User login
Optimizing ‘optimal’ in ovarian cancer cytoreduction
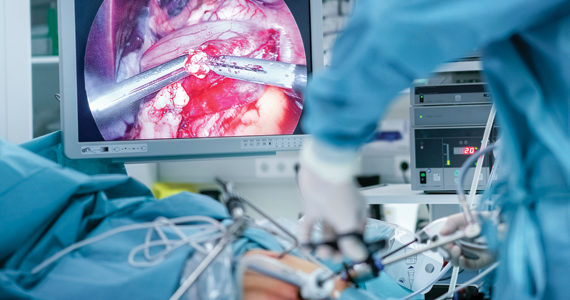
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
The goal of advanced ovarian cancer surgery is to remove all gross disease, or all visible and palpable disease implants. This became the established standard when improved survival was consistently observed among patients who had undergone complete surgical resection. Traditionally, definitions of no gross residual disease have been left in the hands, and eyes, of the surgeon. However, new technology has emerged which affords surgeons the ability to visualize ovarian cancer deposits that are imperceptible to the naked eye. But will this improve upon the poor cure rates for advanced ovarian cancer?
Many are familiar with the traditional definitions of “optimal” (less than 1 cm–sized deposits at any one location) and “suboptimal” (greater than 1 cm–sized deposits remaining) when referring to surgical cytoreduction of ovarian cancer. This nomenclature was introduced to define, categorize, and prognosticate patient groups after surgery. In recent years we have moved away from these descriptive definitions of ovarian cancer resection, borrowing from surgical oncology measures of surgical outcomes where “R0” defines surgical resection with negative margins, “R1” includes resection with positive microscopic margins (negative for tumor intraoperatively, but positive on microscopic pathology), and “R2” refers to macroscopic residual disease remaining.1
In ovarian cancer, surgeons have adopted the expression R0 to include patients in whom there is no gross visible or palpable residual disease, a special, favorable subgrouping of the previous “optimal” group. R1 is applied to patients with macroscopic, residual disease that fits within the traditional “optimal” cytoreduction classification (<1 cm in any one location). Obviously, these are significant variations to the traditional surgical oncology definitions, but not without supporting data. For example, patients with no gross residual disease (now defined as “R0”) have been observed to have improved survival, compared with patients who are “optimally” debulked but with R1 (<1 cm) residual disease.2 Therefore, this new goal of complete surgical resection has replaced the previous standard of “optimal” cytoreduction in which small macroscopic residual disease was acceptable.
Whether or not a surgery is completed with no gross residual disease is a subjective assessment made by the surgeon, and in practice, highly inaccurate. When a posttrial ad hoc analysis of 1,873 patients with advanced ovarian cancer who had been enrolled in a Gynecologic Oncology Group cooperative trial correlated surgeons’ assessments of “optimal” cytoreduction with objective postoperative radiographic findings (performed, on average, less than 1 month postoperatively) they found that postoperative CT scans identified lesions >1 cm in 40% of cases that had been characterized by surgeons as an “optimal” cytoreduction.3 Most commonly, discrepant lesions were identified in the upper abdominal quadrants and retroperitoneal aortic nodal regions. Therefore, surgeons’ subjective assessment of cytoreduction is prone to error, and given how important the completeness of cytoreduction is for clinical outcomes, there is interest in discovering methods to improve upon surgeons’ ability to discriminate volume of disease.
Pafolacianine (Cytalux, On Target Laboratories) is a novel drug that binds a fluorescent molecule to folic acid targeting the folate alpha receptors which are overexpressed on nonmucinous epithelial ovarian cancer cells compared with adjacent nonmalignant tissues.4 The drug is intravenously infused preoperatively and then visualized with companion near-infrared imaging devices during surgery to visualize its fluorescent signal where it is bound to ovarian cancer implants. In a phase 2 study of 178 patients with confirmed or suspected ovarian cancer, pafolacianine was able to detect implants of ovarian cancer in 26.9% of cases where the surgeon’s visual inspection was negative.5 Of note, the false-positive rate of this drug was not trivial, at 20%. Based on this efficacy data, the drug has been granted FDA approved for use in ovarian cancer surgery to augment the surgeon’s visualization of cancer. However, important questions remain unanswered by these preliminary data.
Will removal of additional microscopic ovarian cancer implants, only seen by pafolacianine, improve the survival of patients with ovarian cancer, and what effect will the addition of this extra surgery have on their surgical morbidity and risk? The use of pafolacianine to augment ovarian cancer debulking surgeries pivots on the premise that ovarian cancer outcomes are determined by surgical “effort” more than the biology of the disease. Otherwise said: The more we surgically remove, the more we cure. But this seems an old-fashioned notion, increasingly challenged by data. It has been shown that, when ovarian cancer debulking surgeries are necessarily more radical because of extensive disease distribution, prognosis is worse, compared with those patients with less extensive disease distribution.6 The effect of surgical effort contributes less than that of predetermined patterns of disease presentation. Additionally, genomic traits are different in tumors that are objectively determined to be not amenable to optimal cytoreduction, compared with resectable tumors.7 These data suggest that it is the disease, more than the surgeon, that most influences outcomes.
Additionally, the question of whether surgical removal of microscopic disease improves ovarian cancer survival has already been addressed with negative findings. The LION trial randomized 647 women with advanced ovarian cancer to primary cytoreductive surgery either with or without routine lymphadenectomy of clinically negative nodes.8 This study found no survival benefit to resecting clinically negative, microscopically positive nodes. In light of these data, it is difficult to imagine that there would be different results with the resection of microscopic peritoneal disease implants identified by pafolacianine.
While pafolacianine promises to move us closer to a true “R0” (negative margins) resection of ovarian cancer, is this even a feasible goal in a disease that is widely metastatic, particularly in the peritoneal cavity? What do “negative margins” mean in the peritoneal cavity? The sensitivity of pafolacianine in detecting microscopic disease is obviously not so high that it can guarantee patients a complete resection of a disseminated disease, and we still do not know what absolute benefit is derived from moving a little bit further on the continuum of surgical resection.
Perhaps augmentation of debulking is not the only, or best, use of pafolacianine for ovarian cancer surgery. Perhaps it might serve a role in diagnostics or staging of the disease rather than for a therapeutic purpose. In the meantime, we await ongoing clinical trials in this space to better inform clinicians what benefits, or harms, they might expect from the addition of this new drug as we continue to define the “optimal” surgical procedure for advanced ovarian cancer.
Dr. Emma Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no conflicts of interest.
References
1. Hermanek P, Wittekind C. Semin Surg Oncol 1994;10:12-20.
2. Elattar A et al. Cochrane Database Syst Rev 2011 Aug 10;2011(8):CD007565.
3. Eskander RN et al. Gynecol Oncol 2018;149:525-30.
4. Randall LM et al. Gynecol Oncol 2019;155:63-8.
5. Food and Drug Administration. FDA approves pafolacianine for identifying malignant ovarian cancer lesions. 2021 Dec 1.
6. Horowitz NS et al. J Clin Oncol 2015;33:937-43.
7. Lee S et al. Cell Rep. 2020;31:107502.
8. Harter P et al. N Engl J Med 2019;380:822-32.
Do recent data on use of menopausal HT and subsequent risk of dementia indicate an association?
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
Much interest has surrounded whether the use of menopausal HT impacts future risk of cognitive decline. Recently, Vinogradova and colleagues conducted an observational study using data from 2 large primary care databases, QResearch and the Clinical Practice Research Datalink (CPRD), in the United Kingdom.1 The investigators conducted case-control studies that included women aged 55 and older diagnosed with dementia and up to 5 controls without dementia. Only cases and controls with at least 10 years of medical records prior to the index date (that is, the time of dementia diagnosis in cases) were included. Since early symptoms of dementia prior to diagnosis may cause sleep problems and dysphoria (which also may be symptoms of menopause), HT prescriptions during the 3 years prior to the index date were excluded.
Details of the study
Among 16,291 cases and 68,726 controls, the women’s mean age was approximately 83 years. Cases were identified by using codes for dementia from patients’ clinical records or records of prescriptions for drugs used to treat dementia.
More than half the women were being treated for hypertension, and 14% of women in both groups had used HT. Women were considered users of estrogen-only therapy if they had no prescriptions containing a progestogen after their first prescription for systemic estrogen as the start of exposure to HT. Those with any subsequent prescription containing a progestogen were classified as combined HT users.
Results. In an analysis adjusted for all available potential confounders—including lifestyle factors, ethnicity, family history of dementia, early menopause, oophorectomy/ hysterectomy, comorbidities, and use of other relevant drugs—the use of HT was not associated with risk of dementia.
A reduced risk of dementia was noted among women who had been taking estrogen-only HT for 10 years or more (odds ratio [OR], 0.85; 95% confidence interval [CI], 0.76–0.94). An elevated risk of Alzheimer disease was noted among women who had used estrogen-progestin HT for 5 to 9 years (OR, 1.19; CI, 1.06–1.33).1
Study strengths and limitations
The authors pointed out that this study’s main strengths were that it had a very large sample size representative of the general population and that its design permitted capture of all known cases as well as precision recording for prescribed drugs. On the other hand, the study is limited by the possible lack of data for some older women before the index date; that is, menopause in this latter group started before their registration or before these data were gathered electronically by their practice. ●
The authors of this British large observational study took pains to minimize potential bias. The finding that long-term use of estrogen-only HT may be neuroprotective is consistent with results of recent studies in the United States and Finland,2-4 as well as with the Women’s Health Initiative randomized trial, which found that with 18 years of follow-up, treatment with conjugated estrogen alone was associated with a 26% reduced risk of death from Alzheimer disease.5 Overall, however, the main message we should glean from this important study by Vinogradova and colleagues is that women with bothersome vasomotor symptoms considering use of menopausal HT can be reassured that such therapy has little if any impact on future risk of cognitive decline.
ANDREW M. KAUNITZ, MD, NCMP
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
- Vinogradova Y, Dening T, Hippisley-Cox J, et al. Use of menopausal hormone therapy and risk of dementia: nested case-control studies using QResearch and CPRD databases. BMJ. 2021;374:n2182. doi: 10.1136/bmj.n2182.
- Matyi JM, Rattinger GB, Schwartz S, et al. Lifetime estrogen exposure and cognition in late life: the Cache County study. Menopause. 2019;26:1366-1374. doi: 10.1097 /GME.0000000000001405.
- Liu JH. Does estrogen provide “neuroprotection” for postmenopausal women? Menopause. 2019;26:1361-1362. doi: 10.1097/GME.0000000000001459.
- Imtiaz B, Tuppurainen M, Rikkonen T, et al. Postmenopausal hormone therapy and Alzheimer disease: a prospective cohort study. Neurology. 2017;88:1062-1068. doi: 10.1212 /WNL.0000000000003696.
- Manson JE, Aragaki AK, Rossouw JE, et al; WHI Investigators. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001 /jama.2017.11217.
Are there perinatal benefits to pregnant patients after bariatric surgery?
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
Getahun D, Fassett MJ, Jacobsen SJ, et al. Perinatal outcomes after bariatric surgery. Am J Obstet Gynecol. 2021;S0002-9378(21)00771-7. doi: 10.1016/j.ajog.2021 .06.087.
EXPERT COMMENTARY
Prepregnancy obesity continues to rise in the United States, with a prevalence of 29% among reproductive-age women in 2019, an 11% increase from 2016.1 Pregnant patients with obesity are at increased risk for multiple adverse perinatal outcomes, including gestational diabetes and preeclampsia. Bariatric surgery is effective for weight loss and has been shown to improve comorbidities associated with obesity,2 and it may have potential benefits for pregnancy outcomes, such as reducing rates of gestational diabetes and preeclampsia.3-5 However, little was known about other outcomes as well as other potential factors before a recent study in which investigators examined perinatal outcomes after bariatric surgery.
Details of the study
Getahun and colleagues conducted a population-based, retrospective study of pregnant patients who were eligible for bariatric surgery (body mass index [BMI] ≥40 kg/m2 with no comorbidities or a BMI between 35 and 40 kg/m2 with obesity-related comorbidities, such as diabetes). They aimed to evaluate the association of bariatric surgery with adverse perinatal outcomes.
Results. In a large sample of pregnant patients eligible for bariatric surgery (N = 20,213), the authors found that patients who had bariatric surgery (n = 1,886) had a reduced risk of macrosomia (aOR, 0.24), preeclampsia (aOR, 0.53), gestational diabetes (aOR, 0.60), and cesarean delivery (aOR, 0.65) compared with those who did not have bariatric surgery (n = 18,327). They also found that patients who had bariatric surgery had an increased risk of small-for-gestational age neonates (aOR, 2.46) and postpartum hemorrhage (aOR, 1.79).
These results remained after adjusting for other potential confounders. The authors evaluated the outcomes based on the timing of surgery and the patients’ pregnancy (<1 year, 1-1.5 years, 1.5-2 years, >2 years). The outcomes were more favorable among the patients who had the bariatric surgery regardless of the time interval of surgery to pregnancy than those who did not have the surgery. In addition, the benefits of bariatric surgery did not differ between the 2 most common types of bariatric surgery (Roux-en-Y gastric bypass and vertical sleeve gastrectomy) performed in this study, and both had better outcomes than those who did not have the surgery. Finally, patients with chronic hypertension and pregestational diabetes who had bariatric surgery also had lower risks of adverse outcomes than those without bariatric surgery.
Study strengths and limitations
Given the study’s retrospective design, uncertainties and important confounders could not be addressed, such as why certain eligible patients had the surgery and others did not. However, with its large sample size and an appropriate comparison group, the study findings further support the perinatal benefits of bariatric surgery in obese patients. Of note, this study also had a large sample of Black and Hispanic patients, populations known to have higher rates of obesity1 and pregnancy complications. Subgroup analyses within each racial/ethnic group revealed that those who had the surgery had lower risks of adverse perinatal outcomes than those who did not.
Patients who had the bariatric surgery had an increased risk of postpartum hemorrhage; however, there is no physiologic basis or theory to explain this finding, so further studies are needed. Lastly, although patients who had bariatric surgery had an increased risk of small-for-gestational-age babies and the study was not powered for the risk of stillbirth, the patients who had the surgery had a reduced risk of neonates admitted to the neonatal intensive care unit. More data would have been beneficial to assess if these small-for-gestational-age babies were healthy. In general, obese patients tend to have larger and unhealthy babies; thus, healthier babies, even if small for gestational age, would not be an adverse outcome.
Benefits of bariatric surgery extend to perinatal outcomes
This study reinforces current practice that includes eligible patients being counseled about the health-related benefits of bariatric surgery, which now includes more perinatal outcomes. The finding of the increased risk of small-for-gestational-age fetuses supports the practice of a screening growth ultrasound exam in patients who had bariatric surgery. ●
An important, modifiable risk factor for adverse perinatal outcomes is the patient’s prepregnancy BMI at the time of pregnancy. Bariatric surgery is an effective procedure for weight loss. There are many perinatal benefits for eligible patients who have bariatric surgery before pregnancy. Clinicians should counsel their obese patients who are considering or planning pregnancy about the benefits of bariatric surgery.
RODNEY A. MCLAREN, JR, MD, AND VINCENZO BERGHELLA, MD
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
- Driscoll AK, Gregory ECW. Increases in prepregnancy obesity: United States, 2016-2019. NCHS Data Brief. 2020 Nov;(392):1-8.
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724- 1737. doi: 10.1001/jama.292.14.1724.
- Maggard MA, Yermilov I, Li Z, et al. Pregnancy and fertility following bariatric surgery: a systematic review. JAMA. 2008;300:2286-2296. doi: 10.1001/jama.2008.641.
- Watanabe A, Seki Y, Haruta H, et al. Maternal impacts and perinatal outcomes after three types of bariatric surgery at a single institution. Arch Gynecol Obstet. 2019;300:145-152. doi: 10.1007/s00404-019-05195-9.
- Balestrin B, Urbanetz AA, Barbieri MM, et al. Pregnancy after bariatric surgery: a comparative study of post-bariatric pregnant women versus non-bariatric obese pregnant women. Obes Surg. 2019;29:3142-3148. doi: 10.1007/s11695- 019-03961-x.
Does prophylactic manual rotation of OP and OT positions in early second stage of labor decrease operative vaginal and/or CDs?
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
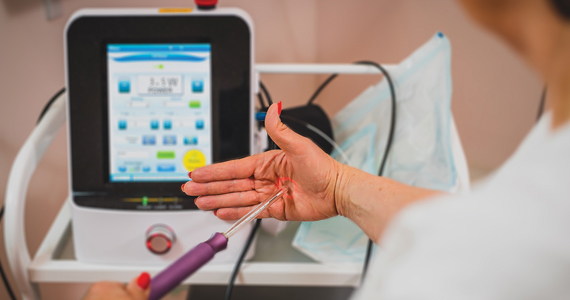
Is vaginal laser therapy more efficacious in improving vaginal menopausal symptoms compared with sham therapy?
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
EXPERT COMMENTARY
Symptomatic vaginal atrophy, also referred to as genitourinary syndrome of menopause (GSM), is common and tends to progress without treatment. When use of over-the-counter lubricants and/or moisturizers are not sufficient to address symptoms, vaginal estrogen has represented the mainstay of treatment for this condition and effectively addresses GSM symptoms.1 In recent years, some physicians have been offering vaginal carbon dioxide (CO2) laser therapy as an alternative to vaginal estrogen in the treatment of GSM; however, the efficacy of laser therapy in this setting has been uncertain.
Li and colleagues conducted a double-blind randomized trial in postmenopausal women with bothersome vaginal symptoms to compare the efficacy of the fractional CO2 vaginal laser with that of sham treatment.
Details of the study
Investigators (who received no funding from any relevant commercial entity) at a teaching hospital in Sydney, Australia, randomly assigned 85 women with menopausal symptoms suggestive of GSM to laser (n = 43) or sham (n = 42) treatment. Participants underwent 3 treatments at monthly intervals. Laser treatments were performed with standard settings (40-watt power), while sham treatments were conducted with low settings that have no tissue effect. Local anesthesia cream was employed for all procedures, and a plume evacuator was used to remove visual and olfactory effects from laser smoke.
To maintain blinding, different clinicians performed assessments and treatments. Symptom severity assessments were based on a visual analog scale (VAS) and the Vulvovaginal Symptom Questionnaire (VSQ), with a minimal clinically important difference specified as a 50% decrease in severity scores of both assessment tools. Change in severity of symptoms, including dyspareunia, dysuria, vaginal dryness, and burning and itching, was assessed at 12 months. Quality of life, the Vaginal Health Index (VHI) score, and vaginal histology were among the secondary outcomes. In addition, vaginal biopsies were performed at baseline and 6 months after study treatment.
Among the 78 women (91.7%) who completed the 12-month evaluations, the mean age was approximately 57, more than 95% were White, and approximately half were sexually active.
Results. For the laser and sham treatment groups, at 12 months no significant differences were noted for change in overall symptoms or in the most severe symptom. Many participants who received laser or sham treatment reported an improvement in vaginal symptoms 12 months following treatment.
The VAS score for a change in symptom severity in the laser-treated group compared with the sham-treated group was -17.2 versus -26.6, a difference of 9.4 (95% confidence interval [CI], -28.6 to 47.5), while the VAS score for the most severe symptom was -24.5 versus -20.4, a difference of -4.1 (95% CI, -32.5 to 24.3). The VSQ score was, respectively, -3.1 versus -1.6 (difference, -1.5 [95% CI, -5.9 to 3.0]). The mean quality of life score showed no significant differences between the laser and the sham group (6.3 vs 1.4, a difference of 4.8 [95% CI, -3.9 to 13.5]). The VHI score was 0.9 in the laser group versus 1.3 in the sham group, for a difference of -0.4 (95% CI, -4.3 to 3.6). Likewise, the proportion of participants who noted a reduction of more than 50% in bother from their most severe symptoms was similar in the 2 groups. Similarly, changes in vaginal histology were similar in the laser and sham groups.
The proportion of participants who reported adverse events, including transient vaginal discomfort, discharge, or urinary tract symptoms, was similar in the 2 groups.
Study strengths and limitations
Although other randomized studies of fractionated laser therapy for GSM have been reported, this Australian trial is the largest and longest to date and also is the first to have used sham-treated controls.
Breast cancer survivors represent a group of patients for whom treatment of GSM can be a major conundrum—induced menopause that often results when combination chemotherapy is employed in premenopausal survivors can result in severe GSM; use of aromatase inhibitors likewise can cause bothersome GSM symptoms. Since the US Food and Drug Administration lists a personal history of breast cancer as a contraindication to use of any estrogen formulation, breast cancer survivors represent a population targeted by physicians offering vaginal laser treatment. Accordingly, that approximately 50% of trial participants were breast cancer survivors means the investigators were assessing the impact of laser therapy in a population of particular clinical relevance. Of note, as with participants overall, laser therapy when employed in breast cancer survivors did not result in outcomes distinct from sham treatments.2 ●
We agree with editorialists that outside of clinical trials, we should not recommend laser for treatment of menopausal vaginal symptoms.3 Currently, a US multisite randomized trial of fractionated laser versus sham for dyspareunia in menopausal women is planned.
ANDREW M. KAUNITZ, MD, NCMP,
AND CHERYL B. IGLESIA, MD
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
- The 2020 genitourinary syndrome of menopause position statement of The North American Menopause Society. Menopause. 2020;27:976- 992. doi: 10.1097/GME.0000000000001609.
- Li FG, Maheux-Lacroix S, Deans R, et al. Effect of fractional carbon dioxide laser vs sham treatment on symptom severity in women with postmenopausal vaginal symptoms: a randomized clinical trial. JAMA. 2021;326:1381-1389. doi: 10.1001/jama.2021.14892.
- Adelman M, Nygaard IE. Time for a “pause” on the use of vaginal laser. JAMA. 2021;326:1378-1380. doi: 10.1001/jama.2021.14809.
Remote and in-home prenatal care: Safe, inclusive, and here to stay

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●

For much of the general public, in-home care from a physician is akin to the rotary telephone: a feature of a bygone age, long since replaced by vastly different systems. While approximately 40% of physician-patient interactions in 1930 were house calls, by the early 1980s this had dwindled to less than 1%,1 with almost all physician-patient encounters taking place in a clinical setting, whether in a hospital or in a free-standing clinic. In the last 2 decades, a smattering of primary care and medical subspecialty clinicians started to incorporate some in-home care into their practices in the form of telemedicine, using video and telephone technology to facilitate care outside of the clinical setting, and by 2016, approximately 15% of physicians reported using some form of telemedicine in their interactions with patients.2
Despite these advances, prior to the COVID-19 pandemic, obstetricians lagged significantly behind in their use of at-home or remote care. Although there were some efforts to promote a hybrid care model that incorporated prenatal telemedicine,3 pre-pandemic ObGyn was one of the least likely fields to offer telemedicine to their patients, with only 9% of practices offering such services.2 In this article, we discuss how the COVID-19 pandemic resulted in a shift from traditional, in-person care to a hybrid remote model and how this may benefit obstetrics patients as well as clinicians.
Pre-pandemic patient management
The traditional model of prenatal care presents a particularly intense time period for patients in terms of its demands. Women who are pregnant and start care in their first trimester typically have 12 to 14 visits during the subsequent 6 to 7 months, with additional visits for those with high-risk pregnancies. Although some of these visits coincide with the need for in-person laboratory work or imaging, many are chiefly oriented around assessment of vital signs or counseling. These frequent prenatal visits represent a significant commitment from patients in terms of transportation, time off work, and childcare resources—all of which may be exacerbated for patients who need to receive their care from overbooked, high-risk specialists.
After delivery, attending an in-person postpartum visit with a newborn can be even more daunting. Despite the increased recognition from professional groups of the importance of postpartum care to support breastfeeding, physical recovery, and mental health, as many as 40% of recently delivered patients do not attend their scheduled postpartum visit(s).4 Still, before 2020, few obstetricians had revised their workflows to “meet patients where they are,” with many continuing to only offer in-person care and assessments.
COVID-19: An impetus for change
As with so many things, the COVID-19 pandemic has challenged our ideas of what is normal. In a sense, the pandemic has catalyzed a revolution in the prenatal care model. The very real risks of exposure and contagion during the pandemic—for clinicians and patients alike—has forced ObGyns to reexamine the actual risks and benefits of in-person and in-clinic prenatal care. As a result, many ObGyns have rapidly adopted telemedicine into practices that were strictly in-person. For example, a national survey of 172 clinicians who offered contraception counseling during the pandemic found that 91% of them were now offering telemedicine services, with 78% of those clinicians new to telemedicine.5 Similarly, although a minority of surveyed obstetricians in New York City reported using telemedicine pre-pandemic, 89% planned to continue using such technology in the future.6
Continue to: Incorporating mobile technology...
Incorporating mobile technology
Obstetricians, forced to consolidate and maximize their in-person care to protect their patients’ safety, have started to realize that many of the conversations and counseling offered to patients can be managed equally effectively with telemedicine. Furthermore, basic home monitoring devices, such as blood pressure machines, can be safely and accurately used by patients without requiring them to come to the office.
More recent research into mobile medical devices suggests that patients can safely and appropriately manage more complex tools. One such example is a mobile, self-operated, ultrasound transducer that is controlled through a smartphone (Instinct, Pulsenmore Ltd). This device was evaluated in an observational, noninterventional trial of 100 women carrying a singleton fetus at 14/0 weeks’ to 39/6 weeks’ gestation. Patients performed 1,360 self-scans, which were reviewed by a clinician in real time online or subsequently off-line. Results showed successful detection rates of 95.3% for fetal heart activity, 88.3% for body movements, 69.4% for tone, 23.8% for breathing movements, and 92.2% for normal amniotic fluid volume.7 The authors concluded that this represents a feasible solution for remote sonographic fetal assessment.
Coordinating care with health care extenders
Remote monitoring options allow patients to be safely monitored during their pregnancies while remaining at home more often, especially when used in conjunction with trained health care extenders such as registered nurses, primary care associates, or “maternity navigators” who can facilitate off-site care. In fact, many aspects of prenatal care are particularly amenable to remote medicine or non–physician-based home care. Different variations of this model of “hybrid” prenatal care may be appropriate depending upon the needs of the patient population served by a given obstetrics practice. Ideally, a prenatal care model personalizes care based on the known risk factors that are identified at the beginning of prenatal care, the anticipated barriers to care, and the patient’s own preferences. As a result, alternatives to the traditional model may be to alternate in-person and telemedicine visits,3,8 to incorporate in-person or remote group prenatal visits,9,10 or to incorporate staff with basic health care skills to serve as health care extenders in the community and provide home visits for basic monitoring, laboratory work, and patient education.11
Benefits of hybrid prenatal models
As we look ahead to the end of the pandemic, how should obstetricians view these hybrid prenatal care models? Are these models safe for patients? Were they only worthwhile to minimize infection risk, or do they have potential benefits for patients going forward?
In fact, data on the use of telemedicine in prenatal care indicate that these models may be equally as safe as the traditional model in terms of clinical outcomes and may have important additional benefits with regard to patient convenience and access to and satisfaction with care. Even audio-only prenatal televisits have been found to be equivalent to in-person visits in terms of serious perinatal outcomes.12 Common pregnancy diagnoses are also well-served by telemedicine. For example, several recent investigations of patients with gestational diabetes have found that telemedicine was as effective as standard care for glucose control.13,14 Management of hypertension during pregnancy, another antenatal condition that is commonly managed with frequent in-person check-ups, also was found to be adequately feasible with telemedicine using home monitors and symptom checklists, with high rates of patient satisfaction.15
With good evidence for safety, the added potential for patients to benefit in such hybrid models is multifactorial. For one, despite our collective hopes, the COVID-19 pandemic may have a long tail. Vaccine hesitancy and COVID-19 variants may mean that clinicians will have to consider the real threat of infection risk in the clinic setting for years to come. In-home prenatal care also provides a wide variety of social, economic, and psychological benefits for pregnant women across various patient populations. The pandemic has introduced many patients to the full potential of working and meeting remotely; pregnant patients are becoming more familiar with these technology platforms and appreciate its incorporation into their busy lives.5 Furthermore, hybrid models actually can provide otherwise “nonadherent” patients with better access to care. From the patient perspective, an in-person 15-minute health care provider visit actually represents a significant commitment of time and resources (ie, hours spent on public transportation, lost wages for those with inflexible work schedules, and childcare costs for patients discouraged from bringing their children to prenatal visits). Especially for patients with fewer socioeconomic resources, these barriers to in-person clinic visits may be daunting, if not insurmountable; the option of remote visits or house calls reduces these barriers and facilitates care.16
Such hybrid models benefit prenatal clinicians as well. In addition to a decreased risk of infection, clinicians may be able to attract a wider potential prenatal patient population with telemedicine by appealing to younger and potentially more technology-savvy patients.17 Importantly, telemedicine is increasingly recognized as on par with in-person visits in many billing algorithms. Changes during the pandemic led Medicare to cover telemedicine visits as well as in-person visits18,19; among other groundbreaking changes, new patients can have an initial billable visit via telemedicine. Although the billing landscape will likely continue to evolve, such changes allow clinicians to focus on patient safety and convenience without financial risk to their practices.
The future of prenatal appointment scheduling
The future of prenatal care certainly doesn’t look like a dozen 15-minute visits in a private physician’s office. While these emerging hybrid models of prenatal care certainly can benefit patients with low-risk uncomplicated pregnancies, they are already being adopted by clinicians who care for patients with antenatal complications that require specialist consultation; for those with conditions that require frequent, low-complexity check-ins (gestational diabetes, chronic hypertension, history of pre-term birth, etc.); and for patients who struggle with financial or logistical barriers to in-person care. Although obstetrics may have lagged behind other subspecialties in revising its traditional health care models, the pandemic has opened up a new world of possibilities of remote and in-home care for this field. ●
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.
- Kao H, Conant R, Soriano T, et al. The past, present, and future of house calls. Clin Geriatr Med. 2009;25:19-34. doi:10.1016/j.cger.2008.10.005.
- Kane CK, Gillis K. The use of telemedicine by physicians: still the exception rather than the rule. Health Aff (Millwood). 2018;37:1923-1930. doi:10.1377/hlthaff.2018.05077.
- Weigel G, Frederiksen B, Ranji U. Telemedicine and pregnancy care. Kaiser Family Foundation website. https://www.kff.org/womens-health-policy/issue-brief/telemedicine-and-pregnancy-care. Accessed August 23, 2021.
- ACOG Committee Opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131:e140-e150. doi:10.1097/AOG.0000000000002633.
- Stifani BM, Avila K, Levi EE. Telemedicine for contraceptive counseling: an exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception. 2021;103:157-162. doi:10.1016/j.contraception.2020.11.006.
- Madden N, Emeruwa UN, Friedman AM, et al. Telehealth uptake into prenatal care and provider attitudes during the COVID-19 pandemic in New York City: a quantitative and qualitative analysis. Am J Perinatol. 2020;37:1005-1014. doi:10.1055/s-0040-1712939.
- Hadar E, Wolff L, Tenenbaum-Gavish K, et al. Mobile self-operated home ultrasound system for remote fetal assessment during pregnancy. Telemed J E Health. 2021. doi:10.1089/tmj.2020.0541.
- Thomas Jefferson University Division of Maternal Fetal Medicine. Jefferson Maternal Fetal Medicine COVID19 Preparedness. Version 2.1. March 19, 2020. https://communities.smfm.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=a109df77-74fe-462b-87fb-895d6ee7d0e6. Accessed August 23, 2021.
- Ickovics JR, Kershaw TS, Westdahl C, et al. Group prenatal care and perinatal outcomes. Obstet Gynecol. 2007;110(2 pt 1):330-339. doi:10.1097/01.AOG.0000275284.24298.23.
- Wicklund M. Oakland launches telehealth program for Black prenatal, postpartum care. Telehealth News. https://mhealthintelligence.com/news/oakland-launches-telehealth-program-for-black-prenatal-postpartum-care. Accessed August 23, 2021.
- Home-based pregnancy care. CayabaCare website. https://www.cayabacare.com. Accessed August 23, 2021.
- Duryea EL, Adhikari EH, Ambia A, et al. Comparison between in-person and audio-only virtual prenatal visits and perinatal outcomes. JAMA Netw Open. 2021;4:e215854. doi:10.1001/jamanetworkopen.2021.5854.
- Ming WK, Mackillop LH, Farmer AJ, et al. Telemedicine technologies for diabetes in pregnancy: a systematic review and meta-analysis. J Med Internet Res. 2016;18:e290. doi:10.2196/jmir.6556.
- Tian Y, Zhang S, Huang F, et al. Comparing the efficacies of telemedicine and standard prenatal care on blood glucose control in women with gestational diabetes mellitus: randomized controlled trial. JMIR Mhealth Uhealth. 2021;9:e22881. doi:10.2196/22881.
- van den Heuvel JFM, Kariman SS, van Solinge WW, et al. SAFE@HOME – feasibility study of a telemonitoring platform combining blood pressure and preeclampsia symptoms in pregnancy care. Eur J Obstet Gynecol Reprod Biol. 2019;240:226-231. doi:10.1016/j.ejogrb.2019.07.012.
- Dixon-Shambley K, Gabbe PT. Using telehealth approaches to address social determinants of health and improve pregnancy and postpartum outcomes. Clin Obstet Gynecol. 2021;64:333-344. doi:10.1097/GRF.0000000000000611.
- Eruchalu CN, Pichardo MS, Bharadwaj M, et al. The expanding digital divide: digital health access inequities during the COVID-19 pandemic in New York City. J Urban Health. 2021;98:183-186. doi:10.1007/s11524-020-00508-9.
- COVID-19 FAQs for obstetrician-gynecologists, telehealth. The American College of Obstetricians and Gynecologists website. https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-telehealth. Accessed August 23, 2021.
- Managing patients remotely: billing for digital and telehealth services. The American College of Obstetricians and Gynecologists website. Updated October 19, 2020. https://www.acog.org/practice-management/coding/coding-library/managing-patients-remotely-billing-for-digital-and-telehealth-services. Accessed August 23, 2021.