User login
ObGyns’ status of Maintenance of Certification now public
The American Board of Medical Specialties (ABMS) sets the standards for physician Board Certification. ABMS has begun to publicly report on its Web site (www.CertificationMatters.org) the status of whether ObGyns meet the ABMS Maintenance of Certification® (ABMS MOC®) program requirements established by the American Board of Obstetrics and Gynecology (ABOG).
There are 24 ABMS Member Boards; 7 began reporting MOC status of the physicians they certify in August 2011, and ABOG is one of 11 boards that began to report MOC status in August 2012. The remaining six ABMS Member Boards expect to make MOC status available by 2014.
The results of a search show the physician’s name, the name of the ABMS Member Board(s) that certify the physician, and a “Yes”, “No,” or “Not Required” response to whether the physician is meeting the MOC requirements of that Member Board. In addition, the physician’s profile contains these features:
- His or her status of all specialty and subspecialty certificates. For example, a physician can be Board Certified in General Obstetrics and Gynecology and the subspecialty of Maternal-Fetal Medicine.
- If a physician became Board Certified before his or her Member Board(s) established their MOC program, and he or she is exempt from participating in MOC, a “Not Required” option is provided. If that physician voluntarily meets the MOC requirements of his or her Member Board(s), the profile will show a “Yes.”
Nearly 800,000 Board Certified physicians voluntarily meet specialty-specific standards beyond what is required for state licensing. Board Certification involves rigorous testing and peer evaluation designed and administered by specialists in a specific medical field. The ABMS MOC program promotes career-long learning, quality improvement activities, and self-assessment. Insurers, hospitals, quality and credentialing organizations, and the federal government recognize ABMS MOC as an important quality marker.
“As the organization that has set the gold standard in physician specialty certification for more than 75 years, this effort further demonstrates ABMS’ commitment to advance quality health care for patients,” said Lois Margaret Nora, MD, JD, MBA, ABMS President and Chief Executive Officer.
Anyone can visit www.CertificationMatters.org to see if a physician is Board Certified by an ABMS Member Board.
We want to hear from you! Tell us what you think.
Reference
1. More doctors to publicly report Maintenance of Certification status in ABMS database [news release]. Chicago, Ill: American Board of Medical Specialties; September 6, 2012. http://www.abms.org/News_and_Events/Media_Newsroom/Releases/release_ABMSDisplayofMOCPRforCredentialers_08212012.aspx. Accessed October 15, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>VTE risk varies by hormone therapy formulation</para></item> <item><para>New molecular cervical cancer test based on NIH’s TERC gene marker</para></item> <item><para>An interview with PAGS course co–chairs Mickey Karram, MD, and Tommaso Falcone, MD</para></item> <item><para>Pregnant women taking antihypertensives—some shown to cause fetal risk—in increasing numbers</para></item> <item><para>Survey: Burnout is widespread among US physicians</para></item> <item><para>UPDATE: CDC publishes final recommendations—baby boomers need a one-time hep C test</para></item> <item><para>Gonorrhea treatment guidelines revised by CDC</para></item> <item><para>Implementation of ICD-10 codes delayed 1 year</para></item> </list>
The American Board of Medical Specialties (ABMS) sets the standards for physician Board Certification. ABMS has begun to publicly report on its Web site (www.CertificationMatters.org) the status of whether ObGyns meet the ABMS Maintenance of Certification® (ABMS MOC®) program requirements established by the American Board of Obstetrics and Gynecology (ABOG).
There are 24 ABMS Member Boards; 7 began reporting MOC status of the physicians they certify in August 2011, and ABOG is one of 11 boards that began to report MOC status in August 2012. The remaining six ABMS Member Boards expect to make MOC status available by 2014.
The results of a search show the physician’s name, the name of the ABMS Member Board(s) that certify the physician, and a “Yes”, “No,” or “Not Required” response to whether the physician is meeting the MOC requirements of that Member Board. In addition, the physician’s profile contains these features:
- His or her status of all specialty and subspecialty certificates. For example, a physician can be Board Certified in General Obstetrics and Gynecology and the subspecialty of Maternal-Fetal Medicine.
- If a physician became Board Certified before his or her Member Board(s) established their MOC program, and he or she is exempt from participating in MOC, a “Not Required” option is provided. If that physician voluntarily meets the MOC requirements of his or her Member Board(s), the profile will show a “Yes.”
Nearly 800,000 Board Certified physicians voluntarily meet specialty-specific standards beyond what is required for state licensing. Board Certification involves rigorous testing and peer evaluation designed and administered by specialists in a specific medical field. The ABMS MOC program promotes career-long learning, quality improvement activities, and self-assessment. Insurers, hospitals, quality and credentialing organizations, and the federal government recognize ABMS MOC as an important quality marker.
“As the organization that has set the gold standard in physician specialty certification for more than 75 years, this effort further demonstrates ABMS’ commitment to advance quality health care for patients,” said Lois Margaret Nora, MD, JD, MBA, ABMS President and Chief Executive Officer.
Anyone can visit www.CertificationMatters.org to see if a physician is Board Certified by an ABMS Member Board.
We want to hear from you! Tell us what you think.
The American Board of Medical Specialties (ABMS) sets the standards for physician Board Certification. ABMS has begun to publicly report on its Web site (www.CertificationMatters.org) the status of whether ObGyns meet the ABMS Maintenance of Certification® (ABMS MOC®) program requirements established by the American Board of Obstetrics and Gynecology (ABOG).
There are 24 ABMS Member Boards; 7 began reporting MOC status of the physicians they certify in August 2011, and ABOG is one of 11 boards that began to report MOC status in August 2012. The remaining six ABMS Member Boards expect to make MOC status available by 2014.
The results of a search show the physician’s name, the name of the ABMS Member Board(s) that certify the physician, and a “Yes”, “No,” or “Not Required” response to whether the physician is meeting the MOC requirements of that Member Board. In addition, the physician’s profile contains these features:
- His or her status of all specialty and subspecialty certificates. For example, a physician can be Board Certified in General Obstetrics and Gynecology and the subspecialty of Maternal-Fetal Medicine.
- If a physician became Board Certified before his or her Member Board(s) established their MOC program, and he or she is exempt from participating in MOC, a “Not Required” option is provided. If that physician voluntarily meets the MOC requirements of his or her Member Board(s), the profile will show a “Yes.”
Nearly 800,000 Board Certified physicians voluntarily meet specialty-specific standards beyond what is required for state licensing. Board Certification involves rigorous testing and peer evaluation designed and administered by specialists in a specific medical field. The ABMS MOC program promotes career-long learning, quality improvement activities, and self-assessment. Insurers, hospitals, quality and credentialing organizations, and the federal government recognize ABMS MOC as an important quality marker.
“As the organization that has set the gold standard in physician specialty certification for more than 75 years, this effort further demonstrates ABMS’ commitment to advance quality health care for patients,” said Lois Margaret Nora, MD, JD, MBA, ABMS President and Chief Executive Officer.
Anyone can visit www.CertificationMatters.org to see if a physician is Board Certified by an ABMS Member Board.
We want to hear from you! Tell us what you think.
Reference
1. More doctors to publicly report Maintenance of Certification status in ABMS database [news release]. Chicago, Ill: American Board of Medical Specialties; September 6, 2012. http://www.abms.org/News_and_Events/Media_Newsroom/Releases/release_ABMSDisplayofMOCPRforCredentialers_08212012.aspx. Accessed October 15, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>VTE risk varies by hormone therapy formulation</para></item> <item><para>New molecular cervical cancer test based on NIH’s TERC gene marker</para></item> <item><para>An interview with PAGS course co–chairs Mickey Karram, MD, and Tommaso Falcone, MD</para></item> <item><para>Pregnant women taking antihypertensives—some shown to cause fetal risk—in increasing numbers</para></item> <item><para>Survey: Burnout is widespread among US physicians</para></item> <item><para>UPDATE: CDC publishes final recommendations—baby boomers need a one-time hep C test</para></item> <item><para>Gonorrhea treatment guidelines revised by CDC</para></item> <item><para>Implementation of ICD-10 codes delayed 1 year</para></item> </list>
Reference
1. More doctors to publicly report Maintenance of Certification status in ABMS database [news release]. Chicago, Ill: American Board of Medical Specialties; September 6, 2012. http://www.abms.org/News_and_Events/Media_Newsroom/Releases/release_ABMSDisplayofMOCPRforCredentialers_08212012.aspx. Accessed October 15, 2012.
More NEWS FOR YOUR PRACTICE…
<list type="bullet"> <item><para>VTE risk varies by hormone therapy formulation</para></item> <item><para>New molecular cervical cancer test based on NIH’s TERC gene marker</para></item> <item><para>An interview with PAGS course co–chairs Mickey Karram, MD, and Tommaso Falcone, MD</para></item> <item><para>Pregnant women taking antihypertensives—some shown to cause fetal risk—in increasing numbers</para></item> <item><para>Survey: Burnout is widespread among US physicians</para></item> <item><para>UPDATE: CDC publishes final recommendations—baby boomers need a one-time hep C test</para></item> <item><para>Gonorrhea treatment guidelines revised by CDC</para></item> <item><para>Implementation of ICD-10 codes delayed 1 year</para></item> </list>
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6
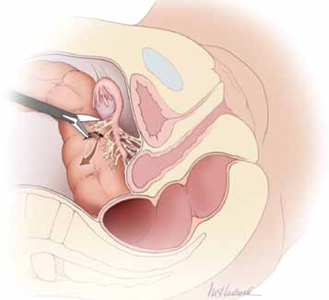
FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.
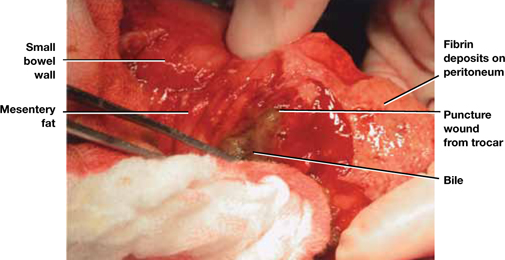
FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
New molecular cervical cancer test based on TERC gene marker
The TERC test, a new molecular assay, examines abnormalities of the telomerase RNA component (TERC) gene based on knowledge about which molecular changes turn cervical dysplasia into malignancy. This knowledge comes from National Institutes of Health (NIH) research demonstrating that the TERC gene is amplified in the precursor cells of cervical cancer.1,2

Neal M. Lonky, MD, MPH
This is the evolution from interpreting anatomical microscopic features to molecular markers associated with cellular proliferation that move our paradigm from “snapshot” diagnosis to a “movie preview” of the prognostic significance of the lesions of the cervix. Like other molecular markers, it will help us assess risk more accurately, preventing unnecessary visits and treatment in the lower risk cohort (with the associated stress and complications), while moving those positive for the markers to appropriate care.
Dr. Lonky is clinical professor of obstetrics and gynecology at the University of California–Irvine and a member of the board of directors of Southern California Permanente Medical Group. Dr. Lonky is an OBG Management contributing editor.
When standard screening like the Pap smear and human papillomavirus (HPV) test do not provide clear results about a patient’s cervical cancer risk, the TERC test will enable detection of specific cancerous characteristics.
Following TERC and HPV testing, women at high risk can undergo additional cervical biopsy under colposcopy and benefit from more aggressive observation and treatment. Women found to be at low risk may not have to undergo extensive follow-up.
It is estimated that almost 12,000 new cases of HPV-associated cervical cancer are diagnosed in the United States each year.3 It is estimated that this test could help detect cervical cancer in more than 1.5 million women with ambiguous Pap test results.2
One of NIH’s goals is to evolve their scientific discoveries into clinically valuable technologies through collaboration with commercial organizations, which is how Quest Diagnostics acquired a nonexclusive patent license for the human TERC gene marker.2
We want to hear from you! Tell us what you think.
1. Heselmever-Haddad K, Sommerfeld K, White NM, et al. Genomic amplification of the human telomerase gene (TERC) in Pap smear predicts the development of cervical cancer. Am J Pathol. 2005;166(4):1229-1238.
2. Quest Diagnostics launches molecular cervical cancer test based on National Institutes of Health’s TERC gene marker [news release]. Madison, NJ: Quest Diagnostics; August 30, 2012. http://newsroom.questdiagnostics.com/2012-08-30-Quest-Diagnostics-Launches-Molecular-Cervical-Cancer-Test-Based-on-National-Institutes-of-Healths-TERC-Gene-Marker. Accessed September 13, 2012.
3. Centers for Disease Control and Prevention. HPV-associated cervical cancer rates by race and ethnicity. http://www.cdc.gov/cancer/hpv/statistics/cervical.htm. Published August 13, 2012. Accessed September 13, 2012.
The TERC test, a new molecular assay, examines abnormalities of the telomerase RNA component (TERC) gene based on knowledge about which molecular changes turn cervical dysplasia into malignancy. This knowledge comes from National Institutes of Health (NIH) research demonstrating that the TERC gene is amplified in the precursor cells of cervical cancer.1,2

Neal M. Lonky, MD, MPH
This is the evolution from interpreting anatomical microscopic features to molecular markers associated with cellular proliferation that move our paradigm from “snapshot” diagnosis to a “movie preview” of the prognostic significance of the lesions of the cervix. Like other molecular markers, it will help us assess risk more accurately, preventing unnecessary visits and treatment in the lower risk cohort (with the associated stress and complications), while moving those positive for the markers to appropriate care.
Dr. Lonky is clinical professor of obstetrics and gynecology at the University of California–Irvine and a member of the board of directors of Southern California Permanente Medical Group. Dr. Lonky is an OBG Management contributing editor.
When standard screening like the Pap smear and human papillomavirus (HPV) test do not provide clear results about a patient’s cervical cancer risk, the TERC test will enable detection of specific cancerous characteristics.
Following TERC and HPV testing, women at high risk can undergo additional cervical biopsy under colposcopy and benefit from more aggressive observation and treatment. Women found to be at low risk may not have to undergo extensive follow-up.
It is estimated that almost 12,000 new cases of HPV-associated cervical cancer are diagnosed in the United States each year.3 It is estimated that this test could help detect cervical cancer in more than 1.5 million women with ambiguous Pap test results.2
One of NIH’s goals is to evolve their scientific discoveries into clinically valuable technologies through collaboration with commercial organizations, which is how Quest Diagnostics acquired a nonexclusive patent license for the human TERC gene marker.2
We want to hear from you! Tell us what you think.
The TERC test, a new molecular assay, examines abnormalities of the telomerase RNA component (TERC) gene based on knowledge about which molecular changes turn cervical dysplasia into malignancy. This knowledge comes from National Institutes of Health (NIH) research demonstrating that the TERC gene is amplified in the precursor cells of cervical cancer.1,2

Neal M. Lonky, MD, MPH
This is the evolution from interpreting anatomical microscopic features to molecular markers associated with cellular proliferation that move our paradigm from “snapshot” diagnosis to a “movie preview” of the prognostic significance of the lesions of the cervix. Like other molecular markers, it will help us assess risk more accurately, preventing unnecessary visits and treatment in the lower risk cohort (with the associated stress and complications), while moving those positive for the markers to appropriate care.
Dr. Lonky is clinical professor of obstetrics and gynecology at the University of California–Irvine and a member of the board of directors of Southern California Permanente Medical Group. Dr. Lonky is an OBG Management contributing editor.
When standard screening like the Pap smear and human papillomavirus (HPV) test do not provide clear results about a patient’s cervical cancer risk, the TERC test will enable detection of specific cancerous characteristics.
Following TERC and HPV testing, women at high risk can undergo additional cervical biopsy under colposcopy and benefit from more aggressive observation and treatment. Women found to be at low risk may not have to undergo extensive follow-up.
It is estimated that almost 12,000 new cases of HPV-associated cervical cancer are diagnosed in the United States each year.3 It is estimated that this test could help detect cervical cancer in more than 1.5 million women with ambiguous Pap test results.2
One of NIH’s goals is to evolve their scientific discoveries into clinically valuable technologies through collaboration with commercial organizations, which is how Quest Diagnostics acquired a nonexclusive patent license for the human TERC gene marker.2
We want to hear from you! Tell us what you think.
1. Heselmever-Haddad K, Sommerfeld K, White NM, et al. Genomic amplification of the human telomerase gene (TERC) in Pap smear predicts the development of cervical cancer. Am J Pathol. 2005;166(4):1229-1238.
2. Quest Diagnostics launches molecular cervical cancer test based on National Institutes of Health’s TERC gene marker [news release]. Madison, NJ: Quest Diagnostics; August 30, 2012. http://newsroom.questdiagnostics.com/2012-08-30-Quest-Diagnostics-Launches-Molecular-Cervical-Cancer-Test-Based-on-National-Institutes-of-Healths-TERC-Gene-Marker. Accessed September 13, 2012.
3. Centers for Disease Control and Prevention. HPV-associated cervical cancer rates by race and ethnicity. http://www.cdc.gov/cancer/hpv/statistics/cervical.htm. Published August 13, 2012. Accessed September 13, 2012.
1. Heselmever-Haddad K, Sommerfeld K, White NM, et al. Genomic amplification of the human telomerase gene (TERC) in Pap smear predicts the development of cervical cancer. Am J Pathol. 2005;166(4):1229-1238.
2. Quest Diagnostics launches molecular cervical cancer test based on National Institutes of Health’s TERC gene marker [news release]. Madison, NJ: Quest Diagnostics; August 30, 2012. http://newsroom.questdiagnostics.com/2012-08-30-Quest-Diagnostics-Launches-Molecular-Cervical-Cancer-Test-Based-on-National-Institutes-of-Healths-TERC-Gene-Marker. Accessed September 13, 2012.
3. Centers for Disease Control and Prevention. HPV-associated cervical cancer rates by race and ethnicity. http://www.cdc.gov/cancer/hpv/statistics/cervical.htm. Published August 13, 2012. Accessed September 13, 2012.
An interview with PAGS course co–chairs Mickey M. Karram, MD, and Tommaso Falcone, MD
| December 13–15, 2012 at the Bellagio, Las Vegas For more information or to register for 2012 PAGS, visit www.PAGS-CME.org |
OBG Management: As Co-Chairs of this meeting, what are your goals for this year’s conference?
Dr. Falcone: Our primary goal is to discuss important—and proven—concepts and potential new approaches to and technology for, gynecologic surgery.
Dr. Karram: Another goal is to update participants on new and challenging issues related to pelvic surgery.
OBG Management: What are some of the specific offerings you are looking forward to this year?
Dr. Falcone: Two sessions will focus on the surgical management of endometriosis. In addition, many attendees enjoy our hysterectomy sessions, in which we offer the latest information on all surgical approaches. We also invite audience participation to our case presentations and always enjoy a lively discussion of individual approaches. And not only the audience benefits—even the presenters usually learn a pearl or two.
Dr. Karram: Like Dr. Falcone, I’m looking forward to our discussions of the routes of hysterectomy, as well as offerings on mesh augmentation for prolapse repair and new technologies in gynecologic surgery.
Dr. Falcone: This year, too, we tackle the practice management side of gynecologic surgery—a mini MBA, if you will. (This aspect of PAGS is optional, by the way.) And last but not least, Barbara S. Levy, MD, Vice President of Health Policy at ACOG, will deliver the keynote address.
OBG Management: How does PAGS differ from other meetings?
Dr. Falcone: There are very few gynecologic surgery meetings in the United States. Most ObGyn meetings attempt to cover the full range of the specialty, most of which is nonsurgical in nature. Other meetings focus only on a single type of surgery or surgical approach. We’re different; we cover all aspects of gynecologic surgery.
Dr. Karram: We also offer objective, data-driven discussions about surgical decisions that need to be made on a daily basis.
OBG Management: What do you expect attendees to take away from the meeting?
Dr. Karram: A better understanding of the indications for and technical aspects of a variety of surgical procedures, as well as a greater understanding of how to avoid and manage surgical complications.
Dr. Falcone: I also expect them to walk away with a better appreciation of the challenges involved in various gynecologic surgeries—new and old—and a greater sense of the evidence backing proven surgical approaches. They will also benefit from the opportunity to meet and talk with their peers and faculty. And, of course, there are the CME credits—as many as 19.5 offered this year!
We want to hear from you! Tell us what you think.
| December 13–15, 2012 at the Bellagio, Las Vegas For more information or to register for 2012 PAGS, visit www.PAGS-CME.org |
OBG Management: As Co-Chairs of this meeting, what are your goals for this year’s conference?
Dr. Falcone: Our primary goal is to discuss important—and proven—concepts and potential new approaches to and technology for, gynecologic surgery.
Dr. Karram: Another goal is to update participants on new and challenging issues related to pelvic surgery.
OBG Management: What are some of the specific offerings you are looking forward to this year?
Dr. Falcone: Two sessions will focus on the surgical management of endometriosis. In addition, many attendees enjoy our hysterectomy sessions, in which we offer the latest information on all surgical approaches. We also invite audience participation to our case presentations and always enjoy a lively discussion of individual approaches. And not only the audience benefits—even the presenters usually learn a pearl or two.
Dr. Karram: Like Dr. Falcone, I’m looking forward to our discussions of the routes of hysterectomy, as well as offerings on mesh augmentation for prolapse repair and new technologies in gynecologic surgery.
Dr. Falcone: This year, too, we tackle the practice management side of gynecologic surgery—a mini MBA, if you will. (This aspect of PAGS is optional, by the way.) And last but not least, Barbara S. Levy, MD, Vice President of Health Policy at ACOG, will deliver the keynote address.
OBG Management: How does PAGS differ from other meetings?
Dr. Falcone: There are very few gynecologic surgery meetings in the United States. Most ObGyn meetings attempt to cover the full range of the specialty, most of which is nonsurgical in nature. Other meetings focus only on a single type of surgery or surgical approach. We’re different; we cover all aspects of gynecologic surgery.
Dr. Karram: We also offer objective, data-driven discussions about surgical decisions that need to be made on a daily basis.
OBG Management: What do you expect attendees to take away from the meeting?
Dr. Karram: A better understanding of the indications for and technical aspects of a variety of surgical procedures, as well as a greater understanding of how to avoid and manage surgical complications.
Dr. Falcone: I also expect them to walk away with a better appreciation of the challenges involved in various gynecologic surgeries—new and old—and a greater sense of the evidence backing proven surgical approaches. They will also benefit from the opportunity to meet and talk with their peers and faculty. And, of course, there are the CME credits—as many as 19.5 offered this year!
We want to hear from you! Tell us what you think.
| December 13–15, 2012 at the Bellagio, Las Vegas For more information or to register for 2012 PAGS, visit www.PAGS-CME.org |
OBG Management: As Co-Chairs of this meeting, what are your goals for this year’s conference?
Dr. Falcone: Our primary goal is to discuss important—and proven—concepts and potential new approaches to and technology for, gynecologic surgery.
Dr. Karram: Another goal is to update participants on new and challenging issues related to pelvic surgery.
OBG Management: What are some of the specific offerings you are looking forward to this year?
Dr. Falcone: Two sessions will focus on the surgical management of endometriosis. In addition, many attendees enjoy our hysterectomy sessions, in which we offer the latest information on all surgical approaches. We also invite audience participation to our case presentations and always enjoy a lively discussion of individual approaches. And not only the audience benefits—even the presenters usually learn a pearl or two.
Dr. Karram: Like Dr. Falcone, I’m looking forward to our discussions of the routes of hysterectomy, as well as offerings on mesh augmentation for prolapse repair and new technologies in gynecologic surgery.
Dr. Falcone: This year, too, we tackle the practice management side of gynecologic surgery—a mini MBA, if you will. (This aspect of PAGS is optional, by the way.) And last but not least, Barbara S. Levy, MD, Vice President of Health Policy at ACOG, will deliver the keynote address.
OBG Management: How does PAGS differ from other meetings?
Dr. Falcone: There are very few gynecologic surgery meetings in the United States. Most ObGyn meetings attempt to cover the full range of the specialty, most of which is nonsurgical in nature. Other meetings focus only on a single type of surgery or surgical approach. We’re different; we cover all aspects of gynecologic surgery.
Dr. Karram: We also offer objective, data-driven discussions about surgical decisions that need to be made on a daily basis.
OBG Management: What do you expect attendees to take away from the meeting?
Dr. Karram: A better understanding of the indications for and technical aspects of a variety of surgical procedures, as well as a greater understanding of how to avoid and manage surgical complications.
Dr. Falcone: I also expect them to walk away with a better appreciation of the challenges involved in various gynecologic surgeries—new and old—and a greater sense of the evidence backing proven surgical approaches. They will also benefit from the opportunity to meet and talk with their peers and faculty. And, of course, there are the CME credits—as many as 19.5 offered this year!
We want to hear from you! Tell us what you think.
Pregnant women taking antihypertensives—some shown to cause fetal risk—in increasing numbers
New research in the American Heart Association journal Hypertension indicates that almost 5% of pregnant women are prescribed antihypertensives, including some drugs that are contraindicated for mothers or their babies.
“Use of high blood pressure drugs during pregnancy is becoming increasingly common,” said Brian T. Bateman, MD, lead author and Assistant Professor of Anesthesia at Harvard Medical School in Boston, Massachusetts. "While we know high blood pressure, or hypertension, occurs in about 6% to 8% of all pregnancies, we know little about how women and their doctors treat the condition."1
A database of more than 1 million Medicaid patients was studied. Of those patients, 48,453 (4.4%) filled prescriptions for high blood pressure drugs during their pregnancies.1
- Antihypertensive drug use increased from 3.5% to 4.9% between 2000 and 2006.
- Antihypertensive drug users were older than nonusers, more likely to have diabetes or kidney disease, and more likely to be white or black than Hispanic or Asian.
- Nearly 2% of pregnant women filled prescriptions for antihypertensive drugs during the first trimester; 1.7% during the second trimester; and 3.2% during the third trimester.
- Angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers—both of which have been shown to have harmful side effects during pregnancy—were used by 4.9% of users in the second trimester, and 1.1% in the third trimester.
Research is needed because there are limited data on the safety, efficacy, and proper use of antihypertensive drugs during pregnancy.
“These drugs can cause poor growth, kidney problems, and even death of the newborn, wrote Bateman. “If women are taking one of these blood pressure medications and they become pregnant or plan to do so, they and their doctors should discuss treatment choices during pregnancy.”1
For access to the abstract, click here.
We want to hear from you! Tell us what you think.
1. More pregnant women taking high blood pressure drugs, yet safety unclear (news release). GlobeNewswire.com. http://www.globenewswire.com/newsroom/news.html?d=10004131. Published September 10, 2012. Accessed September 11, 2012.
2. Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population [published online ahead of print September 10, 2012]. Hypertension. doi: 10.1161/HYPERTENSIONAHA.112.197095.
New research in the American Heart Association journal Hypertension indicates that almost 5% of pregnant women are prescribed antihypertensives, including some drugs that are contraindicated for mothers or their babies.
“Use of high blood pressure drugs during pregnancy is becoming increasingly common,” said Brian T. Bateman, MD, lead author and Assistant Professor of Anesthesia at Harvard Medical School in Boston, Massachusetts. "While we know high blood pressure, or hypertension, occurs in about 6% to 8% of all pregnancies, we know little about how women and their doctors treat the condition."1
A database of more than 1 million Medicaid patients was studied. Of those patients, 48,453 (4.4%) filled prescriptions for high blood pressure drugs during their pregnancies.1
- Antihypertensive drug use increased from 3.5% to 4.9% between 2000 and 2006.
- Antihypertensive drug users were older than nonusers, more likely to have diabetes or kidney disease, and more likely to be white or black than Hispanic or Asian.
- Nearly 2% of pregnant women filled prescriptions for antihypertensive drugs during the first trimester; 1.7% during the second trimester; and 3.2% during the third trimester.
- Angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers—both of which have been shown to have harmful side effects during pregnancy—were used by 4.9% of users in the second trimester, and 1.1% in the third trimester.
Research is needed because there are limited data on the safety, efficacy, and proper use of antihypertensive drugs during pregnancy.
“These drugs can cause poor growth, kidney problems, and even death of the newborn, wrote Bateman. “If women are taking one of these blood pressure medications and they become pregnant or plan to do so, they and their doctors should discuss treatment choices during pregnancy.”1
For access to the abstract, click here.
We want to hear from you! Tell us what you think.
New research in the American Heart Association journal Hypertension indicates that almost 5% of pregnant women are prescribed antihypertensives, including some drugs that are contraindicated for mothers or their babies.
“Use of high blood pressure drugs during pregnancy is becoming increasingly common,” said Brian T. Bateman, MD, lead author and Assistant Professor of Anesthesia at Harvard Medical School in Boston, Massachusetts. "While we know high blood pressure, or hypertension, occurs in about 6% to 8% of all pregnancies, we know little about how women and their doctors treat the condition."1
A database of more than 1 million Medicaid patients was studied. Of those patients, 48,453 (4.4%) filled prescriptions for high blood pressure drugs during their pregnancies.1
- Antihypertensive drug use increased from 3.5% to 4.9% between 2000 and 2006.
- Antihypertensive drug users were older than nonusers, more likely to have diabetes or kidney disease, and more likely to be white or black than Hispanic or Asian.
- Nearly 2% of pregnant women filled prescriptions for antihypertensive drugs during the first trimester; 1.7% during the second trimester; and 3.2% during the third trimester.
- Angiotensin-converting-enzyme (ACE) inhibitors and angiotensin receptor blockers—both of which have been shown to have harmful side effects during pregnancy—were used by 4.9% of users in the second trimester, and 1.1% in the third trimester.
Research is needed because there are limited data on the safety, efficacy, and proper use of antihypertensive drugs during pregnancy.
“These drugs can cause poor growth, kidney problems, and even death of the newborn, wrote Bateman. “If women are taking one of these blood pressure medications and they become pregnant or plan to do so, they and their doctors should discuss treatment choices during pregnancy.”1
For access to the abstract, click here.
We want to hear from you! Tell us what you think.
1. More pregnant women taking high blood pressure drugs, yet safety unclear (news release). GlobeNewswire.com. http://www.globenewswire.com/newsroom/news.html?d=10004131. Published September 10, 2012. Accessed September 11, 2012.
2. Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population [published online ahead of print September 10, 2012]. Hypertension. doi: 10.1161/HYPERTENSIONAHA.112.197095.
1. More pregnant women taking high blood pressure drugs, yet safety unclear (news release). GlobeNewswire.com. http://www.globenewswire.com/newsroom/news.html?d=10004131. Published September 10, 2012. Accessed September 11, 2012.
2. Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population [published online ahead of print September 10, 2012]. Hypertension. doi: 10.1161/HYPERTENSIONAHA.112.197095.
Survey: Burnout is widespread among US physicians
A new survey confirms what many clinicians already know firsthand: Burnout is common among physicians in the United States. Almost half (45.8%) of a large sample of physicians (n = 7,288) reported at least one symptom of burnout, which included high emotional exhaustion (37.9%), a high level of depersonalization (29.4%), and a low sense of personal accomplishment (12.4%).1
Compared with a sample of working US adults (n = 3,442), physicians were more likely to report symptoms of burnout (37.9% vs 27.8%) and to be dissatisfied with their work-life balance (40.2% vs 23.2%).
These findings are disturbing because, as the investigators noted in their report, “burnout may erode professionalism, influence quality of care, increase the risk for medical errors, and promote early retirement. Burnout also seems to have adverse personal consequences for physicians, including contributions to broken relationships, problematic alcohol use, and suicidal ideation.”1
How ObGyns responded
Specialties reporting the highest level of burnout were emergency medicine, general internal medicine, neurology, and family medicine. Those with the lowest level of burnout were pathology, dermatology, general pediatrics, and preventive medicine.
The ObGyn specialty ranked near the top of the list in terms of burnout, with more than 45% of respondents reporting it. The specialty ranked near the bottom of the list (along with general surgery and general surgery subspecialties) in its level of satisfaction with work-life balance (just over 40%).
Some reasons for the high rate of burnout among ObGyns may be the need for extended call, low reimbursement for many procedures, and the high exposure to medicolegal risk among obstetricians.
Mitigating factors
When investigators analyzed data—adjusting for age, sex, relationship status, hours worked per week, and education—they found that burnout was less likely among physicians who were older and those who were married. The more hours worked per week, the greater the risk of burnout.
Education benefitted nonphysicians but tended to increase the likelihood of burnout among physicians. For example, compared with high school graduates, nonphysicians with higher levels of education had a lower risk of burnout (odds ratio [OR] for a bachelor’s degree, 0.80 [P = .048]; OR for a master’s degree, 0.71 [P = .01]; OR for a professional or doctoral degree other than an MD or DO degree, 0.64 [P = .04]). Among physicians, however, an MD or DO degree increased the risk of burnout (OR, 1.36; P <.001).
Data came from AMA Physician Masterfile
Investigators conducted their national survey of physicians in June 2011, with representation from all specialties. In addition, they surveyed a probability-based sample of the general US population for comparison.
The physician sample was compiled from data in the American Medical Association Physician Masterfile (PMF), “an almost complete record of all US physicians, independent of American Medical Association membership, that is primarily used for estimating the size of the physician workforce and for verifying professional credentials.”1
A systemic problem
In commenting on their findings, the authors suggested:
- When considered with the mounting evidence that physician burnout adversely affects quality of care, these findings suggest a highly prevalent and systemic problem threatening the foundation of the US medical care system. The fact that almost 1 in 2 US physicians has symptoms of burnout implies that the origins of this problem are rooted in the environment and care delivery system rather than in the personal characteristics of a few susceptible individuals. Policy makers and health care organizations must address the problem of physician burnout for the sake of physicians and their patients.1
ObGyn Louis Weinstein, MD, agrees that the problem is pervasive. Dr. Weinstein has written extensively about the problems of burnout and physician shortages.2-4
“These issues were recognized in obstetrics and gynecology years ago, but nothing has been done to improve the situation by any of our professional organizations,” Dr. Weinstein says.
“The physician shortage is being addressed by an increase in the size of medical school classes and by the opening of several new medical schools,” Dr. Weinstein continues. “However, these strategies will not solve the epidemic of physician dissatisfaction. There is a very real shortage—which will continue to worsen—of working physicians, and new recruits to medicine will not be immune to the problems of burnout and dissatisfaction. The medical profession must recognize the need to develop and adopt newer models of practice (for example, the laborist model) that will improve physician well-being, decrease physician dissatisfaction, and markedly improve patient safety.”
We want to hear from you! Tell us what you think.
1. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. Published online August 20, 2012. doi:10.1001/archinternmed.2012.3199.
2. Weinstein L, Wolfe HM. The downward spiral of physician satisfaction—an attempt to avert a crisis within the medical profession. Obstet Gynecol. 2007;109(5):1181-1183.
3. Weinstein L. The perinatologist’s lament. Obstet Gynecol. 2008;111(5):1201.-
4. Weinstein L. The unbearable unhappiness of the ObGyn: a crisis looms. OBG Manage. 2008;20(12):34-42.
A new survey confirms what many clinicians already know firsthand: Burnout is common among physicians in the United States. Almost half (45.8%) of a large sample of physicians (n = 7,288) reported at least one symptom of burnout, which included high emotional exhaustion (37.9%), a high level of depersonalization (29.4%), and a low sense of personal accomplishment (12.4%).1
Compared with a sample of working US adults (n = 3,442), physicians were more likely to report symptoms of burnout (37.9% vs 27.8%) and to be dissatisfied with their work-life balance (40.2% vs 23.2%).
These findings are disturbing because, as the investigators noted in their report, “burnout may erode professionalism, influence quality of care, increase the risk for medical errors, and promote early retirement. Burnout also seems to have adverse personal consequences for physicians, including contributions to broken relationships, problematic alcohol use, and suicidal ideation.”1
How ObGyns responded
Specialties reporting the highest level of burnout were emergency medicine, general internal medicine, neurology, and family medicine. Those with the lowest level of burnout were pathology, dermatology, general pediatrics, and preventive medicine.
The ObGyn specialty ranked near the top of the list in terms of burnout, with more than 45% of respondents reporting it. The specialty ranked near the bottom of the list (along with general surgery and general surgery subspecialties) in its level of satisfaction with work-life balance (just over 40%).
Some reasons for the high rate of burnout among ObGyns may be the need for extended call, low reimbursement for many procedures, and the high exposure to medicolegal risk among obstetricians.
Mitigating factors
When investigators analyzed data—adjusting for age, sex, relationship status, hours worked per week, and education—they found that burnout was less likely among physicians who were older and those who were married. The more hours worked per week, the greater the risk of burnout.
Education benefitted nonphysicians but tended to increase the likelihood of burnout among physicians. For example, compared with high school graduates, nonphysicians with higher levels of education had a lower risk of burnout (odds ratio [OR] for a bachelor’s degree, 0.80 [P = .048]; OR for a master’s degree, 0.71 [P = .01]; OR for a professional or doctoral degree other than an MD or DO degree, 0.64 [P = .04]). Among physicians, however, an MD or DO degree increased the risk of burnout (OR, 1.36; P <.001).
Data came from AMA Physician Masterfile
Investigators conducted their national survey of physicians in June 2011, with representation from all specialties. In addition, they surveyed a probability-based sample of the general US population for comparison.
The physician sample was compiled from data in the American Medical Association Physician Masterfile (PMF), “an almost complete record of all US physicians, independent of American Medical Association membership, that is primarily used for estimating the size of the physician workforce and for verifying professional credentials.”1
A systemic problem
In commenting on their findings, the authors suggested:
- When considered with the mounting evidence that physician burnout adversely affects quality of care, these findings suggest a highly prevalent and systemic problem threatening the foundation of the US medical care system. The fact that almost 1 in 2 US physicians has symptoms of burnout implies that the origins of this problem are rooted in the environment and care delivery system rather than in the personal characteristics of a few susceptible individuals. Policy makers and health care organizations must address the problem of physician burnout for the sake of physicians and their patients.1
ObGyn Louis Weinstein, MD, agrees that the problem is pervasive. Dr. Weinstein has written extensively about the problems of burnout and physician shortages.2-4
“These issues were recognized in obstetrics and gynecology years ago, but nothing has been done to improve the situation by any of our professional organizations,” Dr. Weinstein says.
“The physician shortage is being addressed by an increase in the size of medical school classes and by the opening of several new medical schools,” Dr. Weinstein continues. “However, these strategies will not solve the epidemic of physician dissatisfaction. There is a very real shortage—which will continue to worsen—of working physicians, and new recruits to medicine will not be immune to the problems of burnout and dissatisfaction. The medical profession must recognize the need to develop and adopt newer models of practice (for example, the laborist model) that will improve physician well-being, decrease physician dissatisfaction, and markedly improve patient safety.”
We want to hear from you! Tell us what you think.
A new survey confirms what many clinicians already know firsthand: Burnout is common among physicians in the United States. Almost half (45.8%) of a large sample of physicians (n = 7,288) reported at least one symptom of burnout, which included high emotional exhaustion (37.9%), a high level of depersonalization (29.4%), and a low sense of personal accomplishment (12.4%).1
Compared with a sample of working US adults (n = 3,442), physicians were more likely to report symptoms of burnout (37.9% vs 27.8%) and to be dissatisfied with their work-life balance (40.2% vs 23.2%).
These findings are disturbing because, as the investigators noted in their report, “burnout may erode professionalism, influence quality of care, increase the risk for medical errors, and promote early retirement. Burnout also seems to have adverse personal consequences for physicians, including contributions to broken relationships, problematic alcohol use, and suicidal ideation.”1
How ObGyns responded
Specialties reporting the highest level of burnout were emergency medicine, general internal medicine, neurology, and family medicine. Those with the lowest level of burnout were pathology, dermatology, general pediatrics, and preventive medicine.
The ObGyn specialty ranked near the top of the list in terms of burnout, with more than 45% of respondents reporting it. The specialty ranked near the bottom of the list (along with general surgery and general surgery subspecialties) in its level of satisfaction with work-life balance (just over 40%).
Some reasons for the high rate of burnout among ObGyns may be the need for extended call, low reimbursement for many procedures, and the high exposure to medicolegal risk among obstetricians.
Mitigating factors
When investigators analyzed data—adjusting for age, sex, relationship status, hours worked per week, and education—they found that burnout was less likely among physicians who were older and those who were married. The more hours worked per week, the greater the risk of burnout.
Education benefitted nonphysicians but tended to increase the likelihood of burnout among physicians. For example, compared with high school graduates, nonphysicians with higher levels of education had a lower risk of burnout (odds ratio [OR] for a bachelor’s degree, 0.80 [P = .048]; OR for a master’s degree, 0.71 [P = .01]; OR for a professional or doctoral degree other than an MD or DO degree, 0.64 [P = .04]). Among physicians, however, an MD or DO degree increased the risk of burnout (OR, 1.36; P <.001).
Data came from AMA Physician Masterfile
Investigators conducted their national survey of physicians in June 2011, with representation from all specialties. In addition, they surveyed a probability-based sample of the general US population for comparison.
The physician sample was compiled from data in the American Medical Association Physician Masterfile (PMF), “an almost complete record of all US physicians, independent of American Medical Association membership, that is primarily used for estimating the size of the physician workforce and for verifying professional credentials.”1
A systemic problem
In commenting on their findings, the authors suggested:
- When considered with the mounting evidence that physician burnout adversely affects quality of care, these findings suggest a highly prevalent and systemic problem threatening the foundation of the US medical care system. The fact that almost 1 in 2 US physicians has symptoms of burnout implies that the origins of this problem are rooted in the environment and care delivery system rather than in the personal characteristics of a few susceptible individuals. Policy makers and health care organizations must address the problem of physician burnout for the sake of physicians and their patients.1
ObGyn Louis Weinstein, MD, agrees that the problem is pervasive. Dr. Weinstein has written extensively about the problems of burnout and physician shortages.2-4
“These issues were recognized in obstetrics and gynecology years ago, but nothing has been done to improve the situation by any of our professional organizations,” Dr. Weinstein says.
“The physician shortage is being addressed by an increase in the size of medical school classes and by the opening of several new medical schools,” Dr. Weinstein continues. “However, these strategies will not solve the epidemic of physician dissatisfaction. There is a very real shortage—which will continue to worsen—of working physicians, and new recruits to medicine will not be immune to the problems of burnout and dissatisfaction. The medical profession must recognize the need to develop and adopt newer models of practice (for example, the laborist model) that will improve physician well-being, decrease physician dissatisfaction, and markedly improve patient safety.”
We want to hear from you! Tell us what you think.
1. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. Published online August 20, 2012. doi:10.1001/archinternmed.2012.3199.
2. Weinstein L, Wolfe HM. The downward spiral of physician satisfaction—an attempt to avert a crisis within the medical profession. Obstet Gynecol. 2007;109(5):1181-1183.
3. Weinstein L. The perinatologist’s lament. Obstet Gynecol. 2008;111(5):1201.-
4. Weinstein L. The unbearable unhappiness of the ObGyn: a crisis looms. OBG Manage. 2008;20(12):34-42.
1. Shanafelt TD, Boone S, Tan L, et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. Published online August 20, 2012. doi:10.1001/archinternmed.2012.3199.
2. Weinstein L, Wolfe HM. The downward spiral of physician satisfaction—an attempt to avert a crisis within the medical profession. Obstet Gynecol. 2007;109(5):1181-1183.
3. Weinstein L. The perinatologist’s lament. Obstet Gynecol. 2008;111(5):1201.-
4. Weinstein L. The unbearable unhappiness of the ObGyn: a crisis looms. OBG Manage. 2008;20(12):34-42.
Implementation of ICD-10 codes delayed 1 year
Change has come again to ICD-9 diagnostic codes
Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Advisor, November 2011)
Health and Human Services (HHS) Secretary Kathleen G. Sebelius recently announced that the date for compliance with International Classification of Diseases, 10th Edition, diagnosis and procedure codes, or ICD-10, has been changed from October 1, 2013, to October 1, 2014.
Secretary Sebelius noted that the date change has been made in reaction to many providers’ concerns about the administrative burdens they will face in years ahead due to implementation of electronic health records and the Affordable Care Act.1
“ICD-10 codes provide more robust and specific data that will help improve patient care and enable the exchange of our health-care data with that of the rest of the world that has long been using ICD-10. Entities covered under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) will be required to use the ICD-10 diagnostic and procedure codes,” said Secretary Sebelius.”1
The final rule was issued by the Centers for Medicare & Medicaid Services, and published in the Federal Register on September 5, 2012. The rule states that, “The change in the compliance date is intended to give covered health-care providers and other covered entities more time to prepare and fully test their systems to ensure a smooth and coordinated transition by all covered entities.”2
Adoption will “increase standardization within HIPAA standard transactions and provide a platform for other regulatory and industry initiatives. Their adoption will allow for a higher level of automation for health-care provider offices, particularly for provider processing of billing and insurance related tasks, eligibility responses from health plans, and remittance advice that describes health-care claim payments.”2
Decision based on costs versus savings
HHS is aware that health-care plans, hospitals, and physician practices are currently in the process of implementing the ICD-10 due to the anticipated 2013 compliance date. It is estimated that the 1-year delay may cost the entire health-care industry $1 billion to $6.6 billion. However, HHS also anticipates substantial savings ($3.6 billion to $8 billion) by avoiding costs that could incur if a significant number of providers are unprepared for the transition to ICD-10. Possible consequences of implementing ICD-10 in 2013 are: 1) health-care providers and plans could have to manually process claims for claims to be paid and 2) small health-care providers might have to arrange for loans or lines of credit to continue to provide health-care services because of delayed payments. The decision to delay administration of ICD-10 was based on these factors.3
The American Health Information Management Association (AHIMA) will continue to assist the industry through the implementation process. AHIMA CEO Lynne Thomas Gordon, MBA, RHIA, FACHE, CAE, wrote on August 24, 2012, “ICD-10-CM/PCS implementation is inevitable, but today’s news gives the health-care community the certainty and clarity it needs to move forward with implementation, testing, and training. We realize that a few are still apprehensive about the implementation process, and that is why AHIMA remains committed to assisting the health-care community with its transition to a new code set that will lead to improved patient care and reduced costs.”3
We want to hear from you! Tell us what you think.
Change has come again to ICD-9 diagnostic codes
Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Advisor, November 2011)
Health and Human Services (HHS) Secretary Kathleen G. Sebelius recently announced that the date for compliance with International Classification of Diseases, 10th Edition, diagnosis and procedure codes, or ICD-10, has been changed from October 1, 2013, to October 1, 2014.
Secretary Sebelius noted that the date change has been made in reaction to many providers’ concerns about the administrative burdens they will face in years ahead due to implementation of electronic health records and the Affordable Care Act.1
“ICD-10 codes provide more robust and specific data that will help improve patient care and enable the exchange of our health-care data with that of the rest of the world that has long been using ICD-10. Entities covered under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) will be required to use the ICD-10 diagnostic and procedure codes,” said Secretary Sebelius.”1
The final rule was issued by the Centers for Medicare & Medicaid Services, and published in the Federal Register on September 5, 2012. The rule states that, “The change in the compliance date is intended to give covered health-care providers and other covered entities more time to prepare and fully test their systems to ensure a smooth and coordinated transition by all covered entities.”2
Adoption will “increase standardization within HIPAA standard transactions and provide a platform for other regulatory and industry initiatives. Their adoption will allow for a higher level of automation for health-care provider offices, particularly for provider processing of billing and insurance related tasks, eligibility responses from health plans, and remittance advice that describes health-care claim payments.”2
Decision based on costs versus savings
HHS is aware that health-care plans, hospitals, and physician practices are currently in the process of implementing the ICD-10 due to the anticipated 2013 compliance date. It is estimated that the 1-year delay may cost the entire health-care industry $1 billion to $6.6 billion. However, HHS also anticipates substantial savings ($3.6 billion to $8 billion) by avoiding costs that could incur if a significant number of providers are unprepared for the transition to ICD-10. Possible consequences of implementing ICD-10 in 2013 are: 1) health-care providers and plans could have to manually process claims for claims to be paid and 2) small health-care providers might have to arrange for loans or lines of credit to continue to provide health-care services because of delayed payments. The decision to delay administration of ICD-10 was based on these factors.3
The American Health Information Management Association (AHIMA) will continue to assist the industry through the implementation process. AHIMA CEO Lynne Thomas Gordon, MBA, RHIA, FACHE, CAE, wrote on August 24, 2012, “ICD-10-CM/PCS implementation is inevitable, but today’s news gives the health-care community the certainty and clarity it needs to move forward with implementation, testing, and training. We realize that a few are still apprehensive about the implementation process, and that is why AHIMA remains committed to assisting the health-care community with its transition to a new code set that will lead to improved patient care and reduced costs.”3
We want to hear from you! Tell us what you think.
Change has come again to ICD-9 diagnostic codes
Melanie Witt, RN, CPC, COBGC, MA (Reimbursement Advisor, November 2011)
Health and Human Services (HHS) Secretary Kathleen G. Sebelius recently announced that the date for compliance with International Classification of Diseases, 10th Edition, diagnosis and procedure codes, or ICD-10, has been changed from October 1, 2013, to October 1, 2014.
Secretary Sebelius noted that the date change has been made in reaction to many providers’ concerns about the administrative burdens they will face in years ahead due to implementation of electronic health records and the Affordable Care Act.1
“ICD-10 codes provide more robust and specific data that will help improve patient care and enable the exchange of our health-care data with that of the rest of the world that has long been using ICD-10. Entities covered under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) will be required to use the ICD-10 diagnostic and procedure codes,” said Secretary Sebelius.”1
The final rule was issued by the Centers for Medicare & Medicaid Services, and published in the Federal Register on September 5, 2012. The rule states that, “The change in the compliance date is intended to give covered health-care providers and other covered entities more time to prepare and fully test their systems to ensure a smooth and coordinated transition by all covered entities.”2
Adoption will “increase standardization within HIPAA standard transactions and provide a platform for other regulatory and industry initiatives. Their adoption will allow for a higher level of automation for health-care provider offices, particularly for provider processing of billing and insurance related tasks, eligibility responses from health plans, and remittance advice that describes health-care claim payments.”2
Decision based on costs versus savings
HHS is aware that health-care plans, hospitals, and physician practices are currently in the process of implementing the ICD-10 due to the anticipated 2013 compliance date. It is estimated that the 1-year delay may cost the entire health-care industry $1 billion to $6.6 billion. However, HHS also anticipates substantial savings ($3.6 billion to $8 billion) by avoiding costs that could incur if a significant number of providers are unprepared for the transition to ICD-10. Possible consequences of implementing ICD-10 in 2013 are: 1) health-care providers and plans could have to manually process claims for claims to be paid and 2) small health-care providers might have to arrange for loans or lines of credit to continue to provide health-care services because of delayed payments. The decision to delay administration of ICD-10 was based on these factors.3
The American Health Information Management Association (AHIMA) will continue to assist the industry through the implementation process. AHIMA CEO Lynne Thomas Gordon, MBA, RHIA, FACHE, CAE, wrote on August 24, 2012, “ICD-10-CM/PCS implementation is inevitable, but today’s news gives the health-care community the certainty and clarity it needs to move forward with implementation, testing, and training. We realize that a few are still apprehensive about the implementation process, and that is why AHIMA remains committed to assisting the health-care community with its transition to a new code set that will lead to improved patient care and reduced costs.”3
We want to hear from you! Tell us what you think.
CDC: Stop using oral cephalosporins to treat gonorrhea
Gonorrhea, the second most commonly reported infectious disease in the United States, is increasing in incidence because Neisseria gonorrhoeae is progressively developing antibiotic resistance. Results of laboratory studies have provoked growing concern that cephalosporins, the only class of antibiotics that meets the current Centers for Disease Control and Prevention (CDC) efficacy standards, are also becoming ineffective in the treatment of gonorrhea.1
CDC updated its guidelines, as reported in Morbidity and Mortality Weekly Report (MMWR), recommending combination therapy with ceftriaxone and either azithromycin or doxycycline for uncomplicated gonorrhea. By revising the current treatment recommendations, the CDC hopes to delay cephalosporin resistance until new treatment options are developed.1
Gonorrhea is a major cause of pelvic inflammatory disease, which can lead to tubal infertility, ectopic pregnancy, and chronic pelvic pain. A pregnant woman with untreated gonorrhea has a higher risk for miscarriage, preterm birth, or premature rupture of membranes. An infected mother can transmit the disease to her child, with risk of blindness, joint infection, and sepsis in the baby.2 There is also strong epidemiologic and biologic evidence that N. gonorrhoeae infections enable HIV infection transmission.1,3
INCREASING INFECTION RATES
After a decline in reported gonorrhea rates to 98.1 cases per 100,000 population in 2009, the rate increased slightly in 2010 to 100.8 per 100,000, with 309,341 cases reported in the United States. In 2010, the reported gonorrhea rate for women was 106.5 per 100,000, slightly higher than for men (94.1 per 100,000). Reported gonorrhea rates were highest among adolescent girls ages 15 to19 years (570.9 per 100,000) and young women ages 20 to 24 years (560.7 per 100,000). The largest increases were observed among men and woman ages 20 to 24 years (4.9%) and 30 to 34 years (3.2%).3
“In the United States, about 300,000 cases of gonorrhea are reported each year, but because infected people often have no symptoms, the actual number of cases is probably closer to 700,000,” reported Gail Bolan, director of the CDC’s Sexually Transmitted Disease Prevention Division.4
GROWING CONCERN OVER RESISTANCE
Signs of growing resistance have only been seen in laboratory studies; there are no reported cases of treatment-resistant gonorrhea in the Unites States. However, the evidence of emerging cephalosporin resistance is following a similar pattern to that seen in 2007, when gonorrhea became fluoroquinolone-resistant.1,5
“The challenge is that there is not a well-studied second antibiotic we can turn to even when cephalosporin resistance does emerge,” said Robert D. Kirkcaldy, a medical epidemiologist at the CDC. “What we’ve been noticing is really since 2009 and 2010, it’s taking higher concentrations of antibiotic to kill the bacteria. This could mean resistance to the last antibiotic we have for gonorrhea could be on the horizon.”5
NEW CDC TREATMENT RECOMMENDATIONS
The CDC’s updated guidelines include treatment plans for uncomplicated disease; specific alternatives if ceftriaxone cannot be used; test-of-cure procedures; treatment failure strategies; and recommendations for sexual partners.1
Uncomplicated disease
To treat uncomplicated urogenital, anorectal, and pharyngeal gonorrhea, the CDC now recommends combination therapy with a single intramuscular dose of ceftriaxone 250 mg plus either a single dose of azithromycin 1 g orally or doxycycline 100 mg orally twice a day for 7 days.
Ceftriaxone, as a single 250-mg intramuscular injection, provides high and sustained bactericidal blood levels and is highly efficacious at all anatomic sites of N. gonorrhoeae infection currently circulating in the US. Clinical data are not available that support the use of an increased dose.
The percentage of isolates exhibiting tetracycline resistance was high but remained stable from 2006 (20.6%) to 2011 (21.6%).
Alternatives
When ceftriaxone cannot be used to treat urogenital or rectal gonorrhea, there are two options:
- if ceftriaxone is not readily available, give cefixime 400 mg orally plus either azithromycin 1 g orally or doxycycline 100 mg twice daily orally for 7 days
- if ceftriaxone cannot be given because of severe allergy, give azithromycin 2 g orally in a single dose.
A patient with gonorrhea treated with an alternative regimen should return 1 week after treatment for a test-of-cure at the infected anatomic site.
Test-of-cure—specimen culture is essential
The ideal test-of-cure is performed with culture or, if culture is not readily available, with a nucleic acid amplification testing (NAAT). If the NAAT is positive, make every effort to perform a confirmatory culture. All positive cultures for test-of-cure should undergo phenotypic antimicrobial susceptibility testing.
Unfortunately, the capacity of US laboratories to isolate N. gonorrhoeae by culture is declining rapidly because of the widespread use of NAATs for diagnosing gonorrhea. CDC reporters del Rio and colleagues write in MMWR:1
- It is essential that culture capacity for N. gonorrhoeae be maintained to monitor antimicrobial resistance trends and determine susceptibility to guide treatment following treatment failure. To help control gonorrhea in the United States, health-care providers must maintain the ability to collect specimens for culture and be knowledgeable of laboratories to which they can send specimens for culture. Health-care systems and health departments must support access to culture, and laboratories must maintain culture capacity or develop partnerships with laboratories that can perform culture.
Treatment failure
Clinicians treating a patient with persistent infection after treatment with the recommended therapy should culture relevant clinical specimens and perform antimicrobial susceptibility testing of N. gonorrhoeae isolates. Phenotypic antimicrobial susceptibility testing should be performed using disk diffusion, Etest (BioMerieux), or agar dilution. Data are limited on the use of NAAT-based antimicrobial susceptibility testing for genetic mutations.
Patients who experience treatment failure after treatment with alternative regimens should be treated with ceftriaxone 250 mg as a single intramuscular dose and azithromycin 2 g orally as a single dose and should receive infectious disease consultation.
Consult an infectious disease specialist, an STD/HIV Prevention Training Center, or the CDC for treatment advice, and report the case to the CDC through a health department within 24 hours of diagnosis. Conduct test-of-cure 7 days after re-treatment.
Sexual partners
Clinicians should make every effort to ensure that the patient’s sex partners from the preceding 60 days are evaluated promptly and treated as indicated.
“Treatment of patients with gonorrhea with the most effective therapy will limit the transmission of the disease, prevent complications, and slow the emergence of resistance,” wrote CDC reporters. “However, resistance to cephalosporins, including ceftriaxone, is expected to emerge. Reinvestment in gonorrhea prevention and control is warranted. New treatment options for gonorrhea are urgently needed.”1
For the full MMWR article on new gonorrhea treatment guidelines, visit http://1.usa.gov/OxjuaQ
We want to hear from you! Tell us what you think.
1. del Rio C, Hall G, Holmes K, et al. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR. 2012;61(31):590-594.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a3.htm. Published August 10, 2012. Accessed August 13, 2012.
2. Gonorrhea fact sheet. Womenshealth.gov. http://womenshealth.gov/publications/our-publications/fact-sheet/gonorrhea.cfm#h. Updated July 8, 2011. Accessed August 13, 2012.
3. Centers for Disease Control and Prevention. 2010 sexually transmitted diseases surveillance: Gonorrhea. CDC Web site. http://www.cdc.gov/std/stats10/gonorrhea.htm. Updated November 17, 2011. Accessed August 13, 2012.
4. The Washington Post. Only one gonorrhea drug remains for routine cases, CDC says. http://www.washingtonpost.com/national/health-science/gonorrhea-drug-is-becoming-less-effective-cdc-says/2012/08/09/874751e6-e256-11e1-ae7f-d2a13e249eb2_story.html. Published August 10, 2012. Accessed August 13, 2012.
5. Parker-Pope T. Worries about a gonorrhea “superbug”. NY Times Health Science. http://well.blogs.nytimes.com/2011/07/12/worries-about-a-gonorrhea-superbug/ Published July 12, 2011. Accessed August 13, 2012.
Gonorrhea, the second most commonly reported infectious disease in the United States, is increasing in incidence because Neisseria gonorrhoeae is progressively developing antibiotic resistance. Results of laboratory studies have provoked growing concern that cephalosporins, the only class of antibiotics that meets the current Centers for Disease Control and Prevention (CDC) efficacy standards, are also becoming ineffective in the treatment of gonorrhea.1
CDC updated its guidelines, as reported in Morbidity and Mortality Weekly Report (MMWR), recommending combination therapy with ceftriaxone and either azithromycin or doxycycline for uncomplicated gonorrhea. By revising the current treatment recommendations, the CDC hopes to delay cephalosporin resistance until new treatment options are developed.1
Gonorrhea is a major cause of pelvic inflammatory disease, which can lead to tubal infertility, ectopic pregnancy, and chronic pelvic pain. A pregnant woman with untreated gonorrhea has a higher risk for miscarriage, preterm birth, or premature rupture of membranes. An infected mother can transmit the disease to her child, with risk of blindness, joint infection, and sepsis in the baby.2 There is also strong epidemiologic and biologic evidence that N. gonorrhoeae infections enable HIV infection transmission.1,3
INCREASING INFECTION RATES
After a decline in reported gonorrhea rates to 98.1 cases per 100,000 population in 2009, the rate increased slightly in 2010 to 100.8 per 100,000, with 309,341 cases reported in the United States. In 2010, the reported gonorrhea rate for women was 106.5 per 100,000, slightly higher than for men (94.1 per 100,000). Reported gonorrhea rates were highest among adolescent girls ages 15 to19 years (570.9 per 100,000) and young women ages 20 to 24 years (560.7 per 100,000). The largest increases were observed among men and woman ages 20 to 24 years (4.9%) and 30 to 34 years (3.2%).3
“In the United States, about 300,000 cases of gonorrhea are reported each year, but because infected people often have no symptoms, the actual number of cases is probably closer to 700,000,” reported Gail Bolan, director of the CDC’s Sexually Transmitted Disease Prevention Division.4
GROWING CONCERN OVER RESISTANCE
Signs of growing resistance have only been seen in laboratory studies; there are no reported cases of treatment-resistant gonorrhea in the Unites States. However, the evidence of emerging cephalosporin resistance is following a similar pattern to that seen in 2007, when gonorrhea became fluoroquinolone-resistant.1,5
“The challenge is that there is not a well-studied second antibiotic we can turn to even when cephalosporin resistance does emerge,” said Robert D. Kirkcaldy, a medical epidemiologist at the CDC. “What we’ve been noticing is really since 2009 and 2010, it’s taking higher concentrations of antibiotic to kill the bacteria. This could mean resistance to the last antibiotic we have for gonorrhea could be on the horizon.”5
NEW CDC TREATMENT RECOMMENDATIONS
The CDC’s updated guidelines include treatment plans for uncomplicated disease; specific alternatives if ceftriaxone cannot be used; test-of-cure procedures; treatment failure strategies; and recommendations for sexual partners.1
Uncomplicated disease
To treat uncomplicated urogenital, anorectal, and pharyngeal gonorrhea, the CDC now recommends combination therapy with a single intramuscular dose of ceftriaxone 250 mg plus either a single dose of azithromycin 1 g orally or doxycycline 100 mg orally twice a day for 7 days.
Ceftriaxone, as a single 250-mg intramuscular injection, provides high and sustained bactericidal blood levels and is highly efficacious at all anatomic sites of N. gonorrhoeae infection currently circulating in the US. Clinical data are not available that support the use of an increased dose.
The percentage of isolates exhibiting tetracycline resistance was high but remained stable from 2006 (20.6%) to 2011 (21.6%).
Alternatives
When ceftriaxone cannot be used to treat urogenital or rectal gonorrhea, there are two options:
- if ceftriaxone is not readily available, give cefixime 400 mg orally plus either azithromycin 1 g orally or doxycycline 100 mg twice daily orally for 7 days
- if ceftriaxone cannot be given because of severe allergy, give azithromycin 2 g orally in a single dose.
A patient with gonorrhea treated with an alternative regimen should return 1 week after treatment for a test-of-cure at the infected anatomic site.
Test-of-cure—specimen culture is essential
The ideal test-of-cure is performed with culture or, if culture is not readily available, with a nucleic acid amplification testing (NAAT). If the NAAT is positive, make every effort to perform a confirmatory culture. All positive cultures for test-of-cure should undergo phenotypic antimicrobial susceptibility testing.
Unfortunately, the capacity of US laboratories to isolate N. gonorrhoeae by culture is declining rapidly because of the widespread use of NAATs for diagnosing gonorrhea. CDC reporters del Rio and colleagues write in MMWR:1
- It is essential that culture capacity for N. gonorrhoeae be maintained to monitor antimicrobial resistance trends and determine susceptibility to guide treatment following treatment failure. To help control gonorrhea in the United States, health-care providers must maintain the ability to collect specimens for culture and be knowledgeable of laboratories to which they can send specimens for culture. Health-care systems and health departments must support access to culture, and laboratories must maintain culture capacity or develop partnerships with laboratories that can perform culture.
Treatment failure
Clinicians treating a patient with persistent infection after treatment with the recommended therapy should culture relevant clinical specimens and perform antimicrobial susceptibility testing of N. gonorrhoeae isolates. Phenotypic antimicrobial susceptibility testing should be performed using disk diffusion, Etest (BioMerieux), or agar dilution. Data are limited on the use of NAAT-based antimicrobial susceptibility testing for genetic mutations.
Patients who experience treatment failure after treatment with alternative regimens should be treated with ceftriaxone 250 mg as a single intramuscular dose and azithromycin 2 g orally as a single dose and should receive infectious disease consultation.
Consult an infectious disease specialist, an STD/HIV Prevention Training Center, or the CDC for treatment advice, and report the case to the CDC through a health department within 24 hours of diagnosis. Conduct test-of-cure 7 days after re-treatment.
Sexual partners
Clinicians should make every effort to ensure that the patient’s sex partners from the preceding 60 days are evaluated promptly and treated as indicated.
“Treatment of patients with gonorrhea with the most effective therapy will limit the transmission of the disease, prevent complications, and slow the emergence of resistance,” wrote CDC reporters. “However, resistance to cephalosporins, including ceftriaxone, is expected to emerge. Reinvestment in gonorrhea prevention and control is warranted. New treatment options for gonorrhea are urgently needed.”1
For the full MMWR article on new gonorrhea treatment guidelines, visit http://1.usa.gov/OxjuaQ
We want to hear from you! Tell us what you think.
Gonorrhea, the second most commonly reported infectious disease in the United States, is increasing in incidence because Neisseria gonorrhoeae is progressively developing antibiotic resistance. Results of laboratory studies have provoked growing concern that cephalosporins, the only class of antibiotics that meets the current Centers for Disease Control and Prevention (CDC) efficacy standards, are also becoming ineffective in the treatment of gonorrhea.1
CDC updated its guidelines, as reported in Morbidity and Mortality Weekly Report (MMWR), recommending combination therapy with ceftriaxone and either azithromycin or doxycycline for uncomplicated gonorrhea. By revising the current treatment recommendations, the CDC hopes to delay cephalosporin resistance until new treatment options are developed.1
Gonorrhea is a major cause of pelvic inflammatory disease, which can lead to tubal infertility, ectopic pregnancy, and chronic pelvic pain. A pregnant woman with untreated gonorrhea has a higher risk for miscarriage, preterm birth, or premature rupture of membranes. An infected mother can transmit the disease to her child, with risk of blindness, joint infection, and sepsis in the baby.2 There is also strong epidemiologic and biologic evidence that N. gonorrhoeae infections enable HIV infection transmission.1,3
INCREASING INFECTION RATES
After a decline in reported gonorrhea rates to 98.1 cases per 100,000 population in 2009, the rate increased slightly in 2010 to 100.8 per 100,000, with 309,341 cases reported in the United States. In 2010, the reported gonorrhea rate for women was 106.5 per 100,000, slightly higher than for men (94.1 per 100,000). Reported gonorrhea rates were highest among adolescent girls ages 15 to19 years (570.9 per 100,000) and young women ages 20 to 24 years (560.7 per 100,000). The largest increases were observed among men and woman ages 20 to 24 years (4.9%) and 30 to 34 years (3.2%).3
“In the United States, about 300,000 cases of gonorrhea are reported each year, but because infected people often have no symptoms, the actual number of cases is probably closer to 700,000,” reported Gail Bolan, director of the CDC’s Sexually Transmitted Disease Prevention Division.4
GROWING CONCERN OVER RESISTANCE
Signs of growing resistance have only been seen in laboratory studies; there are no reported cases of treatment-resistant gonorrhea in the Unites States. However, the evidence of emerging cephalosporin resistance is following a similar pattern to that seen in 2007, when gonorrhea became fluoroquinolone-resistant.1,5
“The challenge is that there is not a well-studied second antibiotic we can turn to even when cephalosporin resistance does emerge,” said Robert D. Kirkcaldy, a medical epidemiologist at the CDC. “What we’ve been noticing is really since 2009 and 2010, it’s taking higher concentrations of antibiotic to kill the bacteria. This could mean resistance to the last antibiotic we have for gonorrhea could be on the horizon.”5
NEW CDC TREATMENT RECOMMENDATIONS
The CDC’s updated guidelines include treatment plans for uncomplicated disease; specific alternatives if ceftriaxone cannot be used; test-of-cure procedures; treatment failure strategies; and recommendations for sexual partners.1
Uncomplicated disease
To treat uncomplicated urogenital, anorectal, and pharyngeal gonorrhea, the CDC now recommends combination therapy with a single intramuscular dose of ceftriaxone 250 mg plus either a single dose of azithromycin 1 g orally or doxycycline 100 mg orally twice a day for 7 days.
Ceftriaxone, as a single 250-mg intramuscular injection, provides high and sustained bactericidal blood levels and is highly efficacious at all anatomic sites of N. gonorrhoeae infection currently circulating in the US. Clinical data are not available that support the use of an increased dose.
The percentage of isolates exhibiting tetracycline resistance was high but remained stable from 2006 (20.6%) to 2011 (21.6%).
Alternatives
When ceftriaxone cannot be used to treat urogenital or rectal gonorrhea, there are two options:
- if ceftriaxone is not readily available, give cefixime 400 mg orally plus either azithromycin 1 g orally or doxycycline 100 mg twice daily orally for 7 days
- if ceftriaxone cannot be given because of severe allergy, give azithromycin 2 g orally in a single dose.
A patient with gonorrhea treated with an alternative regimen should return 1 week after treatment for a test-of-cure at the infected anatomic site.
Test-of-cure—specimen culture is essential
The ideal test-of-cure is performed with culture or, if culture is not readily available, with a nucleic acid amplification testing (NAAT). If the NAAT is positive, make every effort to perform a confirmatory culture. All positive cultures for test-of-cure should undergo phenotypic antimicrobial susceptibility testing.
Unfortunately, the capacity of US laboratories to isolate N. gonorrhoeae by culture is declining rapidly because of the widespread use of NAATs for diagnosing gonorrhea. CDC reporters del Rio and colleagues write in MMWR:1
- It is essential that culture capacity for N. gonorrhoeae be maintained to monitor antimicrobial resistance trends and determine susceptibility to guide treatment following treatment failure. To help control gonorrhea in the United States, health-care providers must maintain the ability to collect specimens for culture and be knowledgeable of laboratories to which they can send specimens for culture. Health-care systems and health departments must support access to culture, and laboratories must maintain culture capacity or develop partnerships with laboratories that can perform culture.
Treatment failure
Clinicians treating a patient with persistent infection after treatment with the recommended therapy should culture relevant clinical specimens and perform antimicrobial susceptibility testing of N. gonorrhoeae isolates. Phenotypic antimicrobial susceptibility testing should be performed using disk diffusion, Etest (BioMerieux), or agar dilution. Data are limited on the use of NAAT-based antimicrobial susceptibility testing for genetic mutations.
Patients who experience treatment failure after treatment with alternative regimens should be treated with ceftriaxone 250 mg as a single intramuscular dose and azithromycin 2 g orally as a single dose and should receive infectious disease consultation.
Consult an infectious disease specialist, an STD/HIV Prevention Training Center, or the CDC for treatment advice, and report the case to the CDC through a health department within 24 hours of diagnosis. Conduct test-of-cure 7 days after re-treatment.
Sexual partners
Clinicians should make every effort to ensure that the patient’s sex partners from the preceding 60 days are evaluated promptly and treated as indicated.
“Treatment of patients with gonorrhea with the most effective therapy will limit the transmission of the disease, prevent complications, and slow the emergence of resistance,” wrote CDC reporters. “However, resistance to cephalosporins, including ceftriaxone, is expected to emerge. Reinvestment in gonorrhea prevention and control is warranted. New treatment options for gonorrhea are urgently needed.”1
For the full MMWR article on new gonorrhea treatment guidelines, visit http://1.usa.gov/OxjuaQ
We want to hear from you! Tell us what you think.
1. del Rio C, Hall G, Holmes K, et al. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR. 2012;61(31):590-594.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a3.htm. Published August 10, 2012. Accessed August 13, 2012.
2. Gonorrhea fact sheet. Womenshealth.gov. http://womenshealth.gov/publications/our-publications/fact-sheet/gonorrhea.cfm#h. Updated July 8, 2011. Accessed August 13, 2012.
3. Centers for Disease Control and Prevention. 2010 sexually transmitted diseases surveillance: Gonorrhea. CDC Web site. http://www.cdc.gov/std/stats10/gonorrhea.htm. Updated November 17, 2011. Accessed August 13, 2012.
4. The Washington Post. Only one gonorrhea drug remains for routine cases, CDC says. http://www.washingtonpost.com/national/health-science/gonorrhea-drug-is-becoming-less-effective-cdc-says/2012/08/09/874751e6-e256-11e1-ae7f-d2a13e249eb2_story.html. Published August 10, 2012. Accessed August 13, 2012.
5. Parker-Pope T. Worries about a gonorrhea “superbug”. NY Times Health Science. http://well.blogs.nytimes.com/2011/07/12/worries-about-a-gonorrhea-superbug/ Published July 12, 2011. Accessed August 13, 2012.
1. del Rio C, Hall G, Holmes K, et al. Update to CDC’s Sexually Transmitted Diseases Treatment Guidelines, 2010: Oral cephalosporins no longer a recommended treatment for gonococcal infections. MMWR. 2012;61(31):590-594.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a3.htm. Published August 10, 2012. Accessed August 13, 2012.
2. Gonorrhea fact sheet. Womenshealth.gov. http://womenshealth.gov/publications/our-publications/fact-sheet/gonorrhea.cfm#h. Updated July 8, 2011. Accessed August 13, 2012.
3. Centers for Disease Control and Prevention. 2010 sexually transmitted diseases surveillance: Gonorrhea. CDC Web site. http://www.cdc.gov/std/stats10/gonorrhea.htm. Updated November 17, 2011. Accessed August 13, 2012.
4. The Washington Post. Only one gonorrhea drug remains for routine cases, CDC says. http://www.washingtonpost.com/national/health-science/gonorrhea-drug-is-becoming-less-effective-cdc-says/2012/08/09/874751e6-e256-11e1-ae7f-d2a13e249eb2_story.html. Published August 10, 2012. Accessed August 13, 2012.
5. Parker-Pope T. Worries about a gonorrhea “superbug”. NY Times Health Science. http://well.blogs.nytimes.com/2011/07/12/worries-about-a-gonorrhea-superbug/ Published July 12, 2011. Accessed August 13, 2012.
New data: HIV-infected women do not have an elevated risk of cervical cancer
New cervical cancer screening guidelines recommend
less frequent assessment
Janelle Yates (June 2012)
HIV-infected women who have normal cervical cytology and who test negative for oncogenic types of human papillomavirus (HPV) have a 5-year risk of cervical precancer and cancer that is equivalent to the risk among women without HIV infection, according to a cohort study published in July in JAMA.1
Howard D. Strickler, MD, MPH, of Albert Einstein College of Medicine of Yeshiva University, New York, and colleagues conducted the study to explore the 3- and 5-year risk of cervical precancer and cancer in 420 HIV-infected women and 279 women without HIV infection. Precancer and cancer were defined on the basis of cytology or histology:
- cytology: a high-grade squamous intraepithelial lesion (HSIL) or more severe finding
- histology: cervical intraepithelial neoplasia (CIN) grade 2 or higher.
Participants in the study underwent semiannual visits, including Pap testing and cervical biopsy, if indicated.
Two cases of HSIL or more severe cytology were observed during the 5 years of follow-up—one among HIV-infected women who had a CD4 cell count of at least 500 cells/μL and one among HIV-negative women. Overall, the cumulative incidence of HSIL or more severe cytology was 0.3% among HIV-infected women and 0.4% among HIV-negative women.
Based on a total of 15 cases, investigators determined that the cumulative incidence of CIN 2+ histology over 5 years was:
- 2% among HIV-infected women who had a CD4 cell count below 350 cells/μL
- 2% among HIV-infected women who had a CD4 cell count of 350 to 499 cells/μL
- 6% among HIV-infected women who had a CD4 cell count of 500 cells/μL or higher
- 5% in women without HIV infection.
When researchers combined the data among HIV-infected women, they found an overall 5-year cumulative incidence of CIN 2+ of 5%.
Screening intervals may one day be extended for HIV-infected women
These findings are important because cervical cancer screening guidelines for women who are not infected with HIV were recently updated to increase the co-testing interval from 3 to 5 years among women who have normal cytology and who test negative for oncogenic HPV. “Whether a 3-year or 5-year screening interval could be used in HIV-infected women who are cytologically normal and oncogenic HPV-negative is unknown,” wrote Strickler and colleagues as background to their study.
“The results of this prospective study suggest that HIV-infected women undergoing long-term clinical follow-up who are cytologically normal and oncogenic HPV-negative have a risk of cervical precancer similar to that in HIV-uninfected women through 5 years of follow-up. Additional observational studies or a randomized clinical trial may be necessary before clinical guideline committees consider whether to expand current recommendations regarding HPV co-testing to HIV-infected women. More broadly, the current investigation highlights the potential for a new era of molecular testing, including HPV as well as other biomarkers, to improve cervical cancer screening in HIV-infected women,” the investigators concluded.
We want to hear from you! Tell us what you think.
Reference
1. Keller MJ, Burk RD, Xie X, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012;308(4):362-369.
New cervical cancer screening guidelines recommend
less frequent assessment
Janelle Yates (June 2012)
HIV-infected women who have normal cervical cytology and who test negative for oncogenic types of human papillomavirus (HPV) have a 5-year risk of cervical precancer and cancer that is equivalent to the risk among women without HIV infection, according to a cohort study published in July in JAMA.1
Howard D. Strickler, MD, MPH, of Albert Einstein College of Medicine of Yeshiva University, New York, and colleagues conducted the study to explore the 3- and 5-year risk of cervical precancer and cancer in 420 HIV-infected women and 279 women without HIV infection. Precancer and cancer were defined on the basis of cytology or histology:
- cytology: a high-grade squamous intraepithelial lesion (HSIL) or more severe finding
- histology: cervical intraepithelial neoplasia (CIN) grade 2 or higher.
Participants in the study underwent semiannual visits, including Pap testing and cervical biopsy, if indicated.
Two cases of HSIL or more severe cytology were observed during the 5 years of follow-up—one among HIV-infected women who had a CD4 cell count of at least 500 cells/μL and one among HIV-negative women. Overall, the cumulative incidence of HSIL or more severe cytology was 0.3% among HIV-infected women and 0.4% among HIV-negative women.
Based on a total of 15 cases, investigators determined that the cumulative incidence of CIN 2+ histology over 5 years was:
- 2% among HIV-infected women who had a CD4 cell count below 350 cells/μL
- 2% among HIV-infected women who had a CD4 cell count of 350 to 499 cells/μL
- 6% among HIV-infected women who had a CD4 cell count of 500 cells/μL or higher
- 5% in women without HIV infection.
When researchers combined the data among HIV-infected women, they found an overall 5-year cumulative incidence of CIN 2+ of 5%.
Screening intervals may one day be extended for HIV-infected women
These findings are important because cervical cancer screening guidelines for women who are not infected with HIV were recently updated to increase the co-testing interval from 3 to 5 years among women who have normal cytology and who test negative for oncogenic HPV. “Whether a 3-year or 5-year screening interval could be used in HIV-infected women who are cytologically normal and oncogenic HPV-negative is unknown,” wrote Strickler and colleagues as background to their study.
“The results of this prospective study suggest that HIV-infected women undergoing long-term clinical follow-up who are cytologically normal and oncogenic HPV-negative have a risk of cervical precancer similar to that in HIV-uninfected women through 5 years of follow-up. Additional observational studies or a randomized clinical trial may be necessary before clinical guideline committees consider whether to expand current recommendations regarding HPV co-testing to HIV-infected women. More broadly, the current investigation highlights the potential for a new era of molecular testing, including HPV as well as other biomarkers, to improve cervical cancer screening in HIV-infected women,” the investigators concluded.
We want to hear from you! Tell us what you think.
New cervical cancer screening guidelines recommend
less frequent assessment
Janelle Yates (June 2012)
HIV-infected women who have normal cervical cytology and who test negative for oncogenic types of human papillomavirus (HPV) have a 5-year risk of cervical precancer and cancer that is equivalent to the risk among women without HIV infection, according to a cohort study published in July in JAMA.1
Howard D. Strickler, MD, MPH, of Albert Einstein College of Medicine of Yeshiva University, New York, and colleagues conducted the study to explore the 3- and 5-year risk of cervical precancer and cancer in 420 HIV-infected women and 279 women without HIV infection. Precancer and cancer were defined on the basis of cytology or histology:
- cytology: a high-grade squamous intraepithelial lesion (HSIL) or more severe finding
- histology: cervical intraepithelial neoplasia (CIN) grade 2 or higher.
Participants in the study underwent semiannual visits, including Pap testing and cervical biopsy, if indicated.
Two cases of HSIL or more severe cytology were observed during the 5 years of follow-up—one among HIV-infected women who had a CD4 cell count of at least 500 cells/μL and one among HIV-negative women. Overall, the cumulative incidence of HSIL or more severe cytology was 0.3% among HIV-infected women and 0.4% among HIV-negative women.
Based on a total of 15 cases, investigators determined that the cumulative incidence of CIN 2+ histology over 5 years was:
- 2% among HIV-infected women who had a CD4 cell count below 350 cells/μL
- 2% among HIV-infected women who had a CD4 cell count of 350 to 499 cells/μL
- 6% among HIV-infected women who had a CD4 cell count of 500 cells/μL or higher
- 5% in women without HIV infection.
When researchers combined the data among HIV-infected women, they found an overall 5-year cumulative incidence of CIN 2+ of 5%.
Screening intervals may one day be extended for HIV-infected women
These findings are important because cervical cancer screening guidelines for women who are not infected with HIV were recently updated to increase the co-testing interval from 3 to 5 years among women who have normal cytology and who test negative for oncogenic HPV. “Whether a 3-year or 5-year screening interval could be used in HIV-infected women who are cytologically normal and oncogenic HPV-negative is unknown,” wrote Strickler and colleagues as background to their study.
“The results of this prospective study suggest that HIV-infected women undergoing long-term clinical follow-up who are cytologically normal and oncogenic HPV-negative have a risk of cervical precancer similar to that in HIV-uninfected women through 5 years of follow-up. Additional observational studies or a randomized clinical trial may be necessary before clinical guideline committees consider whether to expand current recommendations regarding HPV co-testing to HIV-infected women. More broadly, the current investigation highlights the potential for a new era of molecular testing, including HPV as well as other biomarkers, to improve cervical cancer screening in HIV-infected women,” the investigators concluded.
We want to hear from you! Tell us what you think.
Reference
1. Keller MJ, Burk RD, Xie X, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012;308(4):362-369.
Reference
1. Keller MJ, Burk RD, Xie X, et al. Risk of cervical precancer and cancer among HIV-infected women with normal cervical cytology and no evidence of oncogenic HPV infection. JAMA. 2012;308(4):362-369.
New overactive bladder treatment approved by FDA
The US Food and Drug Administration (FDA) has approved Myrbetriq™ (mirabegron) extended-release tablets for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency.
“Myrbetriq is the first oral OAB treatment with a distinct mechanism of action since the launch of anticholinergic agents 30 years ago,” said Steven Ryder, MD, president of Astellas Pharma Global Development. “The approval of Myrbetriq represents an important milestone in OAB treatment and in our ongoing commitment to advancing urological health.” Astellas Pharma US, Inc., is a subsidiary of Tokyo-based Astellas Pharma Inc.
Myrbetriq, a once-daily oral beta-3 adrenergic agonist, offers a new treatment option for patients with OAB. Antimuscarinics, the current OAB treatment standard, work by binding to muscarinic receptors in the bladder and inhibiting involuntary bladder contractions. Myrbetriq relaxes the detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle by activation of beta-3 adrenergic receptors, therefore increasing bladder capacity.
Myrbetriq has been studied in more than 10,000 individuals over 10 years. FDA approval was based on safety and efficacy data from three placebo-controlled Phase 3 studies in which treatment with Myrbetriq 25 mg and 50 mg resulted in statistically significantly improvement in efficacy parameters of incontinence episodes and number of urinations per 24 hours.
The recommended starting dose for Myrbetriq is 25 mg once daily with or without food. The dose may be increased to 50 mg. Most commonly reported adverse reactions were hypertension, nasopharyngitis, urinary tract infection, and headache. Myrbetriq will be available in pharmacies in the fourth quarter of 2012.
For additional information, visit www.myrbetriq.com.
Update on Urinary Incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
We want to hear from you! Tell us what you think.
The US Food and Drug Administration (FDA) has approved Myrbetriq™ (mirabegron) extended-release tablets for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency.
“Myrbetriq is the first oral OAB treatment with a distinct mechanism of action since the launch of anticholinergic agents 30 years ago,” said Steven Ryder, MD, president of Astellas Pharma Global Development. “The approval of Myrbetriq represents an important milestone in OAB treatment and in our ongoing commitment to advancing urological health.” Astellas Pharma US, Inc., is a subsidiary of Tokyo-based Astellas Pharma Inc.
Myrbetriq, a once-daily oral beta-3 adrenergic agonist, offers a new treatment option for patients with OAB. Antimuscarinics, the current OAB treatment standard, work by binding to muscarinic receptors in the bladder and inhibiting involuntary bladder contractions. Myrbetriq relaxes the detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle by activation of beta-3 adrenergic receptors, therefore increasing bladder capacity.
Myrbetriq has been studied in more than 10,000 individuals over 10 years. FDA approval was based on safety and efficacy data from three placebo-controlled Phase 3 studies in which treatment with Myrbetriq 25 mg and 50 mg resulted in statistically significantly improvement in efficacy parameters of incontinence episodes and number of urinations per 24 hours.
The recommended starting dose for Myrbetriq is 25 mg once daily with or without food. The dose may be increased to 50 mg. Most commonly reported adverse reactions were hypertension, nasopharyngitis, urinary tract infection, and headache. Myrbetriq will be available in pharmacies in the fourth quarter of 2012.
For additional information, visit www.myrbetriq.com.
Update on Urinary Incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
We want to hear from you! Tell us what you think.
The US Food and Drug Administration (FDA) has approved Myrbetriq™ (mirabegron) extended-release tablets for the treatment of overactive bladder (OAB) with symptoms of urge urinary incontinence, urgency, and urinary frequency.
“Myrbetriq is the first oral OAB treatment with a distinct mechanism of action since the launch of anticholinergic agents 30 years ago,” said Steven Ryder, MD, president of Astellas Pharma Global Development. “The approval of Myrbetriq represents an important milestone in OAB treatment and in our ongoing commitment to advancing urological health.” Astellas Pharma US, Inc., is a subsidiary of Tokyo-based Astellas Pharma Inc.
Myrbetriq, a once-daily oral beta-3 adrenergic agonist, offers a new treatment option for patients with OAB. Antimuscarinics, the current OAB treatment standard, work by binding to muscarinic receptors in the bladder and inhibiting involuntary bladder contractions. Myrbetriq relaxes the detrusor smooth muscle during the storage phase of the urinary bladder fill-void cycle by activation of beta-3 adrenergic receptors, therefore increasing bladder capacity.
Myrbetriq has been studied in more than 10,000 individuals over 10 years. FDA approval was based on safety and efficacy data from three placebo-controlled Phase 3 studies in which treatment with Myrbetriq 25 mg and 50 mg resulted in statistically significantly improvement in efficacy parameters of incontinence episodes and number of urinations per 24 hours.
The recommended starting dose for Myrbetriq is 25 mg once daily with or without food. The dose may be increased to 50 mg. Most commonly reported adverse reactions were hypertension, nasopharyngitis, urinary tract infection, and headache. Myrbetriq will be available in pharmacies in the fourth quarter of 2012.
For additional information, visit www.myrbetriq.com.
Update on Urinary Incontinence
Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
We want to hear from you! Tell us what you think.
