User login
Severe rash after COVID-19 vaccination
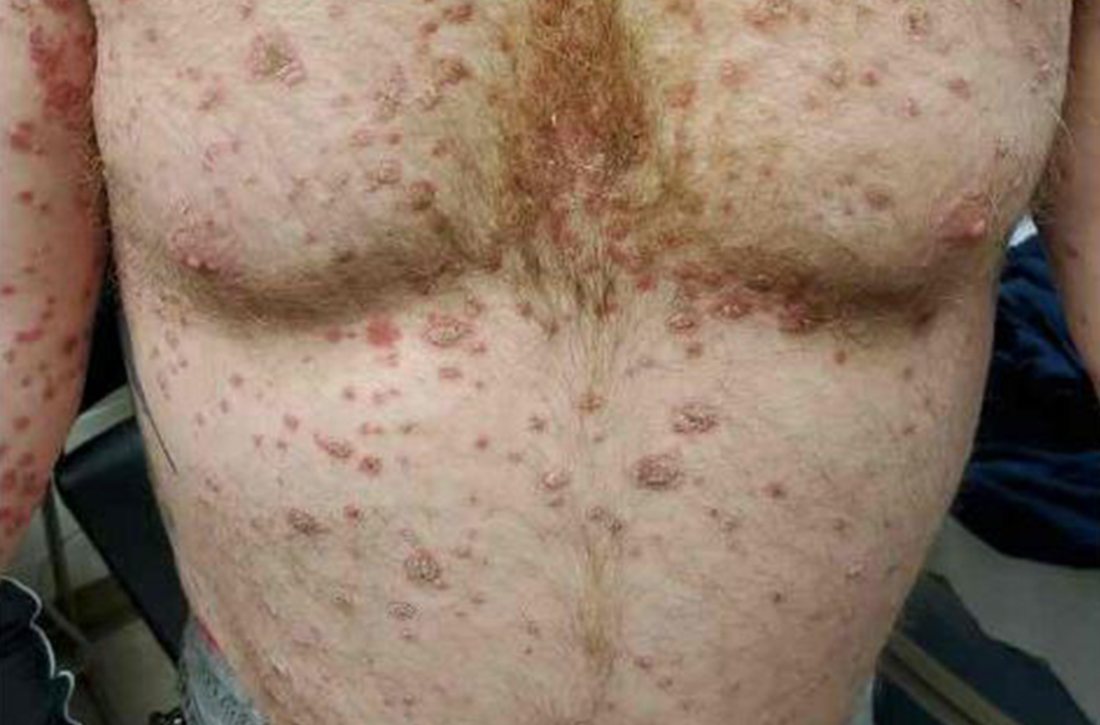
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
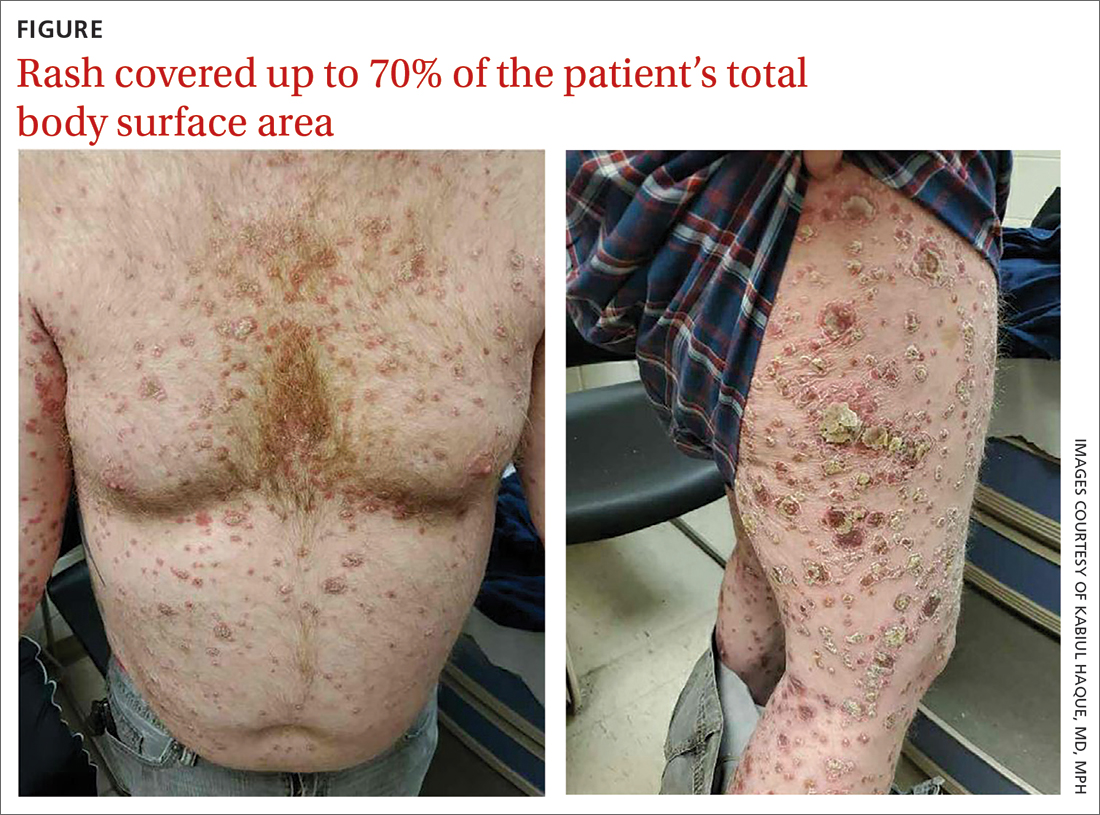
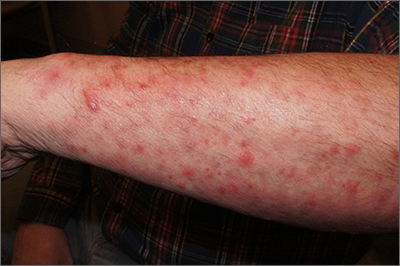
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
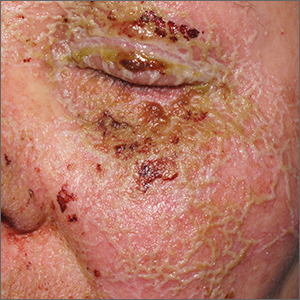
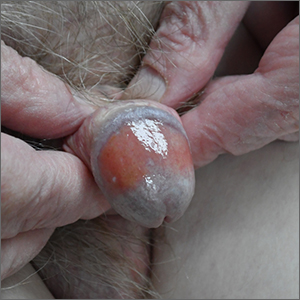
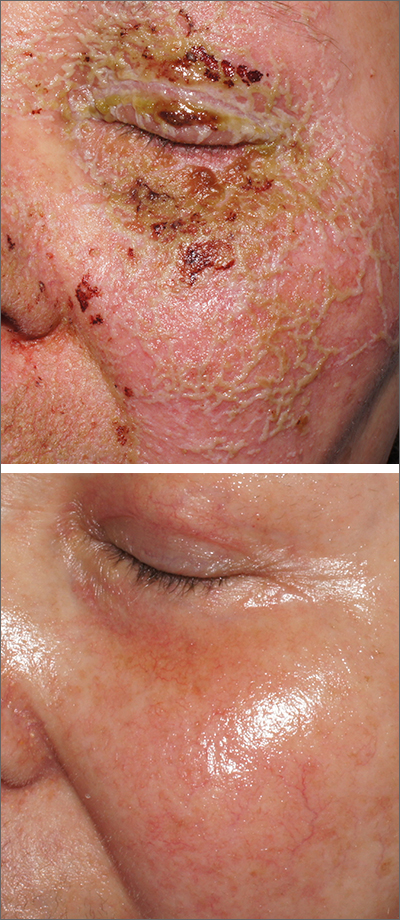
Itchy pustules over hair follicles
A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A potassium hydroxide (KOH) preparation of pus and dry superficial skin taken from 1 of the pustules revealed multiple hyphae and confirmed a diagnosis of nodular granulomatous perifolliculitis, also called Majocchi granuloma.
Majocchi granuloma is a reactive process of inflammation caused by infection of the follicular unit(s) by a dermatophyte—most often the same Trichophyton species responsible for more superficial tinea. On exam, there may be a solitary papule, pustule, or nodule. More often, there are multiple papules and pustules grouped within an annular plaque in hair-bearing areas on the head, trunk, or extremities. Majocchi granuloma can occur in patients who are healthy and those who are immunosuppressed.1 It can also occur when a topical steroid is applied to unsuspected tinea, as occurred here. In this case, the patient was accustomed to having multiple skin plaques of psoriasis and assumed this was a stubborn manifestation of that.
Because the infection penetrates deeper than most topical therapies can effectively reach at adequate concentrations, systemic medications are the treatments of choice. Terbinafine, itraconazole, and fluconazole are all effective options but need to be used for several weeks to be effective.
This patient received terbinafine 250 mg/d for 6 weeks and the pustules cleared completely. He continued with his other psoriasis medications throughout his treatment.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
1. İlkit M, Durdu M, Karakaş M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457. doi: 10.3109/13693786.2012.669503
Pustules on face
A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

A review of the patient’s chemotherapy medications revealed that 4 weeks earlier, panitumumab had been added to her folinic acid, fluorouracil, and irinotecan (FOLFIRI) regimen. The physician diagnosed this acneiform eruption as an adverse effect of the panitumumab.
Panitumumab is a monoclonal antibody that works to inhibit epidermal growth factor receptor (EGFR) proteins that are overexpressed on some solid tumors and responsible for cancer cell proliferation. EGFR inhibitor–induced acneiform eruptions are common in patients receiving panitumumab.
EGFR proteins have been a target of chemotherapy since the approval of the small molecule erlotinib in 2004. Panitumumab and cetuximab are monoclonal antibodies targeting EGFR and improve long-term survival in patients with metastatic colorectal cancer when added to other standard chemotherapy regimens. EGFR is found throughout the epidermis and all EGFR inhibitors may cause unique skin toxicity not seen with other chemotherapy agents. In 1 study of 229 patients, 59% of patients exhibited skin toxicity at Day 15; the most common examples included widespread acne-like papules and pustules or an eczema-like manifestation.1 Eruptions may be worsened by significant sun exposure while on panitumumab. In this case, the acneiform eruption occurred more intensely along visible facial telangiectasias.
When EGFR inhibitor–induced acneiform eruption occurs, patients commonly develop skin toxicity within the first 2 to 4 weeks of therapy. Pre-therapy doxycycline or minocycline and/or topical steroids may help prevent toxicities from occurring. These same therapies may be used to treat symptoms after they have occurred. More severe cases with systemic symptoms or failure to improve with the above measures may need prednisone or cessation of therapy.
This patient was started on topical hydrocortisone 2.5% ointment twice daily and oral doxycycline 100 mg bid for 6 weeks. She had dramatic improvement within 3 weeks. Doxycycline was subsequently continued at a dose of 100 mg/d and the patient was able to continue with her chemotherapy combination for several more months. Unfortunately, her colon cancer progressed despite therapy and she ultimately died from cancer-related complications.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
1. Bouché O, Ben Abdelghani M, Labourey JL, et al. Management of skin toxicities during panitumumab treatment in metastatic colorectal cancer. World J Gastroenterol. 2019;25:4007-4018. doi: 10.3748/wjg.v25.i29.4007
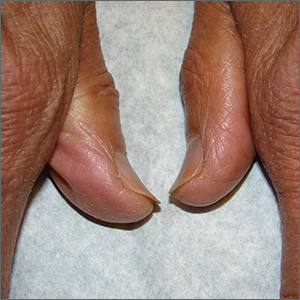
Brittle fingernails
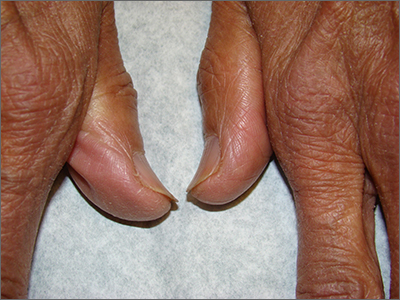
The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610

The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The abnormal upward curve to the fingernails was consistent with a diagnosis of koilonychia—otherwise known as spoon nails.
Koilonychia is an abnormal nail growth pattern where the distal nail matrix is depressed below its normal level, resulting in the spoon shape. The reverse, where the distal nail matrix is elevated in contrast to the proximal nail matrix, results in clubbing.1
There are multiple factors and diseases that result in koilonychia, including lichen planus, psoriasis, nutritional deficiencies (including iron deficiency anemia), and endocrinopathies.1 Lichen planus, which can cause koilonychia, often affects multiple nails and can also cause an associated central ridge pattern. Psoriasis may display a range of nail abnormalities; these include koilonychia, pitting onycholysis, and oil staining.
This patient did not have any signs or symptoms of psoriasis or lichen planus of her nails or skin. A review of her laboratory tests on file made no mention of anemia. Her chemistry profile—including liver tests, renal function tests, and protein levels—were all normal except for glucose levels, which was consistent with her prediabetes. Her thyroid function was also normal. No additional testing was performed since she had no symptoms, physical exam findings, or laboratory clues that pointed to other diseases or systemic processes.
The patient was advised to pick up over-the-counter nail strengtheners and to keep her fingernails trimmed short to minimize the likelihood of painful distal splitting that often occurs with brittle nails. Her physician advised her to follow up with the primary care team if she developed any new signs or symptoms.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610
1. Walker J, Baran R, Vélez N, et al. Koilonychia: an update on pathophysiology, differential diagnosis and clinical relevance. J Eur Acad Dermatol Venereol. 2016;30:1985-1991. doi: 10.1111/jdv.13610
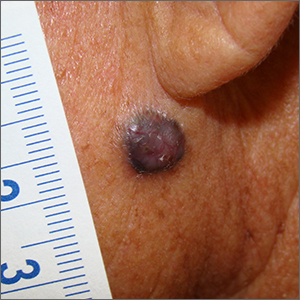
Dark facial lesion
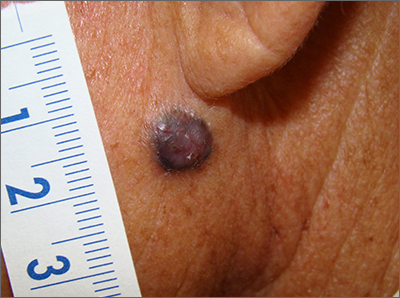
Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Although an elevated and pigmented lesion should be considered for possible melanoma, this one had prominent telangiectasias and was proven to be a basal cell carcinoma (BCC) on biopsy.
While the literature often focuses on light-colored skin types and the high risk of skin cancers, individuals with darker skin can also get melanoma and nonmelanoma skin cancer. Half of the BCCs in African American people are pigmented BCCs, compared to less than 10% for Caucasian individuals. Individuals who are Hispanic have twice the likelihood of pigmented BCCs as those who are Caucasian.1 Pigmented BCCs manifest as darker lesions, as occurred in this individual. Nonpigmented BCCs tend to be pink or pale in color.
Typically, superficial and very small, nodular BCCs can be successfully treated with 2 cycles of electrodesiccation and curettage. EDC should, however, be avoided in low-risk BCCs when these lesions occur in areas of secondary hair growth, such as the beard or scalp. This is because the epidermis follows the hair follicle, and in sites with deep hair follicles, EDC would have to get down to the subcutis to effectively clear the tumor.
For larger, nodular BCCs, full-thickness excision with adequate margins is warranted. For high-risk types, and those in high-risk areas near the nose, eyes, mouth, and ears, Mohs micrographic surgery is recommended to maximize the likelihood of complete excision while minimizing the loss of normal tissue.
Since the physician suspected this was a pigmented BCC, he performed a superficial shave biopsy on a small representative area of the lesion for diagnosis. This patient’s biopsy confirmed a nodular-type pigmented BCC. The lesion was removed in the office with 5-mm margins oriented along the resting skin tension lines with good closure and cosmetic results.
The patient was advised to have routine skin evaluations every 6 months due to the high risk of additional cancers. He was also advised to take oral niacinamide 500 mg twice daily, which can reduce the risk of actinic keratoses and nonmelanoma skin cancers by 15% and 23%, respectively, in those who have had lesions.2
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
1. Higgins S, Nazemi A, Chow M, et al. Review of nonmelanoma skin cancer in African Americans, Hispanics, and Asians. Dermatol Surg. 2018;44:903-910. doi: 10.1097/DSS.0000000000001547
2. Starr P. Oral nicotinamide prevents common skin cancers in high-risk patients, reduces costs. Am Health Drug Benefits. 2015;8(spec issue):13-14.
Acute unilateral visual disturbance
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
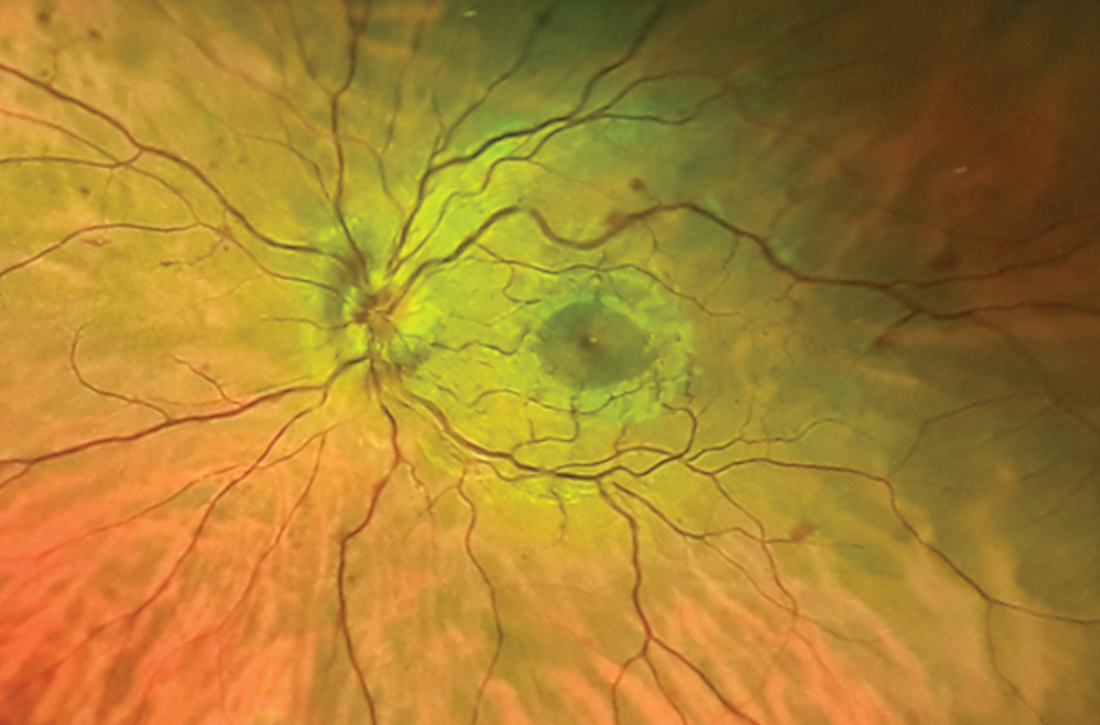
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).
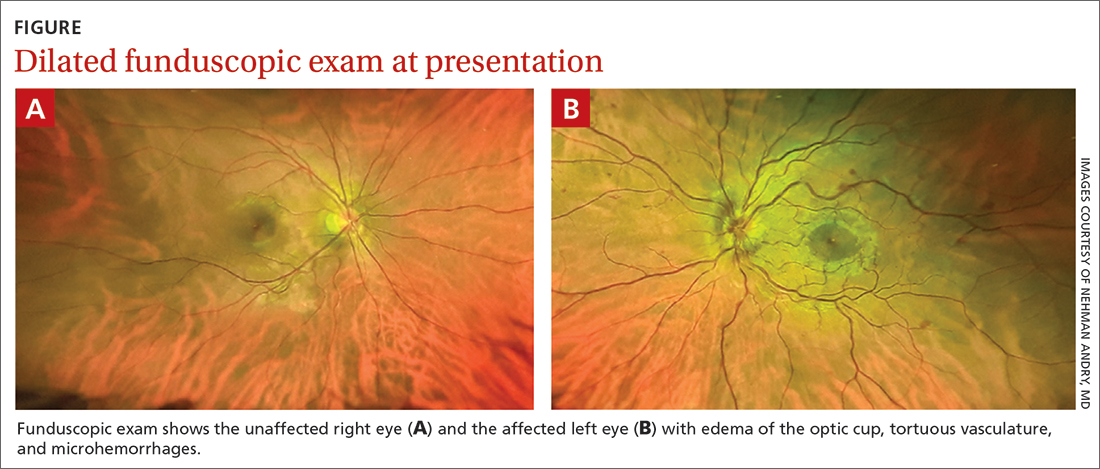
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
A previously healthy 37-year-old runner presented to his primary care physician with acute-onset floaters and scotoma in his left eye, which he first noticed less than 24 hours earlier. He denied eye pain, diplopia, headache, fever, chills, slurred speech, weakness, or other focal neurologic deficits. His vital signs were normal.
Despite the acute visual disturbances, visual acuity was 20/20 in both eyes with corrective lenses; pupils were equal, round, and reactive to light and accommodation; and extraocular movements were intact. On a dilated funduscopic exam, the physician discovered edema of the optic cup, tortuous vasculature, and microhemorrhages in the left eye (FIGURE).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Central retinal vein occlusion
The patient was given a diagnosis of central retinal vein occlusion (CRVO). In this condition, a blockage causes the central retinal vein to leak blood and excess fluid into the retina. This fluid can collect in the macula, leading to visual disturbance.
Retinal vein occlusion is the second most common retinal vascular disease in the United States and is one of the most common causes of vision loss in the elderly.1 Advancing age (≥ 70 years), increasing mean arterial blood pressure, and retinal atherosclerotic signs (focal narrowing, arteriovenous nicking, and opacification) are significant predictors of retinal vein occlusion.2 Other risk factors include diabetes, hyperlipidemia, cardiovascular disease, smoking, obesity, hypercoagulable state, and glaucoma.3-7 However, retinal vein occlusion may also occur in younger, healthier patients who lack the aforementioned risk factors. In such cases, thrombophilic risk factors should be considered.8
CRVO is classified as either ischemic or nonischemic (perfused) based on retinal angiography. More than 80% of CRVO cases are nonischemic,9 of which the majority has visual acuity better than 20/400, mild or no pupillary defect, and mild, unilateral visual changes.10 Nonischemic CRVO can progress to ischemic CRVO, which can result in permanent vision loss. Visual outcome is good in nonischemic CRVO and poor in ischemic CRVO.11 Early detection of poor prognostic features, such as macular edema and neovascularization, is essential for minimizing the risk for permanent damage.12
Dilated funduscopic exam of a patient with CRVO may reveal widespread retinal hemorrhages, markedly dilated and tortuous retinal vessels, cotton wool spots, optic disc or macular edema, and/or vitreous hemorrhages.10
Differential includes varied conditions that can affect vision
CRVO may manifest similarly to the following:
Proliferative diabetic retinopathy can manifest with retinal edema or vitreous and retinal hemorrhages, which also are seen in CRVO.13 Macular edema, retinal hemorrhage, and neovascularization on the optic disc or retinal surface also may be seen on funduscopy in proliferative diabetic retinopathy.14 However, proliferative diabetic retinopathy is often bilateral and gradual in onset in patients with longstanding, uncontrolled diabetes.
Continue to: Hyperviscosity retinopathy
Hyperviscosity retinopathy, which is commonly caused by plasma cell and erythrocyte disorders, also manifests similarly to CRVO. Two noticeable differences include its bilateral presentation and Roth spots, neither of which are commonly seen in CRVO. In addition to visual abnormalities, mucosal bleeding and neurologic abnormalities complete the classic triad of hyperviscosity.15
Ocular ischemic syndrome is often confused with diabetic retinopathies and CRVO on funduscopy. However, patients with this condition may have narrowed retinal arteries, perifoveal telangiectasias, and periorbital pain—findings rarely seen in CRVO.16 Because ocular ischemic syndrome is a manifestation of severe carotid artery atherosclerosis, constitutional symptoms also may be present.
The work-up
When CRVO is suspected, an extensive laboratory work-up is necessary to determine the underlying etiology, including: blood pressure, electrocardiogram, complete blood count, random glucose level, electrolytes, lipid panel, plasma protein electrophoresis, thyroid function tests, and inflammatory markers.1
Additional testing may be required for younger patients who lack vasculopathic risk factors, who have bilateral CRVO, or who have a personal or family history of thrombosis.1 These patients should be screened for thrombophilia, hypercoagulable disorders, and homocysteinuria.1
Cases of CRVO have been linked to dehydration as well, with acute vision changes occurring after strenuous exercise, excessive vomiting, or extended periods of fasting.17-19
Continue to: Treatment may include injections, surgery, or nothing at all
Treatment may include injections, surgery, or nothing at all
Currently, there are no proven treatments to reopen occluded retinal veins. Thus, management is directed at complications that contribute to vision loss, including macular edema and neovascularization.20-21 Intravitreal anti-vascular endothelial growth factor (VEGF) agents are recognized as first-line therapy for macular edema in numerous studies.22-26 Intravitreal corticosteroids are an alternative treatment for patients with macular edema who do not respond to anti-VEGF therapy; however, monitoring is required as these corticosteroids increase the risk for glaucoma and cataract formation.27 In patients with CRVO with neovascularization, panretinal laser photocoagulation may be used.28
Observation and monitoring for the development of complications, rather than initiation of treatment, is appropriate for patients with CRVO without macular edema or neovascularization, with follow-up intervals and duration dictated by the severity of visual loss and whether the CRVO was ischemic or nonischemic.
Our patient’s diagnosis was confirmed by retinal specialists with optic coherence tomography, gonioscopy, and fluorescein angiography. He underwent an extensive laboratory work-up and hypercoagulation studies to determine the etiology. All results returned within normal limits with the exception of a nonspecific pattern found on serum protein electrophoresis that suggested dehydration.
Given his negative hypercoagulation studies, normal laboratory values, and new exercise regimen, dehydration was concluded to be the likely etiology. Since his visual acuity was not affected, observation with bimonthly follow-up for 6 months was the management strategy. He was also encouraged to maintain adequate hydration during exercise. His vision returned to normal 2 weeks after the initial event, and he did not have recurrence during the monitoring period.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
1. Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031-1038. doi: 10.1038/eye.2016.111
2. Cugati S, Wang JJ, Rochtchina E, et al. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726. doi: 10.1001/archopht.124.5.726
3. O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;126:692-699. doi: 10.1001/archopht.126.5.692
4. Hayreh SS, Zimmerman B, McCarthy MJ, et al. Systemic diseases associated with various types of retinal vein occlusion. Am J Ophthalmol. 2001;131:61-77. doi: 10.1016/s0002-9394(00)00709-1
5. Janssen MC, den Heijer M, Cruysberg JR, et al. Retinal vein occlusion: a form of venous thrombosis or a complication of atherosclerosis? A meta-analysis of thrombophilic factors. Thromb Haemost. 2005;93:1021-1026. doi: 10.1160/TH04-11-0768
6. Rehak M, Rehak J, Müller M, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors. Case-control study and meta-analysis. Thromb Haemost. 2008;99:925-929. doi: 10.1160/TH07-11-0658
7. Yin X, Li J, Zhang B, et al. Association of glaucoma with risk of retinal vein occlusion: a meta-analysis. Acta Ophthalmol. 2019;97:652-659. doi: 10.1111/aos.14141
8. Rehak M, Krcova V, Slavik L, et al. The role of thrombophilia in patients with retinal vein occlusion and no systemic risk factors. Can J Ophthalmol. 2010;45:171-175. doi: 10.3129/i09-273
9. Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrent and demographic characteristics. Am J Ophthalmol. 1994;117:429-441. doi: 10.1016/s0002-9394(14)70001-7
10. Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201-217. doi: 10.1007/BF00920022
11. Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011;118:119-133. doi: 10.1016/j.ophtha.2010.04.019
12. Bakri SJ, Berrocal A, Capone A, et al. Retina health series: central retinal vein occlusion. American Society of Retina Specialists. January 2020. Accessed April 16, 2021. www.asrs.org/content/documents/fact-sheet-21-central-retinal-vein-occlusion-2020_1_asrs.pdf
13. Columbia University Department of Ophthalmology. Proliferative diabetic retinopathy (PDR). Accessed July 2, 2021. www.columbiaeye.org/education/digital-reference-of-ophthalmology/vitreous-retina/retinal-vascular-diseases/proliferative-diabetic-retinopathy-pdr
14. Mehta S. Diabetic retinopathy. Merck Manual Professional Version. Updated June 2021. Accessed July 11, 2021. www.merckmanuals.com/professional/eye-disorders/retinal-disorders/diabetic-retinopathy
15. Gertz MA. Acute hyperviscosity: syndromes and management. Blood 2018;132:1379-1385. doi: 10.1182/blood-2018-06-846816
16. Terelak-Borys B, Skonieczna K, Grabska-Liberek I. Ocular ischemic syndrome—a systematic review. Med Sci Monit. 2012;18: RA138-RA144. doi: 10.12659/msm.883260
17. Moisseiev E, Sagiv O, Lazar M. Intense exercise causing central retinal vein occlusion in a young patient: case report and review of the literature. Case Rep Ophthalmol. 2014;5:116-120. doi: 10.1159/000360904.
18. Weiss KD, Kuriyan AE, Flynn HW Jr. Central retinal vein occlusion after prolonged vomiting and repeated valsalva maneuvers associated with gastroenteritis and dehydration. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e23-e25. doi: 10.3928/23258160-20140331-03
19. Jacobs DJ, Flynn HW, Pathengay A, et al. Central retinal vein occlusion after intense exercise: response to intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2011;42:e59-e62. doi: 10.3928/15428877-20110623-02
20. Mohamed Q, McIntosh RL, Saw SM, et al. Interventions for central retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2007;114:507-524. doi: 10.1016/j.ophtha. 2006.11.011
21. Berker N, Batman C. Surgical treatment of central retinal vein occlusion. Acta Ophthalmol. 2008;86:245-252. doi: 10.1111/j.1755-3768.2007.01144.x
22. Braithwaite T, Nanji AA, Greenberg PB. Anti-vascular endothelial growth factor for macular edema secondary to central retinal vein occlusion. Cochrane Database Syst Rev. 2010;10:CD007325. doi: 10.1002/14651858.CD007325.pub2
23. Brown DM, Campochiaro PA, Singh RP, et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1124-1133. doi: 10.1016/j.ophtha.2010.02.022
24. Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118:2041-2049. doi: 10.1016/j.ophtha.2011. 02.038
25. Prasad AG, Schadlu R, Apte RS. Intravitreal pharmacotherapy: applications in retinal disease. Compr Ophthalmol Update. 2007; 8:259-269.
26. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429-437. doi: 10.1016/j.ajo.2012.09.026
27. Ip MS, Scott IU, VanVeldhuisen PC, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 5. Arch Ophthalmol. 2009;127:1101-1114. doi: 10.1001/archophthalmol.2009.234
28. The Central Vein Occlusion Study Group. A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion. The Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434-1444.
Painful axillary lesions
The patient’s recurrent indurated nodules under her arms and other intertriginous areas, often draining pus, are consistent with a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic inflammatory and suppurative skin condition that primarily involves the sweat glands.1 The most commonly affected sites are intertriginous areas that include the axillae, groin, and perianal and inframammary regions.2 Prevalence of this disorder ranges from 0.05% to 4.1% of the population with an onset from puberty to adulthood, usually at around 40 years of age.3 Its incidence is twice as high in women as men and is more common in Black individuals.4,5
While its pathogenesis is not fully understood, it’s believed that excess proliferation of keratinocytes contributes to occlusion, leading to plugging of hair follicle ducts. Hormones, smoking, and obesity may contribute to and exacerbate HS. Intertriginous areas are prone to friction, leading to inflammation and further clogging.
The inflammation evolves into a chronic foreign body-type granulomatous inflammation with the potential for rupture, tunneling, and draining sinuses, which, although malodorous, are sterile, separating HS from an infected abscess.5 The result is thick, dense, scarred tissue.
The diagnosis is clinical in nature, with the history and physical exam distinguishing it from other skin disorders. In addition to the recurring physical pain, there is the emotional distress and self-consciousness about the drainage, odor, and scarring. This particular patient said that she avoided wearing sleeveless shirts due to the lesions’ appearance.
Treatment is multifactorial. Smoking and obesity are contributory factors, so smoking cessation and weight loss are recommended. For very mild HS, topical clindamycin 1% twice daily may suffice, but usually, due to the amount of inflammation, oral antibiotics are the initial therapy. (The use of antibiotics is for their anti-inflammatory component, as the nodules and unruptured tracts are sterile.)
Doxycycline 100 mg twice daily is the usual starting systemic antibiotic. In more severe or resistant cases, a combination of clindamycin and rifampin 300 mg each twice daily is used. (Worth noting: Rifampin interacts with oral contraceptives and many of these patients are women of reproductive age.) Treatment length is usually long (10 to 12 weeks) and recurrence is common.3
Spironolactone 100 mg daily and metformin 1000 mg extended release daily, which reduces insulin resistance, may be helpful. Intralesional injections of 10 mg/mL of triamcinolone in sterile saline can relieve the painful inflamed tracts. Referral for biologic agents, including infliximab, may be needed in severe cases that do not respond to other measures. Although invasive, wide debridement of the diseased tissue can reduce the disease burden.6
This particular patient said that she’d stopped smoking 3 years earlier and would work on losing weight. She was prescribed topical clindamycin 1% lotion twice daily along with oral clindamycin and rifampin dosed as above for 3 months. She declined metformin and intralesional injections. At a follow-up appointment 3 weeks later, she was pleased with the decrease in inflammation and had only 1 remaining tender area of fluctuance. She again declined injections and planned to continue on her oral and topical antibiotics.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Derissa F. Raynold, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi: 10.1016/j.jaad.2006.08.061
2. Storer MA, Danesh MJ, Sandhu ME, et al. An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int J Womens Dermatol. 2018;4:198-202. doi: 10.1016/j.ijwd.2018.08.009
3. Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi: 10.1001/jama.2017.16691
4. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268. doi: 10.2340/00015555-0866
5. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332. doi: 10.2340/00015555-1082
6. Caposiena Caro RD, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035

The patient’s recurrent indurated nodules under her arms and other intertriginous areas, often draining pus, are consistent with a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic inflammatory and suppurative skin condition that primarily involves the sweat glands.1 The most commonly affected sites are intertriginous areas that include the axillae, groin, and perianal and inframammary regions.2 Prevalence of this disorder ranges from 0.05% to 4.1% of the population with an onset from puberty to adulthood, usually at around 40 years of age.3 Its incidence is twice as high in women as men and is more common in Black individuals.4,5
While its pathogenesis is not fully understood, it’s believed that excess proliferation of keratinocytes contributes to occlusion, leading to plugging of hair follicle ducts. Hormones, smoking, and obesity may contribute to and exacerbate HS. Intertriginous areas are prone to friction, leading to inflammation and further clogging.
The inflammation evolves into a chronic foreign body-type granulomatous inflammation with the potential for rupture, tunneling, and draining sinuses, which, although malodorous, are sterile, separating HS from an infected abscess.5 The result is thick, dense, scarred tissue.
The diagnosis is clinical in nature, with the history and physical exam distinguishing it from other skin disorders. In addition to the recurring physical pain, there is the emotional distress and self-consciousness about the drainage, odor, and scarring. This particular patient said that she avoided wearing sleeveless shirts due to the lesions’ appearance.
Treatment is multifactorial. Smoking and obesity are contributory factors, so smoking cessation and weight loss are recommended. For very mild HS, topical clindamycin 1% twice daily may suffice, but usually, due to the amount of inflammation, oral antibiotics are the initial therapy. (The use of antibiotics is for their anti-inflammatory component, as the nodules and unruptured tracts are sterile.)
Doxycycline 100 mg twice daily is the usual starting systemic antibiotic. In more severe or resistant cases, a combination of clindamycin and rifampin 300 mg each twice daily is used. (Worth noting: Rifampin interacts with oral contraceptives and many of these patients are women of reproductive age.) Treatment length is usually long (10 to 12 weeks) and recurrence is common.3
Spironolactone 100 mg daily and metformin 1000 mg extended release daily, which reduces insulin resistance, may be helpful. Intralesional injections of 10 mg/mL of triamcinolone in sterile saline can relieve the painful inflamed tracts. Referral for biologic agents, including infliximab, may be needed in severe cases that do not respond to other measures. Although invasive, wide debridement of the diseased tissue can reduce the disease burden.6
This particular patient said that she’d stopped smoking 3 years earlier and would work on losing weight. She was prescribed topical clindamycin 1% lotion twice daily along with oral clindamycin and rifampin dosed as above for 3 months. She declined metformin and intralesional injections. At a follow-up appointment 3 weeks later, she was pleased with the decrease in inflammation and had only 1 remaining tender area of fluctuance. She again declined injections and planned to continue on her oral and topical antibiotics.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Derissa F. Raynold, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The patient’s recurrent indurated nodules under her arms and other intertriginous areas, often draining pus, are consistent with a diagnosis of hidradenitis suppurativa (HS).
HS is a chronic inflammatory and suppurative skin condition that primarily involves the sweat glands.1 The most commonly affected sites are intertriginous areas that include the axillae, groin, and perianal and inframammary regions.2 Prevalence of this disorder ranges from 0.05% to 4.1% of the population with an onset from puberty to adulthood, usually at around 40 years of age.3 Its incidence is twice as high in women as men and is more common in Black individuals.4,5
While its pathogenesis is not fully understood, it’s believed that excess proliferation of keratinocytes contributes to occlusion, leading to plugging of hair follicle ducts. Hormones, smoking, and obesity may contribute to and exacerbate HS. Intertriginous areas are prone to friction, leading to inflammation and further clogging.
The inflammation evolves into a chronic foreign body-type granulomatous inflammation with the potential for rupture, tunneling, and draining sinuses, which, although malodorous, are sterile, separating HS from an infected abscess.5 The result is thick, dense, scarred tissue.
The diagnosis is clinical in nature, with the history and physical exam distinguishing it from other skin disorders. In addition to the recurring physical pain, there is the emotional distress and self-consciousness about the drainage, odor, and scarring. This particular patient said that she avoided wearing sleeveless shirts due to the lesions’ appearance.
Treatment is multifactorial. Smoking and obesity are contributory factors, so smoking cessation and weight loss are recommended. For very mild HS, topical clindamycin 1% twice daily may suffice, but usually, due to the amount of inflammation, oral antibiotics are the initial therapy. (The use of antibiotics is for their anti-inflammatory component, as the nodules and unruptured tracts are sterile.)
Doxycycline 100 mg twice daily is the usual starting systemic antibiotic. In more severe or resistant cases, a combination of clindamycin and rifampin 300 mg each twice daily is used. (Worth noting: Rifampin interacts with oral contraceptives and many of these patients are women of reproductive age.) Treatment length is usually long (10 to 12 weeks) and recurrence is common.3
Spironolactone 100 mg daily and metformin 1000 mg extended release daily, which reduces insulin resistance, may be helpful. Intralesional injections of 10 mg/mL of triamcinolone in sterile saline can relieve the painful inflamed tracts. Referral for biologic agents, including infliximab, may be needed in severe cases that do not respond to other measures. Although invasive, wide debridement of the diseased tissue can reduce the disease burden.6
This particular patient said that she’d stopped smoking 3 years earlier and would work on losing weight. She was prescribed topical clindamycin 1% lotion twice daily along with oral clindamycin and rifampin dosed as above for 3 months. She declined metformin and intralesional injections. At a follow-up appointment 3 weeks later, she was pleased with the decrease in inflammation and had only 1 remaining tender area of fluctuance. She again declined injections and planned to continue on her oral and topical antibiotics.
Photo courtesy of Daniel Stulberg, MD, FAAFP. Text courtesy of Derissa F. Raynold, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi: 10.1016/j.jaad.2006.08.061
2. Storer MA, Danesh MJ, Sandhu ME, et al. An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int J Womens Dermatol. 2018;4:198-202. doi: 10.1016/j.ijwd.2018.08.009
3. Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi: 10.1001/jama.2017.16691
4. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268. doi: 10.2340/00015555-0866
5. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332. doi: 10.2340/00015555-1082
6. Caposiena Caro RD, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
1. Wolkenstein P, Loundou A, Barrau K, et al. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol. 2007;56:621-623. doi: 10.1016/j.jaad.2006.08.061
2. Storer MA, Danesh MJ, Sandhu ME, et al. An assessment of the relative impact of hidradenitis suppurativa, psoriasis, and obesity on quality of life. Int J Womens Dermatol. 2018;4:198-202. doi: 10.1016/j.ijwd.2018.08.009
3. Saunte DML, Jemec GBE. Hidradenitis suppurativa: advances in diagnosis and treatment. JAMA. 2017;318:2019-2032. doi: 10.1001/jama.2017.16691
4. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90:264-268. doi: 10.2340/00015555-0866
5. Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol. 2011;91:328-332. doi: 10.2340/00015555-1082
6. Caposiena Caro RD, Cannizzaro MV, Botti E, et al. Clindamycin versus clindamycin plus rifampicin in hidradenitis suppurativa treatment: clinical and ultrasound observations. J Am Acad Dermatol. 2019;80:1314-1321. doi: 10.1016/j.jaad.2018.11.035
Penile rash
Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32

Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

Biopsy revealed a lichenoid infiltrate in the upper dermis with thinning of the epidermis and a predominance of plasma cells. This finding, along with a red-orange, glossy lesion on the glans penis in an uncircumcised man is a classic presentation of Zoon balanitis.
Zoon balanitis is a chronic, idiopathic disorder that affects uncircumcised men who are middle-aged and older.1 Although the exact pathogenesis is unknown, it is hypothesized to be the result of chronic irritation due to poor hygiene/urine retention with prepuce dysfunction. The classic clinical presentation is an asymptomatic, well-defined, orange-to-red glazed patch with symmetric small red “cayenne pepper” spots contained within the glans penis and/or prepuce. Often there are symmetric “kissing lesions” where a second lesion of similar morphology is apparent on the prepuce where it meets the glans penis. While this disorder is typically asymptomatic, it can be associated with itching and/or burning.
There are several other inflammatory disorders in the differential for Zoon balanitis (erosive lichen planus, lichen sclerosus, psoriasis), but the most important consideration is penile intraepithelial carcinoma, specifically erythroplasia of Queyrat. Erythroplasia of Queyrat can be difficult to distinguish clinically and often requires a biopsy. Zoon balanitis will show a plasma cell predominant lichenoid infiltrate, whereas erythroplasia of Queyrat will show squamous cell carcinoma in situ.
It is important to note that Zoon balanitis is a clinicopathologic diagnosis and that zoonoid inflammation on biopsy is not pathognomonic, as this can also be seen in other inflammatory and neoplastic conditions. It is, therefore, advisable to follow up with patients with Zoon balanitis to ensure that the lesion is resolving and/or not getting worse.
Improved hygiene measures are the mainstay of treatment; circumcision is also effective.2 Both can help keep the glans clean and dry. In those with symptomatic disease, low-strength topical steroids including 1% hydrocortisone ointment or cream twice daily or topical calcineurin inhibitors (pimecrolimus 1% or tacrolimus 0.1%) twice daily can be used for symptom management.
Because this patient’s disease was asymptomatic, treatment was deferred. He was counseled to draw back the foreskin when urinating and to do the same while showering so that he could wash, then dry, the glans before returning the foreskin to its normal position. The patient is being monitored clinically.
Photo courtesy of Drew Mitchell, MD. Text courtesy of Drew Mitchell, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32
1. Mallon E, Hawkins D, Dinneen M, et al. Circumcision and genital dermatoses. Arch Dermatol. 2000;136:350-354. doi: 10.1001/archderm.136.3.350
2. Kumar B, Sharma R, Rajagopalan M, et al. Plasma cell balanitis: clinical and histopathological features—response to circumcision. Genitourin Med. 1995;71:32-34. doi: 10.1136/sti.71.1.32