diseases ranging from Crohn disease to multiple sclerosis.1 There are limited data about the use of LDN in dermatology, but reports regarding how it works as an anti-inflammatory agent have been published.1,2
Naltrexone is an opioid receptor antagonist that originally was approved by the US Food and Drug Administration to treat addiction to alcohol, opiates, and heroin.2 The dose of naltrexone to treat addiction ranges from 50 to 100 mg/d, and at these levels the effects of opioids are blocked for 24 hours; however, the dosing for LDN is much lower, ranging from 1.5 to 4.5 mg/d.3 At this low dose, naltrexone partially binds to various opioid receptors, leading to a temporary blockade.4 One of the downstream effects of this opioid receptor blockade is a paradoxical increase in endogenous endorphins.3
In addition to opioid blockage, lower doses of naltrexone have anti-inflammatory effects by inhibiting nonopioid receptors. Naltrexone blocks toll-like receptor 4, which is found on keratinocytes and also on macrophages such as microglia.5 These macrophages also contain inflammatory compounds such as tumor necrosis factor α and IL-6. Low-dose naltrexone can suppress levels of these inflammatory markers. It is important to note that these anti-inflammatory effects have not been observed at the standard higher doses of naltrexone.1
When to Use
Low-dose naltrexone is a treatment option for inflammatory dermatologic conditions. A recent review of the literature outlined the use of LDN in a variety of inflammatory skin conditions. Improvement was noted in patients with Hailey-Hailey disease, lichen planopilaris, and various types of pruritus (ie, aquagenic, cholestatic, uremic, atopic dermatitis related).3 A case report of LDN successfully treating a patient with psoriasis also has been published.6 We often use LDN at the University of Wisconsin (Madison, Wisconsin) to treat patients with psoriasis. Ekelem et al3 also discussed patients with skin conditions that either had no response or worsened with naltrexone treatment, including various types of pruritus (ie, uremic, mycosis fungoides related, other causes of pruritus). Importantly, in the majority of cases without an improved response, the dose used was 50 mg/d.3 Higher doses of naltrexone are not known to have anti-inflammatory effects.
Low-dose naltrexone can be considered as a treatment option in patients with contraindications to other systemic anti-inflammatory treatments; for example, patients with a history of malignancy may prefer to avoid treatment with biologic agents. Low-dose naltrexone also can be considered as a treatment option in patients who are uncomfortable with the side-effect profiles of other systemic anti-inflammatory treatments, such as the risk for leukemias and lymphomas associated with biologic agents, the risk for liver toxicity with methotrexate, or the risk for hyperlipidemia with acitretin.
How to Monitor
The following monitoring information is adapted from the practice of Apple Bodemer, MD, a board-certified dermatologist at the University of Wisconsin (Madison, Wisconsin) who also is fellowship trained in integrative medicine.
There is a paucity of published data about LDN dosing for inflammatory skin diseases. However, prescribers should be aware that LDN can alter thyroid hormone levels, especially in patients with autoimmune thyroid disease. If a thyroid-stimulating hormone (TSH) level within reference range has not been noted in the last year, consider screening with a TSH test and also assessing for a personal or family history of thyroid disease. If the TSH level is within reference range, there generally is no need to monitor while treating with LDN. Consider checking TSH levels every 4 months in patients with thyroid disease while they are on LDN therapy and be sure to educate them about symptoms of hyperthyroidism.
Side Effects
Low-dose naltrexone has a minimal side-effect profile with self-limited side effects that often resolve within approximately 1 week. One of the most commonly reported side effects is sleep disturbance with vivid dreams, which has been reported in 37% of participants.1 If your patients experience this side effect, you can reassure them that it improves with time. You also can switch to morning dosing to try and alleviate sleep disturbances at night. Another possible side effect is gastrointestinal tract upset. Importantly, there is no known abuse potential for LDN.1 To stop LDN, patients should be stable for 6 to 12 months, and there is no need to wean them off it.
Cost and Availability
Because use of LDN in dermatology is considered off label and it is not approved by the US Food and Drug Administration to treat any medical conditions, it must be prescribed through a compounding pharmacy, usually without insurance coverage. The monthly cost is approximately $30 depending on the pharmacy (unpublished data), which may be cost prohibitive for patients, so it is important to counsel them about price
Final Thoughts
Low-dose naltrexone is an alternative treatment option that can be considered in patients with inflammatory skin diseases. It has a favorable side-effect profile, especially compared to other systemic anti-inflammatory agents; however, additional studies are needed to learn more about its safety and efficacy. If patients ask you about LDN, the information provided here can guide you with how it works and how to prescribe it.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
- Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451-459.
- Brown N, Panksepp J. Low-dose naltrexone for disease prevention and quality of life. Med Hypotheses. 2009;72:333-337.
- Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236.
- Bihari B. Efficacy of low dose naltrexone as an immune stabilizing agent for the treatment of HIV/AIDS. AIDS Patient Care. 1995;9:3.
- Lee B, Elston DM. The uses of naltrexone in dermatologic conditions [published online December 21, 2018]. J Am Acad Dermatol. 2019;80:1746-1752.
- Bridgman AC, Kirchhof MG. Treatment of psoriasis vulgaris using low-dose naltrexone. JAAD Case Rep. 2018;4:827-829.
Resident Pearl
- Low-dose naltrexone is an alternative antiinflammatory treatment to consider in patients with inflammatory skin diseases, with a minimal side-effect profile.
A Comparison of Knowledge Acquisition and Perceived Efficacy of a Traditional vs Flipped Classroom–Based Dermatology Residency Curriculum
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).
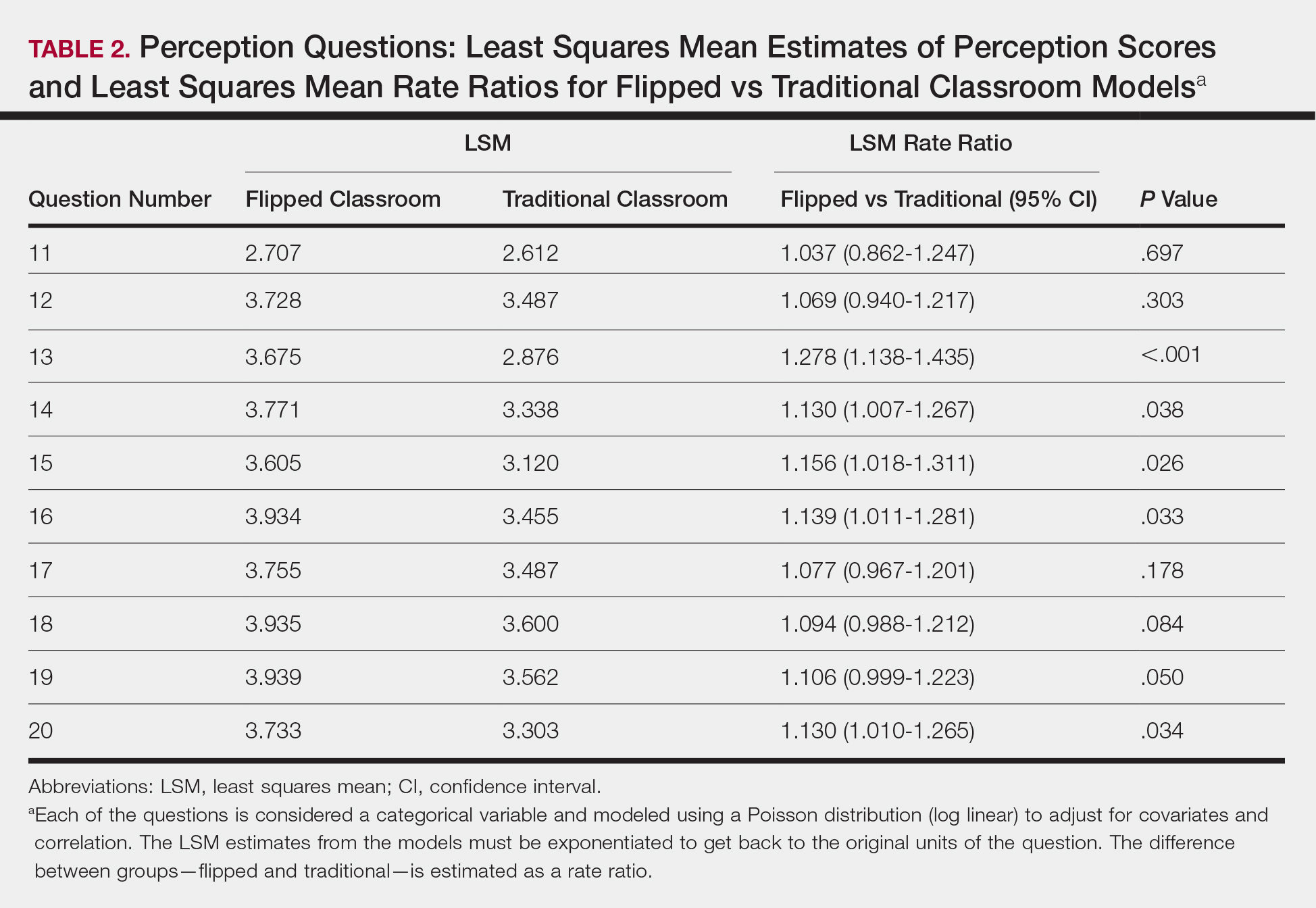
Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
The ideal method of resident education is a subject of great interest within the medical community, and many dermatology residency programs utilize a traditional classroom model for didactic training consisting of required textbook reading completed at home and classroom lectures that often include presentations featuring text, dermatology images, and questions throughout the lecture. A second teaching model is known as the flipped, or inverted, classroom. This model moves the didactic material that typically is covered in the classroom into the realm of home study or homework and focuses on application and clarification of the new material in the classroom. 1 There is an emphasis on completing and understanding course material prior to the classroom session. Students are expected to be prepared for the lesson, and the classroom session can include question review and deeper exploration of the topic with a focus on subject mastery. 2
In recent years, the flipped classroom model has been used in elementary education, due in part to the influence of teachers Bergmann and Sams,3 as described in their book Flip Your Classroom: Reach Every Student in Every Class Every Day. More recently, Prober and Khan4 argued for its use in medical education, and this model has been utilized in medical school curricula to teach specialty subjects, including medical dermatology.5
Given the increasing popularity and use of the flipped classroom, the primary objective of this study was to determine if a difference in knowledge acquisition and resident perception exists between the traditional and flipped classrooms. If differences do exist, the secondary aim was to quantify them. We hypothesized that the flipped classroom actively engages residents and would improve both knowledge acquisition and resident sentiment toward the residency program curriculum compared to the traditional model.
Methods
The Duke Health (Durham, North Carolina) institutional review board granted approval for this study. All of the dermatology residents from Duke University Medical Center for the 2014-2015 academic year participated in this study. Twelve individual lectures chosen by the dermatology residency program director were included: 6 traditional lectures and 6 flipped lectures. The lectures were paired for similar content.
Survey Administration
Each resident was assigned a unique 4-digit numeric code that was unknown to the investigators and recorded at the beginning of each survey. The residents expected flipped lectures for each session and were blinded as to when a traditional lecture and quiz would occur, with the exception of the resident providing the lecture. Classroom presentations were immediately followed by a voluntary survey administered through Qualtrics.6 Consent was given at the beginning of each survey, followed by 10 factual questions and 10 perception questions. The factual questions varied based on the lecture topic and were multiple-choice questions written by the program director, associate program director, and faculty. Each factual question was worth 10 points, and the scaled score for each quiz had a maximum value of 100. The perception questions were developed by the authors (J.H. and A.R.A.) in consultation with a survey methodology expert at the Duke Social Science Research Institute. These questions remained constant across each survey and were descriptive based on standard response scales. The data were extracted from Qualtrics for statistical analysis.
Statistical Analysis
The mean score with the standard deviation for each factual question quiz was calculated and plotted. A generalized linear mixed model was created to study the difference in quiz scores between the 2 classroom models after adjusting for other covariates, including resident, the interaction between resident and class type, quiz time, and the interaction between class type and quiz time. The variable resident was specified as a random variable, and a variance components covariance structure was used. For the perception questions, the frequency and percentage of each answer for a question was counted. Generalized linear mixed models with a Poisson distribution were created to study the difference in answers for each survey question between the 2 curriculum types after adjusting for other covariates, including scores for factual questions, quiz time, and the interaction between class type and quiz time. The variable resident was again specified as a random variable, and a diagonal covariance structure was used. All statistical analyses were carried out using SAS software package version 9.4 (SAS Institute) by the Duke University Department of Biostatistics and Bioinformatics. P<.05 was considered statistically significant.
Results
All 9 of the department’s residents were included and participated in this study. Mean score with standard deviation for each factual quiz is plotted in the Figure. Across all residents, the mean factual quiz score was slightly higher but not statistically significant in the flipped vs traditional classrooms (67.5% vs 65.4%; P=.448)(data not shown). When comparing traditional and flipped factual quiz scores by individual resident, there was not a significant difference in quiz performance (P=.166)(data not shown). However, there was a significant difference in the factual quiz scores among residents for all quizzes (P=.005) as well as a significant difference in performance between each individual quiz over time (P<.001)(data not shown). In the traditional classroom, residents demonstrated a trend in variable performance with each factual quiz. In the flipped classroom, residents also had variable performance, with wide-ranging scores (P=.008)(data not shown).
Each resident also answered 10 perception questions (Table 1). When comparing the responses by quiz type (Table 2), there was a significant difference for several questions in favor of the flipped classroom: how actively residents thought their co-residents participated in the lecture (P<.001), how much each resident enjoyed the session (P=.038), and how much each resident believed their co-residents enjoyed the session (P=.026). Additionally, residents thought that the flipped classroom sessions were more efficient (P=.033), better prepared them for boards (P=.050), and better prepared them for clinical practice (P=.034). There was not a significant difference in the amount of reading and preparation residents did for class (P=.697), how actively the residents thought they participated in the lecture (P=.303), the effectiveness of the day’s curriculum structure (P=.178), or whether residents thought the lesson increased their knowledge on the topic (P=.084).

Comment
The traditional model in medical education has undergone changes in recent years, and researchers have been looking for new ways to convey more information in shorter periods of time, especially as the field of medicine continues to expand. Despite the growing popularity and adoption of the flipped classroom, studies in dermatology have been limited. In this study, we compared a traditional classroom model with the flipped model, assessing both knowledge acquisition and resident perception of the experience.
There was not a significant difference in mean objective quiz scores when comparing the 2 curricula. The flipped model was not better or worse than the traditional teaching model at relaying information and promoting learning. Rather, there was a significant difference in quiz scores based on the individual resident and on the individual quiz. Individual performance was not affected by the teaching model but rather by the individual resident and lecture topic.
These findings differ from a study of internal medicine residents, which revealed that trainees in a quality-improvement flipped classroom had greater increases in knowledge than a traditional cohort.7 It is difficult to make direct comparisons to this group, given the difference in specialty and subject content. In comparison, an emergency medicine program completed a cross-sectional cohort study of in-service examination scores in the setting of a traditional curriculum (2011-2012) vs a flipped curriculum (2015-2016) and found that there was no statistical difference in average in-service examination scores.8 The type of examination content in this study may be more similar to the quizzes that our residents experienced (ie, fact-based material based on traditional medical knowledge).
The dermatology residents favored the flipped curriculum for 6 of 10 perception questions, which included areas of co-resident participation, personal and co-resident enjoyment, efficiency, boards preparation, and preparation for clinical practice. They did not favor the flipped classroom for prelecture preparation, personal participation, lecture effectiveness, or knowledge acquisition. They perceived their peers as being more engaged and found the flipped classroom to be a more positive experience. The residents thought that the flipped lectures were more time efficient, which could have contributed to overall learner satisfaction. Additionally, they thought that the flipped model better prepared them for both the boards and clinical practice, which are markers of future performance.
These findings are consistent with other studies that revealed improved postcourse perception scores for a quality improvement emergency medicine–flipped classroom. Most of this group preferred the flipped classroom over the traditional after completion of the flipped curriculum.9 A neurosurgery residency program also reported increased resident engagement and resident preference for a newly designed flipped curriculum.10
Overall, our data indicate that there was no objective change in knowledge acquisition at the time of the quiz, but learner satisfaction was significantly greater in the flipped classroom model.
Limitations
This study was comprised of a small number of residents from a single institution and was based on a limited number of lectures given throughout the year. All lectures during the study year were flipped with the exception of the 6 traditional study lectures. Therefore, each resident who presented a traditional lecture was not blinded for her individual assigned lecture. In addition, because traditional lectures only occurred on study days, once the lectures started, all trainees could predict that a content quiz would occur at the end of the session, which could potentially introduce bias toward better quiz performance for the traditional lectures.
Conclusion
When comparing traditional and flipped classroom models, we found no difference in knowledge acquisition. Rather, the difference in quiz scores was among individual residents. There was a significant positive difference in how residents perceived these teaching models, including enjoyment and feeling prepared for the boards. The flipped classroom model provides another opportunity to better engage residents during teaching and should be considered as part of dermatology residency education.
Acknowledgments
Duke Social Sciences Institute postdoctoral fellow Scott Clifford, PhD, and Duke Dermatology residents Daniel Chang, MD; Sinae Kane, MD; Rebecca Bialas, MD; Jolene Jewell, MD; Elizabeth Ju, MD; Michael Raisch, MD; Reed Garza, MD; Joanna Hooten, MD; and E. Schell Bressler, MD (all Durham, North Carolina)
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
- Lage MJ, Platt GJ, Treglia M. Inverting the classroom: a gateway to creating an inclusive learning environment. J Economic Educ. 2000;31:30-43.
- Gillispie V. Using the flipped classroom to bridge the gap to generation Y. Ochsner J. 2016;16:32-36.
- Bergmann J, Sams A. Flip Your Classroom: Reach Every Student in Every Class Every Day. Alexandria, VA: International Society for Technology in Education; 2012.
- Prober CG, Khan S. Medical education reimagined: a call to action. Acad Med. 2013;88:1407-1410.
- Aughenbaugh WD. Dermatology flipped, blended and shaken: a comparison of the effect of an active learning modality on student learning, satisfaction, and teaching. Paper presented at: Dermatology Teachers Exchange Group 2013; September 27, 2013; Chicago, IL.
- Oppenheimer AJ, Pannucci CJ, Kasten SJ, et al. Survey says? A primer on web-based survey design and distribution. Plast Reconstr Surg. 2011;128:299-304.
- Bonnes SL, Ratelle JT, Halvorsen AJ, et al. Flipping the quality improvement classroom in residency education. Acad Med. 2017;92:101-107.
- King AM, Mayer C, Barrie M, et al. Replacing lectures with small groups: the impact of flipping the residency conference day. West J Emerg Med. 2018;19:11-17.
- Young TP, Bailey CJ, Guptill M, et al. The flipped classroom: a modality for mixed asynchronous and synchronous learning in a residency program. Western J Emerg Med. 2014;15:938-944.
- Girgis F, Miller JP. Implementation of a “flipped classroom” for neurosurgery resident education. Can J Neurol Sci. 2018;45:76-82.
Practice Points
- There was not a significant difference in dermatology resident factual quiz scores when comparing flipped vs traditional classroom teaching sessions.
- There was a significant difference between the flipped vs traditional teaching models, with dermatology residents favoring the flipped classroom, for co-resident lecture participation and individual and co-resident enjoyment of the lecture.
- Residents also perceived that the flipped classroom sessions were more efficient, better prepared them for boards, and better prepared them for clinical practice.
Annual Skin Check: Examining the Dermatology Headlines of 2019
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
From chemical sunscreen to the measles outbreak and drug approvals to product recalls, dermatology experienced its share of firsts and controversies in 2019.
Chemical Sunscreen Controversies
Controversial concerns about the effects of chemical sunscreen on coral reefs took an unprecedented turn in the United States this last year. On February 5, 2019, an ordinance was passed in Key West, Florida, prohibiting the sale of sunscreen containing the organic UV filters oxybenzone and/or octinoxate within city limits.1 On June 25, 2019, a similar law that also included octocrylene was passed in the US Virgin Islands.2 In so doing, these areas joined Hawaii, the Republic of Palau, and parts of Mexico in restricting chemical sunscreen sales.1 Although the Key West ordinance is set to take effect in January 2021, opponents, including dermatologists who believe it will discourage sunscreen use, currently are trying to overturn the ban.3 In the US Virgin Islands, part of the ban went into effect in September 2019, with the rest of the ban set to start in March 2020.2 Companies have started to follow suit. On August 1, 2019, CVS Pharmacy announced that, by the end of 2020, it will remove oxybenzone and octinoxate from some of its store-brand chemical sunscreens.4
On February 26, 2019, the US Food and Drug Administration (FDA) proposed that there are insufficient data to determine if 12 organic UV filters—including the aforementioned oxybenzone, octinoxate, and octocrylene—are generally recognized as safe and effective (GRASE).5 Although these ingredients were listed as GRASE by the FDA in 2011, the rise in sunscreen use since then, as well as changes in sunscreen formulations, prompted the FDA to ask manufacturers to perform additional studies on safety parameters such as systemic absorption.5,6 One study conducted by the FDA itself was published in May 2019 and showed that maximal use of 4 sunscreens resulted in systemic absorption of 4 organic UV filters above 0.5 ng/mL, the FDA’s threshold for requiring nonclinical toxicology assessment. The study authors concluded that “further studies [are needed] to determine the clinical significance of these findings. [But] These results do not indicate that individuals should refrain from the use of
End of the New York City Measles Outbreak
On September 3, 2019, New York City’s largest measles outbreak in nearly 30 years was declared over. This announcement reflected the fact that 2 incubation periods for measles—42 days—had passed since the last measles patient was considered contagious. In total, there were 654 cases of measles and 52 associated hospitalizations, including 16 admissions to the intensive care unit. Most patients were younger than 18 years and unvaccinated.8
The outbreak began in October 2018 after Orthodox Jewish children from Brooklyn became infected while visiting Israel and imported the measles virus upon their return home.8,9 All 5 boroughs in New York City were ultimately affected, although 4 zip codes in Williamsburg, a neighborhood in Brooklyn with an undervaccinated Orthodox Jewish community, accounted for 72% of cases.8,10 As part of a $6 million effort to stop the outbreak, an emergency order was placed on these 4 zip codes, posing potential fines on people living or working there if they were unvaccinated.8 In addition, a bill was passed and signed into law in New York State that eliminated religious exemptions for immunizations.11 In collaboration with Jewish leaders, these efforts increased the administration of measles-mumps-rubella vaccines by 41% compared with the year before in Williamsburg and Borough Park, another heavily Orthodox Jewish neighborhood in Brooklyn.8
Drug Approvals for Pediatric Dermatology
On March 11, 2019, the IL-4/IL-13 inhibitor dupilumab became the third biologic with a pediatric dermatology indication when the FDA extended its approval to adolescents for the treatment of atopic dermatitis.12 The FDA approval was based on a randomized, double-blind, placebo-controlled trial in which 42% (34/82) of adolescents treated with dupilumab monotherapy every other week achieved 75% or more improvement in the Eczema Area and Severity Index at week 16 compared with 8% (7/85) in the placebo group (P<.001).13
In October 2019, trifarotene cream and minocycline foam were approved by the FDA for the treatment of acne in patients 9 years and older.14,15 As such, both became the first acne therapies to include patients as young as 9 years in their studies and indication—a milestone, considering the fact that children have historically been excluded from clinical trials.16 The 2 topical treatments also are noteworthy for being first in class: trifarotene cream is the only topical retinoid to selectively target the retinoic acid receptor γ and to have been studied specifically for both facial and truncal acne,14,17 and minocycline foam is the first topical tetracycline.15
Drug Approvals for Rare Dermatologic Diseases
On July 19, 2019, apremilast, a phosphodiesterase 4 inhibitor, became the first medication approved by the FDA for the treatment of adults with oral ulcers due to Behçet disease, a rare multisystem inflammatory disease.18 The FDA approval was based on a double-blind, randomized, placebo-controlled trial in which 53% (55/104) of patients receiving apremilast monotherapy were ulcer free at week 12 compared to 22% (23/103) receiving placebo (P<.0001)(ClinicalTrials.gov Identifier NCT02307513).19
On October 8, 2019, afamelanotide was approved by the FDA to increase pain-free light exposure in adults with erythropoietic protoporphyria, a rare metabolic disorder associated with photosensitivity.20 A melanocortin receptor agonist, afamelanotide is believed to confer photoprotection by increasing the production of eumelanin in the epidermis. The FDA approval was based on 2 randomized, double-blind, placebo-controlled trials, both of which found that patients given afamelanotide spent significantly more time in direct sunlight without pain compared to patients in the placebo group (P=.005 and P=.04).21
Recalls of Popular Skin Products
On July 5, 2019, Neutrogena recalled its cult-favorite Light Therapy Acne Mask. The recall was driven by rare reports of transient visual side effects due to insufficient eye protection from the mask’s light-emitting diodes.22,23 Reported in association with 0.02% of masks sold at the time of the recall, these side effects included eye pain, irritation, tearing, blurry vision, seeing spots, and changes in color vision.24 In addition, a risk for potentially irreversible eye injury from the mask was cited in people taking photosensitizing medications, such as doxycycline, and people with certain underlying eye conditions, such as retinitis pigmentosa and ocular albinism.22,24,25
Following decades of asbestos-related controversy, 1 lot of the iconic Johnson’s Baby Powder was recalled for the first time on October 18, 2019, after the FDA found subtrace levels of asbestos in 1 of the lot’s bottles.26 After the recall, Johnson & Johnson reported that 2 third-party laboratories did not ultimately find asbestos when they tested the bottle of interest as well as other bottles from the recalled lot. Three of 5 samples prepared in 1 room by the third-party laboratories initially did test positive for asbestos, but this result was attributed to the room’s air conditioner, which was found to be contaminated with asbestos. When the same samples were prepared in another room, no asbestos was detected.27 The FDA maintained there was “no indication of cross-contamination” when they originally tested the implicated bottle.28
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
- Zraick K. Key West bans sunscreen containing chemicals believed to harm coral reefs. New York Times. February 7, 2019. https://www.nytimes.com/2019/02/07/us/sunscreen-coral-reef-key-west.html. Accessed December 23, 2019.
- Gies H. The U.S. Virigin Islands becomes the first American jurisdiction to ban common chemical sunscreens. Pacific Standard. July 18, 2019. https://psmag.com/environment/sunscreen-is-corals-biggest-anemone. Accessed December 23, 2019.
- Luscombe R. Republicans seek to overturn Key West ban on coral-damaging sunscreens. The Guardian. November 9, 2019. https://www.theguardian.com/us-news/2019/nov/09/key-west-sunscreen-coral-reef-backlash-skin-cancer. Accessed December 23, 2019.
- Salazar D. CVS to remove 2 chemicals from 60 store-brand sunscreens. Drug Store News. August 2, 2019. https://drugstorenews.com/retail-news/cvs-to-remove-2-chemicals-from-60-store-brand-sunscreens. Accessed December 23, 2019.
- Sunscreen drug products for over-the-counter human use. Fed Registr. 2019;84(38):6204-6275. To be codified at 21 CFR §201, 310, 347, and 352.
- DeLeo VA. Sunscreen regulations and advice for your patients. Cutis. 2019;103:251-253.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Mayor de Blasio, health officials declare end of measles outbreak in New York City [news release]. New York, NY: City of New York; September 3, 2019. https://www1.nyc.gov/office-of-the-mayor/news/409-19/mayor-de-blasio-health-officials-declare-end-measles-outbreak-new-york-city. Accessed December 23, 2019.
- Health department reports eleven new cases of measles in Brooklyn’s Orthodox Jewish community, urges on time vaccination for all children, especially before traveling to Israel and other countries experiencing measles outbreaks [news release]. New York, NY: City of New York; November 2, 2018. https://www1.nyc.gov/site/doh/about/press/pr2018/pr091-18.page. Accessed December 23, 2019.
- Centers for Disease Control and Prevention. Measles elimination. https://www.cdc.gov/measles/elimination.html. Updated October 4, 2019. Accessed December 23, 2019.
- McKinley J. Measles outbreak: N.Y. eliminates religious exemptions for vaccinations. New York Times. June 13, 2019. https://www.nytimes.com/2019/06/13/nyregion/measles-vaccinations-new-york.html. Accessed December 23, 2019.
- FDA approves Dupixent® (dupilumab) for moderate-to-severe atopic dermatitis in adolescents [news release]. Cambridge, MA: Sanofi; March 11, 2019. http://www.news.sanofi.us/2019-03-11-FDA-approves-Dupixent-R-dupilumab-for-moderate-to-severe-atopic-dermatitis-in-adolescents. Accessed December 23, 2019.
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial [published online ahead of print November 6, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.3336.
- Galderma receives FDA approval for AKLIEF® (trifarotene) cream, 0.005%, the first new retinoid molecule for the treatment of acne in over 20 years [news release]. Fort Worth, TX: Galderma Laboratories, LP; October 4, 2019. https://www.multivu.com/players/English/8613051-galderma-aklief-retinoid-molecule-acne-treatment/. Accessed December 23, 2019.
- Update—Foamix receives FDA approval of AMZEEQ™ topical minocycline treatment for millions of moderate to severe acne sufferers [news release]. Bridgewater, NJ: Foamix Pharmaceuticals Ltd; October 18, 2019. http://www.foamix.com/news-releases/news-release-details/update-foamix-receives-fda-approval-amzeeqtm-topical-minocycline. Accessed December 23, 2019.
- Redfearn S. Clinical trial patient inclusion and exclusion criteria need an overhaul, say experts. CenterWatch website. April 23, 2018. https://www.centerwatch.com/cwweekly/2018/04/23/clinical-trial-patient-inclusion-and-exclusion-criteria-need-an-overhaul-say-experts. Accessed December 23, 2019.
- Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 mug/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80:1691-1699.
- FDA approves OTEZLA® (apremilast) for the treatment of oral ulcers associated with Behçet’s disease [news release]. Summit, NJ: Celgene; July 19, 2019. https://ir.celgene.com/press-releases/press-release-details/2019/FDA-Approves-OTEZLA-apremilast-for-the-Treatment-of-Oral-Ulcers-Associated-with-Behets-Disease/default.aspx. Accessed December 23, 2019.
- Apremilast [package insert]. Summit, NJ: Celgene Corporation; 2019.
- FDA approves first treatment to increase pain-free light exposure in patients with a rare disorder [news release]. Silver Spring, MD: US Food and Drug Administration; October 8, 2019. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-increase-pain-free-light-exposure-patients-rare-disorder. Accessed December 23, 2019.
- Langendonk JG, Balwani M, Anderson KE, et al. Afamelanotide for erythropoietic protoporphyria. N Engl J Med. 2015;373:48-59.
- Light Therapy Mask recall statement. Neutrogena website. https://www.neutrogena.com/light-therapy-statement.html. Accessed December 23, 2019.
- Bromwich JE. Neutrogena recalls Light Therapy Masks, citing risk of eye injury. New York Times. July 18, 2019. https://www.nytimes.com/2019/07/18/style/neutrogena-light-therapy-mask-recall.html. Accessed December 23, 2019, 2019.
- Nguyen T. Neutrogena recalls acne mask over concerns about blue light. Chemical & Engineering News. August 6, 2019. https://cen.acs.org/safety/lab-safety/Neutrogena-recalls-acne-mask-over-concerns-about-blue-light/97/web/2019/08. Accessed November 16, 2019.
- Australian Government Department of Health, Therapeutic Goods Administration. Neutrogena Visibly Clear Light Therapy Acne Mask and Activator: Recall - potential for eye damage. https://www.tga.gov.au/alert/neutrogena-visibly-clear-light-therapy-acne-mask-and-activator. Published July 17, 2019. Accessed December 23, 2019.
- Johnson & Johnson Consumer Inc. to voluntarily recall a single lot of Johnson’s Baby Powder in the United States [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 18, 2019. https://www.factsabouttalc.com/_document/15-new-tests-from-the-same-bottle-of-johnsons-baby-powder-previously-tested-by-fda-find-no-asbestos?id=0000016e-1915-dc68-af7e-df3f147c0000. Accessed December 23, 2019.
- 15 new tests from the same bottle of Johnson’s Baby Powder previously tested by FDA find no asbestos [press release]. New Brunswick, NJ: Johnson & Johnson Consumer Inc; October 29, 2019. https://www.factsabouttalc.com/_document/johnson-johnson-consumer-inc-to-voluntarily-recall-a-single-lot-of-johnsons-baby-powder-in-the-united-states?id=0000016d-debf-d71d-a77d-dfbfebeb0000. Accessed December 23, 2019.
- Hsu T. Johnson & Johnson says recalled baby powder doesn’t have asbestos. New York Times. October 29, 2019. https://www.nytimes.com/2019/10/29/business/johnson-baby-powder-asbestos.html. Accessed December 23, 2019.
Resident Pearls
- Chemical sunscreen made headlines in 2019 due to concerns over coral reef toxicity and systemic absorption in humans.
- With a total of 654 cases, New York City’s largest measles outbreak in nearly 30 years ended in September 2019.
- From dupilumab for adolescent atopic dermatitis to apremilast for Behçet disease, the US Food and Drug Administration approved several therapies for pediatric dermatology and rare dermatologic conditions in 2019.
- Two popular skin care products—the Neutrogena Light Therapy Acne Mask and Johnson’s Baby Powder—were involved in recalls in 2019.
Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
Another Match Day is approaching. Students find themselves paying more each year to apply to one of the most competitive fields, while program directors struggle to sort through hundreds of stellar applications to invite a handful of candidates for interviews. Estimates place the cost of the application process at $5 million in total for all medical school seniors, or roughly $10,000 per applicant.1 Approximately 60% of these costs occur during the interview process.1,2 In an era in which students routinely graduate medical school with hundreds of thousands of dollars of debt, these costs must be addressed as soon as possible.
This problem is not unique to dermatology; otolaryngology, another especially competitive field, has considered various changes to the match process based on applicants’ feedback.3 As an applicant during the 2018-2019 match cycle for dermatology, I offer 2 solutions that are a starting point aimed at streamlining the application process for both applicants and program directors: regional interview coordination and a cap on the number of residency applications.
Regional Interview Coordination
Regional interview coordination would reduce travel costs and facilitate greater predictability in scheduling clinical rotations. In the current climate, it is not uncommon for applicants to make multiple cross-country round trips in the same week, especially given that the interview season for dermatology, including interviews for preliminary programs, now ranges from mid-October to early February. Although affluent applicants may not be concerned with financial costs of frequent travel, all applicants face travel inconveniences that could be mitigated through regional coordination. For example, an applicant invited to multiple interviews in the New York City area could reserve a room in a single hotel over a period of several days. During each interview day, he/she could travel back and forth from that accommodation to each institution without needing to bring luggage, worrying about reaching the airport on time, or missing a pre-interview dinner at a program in a faraway city.
Given the amount of coordination required among programs, it may lead to more positive working relationships among regional dermatology programs. One limitation of this approach is that competitive programs may be unwilling to cooperate. If even one program deviates from the interview time frame, it reduces the incentive for others to participate. Programs must be willing to sacrifice short-term autonomy in interview scheduling for their long-term shared interest in reducing the application burden for students, which is known as a commitment problem in game theory, and could be addressed through joint decision-making that incorporates the time frame preferences of all programs as well as binding commitments on interview dates that are decided before the process begins.4 Another limitation is that inclement weather could affect all regional programs simultaneously. In this case, offering interviews via video conference for affected students may be a solution.
Capping the Number of Applications
A second method of reducing interview costs would be capping the number of applications. Although matched seniors applied to a median of 72 programs, the Association of American Medical Colleges suggests that dermatology applicants can maximize their return on investment (ie, ratio of interviews to applications) by sending 35 to 55 applications depending on US Medical Licensing Examination scores. Attending more than 10 interviews does not meaningfully improve the chance of matching.5,6
Programs have limited capacity for interviews and must judiciously allocate invitations based solely on the information provided through the Electronic Residency Application Service (ERAS). Given the competitiveness of dermatology, applicants usually will accept every interview invitation. Therefore, applicants who are not genuinely interested in a program may crowd out others who are interested. In a survey of otolaryngology applicants (N=150), 90.6% of respondents admitted applying to programs in which they had no specific interest, simply to increase their chance of matching.3 Capping application numbers would force students to apply more selectively and enable residencies to gauge students’ true interest more effectively. In contrast to regional interview coordination, this policy change would be easy to enforce. It also may be popular; nearly two-thirds of otolaryngology applicants agreed to a hypothetical cap on residency applications to reduce the burden on students and programs.3
An alternative to a hard cap on applications could be restructuring the ERAS application fee to incentivize students to apply to fewer programs. For example, a flat fee might cover application numbers up to the point of diminishing returns, after which the price per application could increase exponentially. This approach would have a similar effect of a hard cap and cause many students to apply to fewer programs; however, one notable drawback is that highly affluent applicants would simply absorb the extra cost and still gain a competitive advantage in applying to more programs, which might further decrease the number of lower-income individuals successfully matching into dermatology.
A benefit of decreased application numbers to program directors would be giving them more time to conduct a holistic review of applicants, rather than attempting to weed out candidates through arbitrary cutoffs for US Medical Licensing Examination scores or Alpha Omega Alpha Honor Medical Society membership. The ERAS could allow applicants the option of stating preferences for geographic regions, desired fellowships, areas of research interest, and other intangible metrics. Selection committees could filter their candidate search by different variables and then look at each candidate holistically.
Limitations of capping application numbers include the risk that such a cap would harm less-competitive applicants while failing to address the primary cost drivers (ie, travel costs). The specific cap number would be controversial and may need to be adjusted higher for special cases such as couples matching and international applicants, thus making a cap seem arbitrary.
Final Thoughts
The dermatology residency match can be streamlined to the benefit of both applicants and selection committees. Regional interview coordination would reduce both financial and logistical barriers for applicants but may be difficult to enforce without cooperation from multiple programs. Capping the number of applications, either through a hard cap or an increased financial barrier, would be relatively easy to enforce and might empower selection committees to conduct more detailed, holistic reviews of applicants; however, certain types of applicants may find the application limits detrimental to their chances of matching. These policy recommendations are meant to be a starting point for discussion. Streamlining the application process is critical to improving the diversity of dermatology residencies.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
- Tichy AL, Peng DH, Lane AT. Applying for dermatology residency is difficult and expensive. J Am Acad Dermatol. 2012;66:696-697.
- Ward M, Pingree C, Laury AM, et al. Applicant perspectives on the otolaryngology residency application process. JAMA Otolaryngol Head Neck Surg. 2017;143:782-787.
- North DC. Institutions and credible commitment. J Inst Theor Econ. 1993;149:11-23.
- Association of American Medical Colleges. Apply smart: data to consider when applying to residency. https://students-residents.aamc.org/applying-residency/filteredresult/apply-smart-data-consider-when-applying-residency/. Accessed November 12, 2019.
- Charting Outcomes in the Match: Characteristics of U.S. Allopathic Seniors Who Matched to Their Preferred Specialty in the 2018 Main Residency Match. 2nd ed. Washington, DC: National Resident Matching Program; July 2018. https://www.nrmp.org/wp-content/uploads/2018/06/Charting-Outcomes-in-the-Match-2018-Seniors.pdf. Accessed November 11, 2019.
Don’t Forget These 5 Things When Treating Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a common and debilitating inflammatory disorder of the pilosebaceous unit that presents with recurrent scarring inflammatory nodules and sinus tracts in the intertriginous folds of the body. It is a complex condition that requires multimodal management to address the medical, surgical, and psychosocial needs of affected patients. However, it can be difficult to coordinate all that goes into HS management beyond the standard therapeutic ladder of topical and oral antimicrobials, intralesional corticosteroids, biologics, and surgery. In this article, I will outline 5 important aspects of HS treatment that often are overlooked.
Talk About Pathophysiology
Patients with HS often have limited understanding of their condition. One common misperception is that HS is an infectious disease and that disease activity is associated with poor hygiene.1 Dispelling this myth may help patients avoid unnecessary hygiene practices, decrease perceived stigma, and enhance your therapeutic alliance.
The current model of HS pathophysiology implicates an aberrant inflammatory response to the cutaneous bacterial microbiome, which leads to follicular occlusion and then rupture of debris and bacteria into the surrounding dermis. Immune cells and inflammatory mediators such as nuclear factor κB and tumor necrosis factor α respond to the disruption. Chronic lesions develop due to tissue repair with scarring and re-epithelialization.2,3 Although most patients probably are not interested in the esoteric details, I typically make a point of explaining to patients that HS is a chronic inflammatory disease and provide reassurance that it is not a sign of poor hygiene.
Counsel on Smoking Cessation
Most HS patients use tobacco. As many as 75% of HS patients are active smokers and another 10% to 15% are former smokers. Although there is mixed evidence that disease activity correlates with smoking status, the Hidradenitis Suppurativa Foundation in the United States and Canada concluded in the 2019 North American Clinical Management Guidelines for Hidradenitis Suppurativa that due to the overall health risks of smoking, we should recommend cessation to our patients.4
Laser Hair Removal Works
Don’t forget about laser hair removal! Evidence from randomized controlled trials supports the use of the Nd:YAG laser in the treatment of HS. Treat the entire affected anatomic area and use stacked double pulses on active nodules (typical settings: 10-mm spot size; 10-millisecond pulse duration and 35–50 J/cm2 in Fitzpatrick skin types I–III; 20-millisecond pulse duration and 25–40 J/cm2 in Fitzpatrick skin types IV–VI).4 Especially if it is covered by your patient’s insurance, Nd:YAG is a great adjunctive treatment to consider. The guidelines also recommend long-pulsed alexandrite and diode lasers as well as intense pulsed light, all of which result in follicular destruction, though these treatments have less supporting evidence.4
Have a Plan for Flares
Intralesional injection of triamcinolone is a mainstay of HS treatment and provides patients with rapid relief of symptoms during a flare.5 One case series found that there was a notable decrease in pain, size, and drainage after just 1 day of treatment with intralesional triamcinolone 10 mg/mL (0.2–2.0 mL).6
Intralesional steroid injection is a great tool for quieting an active disease flare while simultaneously instating ongoing treatment for preventive management. However, even when disease control is optimized, patients may still experience intermittent flares of disease. For some patients, it may be appropriate to have a plan in place for a return to clinic during the beginning of a flare to obtain intralesional steroids. The ability to come in on short notice may help avoid visits to the emergency department and urgent care where your patients may receive treatments such as short courses of antibiotics or incision and drainage that may deviate from your overall treatment plan.
Consider Childbearing Status
Don’t forget to consider childbearing plans and childbearing potential when treating female patients with HS. Pregnancy is a frequent consideration in HS patients, as HS affects 3 to 4 times more women than men and typically presents after puberty (second or third decades of life). Many of the medications in the HS armamentarium are contraindicated in pregnancy including tetracyclines, retinoids, and hormonal agents. Surgery should be avoided in pregnant patients whenever possible, particularly in the first trimester. Relatively safe options include topical antibiotics such as clindamycin and metronidazole, as well as tumor necrosis factor α inhibitors, which are classified as category B in pregnancy.5
Before making treatment decisions in pregnant and breastfeeding patients, consult the US Food and Drug Administration recommendations. Perng et al7 reviewed current management strategies for HS in pregnant and breastfeeding women, and their review article in the Journal of the American Academy of Dermatology is an excellent resource.
Final Thoughts
Comprehensive management of HS may include a combination of medication and procedures, lifestyle modification, management of comorbidities, and social support. Formulating a good treatment plan may be a challenge but can drastically improve your patient’s quality of life.
- What is hidradenitis suppurativa? Hidradenitis Suppurativa Foundation website. https://www.hs-foundation.org/what-is-hs. Accessed October 9, 2019.
- Frew JW, Hawkes JE, Krueger JG. Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms. Ther Adv Chronic Dis. 2019;10:2040622319830646. doi:10.1177/2040622319830646
- Lacarrubba F, Musumeci ML, Nasca MR, et al. Double-ended pseudocomedones in hidradenitis suppurativa: clinical, dermoscopic, and histopathological correlation. Acta Derm Venereol. 2017;97:763-764.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989.
Hidradenitis suppurativa (HS) is a common and debilitating inflammatory disorder of the pilosebaceous unit that presents with recurrent scarring inflammatory nodules and sinus tracts in the intertriginous folds of the body. It is a complex condition that requires multimodal management to address the medical, surgical, and psychosocial needs of affected patients. However, it can be difficult to coordinate all that goes into HS management beyond the standard therapeutic ladder of topical and oral antimicrobials, intralesional corticosteroids, biologics, and surgery. In this article, I will outline 5 important aspects of HS treatment that often are overlooked.
Talk About Pathophysiology
Patients with HS often have limited understanding of their condition. One common misperception is that HS is an infectious disease and that disease activity is associated with poor hygiene.1 Dispelling this myth may help patients avoid unnecessary hygiene practices, decrease perceived stigma, and enhance your therapeutic alliance.
The current model of HS pathophysiology implicates an aberrant inflammatory response to the cutaneous bacterial microbiome, which leads to follicular occlusion and then rupture of debris and bacteria into the surrounding dermis. Immune cells and inflammatory mediators such as nuclear factor κB and tumor necrosis factor α respond to the disruption. Chronic lesions develop due to tissue repair with scarring and re-epithelialization.2,3 Although most patients probably are not interested in the esoteric details, I typically make a point of explaining to patients that HS is a chronic inflammatory disease and provide reassurance that it is not a sign of poor hygiene.
Counsel on Smoking Cessation
Most HS patients use tobacco. As many as 75% of HS patients are active smokers and another 10% to 15% are former smokers. Although there is mixed evidence that disease activity correlates with smoking status, the Hidradenitis Suppurativa Foundation in the United States and Canada concluded in the 2019 North American Clinical Management Guidelines for Hidradenitis Suppurativa that due to the overall health risks of smoking, we should recommend cessation to our patients.4
Laser Hair Removal Works
Don’t forget about laser hair removal! Evidence from randomized controlled trials supports the use of the Nd:YAG laser in the treatment of HS. Treat the entire affected anatomic area and use stacked double pulses on active nodules (typical settings: 10-mm spot size; 10-millisecond pulse duration and 35–50 J/cm2 in Fitzpatrick skin types I–III; 20-millisecond pulse duration and 25–40 J/cm2 in Fitzpatrick skin types IV–VI).4 Especially if it is covered by your patient’s insurance, Nd:YAG is a great adjunctive treatment to consider. The guidelines also recommend long-pulsed alexandrite and diode lasers as well as intense pulsed light, all of which result in follicular destruction, though these treatments have less supporting evidence.4
Have a Plan for Flares
Intralesional injection of triamcinolone is a mainstay of HS treatment and provides patients with rapid relief of symptoms during a flare.5 One case series found that there was a notable decrease in pain, size, and drainage after just 1 day of treatment with intralesional triamcinolone 10 mg/mL (0.2–2.0 mL).6
Intralesional steroid injection is a great tool for quieting an active disease flare while simultaneously instating ongoing treatment for preventive management. However, even when disease control is optimized, patients may still experience intermittent flares of disease. For some patients, it may be appropriate to have a plan in place for a return to clinic during the beginning of a flare to obtain intralesional steroids. The ability to come in on short notice may help avoid visits to the emergency department and urgent care where your patients may receive treatments such as short courses of antibiotics or incision and drainage that may deviate from your overall treatment plan.
Consider Childbearing Status
Don’t forget to consider childbearing plans and childbearing potential when treating female patients with HS. Pregnancy is a frequent consideration in HS patients, as HS affects 3 to 4 times more women than men and typically presents after puberty (second or third decades of life). Many of the medications in the HS armamentarium are contraindicated in pregnancy including tetracyclines, retinoids, and hormonal agents. Surgery should be avoided in pregnant patients whenever possible, particularly in the first trimester. Relatively safe options include topical antibiotics such as clindamycin and metronidazole, as well as tumor necrosis factor α inhibitors, which are classified as category B in pregnancy.5
Before making treatment decisions in pregnant and breastfeeding patients, consult the US Food and Drug Administration recommendations. Perng et al7 reviewed current management strategies for HS in pregnant and breastfeeding women, and their review article in the Journal of the American Academy of Dermatology is an excellent resource.
Final Thoughts
Comprehensive management of HS may include a combination of medication and procedures, lifestyle modification, management of comorbidities, and social support. Formulating a good treatment plan may be a challenge but can drastically improve your patient’s quality of life.
Hidradenitis suppurativa (HS) is a common and debilitating inflammatory disorder of the pilosebaceous unit that presents with recurrent scarring inflammatory nodules and sinus tracts in the intertriginous folds of the body. It is a complex condition that requires multimodal management to address the medical, surgical, and psychosocial needs of affected patients. However, it can be difficult to coordinate all that goes into HS management beyond the standard therapeutic ladder of topical and oral antimicrobials, intralesional corticosteroids, biologics, and surgery. In this article, I will outline 5 important aspects of HS treatment that often are overlooked.
Talk About Pathophysiology
Patients with HS often have limited understanding of their condition. One common misperception is that HS is an infectious disease and that disease activity is associated with poor hygiene.1 Dispelling this myth may help patients avoid unnecessary hygiene practices, decrease perceived stigma, and enhance your therapeutic alliance.
The current model of HS pathophysiology implicates an aberrant inflammatory response to the cutaneous bacterial microbiome, which leads to follicular occlusion and then rupture of debris and bacteria into the surrounding dermis. Immune cells and inflammatory mediators such as nuclear factor κB and tumor necrosis factor α respond to the disruption. Chronic lesions develop due to tissue repair with scarring and re-epithelialization.2,3 Although most patients probably are not interested in the esoteric details, I typically make a point of explaining to patients that HS is a chronic inflammatory disease and provide reassurance that it is not a sign of poor hygiene.
Counsel on Smoking Cessation
Most HS patients use tobacco. As many as 75% of HS patients are active smokers and another 10% to 15% are former smokers. Although there is mixed evidence that disease activity correlates with smoking status, the Hidradenitis Suppurativa Foundation in the United States and Canada concluded in the 2019 North American Clinical Management Guidelines for Hidradenitis Suppurativa that due to the overall health risks of smoking, we should recommend cessation to our patients.4
Laser Hair Removal Works
Don’t forget about laser hair removal! Evidence from randomized controlled trials supports the use of the Nd:YAG laser in the treatment of HS. Treat the entire affected anatomic area and use stacked double pulses on active nodules (typical settings: 10-mm spot size; 10-millisecond pulse duration and 35–50 J/cm2 in Fitzpatrick skin types I–III; 20-millisecond pulse duration and 25–40 J/cm2 in Fitzpatrick skin types IV–VI).4 Especially if it is covered by your patient’s insurance, Nd:YAG is a great adjunctive treatment to consider. The guidelines also recommend long-pulsed alexandrite and diode lasers as well as intense pulsed light, all of which result in follicular destruction, though these treatments have less supporting evidence.4
Have a Plan for Flares
Intralesional injection of triamcinolone is a mainstay of HS treatment and provides patients with rapid relief of symptoms during a flare.5 One case series found that there was a notable decrease in pain, size, and drainage after just 1 day of treatment with intralesional triamcinolone 10 mg/mL (0.2–2.0 mL).6
Intralesional steroid injection is a great tool for quieting an active disease flare while simultaneously instating ongoing treatment for preventive management. However, even when disease control is optimized, patients may still experience intermittent flares of disease. For some patients, it may be appropriate to have a plan in place for a return to clinic during the beginning of a flare to obtain intralesional steroids. The ability to come in on short notice may help avoid visits to the emergency department and urgent care where your patients may receive treatments such as short courses of antibiotics or incision and drainage that may deviate from your overall treatment plan.
Consider Childbearing Status
Don’t forget to consider childbearing plans and childbearing potential when treating female patients with HS. Pregnancy is a frequent consideration in HS patients, as HS affects 3 to 4 times more women than men and typically presents after puberty (second or third decades of life). Many of the medications in the HS armamentarium are contraindicated in pregnancy including tetracyclines, retinoids, and hormonal agents. Surgery should be avoided in pregnant patients whenever possible, particularly in the first trimester. Relatively safe options include topical antibiotics such as clindamycin and metronidazole, as well as tumor necrosis factor α inhibitors, which are classified as category B in pregnancy.5
Before making treatment decisions in pregnant and breastfeeding patients, consult the US Food and Drug Administration recommendations. Perng et al7 reviewed current management strategies for HS in pregnant and breastfeeding women, and their review article in the Journal of the American Academy of Dermatology is an excellent resource.
Final Thoughts
Comprehensive management of HS may include a combination of medication and procedures, lifestyle modification, management of comorbidities, and social support. Formulating a good treatment plan may be a challenge but can drastically improve your patient’s quality of life.
- What is hidradenitis suppurativa? Hidradenitis Suppurativa Foundation website. https://www.hs-foundation.org/what-is-hs. Accessed October 9, 2019.
- Frew JW, Hawkes JE, Krueger JG. Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms. Ther Adv Chronic Dis. 2019;10:2040622319830646. doi:10.1177/2040622319830646
- Lacarrubba F, Musumeci ML, Nasca MR, et al. Double-ended pseudocomedones in hidradenitis suppurativa: clinical, dermoscopic, and histopathological correlation. Acta Derm Venereol. 2017;97:763-764.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989.
- What is hidradenitis suppurativa? Hidradenitis Suppurativa Foundation website. https://www.hs-foundation.org/what-is-hs. Accessed October 9, 2019.
- Frew JW, Hawkes JE, Krueger JG. Topical, systemic and biologic therapies in hidradenitis suppurativa: pathogenic insights by examining therapeutic mechanisms. Ther Adv Chronic Dis. 2019;10:2040622319830646. doi:10.1177/2040622319830646
- Lacarrubba F, Musumeci ML, Nasca MR, et al. Double-ended pseudocomedones in hidradenitis suppurativa: clinical, dermoscopic, and histopathological correlation. Acta Derm Venereol. 2017;97:763-764.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90.
- Alikhan A, Sayed C, Alavi A, et al. North American Clinical Management Guidelines for Hidradenitis Suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations. part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
- Riis PT, Boer J, Prens EP, et al. Intralesional triamcinolone for flares of hidradenitis suppurativa (HS): a case series. J Am Acad Dermatol. 2016;75:1151-1155.
- Perng P, Zampella JG, Okoye GA. Management of hidradenitis suppurativa in pregnancy. J Am Acad Dermatol. 2017;76:979-989.
Resident Pearls
- Medical treatment of hidradenitis suppurativa (HS) can be relatively straightforward, but optimal comprehensive management is multifaceted.
- Educate patients about pathophysiology, counsel on smoking cessation, remember laser hair removal, consider an ongoing plan for addressing flares, and think about childbearing status when treating HS patients.
Current Controversies in Mohs Micrographic Surgery
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Resident Pearl:
• Further investigation is needed to elucidate and optimize solutions to current controversies in Mohs micrographic surgery.