User login
Things We Do for No Reason™: Routinely Prescribing Transfusion Premedication To Prevent Acute Transfusion Reactions

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Thyroid-Stimulating Hormone Testing in the Hospital

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
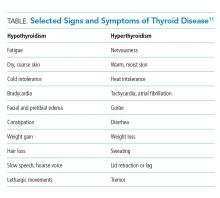
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Lumbar Punctures in Low-Risk Febrile Infants with Bronchiolitis

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
© 2019 Society of Hospital Medicine
Things We Do for No Reason™: Supplemental Oxygen for Patients without Hypoxemia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Routine Blood Culture Acquisition for Children Hospitalized with Community-Acquired Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 4-year-old previously healthy, fully immunized boy presented to the emergency department (ED) with three days of worsening cough, fever to 103oF, dyspnea, and decreased oral intake. In the ED, he was febrile, temperature 102.7oF, heart rate 115 beats/min, respiratory rate 30 breaths/min, and O2 saturation 86%. Pertinent findings identified on examination included tachypnea, dry mucous membranes, and decreased breath sounds in the posterior right lung fields. Chest radiograph revealed a right lower lobe opacification concerning for community-acquired pneumonia (CAP). He was admitted to the hospital due to hypoxemia and dehydration. A blood culture was obtained, and treatment with ampicillin was initiated. The following morning, he was afebrile, clinically improved, and no longer hypoxemic, but the blood culture grew Gram-positive cocci. Another blood culture was performed, and he was switched to vancomycin. The next day, penicillin-susceptible Streptococcus pneumoniae was confirmed from the original culture, and he was discharged home on high-dose amoxicillin.
WHY YOU MIGHT THINK A BLOOD CULTURE IS HELPFUL
CAP is a prominent cause of childhood morbidity and among the most common causes for acute childhood hospitalizations in the United States, with 124,900 hospital stays documented in 2012.1 In 2011, the Infectious Diseases Society of America (IDSA) released recommendations for pediatric CAP in immunocompetent children aged >3 months without chronic medical conditions. The recommendations clearly discourage blood cultures in the outpatient setting but are less direct in the inpatient setting. The recommendations state that providers should obtain blood cultures “in children requiring hospitalization for presumed bacterial CAP that is moderate to severe, particularly those with complicated pneumonia.”2 The recommendation is graded as “strong”, though the IDSA acknowledged the “low” quality of supporting evidence. Although the organization provides a classification for “complicated pneumonia,” it does not define what constitutes mild versus moderate or severe pneumonia.
Without clear recommendations, decisions to obtain blood cultures for hospitalized children with CAP vary among providers and institutions, with the reported hospital-to-hospital variation being as large as 0%-78.7%.3 Some believe that any child hospitalized with CAP meets the definition of moderate to severe pneumonia and have implemented projects to increase blood culture acquisition for this population.4 The decision to err on the side of routinely obtaining a blood culture may come from providers’ prevalent worry of “missing” a diagnosis, desire to target antibacterial therapy, and assumption that it will provide additional information for patients lacking improvement.
WHY A ROUTINE BLOOD CULTURE ON PEDIATRIC CAP ADMISSIONS IS NOT HELPFUL
Since the publication of the 2011 IDSA guidelines, new evidence has revealed a decreasing incidence of bacteremia in pediatric populations.5 Moreover, viruses were the most frequently identified pathogens in children hospitalized with CAP in a large study, which were isolated in 66% of patients, whereas typical bacteria (either alone or in combination with a virus) were identified in only 7% of cases.6 When blood cultures are obtained for pediatric CAP, the incidence of a true bacterial bloodstream pathogen is 1.4%-7% of patients in the United States in the conjugate vaccine era.7-11 Given that the practice of obtaining blood cultures varies widely among hospitalized patients and that cultures are often obtained based on perceived severity of presentation,8,9,12 the true incidence of bacteremia in children with CAP would likely be lower if blood cultures were performed in all patients.
Since the introduction of the first conjugated pneumococcal vaccine, the prevalence of penicillin resistance among pneumococcal isolates dramatically declined,13 though with geographic variability.14 Therefore, when we isolate pneumococcus strains, resistance prevalence requires that we alter treatment much less frequently in the majority of patients with CAP receiving IDSA-recommended ampicillin/amoxicillin.2 In a large six-center, geographically dispersed retrospective cohort study, Neuman et al. reported a rate of true bacteremia of 2.53%; 82% of all pathogens and 92% of pneumococcal isolates were susceptible to penicillin. Therefore, the authors estimated that 667 children hospitalized with CAP would need blood cultures to identify one child requiring an antibiotic other than an aminopenicillin.9 Staphylococcus aureus was identified only in 1% (23/2,138) of patients in the EPIC cohort; the pathogen was identified via blood culture in only 26% (6/23) of these patients.15 Therefore, the concern about the possibility of S. aureus may be a common reason for physicians straying from IDSA-recommended therapy, but it is an uncommon cause of CAP and infrequently identified via blood culture.
Blood culture contaminants have been reported to approach the rate of true pathogens in some studies8,9,11 and be equal or exceed the rates in others.7,16 While awaiting bacterial speciation, antibiotic coverage is often broadened, even for contaminants,8 which can result in unnecessary exposure to nephrotoxic agents such as vancomycin, cause rare adverse events such as Stevens-Johnson syndrome, contribute to antibiotic resistance and unnecessary costs, and increase the length of stay and laboratory utilization.17-19
WHEN MIGHT A BLOOD CULTURE BE HELPFUL
Given the low penicillin resistance prevalence among pneumococcal isolates in several parts of the United States, blood cultures should be used to identify patients with nonpneumococcal CAP as these patients are more likely to require antibiotics other than penicillin or aminopenicillin. Children with complicated pneumonia are more likely to have nonpneumococcal etiologies than children with uncomplicated pneumonia.2 Moreover, literature published since the IDSA guidelines continues to indicate that the incidence of bacteremia in complicated pneumonia is significantly higher than that in uncomplicated pneumonia (Table). This further supports the IDSA guideline recommendation for blood culture acquisition in children with complicated pneumonia.2
One difficulty in interpreting these data is that each publication used a different definition of “complicated” pneumonia, probably due to differences in data sources. Neuman et al. incorporated the narrowest definition of severe and complicated pneumonia as patients who were either admitted to an intensive care unit (ICU) or who underwent a pleural drainage procedure.9 Myers’ and Shah’s definitions were similar to each other but much broader than that of Neuman et al. Shah et al. included lung abscess/necrosis, parapneumonic effusion/empyema, or bronchopleural fistula.11 Myers et al. included the same indications but qualified their pleural fluid effusions as “moderate-to-large” and any effusion that required pleural drainage procedure.8 Myers et al. also reported bacteremia in 75% of patients with metastatic complications, including osteomyelitis.8 These definitions of complicated pneumonia may at least partially explain the differences noted in the rates of bacteremia in complicated pneumonia, with the patients in the study of Myers et al. potentially representing the most severe cohort and with the highest rate of bacteremia8,9 (Table).
These studies not only support the definition of complicated pneumonia put forward by the IDSA but also provide further information, though imperfect, on how to define “moderate to severe.” All the patients with bacteremia in the report of Heine et al. had complicated pneumonia and were described on chart review as either toxic-appearing or requiring ICU care.7 This, in addition to the inclusion of ICU care in the definition of complicated pneumonia of Neuman et al.,9 indicates that children with CAP requiring ICU care may be at higher risk of bacteremia. In fact, the British Thoracic Society guidelines do not recommend microbiological investigations of children with CAP, including blood culture, unless a child requires ICU care.20
WHAT YOU SHOULD DO INSTEAD
Given the low rate of bacteremia in CAP, the risk of blood culture contaminants, and the small likelihood that isolation of a pathogen alters treatment for children, we recommend not using hospital admission as the determining factor for blood culture acquisition. Instead, we recommend a more targeted approach. To achieve a higher rate of true-positive bacteremia in immunocompetent children with up-to-date vaccinations, we recommend acquiring a blood culture in children with complicated pneumonia, metastatic complications, or with ICU needs. By initiating the IDSA-recommended ampicillin/amoxicillin in the remaining hospitalized patients and acquiring blood cultures for the minority of patients who do not improve, we can increase the likelihood of isolating penicillin-resistant bacteria.
Attempting to balance the importance of identifying clinically important bacteremia for children hospitalized with CAP and the inherent risks of obtaining blood cultures for all hospitalized patients, Andrews et al. created and analyzed a cost-effectiveness model. The authors compared universal acquisition of blood cultures for hospitalized children with CAP versus a targeted approach with blood cultures obtained in patients with effusion or empyema, requiring ICU care, or who are immunosuppressed. Based on this model, a targeted approach could save more than $187 million annually, reduce the number of cultures needed to result in a meaningful change in antibiotic therapy for one patient from 122 to 42, and would “miss” only approximately one case of bacteremia resulting in treatment failure per 1,400 patients.17
RECOMMENDATIONS
- Do not obtain blood culture routinely for children aged >3 months hospitalized for uncomplicated CAP.
- Obtain a blood culture for the following hospitalized patients with CAP:
a. Patients with complicated CAP as defined by the IDSA, particularly those with empyema, abscess, or fistula, or metastatic complications of pneumonia (Table); or
b. Patients with CAP requiring ICU care20 for the management of shock and/or advanced respiratory support.
c. Patients with CAP judged to need antibiotic treatment with an agent other than the IDSA-recommended ampicillin/penicillin (concern for pathogens other than penicillin-sensitive S. pneumonia, immunocompromised or under-immunized status, or inadequate clinical response to empiric ampicillin therapy).
CONCLUSION
Implementing a more targeted approach to blood culture acquisition for hospitalized children with CAP will hopefully increase the yield of true bacterial pathogens that alter management decisions. A targeted approach for the child in the opening vignette would have saved him from the pain of unnecessary phlebotomy (repeat culture), exposure to vancomycin as a nephrotoxic agent, and an additional hospital day.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?™” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by e-mailing TWDFNR@hospitalmedicine.org.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
1. Whitney P, Whitt AJW, Elixhauser A. Overview of hospital stays for children in the United States, 2012. Statistical Brief 187. 2014;187. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb187-Hospital-Stays-Children-2012.jsp. Accessed December 21, 2017.
2. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76. https://doi.org/10.1093/cid/cir531.
3. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. https://doi.org/10.1097/INF.0b013e31825f2b10.
4. Murtagh Kurowski E, Shah SS, Thomson J, et al. Improvement methodology increases guideline recommended blood cultures in children with pneumonia. Pediatrics. 2015;135(4):e1052-e1059. https://doi.org/10.1542/peds.2014-2077.
5. Greenhow TL, Hung YY, Herz A. Bacteremia in children 3 to 36 months old after introduction of conjugated pneumococcal vaccines. Pediatrics. 2017;139(4):e20162098. https://doi.org/10.1542/peds.2016-2098.
6. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. https://doi.org/10.1056/NEJMoa1405870.
7. Heine D, Cochran C, Moore M, Titus MO, Andrews AL. The prevalence of bacteremia in pediatric patients with community-acquired pneumonia: guidelines to reduce the frequency of obtaining blood cultures. Hosp Pediatr. 2013;3(2):92-96. https://doi.org/10.1542/hpeds.2012-0050.
8. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. https://doi.org/10.1097/INF.0b013e318290bf63.
9. Neuman MI, Hall M, Lipsett SC, et al. Utility of blood culture among children hospitalized with community-acquired pneumonia. Pediatrics. 2017;140(3). https://doi.org/10.1542/peds.2017-1013.
10. Sandora TJ, Desai R, Miko BA, Harper MB. Assessing quality indicators for pediatric community-acquired pneumonia. Am J Med Qual. 2009;24(5):419-427. https://doi.org/10.1177/1062860609337900.
11. Shah SS, Dugan MH, Bell LM, et al. Blood cultures in the emergency department evaluation of childhood pneumonia. Pediatr Infect Dis J. 2011;30(6):475-479. https://doi.org/10.1097/INF.0b013e31820a5adb.
12. Davis TR, Evans HR, Murtas J et al. Utility of blood cultures in children admitted to hospital with community-acquired pneumonia. J Paediatr Child Health. 2017;53(3):232-236. https://doi.org/10.1111/jpc.13376.
13. Williams DJ, Shah SS. Community-acquired pneumonia in the conjugate vaccine era. J Pediatr Infect Dis Soc. 2012;1(4):314-328. https://doi.org/10.1093/jpids/pis101.
14. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354(14):1455-1463. https://doi.org/10.1056/NEJMoa051642.
15. Frush JM, Zhu Y, Edwards KM, et al. Prevalence of Staphylococcus aureus and use of antistaphylococcal therapy in children hospitalized with pneumonia. J Hosp Med. 2018;13(12):848-852. https://doi.org/10.12788/jhm.3093.
16. Mendoza-Paredes A, Bastos J, Leber M, Erickson E, Waseem M. Utility of blood culture in uncomplicated pneumonia in children. Clin Med Insights Pediatr. 2013;7:1-5. https://doi.org/10.4137/CMPed.S8051.
17. Andrews AL, Simpson AN, Heine D, Teufel II RJ. A cost-effectiveness analysis of obtaining blood cultures in children hospitalized for community-acquired pneumonia. J Pediatr. 2015;167(6):1280-1286. https://doi.org/10.1016/j.jpeds.2015.09.025.
18. Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK. Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr. 2014;165(3):585-591. https://doi.org/10.1016/j.jpeds.2014.04.062.
19. McCulloh RJ, Koster MP, Yin DE, et al. Evaluating the use of blood cultures in the management of children hospitalized for community-acquired pneumonia. PloS One. 2015;10(2):e0117462. https://doi.org/10.1371/journal.pone.0117462.
20. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax. 2011;66(2):ii1-ii23. https://doi.org/10.1136/thoraxjnl-2011-200598.
© 2020 Society of Hospital Medicine