User login
FDA, hospitals caution against laparoscopic power morcellation during hysterectomy and myomectomy
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, and the city and state in which you practice.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, and the city and state in which you practice.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com Please include your name, and the city and state in which you practice.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
To access articles, videos, and audiocasts in the Morcellation Topic Collection, click here.
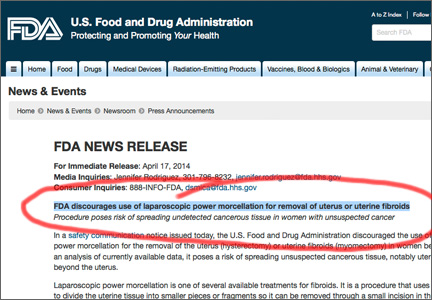
FDA discourages use of laparoscopic power morcellation during hysterectomy and myomectomy
On April 17, 2014, the US Food and Drug Administration (FDA) issued a Safety Communication discouraging the use of laparoscopic power morcellation in hysterectomy and myomectomy for uterine fibroids.
Based on an FDA analysis of current data, “… it is estimated that 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma. If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient’s likelihood of long-term survival.”1
FDA recommendations
The FDA posted the following recommendations for health-care providers1:
- Laparoscopic uterine power morcellation should not be used in women with suspected or known uterine cancer
- All available treatment options should be considered for women with symptomatic uterine fibroids
- The benefits and risks of all treatments should be discussed thoroughly with each patient
- If, after a careful benefit-risk evaluation, laparoscopic power morcellation is considered the best therapeutic option for an individual patient, then:
- Inform the patient that her fibroid(s) may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening her prognosis
- Some clinicians and medical institutions now advocate using a specimen “bag” during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.
Although many women choose laparoscopic hysterectomy or myomectomy because of the associated benefits, there are other treatments available, including vaginal or abdominal hysterectomy and myomectomy; laparoscopic hysterectomy or myomectomy without morcellation; minilaparotomy; uterine artery embolization; high-intensity focused ultrasound; and drug therapy.
FDA actions
To reduce the risk of inadvertent spread of unsuspected cancer to the abdomen and pelvis, the FDA has instructed manufacturers of power morcellators used during laparoscopic hysterectomy and myomectomy to immediately review labeling for accurate risk information.
A to-be-convened public meeting of the FDA’s Obstetrics and Gynecological Medical Device Advisory Committee will discuss1:
- the clinical role of laparoscopic power morcellation in the treatment of uterine fibroids
- whether surgical techniques and/or use of accessories, such as morcellation and/or specimen bags, can enhance the safe and effective use of these devices
- if a boxed warning relating the risk of cancer spread should be required for laparoscopic power morcellators.
The FDA will continue to review adverse event reports and peer-reviewed literature, as well as patient information and evidence from health-care providers, gynecologic and surgical professional societies, and medical device manufacturers.
Adverse events should promptly be reported to the FDA by filing a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
Institution reaction
Brigham and Women's Hospital had banned the use of open power morcellation on March 31, 2014, allowing for the use of power morcellators within a containment system. In light of the FDA notice that discourages the use of power morcellators during hysterectomy or myomectomy for the treatment of uterine fibroids, however, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s advised surgical staff to "immediately suspend use of power morcellators in all cases until further notice."
Massachusetts General, which also had placed restrictions on the use of power morcellators prior to the FDA communication, suspended the use of power morcellation.
“I have asked our doctors to stop the procedure immediately until more information is available,’’ Dr. Isaac Schiff, Massachusetts General’s chief of obstetrics and gynecology, told the Boston Globe.
Reference
- US Food and Drug Administration. Laparoscopic uterine power morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed April 17, 2014.
On April 17, 2014, the US Food and Drug Administration (FDA) issued a Safety Communication discouraging the use of laparoscopic power morcellation in hysterectomy and myomectomy for uterine fibroids.
Based on an FDA analysis of current data, “… it is estimated that 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma. If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient’s likelihood of long-term survival.”1
FDA recommendations
The FDA posted the following recommendations for health-care providers1:
- Laparoscopic uterine power morcellation should not be used in women with suspected or known uterine cancer
- All available treatment options should be considered for women with symptomatic uterine fibroids
- The benefits and risks of all treatments should be discussed thoroughly with each patient
- If, after a careful benefit-risk evaluation, laparoscopic power morcellation is considered the best therapeutic option for an individual patient, then:
- Inform the patient that her fibroid(s) may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening her prognosis
- Some clinicians and medical institutions now advocate using a specimen “bag” during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.
Although many women choose laparoscopic hysterectomy or myomectomy because of the associated benefits, there are other treatments available, including vaginal or abdominal hysterectomy and myomectomy; laparoscopic hysterectomy or myomectomy without morcellation; minilaparotomy; uterine artery embolization; high-intensity focused ultrasound; and drug therapy.
FDA actions
To reduce the risk of inadvertent spread of unsuspected cancer to the abdomen and pelvis, the FDA has instructed manufacturers of power morcellators used during laparoscopic hysterectomy and myomectomy to immediately review labeling for accurate risk information.
A to-be-convened public meeting of the FDA’s Obstetrics and Gynecological Medical Device Advisory Committee will discuss1:
- the clinical role of laparoscopic power morcellation in the treatment of uterine fibroids
- whether surgical techniques and/or use of accessories, such as morcellation and/or specimen bags, can enhance the safe and effective use of these devices
- if a boxed warning relating the risk of cancer spread should be required for laparoscopic power morcellators.
The FDA will continue to review adverse event reports and peer-reviewed literature, as well as patient information and evidence from health-care providers, gynecologic and surgical professional societies, and medical device manufacturers.
Adverse events should promptly be reported to the FDA by filing a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
Institution reaction
Brigham and Women's Hospital had banned the use of open power morcellation on March 31, 2014, allowing for the use of power morcellators within a containment system. In light of the FDA notice that discourages the use of power morcellators during hysterectomy or myomectomy for the treatment of uterine fibroids, however, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s advised surgical staff to "immediately suspend use of power morcellators in all cases until further notice."
Massachusetts General, which also had placed restrictions on the use of power morcellators prior to the FDA communication, suspended the use of power morcellation.
“I have asked our doctors to stop the procedure immediately until more information is available,’’ Dr. Isaac Schiff, Massachusetts General’s chief of obstetrics and gynecology, told the Boston Globe.
On April 17, 2014, the US Food and Drug Administration (FDA) issued a Safety Communication discouraging the use of laparoscopic power morcellation in hysterectomy and myomectomy for uterine fibroids.
Based on an FDA analysis of current data, “… it is estimated that 1 in 350 women undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma, a type of uterine cancer that includes leiomyosarcoma. If laparoscopic power morcellation is performed in women with unsuspected uterine sarcoma, there is a risk that the procedure will spread the cancerous tissue within the abdomen and pelvis, significantly worsening the patient’s likelihood of long-term survival.”1
FDA recommendations
The FDA posted the following recommendations for health-care providers1:
- Laparoscopic uterine power morcellation should not be used in women with suspected or known uterine cancer
- All available treatment options should be considered for women with symptomatic uterine fibroids
- The benefits and risks of all treatments should be discussed thoroughly with each patient
- If, after a careful benefit-risk evaluation, laparoscopic power morcellation is considered the best therapeutic option for an individual patient, then:
- Inform the patient that her fibroid(s) may contain unexpected cancerous tissue and that laparoscopic power morcellation may spread the cancer, significantly worsening her prognosis
- Some clinicians and medical institutions now advocate using a specimen “bag” during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.
Although many women choose laparoscopic hysterectomy or myomectomy because of the associated benefits, there are other treatments available, including vaginal or abdominal hysterectomy and myomectomy; laparoscopic hysterectomy or myomectomy without morcellation; minilaparotomy; uterine artery embolization; high-intensity focused ultrasound; and drug therapy.
FDA actions
To reduce the risk of inadvertent spread of unsuspected cancer to the abdomen and pelvis, the FDA has instructed manufacturers of power morcellators used during laparoscopic hysterectomy and myomectomy to immediately review labeling for accurate risk information.
A to-be-convened public meeting of the FDA’s Obstetrics and Gynecological Medical Device Advisory Committee will discuss1:
- the clinical role of laparoscopic power morcellation in the treatment of uterine fibroids
- whether surgical techniques and/or use of accessories, such as morcellation and/or specimen bags, can enhance the safe and effective use of these devices
- if a boxed warning relating the risk of cancer spread should be required for laparoscopic power morcellators.
The FDA will continue to review adverse event reports and peer-reviewed literature, as well as patient information and evidence from health-care providers, gynecologic and surgical professional societies, and medical device manufacturers.
Adverse events should promptly be reported to the FDA by filing a voluntary report through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
Institution reaction
Brigham and Women's Hospital had banned the use of open power morcellation on March 31, 2014, allowing for the use of power morcellators within a containment system. In light of the FDA notice that discourages the use of power morcellators during hysterectomy or myomectomy for the treatment of uterine fibroids, however, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s advised surgical staff to "immediately suspend use of power morcellators in all cases until further notice."
Massachusetts General, which also had placed restrictions on the use of power morcellators prior to the FDA communication, suspended the use of power morcellation.
“I have asked our doctors to stop the procedure immediately until more information is available,’’ Dr. Isaac Schiff, Massachusetts General’s chief of obstetrics and gynecology, told the Boston Globe.
Reference
- US Food and Drug Administration. Laparoscopic uterine power morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed April 17, 2014.
Reference
- US Food and Drug Administration. Laparoscopic uterine power morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed April 17, 2014.
Click here to access articles, videos, and audiocasts in the Morcellation Topic Collection
Type 2 diabetes begins in utero?
A new study1 provides the first evidence of a direct effect of maternal metabolism on fetal brain activity, suggesting that insulin resistance, the precursor to type 2 diabetes, begins its formation prenatally.
DETAILS OF THE STUDY
Dr. Katarzyna Linder from the University Hospital Tübingen in Germany and colleagues included in their study 13 healthy pregnant women with normal, singleton pregnancies of between 27 and 36 weeks. All of the women underwent an oral glucose tolerance test, meaning that after a 5-hour overnight fast, each woman drank a solution containing 75 g glucose. The investigators ascertained blood glucose and plasma insulin levels from blood samples taken at 0, 60, and 120 minutes.
At approximately the same time points, but before they drew each blood sample, the authors obtained a fetal magnetoencephalography (fMEG) measurement in an effort to noninvasively record brain activity in utero. During each measurement, they presented an auditory sequence to the fetus. Most (75%) of the time, the sound presented had a frequency of 500 Hz, but 25% of the time the researchers presented a deviant tone with a frequency of 750 Hz to prevent habituation.
The researchers found that maternal insulin sensitivity significantly correlated with response latency of the fetus at the 60-minute time point, so that the higher the insulin sensitivity of the mother, the shorter the response time of the fetus to the sound. The association remained significant even after the investigators controlled for relative maternal weight gain, gestational age, and the child’s birth weight. No significant correlation existed at baseline or at 120 minutes.
The investigators then split the women into 2 groups: those who were insulin-resistant and those who were insulin-sensitive. They found that the fetuses of the insulin-resistant moms were almost 40% slower to respond to the auditory stimuli than those of the insulin-sensitive moms (mean [SD], 283 [79] ms vs 178 [46] ms; P=.03).
INTERPRETING THE FINDINGS
According to the US Centers for Disease Control and Prevention, almost one-third (30.3%) of US adults between the ages of 20 and 39 years—the primary child-bearing years—are obese,2 as are 17% of our children and adolescents—triple the rate of 1 generation previous.3 Furthermore, 25.8 million people in the United States have diabetes, including 1 in every 400 children and adolescents.4
Experts know that the children of obese or diabetic mothers are at increased risk for obesity and type 2 diabetes as adults, and that the connection is not purely genetic; environmental and epigenetic (environmental elements that affect genetics) factors also play key roles. The latter is the basis for the fetal or developmental origins hypothesis,5 which posits that a pregnant woman’s exposure to certain environmental factors can affect the programming of her unborn child and impact adult health.
The authors of the current study demonstrate that the metabolism of a pregnant woman after a sugar load directly affects the response time and brain activity of her developing fetus. They suggest as a mechanism for the effect that “insulin-resistant mothers have higher glucose levels accompanied by increased insulin levels in the postprandial state. As glucose passes the placenta, these postprandially increased glucose levels induce hyperinsulinaemia in the fetus.” The resulting chronic hyperinsulinemia “might induce insulin resistance in the fetal brain.”
1. Linder K, Schleger F, Ketterer C, et al. Maternal insulin sensitivity is associated with oral glucose-induced changes in fetal brain activity. Diabetologia. 2014. [Epub ahead of print]
2. Adult obesity facts. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/data/adult.html. Accessed April 12, 2014.
3. Childhood overweight and obesity. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/childhood/index.html. Accessed April 12, 2014.
4. Statistics about diabetes. American Diabetes Association Web site. http://www.diabetes.org/diabetes-basics/statistics/. Accessed April 12, 2014.
5. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368.
A new study1 provides the first evidence of a direct effect of maternal metabolism on fetal brain activity, suggesting that insulin resistance, the precursor to type 2 diabetes, begins its formation prenatally.
DETAILS OF THE STUDY
Dr. Katarzyna Linder from the University Hospital Tübingen in Germany and colleagues included in their study 13 healthy pregnant women with normal, singleton pregnancies of between 27 and 36 weeks. All of the women underwent an oral glucose tolerance test, meaning that after a 5-hour overnight fast, each woman drank a solution containing 75 g glucose. The investigators ascertained blood glucose and plasma insulin levels from blood samples taken at 0, 60, and 120 minutes.
At approximately the same time points, but before they drew each blood sample, the authors obtained a fetal magnetoencephalography (fMEG) measurement in an effort to noninvasively record brain activity in utero. During each measurement, they presented an auditory sequence to the fetus. Most (75%) of the time, the sound presented had a frequency of 500 Hz, but 25% of the time the researchers presented a deviant tone with a frequency of 750 Hz to prevent habituation.
The researchers found that maternal insulin sensitivity significantly correlated with response latency of the fetus at the 60-minute time point, so that the higher the insulin sensitivity of the mother, the shorter the response time of the fetus to the sound. The association remained significant even after the investigators controlled for relative maternal weight gain, gestational age, and the child’s birth weight. No significant correlation existed at baseline or at 120 minutes.
The investigators then split the women into 2 groups: those who were insulin-resistant and those who were insulin-sensitive. They found that the fetuses of the insulin-resistant moms were almost 40% slower to respond to the auditory stimuli than those of the insulin-sensitive moms (mean [SD], 283 [79] ms vs 178 [46] ms; P=.03).
INTERPRETING THE FINDINGS
According to the US Centers for Disease Control and Prevention, almost one-third (30.3%) of US adults between the ages of 20 and 39 years—the primary child-bearing years—are obese,2 as are 17% of our children and adolescents—triple the rate of 1 generation previous.3 Furthermore, 25.8 million people in the United States have diabetes, including 1 in every 400 children and adolescents.4
Experts know that the children of obese or diabetic mothers are at increased risk for obesity and type 2 diabetes as adults, and that the connection is not purely genetic; environmental and epigenetic (environmental elements that affect genetics) factors also play key roles. The latter is the basis for the fetal or developmental origins hypothesis,5 which posits that a pregnant woman’s exposure to certain environmental factors can affect the programming of her unborn child and impact adult health.
The authors of the current study demonstrate that the metabolism of a pregnant woman after a sugar load directly affects the response time and brain activity of her developing fetus. They suggest as a mechanism for the effect that “insulin-resistant mothers have higher glucose levels accompanied by increased insulin levels in the postprandial state. As glucose passes the placenta, these postprandially increased glucose levels induce hyperinsulinaemia in the fetus.” The resulting chronic hyperinsulinemia “might induce insulin resistance in the fetal brain.”
A new study1 provides the first evidence of a direct effect of maternal metabolism on fetal brain activity, suggesting that insulin resistance, the precursor to type 2 diabetes, begins its formation prenatally.
DETAILS OF THE STUDY
Dr. Katarzyna Linder from the University Hospital Tübingen in Germany and colleagues included in their study 13 healthy pregnant women with normal, singleton pregnancies of between 27 and 36 weeks. All of the women underwent an oral glucose tolerance test, meaning that after a 5-hour overnight fast, each woman drank a solution containing 75 g glucose. The investigators ascertained blood glucose and plasma insulin levels from blood samples taken at 0, 60, and 120 minutes.
At approximately the same time points, but before they drew each blood sample, the authors obtained a fetal magnetoencephalography (fMEG) measurement in an effort to noninvasively record brain activity in utero. During each measurement, they presented an auditory sequence to the fetus. Most (75%) of the time, the sound presented had a frequency of 500 Hz, but 25% of the time the researchers presented a deviant tone with a frequency of 750 Hz to prevent habituation.
The researchers found that maternal insulin sensitivity significantly correlated with response latency of the fetus at the 60-minute time point, so that the higher the insulin sensitivity of the mother, the shorter the response time of the fetus to the sound. The association remained significant even after the investigators controlled for relative maternal weight gain, gestational age, and the child’s birth weight. No significant correlation existed at baseline or at 120 minutes.
The investigators then split the women into 2 groups: those who were insulin-resistant and those who were insulin-sensitive. They found that the fetuses of the insulin-resistant moms were almost 40% slower to respond to the auditory stimuli than those of the insulin-sensitive moms (mean [SD], 283 [79] ms vs 178 [46] ms; P=.03).
INTERPRETING THE FINDINGS
According to the US Centers for Disease Control and Prevention, almost one-third (30.3%) of US adults between the ages of 20 and 39 years—the primary child-bearing years—are obese,2 as are 17% of our children and adolescents—triple the rate of 1 generation previous.3 Furthermore, 25.8 million people in the United States have diabetes, including 1 in every 400 children and adolescents.4
Experts know that the children of obese or diabetic mothers are at increased risk for obesity and type 2 diabetes as adults, and that the connection is not purely genetic; environmental and epigenetic (environmental elements that affect genetics) factors also play key roles. The latter is the basis for the fetal or developmental origins hypothesis,5 which posits that a pregnant woman’s exposure to certain environmental factors can affect the programming of her unborn child and impact adult health.
The authors of the current study demonstrate that the metabolism of a pregnant woman after a sugar load directly affects the response time and brain activity of her developing fetus. They suggest as a mechanism for the effect that “insulin-resistant mothers have higher glucose levels accompanied by increased insulin levels in the postprandial state. As glucose passes the placenta, these postprandially increased glucose levels induce hyperinsulinaemia in the fetus.” The resulting chronic hyperinsulinemia “might induce insulin resistance in the fetal brain.”
1. Linder K, Schleger F, Ketterer C, et al. Maternal insulin sensitivity is associated with oral glucose-induced changes in fetal brain activity. Diabetologia. 2014. [Epub ahead of print]
2. Adult obesity facts. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/data/adult.html. Accessed April 12, 2014.
3. Childhood overweight and obesity. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/childhood/index.html. Accessed April 12, 2014.
4. Statistics about diabetes. American Diabetes Association Web site. http://www.diabetes.org/diabetes-basics/statistics/. Accessed April 12, 2014.
5. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368.
1. Linder K, Schleger F, Ketterer C, et al. Maternal insulin sensitivity is associated with oral glucose-induced changes in fetal brain activity. Diabetologia. 2014. [Epub ahead of print]
2. Adult obesity facts. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/data/adult.html. Accessed April 12, 2014.
3. Childhood overweight and obesity. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/obesity/childhood/index.html. Accessed April 12, 2014.
4. Statistics about diabetes. American Diabetes Association Web site. http://www.diabetes.org/diabetes-basics/statistics/. Accessed April 12, 2014.
5. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368.
Beware of invasive H influenzae disease among pregnant women
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Experts generally have not considered H influenzae disease to be a substantial contributor to severely adverse fetal outcomes. Yet new research from England and Wales finds that even though pregnant women in their study tended to be younger and healthier than their nonpregnant counterparts, they were far more likely to develop the invasive unencapsulated form of disease and far more likely to present with bacteremia. Not to mention that almost all of their pregnancies resulted in miscarriage, stillbirth, extremely preterm birth, or, at best, the birth of infants with respiratory distress with or without sepsis.
DETAILS OF THE STUDY
Collins and colleagues queried prospectively general practitioners who cared for women of reproductive age (15 to 44 years) with invasive H influenzae disease during the 4-year period of 2009 to 2012. One hundred percent of those queried responded. The study encompassed more than 45 million woman-years of follow-up.
Of the 171 women who developed the infection (confirmed by positive culture from a normally sterile site), approximately 84% (144) had unencapsulated disease. Not quite half (44% or 75) of the 171 women were pregnant at the time of infection. The overall incidence of confirmed invasive H influenzae disease was low at 0.50 per 100,000 women of reproductive age, but the 75 pregnant women were 17.2 (95% confidence interval [CI], 12.2-24.1; P<.001) times as likely to develop the infection as the 96 nonpregnant women (2.98/100,000 woman-years versus 0.17/100,000 woman-years, respectively). And, despite being previously healthy, almost three times as many pregnant as nonpregnant women presented with bacteremia (90.3% versus 33.3%, respectively).
Of 47 women who developed unencapsulated H influenzae infection during the first 24 weeks of pregnancy, 44 lost the fetus and three had extremely preterm births. Of 28 women who developed the infection during the second half of their pregnancy, two had stillbirths. Eight of the 26 live births were preterm, and 21 of the 26 infants had respiratory distress with or without sepsis at birth. The researchers calculated that the rate of pregnancy loss following invasive H influenzae disease was almost 3 times higher than the average rate of pregnancy loss in England and Wales.
CLINICAL RECOMMENDATIONS
It is generally reported that up to one-quarter of the approximately 26,000 stillbirths occurring yearly in the United States are due to some type of maternal or fetal infection.2,3 Dr. Morven S. Edwards, in an editorial4 appearing in the same issue of JAMA as the study, comments, “Given the magnitude of the burden of perinatal deaths, clarifying the extent that bacterial infections result in stillbirth and preterm delivery could potentially inform interventions to improve child and maternal health globally.”
Open power morcellation of uterine tumors during hysterectomy banned at two Boston hospitals
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy may be riskier than many have thought, especially when morcellation is performed in an “open” fashion (without use of a protective bag) in the peritoneal cavity. That’s the conclusion reached by two top Boston hospitals recently, when Brigham and Women’s and Massachusetts General both banned use of open power morcellation in gynecologic surgery.
Both hospitals assert that, when used outside of a containment system such as a morcellation bag, intraperitoneal open morcellation can spread tumor tissue throughout the peritoneal cavity. Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Related article: Options for reducing the use of open power morcellation of uterine tumors Robert L. Barbieri, MD (Editorial, March 2014)
The two Boston hospitals are not the only institutions reconsidering the use of open power morcellation. Temple University Hospital in Philadelphia banned the procedure in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also of dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
The most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”4
FILLING THE TECHNOLOGY GAP
Now that open power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation. His strategy involves utilization of a large bowel isolation bag. For more on this approach, click here.
AAGL is in the process of formulating a policy on the use of open power morcellation. ACOG has not signaled its intent to weigh in on the issue.
Brigham and Women’s Hospital intends to carefully review requests for permission to utilize open power morcellation on a case-by-case basis, provided the surgeon presents all case details and a rationale for exemption from the new rule.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL. AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy may be riskier than many have thought, especially when morcellation is performed in an “open” fashion (without use of a protective bag) in the peritoneal cavity. That’s the conclusion reached by two top Boston hospitals recently, when Brigham and Women’s and Massachusetts General both banned use of open power morcellation in gynecologic surgery.
Both hospitals assert that, when used outside of a containment system such as a morcellation bag, intraperitoneal open morcellation can spread tumor tissue throughout the peritoneal cavity. Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Related article: Options for reducing the use of open power morcellation of uterine tumors Robert L. Barbieri, MD (Editorial, March 2014)
The two Boston hospitals are not the only institutions reconsidering the use of open power morcellation. Temple University Hospital in Philadelphia banned the procedure in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also of dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
The most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”4
FILLING THE TECHNOLOGY GAP
Now that open power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation. His strategy involves utilization of a large bowel isolation bag. For more on this approach, click here.
AAGL is in the process of formulating a policy on the use of open power morcellation. ACOG has not signaled its intent to weigh in on the issue.
Brigham and Women’s Hospital intends to carefully review requests for permission to utilize open power morcellation on a case-by-case basis, provided the surgeon presents all case details and a rationale for exemption from the new rule.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy may be riskier than many have thought, especially when morcellation is performed in an “open” fashion (without use of a protective bag) in the peritoneal cavity. That’s the conclusion reached by two top Boston hospitals recently, when Brigham and Women’s and Massachusetts General both banned use of open power morcellation in gynecologic surgery.
Both hospitals assert that, when used outside of a containment system such as a morcellation bag, intraperitoneal open morcellation can spread tumor tissue throughout the peritoneal cavity. Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Related article: Options for reducing the use of open power morcellation of uterine tumors Robert L. Barbieri, MD (Editorial, March 2014)
The two Boston hospitals are not the only institutions reconsidering the use of open power morcellation. Temple University Hospital in Philadelphia banned the procedure in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also of dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
The most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”4
FILLING THE TECHNOLOGY GAP
Now that open power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation. His strategy involves utilization of a large bowel isolation bag. For more on this approach, click here.
AAGL is in the process of formulating a policy on the use of open power morcellation. ACOG has not signaled its intent to weigh in on the issue.
Brigham and Women’s Hospital intends to carefully review requests for permission to utilize open power morcellation on a case-by-case basis, provided the surgeon presents all case details and a rationale for exemption from the new rule.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL. AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL. AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
RELATED ARTICLES
![]()
![]()
Options for reducing the use of open power morcellation of uterine tumors Robert L. Barbieri, MD (Editorial, March 2014)
How I avoid open power morcellation
Morcellated leiomyosarcoma is a very real risk
(Comment & Controversy, March 2014)

![]()
Benefits and pitfalls of open power morcellation of uterine fibroids Robert L. Barbieri, MD (Editorial, February 2014)
On-site reporting from the Society of Gynecologic Surgeons (SGS) Scientific Meeting
3/26/14. Day 3 at SGS
Debates, rebuttals, and relaxation
The morning at SGS was divided up between the small-group academic roundtables with experts in the fields. Topics ranged from mesh complications to coding and billing, and even a primer on urology for the gynecologist.
In the main hall, Drs. Dee Feener and Mark Walters outlined the challenges and opportunities for training the next generation of gynecologic surgeons. Dr. Feener argued that there simply are not enough cases, not enough time, and not enough people to train excellent surgeons. A perfect storm. Dr. Walters outlined his program and resident support at the Cleveland Clinic, showing how to provide robust experience and feedback to residents and fellows. Questions from the audience were pointed, and questioned the need to track obstetrics and gynecology separately for trainees.
Oral posters today also added to the debate with Vanderbilt sharing their hysterectomy training experience both before and after adding a fellowship. They did not see any change in vaginal hysterectomy participation over time. Most interesting was a study looking at abstract acceptance rates if an institution, research network, or author were disclosed in the body of a blinded abstract. They saw a much higher rate of acceptance if the source of the research was known by the reviewer. In his discussion, Dr. John Gebhart mused if the quality of these studies were somehow better, or if this perceived association resulted in any true bias. Nevertheless, the audience was actively engaged in the discussion.
The morning's highlight was certainly the debate over cosmetic gynecologic surgery. Dr. Rachel Pauls advocated FOR labiaplasty and Dr. Becky Rogers AGAINST. Though spirited and based largely on the principals of medical ethics, the final blow came from Dr. Rogers as she distributed Love Our Labia (LOL) buttons to the audience and presented Dr. Pauls with a pink LOL t-shirt. The Twitter feed exploded after this.
Follow us on Twitter @obgmanagement #SGS14.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
We were also honored to have Dr. Clifford Ko, director of the American College of Surgeons Quality Improvement Program, as the esteemed Telinde lecturer. This robust and data-filled talk underlines his thesis that accurate, believable, and actionable data can be used to create quality in surgery. Quality improvement is local, he stated, and culture is the hardest institutional characteristic to change. Though any team working together on quality will elevate their culture if the data are good and the benefit to patients is clear. Dr. Ko, a colorectal surgeon at UCLA, is also now an honorary member of SGS.
The afternoon adjourned after the business meeting, and members were able to play golf, tour the desert in 4-wheel drive, or just relax in the lazy river by the pool. Activities were threatened by a large dust storm in Phoenix, but I have heard of no reports of problems.
Everyone convened at the outside terrace for the evening Fiesta Margarita reception. Over drinks and Southwest-themed sombreros, the new Michael Aronson Fund was announced to support Surgeons Helping Advance Research and Education (SHARE). This was the result of more than $25,000 raised by the program committee and SGS Board. Tomorrow looks to be an excellent conclusion to a well-planned and very well-executed meeting.
Follow us on Twitter @obgmanagement #SGS14.
3/25/14. Day 2 at SGS
Scientific sessions and socializing
The first day of the SGS scientific sessions was another energetic and interactive day. Oral posters stimulated heated debates on uterine morcellation, asymptomatic prolapse, and resident training. The Fellows Pelvic Research Network (FPRN) presented their work on the introduction of robotic hysterectomies to training centers. They showed that number of hysterectomies went down, and participation in robotic cases was poor.
This was followed by the exceptional keynote address by Dr. Barbara Levy. She shared her expertise of health policy and described the coming of quality-based payment, value in Supervises, and the need to protect resources. She predicted that hospitals need to cut costs by 25% to 30% in the next 5 years just to survive.
The afternoon videofest included surgical techniques, anatomy instruction, and a comprehensive review on bowel surgery for the practicing gynecologist.
For the second year running, the SGS hosted a mock NIH study session. Dr. Katherine Hartmann of Vanderbilt University provided background prep to prepare fellows for a K or R award application. Combined with a most-study section review of two actual applications demystified the process of grant review (and rejection).
The FPRN met to update their ongoing projects and to review new proposals. This was an enlightening and engaging session which should give everyone great hope to see the creativity and energy of the next generation of researchers.
SGS President Dr. Holly Richter had the great honor to present the best poster and video awards, as well as recognize the largest new-member class in the history of SGS. Dr. Norton was recognized for the best member presentation, which was on long-term prolapse follow-up in the TOMUS trial cohort. The FPRN was also recognized for their work on the impact of robotic hysterectomy in training.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
3/24/14. Day 1 at SGS
Postgraduate course examines cautions and takeaways from published research
Our first day at the annual Society of Gynecologic Surgeons Scientific Meeting was off to a running start at the Postgraduate Courses. Program Chair Dr. Cheryl Iglesia joined me for a rapid-fire account of the evidence-based medicine course on social media.
The SGS birth on Twitter was explosive, with our four social media Fellow Scholars linking real-time comments to the courses. Dr. Vivian Sung put together an amazing team to review and apply the principles of evidence-based medicine for the course attendees. Once we accepted that most published research was bad and not terribly generalizable, small break-out groups were quick to use the PICO-S model to define (or try to define) a Population, Intervention, Comparator, Outcomes, and Study design.
This was followed by Dr. Ethan Balk of Tufts Center for Clinical Evidence Synthesis helping us wrap our heads around the randomized controlled trial (RCT). His caution was to consider the costly and underpowered trial, and lack of generalizability needed to define rigorous study inclusion and outcome criteria.
More bad news followed when Dr. Sung reviewed the cautionary tale of surrogate outcomes. While the perfect surrogate would allow us to shorten studies and save money, the seduction of association and causation can lead to some questionable conclusions. Are anatomical and urodynamic outcomes the same as patient perception of cure and improvement?
It wasn't all doom and gloom, as reflected in the lively tweets and posts by @obgmanagement and @gynsurgery. The strong work of the SGS Systemic Review Committee was lauded by Dr. Miles Murphy in his "How to Use a Clinical Practice Guideline." A systematic review needs to be included, though a meta-analysis is not always required, he said. What limits us is the poor quality and paucity of randomized trials for most patient populations. Treatment effect is best shown in RCTs, but minimizes harm; cohort and case series are better. Patient registries may allow for better determining a denominator and harm "rates," though they will miss clinical patient-based outcomes. With the coming of comparative effectiveness, these registries will be online quickly. Further, Dr. Balk showed us that, with more than 13,000 gynecologic research papers published each year, no one could ever keep track.
Dr. Ike Rahn gave an excellent presentation of subgroup analysis. To summarize: do it cautiously, describe which groups you analyze and have statistical back-up for your power and P-value calculations.
To round out the course, Dr. John Wong took us through his crystal ball on the future of evidence-based medicine. Because RCTs are expensive and comprise less than 2.5% of published studies, he proposed the analysis of observational studies as RCTs. Using patient-centered outcomes, efficacy data, and multiple providers, we will be better able to inform our patient and our colleagues on the best treatments. Again, as comparative effectiveness broadens policy decision, we must be agile, adaptive, and accountable.
Follow us on Twitter @obgmanagement #SGS14
3/26/14. Day 3 at SGS
Debates, rebuttals, and relaxation
The morning at SGS was divided up between the small-group academic roundtables with experts in the fields. Topics ranged from mesh complications to coding and billing, and even a primer on urology for the gynecologist.
In the main hall, Drs. Dee Feener and Mark Walters outlined the challenges and opportunities for training the next generation of gynecologic surgeons. Dr. Feener argued that there simply are not enough cases, not enough time, and not enough people to train excellent surgeons. A perfect storm. Dr. Walters outlined his program and resident support at the Cleveland Clinic, showing how to provide robust experience and feedback to residents and fellows. Questions from the audience were pointed, and questioned the need to track obstetrics and gynecology separately for trainees.
Oral posters today also added to the debate with Vanderbilt sharing their hysterectomy training experience both before and after adding a fellowship. They did not see any change in vaginal hysterectomy participation over time. Most interesting was a study looking at abstract acceptance rates if an institution, research network, or author were disclosed in the body of a blinded abstract. They saw a much higher rate of acceptance if the source of the research was known by the reviewer. In his discussion, Dr. John Gebhart mused if the quality of these studies were somehow better, or if this perceived association resulted in any true bias. Nevertheless, the audience was actively engaged in the discussion.
The morning's highlight was certainly the debate over cosmetic gynecologic surgery. Dr. Rachel Pauls advocated FOR labiaplasty and Dr. Becky Rogers AGAINST. Though spirited and based largely on the principals of medical ethics, the final blow came from Dr. Rogers as she distributed Love Our Labia (LOL) buttons to the audience and presented Dr. Pauls with a pink LOL t-shirt. The Twitter feed exploded after this.
Follow us on Twitter @obgmanagement #SGS14.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
We were also honored to have Dr. Clifford Ko, director of the American College of Surgeons Quality Improvement Program, as the esteemed Telinde lecturer. This robust and data-filled talk underlines his thesis that accurate, believable, and actionable data can be used to create quality in surgery. Quality improvement is local, he stated, and culture is the hardest institutional characteristic to change. Though any team working together on quality will elevate their culture if the data are good and the benefit to patients is clear. Dr. Ko, a colorectal surgeon at UCLA, is also now an honorary member of SGS.
The afternoon adjourned after the business meeting, and members were able to play golf, tour the desert in 4-wheel drive, or just relax in the lazy river by the pool. Activities were threatened by a large dust storm in Phoenix, but I have heard of no reports of problems.
Everyone convened at the outside terrace for the evening Fiesta Margarita reception. Over drinks and Southwest-themed sombreros, the new Michael Aronson Fund was announced to support Surgeons Helping Advance Research and Education (SHARE). This was the result of more than $25,000 raised by the program committee and SGS Board. Tomorrow looks to be an excellent conclusion to a well-planned and very well-executed meeting.
Follow us on Twitter @obgmanagement #SGS14.
3/25/14. Day 2 at SGS
Scientific sessions and socializing
The first day of the SGS scientific sessions was another energetic and interactive day. Oral posters stimulated heated debates on uterine morcellation, asymptomatic prolapse, and resident training. The Fellows Pelvic Research Network (FPRN) presented their work on the introduction of robotic hysterectomies to training centers. They showed that number of hysterectomies went down, and participation in robotic cases was poor.
This was followed by the exceptional keynote address by Dr. Barbara Levy. She shared her expertise of health policy and described the coming of quality-based payment, value in Supervises, and the need to protect resources. She predicted that hospitals need to cut costs by 25% to 30% in the next 5 years just to survive.
The afternoon videofest included surgical techniques, anatomy instruction, and a comprehensive review on bowel surgery for the practicing gynecologist.
For the second year running, the SGS hosted a mock NIH study session. Dr. Katherine Hartmann of Vanderbilt University provided background prep to prepare fellows for a K or R award application. Combined with a most-study section review of two actual applications demystified the process of grant review (and rejection).
The FPRN met to update their ongoing projects and to review new proposals. This was an enlightening and engaging session which should give everyone great hope to see the creativity and energy of the next generation of researchers.
SGS President Dr. Holly Richter had the great honor to present the best poster and video awards, as well as recognize the largest new-member class in the history of SGS. Dr. Norton was recognized for the best member presentation, which was on long-term prolapse follow-up in the TOMUS trial cohort. The FPRN was also recognized for their work on the impact of robotic hysterectomy in training.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
3/24/14. Day 1 at SGS
Postgraduate course examines cautions and takeaways from published research
Our first day at the annual Society of Gynecologic Surgeons Scientific Meeting was off to a running start at the Postgraduate Courses. Program Chair Dr. Cheryl Iglesia joined me for a rapid-fire account of the evidence-based medicine course on social media.
The SGS birth on Twitter was explosive, with our four social media Fellow Scholars linking real-time comments to the courses. Dr. Vivian Sung put together an amazing team to review and apply the principles of evidence-based medicine for the course attendees. Once we accepted that most published research was bad and not terribly generalizable, small break-out groups were quick to use the PICO-S model to define (or try to define) a Population, Intervention, Comparator, Outcomes, and Study design.
This was followed by Dr. Ethan Balk of Tufts Center for Clinical Evidence Synthesis helping us wrap our heads around the randomized controlled trial (RCT). His caution was to consider the costly and underpowered trial, and lack of generalizability needed to define rigorous study inclusion and outcome criteria.
More bad news followed when Dr. Sung reviewed the cautionary tale of surrogate outcomes. While the perfect surrogate would allow us to shorten studies and save money, the seduction of association and causation can lead to some questionable conclusions. Are anatomical and urodynamic outcomes the same as patient perception of cure and improvement?
It wasn't all doom and gloom, as reflected in the lively tweets and posts by @obgmanagement and @gynsurgery. The strong work of the SGS Systemic Review Committee was lauded by Dr. Miles Murphy in his "How to Use a Clinical Practice Guideline." A systematic review needs to be included, though a meta-analysis is not always required, he said. What limits us is the poor quality and paucity of randomized trials for most patient populations. Treatment effect is best shown in RCTs, but minimizes harm; cohort and case series are better. Patient registries may allow for better determining a denominator and harm "rates," though they will miss clinical patient-based outcomes. With the coming of comparative effectiveness, these registries will be online quickly. Further, Dr. Balk showed us that, with more than 13,000 gynecologic research papers published each year, no one could ever keep track.
Dr. Ike Rahn gave an excellent presentation of subgroup analysis. To summarize: do it cautiously, describe which groups you analyze and have statistical back-up for your power and P-value calculations.
To round out the course, Dr. John Wong took us through his crystal ball on the future of evidence-based medicine. Because RCTs are expensive and comprise less than 2.5% of published studies, he proposed the analysis of observational studies as RCTs. Using patient-centered outcomes, efficacy data, and multiple providers, we will be better able to inform our patient and our colleagues on the best treatments. Again, as comparative effectiveness broadens policy decision, we must be agile, adaptive, and accountable.
Follow us on Twitter @obgmanagement #SGS14
3/26/14. Day 3 at SGS
Debates, rebuttals, and relaxation
The morning at SGS was divided up between the small-group academic roundtables with experts in the fields. Topics ranged from mesh complications to coding and billing, and even a primer on urology for the gynecologist.
In the main hall, Drs. Dee Feener and Mark Walters outlined the challenges and opportunities for training the next generation of gynecologic surgeons. Dr. Feener argued that there simply are not enough cases, not enough time, and not enough people to train excellent surgeons. A perfect storm. Dr. Walters outlined his program and resident support at the Cleveland Clinic, showing how to provide robust experience and feedback to residents and fellows. Questions from the audience were pointed, and questioned the need to track obstetrics and gynecology separately for trainees.
Oral posters today also added to the debate with Vanderbilt sharing their hysterectomy training experience both before and after adding a fellowship. They did not see any change in vaginal hysterectomy participation over time. Most interesting was a study looking at abstract acceptance rates if an institution, research network, or author were disclosed in the body of a blinded abstract. They saw a much higher rate of acceptance if the source of the research was known by the reviewer. In his discussion, Dr. John Gebhart mused if the quality of these studies were somehow better, or if this perceived association resulted in any true bias. Nevertheless, the audience was actively engaged in the discussion.
The morning's highlight was certainly the debate over cosmetic gynecologic surgery. Dr. Rachel Pauls advocated FOR labiaplasty and Dr. Becky Rogers AGAINST. Though spirited and based largely on the principals of medical ethics, the final blow came from Dr. Rogers as she distributed Love Our Labia (LOL) buttons to the audience and presented Dr. Pauls with a pink LOL t-shirt. The Twitter feed exploded after this.
Follow us on Twitter @obgmanagement #SGS14.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
We were also honored to have Dr. Clifford Ko, director of the American College of Surgeons Quality Improvement Program, as the esteemed Telinde lecturer. This robust and data-filled talk underlines his thesis that accurate, believable, and actionable data can be used to create quality in surgery. Quality improvement is local, he stated, and culture is the hardest institutional characteristic to change. Though any team working together on quality will elevate their culture if the data are good and the benefit to patients is clear. Dr. Ko, a colorectal surgeon at UCLA, is also now an honorary member of SGS.
The afternoon adjourned after the business meeting, and members were able to play golf, tour the desert in 4-wheel drive, or just relax in the lazy river by the pool. Activities were threatened by a large dust storm in Phoenix, but I have heard of no reports of problems.
Everyone convened at the outside terrace for the evening Fiesta Margarita reception. Over drinks and Southwest-themed sombreros, the new Michael Aronson Fund was announced to support Surgeons Helping Advance Research and Education (SHARE). This was the result of more than $25,000 raised by the program committee and SGS Board. Tomorrow looks to be an excellent conclusion to a well-planned and very well-executed meeting.
Follow us on Twitter @obgmanagement #SGS14.
3/25/14. Day 2 at SGS
Scientific sessions and socializing
The first day of the SGS scientific sessions was another energetic and interactive day. Oral posters stimulated heated debates on uterine morcellation, asymptomatic prolapse, and resident training. The Fellows Pelvic Research Network (FPRN) presented their work on the introduction of robotic hysterectomies to training centers. They showed that number of hysterectomies went down, and participation in robotic cases was poor.
This was followed by the exceptional keynote address by Dr. Barbara Levy. She shared her expertise of health policy and described the coming of quality-based payment, value in Supervises, and the need to protect resources. She predicted that hospitals need to cut costs by 25% to 30% in the next 5 years just to survive.
The afternoon videofest included surgical techniques, anatomy instruction, and a comprehensive review on bowel surgery for the practicing gynecologist.
For the second year running, the SGS hosted a mock NIH study session. Dr. Katherine Hartmann of Vanderbilt University provided background prep to prepare fellows for a K or R award application. Combined with a most-study section review of two actual applications demystified the process of grant review (and rejection).
The FPRN met to update their ongoing projects and to review new proposals. This was an enlightening and engaging session which should give everyone great hope to see the creativity and energy of the next generation of researchers.
SGS President Dr. Holly Richter had the great honor to present the best poster and video awards, as well as recognize the largest new-member class in the history of SGS. Dr. Norton was recognized for the best member presentation, which was on long-term prolapse follow-up in the TOMUS trial cohort. The FPRN was also recognized for their work on the impact of robotic hysterectomy in training.
The evening wrapped up with a lively social event in the exhibit hall with the meeting sponsors, colleagues, and friends.
3/24/14. Day 1 at SGS
Postgraduate course examines cautions and takeaways from published research
Our first day at the annual Society of Gynecologic Surgeons Scientific Meeting was off to a running start at the Postgraduate Courses. Program Chair Dr. Cheryl Iglesia joined me for a rapid-fire account of the evidence-based medicine course on social media.
The SGS birth on Twitter was explosive, with our four social media Fellow Scholars linking real-time comments to the courses. Dr. Vivian Sung put together an amazing team to review and apply the principles of evidence-based medicine for the course attendees. Once we accepted that most published research was bad and not terribly generalizable, small break-out groups were quick to use the PICO-S model to define (or try to define) a Population, Intervention, Comparator, Outcomes, and Study design.
This was followed by Dr. Ethan Balk of Tufts Center for Clinical Evidence Synthesis helping us wrap our heads around the randomized controlled trial (RCT). His caution was to consider the costly and underpowered trial, and lack of generalizability needed to define rigorous study inclusion and outcome criteria.
More bad news followed when Dr. Sung reviewed the cautionary tale of surrogate outcomes. While the perfect surrogate would allow us to shorten studies and save money, the seduction of association and causation can lead to some questionable conclusions. Are anatomical and urodynamic outcomes the same as patient perception of cure and improvement?
It wasn't all doom and gloom, as reflected in the lively tweets and posts by @obgmanagement and @gynsurgery. The strong work of the SGS Systemic Review Committee was lauded by Dr. Miles Murphy in his "How to Use a Clinical Practice Guideline." A systematic review needs to be included, though a meta-analysis is not always required, he said. What limits us is the poor quality and paucity of randomized trials for most patient populations. Treatment effect is best shown in RCTs, but minimizes harm; cohort and case series are better. Patient registries may allow for better determining a denominator and harm "rates," though they will miss clinical patient-based outcomes. With the coming of comparative effectiveness, these registries will be online quickly. Further, Dr. Balk showed us that, with more than 13,000 gynecologic research papers published each year, no one could ever keep track.
Dr. Ike Rahn gave an excellent presentation of subgroup analysis. To summarize: do it cautiously, describe which groups you analyze and have statistical back-up for your power and P-value calculations.
To round out the course, Dr. John Wong took us through his crystal ball on the future of evidence-based medicine. Because RCTs are expensive and comprise less than 2.5% of published studies, he proposed the analysis of observational studies as RCTs. Using patient-centered outcomes, efficacy data, and multiple providers, we will be better able to inform our patient and our colleagues on the best treatments. Again, as comparative effectiveness broadens policy decision, we must be agile, adaptive, and accountable.
Follow us on Twitter @obgmanagement #SGS14
The optimal time for scheduled repeat cesarean may be less than 39 weeks
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
Current recommendations1,2 are for women to schedule CDs no earlier than 39 weeks’ gestation, regardless of the number of prior CDs they have had. But new research3 suggests that delivery during the 38th week, for women who have had two prior CDs, and during the 37th week, for women who have had at least three prior CDs, is the best way to minimize maternal complications without compromising perinatal outcomes.
DETAILS OF THE STUDY
Researchers from the University of Texas Medical School at Houston presented their findings at the 34th annual meeting of the Society for Maternal–Fetal Medicine, the Pregnancy Meeting, held February 3–8, 2014, in New Orleans, LA. They studied 6,435 women who had at least 2 prior CDs and were at least 37 weeks pregnant. None of the women had any underlying medical or obstetric conditions requiring delivery prior to 39 weeks.
The investigators looked at the occurrence of the following maternal complications: transfusion, hysterectomy, operative injury (cystotomy, ureteral injury, or bowel injury), coagulopathy, thromboembolic event, pulmonary edema, and death. Adverse perinatal outcomes included in the study were respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage grades 3 or 4, seizures, and fetal or neonatal death.
Complication rates were significantly different across gestational ages for both maternal (P <.05) and neonatal outcomes (P <.05). Among the women who had two prior CDs, the risk of a maternal complication was three times higher for the women who delivered at or after 39 weeks than for those who delivered at 38 weeks, and there was a concomitant rise in the risk of a perinatal complication.
Among the group of women with at least three prior CDs, those who gave birth at or after 39 weeks’ gestation had an eightfold higher risk of a maternal complication than those who delivered at 37 weeks. Even those who delivered at 38 weeks had a fourfold greater risk than those who delivered at 37 weeks.
CLINICAL RECOMMENDATIONS
The authors of the study concluded that the optimal time for scheduling delivery for women with 2 previous CDs is between 38 weeks 0 days and 38 weeks 6 days. For women with at least 3 previous CDs, optimal timing is between 37 weeks 0 days and 37 weeks 6 days.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
1. Toward Improving the Outcome of Pregnancy III. White Plains, NY: March of Dimes; 2010.
2. American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin #107: Induction of Labor. Obstet Gynecol. 2009;114:386–397.
3. Hart L, Refuerzo J, Sibai B, Blackwell S. Should the “39 week rule” apply to women with multiple prior cesarean deliveries? [SMFM abstract 40]. Am J Obstet Gynecol. 2014;210(suppl):S27.
FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
The US Food and Drug Administration (FDA) Microbiology Devices Panel of the Medical Devices Advisory Committee has unanimously recommended that the cobas HPV (human papillomavirus) test be used as a first-line primary screening tool in women aged 25 years and older to assess their risk of cervical cancer based on the presence of clinically relevant high-risk HPV DNA. The committee’s recommendation indicates that the benefits outweigh the risks of the test, and that the cobas HPV test is safe and effective for the proposed indication for use.1
If approved by the FDA, the cobas HPV test would become the “first and only HPV test indicated as the first-line primary screen of cervical cancer in the United States.”2 Although the FDA is not required to follow the Advisory Committee’s recommendation, it takes the advice into consideration.
Data behind the recommendation
The Advisory Committee’s recommendation is supported by data from the ATHENA study, which included more than 47,000 women – the largest US-based registration study for cervical cancer screening. Data show that when the cobas HPV test was used as the primary test and Pap cytology as a secondary test, significantly more cervical disease was detected compared with Pap screening alone.2
“Through technological and scientific advancement, we now have a better screening tool for cervical cancer. Women around the world deserve the best tool to know their risk and reduce their chances of developing cervical cancer,” said Roland Diggelmann, COO for the Division of Roche Diagnostics, the company who developed and manufactures the test. “We look forward to working with the FDA and medical community to support the growing understanding and awareness of the role that HPV plays in cervical disease, and the importance of the cobas HPV test, which provides the necessary medical benefit to become the first-line test in a cervical cancer screening strategy.”2
How could current practice change as a result of final FDA approval?
The cobas HPV test is currently FDA-approved for co-testing with the Pap smear in women older than age 30 for cervical cancer screening, and for screening patients aged 21 and older with abnormal cervical cytology results.
Mark H. Einstein, MD, MS, chair of the cervical cancer education efforts of the Foundation for Women’s Cancer and professor of obstetrics and gynecology at Albert Einstein Cancer Center and Montefiore Medical Center in Bronx, New York, says final approval of this testing as a primary screening tool represents significant changes to clinical practice. However, “similar to what happened when co-testing [with the cobas HPV test] was approved, it took time for scientific stakeholding groups to update clinical guidelines, then years before clinicians adopted it into routine practice.”
“Unlike a new prescription, clinical algorithms tend to be 'hard-wired' into clinicians heads, and adopting significant change is a process,” Einstein says. “It’s likely that a new cervical cancer screening testing clinical algorithm would be adopted by some clinicians early and by many clinicians over time.” He added that the Society of Gynecologic Oncologists and the American Society for Colposcopy and Cervical Pathology have an interim clinical guidance document currently drafted, and those guidelines will be released soon after any decisions by the FDA.
When that time comes (assuming final FDA approval is received), Einstein says, “some clinical settings will be able to start with the more sensitive HPV test. For some patients, this will be followed by genotyping or cytology. This has been shown to be an effective strategy for honing in on the most at-risk women in a screening population.”
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
- FDA Executive Summary: March 12, 2014. 2014 Meeting Materials of the Microbiology Devices Panel. FDA Web site. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed March 14, 2014.
- FDA Advisory Committee unanimously recommends Roche's HPV Test as primary screening tool for detection of women at high risk for cervical cancer [media release]. http://www.roche.com/media/media_releases/med-cor-2014-03-13.htm. Published March 13, 2014. Accessed March 14, 2014.
The intrauterine ball: The IUD goes 3D
Most intrauterine devices (IUDs) worldwide, and all IUDs currently available in the United States have a 2-dimensional T-shaped design. A new device, called the intrauterine ball (IUB), consists of 17 copper spheres threaded over a plastic-coated wire constructed of nitinol, an alloy with elastic characteristics.1,2 The wire and copper spheres are placed in the uterus using a 3.2-mm outer-diameter tube. When released from the tube, the wire and spheres assume a circular configuration within the endometrial cavity that is larger than the cervical canal.1,2
Details of a small study
Dr. Ilan Baram and colleagues recently published a case series in the journal Contraception in which 15 women (mean age 33.9 years; one woman was nulliparous) were followed for one year. The study was supported in part by the National Institutes of Health (NIH) and also by Ocon Medical, the device manufacturer (two of the authors reported being officers of Ocon Medical).1
The report authors noted that the IUB was easily inserted in each woman. No perforations, expulsions, malpositioned devices, or pregnancies were observed. Due to heavy uterine bleeding, the IUB was removed from one woman at 17 weeks; curettage revealed simple endometrial hyperplasia. Three women reported transient abdominal discomfort. At one year, 12 women indicated they were “very satisfied” with this contraceptive; one indicated she was “satisfied”; and one indicated she was “moderately satisfied”.1 (One woman discontinued the study at 119 days after insertion for a reason not related to the device.)
What is a practitioner’s point of view regarding this new IUD design? Andrew M. Kaunitz, MD, Professor and Associate Chairman of the Department of Obstetrics and Gynecology at the University of Florida College of Medicine in Jacksonville, and member of the OBG Management Board of Editors, says, “Although the design of the IUB as well as the findings from this small study are intriguing, it will be important to learn how the IUB’s performance (including rates of failure, infection, perforation, expulsion, device malposition, and continuation as well as insertion pain and impact on bleeding) compare with existing IUDs.”
Additional trials planned
The study authors indicate that a larger randomized trial comparing the IUB with the Copper T380 is now underway in Europe.1
A North American clinical trial3 has begun to evaluate early expulsion rates of Ocon’s second product, the IUB SCu380A, with a larger copper surface area (380 mm2 copper surface area) than the original SCu300A (300 mm2 copper surface area).2 The trial site, located in Vancouver, Canada, will recruit 50 women. The trial will examine expulsion rates after 2 months and will continue to follow these women for 12 months.
According to a press release, Ocon hopes to gather clinical evidence to understand the IUB’s advantages over current IUDs, especially with respect to its safety profile and quality of life attributes. To accomplish this, Ocon will initiate additional multinational clinical trials in the next several months.3,4
To watch a video that highlights the design of the Ocon Medical IUB, click here.
- Baram I, Weinstein A, Trussell J. The IUB, a newly invented IUD: a brief report. Contraception. 2014;89(2):139–141.
- Devices. Ocon Medical Web site. http://oconmed.com/devices.htm. Accessed March 13, 2014.
- North American clinical trial of the IUB SC380A intrauterine contraceptive device initiated with positive initial results [press release]. Evaluategroup.com. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=484094. Published January 21, 2014. Accessed March 13, 2014.
- Press releases. Ocon Medical Web site. http://oconmed.com. Accessed March 13, 2014.
Most intrauterine devices (IUDs) worldwide, and all IUDs currently available in the United States have a 2-dimensional T-shaped design. A new device, called the intrauterine ball (IUB), consists of 17 copper spheres threaded over a plastic-coated wire constructed of nitinol, an alloy with elastic characteristics.1,2 The wire and copper spheres are placed in the uterus using a 3.2-mm outer-diameter tube. When released from the tube, the wire and spheres assume a circular configuration within the endometrial cavity that is larger than the cervical canal.1,2
Details of a small study
Dr. Ilan Baram and colleagues recently published a case series in the journal Contraception in which 15 women (mean age 33.9 years; one woman was nulliparous) were followed for one year. The study was supported in part by the National Institutes of Health (NIH) and also by Ocon Medical, the device manufacturer (two of the authors reported being officers of Ocon Medical).1
The report authors noted that the IUB was easily inserted in each woman. No perforations, expulsions, malpositioned devices, or pregnancies were observed. Due to heavy uterine bleeding, the IUB was removed from one woman at 17 weeks; curettage revealed simple endometrial hyperplasia. Three women reported transient abdominal discomfort. At one year, 12 women indicated they were “very satisfied” with this contraceptive; one indicated she was “satisfied”; and one indicated she was “moderately satisfied”.1 (One woman discontinued the study at 119 days after insertion for a reason not related to the device.)
What is a practitioner’s point of view regarding this new IUD design? Andrew M. Kaunitz, MD, Professor and Associate Chairman of the Department of Obstetrics and Gynecology at the University of Florida College of Medicine in Jacksonville, and member of the OBG Management Board of Editors, says, “Although the design of the IUB as well as the findings from this small study are intriguing, it will be important to learn how the IUB’s performance (including rates of failure, infection, perforation, expulsion, device malposition, and continuation as well as insertion pain and impact on bleeding) compare with existing IUDs.”
Additional trials planned
The study authors indicate that a larger randomized trial comparing the IUB with the Copper T380 is now underway in Europe.1
A North American clinical trial3 has begun to evaluate early expulsion rates of Ocon’s second product, the IUB SCu380A, with a larger copper surface area (380 mm2 copper surface area) than the original SCu300A (300 mm2 copper surface area).2 The trial site, located in Vancouver, Canada, will recruit 50 women. The trial will examine expulsion rates after 2 months and will continue to follow these women for 12 months.
According to a press release, Ocon hopes to gather clinical evidence to understand the IUB’s advantages over current IUDs, especially with respect to its safety profile and quality of life attributes. To accomplish this, Ocon will initiate additional multinational clinical trials in the next several months.3,4
To watch a video that highlights the design of the Ocon Medical IUB, click here.
Most intrauterine devices (IUDs) worldwide, and all IUDs currently available in the United States have a 2-dimensional T-shaped design. A new device, called the intrauterine ball (IUB), consists of 17 copper spheres threaded over a plastic-coated wire constructed of nitinol, an alloy with elastic characteristics.1,2 The wire and copper spheres are placed in the uterus using a 3.2-mm outer-diameter tube. When released from the tube, the wire and spheres assume a circular configuration within the endometrial cavity that is larger than the cervical canal.1,2
Details of a small study
Dr. Ilan Baram and colleagues recently published a case series in the journal Contraception in which 15 women (mean age 33.9 years; one woman was nulliparous) were followed for one year. The study was supported in part by the National Institutes of Health (NIH) and also by Ocon Medical, the device manufacturer (two of the authors reported being officers of Ocon Medical).1
The report authors noted that the IUB was easily inserted in each woman. No perforations, expulsions, malpositioned devices, or pregnancies were observed. Due to heavy uterine bleeding, the IUB was removed from one woman at 17 weeks; curettage revealed simple endometrial hyperplasia. Three women reported transient abdominal discomfort. At one year, 12 women indicated they were “very satisfied” with this contraceptive; one indicated she was “satisfied”; and one indicated she was “moderately satisfied”.1 (One woman discontinued the study at 119 days after insertion for a reason not related to the device.)
What is a practitioner’s point of view regarding this new IUD design? Andrew M. Kaunitz, MD, Professor and Associate Chairman of the Department of Obstetrics and Gynecology at the University of Florida College of Medicine in Jacksonville, and member of the OBG Management Board of Editors, says, “Although the design of the IUB as well as the findings from this small study are intriguing, it will be important to learn how the IUB’s performance (including rates of failure, infection, perforation, expulsion, device malposition, and continuation as well as insertion pain and impact on bleeding) compare with existing IUDs.”
Additional trials planned
The study authors indicate that a larger randomized trial comparing the IUB with the Copper T380 is now underway in Europe.1
A North American clinical trial3 has begun to evaluate early expulsion rates of Ocon’s second product, the IUB SCu380A, with a larger copper surface area (380 mm2 copper surface area) than the original SCu300A (300 mm2 copper surface area).2 The trial site, located in Vancouver, Canada, will recruit 50 women. The trial will examine expulsion rates after 2 months and will continue to follow these women for 12 months.
According to a press release, Ocon hopes to gather clinical evidence to understand the IUB’s advantages over current IUDs, especially with respect to its safety profile and quality of life attributes. To accomplish this, Ocon will initiate additional multinational clinical trials in the next several months.3,4
To watch a video that highlights the design of the Ocon Medical IUB, click here.
- Baram I, Weinstein A, Trussell J. The IUB, a newly invented IUD: a brief report. Contraception. 2014;89(2):139–141.
- Devices. Ocon Medical Web site. http://oconmed.com/devices.htm. Accessed March 13, 2014.
- North American clinical trial of the IUB SC380A intrauterine contraceptive device initiated with positive initial results [press release]. Evaluategroup.com. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=484094. Published January 21, 2014. Accessed March 13, 2014.
- Press releases. Ocon Medical Web site. http://oconmed.com. Accessed March 13, 2014.
- Baram I, Weinstein A, Trussell J. The IUB, a newly invented IUD: a brief report. Contraception. 2014;89(2):139–141.
- Devices. Ocon Medical Web site. http://oconmed.com/devices.htm. Accessed March 13, 2014.
- North American clinical trial of the IUB SC380A intrauterine contraceptive device initiated with positive initial results [press release]. Evaluategroup.com. http://www.evaluategroup.com/Universal/View.aspx?type=Story&id=484094. Published January 21, 2014. Accessed March 13, 2014.
- Press releases. Ocon Medical Web site. http://oconmed.com. Accessed March 13, 2014.
Abortion rates decline in the United States
Results from a recent report by the Guttmacher Institute indicate that the abortion rate continued to decline through 2011, without evidence that the drop was related to the decrease in providers or to restrictions implemented between 2008 and 2011.1
Results of the study
Researchers from the Guttmacher Institute estimate 1.1 million abortions were performed in the United States in 2011, representing a drop of 13% since 2008 (16.9 vs 19.4 per 1,000 women ages 15–44).
Compared with 2008, 2011 data show a 4% decline in the number of abortion providers, and a 1% reduction in the number of abortion clinics. Early medication abortions accounted for a larger proportion of nonhospital abortions in 2011 than in 2008 (23% versus 17%, respectively). Of the 106 new abortion restrictions implemented during the 2010–2011 study period, few or none appeared to be related to state-level patterns in abortion rates or number of providers, according to the study authors.1
The report is based on abortion statistics compiled annually by the Centers for Disease Control and Prevention (CDC). Although the data do not include California, they can be used to illustrate trends, say the researchers.1
Conclusions
No evidence was found to indicate the overall drop in abortion incidence was related to the decrease in providers or to abortion restrictions implemented between 2008 and 2011. The national abortion rate is in decline, the researchers conclude. In fact, they say significantly fewer pregnancies, births, and abortions happened in 2011 than in 2008, partly because of an improvement in contraceptive use.
“If fewer women were experiencing unintended pregnancies because they were using more effective methods, or were using methods more consistently, this would suggest that—after a decade of stalled progress—the United States has made headway in the public health goal of reducing the rate of unintended pregnancy.”1
The study authors add, “Because state legislatures continued to debate and enact more restrictive abortion measures throughout 2011, 2012, and 2013, future research will need to examine whether and to what extent these laws affect abortion incidence and access to services.”1
Reference
Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2011 [published online ahead of print February 3, 2014]. Perspect Sex Reprod Health. doi:10.1363/46e0414. http://www.guttmacher.org/pubs/journals/psrh.46e0414.pdf. Accessed February 13, 2014.
Results from a recent report by the Guttmacher Institute indicate that the abortion rate continued to decline through 2011, without evidence that the drop was related to the decrease in providers or to restrictions implemented between 2008 and 2011.1
Results of the study
Researchers from the Guttmacher Institute estimate 1.1 million abortions were performed in the United States in 2011, representing a drop of 13% since 2008 (16.9 vs 19.4 per 1,000 women ages 15–44).
Compared with 2008, 2011 data show a 4% decline in the number of abortion providers, and a 1% reduction in the number of abortion clinics. Early medication abortions accounted for a larger proportion of nonhospital abortions in 2011 than in 2008 (23% versus 17%, respectively). Of the 106 new abortion restrictions implemented during the 2010–2011 study period, few or none appeared to be related to state-level patterns in abortion rates or number of providers, according to the study authors.1
The report is based on abortion statistics compiled annually by the Centers for Disease Control and Prevention (CDC). Although the data do not include California, they can be used to illustrate trends, say the researchers.1
Conclusions
No evidence was found to indicate the overall drop in abortion incidence was related to the decrease in providers or to abortion restrictions implemented between 2008 and 2011. The national abortion rate is in decline, the researchers conclude. In fact, they say significantly fewer pregnancies, births, and abortions happened in 2011 than in 2008, partly because of an improvement in contraceptive use.
“If fewer women were experiencing unintended pregnancies because they were using more effective methods, or were using methods more consistently, this would suggest that—after a decade of stalled progress—the United States has made headway in the public health goal of reducing the rate of unintended pregnancy.”1
The study authors add, “Because state legislatures continued to debate and enact more restrictive abortion measures throughout 2011, 2012, and 2013, future research will need to examine whether and to what extent these laws affect abortion incidence and access to services.”1
Results from a recent report by the Guttmacher Institute indicate that the abortion rate continued to decline through 2011, without evidence that the drop was related to the decrease in providers or to restrictions implemented between 2008 and 2011.1
Results of the study
Researchers from the Guttmacher Institute estimate 1.1 million abortions were performed in the United States in 2011, representing a drop of 13% since 2008 (16.9 vs 19.4 per 1,000 women ages 15–44).
Compared with 2008, 2011 data show a 4% decline in the number of abortion providers, and a 1% reduction in the number of abortion clinics. Early medication abortions accounted for a larger proportion of nonhospital abortions in 2011 than in 2008 (23% versus 17%, respectively). Of the 106 new abortion restrictions implemented during the 2010–2011 study period, few or none appeared to be related to state-level patterns in abortion rates or number of providers, according to the study authors.1
The report is based on abortion statistics compiled annually by the Centers for Disease Control and Prevention (CDC). Although the data do not include California, they can be used to illustrate trends, say the researchers.1
Conclusions
No evidence was found to indicate the overall drop in abortion incidence was related to the decrease in providers or to abortion restrictions implemented between 2008 and 2011. The national abortion rate is in decline, the researchers conclude. In fact, they say significantly fewer pregnancies, births, and abortions happened in 2011 than in 2008, partly because of an improvement in contraceptive use.
“If fewer women were experiencing unintended pregnancies because they were using more effective methods, or were using methods more consistently, this would suggest that—after a decade of stalled progress—the United States has made headway in the public health goal of reducing the rate of unintended pregnancy.”1
The study authors add, “Because state legislatures continued to debate and enact more restrictive abortion measures throughout 2011, 2012, and 2013, future research will need to examine whether and to what extent these laws affect abortion incidence and access to services.”1
Reference
Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2011 [published online ahead of print February 3, 2014]. Perspect Sex Reprod Health. doi:10.1363/46e0414. http://www.guttmacher.org/pubs/journals/psrh.46e0414.pdf. Accessed February 13, 2014.
Reference
Jones RK, Jerman J. Abortion incidence and service availability in the United States, 2011 [published online ahead of print February 3, 2014]. Perspect Sex Reprod Health. doi:10.1363/46e0414. http://www.guttmacher.org/pubs/journals/psrh.46e0414.pdf. Accessed February 13, 2014.