User login
Occipital Scalp Nodule in a Newborn
The Diagnosis: Subcutaneous Fat Necrosis
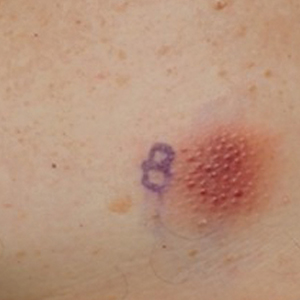
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.
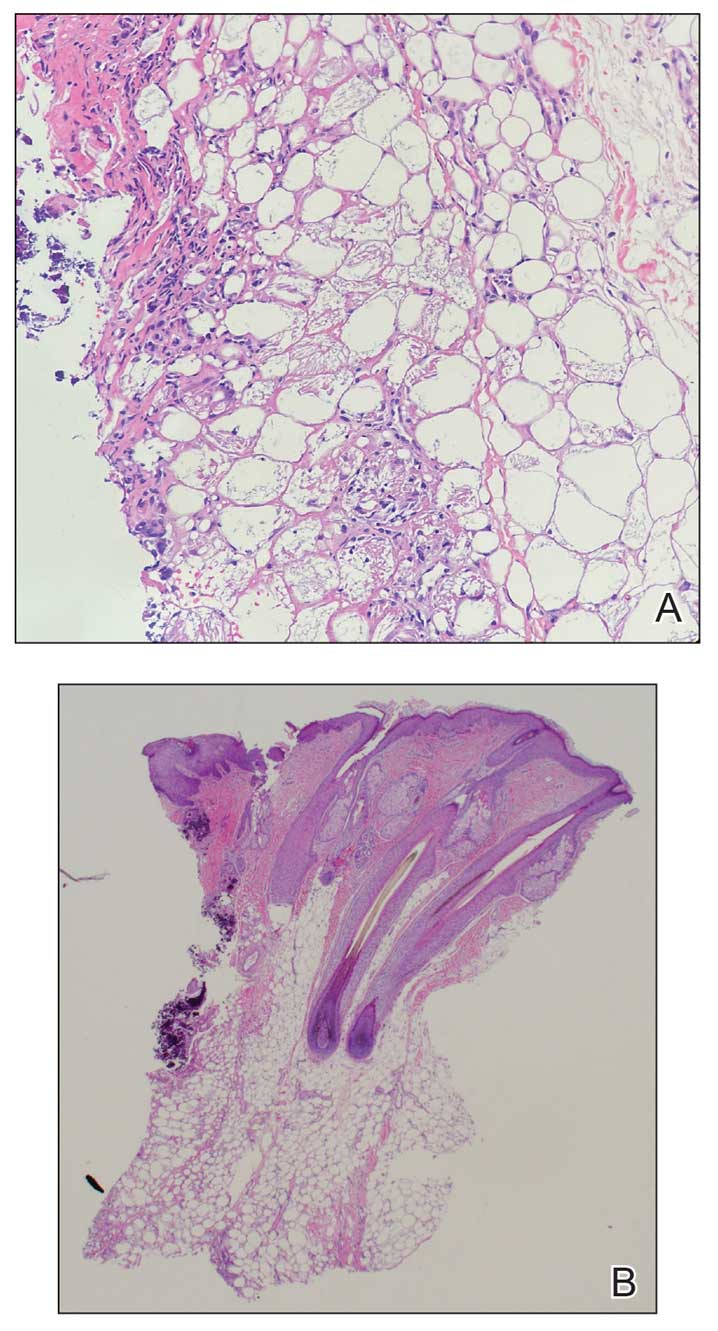
Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
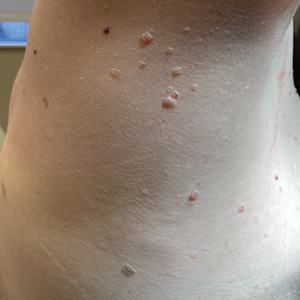
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
The Diagnosis: Subcutaneous Fat Necrosis
Histopathology revealed lobular panniculitis with lymphohistiocytic inflammation, lipid crystals, and calcifications in our patient (Figure). Subcutaneous fat necrosis (SCFN) was diagnosed based on these characteristic histopathologic findings. No further treatment was pursued.

Subcutaneous fat necrosis is a rare, self-limiting panniculitis that typically resolves within several weeks to months without scarring. It manifests as red or violaceous subcutaneous nodules or plaques most commonly on the buttocks, trunk, proximal arms and legs, and cheeks.1 Histopathology reveals lobular panniculitis with dense granulomatous infiltrates of histiocytes, eosinophils, and multinucleated giant cells with needle-shaped crystals. Focal areas of fat necrosis with calcification also can be seen.2
The epidemiology of SCFN is unknown. Most cases occur in healthy full-term to postterm neonates who experience hypoxia, other prenatal stressors, or therapeutic hypothermia for the treatment of hypoxic-ischemic encephalopathy.3 Although the etiology is unclear, certain inciting factors such as local tissue hypoxia, cold exposure, meconium aspiration, maternal diabetes, preeclampsia, and mechanical pressure have been proposed. Our patient underwent hypothermic cooling protocol, and it has been suggested that the increased saturated to unsaturated fat concentration in the skin of newborns increases the melting point, thus predisposing them to fat crystalization.4 Cases of SCFN involving the scalp are rare; therefore, any newborns receiving hypothermic therapy for hypoxic-ischemic encephalopathy should have a thorough skin examination with possible biopsy of lesions that are characteristic of SCFN, such as red or violaceous subcutaneous nodules or plaques, for specific disease identification.
The main complication of SCFN is hypercalcemia, which occurs in approximately 50% of cases. Other serum abnormalities include hyperglycemia, hypertriglyceridemia, and thrombocytopenia, though these findings are not as well associated.4 Patients with associated hypercalcemia may be asymptomatic, as in our patient, but other presentations include irritability, weakness, anorexia, vomiting, renal failure, failure to thrive, and encephalopathy. Nephrocalcinosis is a common complication of severe hypercalcemia; however, there is little evidence of associated major renal dysfunction.5 The exact mechanism of hypercalcemia is poorly understood. A widely accepted theory postulates that a granulomatous inflammatory infiltrate upregulates 1-α-hydroxylase activity, which enzymatically converts 25-hydroxyvitamin D to its active form, 1,25-dihydroxycholecalciferol, which increases bone resorption and calcium absorption through the gastrointestinal tract and renal systems. Treatments for hypercalcemia include hyperhydration, calcium-wasting diuretics, and low calcium intake.6 Furthermore, calcium levels should be obtained at the time of diagnosis and 30, 45, and 60 days after the lesions resolve.4
Subcutaneous fat necrosis needs to be differentiated from the more severe panniculitis, sclerema neonatorum (SN), which typically affects critically ill, preterm, and small-for-gestational-age newborns. It is associated with a high mortality rate and is characterized by skin and subadjacent tissue structures. The process typically begins in the thighs, buttocks, or trunk and spreads diffusely, sparing the fat-free palms, soles, and genitalia.7 Although our patient was born preterm, the physical characteristics of the nodule and the lack of severe illness placed SN lower on our differential. Histopathologic differences between SCFN and SN involve the extent of tissue fibrosis and presence of inflammatory cells. Sclerema neonatorum typically manifests with thickened connective tissue with a sparse inflammatory infiltrate, including lymphocytes, histiocytes, and multinucleated giant cells.7 Conversely, SCFN manifests with fat necrosis with an extensive inflammatory infiltrate. It is important to be able to distinguish between these 2 conditions, as both have vastly different prognoses.
Cold panniculitis, sometimes called “popsicle panniculitis,” is a phenomenon in which cold contact with the skin causes eruption of firm, erythematous, indurated plaques at the site of exposure. This self-limiting condition typically appears hours to days after cold exposure and spontaneously resolves in a few weeks.8 Therapeutic hypothermic protocol treatment involves using cooling devices to lower the body temperature for a short duration. The temperature typically is lowered to approximately 32 °C to 36 °C. These temperatures are not low enough to induce cold panniculitis, which is more commonly seen in facial ice applications when managing supraventricular tachycardia in neonates.
Cephalohematoma is a birthing injury that causes blood accumulation within the subperiosteal space. During parturition, the compressive and sheering forces on the calvarium rupture the vessels passing through the periosteum, causing blood to pool slowly into the subperiostium; thus, a cephalohematoma usually manifests later at 1 to 3 days of life as localized head swelling.9 The bleeding typically does not cross suture lines and is primarily found in the occipital or parietal regions. The incidence has been reported to be 0.4% to 2.5% of all live births.10 Although the location of the nodule in our patient was in the occipital region, imaging and biopsy results did not show hemorrhagic findings consistent with cephalohematoma. Management of cephalohematoma mainly is observational, as the mass slowly regresses and the accumulated blood gradually is reabsorbed.
Fungal scalp infections (tinea capitis) are common in the pediatric population. The peak incidence of this infection has been reported in children aged 3 to 7 years, with Trichophyton tonsurans and Microsporum canis as the usual causative organisms.11 Clinical features of tinea capitis include scaly patches with hair loss, hair loss with black pigmented dots at the follicular openings, diffuse scalp scaling with subtle hair loss, and cervical lymphadenopathy.12 Although less common, tinea capitis can progress to a more severe form known as a kerion, which is characterized by a tender plaque with pustules and crusting. A kerion can result in permanent scarring and alopecia if left untreated.12 In our patient, a nodule with scaling and faint erythema was observed, but no black pigmented dots at the follicular orifices were present. Therefore, a potassium hydroxide wet mount preparation used to diagnose tinea capitis was unnecessary. Systemic oral antifungal therapy such as fluconazole or terbinafine is the standard treatment for tinea capitis.
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
- Coondoo A, Lahiry R, Choudhury A, et al. Tender skin nodules in a newborn. Indian J Dermatol. 2013;58:328. doi:10.4103/0019-5154.113983
- Mitra S, Dove J, Somisetty SK. Subcutaneous fat necrosis in newbornan unusual case and review of literature. Eur J Pediatr. 2011;170:1107- 1110. doi:10.1007/s00431-011-1405-x
- Velasquez JH, Mendez MD. Newborn subcutaneous fat necrosis. In: StatPearls. StatPearls Publishing; 2022.
- Stefanko NS, Drolet BA. Subcutaneous fat necrosis of the newborn and associated hypercalcemia: a systematic review of the literature. Pediatr Dermatol. 2019;36:24-30. doi:10.1111/pde.13640
- Shumer DE, Thaker V, Taylor GA, et al. Severe hypercalcaemia due to subcutaneous fat necrosis: presentation, management and complications. Arch Dis Child Fetal Neonatal Ed. 2014;99:F419-F421. doi:10.1136/ archdischild-2014-306069
- Farooque A, Moss C, Zehnder D, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in subcutaneous fat necrosis. Br J Dermatol. 2009;160:423-425. doi:10.1111/j.1365-2133.2008.08844.x
- Zeb A, Darmstadt GL. Sclerema neonatorum: a review of nomenclature, clinical presentation, histological features, differential diagnoses and management. J Perinatol. 2008;28:453-460. doi:10.1038/jp.2008.33
- Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489, vii. doi:10.1016 /j.det.2008.05.015
- Raines DA, Krawiec C, Jain S. Cephalohematoma. In: StatPearls. StatPearls Publishing; 2023.
- Chung HY, Chung JY, Lee DG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004;15:774-779. doi:10.1097/00001665- 200409000-00015
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68. doi:10.2174/1872 213x14666200106145624
- Kovitwanichkanont T, Chong A. Superficial fungal infections. Aust J Gen Pract. 2019;48:706-711. doi:10.31128/ajgp-05-19-4930
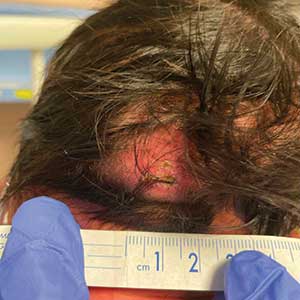
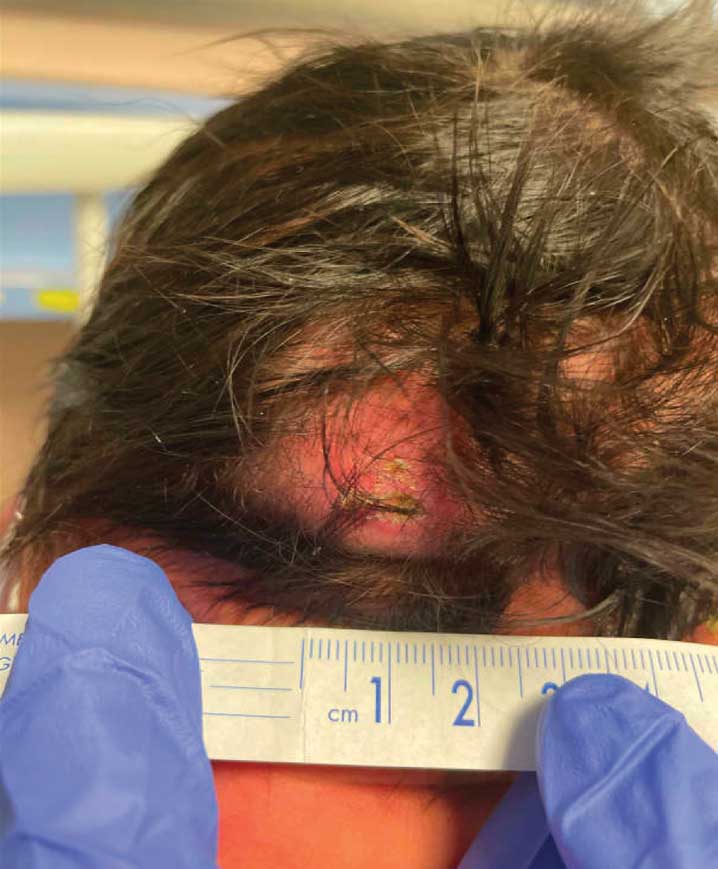
A 4-week-old male infant was referred to dermatology for evaluation of a nodule on the occipital protuberance of 2 weeks’ duration. The patient was born at 36 weeks and 6 days’ gestation via an emergency cesarean delivery due to fetal distress. He later was found to have hypoxic-ischemic encephalopathy, pulmonary hypertension, and hypertrophic cardiomyopathy. He underwent therapeutic hypothermia protocol treatment starting at less than 6 hours after birth. At the current presentation, physical examination showed a 2.5-cm, erythematous, firm, mobile nodule on the occipital scalp with some overlying crusting and minimal surrounding erythema. No other cutaneous features or lesions were present. Initial laboratory findings were remarkable for hypercalcemia at 11 mg/dL (reference range, 8.5-10.5 mg/dL). Magnetic resonance imaging showed a faint abnormality in the subcutaneous tissue in this region without a noted connection to the underlying brain/meningeal matter. A punch biopsy was performed.
Progressively Worsening Scaly Patches and Plaques in an Infant
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
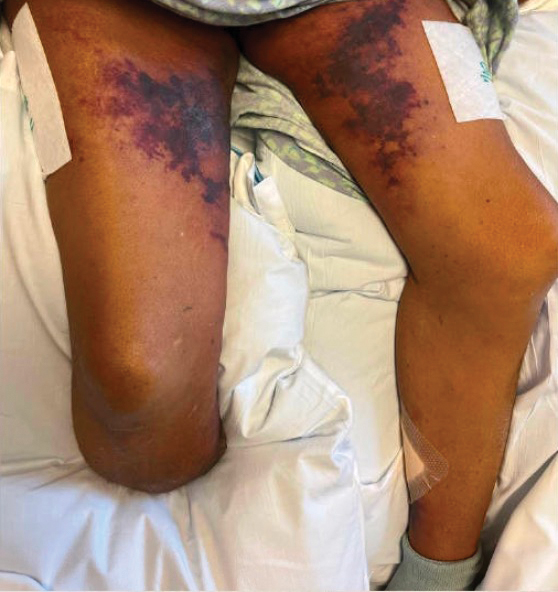
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
The Diagnosis: Erythrodermic Allergic Contact Dermatitis
The worsening symptoms in our patient prompted intervention rather than observation and reassurance. Contact allergy to lanolin was suspected given the worsening presentation after the addition of Minerin, which was immediately discontinued. The patient’s family applied betamethasone cream 0.1% twice daily to severe plaques, pimecrolimus cream 1% to the face, and triamcinolone cream 0.1% to the rest of the body. At follow-up 1 week later, he experienced complete resolution of symptoms, which supported the diagnosis of erythrodermic allergic contact dermatitis (ACD).
The prevalence of ACD caused by lanolin varies among the general population from 1.2% to 6.9%.1 Lanolin recently was named Allergen of the Year in 2023 by the American Contact Dermatitis Society.2 It can be found in various commercial products, including creams, soaps, and ointments. Atopic dermatitis (AD) is a common pediatric inflammatory skin disorder that typically is treated with these products.3 In a study analyzing 533 products, up to 6% of skin care products for babies and children contained lanolin.4 Therefore, exposure to lanolin-containing products may be fairly common in the pediatric population.
Lanolin is a fatlike substance derived from sheep sebaceous gland secretions and extracted from sheep’s wool. Its composition varies by sheep breed, location, and extraction and purification methods. The most common allergens involve the alcoholic fraction produced by hydrolysis of lanolin.4 In 1996, Wolf5 described the “lanolin paradox,” which argued the difficulty with identifying lanolin as an allergen (similar to Fisher’s “paraben paradox”) based on 4 principles: (1) lanolin-containing topical medicaments tend to be more sensitizing than lanolin-containing cosmetics; (2) patients with ACD after applying lanolin-containing topical medicaments to damaged or ulcerated skin often can apply lanolin-containing cosmetics to normal or unaffected skin without a reaction; (3) false-negative patch test results often occur in lanolin-sensitive patients; and (4) patch testing with a single lanolin-containing agent (lanolin alcohol [30% in petrolatum]) is an unreliable and inadequate method of detecting lanolin allergy.6,7 This theory elucidates the challenge of diagnosing contact allergies, particularly lanolin contact allergies.
Clinical features of acute ACD vary by skin type. Lighter skin types may have well-demarcated, pruritic, eczematous patches and plaques affecting the flexor surfaces. Asian patients may present with psoriasiform plaques with more well-demarcated borders and increased scaling and lichenification. In patients with darker skin types, dermatitis may manifest as papulation, lichenification, and color changes (violet, gray, or darker brown) along extensor surfaces.8 Chronic dermatitis manifests as lichenified scaly plaques. Given the diversity in dermatitis manifestation and the challenges of identifying erythema, especially in skin of color, clinicians may misidentify disease severity. These features aid in diagnosing and treating patients presenting with diffuse erythroderma and worsening eczematous patches and plaques despite use of typical topical treatments.
The differential diagnosis includes irritant contact dermatitis, AD, seborrheic dermatitis, and chronic plaque psoriasis. Negative patch testing suggests contact dermatitis based on exposure to a product. A thorough medication and personal history helps distinguish ACD from AD. Atopic dermatitis classically appears on the flexural areas, face, eyelids, and hands of patients with a personal or family history of atopy. Greasy scaly plaques on the central part of the face, eyelids, and scalp commonly are found in seborrheic dermatitis. In chronic plaque psoriasis, lesions typically are described as welldemarcated, inflamed plaques with notable scale located primarily in the scalp and diaper area in newborns and children until the age of 2 years. Our patient presented with scaly plaques throughout most of the body. The history of Minerin use over the course of 3 to 5 months and worsening skin eruptions involving a majority of the skin surface suggested continued exposure.
Patch testing assists in the diagnosis of ACD, with varying results due to manufacturing and processing inconsistencies in the composition of various substances used in the standard test sets, often making it difficult to diagnose lanolin as an allergen. According to Lee and Warshaw,6 the lack of uniformity within testing of lanolin-containing products may cause false-positive results, poor patch-test reproducibility, and loss of allergic contact response. A 2019 study utilized a combination of Amerchol L101 and lanolin alcohol to improve the diagnosis of lanolin allergy, as standard testing may not identify patients with lanolin sensitivities.1 A study with the North American Contact Dermatitis Group from 2005 to 2012 demonstrated that positive patch testing among children was the most consistent method for diagnosing ACD, and results were clinically relevant.9 However, the different lanolin-containing products are not standardized in patch testing, which often causes mixed reactions and does not definitely demonstrate classic positive results, even with the use of repeated open application tests.2 Although there has been an emphasis on refining the standardization of the lanolin used for patch testing, lanolin contact allergy remains a predominantly clinical diagnosis.
Both AD and ACD are common pediatric skin findings, and mixed positive and neutral associations between AD and allergy to lanolin have been described in a few studies.1,3,9,10 A history of atopy is more notable in a pediatric patient vs an adult, as sensitivities tend to subside into adulthood.9 Further studies and more precise testing are needed to investigate the relationship between AD and ACD.
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
- Knijp J, Bruynzeel DP, Rustemeyer T. Diagnosing lanolin contact allergy with lanolin alcohol and Amerchol L101. Contact Dermatitis. 2019;80:298-303. doi:10.1111/cod.13210
- Jenkins BA, Belsito DV. Lanolin. Dermatitis. 2023;34:4-12. doi:10.1089 /derm.2022.0002
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol .2016.6136
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018; 29:81-84. doi:10.1097/DER.0000000000000348
- Wolf R. The lanolin paradox. Dermatology. 1996;192:198-202. doi:10.1159/000246365
- Lee B, Warshaw E. Lanolin allergy: history, epidemiology, responsible allergens, and management. Dermatitis. 2008;19:63-72.
- Miest RY, Yiannias JA, Chang YH, et al. Diagnosis and prevalence of lanolin allergy. Dermatitis. 2013;24:119-123. doi:10.1097 /DER.0b013e3182937aa4
- Sangha AM. Dermatological conditions in SKIN OF COLOR-: managing atopic dermatitis. J Clin Aesthet Dermatol. 2021;14(3 Suppl 1):S20-S22.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Wakelin SH, Smith H, White IR, et al. A retrospective analysis of contact allergy to lanolin. Br J Dermatol. 2001;145:28-31. doi:10.1046 /j.1365-2133.2001.04277.x
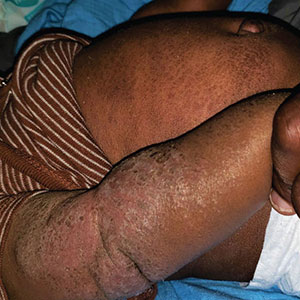
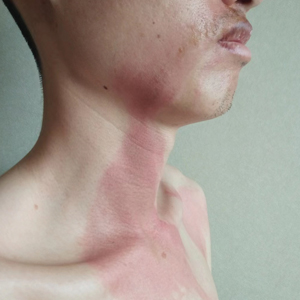
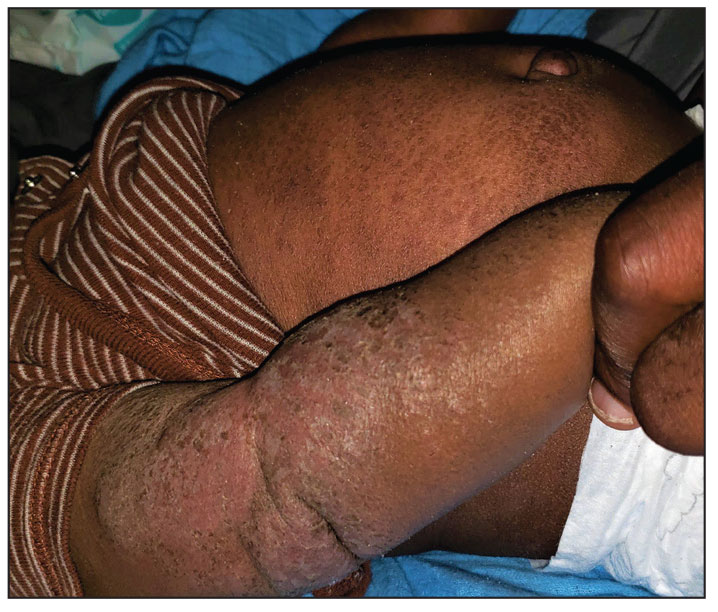
A 5-month-old male with moderately brown skin that rarely burns and tans profusely presented to the emergency department with a worsening red rash of more than 4 months’ duration. The patient had diffuse erythroderma and eczematous patches and plaques covering 95% of the total body surface area, including lichenified plaques on the arms and elbows, with no signs of infection. He initially presented for his 1-month appointment at the pediatric clinic with scaly patches and plaques on the face and trunk as well as diffuse xerosis. He was prescribed daily oatmeal baths and topical Minerin (Major Pharmaceuticals)—containing water, petrolatum, mineral oil, mineral wax, lanolin alcohol, methylchloroisothiazolinone, and methylisothiazolinone—to be applied to the whole body twice daily. At the patient’s 2-month well visit, symptoms persisted. The patient’s pediatrician increased application of Minerin to 2 to 3 times daily, and hydrocortisone cream 2.5% application 2 to 3 times daily was added.
Lichenoid Dermatosis on the Feet
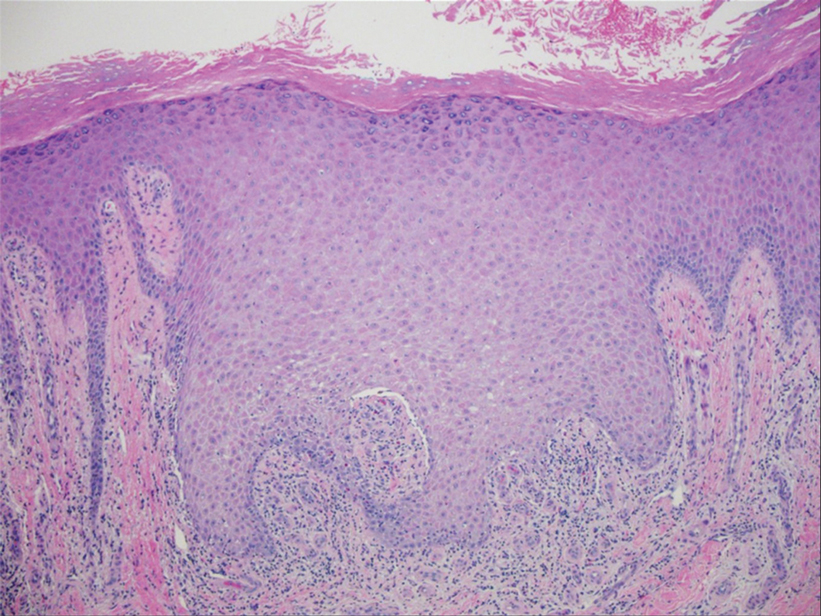
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).
Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.
The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
The Diagnosis: Hypertrophic Lichen Planus
Two biopsies from the left lateral foot revealed hyperkeratosis, wedge-shaped hypergranulosis, irregular acanthosis, and a bandlike lymphocytic infiltrate in the superficial dermis with a classic sawtooth pattern of the rete ridges (Figure 1). Based on the clinical findings and histopathology, the patient was diagnosed with hypertrophic lichen planus (LP) and was treated with clobetasol ointment 0.05%, which resulted in progression of the symptoms. She experienced notable improvement 3 months after adding methotrexate 12.5 mg weekly (Figure 2).

Lichen planus is an idiopathic chronic inflammatory condition of the skin and mucous membranes that classically manifests as pruritic violaceous papules and plaques, which commonly are found on the wrists, lower back, and ankles.1 The most common variants of LP are hypertrophic, linear, mucosal, actinic, follicular, pigmented, annular, atrophic, and guttate.2 The clinical presentation and biopsy results in our patient were consistent with the hypertrophic variant of LP, which is a chronic condition that most often manifests on the lower legs, especially around the ankles, as hyperkeratotic papules, plaques, and nodules.2,3 The exact pathophysiology of hypertrophic LP is unknown, but there is evidence that the immune system plays a role in its development and that the Koebner phenomenon may contribute to its exacerbation.4 There is a well-known association between LP and hepatitis. Patients with chronic LP may develop squamous cell carcinoma.4 The variants of LP can overlap and do not exist independent of one another. Recognizing the overlap in these variants allows for earlier diagnosis and therapeutic intervention of the disease process to limit disease progression and patient clinic visits and to improve patient quality of life.

The differential diagnosis for hyperkeratotic plaques of the feet and ankles can be broad and may include keratosis lichenoides chronica, palmoplantar keratoderma, palmoplantar psoriasis, or lichen amyloidosis. These conditions are classified based on various criteria that include extent of disease manifestations, morphology of palmoplantar skin involvement, inheritance patterns, and molecular pathogenesis.5 Keratosis lichenoides chronica is a rare dermatosis that presents as a distinctive seborrheic dermatitis–like facial eruption. The facial eruption is accompanied by violaceous papular and nodular lesions that appear on the extremities and trunk, typically arranged in a linear or reticular pattern.6 Palmoplantar keratoderma represents a group of acquired and hereditary conditions that are characterized by excessive thickening of the palms and soles.5 Palmoplantar psoriasis is a variant of psoriasis that affects the palms and soles and can manifest as hyperkeratosis, pustular, or mixed morphology.7 Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis that manifests as multiple pruritic, firm, hyperpigmented, hyperkeratotic papules on the shins that later coalesce in a rippled pattern.8,9
The first-line treatment for hypertrophic LP is topical corticosteroids. Alternative therapies include mycophenolate mofetil, acitretin, and intralesional corticosteroid injections.4 Treatment is similar for all of the LP variants.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
- Arnold DL, Krishnamurthy K. Lichen planus. In: StatPearls. StatPearls Publishing; 2022.
- Namazi MR, Bahmani M. Diagnosis: hypertrophic lichen planus. Ann Saudi Med. 2008;28:1-2. doi:10.5144/0256-4947.2008.222
- Riahi RR, Cohen PR. Hypertrophic lichen planus mimicking verrucous lupus erythematosus. Cureus. 2018;10:e3555. doi:10.7759 /cureus.3555
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j .ijwd.2015.04.001
- Has C, Technau-Hafsi K. Palmoplantar keratodermas: clinical and genetic aspects. J Dtsch Dermatol Ges. 2016;14:123-139; quiz 140. doi:10.1111/ddg.12930
- Konstantinov KN, Søndergaard J, Izuno G, et al. Keratosis lichenoides chronica. J Am Acad Dermatol. 1998;38(2 Pt 2):306-309. doi:10.1016 /s0190-9622(98)70570-5
- Miceli A, Schmieder GJ. Palmoplantar psoriasis. In: StatPearls. StatPearls Publishing; 2023.
- Tay CH, Dacosta JL. Lichen amyloidosis—clinical study of 40 cases. Br J Dermatol. 1970;82:129-136.
- Salim T, Shenoi SD, Balachandran C, et al. Lichen amyloidosis: a study of clinical, histopathologic and immunofluorescence findings in 30 cases. Indian J Dermatol Venereol Leprol. 2005;71:166-169.
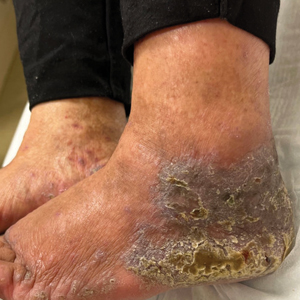
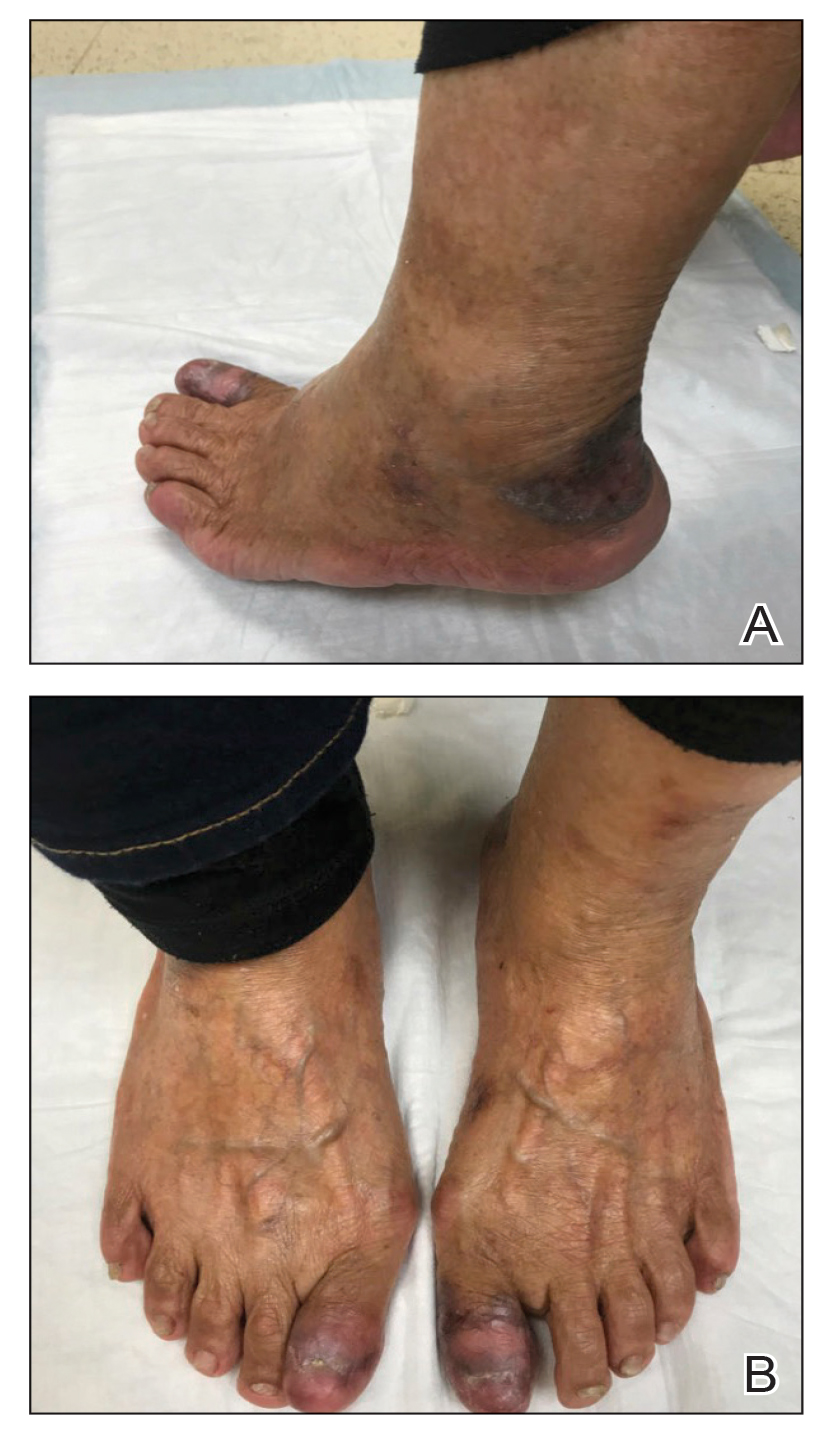
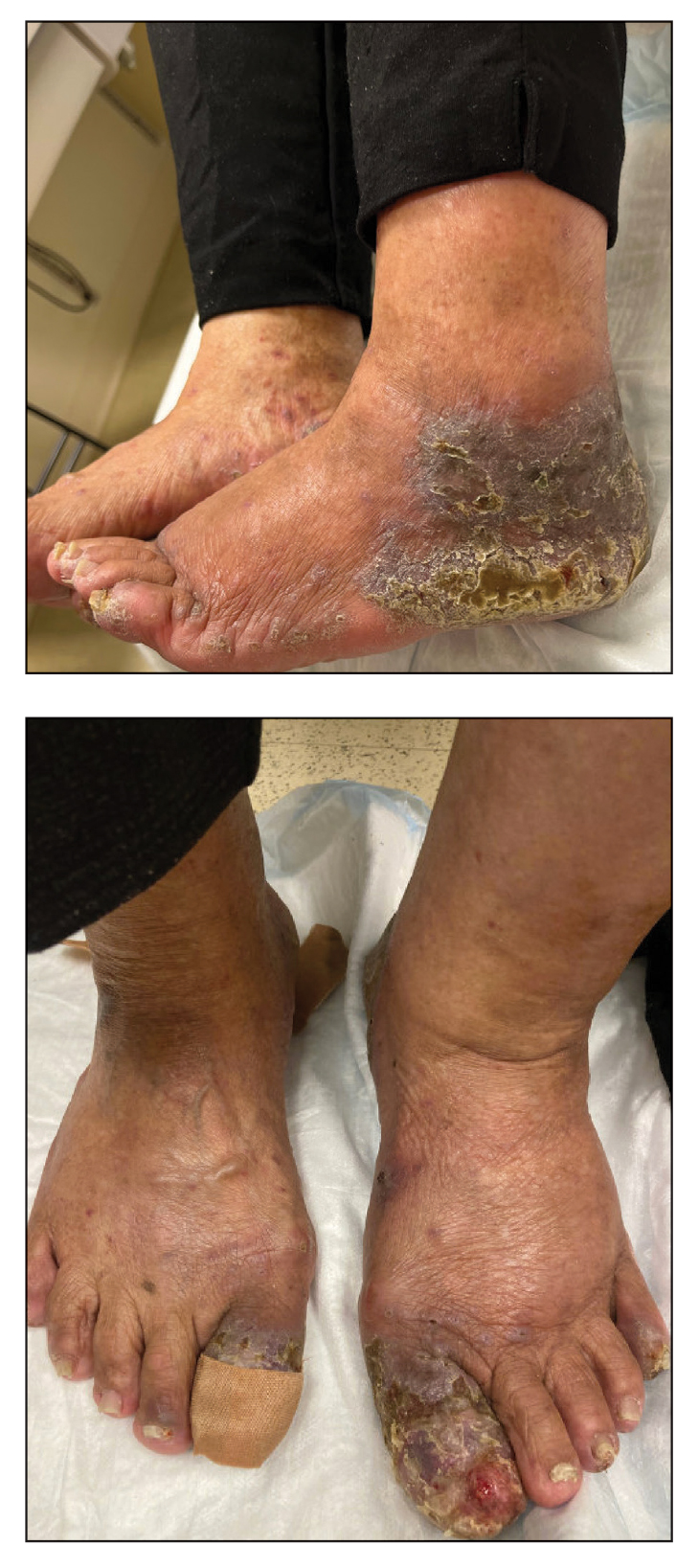
An 83-year-old woman presented for evaluation of hyperkeratotic plaques on the medial and lateral aspects of the left heel (top). Physical examination also revealed onychodystrophy of the toenails on the halluces (bottom). A crusted friable plaque on the lower lip and white plaques with peripheral reticulation and erosions on the buccal mucosa also were present. The patient had a history of nummular eczema, stasis dermatitis, and hand dermatitis. She denied a history of cold sores.
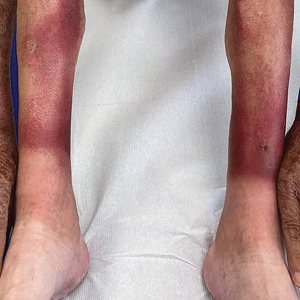
Asymptomatic Erythematous Plaque in an Outdoorsman
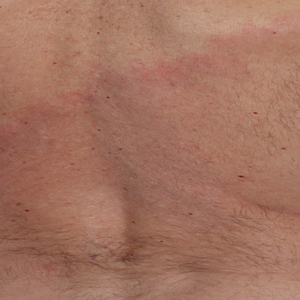
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
A middle-aged man presented with a well-demarcated, hyperpigmented, erythematous patch with an annular erythematous border that extended from the mid-back to the lower back. The patient was otherwise asymptomatic. He was an avid gardener who resided in South Carolina and had recently adopted 2 puppies.
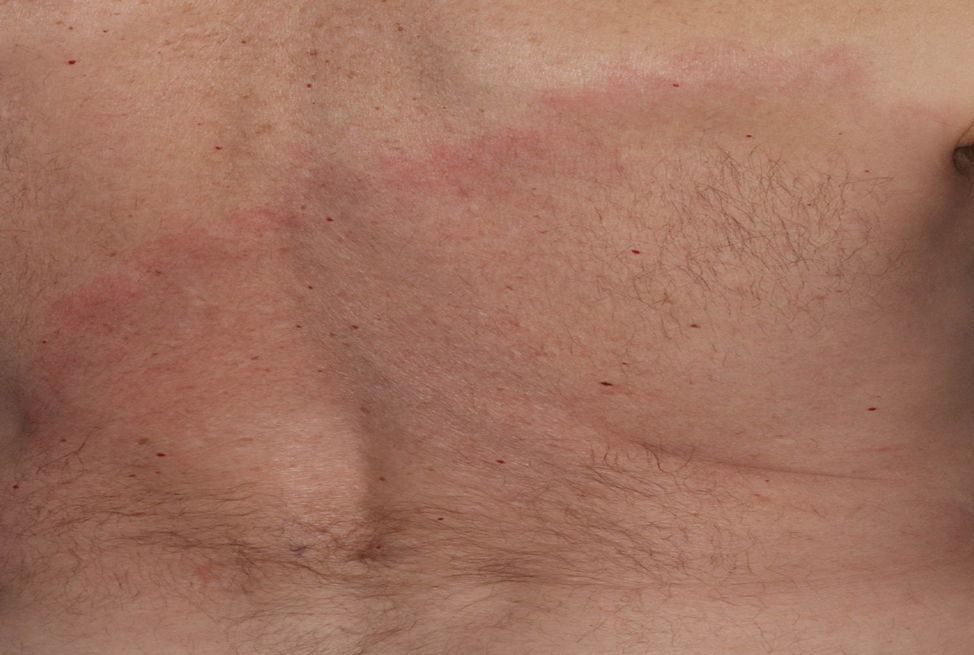
Skin Lesions on the Face and Chest
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
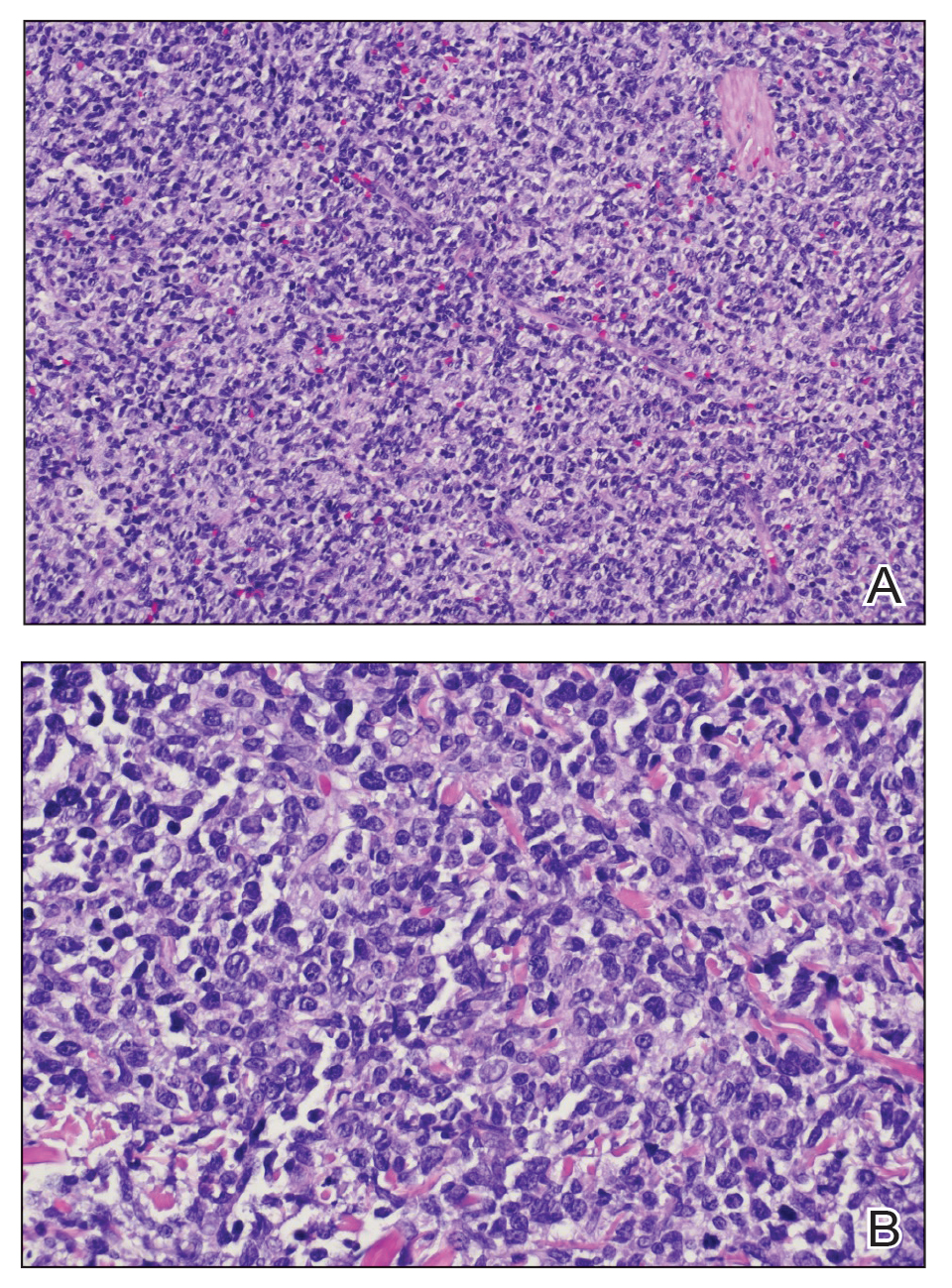
Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
A 79-year-old man presented to the dermatology clinic with multiple skin lesions of 4 months’ duration. The patient had a history of monoclonal gammopathy and reported no changes in medication, travel, or trauma. He reported tenderness only when trying to comb hair over the left occipital nodule. He denied fevers, night sweats, weight loss, or poor appetite. Physical examination revealed 4 concerning skin lesions: a 3×3-cm violaceous nodule with underlying ecchymosis on the right medial jaw (top), a 3×2.5-cm violaceous nodule on the posterior occiput, a pink plaque with 1-mm vascular papules on the right mid-chest (bottom), and a 4×2.5-cm oval pink patch on the left side of the lower back. Punch biopsies were performed on the right medial jaw nodule and right mid-chest plaque.
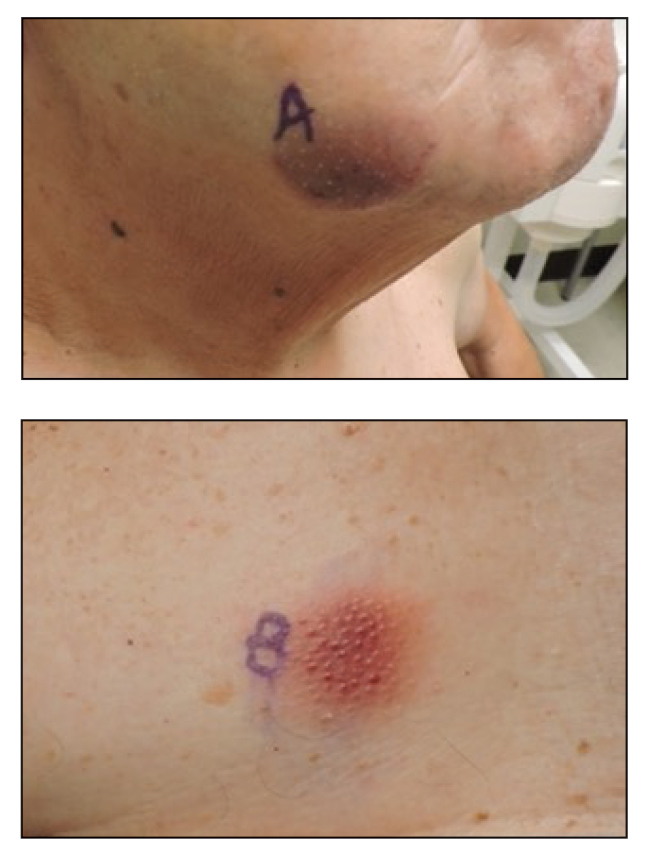
Papules on the Breast, Flank, and Arm Following Breast Cancer Treatment
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.
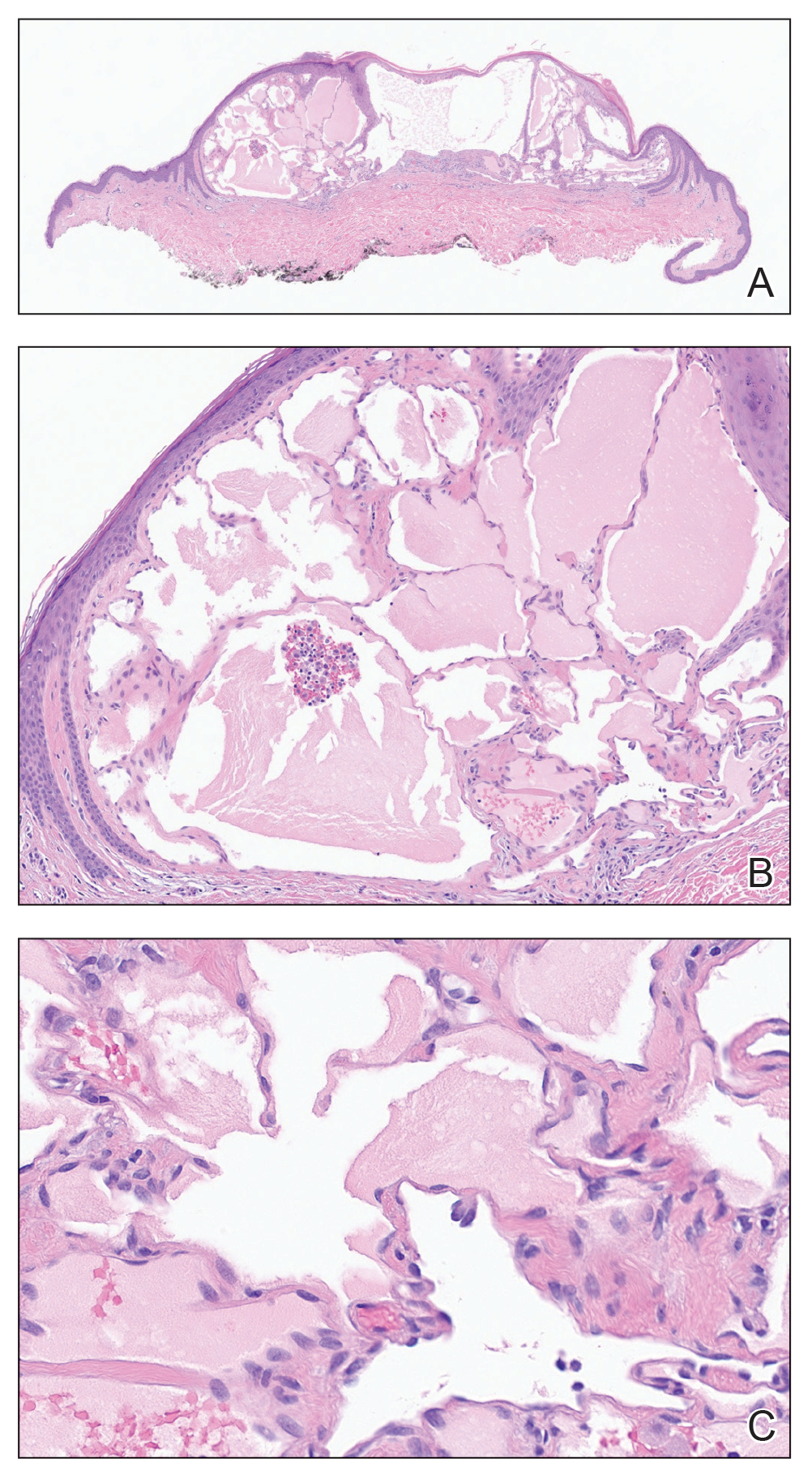
Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
The Diagnosis: Acquired Cutaneous Lymphangiectasia
Histopathology showed a cluster of widely ectatic, thin-walled lymphatic spaces immediately subjacent to the epidermis and flanked by an epidermal collarette (Figure, A). The vessels did not extend any further than the papillary dermis and were not accompanied by any notable inflammation (Figure, B). A single layer of bland endothelial cells lined each lymphatic space (Figure, C). A diagnosis of acquired cutaneous lymphangiectasia secondary to surgical and radiation treatment of breast cancer was made. Clinical monitoring was recommended, but no treatment was required unless symptoms arose. At 2-year follow-up, she continued to do well.

Acquired cutaneous lymphangiectasia is characterized by benign dilations of surface lymphatic vessels, likely resulting from disruption of the lymphatic system.1 This finding most commonly occurs on the external genitalia following combined surgical and radiation treatment of malignancy, though in a minority of cases it is seen with surgical or radiation treatment alone.2 Acquired cutaneous lymphangiectasia secondary to radical mastectomy for breast cancer was first reported in 1956 in a patient with persistent ipsilateral lymphadenopathy.3 The presentation in a patient with Cowden syndrome is rare. Cowden syndrome (also called PTEN hamartoma tumor syndrome) is a rare autosomal-dominant disorder caused by mutations in the tumor suppressor phosphatase and tensin homolog gene, PTEN. It is characterized by multiple hamartomas and substantially increased risk for breast, endometrial, and thyroid malignancy.4 In addition to breast cancer, our patient had a history of papillary thyroid carcinoma, cerebellar dysplastic gangliocytoma, and multiple cutaneous fibromas and angiolipomas.
A diagnosis of syringomas—benign tumors that arise from the intraepidermal aspect of eccrine sweat ducts— could be considered in the differential diagnosis. Cases of eruptive syringoma on the breast have been reported, but the biopsy would show a circumscribed proliferation of tadpole-shaped tubules comprised of secretory cells in a sclerotic stroma.5 Hidrocystomas are benign sweat gland cysts that present on the face, especially around the eyes, but rarely have been reported on the trunk, particularly the axillae.6 Although they clinically manifest as translucent papules, histopathology shows fluid-filled cysts lined by a layer of secretory columnar epithelium.7 Metastatic breast carcinoma was considered, given the patient’s history of breast cancer. Cutaneous metastases often are found on the chest wall but also can occur at distant sites. Histopathology can reveal various patterns, including islands of tumor cells with glandular formation or single files of cells infiltrating through dermal collagen.
Angiosarcoma also must be considered in the setting of any vasoformative proliferation arising on previously irradiated skin. Angiosarcomas can sometimes be well differentiated with paradoxically bland cytomorphology but characteristically have anastomosing vessels and infiltrative architecture, which were not identified in our patient. Other diagnostic features of angiosarcoma include endothelial nuclear atypia, multilayering, and mitoses. Radiation-associated angiosarcomas amplify MYC, a transcription factor that affects multiple aspects of the cell cycle and is an oncogene implicated in several different types of malignancy.8MYC immunohistochemistry testing should be performed whenever a vasoformative proliferation on irradiated skin is partially sampled or shows any features concerning for angiosarcoma. Lastly, the term postradiation atypical vascular lesion has been introduced to describe discrete papular proliferations that show close histopathologic overlap with lymphangioma/lymphatic malformations. In contrast, atypical vascular lesions show wedge-shaped intradermal growth that can cause diagnostic confusion with well-differentiated angiosarcoma. Unlike angiosarcomas, they do not express MYC. Postradiation atypical vascular lesions sometimes have an associated inflammatory infiltrate.9 Considerable histomorphologic overlap among lymphangiomas, atypical vascular lesions, and well-differentiated angiosarcomas exists; thus, lesions should be removed in their perceived totality whenever possible to help permit diagnostic distinction. In our patient, the abrupt discontinuation of vessels at the interface of the papillary and reticular dermis was reassuring of benignancy.
Our patient’s diagnosis of acquired cutaneous lymphangiectasia was a benign adverse effect of prior breast cancer treatments. This case demonstrates a rare dermatologic sequela that may arise in patients who receive surgical or radiation treatment of breast cancer. Given the heightened risk for angiosarcoma after radiation therapy as well as the increased risk for malignancy in patients with Cowden syndrome, biopsy can be an important diagnostic step in the management of these patients.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Valdés F, Peteiro C, Toribio J. Acquired lymphangiectases and breast cancer. Actas Dermosifiliogr (Engl Ed). 2007;98:347-350.
- Chiyomaru K, Nishigori C. Acquired lymphangiectasia associated with treatment for preceding malignant neoplasm: a retrospective series of 73 Japanese patients. AMA Arch Derm. 2009;145:841-842.
- Plotnick H, Richfield D. Tuberous lymphangiectatic varices secondary to radical mastectomy. AMA Arch Derm. 1956;74:466-468.
- Pilarski R, Burt R, Kohlman W, et al. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607-1616.
- Müller CSL, Tilgen W, Pföhler C. Clinicopathological diversity of syringomas: a study on current clinical and histopathologic concepts. Dermatoendocrinol. 2009;1:282-288.
- Anzai S, Goto M, Fujiwara S, et al. Apocrine hidrocystoma: a case report and analysis of 167 Japanese cases. Int J Dermatol. 2005;44:702-703.
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. MedGenMed. 2006;8:57.
- Ahmadi SE, Rahimi S, Zarandi B, et al. MYC: a multipurpose oncogene with prognostic and therapeutic implications in blood malignancies. J Hematol Oncol. 2021;14:121. doi:10.1186/s13045-021-01111-4
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post-radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
A 47-year-old woman with Cowden syndrome presented to the dermatology clinic with asymptomatic papules on and near the right breast that had increased in number over the last year. She had a medical history of breast cancer treated with mastectomy, chemotherapy, and radiation; papillary thyroid carcinoma treated with thyroidectomy and subsequent thyroid hormone replacement; dysplastic cerebellar gangliocytoma treated with surgical excision; and multiple cutaneous fibromas and angiolipomas. Physical examination revealed multiple clustered, 1- to 5-mm, translucent to red papules on the right breast, flank, and upper arm. A shave biopsy of a papule from the right lateral breast was performed.
Burning Skin Patches on the Face, Neck, and Chest
The Diagnosis: Gastric Acid Dermatitis
After further discussion, the patient indicated that he had vomited during the night of alcohol consumption, and the vomitus remained on the affected areas until the next morning, indicating that excessive alcohol ingestion stimulated abundant secretion of gastric acid, which caused the symptoms. Additionally, the presence of clothing acted as a buffer in the unaffected areas, which helped make the final diagnosis of gastric acid dermatitis. The patient was treated with external application of recombinant bovine basic fibroblast growth factor gel (21,000 IU/5 g) once daily, and the lesions greatly improved within 7 days. The burning pain of the throat, stomach, and esophagus resolved after consultation with an otolaryngologist and a gastroenterologist.
Gastric acid dermatitis is a new term used to describe an acute skin burn caused by the patient's own gastric acid. Generally, the pH of human gastric acid is between 0.9 and 1.8 but will be diluted after eating and will gradually increase to approximately 3.5, which is not enough to induce burns on the skin.1 In addition, the skin barrier is capable of preventing transient gastric acid corrosion.2,3 However, the release of a large amount of gastric acid after excessive alcohol ingestion coupled with 1 night of lethargy left enough acid and time to induce skin burns in our patient.
Dermatitis caused by other allergic or chemical factors, such as Paederus dermatitis, was excluded, as the patient’s manifestation occurred during the inactive period of Paederus fuscipes. Furthermore, the patient denied any history of contact with chemicals in the last month. Food eruptions primarily manifest as systemic anaphylaxis with eruptive and pruritic rashes after consumption of seafood, eggs, milk, or other proteins, while alcoholic contact dermatitis is a form of irritating dermatitis that could be easily induced again by direct skin contact with alcohol.
Management of gastric acid dermatitis is similar to that for other chemical burns. Because scarring seldom occurs, the central issue is to restore the skin barrier as quickly as possible and to avoid or alleviate postinflammatory hyperpigmentation. Treatments to restore the skin barrier include recombinant bovine or human-derived basic fibroblast growth factor gel, moist exposed burn ointment, and medical sodium hyaluronate gelatin. To treat postinflammatory hyperpigmentation, some whitening agents such as compound superoxide dismutase arbutin cream and hydroquinone cream as well as the Q-switched Nd:YAG laser are effective to ameliorate the skin condition. If skin burns are on sun-exposed areas, photoprotection is necessary to prevent hyperpigmentation.
Acknowledgment—We thank the patient for granting permission to publish this information.
- Ergun P, Kipcak S, Dettmar PW, et al. Pepsin and pH of gastric juice in patients with gastrointestinal reflux disease and subgroups. J Clin Gastroenterol. 2022;56:512-517. doi:10.1097 /MCG.0000000000001560
- Mitamura Y, Ogulur I, Pat Y, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85:615-626. doi:10.1111/cod.13959
- Kuo SH, Shen CJ, Shen CF, et al. Role of pH value in clinically relevant diagnosis. Diagnostics (Basel). 2020;10:107. doi:10.3390 /diagnostics10020107
The Diagnosis: Gastric Acid Dermatitis
After further discussion, the patient indicated that he had vomited during the night of alcohol consumption, and the vomitus remained on the affected areas until the next morning, indicating that excessive alcohol ingestion stimulated abundant secretion of gastric acid, which caused the symptoms. Additionally, the presence of clothing acted as a buffer in the unaffected areas, which helped make the final diagnosis of gastric acid dermatitis. The patient was treated with external application of recombinant bovine basic fibroblast growth factor gel (21,000 IU/5 g) once daily, and the lesions greatly improved within 7 days. The burning pain of the throat, stomach, and esophagus resolved after consultation with an otolaryngologist and a gastroenterologist.
Gastric acid dermatitis is a new term used to describe an acute skin burn caused by the patient's own gastric acid. Generally, the pH of human gastric acid is between 0.9 and 1.8 but will be diluted after eating and will gradually increase to approximately 3.5, which is not enough to induce burns on the skin.1 In addition, the skin barrier is capable of preventing transient gastric acid corrosion.2,3 However, the release of a large amount of gastric acid after excessive alcohol ingestion coupled with 1 night of lethargy left enough acid and time to induce skin burns in our patient.
Dermatitis caused by other allergic or chemical factors, such as Paederus dermatitis, was excluded, as the patient’s manifestation occurred during the inactive period of Paederus fuscipes. Furthermore, the patient denied any history of contact with chemicals in the last month. Food eruptions primarily manifest as systemic anaphylaxis with eruptive and pruritic rashes after consumption of seafood, eggs, milk, or other proteins, while alcoholic contact dermatitis is a form of irritating dermatitis that could be easily induced again by direct skin contact with alcohol.
Management of gastric acid dermatitis is similar to that for other chemical burns. Because scarring seldom occurs, the central issue is to restore the skin barrier as quickly as possible and to avoid or alleviate postinflammatory hyperpigmentation. Treatments to restore the skin barrier include recombinant bovine or human-derived basic fibroblast growth factor gel, moist exposed burn ointment, and medical sodium hyaluronate gelatin. To treat postinflammatory hyperpigmentation, some whitening agents such as compound superoxide dismutase arbutin cream and hydroquinone cream as well as the Q-switched Nd:YAG laser are effective to ameliorate the skin condition. If skin burns are on sun-exposed areas, photoprotection is necessary to prevent hyperpigmentation.
Acknowledgment—We thank the patient for granting permission to publish this information.
The Diagnosis: Gastric Acid Dermatitis
After further discussion, the patient indicated that he had vomited during the night of alcohol consumption, and the vomitus remained on the affected areas until the next morning, indicating that excessive alcohol ingestion stimulated abundant secretion of gastric acid, which caused the symptoms. Additionally, the presence of clothing acted as a buffer in the unaffected areas, which helped make the final diagnosis of gastric acid dermatitis. The patient was treated with external application of recombinant bovine basic fibroblast growth factor gel (21,000 IU/5 g) once daily, and the lesions greatly improved within 7 days. The burning pain of the throat, stomach, and esophagus resolved after consultation with an otolaryngologist and a gastroenterologist.
Gastric acid dermatitis is a new term used to describe an acute skin burn caused by the patient's own gastric acid. Generally, the pH of human gastric acid is between 0.9 and 1.8 but will be diluted after eating and will gradually increase to approximately 3.5, which is not enough to induce burns on the skin.1 In addition, the skin barrier is capable of preventing transient gastric acid corrosion.2,3 However, the release of a large amount of gastric acid after excessive alcohol ingestion coupled with 1 night of lethargy left enough acid and time to induce skin burns in our patient.
Dermatitis caused by other allergic or chemical factors, such as Paederus dermatitis, was excluded, as the patient’s manifestation occurred during the inactive period of Paederus fuscipes. Furthermore, the patient denied any history of contact with chemicals in the last month. Food eruptions primarily manifest as systemic anaphylaxis with eruptive and pruritic rashes after consumption of seafood, eggs, milk, or other proteins, while alcoholic contact dermatitis is a form of irritating dermatitis that could be easily induced again by direct skin contact with alcohol.
Management of gastric acid dermatitis is similar to that for other chemical burns. Because scarring seldom occurs, the central issue is to restore the skin barrier as quickly as possible and to avoid or alleviate postinflammatory hyperpigmentation. Treatments to restore the skin barrier include recombinant bovine or human-derived basic fibroblast growth factor gel, moist exposed burn ointment, and medical sodium hyaluronate gelatin. To treat postinflammatory hyperpigmentation, some whitening agents such as compound superoxide dismutase arbutin cream and hydroquinone cream as well as the Q-switched Nd:YAG laser are effective to ameliorate the skin condition. If skin burns are on sun-exposed areas, photoprotection is necessary to prevent hyperpigmentation.
Acknowledgment—We thank the patient for granting permission to publish this information.
- Ergun P, Kipcak S, Dettmar PW, et al. Pepsin and pH of gastric juice in patients with gastrointestinal reflux disease and subgroups. J Clin Gastroenterol. 2022;56:512-517. doi:10.1097 /MCG.0000000000001560
- Mitamura Y, Ogulur I, Pat Y, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85:615-626. doi:10.1111/cod.13959
- Kuo SH, Shen CJ, Shen CF, et al. Role of pH value in clinically relevant diagnosis. Diagnostics (Basel). 2020;10:107. doi:10.3390 /diagnostics10020107
- Ergun P, Kipcak S, Dettmar PW, et al. Pepsin and pH of gastric juice in patients with gastrointestinal reflux disease and subgroups. J Clin Gastroenterol. 2022;56:512-517. doi:10.1097 /MCG.0000000000001560
- Mitamura Y, Ogulur I, Pat Y, et al. Dysregulation of the epithelial barrier by environmental and other exogenous factors. Contact Dermatitis. 2021;85:615-626. doi:10.1111/cod.13959
- Kuo SH, Shen CJ, Shen CF, et al. Role of pH value in clinically relevant diagnosis. Diagnostics (Basel). 2020;10:107. doi:10.3390 /diagnostics10020107
A 26-year-old man presented with a burning skin rash around the mouth, neck, and chest after 1 night of lethargy due to excessive alcohol consumption 2 days prior. He also reported a sore throat and burning pain in the stomach and esophagus. Physical examination revealed signs of severe epidermal necrosis, including erythema, blisters, serous discharge, and superficial crusts on the perioral region, as well as well-defined erythema on the anterior neck and chest. Gastroscopy and laryngoscopy showed extensive mucosal erosion. A laboratory workup revealed no abnormalities.
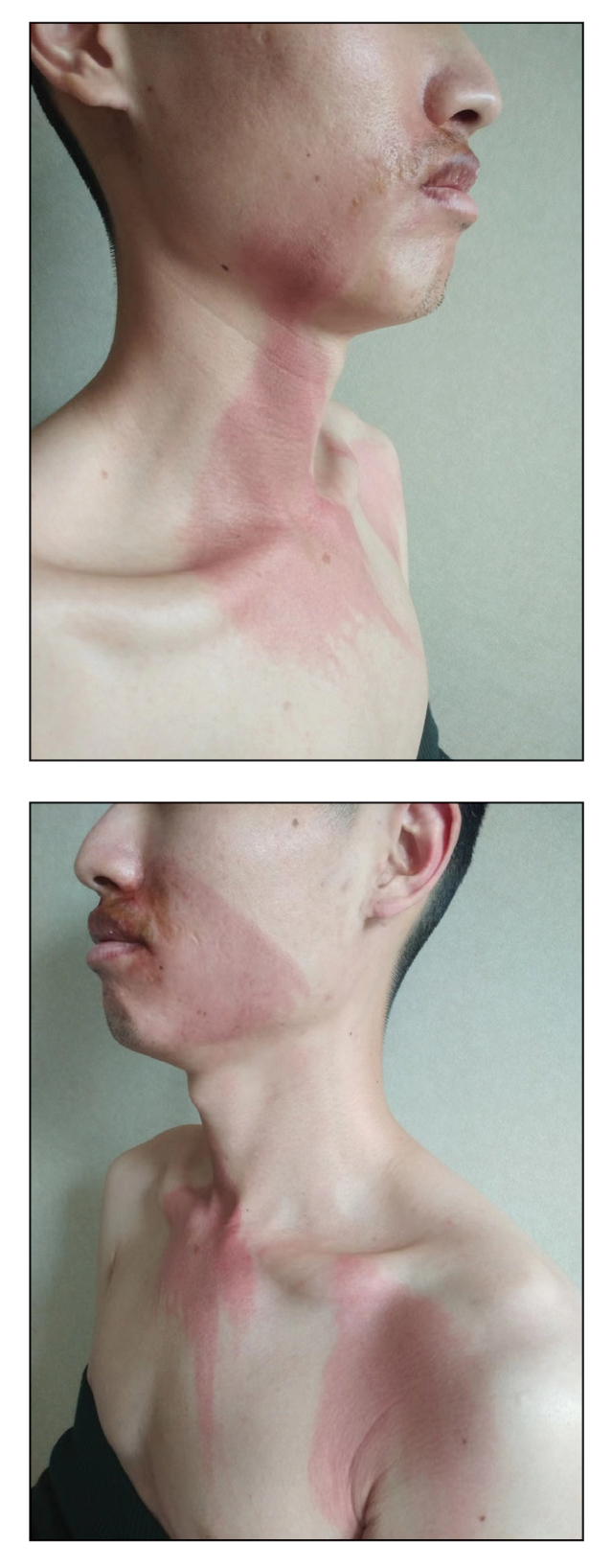
Brown Plaque in the Axilla Following Immobilization of the Arm
The Diagnosis: Granular Parakeratosis
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.
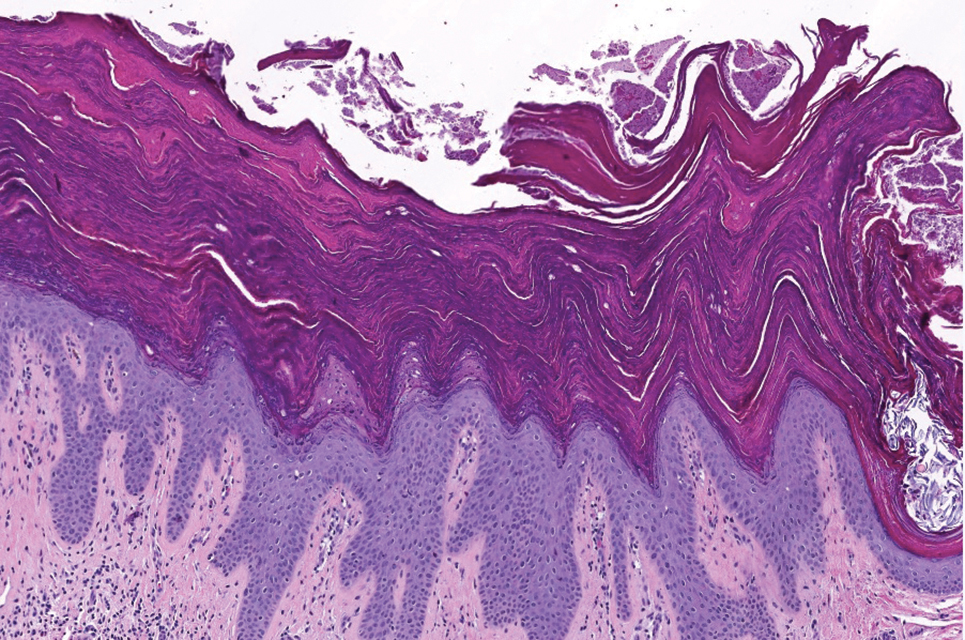
The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
The Diagnosis: Granular Parakeratosis
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.

The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
The Diagnosis: Granular Parakeratosis
Histopathology demonstrated diffuse parakeratosis with retention of keratohyalin granules throughout the stratum corneum consistent with a diagnosis of granular parakeratosis (Figure), a rare benign cutaneous condition that is thought to occur due to a defect in epidermal differentiation. The lesion resolved without additional treatment.

The pathogenesis of granular parakeratosis is unclear, but a reactive process in which locoregional irritation or occlusion prompts increased cell turnover and prevention of profilaggrin breakdown has been proposed.1,2 The diagnosis is linked to various precipitating agents, most commonly topical products (eg, zinc oxide, antiperspirants) and products with benzalkonium chloride (eg, laundry rinses). These agents are thought to cause retention of keratohyalin granules in the stratum corneum during epidermal differentiation.1,2
Most affected patients are middle-aged women (mean age at diagnosis, 37.8 years).2 Patients present with eruptions of erythematous, brown, hyperkeratotic patches and papules that coalesce into plaques.1,2 These lesions can be pruritic and painful or asymptomatic. They often manifest bilaterally in intertriginous sites, most commonly the axillae, groin, or inguinal folds.1,2
Treatment involves identification and removal of potential triggers including changing antiperspirants, limiting use of irritating agents (eg, topical products with strong fragrances), and reducing heat and moisture in the affected areas. If the lesion persists, stepwise treatment can be initiated with topical agents (eg, corticosteroids, vitamin D analogues, retinoids, keratolytics, calcineurin inhibitors) followed by systemic medications (eg, antibiotics, isotretinoin, antifungals, dexamethasone) and procedures (eg, botulinum toxin injections, surgery, laser, cryotherapy).1,2
Unilateral granular parakeratosis, as seen in our patient, is an uncommon manifestation. Our case supports the theory that occlusion is a precipitating factor for this condition, given persistent axillary exposure to heat, sweat, and friction in the setting of limb immobilization.3
Granular parakeratosis is a challenge to diagnose due to clinical overlap with several other cutaneous conditions; histopathologic confirmation is required. Fox- Fordyce disease is a rare condition that is thought to result from keratin buildup or occlusion of apocrine or apoeccrine sweat ducts leading to duct rupture and surrounding inflammation.4 Common triggers include laser hair removal, hormonal changes, and living conditions that promote hot and humid environments.5 It can manifest similarly to granular parakeratosis, with eruptions of multiple red-violet papules that appear bilaterally in aprocine gland–rich areas, including the axillae and less commonly the genital, periareolar, thoracic, abdominal, and facial areas.4,5 However, most patients with Fox-Fordyce disease tend to be younger females (aged 13–35 years) with severely pruritic lesions,4,5 unlike our patient. In addition, histopathology shows hyperkeratosis, hair follicle plugging, and sweat gland and duct dilation.4
Seborrheic keratoses are common benign epidermal tumors caused by an overproliferation of immature keratinocytes.6,7 Similar to granular parakeratosis, they commonly manifest in older adults as hyperpigmented, well-demarcated, verrucous plaques with a hyperkeratotic surface.6 However, they are more common on the face, neck, trunk, and extremities, and they tend to be asymptomatic, differentiating them from granular parakerosis.6 Histopathology demonstrates a papillomatous epidermal surface, large capillaries in the dermal papillae, and intraepidermal and pseudohorn epidermal cysts.7
Inverse lichen planus, a variant of lichen planus, is a rare inflammatory condition that involves the lysis of basal keratinocytes by CD8+ lymphocytes.8 Similar to granular parakeratosis, lichen planus commonly affects middle-aged women (aged 30–60 years), and this particular variant manifests with asymptomatic or mildly pruritic, hyperpigmented patches and plaques in intertriginous areas. Although it also shows hyperkeratosis on histopathology, it can be differentiated from granular parakeratosis by the additional findings of epidermal hypergranulosis, sawtooth acanthosis of rete ridges, apoptotic keratinocytes in the dermoepidermal junction, and lymphocytic infiltrate in the upper dermis.8
Hailey-Hailey disease (also known as familial benign pemphigus) is a rare condition caused by an autosomaldominant mutation affecting intracellular calcium signaling that impairs keratinocyte adhesion.9 Similar to granular parakeratosis, it is most common in middle-aged adults (aged 30–40 years) and manifests as pruritic and burning lesions in symmetric intertriginous areas that also can be triggered by heat and sweating. However, patients present with recurrent blistering and vesicular lesions that may lead to erosions and secondary infections, which reduced clinical suspicion for this diagnosis in our patient. Histopathology shows suprabasilar and intraepidermal clefts, full-thickness acantholysis, protruding dermal papillae, and a perivascular lymphocytic infiltrate in the superficial dermis.9
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
- Ding CY, Liu H, Khachemoune A. Granular parakeratosis: a comprehensive review and a critical reappraisal. Am J Clin Dermatol. 2015;16:495-500. doi:10.1007/s40257-015-0148-2
- Ip KH, Li A. Clinical features, histology, and treatment outcomes of granular parakeratosis: a systematic review. Int J Dermatol. 2022;61:973-978. doi:10.1111/ijd.16107
- Mehregan DA, Thomas JE, Mehregan DR. Intertriginous granular parakeratosis. J Am Acad Dermatol. 1998;39:495-496. doi:10.1016/s0190-9622(98)70333-0
- Kamada A, Saga K, Jimbow K. Apoeccrine sweat duct obstruction as a cause for Fox-Fordyce disease. J Am Acad Dermatol. 2003;48:453-455. doi:10.1067/mjd.2003.93
- Salloum A, Bouferraa Y, Bazzi N, et al. Pathophysiology, clinical findings, and management of Fox-Fordyce disease: a systematic review. J Cosmet Dermatol. 2022;21:482-500. doi:10.1111/jocd.14135
- Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238:205-217. doi:10.1159/000517070
- Minagawa A. Dermoscopy-pathology relationship in seborrheic keratosis. J Dermatol. 2017;44:518-524. doi:10.1111/1346-8138.13657
- Weston G, Payette M. Update on lichen planus and its clinical variants [published online September 16, 2015]. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Ben Lagha I, Ashack K, Khachemoune A. Hailey-Hailey disease: an update review with a focus on treatment data. Am J Clin Dermatol. 2020;21:49-68. doi:10.1007/s40257-019-00477-z
A 62-year-old woman presented to our clinic for evaluation of a brown plaque in the left axilla of 2 weeks’ duration. She had a history of a rotator cuff injury and adhesive capsulitis several months prior that required immobilization of the left arm in a shoulder orthosis for several months. After the sling was removed, she noticed the lesion and reported mild cutaneous pain. Physical examination revealed a 1.5-cm, verrucous, red-brown plaque in the left axillary vault. A shave biopsy of the plaque was performed.
Photoexposed Rash in an Older Adult
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3
The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
The Diagnosis: Pellagra
The patient was diagnosed with pellagra based on the clinical and laboratory findings. He was discharged with nicotinamide 250 mg and folic acid 5 mg supplementation daily. After 3 months, all symptoms resolved.
Pellagra is a condition usually associated with the 4 Ds: dermatitis; diarrhea; dementia; and, if untreated, death.1 The word pellagra is derived from the Italian terms pelle and agra, which mean skin and rough, respectively.2 Spanish physician Gasper Casal first described pellagra in 1762 after observing the disease in poorer peasants in Asturias who mainly relied on maize and rarely consumed fresh meat.1,2 Joseph Goldberger conducted research in the early 20th century, provoking the disease in jail prisoners by modifying their diets. However, it was not until 1926 that Goldberger discovered the true cause of the illness to be a poor diet and named what would become known as nicotinamide as the pellagra preventative factor.1,2 Niacin (vitamin B3), the deficient molecule in pellagra, also is known as nicotinic acid, nicotinamide, or niacinamide. It is a water-soluble vitamin that is converted into nicotinamide-adenine-dinucleotide (NAD) and its phosphate NADP.1,2 It has been hypothesized that pellagra symptoms arise from insufficient amounts of NAD and NADP, making the body unable to support cellular energy transfer processes.3
Pellagra manifests 50 to 60 days after starting a diet low in niacin. Niacin and nicotinamide are absorbed from the digested food to the stomach through a sodiumdependent mechanism, and then nicotinamide may be transformed into nicotinic acid with microsomal deamidation.3 Niacin may be obtained from one’s diet or produced from tryptophan. Foods with the highest amounts of niacin include liver, poultry, fish, eggs, milk, pork, mushrooms, avocados, almonds, and legumes.1,3 Coffee also contains trigonelline, which may be transformed into nicotinic acid when roasted, increasing the niacin level by 30 times.3 Approximately 60 mg of dietary tryptophan is needed to produce up to 1 mg of niacin in the presence of B2 and B6 vitamins. This mechanism provides approximately half of the needs for niacin.3 Insufficient dietary intake of niacin or the essential amino acid tryptophan can cause pellagra (primary pellagra), which is a concern in resource-limited countries. Alternatively, the body may not be able to properly utilize niacin for metabolic processes (secondary pellagra), which occurs more frequently in developed countries.1 Secondary pellagra also may be caused by alcoholism, colitis, cirrhosis, carcinoid tumors, Hartnup disease, or gastrointestinal tuberculosis, as these conditions prevent niacin from being consumed, absorbed, or processed. Certain medications can cause pellagra by interfering with the tryptophan-niacin pathway, including isoniazid, 5-fluorouracil, pyrazinamide, 6-mercaptopurine, hydantoins, ethionamide, phenobarbital, azathioprine, and chloramphenicol.2
The clinical manifestations of pellagra are diverse because it affects tissues with high turnover rates. Clinical features of pellagra include symmetric photosensitive skin eruptions, gastrointestinal tract symptoms, and neurologic and mental disorders.3 The first signs of pellagra may include muscle weakness, digestive concerns, and psychological or emotional discomfort.2 Pellagra dermatitis manifests as an acute or intermittent, bilaterally symmetrical eruption on sun-exposed areas and is markedly distinct from healthy skin.3 Some individuals may experience vesiculation and bullae development (wet pellagra). The erythema is first brilliant red then turns into a cinnamon-brown color. Over time, the skin becomes thickened, scaly, cracked, and hyperpigmented.1 The dryness of the skin likely is due to a remarkable decrease in wax ester and sebaceous gland atrophy seen on histopathology.4 Pellagra most frequently affects the back of the hands (77%–97% of cases), which can extend upward to create the so-called pellagra glove or gauntlet.3 It is common to see symmetrical eruptions in the shape of a butterfly following an anatomical pattern innervated by the trigeminal nerve, which resembles lupus erythematosus on the face. Another common manifestation is Casal necklace, a well-marginated eruption frequently seen on the front of the neck (Figure).2 On the foot, lesions often do not develop close to the malleoli but rather terminate distally on the backs of the toes. Sometimes a boot pattern may form that covers the front and back of the leg.1-3

The pathophysiology of photosensitivity in pellagra was hypothesized by Karthikeyan and Thappa.3 They discovered an excessive synthesis of a phototoxic substance, kynurenic acid, and a deficiency in urocanic acid, which normally protects the skin by absorbing light in the UVB range. Niacin deprivation leads to the production of kynurenic acid through the tryptophan-kynurenine-nicotinic acid pathway and reduces the amount of urocanic acid by affecting the enzyme histidase in the stratum corneum.1-3 In one-third of patients, pellagra affects the oral mucosa, causing characteristic symptoms such as glossitis, angular stomatitis, and cheilitis.2 In nearly 50% of patients, poor appetite, nausea, epigastric discomfort, diarrhea, and excessive salivation are present. Most of the gastrointestinal tract is affected by mucosal inflammation and atrophy, which can cause malnutrition and cachexia due to anorexia and malabsorptive diarrhea.2 Headache, irritability, poor concentration, hallucinations, photophobia, tremor, and depression are some of the neuropsychiatric symptoms. Patients experience delirium and disorientation as pellagra progresses, followed by a comatose state and ultimately death.2
The patient’s history and physical examination are used to make the diagnosis, with particular attention to the patient’s dietary details. The diagnosis is made in part ex juvantibus by seeing how the patient responds to higher niacin doses. Anemia, hypoproteinemia, elevated blood calcium, reduced serum potassium and phosphorus, abnormal liver function tests, and elevated serum porphyrin levels also indicate pellagra. Niacin 300 mg in divided doses for up to 4 weeks has been recommended by the World Health Organization to treat pellagra.5 The flushing seen with niacin administration is not linked to the usage of nicotinamide. The recommended nicotinamide dosage for adults is 100 mg orally every 6 hours until most acute symptoms have disappeared, followed by oral administration of 50 mg every 8 to 12 hours until all skin lesions have healed.2
Among the differential diagnoses, necrolytic migratory erythema is characterized by an episodic eruption of crusted, erosive, annular erythematous plaques with blister development, which occurs in 70% of patients with glucagonoma syndrome. The perioral region, perineum, lower belly, thighs, and distal extremities are the usual locations.6,7 Laboratory test results include elevated fasting serum glucagon (>1000 ng/L) and normocytic anemia, which aided in ruling out this diagnosis in our patient. Generalized acute cutaneous lupus erythematosus may appear as a broad morbilliform eruption. The hands frequently exhibit erythema and edema, especially across the dorsal and interphalangeal regions.8 Other typical findings of systemic lupus erythematosus such as antinuclear antibody were not seen in our patient, making this diagnosis unlikely. Porphyria cutanea tarda also must be considered in the differential diagnosis. The hepatic deficiency of uroporphyrinogen decarboxylase is the primary cause of this condition. Although it is characterized by blistering lesions, patients more frequently describe increased skin fragility in sun-exposed regions. Hypertrichosis, hyperpigmentation or hypopigmentation, hirsutism, or scarring may appear in the later stage of the disease.9 Phototoxic reaction was ruled out because the patient spent most of the time at home, and no new drugs had been prescribed in the previous months.
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
- Prabhu D, Dawe RS, Mponda K. Pellagra a review exploring causes and mechanisms, including isoniazid-induced pellagra. Photodermatol Photoimmunol Photomed. 2021;37:99-104. doi:10.1111 /phpp.12659
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. Int J Dermatol. 2004;43:1-5. doi:10.1111/j.1365-4632.2004.01959.x
- Karthikeyan K, Thappa DM. Pellagra and skin. Int J Dermatol. 2002;41:476-481. doi:10.1046/j.1365-4362.2002.01551.x
- Dogliotti M, Liebowitz M, Downing DT, et al. Nutritional influences of pellagra on sebum composition. Br J Dermatol. 1977;97:25-28. doi:10.1111/j.1365-2133.1977.tb15423.x
- World Health Organization. Pellagra and Its Prevention and Control in Major Emergencies. Published February 23, 2000. Accessed February 15, 2024. https://www.who.int/publications/i/item/WHO-NHD-00.10
- Liu JW, Qian YT, Ma DL. Necrolytic migratory erythema. JAMA Dermatol. 2019;155:1180. doi:10.1001/jamadermatol.2019.1658
- Tolliver S, Graham J, Kaffenberger BH. A review of cutaneous manifestations within glucagonoma syndrome: necrolytic migratory erythema. Int J Dermatol. 2018;57:642-645. doi:10.1111/ijd.13947
- Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365-381. doi:10.2165/11310780-000000000-00000
- Singal AK. Porphyria cutanea tarda: recent update. Mol Genet Metab. 2019;128:271-281. doi:10.1016/j.ymgme.2019.01.004
A 66-year-old man presented with an intermittent pruriginous symmetric rash on the dorsal aspects of the arms, legs, and upper chest of 4 months' duration. The patient’s hands, forearms, and neck were diffusely hyperpigmented, dry, cracked, and scaling with a ring of peripheral erythema. He also experienced recurrent photosensitivity reactions on the legs. His poor clinical condition including confusion and diarrhea hindered intake of a balanced diet. He also reported a history of excessive alcohol use. The patient’s vital signs were normal, and Doppler ultrasonography ruled out deep venous thrombosis of the lower legs. A complete blood cell count showed anemia with decreased hemoglobin levels (117 g/L [reference range, 140–180 g/L]) and increased mean corpuscular volume (107.1 fL [reference range, 80–100 fL]). Additionally, low serum levels of albumin, folate, and vitamin B12 were noted. The patient had been taking hydrochlorothiazide and salicylic acid for hypertension with no recent changes in his medication regimen.
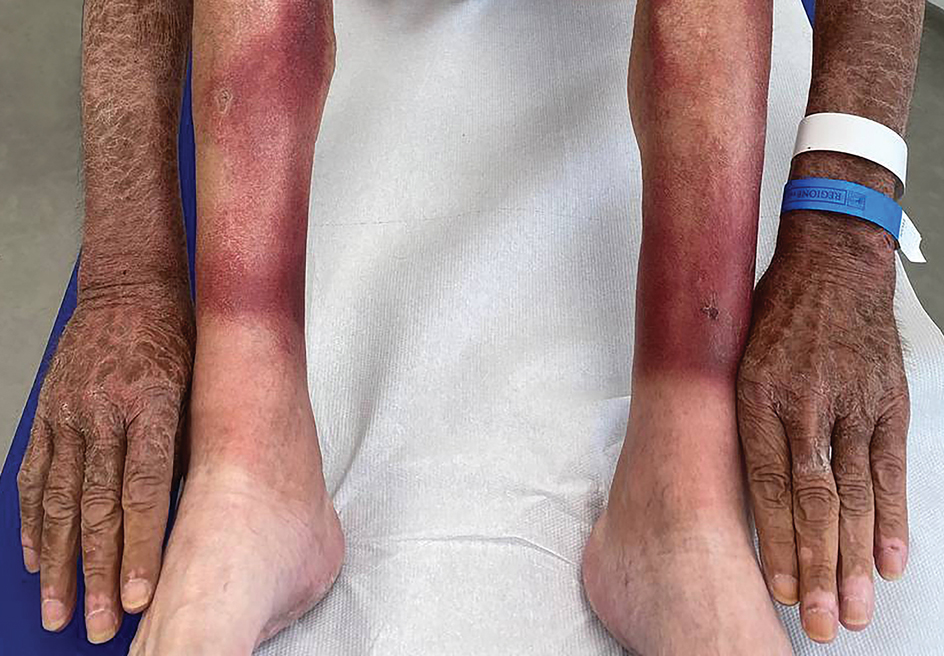
Painful Retiform Purpura in a Peritoneal Dialysis Patient
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.
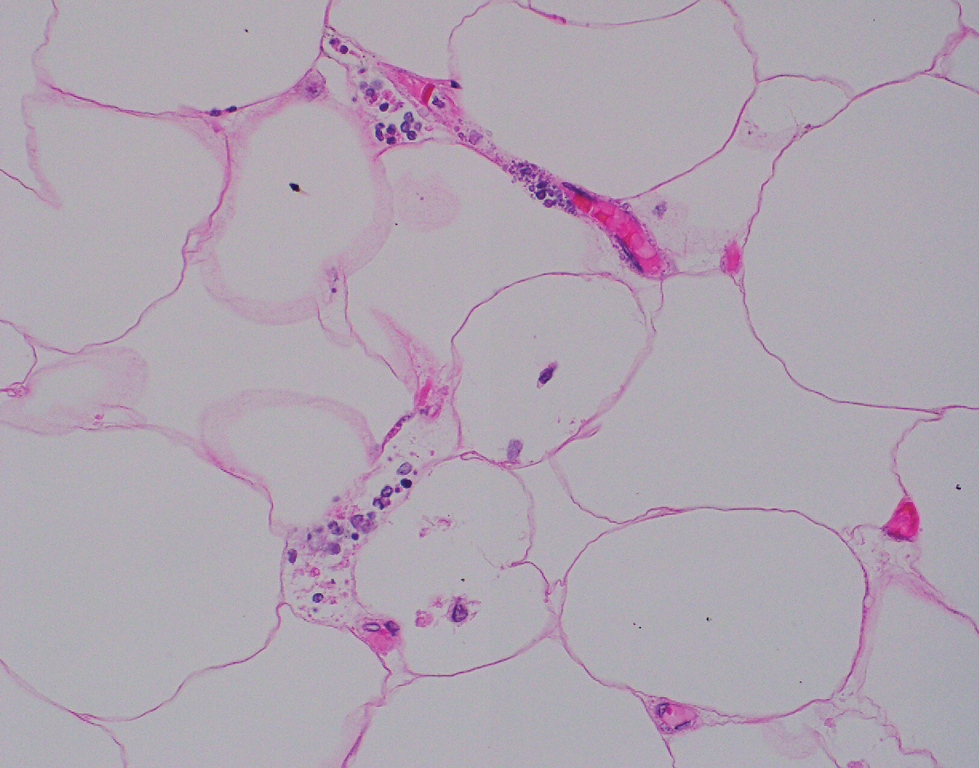
Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
The Diagnosis: Calcific Uremic Arteriolopathy
Computed tomography of the abdomen and pelvis with contrast revealed a right complex renal cyst with peripheral calcification; computed tomography of the head without contrast revealed atherosclerotic changes with calcification of the intracranial arteries, vertebral basilar arteries, and bilateral branches of the ophthalmic artery. Histopathology revealed occlusive vasculopathy with epidermal ischemic changes as well as dermal and subcutaneous vascular congestion and small thrombi. Within the subcutis, there were tiny stippled calcium deposits within very small vascular lumina (Figure). The combination of clinical and histological findings was highly suggestive of calcific uremic arteriolopathy, and the patient was transitioned to hemodialysis against a low-calcium bath to avoid hypercalcemia. Unfortunately, she developed complications related to sepsis and experienced worsening mentation. After a discussion with palliative care, the patient was transitioned to comfort measures and discharged home on hospice 1 week after the biopsy at her family’s request.

Calcific uremic arteriolopathy (also known as calciphylaxis) is a rare, life-threatening syndrome of widespread vascular calcification leading to microvascular occlusion within the dermis and subcutaneous tissues.1 Clinically, it typically manifests as severely painful, purpuric skin lesions that evolve through phases of blistering, ulceration, and ultimately visible skin necrosis.2 The pain likely is a consequence of ischemia and nociceptive activation and often may precede any visibly apparent skin lesions.3 Risk factors associated with the development of this condition include female sex; history of diabetes mellitus, obesity, rapid weight loss, or end-stage renal disease; abnormalities in calcium and phosphorus homeostasis; and vitamin K deficiency.1,3 It is more prevalent in patients on peritoneal dialysis compared to hemodialysis.4
Calciphylaxis is diagnosed with combined clinical and histopathological evidence. Laboratory test abnormalities are not specific for disease; therefore, skin biopsy is the standard confirmatory test, though its practice is contentious due to the risk for nonhealing ulceration and increasing risk for infection.1 Findings suggestive of disease include focal to diffuse calcification (intravascular, extravascular, or perieccrine), superficial fat calcium deposition, mid panniculus calcium deposition, mid panniculus vascular thrombi, and focal to diffuse angioplasia.5 The hallmark feature is diffuse calcification of small capillaries in adipose tissue.6
The mortality rate associated with this disease is high—a 6-month mortality rate of 27% to 43% has been reported from the time of diagnosis7-9—which often is related to subsequent superimposed infections patients acquire from necrotic skin tissue.2 The disease also carries high morbidity, with patients experiencing frequent hospitalizations related to pain, infections, and nonhealing wounds.6 There is no standard treatment, and trials have been limited to small sample sizes. A multidisciplinary treatment approach is essential to maximize outcomes, which includes wound care, risk factor modification, analgesia, and symptomatic management strategies.1,2,6
Some pharmacologic agents have received noteworthy attention in treating calciphylaxis, including sodium thiosulfate (STS), bisphosphonates, and vitamin K supplementation.1 The strongest evidence supporting the use of STS comes from 2 trials involving 53 and 27 dialysis patients, with complete remission in 14 (26%) and 14 (52%) patients, respectively.10,11 However, these trials did not include control groups to compare outcomes, and mortality rates were similarly high among partial responders and nonresponders compared with patients not treated with STS. A 2018 systematic review failed to assess the efficacy of STS alone for the treatment of calciphylaxis but suggested there may be a future role for it, with 251 of 358 patients (70.1%) responding to therapy.12
Erythema ab igne is a cutaneous reaction related to long-term heat exposure, often from electronic devices such as laptops, heating pads, space heaters, or hot-water bottles.13,14 Clinically, this rash appears as an erythematous, purpuric, or hyperpigmented reticular dermatosis that is below the clinical threshold to define a thermal burn.13 Lesions often are seen on the anterior thighs or across the abdomen.15 There usually are no long-term clinical sequelae; however, rare malignant transformation has been documented in cases of atrophy or nonhealing ulceration.16 Treatment is supportive with removal of the offending agent, but hyperpigmentation may persist for months to years.14
Livedo reticularis is a cutaneous pattern of mottled violaceous or hyperpigmented changes that often signifies underlying vascular dermal changes.17 It can be seen in various pathologic states, including vasculitis, autoimmune disease, connective tissue disease, neurologic disease, infection, or malignancy, or it can be drug induced.18 There are no pathognomonic microscopic changes, as the histology will drastically differ based on the etiology. Workup can be extensive; cues to the underlying pathology should be sought based on the patient’s history and concurrent presenting symptoms. Livedo reticularis is the most common dermatologic finding in patients with antiphospholipid syndrome, and workup should include antiphospholipid antibodies (eg, lupus anticoagulant, anticardiolipin, anti–beta-2-glycoproteins) as well as lupus testing (eg, antinuclear antibodies, anti– double-stranded DNA).19 Treatment is targeted at the underlying disease process.
Cryoglobulinemia is a disease characterized by abnormal serum immunoglobulins that precipitate at cold temperatures and is further subcategorized by the type of complexes that are deposited.20 Type I represents purely monoclonal cryoglobulins, type III purely polyclonal, and type II a mixed picture. Clinical manifestations arise from excessive deposition of these proteins in the skin, joints, peripheral vasculature, and kidneys leading to purpuric skin lesions, chronic ulceration, arthralgia, and glomerulonephritis. Cutaneous findings may include erythematous to purpuric macular or papular changes with or without the presence of ulceration, infarction, or hemorrhagic crusting.21 Systemic disease often underlies a diagnosis, and further investigation for hepatitis C virus, connective tissue disease, and hematologic malignancies should be considered.20 Treatment is targeted at underlying systemic disease, such as antiviral treatment for hepatitis or chemotherapeutic regimens for hematologic disease.22
Polyarteritis nodosa is a systemic necrotizing vasculitis that typically involves small- to medium-sized arteries. Cutaneous manifestations often include subcutaneous nodules, livedo reticularis, and ulcerations most found on the lower extremities.23 Systemic symptoms including fever, myalgia, arthralgia, and neuropathy often are present. Characteristic histopathology findings include inflammation and destruction of medium-sized arteries at the junctional zone of the dermis and subcutis along with microaneurysms along the vessels.24 Treatment is based on the severity of disease, with localized cutaneous disease often being controlled with topical steroids and anti-inflammatory agents, while more widespread disease requires immunosuppression with systemic steroids, hydroxychloroquine, azathioprine, methotrexate, mycophenolate mofetil, or intravenous immunoglobulins.23
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
- Nigwekar SU, Thadhani R, Brandenburg VM. Calciphylaxis. N Engl J Med. 2018;378:1704-1714. doi:10.1056/NEJMra1505292
- Nigwekar SU, Kroshinsky D, Nazarian RM, et al. Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis. 2015;66:133-146. doi:10.1053/j.ajkd.2015.01.034
- Chang JJ. Calciphylaxis: diagnosis, pathogenesis, and treatment. Adv Skin Wound Care. 2019;32:205-215. doi:10.1097/01 .ASW.0000554443.14002.13
- Zhang Y, Corapi KM, Luongo M, et al. Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis. 2016;9:235-241. doi:10.2147/ijnrd.S115701
- Chen TY, Lehman JS, Gibson LE, et al. Histopathology of calciphylaxis: cohort study with clinical correlations. Am J Dermatopathol. 2017;39:795-802. doi:10.1097/DAD.0000000000000824
- Kodumudi V, Jeha GM, Mydlo N, et al. Management of cutaneous calciphylaxis. Adv Ther. 2020;37:4797-4807. doi:10.1007 /s12325-020-01504-w
- Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421-3429. doi:10.1681/asn.2015091065
- McCarthy JT, El-Azhary RA, Patzelt MT, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384-1394. doi:10.1016/j.mayocp.2016.06.025
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217. doi:10.1046/j.1523-1755.2002.00375.x
- Nigwekar SU, Brunelli SM, Meade D, et al. Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol. 2013;8:1162-1170. doi:10.2215/cjn.09880912
- Zitt E, König M, Vychytil A, et al. Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant. 2013;28:1232-1240. doi:10.1093/ndt/gfs548
- Peng T, Zhuo L, Wang Y, et al. Systematic review of sodium thiosulfate in treating calciphylaxis in chronic kidney disease patients. Nephrology (Carlton). 2018;23:669-675. doi:10.1111/nep.13081
- Miller K, Hunt R, Chu J, et al. Erythema ab igne. Dermatol Online J. 2011;17:28.
- Kettelhut EA, Traylor J, Sathe NC, et al. Erythema ab igne. StatPearls. StatPearls Publishing; 2022.
- Knöpfel N, Weibel L. Erythema Ab Igne. JAMA Dermatol. 2021;157: 106. doi:10.1001/jamadermatol.2020.3995
- Sigmon JR, Cantrell J, Teague D, et al. Poorly differentiated carcinoma arising in the setting of erythema ab igne. Am J Dermatopathol. 2013;35:676-678. doi:10.1097/DAD.0b013e3182871648
- Rose AE, Sagger V, Boyd KP, et al. Livedo reticularis. Dermatol Online J. 2013;19:20705.
- Sajjan VV, Lunge S, Swamy MB, et al. Livedo reticularis: a review of the literature. Indian Dermatol Online J. 2015;6:315-321. doi:10.4103/2229-5178.164493
- Uthman IW, Khamashta MA. Livedo racemosa: a striking dermatological sign for the antiphospholipid syndrome. J Rheumatol. 2006;33:2379-2382.
- Desbois AC, Cacoub P, Saadoun D. Cryoglobulinemia: an update in 2019. Joint Bone Spine. 2019;86:707-713. doi:10.1016/j .jbspin.2019.01.016
- Cohen SJ, Pittelkow MR, Su WP. Cutaneous manifestations of cryoglobulinemia: clinical and histopathologic study of seventy-two patients. J Am Acad Dermatol. 1991;25(1, pt 1):21-27. doi:10.1016 /0190-9622(91)70168-2
- Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia (review). Mol Med Rep. 2012;6:3-8. doi:10.3892/mmr.2012.861
- Turska M, Parada-Turska J. Cutaneous polyarteritis nodosa. Wiad Lek. 2018;71(1, pt 1):73-77.
- De Virgilio A, Greco A, Magliulo G, et al. Polyarteritis nodosa: a contemporary overview. Autoimmun Rev. 2016;15:564-570. doi:10.1016/j.autrev.2016.02.015
A 72-year-old woman presented to the emergency department with concerns of confusion and lethargy during a session of peritoneal dialysis, which she had been receiving for the last 2 years for end-stage renal disease. She had a history of type 2 diabetes mellitus, diabetic retinopathy, hypertension, coronary artery disease, and peripheral vascular disease preceding a recent right below-knee amputation. A review of systems was positive for a rash on the thighs of several weeks’ duration that was preceded by several days of burning pain in the same distribution. Physical examination revealed retiform purpura with irregular contours and interspersed white stellate patterns scattered across the superomedial thighs, right lower back, and left lower abdomen. An initial laboratory workup revealed an elevated creatinine level of 5.03 mg/dL (reference range, 0.6–1.1 mg/dL; baseline level, 3.0 mg/dL) and mild leukocytosis (12.5 cells/mm3 [reference range, 4.5–11.0 cells/mm3]). Dermatology was consulted, and a 4-mm punch biopsy was obtained from the left medial thigh. Nephrology, infectious disease, and wound care consultations also were placed.