User login
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
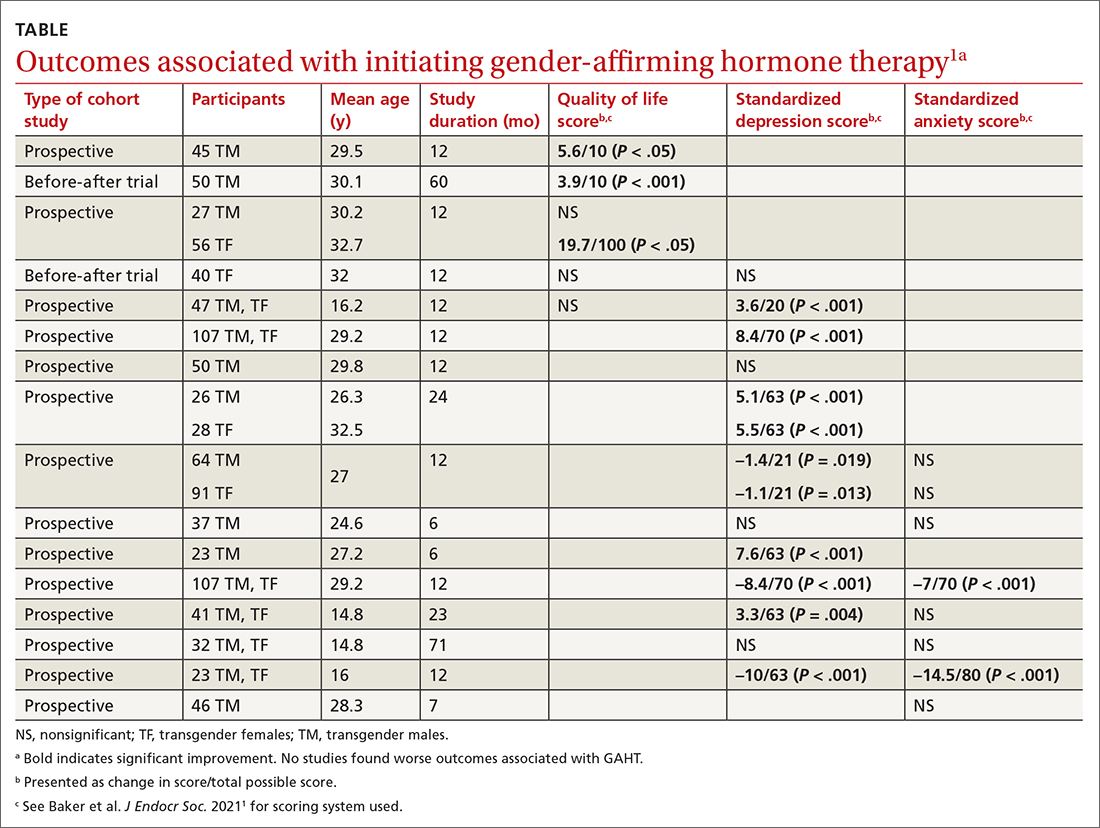
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
Evidence summary
GAHT may improve depression and quality of life, but not anxiety
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.

The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..
An Australian prospective longitudinal controlled study (n = 77 transgender adults; 103 cisgender controls) evaluated GAHT outcomes after 6 months and found a significant reduction in gender dysphoria scores in both transgender males (adjusted mean difference [aMD] = –6.8; 95% CI, –8.7 to –4.9; P < .001) and transgender females (aMD = –4.2; 95% CI, –6.2 to –2.2; P < .001) vs controls. QOL scores (emotional well-being, social functioning) improved only for transgender males (well-being: aMD = +7.5; 95% CI, 1.3 to 13.6; P < .018; social functioning: aMD = +12.5; 95% CI, 2.8 to 22.2; P = .011).2
A US prospective cohort study (n = 104 adolescents; mean age, 16 years) examined the effect of GnRH and/or GAHT over a 12-month period and found significant decreases in standardized scores for depression (adjusted odds ratio [aOR] = 0.4; 95% CI, 0.17-0.95) and suicidality (aOR = 0.27; 95% CI, 0.11-0.65) but not for anxiety. Participants who did not receive hormonal interventions had increased scores for depression and suicidality at 3 and 6 months’ follow-up.3
A prospective cohort study from the UK (n = 178 transgender adults) examined outcomes before and after GAHT treatment over 18 months and found significant decreases in standardized scores for depression (transgender males: –2.1; 95% CI, –3.2 to –1.2; P < .001; transgender females: –1.9; 95% CI, –2.8 to –1.0; P < .001) but not for anxiety.4
A large US study shows GAHT may reduce depression scores
A recent large cross-sectional study from the United States (n = 11,914 transgender or nonbinary youth, ages 13-24 years) found that receiving GAHT was associated with significantly lower odds of recent depression (aOR = 0.73; P < .001) and suicidality (aOR = 0.74; P < .001) compared to those who wanted GAHT but did not receive it. The authors were unable to differentiate the effects of receiving GAHT from the effects of parental support for their child’s gender identity, which may be a confounding factor.5
Recommendations from others
The World Professional Association for Transgender Health Standards of Care state that “gender incongruence that causes clinically significant distress and impairment often requires medically necessary clinical interventions” and recommends “health care professionals initiate and continue gender-affirming hormone therapy … due to demonstrated improvement in psychosocial functioning and quality of life.”6 The Endocrine Society Position Statement on Transgender Health states that “medical intervention for transgender youth and adults (including … hormone therapy) is effective, relatively safe (when appropriately monitored), and has been established as the standard of care.”7 The American Academy of Family Physicians “supports gender-affirming care as an evidence-informed intervention that can promote health equity for gender-diverse individuals.”8
Editor’s takeaway
Family physicians commonly address many factors that can impact the QOL for our patients with gender dysphoria: lack of fixed residence, underemployment, food insecurity, and trauma. GAHT, especially in male-to-female transgender patients, may further improve QOL without evidence of harm.
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
1. Baker KE, Wilson LM, Sharma R, et al. Hormone therapy, mental health, and quality of life among transgender people: a systematic review. J Endocr Soc. 2021;5:bvab011. doi: 10.1210/jendso/bvab011
2. Foster Skewis L, Bretherton I, Leemaqz, SY, et al. Short-term effects of gender-affirming hormone therapy on dysphoria and quality of life in transgender individuals: a prospective controlled study. Front Endocrinol (Lausanne). 2021;12:717766.
3. Tordoff DM, Wanta JW, Collin A, et al. Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Netw Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978
4. Aldridge Z, Patel S, Guo B, et al. Long-term effect of gender-affirming hormone treatment on depression and anxiety symptoms in transgender people: a prospective cohort study. Andrology. 2021;9:1808-1816. doi: 10.1111/andr.12884
5. Green AE, DeChants JP, Price MN, et al. Association of gender-affirming hormone therapy with depression, thoughts of suicide, and attempted suicide among transgender and nonbinary youth. J Adolesc Health. 2022;70:643-649. doi: 10.1016/j.jadohealth.2021.10.036
6. World Professional Association for Transgender Health. Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. 8th version. Published 2022. Accessed November 17, 2022. www.wpath.org/publications/soc
7. Endocrine Society. Transgender health: an Endocrine Society position statement. Updated December 16, 2020. Accessed November 17, 2022. www.endocrine.org/advocacy/position-statements/transgender-health
8. American Academy of Family Physicians. Care for the transgender and gender nonbinary patient. Updated September 2022. Accessed November 17, 2022. www.aafp.org/about/policies/all/transgender-nonbinary.html
EVIDENCE-BASED ANSWER:
There are modest effects on depression but not anxiety. Gender-affirming hormone therapy (GAHT) is associated with modest improvements in standardized scores for quality of life (QOL) and depression in adult male-to-female and female-to-male transgender people and modest improvements in depression scores in transgender adolescents, but the effect on anxiety is uncertain (strength of recommendation [SOR]: B, based on a preponderance of low-quality prospective cohort studies with inconsistent results).
GAHT is associated with reduced gender dysphoria and decreased suicidality (SOR: B, based on a prospective cohort study). However, there is insufficient evidence to determine any effect on suicide completion. No studies associated GAHT with worsened QOL, depression, or anxiety scores.
