User login
How does gender-affirming hormone therapy affect QOL in transgender patients?
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
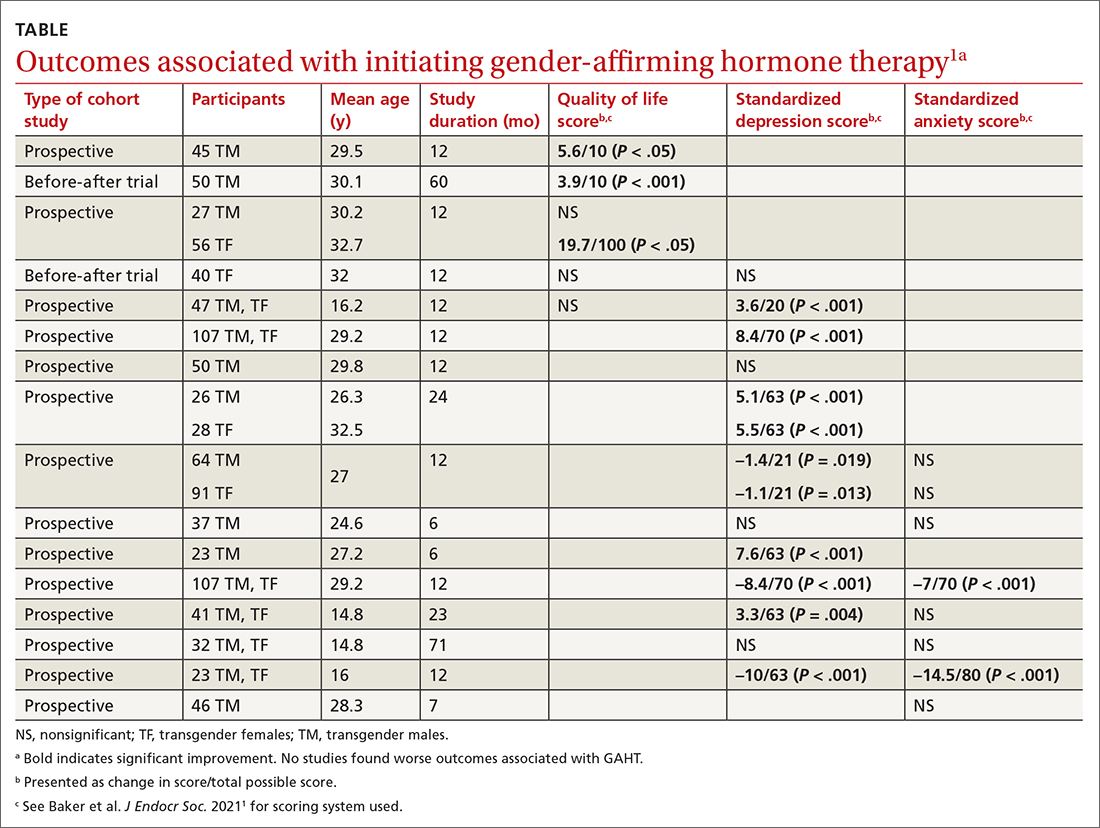
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
EVIDENCE-BASED ANSWER:
There are modest effects on depression but not anxiety. Gender-affirming hormone therapy (GAHT) is associated with modest improvements in standardized scores for quality of life (QOL) and depression in adult male-to-female and female-to-male transgender people and modest improvements in depression scores in transgender adolescents, but the effect on anxiety is uncertain (strength of recommendation [SOR]: B, based on a preponderance of low-quality prospective cohort studies with inconsistent results).
GAHT is associated with reduced gender dysphoria and decreased suicidality (SOR: B, based on a prospective cohort study). However, there is insufficient evidence to determine any effect on suicide completion. No studies associated GAHT with worsened QOL, depression, or anxiety scores.
How accurate is transcutaneous bilirubin testing in newborns with darker skin tones?
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
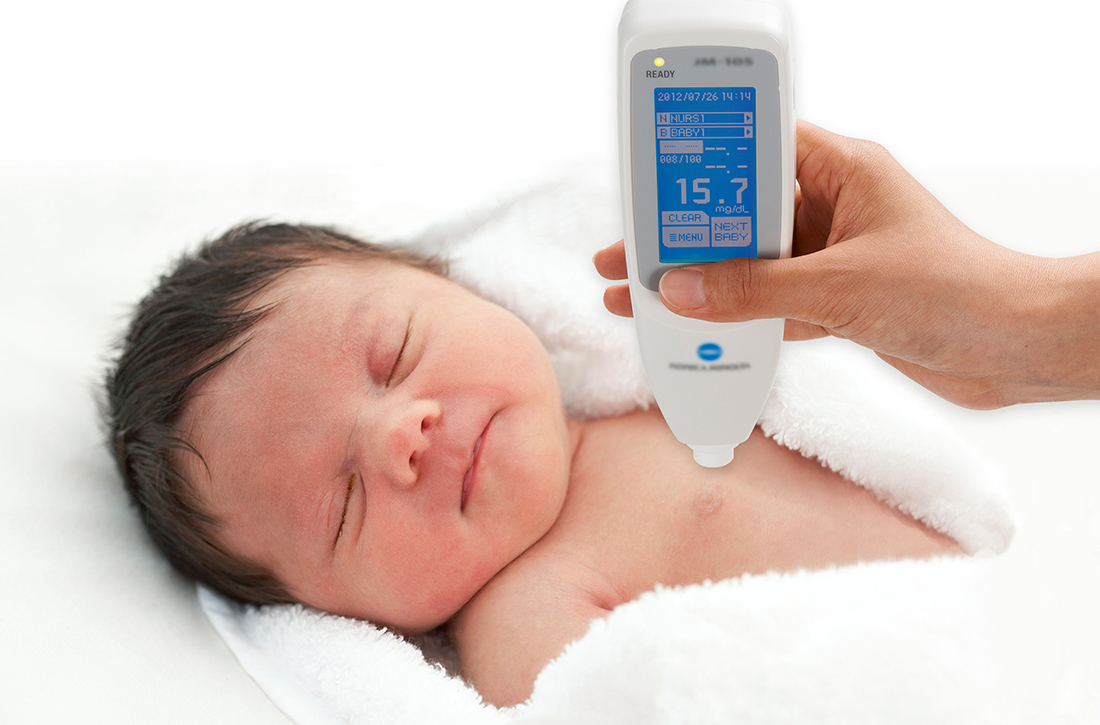
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
EVIDENCE SUMMARY
Some evidence suggests overestimation in all skin tones
In a prospective diagnostic cohort study of 1553 infants in Nigeria, the accuracy of TcB measurement with 2 transcutaneous bilirubinometers (Konica Minolta/Air Shields JM- 103 and Respironics BiliChek) was analyzed. 1 The study population was derived from neonates delivered in a single maternity hospital in Lagos who were ≥ 35 weeks gestational age or ≥ 2.2 kg.
Using a color scale generated for this population, researchers stratified neonates into 1 of 3 skin tone groups: light brown, medium brown, or dark brown. TcB and TSB paired samples were collected in the first 120 hours of life in all patients. JM-103 recordings comprised 71.9% of TcB readings.
Overall, TcB testing overestimated the TSB by ≥ 2 mg/dL in 64.5% of infants, ≥ 3 mg/dL in 42.7%, and > 4 mg/dL in 25.7%. TcB testing underestimated the TSB by ≥ 2 mg/dL in 1.1% of infants, ≥ 3 mg/dL in 0.5%, and > 4 mg/dL in 0.3%.1
Local variation in skin tone was not associated with changes in overestimation, although the researchers noted that a key limitation of the study was a lack of lighttoned infants for comparison.1
A prospective diagnostic cohort study of 1359 infants in Spain compared TcB measurements to TSB levels using the Dräger Jaundice Meter JM-105.2 Patients included all neonates (gestational age, 36.6 to 41.1 weeks) born at a single hospital in Barcelona.
Using a validated skin tone scale, researchers stratified neonates at 24 hours of life to 1 of 4 skin tones: light (n = 337), medium light (n = 750), medium dark (n = 249), and dark (n = 23). They then obtained TSB samples at 48 to 72 hours of life, along with other routine screening labs and midsternal TcB measurements.
TcB testing tended to overestimate TSB (when < 15 mg/dL) for all skin tones, although to a larger degree for neonates with dark skin tones (mean overestimation, 0.7 mg/dL for light; 1.08 mg/dL for medium light; 1.89 mg/dL for medium dark; and 1.86 mg/dL for dark; P < .001 for light vs medium dark or dark).2
Continue to: Stated limitations...
Stated limitations of the study included relatively low numbers of neonates with dark skin tone, no test of interobserver reliability in skin tone assignment, and enrollment of exclusively healthy neonates with low bilirubin levels.2
Other studies report overestimation in infants with darker skin tone
Two Canadian diagnostic cohort studies also found evidence that TcB testing overestimated TSB in infants with darker skin tones, although TcB test characteristics proved stable over a wide range of bilirubin levels.
The first study enrolled 451 neonates ≥ 35 weeks gestational age at a hospital in Ottawa and assessed TcB using the JM-103 meter.3 The neonates were stratified into light (n = 51), medium (n = 326), and dark (n = 74) skin tones using cosmetic reference color swatches. All had a TcB and TSB obtained within 30 minutes of each other.
TcB testing underestimated TSB in infants with light and medium skin tones and overestimated TSB in infants with darker skin tone (mean difference, –0.88 mg/dL for light; –1.1 mg/dL for medium; and 0.68 mg/dL for dark; P not given). The mean area under the curve (AUC) was ≥ 0.94 for all receiver–operator characteristic (ROC) curves across all skin tones and bilirubin thresholds (AUC range, 0-1, with > 0.8 indicating strong modeling).3
Limitations of the study included failure to check interrater reliability for skin tone assessment, low numbers of infants with elevated bilirubin (≥ 13.5 mg/dL), and very few infants in either the dark or light skin tone groups.3
Continue to: The second Canadian study...
The second Canadian study enrolled 774 infants born at ≥ 37 weeks gestational age in Calgary and assessed TcB with the JM-103.4 Infants were categorized as having light (n = 347), medium (n = 412), and dark (n = 15) skin tones by study nurses, based on reference cosmetic colors. All infants had paired TcB and TSB measurements within 60 minutes of each other and before 120 hours of life.
Multivariate linear regression analysis using medium skin tone as the reference group found a tendency toward low TcB levels in infants with light skin tone and a tendency toward high TcB levels in infants with dark skin tone (adjusted R2 = 0.86). The AUC was ≥ 0.95 for all ROC curves for lightand medium-toned infants at key TSB cutoff points; the study included too few infants with dark skin tone to generate ROC curves for that group.4
Recommendations from others
In 2009, the American Academy of Pediatrics (AAP) recommended universal predischarge screening for hyperbilirubinemia in newborns using either TcB testing or TSB. The AAP statement did not address the effect of skin tone on TcB levels, but did advise regular calibration of TcB and TSB results at the hospital level.5
In 2016, the National Institute for Health and Care Excellence (NICE) updated their guideline on jaundice in newborns younger than 28 days old. NICE recommended visual inspection of all babies for jaundice by examining them in bright natural light and looking for jaundice on blanched skin; it specifically advised checking sclera and gums in infants with darker skin tones.6
The Nigerian researchers noted earlier have published an updated TcB nomogram for their patient population.7
Editor’s takeaway
Even with the small variation of 2 mg/dL or less between transcutaneous and serum bilirubin, and the SOR of C due to lab values being labeled disease-oriented evidence, TcB proves to be useful. In practice, concerning TcB values should lead to serum bilirubin confirmation. This evidence indicates we might be ordering TSB measurements more or less often depending on skin tone, reinforcing the need for review and adjustment of TcB cut-off levels based on the local population.
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
1. Olusanya BO, Imosemi DO, Emokpae AA. Differences between transcutaneous and serum bilirubin measurements in Black African neonates. Pediatrics. 2016;138:e20160907. doi: 10.1542/ peds.2016-0907
2. Maya-Enero S, Candel-Pau J, Garcia-Garcia J, et al. Reliability of transcutaneous bilirubin determination based on skin color determined by a neonatal skin color scale of our own. Eur J Pediatr. 2021;180:607-616. doi: 10.1007/s00431-020-03885-0
3. Samiee-Zafarghandy S, Feberova J, Williams K, et al. Influence of skin colour on diagnostic accuracy of the jaundice meter JM 103 in newborns. Arch Dis Child Fetal Neonatal Ed. 2014;99: F480-F484. doi: 10.1136/archdischild-2013-305699
4. Wainer S, Rabi Y, Parmar SM, et al. Impact of skin tone on the performance of a transcutaneous jaundice meter. Acta Paediatr. 2009;98:1909-1915. doi: 10.1111/j.1651-2227.2009.01497.x
5. Maisels MJ, Bhutani VK, Bogen D, et al. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193-1198. doi: 10.1542/peds. 2009-0329
6. Amos RC, Jacob H, Leith W. Jaundice in newborn babies under 28 days: NICE guideline 2016 (CG98). Arch Dis Child Educ Pract Ed. 2017;102:207-209. doi: 10.1136/archdischild-2016-311556
7. Olusanya BO, Mabogunje CA, Imosemi DO, et al. Transcutaneous bilirubin nomograms in African neonates. PloS ONE. 2017; 12:e0172058. doi: 10.1371/journal.pone.0172058
EVIDENCE-BASED ANSWER:
Fairly accurate. Photometric transcutaneous bilirubin (TcB) testing may overestimate total serum bilirubin (TSB) in neonates with darker skin tones by a mean of 0.68 to > 2 mg/dL (strength of recommendation [SOR]: C, diagnostic cohort studies with differing reference standards).
Overall, TcB meters retain acceptable accuracy in infants of all skin tones across a range of bilirubin levels, despite being more likely to underestimate lighter skin tones and overestimate darker ones (SOR: C, diagnostic cohort studies with differing reference standards). It is unclear if the higher readings prompt an increase in blood draws or otherwise alter care.
Does an early COPD diagnosis improve long-term outcomes?
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
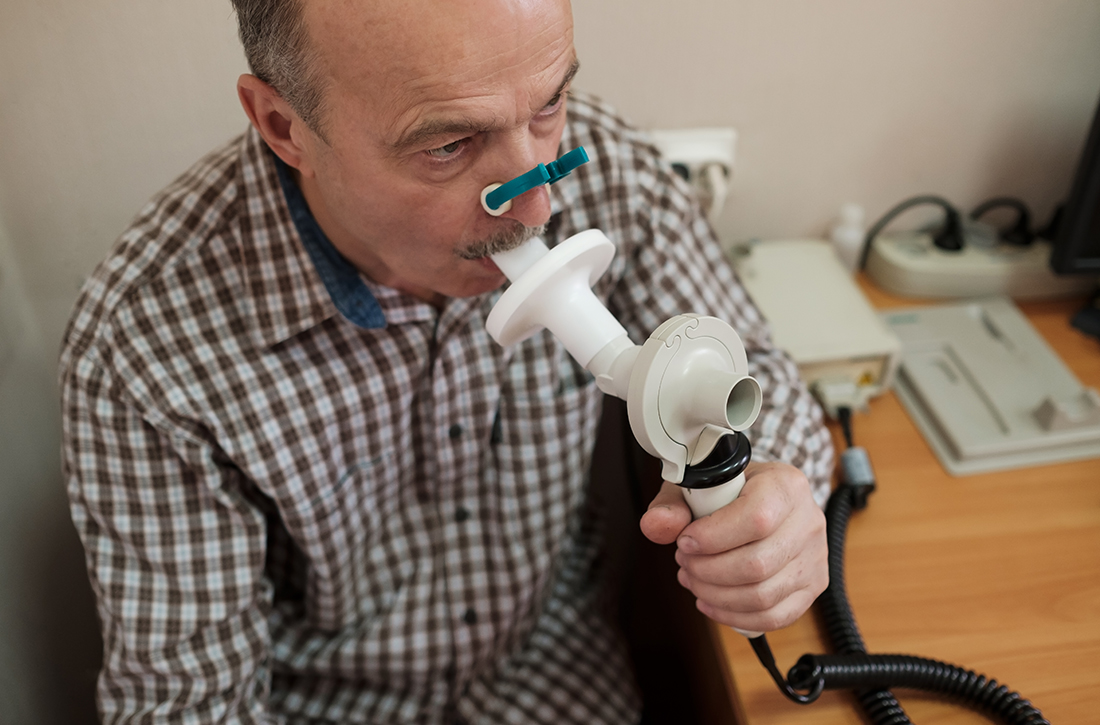
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
EVIDENCE SUMMARY
Early Dx didn’t improve smoking cessation rates or treatment outcomes
A 2016 evidence report and systematic review for the US Preventive Services Task Force (USPSTF) identified no studies directly comparing the effectiveness of COPD screening on patient outcomes, so the authors looked first at studies on the outcomes of screening, followed by studies exploring the effects of early treatment.1
The authors identified 5 fair-quality RCTs (N = 1694) addressing the effect of screening asymptomatic patients for COPD with spirometry on the outcome of smoking cessation. One trial (n = 561) found better 12-month smoking cessation rates in patients who underwent spirometry screening and were given their “lung age” (13.6% vs 6.4% not given a lung age; P < .005; number needed to treat [NNT] = 14). However, a similar study (n = 542) published a year later found no significant difference in quit rates with or without “lung age” discussions (10.9% vs 13%, respectively; P not significant). In the other 3 studies, screening produced no significant effect on smoking cessation rates.1
As for possible early treatment benefits, the review authors identified only 1 RCT (n = 1175) that included any patients with mild COPD (defined as COPD with a forced expiratory volume in 1 second [FEV1] ≥ 80% of predicted normal value). It assessed treatment with inhaled corticosteroids (ICS) in patients with mild COPD who continued to smoke. The trial did not record symptoms (if any) at intake. ICS therapy reduced the frequency of COPD exacerbations (relative risk = 0.63; 95% CI, 0.47-0.85), although patients with milder COPD benefitted little in absolute terms (by 0.02 exacerbations/year).1 The review authors further noted that data were insufficient to make definitive statements about the effect of ICS on dyspnea or health-related quality of life.
But later diagnosis is associated with poorer outcomes
Two recent, large retrospective observational cohort studies, however, have examined the impact of an early vs late COPD diagnosis in patients with dyspnea or other symptoms of COPD.2,3 A later diagnosis was associated with worse outcomes.
In the first study, researchers in Sweden identified patients older than 40 years who had received a new diagnosis of COPD between 2000 and 2014.2 They examined electronic health record data for 6 different “indicators” of COPD during the 5 years prior to date of diagnosis: pneumonia, other respiratory disease, oral steroids, antibiotics for respiratory infection, prescribed drugs for respiratory symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (if they had ≤ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3870), late diagnosis (n = 8827) was associated with
- a higher annual rate of exacerbations within the first 2 years after diagnosis (2.67 vs 1.41; hazard ratio [HR] = 1.89; 95% CI, 1.83-1.96; P < .0001; number of early diagnoses needed to prevent 1 exacerbation in 1 year = 79),
- shorter time to first exacerbation (HR = 1.61; 95% CI, 1.54-1.69; P < .0001), and
- higher direct health care costs (by €1500 per year; no P value given).
Mortality was not different between the groups (HR = 1.04; 95% CI, 0.98-1.11; P = .18).
The second investigation was a similarly designed retrospective observational cohort study using a large UK database.3 Researchers enrolled patients who were at least 40 years old and received a new diagnosis of COPD between 2011 and 2014.
Continue to: Researchers examined electronic...
Researchers examined electronic health record data in the 5 years prior to diagnosis for 7 possible indicators of early COPD: pneumonia, respiratory disease other than pneumonia, chest radiograph, prescription of oral steroids, prescription of antibiotics for lung infection, prescription to manage respiratory disease symptoms, and lung function measurement. Researchers categorized patients as early diagnosis (≥ 2 indicators prior to diagnosis) or late diagnosis (≥ 3 indicators prior to diagnosis). Compared with early diagnosis (n = 3375), late diagnosis (n = 6783) was associated with a higher annual rate of exacerbations over 3-year follow-up (1.09 vs 0.57; adjusted HR = 1.68; 95% CI, 1.59-1.79; P < .0001; or 1 additional exacerbation in 192 patients in 1 year), shorter mean time to first exacerbation (HR = 1.46; 95% CI: 1.38-1.55; P < .0001), and a higher risk of hospitalization within 3 years (rate ratio = 1.18; 95% CI, 1.08-1.28; P = .0001). The researchers did not evaluate for mortality.
Importantly, patients in the late COPD diagnosis group in both trials had higher rates of other severe illnesses that cause dyspnea, including cardiovascular disease and other pulmonary diseases. As a result, dyspnea of other etiologies may have contributed to both the later diagnoses and the poorer clinical outcomes of the late-diagnosis group. Both studies had a high risk of lead-time bias.
Recommendations from others
In 2016, the USPSTF gave a “D” rating (moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits) to screening asymptomatic adults without respiratory symptoms for COPD.4 Likewise, the 2017 Global Initiative for Chronic Obstructive Lung Disease (GOLD) report did not recommend routine screening with spirometry but did advocate trying to make an accurate diagnosis using spirometry in patients with risk factors for COPD and chronic, progressive symptoms.5
Editor’s takeaway
Reasonably good evidence failed to find a benefit from an early COPD diagnosis. Even smoking cessation rates were not improved. Without better disease-modifying treatments, spirometry—the gold standard for confirming a COPD diagnosis—should not be used for screening asymptomatic patients.
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
1. Guirguis-Blake JM, Senger CA, Webber EM, et al. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315:1378-1393. doi:10.1001/jama.2016.2654
2. Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi: 10.2147/COPD.S195382
3. Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729-1738. doi: 10.2147/COPD.S255414
4. US Preventive Services Task Force; Siu AL, Bibbins-Domingo K, Grossman DC, et al. Screening for chronic obstructive pulmonary disease: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:1372-1377. doi: 10.1001/jama.2016.2638
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557-582. doi: 10.1164/rccm.201701-0218PP
EVIDENCE-BASED ANSWER:
It depends. A diagnosis of chronic obstructive pulmonary disease (COPD) made using screening spirometry in patients without symptoms does not change the course of the disease or alter smoking rates (strength of recommendation [SOR]: A, preponderance of evidence from multiple randomized controlled trials [RCTs]). However, once a patient develops symptoms of lung disease, a delayed diagnosis is associated with poorer outcomes (SOR: B, cohort studies). Active case finding (including the use of spirometry) is recommended for patients with risk factors for COPD who present with consistent symptoms (SOR: C, expert opinion).
What barriers delay treatment in patients with hepatitis C?
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.
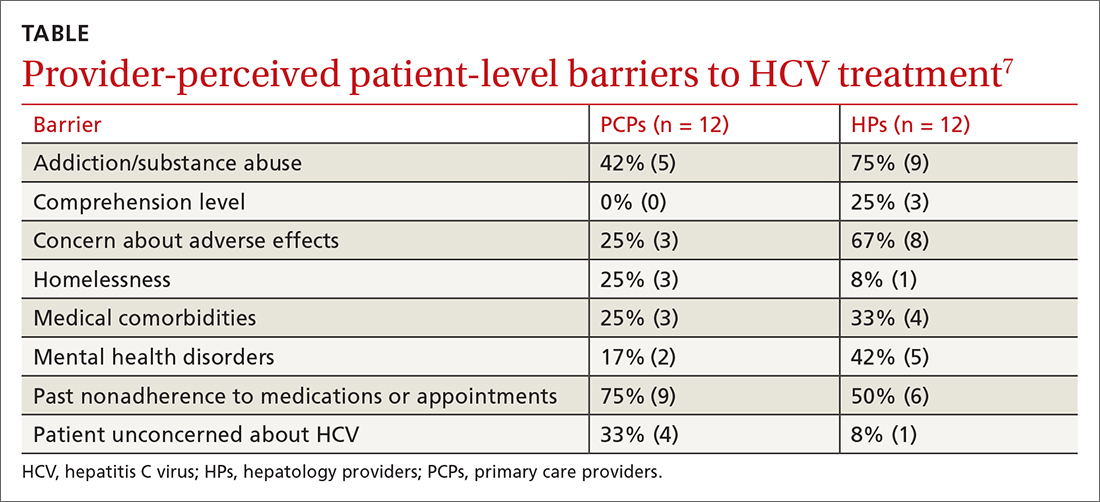
Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
EVIDENCE SUMMARY
Race, gender, and other factors are associated with lack of HCV Tx
A retrospective study (N = 894) assessed factors associated with direct-acting antiviral (DAA) initiation.1 Patients who were HCV+ with at least 1 clinical visit during the study period completed a survey of psychological, behavioral, and social life assessments. The final cohort (57% male; 64% ≥ 61 years old) was divided into patients who initiated DAA treatment (n = 690) and those who did not (n = 204).
In an adjusted multivariable analysis, factors associated with lower odds of DAA initiation included Black race (adjusted odds ratio [aOR] = 0.59 vs White race; 95% CI, 0.36-0.98); perceived difficulty accessing medical care (aOR = 0.48 vs no difficulty; 95% CI, 0.27-0.83); recent intravenous (IV) drug use (aOR = 0.11 vs no use; 95% CI, 0.02-0.54); alcohol use disorder (AUD; aOR = 0.58 vs no AUD; 95% CI, 0.38-0.90); severe depression (aOR = 0.42 vs no depression; 95% CI, 0.2-0.9); recent homelessness (aOR = 0.36 vs no homelessness; 95% CI, 0.14-0.94); and recent incarceration (aOR = 0.34 vs no incarceration; 95% CI, 0.12-0.94).1
A multicenter, observational prospective cohort study (N = 3075) evaluated receipt of HCV treatment for patients co-infected with HCV and HIV.2 The primary outcome was initiation of HCV treatment with DAAs; 1957 patients initiated therapy, while 1118 did not. Significant independent risk factors for noninitiation of treatment included age younger than 50 years, a history of IV drug use, and use of opioid substitution therapy (OST). Other factors included psychiatric comorbidity (odds ratio [OR] = 0.45; 95% CI, 0.27-0.75), incarceration (OR = 0.6; 95% CI, 0.43-0.87), and female gender (OR = 0.80; 95% CI, 0.66-0.98). In a multivariate analysis limited to those with a history of IV drug use, both use of OST (aOR = 0.55; 95% CI, 0.40-0.75) and recent IV drug use (aOR = 0.019; 95% CI, 0.004-0.087) were identified as factors with low odds of treatment implementation.2
A retrospective cohort study (N = 1024) of medical charts examined the barriers to treatment in adults with chronic HCV infection.3 Of the patient population, 208 were treated and 816 were untreated. Patients not receiving DAAs were associated with poor adherence to/loss to follow-up (n = 548; OR = 36.6; 95% CI, 19.6-68.4); significant psychiatric illness (n = 103; OR = 2.02; 95% CI, 1.13-3.71); and coinfection with HIV (n = 188; OR = 4.5; 95% CI, 2.5-8.2).3
A German multicenter retrospective case-control study (N = 793) identified factors in patient and physician decisions to initiate treatment for HCV.4 Patients were ≥ 18 years old, confirmed to be HCV+, and had visited their physician at least 1 time during the observation period. A total of 573 patients received treatment and 220 did not. Patients and clinicians of those who chose not to receive treatment completed a survey that collected reasons for not treating. The most prevalent reason for not initiating treatment was patient wish (42%). This was further delineated to reveal that 17.3% attributed their decision to fear of treatment and 13.2% to fear of adverse events. Other factors associated with nontreatment included IV drug use (aOR = 0.31; 95% CI, 0.16-0.62); HIV coinfection (aOR = 0.19; 95% CI, 0.09-0.40); and use of OST (aOR = 0.37; 95% CI, 0.21-0.68). Patient demographics associated with wish not to be treated included older age (20.2% of those ≥ 40 years old vs 6.4% of those < 40 years old; P = .03) and female gender (51.0% of females vs 35.2% of males; P = .019).4
An analysis of a French insurance database (N = 22,545) evaluated the incidence of HCV treatment with DAAs in patients who inject drugs (PWID) with a diagnosis of alcohol use disorder (AUD).5 All participants (78% male; median age, 49 years) were chronically HCV-infected and covered by national health insurance. Individuals were grouped by AUD status: untreated (n = 5176), treated (n = 3020), and no AUD (n = 14349). After multivariate adjustment, those with untreated AUD had lower uptake of DAAs than those who did not have AUD (adjusted hazard ratio [aHR] = 0.86; 95% CI, 0.78-0.94) and those with treated AUD (aHR = 0.83; 95% CI, 0.74-0.94). There were no differences between those with treated AUD and those who did not have AUD. Other factors associated with lower DAA uptake were access to care (aHR = 0.90; 95% CI, 0.83-0.98) and female gender (aHR = 0.83; 95% CI, 0.76-0.9).5
A 2017 retrospective cohort study evaluated predictors and barriers to follow-up and treatment with DAAs among veterans who were HCV+.6 Patients (94% > 50 years old; 97% male; 48% white) had established HCV care within the US Department of Veterans Affairs system. Of those who followed up with at least 1 visit to an HCV specialty clinic (n = 47,165), 29% received DAAs. Factors associated with lack of treatment included race (Black vs White: OR = 0.77; 95% CI, 0.72-0.82; Hispanic vs White: OR = 0.88; 95% CI, 0.79-0.97); IV drug use (OR = 0.84; 95% CI, 0.80-0.88); AUD (OR = 0.73; 95% CI, 0.70-0.77); medical comorbidities (OR = 0.71; 95% CI, 0.66-0.77); and hepatocellular carcinoma (OR = 0.73; 95% CI, 0.65-0.83).6
Continue to: Providers identify similar barriers to treatment of HCV
Providers identify similar barriers to treatment of HCV
A 2017 prospective qualitative study (N = 24) from a Veterans Affairs health care system analyzed provider-perceived barriers to initiation of and adherence to HCV treatment.7 The analysis focused on differences by provider specialty. Primary care providers (PCPs; n = 12; 17% with > 40 patients with HCV) and hepatology providers (HPs; n = 12; 83% with > 40 patients with HCV) participated in a semi-structured telephone-based interview, providing their perceptions of patient-level barriers to HCV treatment. Eight patient-level barrier themes were identified; these are outlined in the TABLE7 along with data for both PCPs and HPs.

Editor’s takeaway
These 7 cohort studies show us the factors we consider and the reasons we give to not initiate HCV treatment. Some of the factors seem reasonable, but many do not. We might use this list to remind and challenge ourselves to work through barriers to provide the best possible treatment.
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
1. Spradling PR, Zhong Y, Moorman AC, et al. Psychosocial obstacles to hepatitis C treatment initiation among patients in care: a hitch in the cascade of cure. Hepatol Commun. 2021;5:400-411. doi: 10.1002/hep4.1632
2. Rivero-Juarez A, Tellez F, Castano-Carracedo M, et al. Parenteral drug use as the main barrier to hepatitis C treatment uptake in HIV-infected patients. HIV Medicine. 2019;20:359-367. doi: 10.1111/hiv.12715
3. Al-Khazraji A, Patel I, Saleh M, et al. Identifying barriers to the treatment of chronic hepatitis C infection. Dig Dis. 2020;38:46-52. doi: 10.1159/000501821
4. Buggisch P, Heiken H, Mauss S, et al. Barriers to initiation of hepatitis C virus therapy in Germany: a retrospective, case-controlled study. PLoS ONE. 2021;16:3p250833. doi: 10.1371/journal.pone.0250833
5. Barré T, Marcellin F, Di Beo V, et al. Untreated alcohol use disorder in people who inject drugs (PWID) in France: a major barrier to HCV treatment uptake (the ANRS-FANTASIO study). Addiction. 2019;115:573-582. doi: 10.1111/add.14820
6. Lin M, Kramer J, White D, et al. Barriers to hepatitis C treatment in the era of direct acting antiviral agents. Aliment Pharmacol Ther. 2017;46:992-1000. doi: 10.1111/apt.14328
7. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62:1933-1943. doi: 10.1007/s10620-017-4608-9
EVIDENCE-BASED ANSWER:
Multiple patient-specific and provider-perceived factors delay initiation of treatment in patients with hepatitis C. Patient-specific barriers to initiation of treatment for hepatitis C virus (HCV) include age, race, gender, economic status, insurance status, and comorbidities such as HIV coinfection, psychiatric illness, and other psychosocial factors.
Provider-perceived patient factors include substance abuse history, older age, psychiatric illness, medical comorbidities, treatment adverse effect risks, and factors that might limit adherence (eg, comprehension level).
Study limitations included problems with generalizability of the populations studied and variability in reporting or interpreting data associated with substance or alcohol use disorders
Do behavioral interventions improve nighttime sleep in children < 1 year old?
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
EVIDENCE-BASED ANSWER:
YES. Infants respond to behavioral interventions, although objective data are limited. Behavioral interventions include establishing regular daytime and sleep routines for the infant, reducing environmental noises or distractions, and allowing for self-soothing at bedtime (strength of recommendation: B, based on multiple randomized and nonrandomized studies).
Are antipsychotics effective adjunctive Tx for patients with moderate-to-severe depression?
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
EVIDENCE-BASED ANSWER:
YES. Augmentation with second- generation antipsychotics, especially aripiprazole and quetiapine, appears to be effective in patients with moderate-to-severe depression who have had a suboptimal response to a selective serotonin reuptake inhibitor or a serotonin-norepinephrine reuptake inhibitor (strength of recommendation [SOR]: A, based on a systematic review of randomized controlled trials [RCTs] and an individual RCT). Augmenting antidepressant therapy with cariprazine, ziprasidone, or olanzapine also appears to improve depressive symptoms over the short term. All antipsychotics studied carried an increased likelihood of adverse effects that could lead to discontinuation (SOR: A, based on a systematic review of RCTs).