User login
Improving Healthcare Value: COVID-19 Emergency Regulatory Relief and Implications for Post-Acute Skilled Nursing Facility Care
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. Govt doesn’t always make sense. We’re listening to feedback.
—Centers for Medicare & Medicaid Services Administrator Seema Verma, @SeemaCMS, August 4, 2019, via Twitter.1
On March 13, 2020, the president of the United States declared a national health emergency, granting the secretary of the United States Department of Health & Human Services authority to grant waivers intended to ease certain Medicare and Medicaid program requirements.2 Broad waiver categories include those that may be requested by an individual institution, as well as “COVID-19 Emergency Declaration Blanket Waivers,” which automatically apply across all facilities and providers. As stated by the Centers for Medicare & Medicaid Services (CMS), waivers are intended to create “regulatory flexibilities to help healthcare providers contain the spread of 2019 Novel Coronavirus Disease (COVID-19).” These provisions are retroactive to March 1, 2020, expire at the end of the “emergency period or 60 days from the date the waiver . . . is first published” and can be extended by the secretary.2
The issued blanket waivers remove administrative requirements in a wide range of care settings including home health, hospice, hospitals, and skilled nursing facilities (SNF), among others. The waiving of many of these administrative requirements are welcomed by providers and administrators alike in this time of national crisis. For example, relaxation of verbal order signage requirements and expanded coverage of telehealth will, almost certainly, improve accessibility, efficiency, and requisite coordination and care across settings. Emergence of these new “COVID-19” waivers also present rare and valuable opportunities to examine care improvement in areas long believed to need permanent regulatory change. Perhaps the most important of these long over-due changes is the current CMS process for determining Part A eligibility for post-acute skilled nursing facility coverage for traditional Medicare beneficiaries following an inpatient hospitalization. Under COVID-19, CMS has now granted a waiver that “authorizes the Secretary to provide for Skilled Nursing Facilities (SNF) coverage in the absence of a qualifying [three consecutive inpatient midnight] hospital stay. . . .”2 Although demand for SNF placement may shift during the pandemic, hospitals facing capacity issues will more easily be able to discharge Medicare beneficiaries ready for post-acute care.
POST-ACUTE SKILLED NURSING FACILITY COVERAGE
When Medicare was established in 1965, approximately half of Americans over age 65 did not have health insurance, and older adults were the most likely demographic to be living in poverty.3 Originally called “Hospital Insurance” or “Medicare Part A,” these “Inpatient Hospital Services” are described in Social Security statute as “items and services furnished to an inpatient of a hospital” including room and board, nursing services, pharmaceuticals, and medical and surgical services delivered in the hospital.4 In 1967, Medicare beneficiaries staying three consecutive inpatient hospital midnights were also afforded post-acute SNF coverage for up to 100 days. As expected, hospital use increased as seniors had coverage for hospital care and were also, in many cases, able to access higher quality post-hospital care.5
Over the past 50 years, two important changes have shifted Medicare beneficiary SNF coverage. First, due to efficiencies and changes in care delivery, average length of hospital stay for Americans over age 65 has shrunk from 14 days in 1965 to approximately 5 days currently.5,6 Now, fewer beneficiaries spend the necessary three or more nights in the hospital to qualify for post-acute SNF coverage. Second, and most importantly, CMS created “observation status” in the 1980s, which allowed for patients to be observed as “outpatients” in a hospital instead of as inpatients. Notably, these observation nights fall under outpatient status (Part B), and therefore do not count toward the statutory SNF coverage requirement of three inpatient midnights.
According to CMS, observation should be used so that a “decision can be made regarding whether patients will require further treatment as hospital inpatients or if they are able to be discharged from the hospital. . . . In the majority of cases, the decision can be made in less than 48 hours, usually in less than 24 hours.”7 At the time of its development, this concept fit the growing use of Emergency Department observation units, in which patients presented for an acute issue but could usually discharge home in the stated time frame.
OBSERVATION CARE
In reality, outpatient (observation) status is not synonymous with observation units. Because observation is a billing determination, not a specific type of clinical care, observation care may be delivered anywhere in a hospital—including an observation unit, a hospital ward, or even an intensive care unit (ICU). While all hospitals may deliver observation care, only about one-third of hospitals have observation units, and even hospitals with observation units deliver observation care outside of these units. Traditional Medicare beneficiaries who stay three or more nights in the hospital but cannot meet the three inpatient midnight requirement to access their SNF coverage benefits because of outpatient (observation) nights are often left vulnerable and confused, saddling them with an average of $10,503 for each uncovered SNF stay.8 As emergent evidence demonstrates striking racial, geographic, and socioeconomic-based health disparities in COVID-19, renewal of the “three-midnight rule” could have disproportionate and long-lasting ramifications for these populations in particular.9
Hospital observation stays (or observation nights) can look identical to inpatient hospital stays, as defined by the Social Security statute4; yet never count toward the three-inpatient-midnight tally. In 2014, the Office of Inspector General (OIG) found there were 633,148 hospital stays that lasted three midnights or longer but did not contain three consecutive inpatient midnights, which resulted in nonqualifying stays for purposes of SNF coverage, if that coverage was needed.10 A more recent OIG report found that Medicare was paying erroneously for some SNF stays because even CMS could not distinguish between three midnights that were all inpatient or a combination of inpatient and observation.11 Additionally, because care provided is often indistinguishable, status changes between outpatient and inpatient are common; in 2014, 40% of Medicare observation stays occurring within 30 days of an inpatient stay changed to inpatient over the course of a single hospitalization.12 Now, in the time of COVID-19, this untenable decades-long problem has the potential to be definitively addressed by a permanent removal of the three midnight requirement altogether.
PROGRESS TOWARD REFORM
Several recent signals suggest that change is supported by a diverse group of stakeholders. In their 2019 Top 25 Unimplemented Recommendations, the OIG acknowledged the similarity in observation and inpatient care, recommending that “CMS . . . analyze the potential impacts of counting time spent as an outpatient toward the 3-night requirement for skilled nursing facility (SNF) services so that beneficiaries receiving similar hospital care have similar access to these services.”13 The “Improving Access to Medicare Coverage Act of 2019,” reintroduced in the 116th Congress, would count all midnights spent in the hospital, whether those nights are inpatient or observation, toward the three midnight requirement.14 This bill has bipartisan, bicameral support, which demonstrates unified legislative interest across the political spectrum. More recently in March 2020, a federal judge in the class action lawsuit Alexander v Azar determined that Medicare beneficiaries had the right to appeal to Medicare if a physician placed a patient in inpatient status and this decision was overturned administratively by a hospital, resulting in loss of a beneficiary’s SNF coverage.15 Although now under appeal, this judicial decision signals the importance of beneficiary rights to appeal directly to CMS.
Given the mounting support for reform, it is probable that cost concerns and allocation of resources to the Part A vs Part B “buckets” remain the only barrier to permanently reforming the three-midnight inpatient stay policy. Pilot programs testing Medicare SNF waivers more than 30 years ago suggested increased cost and SNF usage.16 However, more contemporary experience from Medicare Advantage programs suggest just the opposite. Grebla et al showed there was no increased SNF use nor SNF length of stay for beneficiaries in Medicare Advantage plans that waived the three inpatient midnight requirement.17
Arguably, the current COVID-19 emergency blanket SNF waiver is not a perfect test of short- or long-term Medicare costs. First, factors such as reduced hospital elective surgeries that may typically drive post-acute SNF admissions, as well as potentially reduced SNF utilization caused by fear of COVID-19 outbreaks, may temporarily lower SNF use and associated Medicare expenditures. The existing waiver of statute is also financially constrained, stipulating that “this action does not increase overall program payments. . . .”2 Longer term, innovations in care delivery prompted by accelerated telehealth reforms may shift more post-acute care from SNFs to the home setting, changing patterns of SNF utilization altogether. Despite these limitations, this regulatory relief will still provide valuable utilization and cost information on SNF use under a system absent the three-midnight requirement.
CONCLUSION
Rarely, if ever, does a national healthcare system experience such a rapid and marked change as that seen with the COVID-19 pandemic. Despite the tragic emergency circumstances prompting CMS’s blanket waivers, it provides CMS and stakeholders with a rare opportunity to evaluate potential improvements revealed by each individual aspect of COVID-19 regulatory relief. CMS has in the past argued the three-midnight SNF requirement is a statutory issue and thus not within their control, yet they have used their regulatory authority to waive this policy to facilitate efficient care in a national health crisis. This is a change that many believe is long overdue, and one that should be maintained even after COVID-19 abates. “Govt doesn’t always make sense,” as Administrator Verma wrote,1 should be a cry for government to make better sense of existing legislation and regulation. Reform of the three-midnight inpatient rule is the right place to start.
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
1. @SeemaCMS. #Medicare beneficiary who requires skilled care in a nursing home? Better be admitted for at least 3 days in the hospital first if you want the nursing home paid for. [Flushed face emoji] Govt doesn’t always make sense. We’re listening to feedback. #RedTapeTales #TheBoldAndTheBureaucratic. August 4, 2019. Accessed April 17, 2020. https://twitter.com/SeemaCMS/status/1158029830056828928
2. COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. Centers for Medicare & Medicaid Services, US Dept of Health & Human Services; 2020. Accessed April 17, 2020. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
3. Medicare & Medicaid Milestones, 1937 to 2015. Centers for Medicare and Medicaid Services, US Dept of Health & Human Services; 2015. Accessed April 17, 2020. https://www.cms.gov/About-CMS/Agency-Information/History/Downloads/Medicare-and-Medicaid-Milestones-1937-2015.pdf
4. Social Security Laws, 42 USC 1395x §1861 (1965). Accessed April 17, 2020. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm
5. Loewenstein R. Early effects of Medicare on the health care of the aged. Social Security Bulletin. April 1971; pp 3-20, 42. Accessed April 14, 2020. https://www.ssa.gov/policy/docs/ssb/v34n4/v34n4p3.pdf
6. Weiss AJ, Elixhauser A. Overview of Hospital Stays in the United States, 2012. Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb180-Hospitalizations-United-States-2012.pdf
7. Medicare Benefits Policy Manual, Internet-Only Manuals. Centers for Medicare & Medicaid Services. Pub. 100-02, Chapter 6, § 20.6. Updated April 5, 2012. Accessed April 17, 2020. http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs.html
8. Wright S. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Office of the Inspector General, US Dept of Health & Human Services; 2014. Accessed April 16, 2020. https://oig.hhs.gov/oei/reports/oei-02-12-00040.asp
9. Yancy CW. COVID-19 and African Americans. JAMA. Published online April 15, 2020. https://doi.org/10.1001/jama.2020.6548
10. Levinson DR. Vulnerabilities Remain Under Medicare’s 2-Midnight Hospital Policy. Office of the Inspector General, US Dept of Health & Human Services; 2016. Accessed April 18, 2020. https://oig.hhs.gov/oei/reports/oei-02-15-00020.pdf
11. Levinson DR. CMS Improperly Paid Millions of Dollars for Skilled Nursing Facility Services When the Medicare 3-Day Inpatient Hospital Stay Requirement Was Not Met. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 16, 2020. https://www.oig.hhs.gov/oas/reports/region5/51600043.pdf
12. Sheehy A, Shi F, Kind A. Identifying observation stays in Medicare data: policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
13. Solutions to Reduce Fraud, Waste, and Abuse in HHS Programs: OIG’s Top Recommendations. Office of the Inspector General, US Dept of Health & Human Services; 2019. Accessed April 18, 2020. https://oig.hhs.gov/reports-and-publications/compendium/files/compendium2019.pdf
14. Improving Access to Medicare Coverage Act of 2019, HR 1682, 116th Congress (2019). Accessed April 16, 2020. https://www.congress.gov/bill/116th-congress/house-bill/1682
15. Alexander v Azar, 396 F Supp 3d 242 (D CT 2019). Accessed May 26, 2020. https://casetext.com/case/alexander-v-azar-1?
16. Lipsitz L. The 3-night hospital stay and Medicare coverage for skilled nursing care. JAMA. 2013;310(14):1441-1442. https://doi.org/10.1001/jama.2013.254845
17. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trevedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Affairs (Millwood). 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054
© 2020 Society of Hospital Medicine
Overlap between Medicare’s Voluntary Bundled Payment and Accountable Care Organization Programs
Voluntary accountable care organizations (ACOs) and bundled payments have concurrently become cornerstone strategies in Medicare’s shift from volume-based fee-for-service toward value-based payment.
Physician practice and hospital participation in Medicare’s largest ACO model, the Medicare Shared Savings Program (MSSP),1 grew to include 561 organizations in 2018. Under MSSP, participants assume financial accountability for the global quality and costs of care for defined populations of Medicare fee-for-service patients. ACOs that manage to maintain or improve quality while achieving savings (ie, containing costs below a predefined population-wide spending benchmark) are eligible to receive a portion of the difference back from Medicare in the form of “shared savings”.
Similarly, hospital participation in Medicare’s bundled payment programs has grown over time. Most notably, more than 700 participants enrolled in the recently concluded Bundled Payments for Care Improvement (BPCI) initiative,2 Medicare’s largest bundled payment program over the past five years.3 Under BPCI, participants assumed financial accountability for the quality and costs of care for all Medicare patients triggering a qualifying “episode of care”. Participants that limit episode spending below a predefined benchmark without compromising quality were eligible for financial incentives.
As both ACOs and bundled payments grow in prominence and scale, they may increasingly overlap if patients attributed to ACOs receive care at bundled payment hospitals. Overlap could create synergies by increasing incentives to address shared processes (eg, discharge planning) or outcomes (eg, readmissions).4 An ACO focus on reducing hospital admissions could complement bundled payment efforts to increase hospital efficiency.
Conversely, Medicare’s approach to allocating savings and losses can penalize ACOs or bundled payment participants.3 For example, when a patient included in an MSSP ACO population receives episodic care at a hospital participating in BPCI, the historical costs of care for the hospital and the episode type, not the actual costs of care for that specific patient and his/her episode, are counted in the performance of the ACO. In other words, in these cases, the performance of the MSSP ACO is dependent on the historical spending at BPCI hospitals—despite it being out of ACO’s control and having little to do with the actual care its patients receive at BPCI hospitals—and MSSP ACOs cannot benefit from improvements over time. Therefore, MSSP ACOs may be functionally penalized if patients receive care at historically high-cost BPCI hospitals regardless of whether they have considerably improved the value of care delivered. As a corollary, Medicare rules involve a “claw back” stipulation in which savings are recouped from hospitals that participate in both BPCI and MSSP, effectively discouraging participation in both payment models.
Although these dynamics are complex, they highlight an intuitive point that has gained increasing awareness,5 ie, policymakers must understand the magnitude of overlap to evaluate the urgency in coordinating between the payment models. Our objective was to describe the extent of overlap and the characteristics of patients affected by it.
METHODS
We used 100% institutional Medicare claims, MSSP beneficiary attribution, and BPCI hospital data to identify fee-for-service beneficiaries attributed to MSSP and/or receiving care at BPCI hospitals for its 48 included episodes from the start of BPCI in 2013 quarter 4 through 2016 quarter 4.
We examined the trends in the number of episodes across the following three groups: MSSP-attributed patients hospitalized at BPCI hospitals for an episode included in BPCI (Overlap), MSSP-attributed patients hospitalized for that episode at non-BPCI hospitals (MSSP-only), and non-MSSP-attributed patients hospitalized at BPCI hospitals for a BPCI episode (BPCI-only). We used Medicare and United States Census Bureau data to compare groups with respect to sociodemographic (eg, age, sex, residence in a low-income area),6 clinical (eg, Elixhauser comorbidity index),7 and prior utilization (eg, skilled nursing facility discharge) characteristics.
Categorical and continuous variables were compared using logistic regression and one-way analysis of variance, respectively. Analyses were performed using Stata (StataCorp, College Station, Texas), version 15.0. Statistical tests were 2-tailed and significant at α = 0.05. This study was approved by the institutional review board at the University of Pennsylvania.
RESULTS
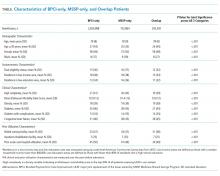
The number of MSSP ACOs increased from 220 in 2013 to 432 in 2016. The number of BPCI hospitals increased from 9 to 389 over this period, peaking at 413 hospitals in 2015. Over our study period, a total of 243,392, 2,824,898, and 702,864 episodes occurred in the Overlap, ACO-only, and BPCI-only groups, respectively (Table). Among episodes, patients in the Overlap group generally showed lower severity than those in other groups, although the differences were small. The BPCI-only, MSSP-only, and Overlap groups also exhibited small differences with respect to other characteristics such as the proportion of patients with Medicare/Medicaid dual-eligibility (15% of individual vs 16% and 12%, respectively) and prior use of skilled nursing facilities (33% vs 34% vs 31%, respectively) and acute care hospitals (45% vs 41% vs 39%, respectively) (P < .001 for all).
The overall overlap facing MSSP patients (overlap as a proportion of all MSSP patients) increased from 0.3% at the end of 2013 to 10% at the end of 2016, whereas over the same period, overlap facing bundled payment patients (overlap as a proportion of all bundled payment patients) increased from 11.9% to 27% (Appendix Figure). Overlap facing MSSP ACOs varied according to episode type, ranging from 3% for both acute myocardial infarction and chronic obstructive pulmonary disease episodes to 18% for automatic implantable cardiac defibrillator episodes at the end of 2016. Similarly, overlap facing bundled payment patients varied from 21% for spinal fusion episodes to 32% for lower extremity joint replacement and automatic implantable cardiac defibrillator episodes.
DISCUSSION
To our knowledge, this is the first study to describe the sizable and growing overlap facing ACOs with attributed patients who receive care at bundled payment hospitals, as well as bundled payment hospitals that treat patients attributed to ACOs.
The major implication of our findings is that policymakers must address and anticipate forthcoming payment model overlap as a key policy priority. Given the emphasis on ACOs and bundled payments as payment models—for example, Medicare continues to implement both nationwide via the Next Generation ACO model8 and the recently launched BPCI-Advanced program9—policymakers urgently need insights about the extent of payment model overlap. In that context, it is notable that although we have evaluated MSSP and BPCI as flagship programs, true overlap may actually be greater once other programs are considered.
Several factors may underlie the differences in the magnitude of overlap facing bundled payment versus ACO patients. The models differ in how they identify relevant patient populations, with patients falling under bundled payments via hospitalization for certain episode types but patients falling under ACOs via attribution based on the plurality of primary care services. Furthermore, BPCI participation lagged behind MSSP participation in time, while also occurring disproportionately in areas with existing MSSP ACOs.
Given these findings, understanding the implications of overlap should be a priority for future research and policy strategies. Potential policy considerations should include revising cost accounting processes so that when ACO-attributed patients receive episodic care at bundled payment hospitals, actual rather than historical hospital costs are counted toward ACO cost performance. To encourage hospitals to assume more accountability over outcomes—the ostensible overarching goal of value-based payment reform—Medicare could elect not to recoup savings from hospitals in both payment models. Although such changes require careful accounting to protect Medicare from financial losses as it forgoes some savings achieved through payment reforms, this may be worthwhile if hospital engagement in both models yields synergies.
Importantly, any policy changes made to address program overlap would need to accommodate ongoing changes in ACO, bundled payments, and other payment programs. For example, Medicare overhauled MSSP in December 2018. Compared to the earlier rules, in which ACOs could avoid downside financial risk altogether via “upside only” arrangements for up to six years, new MSSP rules require all participants to assume downside risk after several years of participation. Separately, forthcoming payment reforms such as direct contracting10 may draw clinicians and hospitals previously not participating in either Medicare fee-for-service or value-based payment models into payment reform. These factors may affect overlap in unpredictable ways (eg, they may increase the overlap by increasing the number of patients whose care is covered by different payment models or they may decrease overlap by raising the financial stakes of payment reforms to a degree that organizations drop out altogether).
This study has limitations. First, generalizability is limited by the fact that our analysis did not include bundled payment episodes assigned to physician group participants in BPCI or hospitals in mandatory joint replacement bundles under the Medicare Comprehensive Care for Joint Replacement model.11 Second, although this study provides the first description of overlap between ACO and bundled payment programs, it was descriptive in nature. Future research is needed to evaluate the impact of overlap on clinical, quality, and cost outcomes. This is particularly important because although we observed only small differences in patient characteristics among MSSP-only, BPCI-only, and Overlap groups, characteristics could change differentially over time. Payment reforms must be carefully monitored for potentially unintended consequences that could arise from differential changes in patient characteristics (eg, cherry-picking behavior that is disadvantageous to vulnerable individuals).
Nonetheless, this study underscores the importance and extent of overlap and the urgency to consider policy measures to coordinate between the payment models.
Acknowledgments
The authors thank research assistance from Sandra Vanderslice who did not receive any compensation for her work. This research was supported in part by The Commonwealth Fund. Rachel Werner was supported in part by K24-AG047908 from the NIA.
1. Centers for Medicare and Medicaid Services. Shared Savings Program. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/sharedsavingsprogram/index.html. Accessed July 22, 2019.
2. Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: General Information. https://innovation.cms.gov/initiatives/bundled-payments/. Accessed July 22, 2019.
3. Mechanic RE. When new Medicare payment systems collide. N Engl J Med. 2016;374(18):1706-1709. https://doi.org/10.1056/NEJMp1601464.
4. Ryan AM, Krinsky S, Adler-Milstein J, Damberg CL, Maurer KA, Hollingsworth JM. Association between hospitals’ engagement in value-based reforms and readmission reduction in the hospital readmission reduction program. JAMA Intern Med. 2017;177(6):863-868. https://doi.org/10.1001/jamainternmed.2017.0518.
5. Liao JM, Dykstra SE, Werner RM, Navathe AS. BPCI Advanced will further emphasize the need to address overlap between bundled payments and accountable care organizations. https://www.healthaffairs.org/do/10.1377/hblog20180409.159181/full/. Accessed May 14, 2019.
6. Census Bureau. United States Census Bureau. https://www.census.gov/. Accessed May 14, 2018.
7. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5.
8. Centers for Medicare and Medicaid Services. Next, Generation ACO Model. https://innovation.cms.gov/initiatives/next-generation-aco-model/. Accessed July 22, 2019.
9. Centers for Medicare and Medicaid Services. BPCI Advanced. https://innovation.cms.gov/initiatives/bpci-advanced. Accessed July 22, 2019.
10. Centers for Medicare and Medicaid Services. Direct Contracting. https://www.cms.gov/newsroom/fact-sheets/direct-contracting. Accessed July 22, 2019.
11. Centers for Medicare and Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed July 22, 2019.
Voluntary accountable care organizations (ACOs) and bundled payments have concurrently become cornerstone strategies in Medicare’s shift from volume-based fee-for-service toward value-based payment.
Physician practice and hospital participation in Medicare’s largest ACO model, the Medicare Shared Savings Program (MSSP),1 grew to include 561 organizations in 2018. Under MSSP, participants assume financial accountability for the global quality and costs of care for defined populations of Medicare fee-for-service patients. ACOs that manage to maintain or improve quality while achieving savings (ie, containing costs below a predefined population-wide spending benchmark) are eligible to receive a portion of the difference back from Medicare in the form of “shared savings”.
Similarly, hospital participation in Medicare’s bundled payment programs has grown over time. Most notably, more than 700 participants enrolled in the recently concluded Bundled Payments for Care Improvement (BPCI) initiative,2 Medicare’s largest bundled payment program over the past five years.3 Under BPCI, participants assumed financial accountability for the quality and costs of care for all Medicare patients triggering a qualifying “episode of care”. Participants that limit episode spending below a predefined benchmark without compromising quality were eligible for financial incentives.
As both ACOs and bundled payments grow in prominence and scale, they may increasingly overlap if patients attributed to ACOs receive care at bundled payment hospitals. Overlap could create synergies by increasing incentives to address shared processes (eg, discharge planning) or outcomes (eg, readmissions).4 An ACO focus on reducing hospital admissions could complement bundled payment efforts to increase hospital efficiency.
Conversely, Medicare’s approach to allocating savings and losses can penalize ACOs or bundled payment participants.3 For example, when a patient included in an MSSP ACO population receives episodic care at a hospital participating in BPCI, the historical costs of care for the hospital and the episode type, not the actual costs of care for that specific patient and his/her episode, are counted in the performance of the ACO. In other words, in these cases, the performance of the MSSP ACO is dependent on the historical spending at BPCI hospitals—despite it being out of ACO’s control and having little to do with the actual care its patients receive at BPCI hospitals—and MSSP ACOs cannot benefit from improvements over time. Therefore, MSSP ACOs may be functionally penalized if patients receive care at historically high-cost BPCI hospitals regardless of whether they have considerably improved the value of care delivered. As a corollary, Medicare rules involve a “claw back” stipulation in which savings are recouped from hospitals that participate in both BPCI and MSSP, effectively discouraging participation in both payment models.
Although these dynamics are complex, they highlight an intuitive point that has gained increasing awareness,5 ie, policymakers must understand the magnitude of overlap to evaluate the urgency in coordinating between the payment models. Our objective was to describe the extent of overlap and the characteristics of patients affected by it.
METHODS
We used 100% institutional Medicare claims, MSSP beneficiary attribution, and BPCI hospital data to identify fee-for-service beneficiaries attributed to MSSP and/or receiving care at BPCI hospitals for its 48 included episodes from the start of BPCI in 2013 quarter 4 through 2016 quarter 4.
We examined the trends in the number of episodes across the following three groups: MSSP-attributed patients hospitalized at BPCI hospitals for an episode included in BPCI (Overlap), MSSP-attributed patients hospitalized for that episode at non-BPCI hospitals (MSSP-only), and non-MSSP-attributed patients hospitalized at BPCI hospitals for a BPCI episode (BPCI-only). We used Medicare and United States Census Bureau data to compare groups with respect to sociodemographic (eg, age, sex, residence in a low-income area),6 clinical (eg, Elixhauser comorbidity index),7 and prior utilization (eg, skilled nursing facility discharge) characteristics.
Categorical and continuous variables were compared using logistic regression and one-way analysis of variance, respectively. Analyses were performed using Stata (StataCorp, College Station, Texas), version 15.0. Statistical tests were 2-tailed and significant at α = 0.05. This study was approved by the institutional review board at the University of Pennsylvania.
RESULTS
The number of MSSP ACOs increased from 220 in 2013 to 432 in 2016. The number of BPCI hospitals increased from 9 to 389 over this period, peaking at 413 hospitals in 2015. Over our study period, a total of 243,392, 2,824,898, and 702,864 episodes occurred in the Overlap, ACO-only, and BPCI-only groups, respectively (Table). Among episodes, patients in the Overlap group generally showed lower severity than those in other groups, although the differences were small. The BPCI-only, MSSP-only, and Overlap groups also exhibited small differences with respect to other characteristics such as the proportion of patients with Medicare/Medicaid dual-eligibility (15% of individual vs 16% and 12%, respectively) and prior use of skilled nursing facilities (33% vs 34% vs 31%, respectively) and acute care hospitals (45% vs 41% vs 39%, respectively) (P < .001 for all).
The overall overlap facing MSSP patients (overlap as a proportion of all MSSP patients) increased from 0.3% at the end of 2013 to 10% at the end of 2016, whereas over the same period, overlap facing bundled payment patients (overlap as a proportion of all bundled payment patients) increased from 11.9% to 27% (Appendix Figure). Overlap facing MSSP ACOs varied according to episode type, ranging from 3% for both acute myocardial infarction and chronic obstructive pulmonary disease episodes to 18% for automatic implantable cardiac defibrillator episodes at the end of 2016. Similarly, overlap facing bundled payment patients varied from 21% for spinal fusion episodes to 32% for lower extremity joint replacement and automatic implantable cardiac defibrillator episodes.
DISCUSSION
To our knowledge, this is the first study to describe the sizable and growing overlap facing ACOs with attributed patients who receive care at bundled payment hospitals, as well as bundled payment hospitals that treat patients attributed to ACOs.
The major implication of our findings is that policymakers must address and anticipate forthcoming payment model overlap as a key policy priority. Given the emphasis on ACOs and bundled payments as payment models—for example, Medicare continues to implement both nationwide via the Next Generation ACO model8 and the recently launched BPCI-Advanced program9—policymakers urgently need insights about the extent of payment model overlap. In that context, it is notable that although we have evaluated MSSP and BPCI as flagship programs, true overlap may actually be greater once other programs are considered.
Several factors may underlie the differences in the magnitude of overlap facing bundled payment versus ACO patients. The models differ in how they identify relevant patient populations, with patients falling under bundled payments via hospitalization for certain episode types but patients falling under ACOs via attribution based on the plurality of primary care services. Furthermore, BPCI participation lagged behind MSSP participation in time, while also occurring disproportionately in areas with existing MSSP ACOs.
Given these findings, understanding the implications of overlap should be a priority for future research and policy strategies. Potential policy considerations should include revising cost accounting processes so that when ACO-attributed patients receive episodic care at bundled payment hospitals, actual rather than historical hospital costs are counted toward ACO cost performance. To encourage hospitals to assume more accountability over outcomes—the ostensible overarching goal of value-based payment reform—Medicare could elect not to recoup savings from hospitals in both payment models. Although such changes require careful accounting to protect Medicare from financial losses as it forgoes some savings achieved through payment reforms, this may be worthwhile if hospital engagement in both models yields synergies.
Importantly, any policy changes made to address program overlap would need to accommodate ongoing changes in ACO, bundled payments, and other payment programs. For example, Medicare overhauled MSSP in December 2018. Compared to the earlier rules, in which ACOs could avoid downside financial risk altogether via “upside only” arrangements for up to six years, new MSSP rules require all participants to assume downside risk after several years of participation. Separately, forthcoming payment reforms such as direct contracting10 may draw clinicians and hospitals previously not participating in either Medicare fee-for-service or value-based payment models into payment reform. These factors may affect overlap in unpredictable ways (eg, they may increase the overlap by increasing the number of patients whose care is covered by different payment models or they may decrease overlap by raising the financial stakes of payment reforms to a degree that organizations drop out altogether).
This study has limitations. First, generalizability is limited by the fact that our analysis did not include bundled payment episodes assigned to physician group participants in BPCI or hospitals in mandatory joint replacement bundles under the Medicare Comprehensive Care for Joint Replacement model.11 Second, although this study provides the first description of overlap between ACO and bundled payment programs, it was descriptive in nature. Future research is needed to evaluate the impact of overlap on clinical, quality, and cost outcomes. This is particularly important because although we observed only small differences in patient characteristics among MSSP-only, BPCI-only, and Overlap groups, characteristics could change differentially over time. Payment reforms must be carefully monitored for potentially unintended consequences that could arise from differential changes in patient characteristics (eg, cherry-picking behavior that is disadvantageous to vulnerable individuals).
Nonetheless, this study underscores the importance and extent of overlap and the urgency to consider policy measures to coordinate between the payment models.
Acknowledgments
The authors thank research assistance from Sandra Vanderslice who did not receive any compensation for her work. This research was supported in part by The Commonwealth Fund. Rachel Werner was supported in part by K24-AG047908 from the NIA.
Voluntary accountable care organizations (ACOs) and bundled payments have concurrently become cornerstone strategies in Medicare’s shift from volume-based fee-for-service toward value-based payment.
Physician practice and hospital participation in Medicare’s largest ACO model, the Medicare Shared Savings Program (MSSP),1 grew to include 561 organizations in 2018. Under MSSP, participants assume financial accountability for the global quality and costs of care for defined populations of Medicare fee-for-service patients. ACOs that manage to maintain or improve quality while achieving savings (ie, containing costs below a predefined population-wide spending benchmark) are eligible to receive a portion of the difference back from Medicare in the form of “shared savings”.
Similarly, hospital participation in Medicare’s bundled payment programs has grown over time. Most notably, more than 700 participants enrolled in the recently concluded Bundled Payments for Care Improvement (BPCI) initiative,2 Medicare’s largest bundled payment program over the past five years.3 Under BPCI, participants assumed financial accountability for the quality and costs of care for all Medicare patients triggering a qualifying “episode of care”. Participants that limit episode spending below a predefined benchmark without compromising quality were eligible for financial incentives.
As both ACOs and bundled payments grow in prominence and scale, they may increasingly overlap if patients attributed to ACOs receive care at bundled payment hospitals. Overlap could create synergies by increasing incentives to address shared processes (eg, discharge planning) or outcomes (eg, readmissions).4 An ACO focus on reducing hospital admissions could complement bundled payment efforts to increase hospital efficiency.
Conversely, Medicare’s approach to allocating savings and losses can penalize ACOs or bundled payment participants.3 For example, when a patient included in an MSSP ACO population receives episodic care at a hospital participating in BPCI, the historical costs of care for the hospital and the episode type, not the actual costs of care for that specific patient and his/her episode, are counted in the performance of the ACO. In other words, in these cases, the performance of the MSSP ACO is dependent on the historical spending at BPCI hospitals—despite it being out of ACO’s control and having little to do with the actual care its patients receive at BPCI hospitals—and MSSP ACOs cannot benefit from improvements over time. Therefore, MSSP ACOs may be functionally penalized if patients receive care at historically high-cost BPCI hospitals regardless of whether they have considerably improved the value of care delivered. As a corollary, Medicare rules involve a “claw back” stipulation in which savings are recouped from hospitals that participate in both BPCI and MSSP, effectively discouraging participation in both payment models.
Although these dynamics are complex, they highlight an intuitive point that has gained increasing awareness,5 ie, policymakers must understand the magnitude of overlap to evaluate the urgency in coordinating between the payment models. Our objective was to describe the extent of overlap and the characteristics of patients affected by it.
METHODS
We used 100% institutional Medicare claims, MSSP beneficiary attribution, and BPCI hospital data to identify fee-for-service beneficiaries attributed to MSSP and/or receiving care at BPCI hospitals for its 48 included episodes from the start of BPCI in 2013 quarter 4 through 2016 quarter 4.
We examined the trends in the number of episodes across the following three groups: MSSP-attributed patients hospitalized at BPCI hospitals for an episode included in BPCI (Overlap), MSSP-attributed patients hospitalized for that episode at non-BPCI hospitals (MSSP-only), and non-MSSP-attributed patients hospitalized at BPCI hospitals for a BPCI episode (BPCI-only). We used Medicare and United States Census Bureau data to compare groups with respect to sociodemographic (eg, age, sex, residence in a low-income area),6 clinical (eg, Elixhauser comorbidity index),7 and prior utilization (eg, skilled nursing facility discharge) characteristics.
Categorical and continuous variables were compared using logistic regression and one-way analysis of variance, respectively. Analyses were performed using Stata (StataCorp, College Station, Texas), version 15.0. Statistical tests were 2-tailed and significant at α = 0.05. This study was approved by the institutional review board at the University of Pennsylvania.
RESULTS
The number of MSSP ACOs increased from 220 in 2013 to 432 in 2016. The number of BPCI hospitals increased from 9 to 389 over this period, peaking at 413 hospitals in 2015. Over our study period, a total of 243,392, 2,824,898, and 702,864 episodes occurred in the Overlap, ACO-only, and BPCI-only groups, respectively (Table). Among episodes, patients in the Overlap group generally showed lower severity than those in other groups, although the differences were small. The BPCI-only, MSSP-only, and Overlap groups also exhibited small differences with respect to other characteristics such as the proportion of patients with Medicare/Medicaid dual-eligibility (15% of individual vs 16% and 12%, respectively) and prior use of skilled nursing facilities (33% vs 34% vs 31%, respectively) and acute care hospitals (45% vs 41% vs 39%, respectively) (P < .001 for all).
The overall overlap facing MSSP patients (overlap as a proportion of all MSSP patients) increased from 0.3% at the end of 2013 to 10% at the end of 2016, whereas over the same period, overlap facing bundled payment patients (overlap as a proportion of all bundled payment patients) increased from 11.9% to 27% (Appendix Figure). Overlap facing MSSP ACOs varied according to episode type, ranging from 3% for both acute myocardial infarction and chronic obstructive pulmonary disease episodes to 18% for automatic implantable cardiac defibrillator episodes at the end of 2016. Similarly, overlap facing bundled payment patients varied from 21% for spinal fusion episodes to 32% for lower extremity joint replacement and automatic implantable cardiac defibrillator episodes.
DISCUSSION
To our knowledge, this is the first study to describe the sizable and growing overlap facing ACOs with attributed patients who receive care at bundled payment hospitals, as well as bundled payment hospitals that treat patients attributed to ACOs.
The major implication of our findings is that policymakers must address and anticipate forthcoming payment model overlap as a key policy priority. Given the emphasis on ACOs and bundled payments as payment models—for example, Medicare continues to implement both nationwide via the Next Generation ACO model8 and the recently launched BPCI-Advanced program9—policymakers urgently need insights about the extent of payment model overlap. In that context, it is notable that although we have evaluated MSSP and BPCI as flagship programs, true overlap may actually be greater once other programs are considered.
Several factors may underlie the differences in the magnitude of overlap facing bundled payment versus ACO patients. The models differ in how they identify relevant patient populations, with patients falling under bundled payments via hospitalization for certain episode types but patients falling under ACOs via attribution based on the plurality of primary care services. Furthermore, BPCI participation lagged behind MSSP participation in time, while also occurring disproportionately in areas with existing MSSP ACOs.
Given these findings, understanding the implications of overlap should be a priority for future research and policy strategies. Potential policy considerations should include revising cost accounting processes so that when ACO-attributed patients receive episodic care at bundled payment hospitals, actual rather than historical hospital costs are counted toward ACO cost performance. To encourage hospitals to assume more accountability over outcomes—the ostensible overarching goal of value-based payment reform—Medicare could elect not to recoup savings from hospitals in both payment models. Although such changes require careful accounting to protect Medicare from financial losses as it forgoes some savings achieved through payment reforms, this may be worthwhile if hospital engagement in both models yields synergies.
Importantly, any policy changes made to address program overlap would need to accommodate ongoing changes in ACO, bundled payments, and other payment programs. For example, Medicare overhauled MSSP in December 2018. Compared to the earlier rules, in which ACOs could avoid downside financial risk altogether via “upside only” arrangements for up to six years, new MSSP rules require all participants to assume downside risk after several years of participation. Separately, forthcoming payment reforms such as direct contracting10 may draw clinicians and hospitals previously not participating in either Medicare fee-for-service or value-based payment models into payment reform. These factors may affect overlap in unpredictable ways (eg, they may increase the overlap by increasing the number of patients whose care is covered by different payment models or they may decrease overlap by raising the financial stakes of payment reforms to a degree that organizations drop out altogether).
This study has limitations. First, generalizability is limited by the fact that our analysis did not include bundled payment episodes assigned to physician group participants in BPCI or hospitals in mandatory joint replacement bundles under the Medicare Comprehensive Care for Joint Replacement model.11 Second, although this study provides the first description of overlap between ACO and bundled payment programs, it was descriptive in nature. Future research is needed to evaluate the impact of overlap on clinical, quality, and cost outcomes. This is particularly important because although we observed only small differences in patient characteristics among MSSP-only, BPCI-only, and Overlap groups, characteristics could change differentially over time. Payment reforms must be carefully monitored for potentially unintended consequences that could arise from differential changes in patient characteristics (eg, cherry-picking behavior that is disadvantageous to vulnerable individuals).
Nonetheless, this study underscores the importance and extent of overlap and the urgency to consider policy measures to coordinate between the payment models.
Acknowledgments
The authors thank research assistance from Sandra Vanderslice who did not receive any compensation for her work. This research was supported in part by The Commonwealth Fund. Rachel Werner was supported in part by K24-AG047908 from the NIA.
1. Centers for Medicare and Medicaid Services. Shared Savings Program. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/sharedsavingsprogram/index.html. Accessed July 22, 2019.
2. Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: General Information. https://innovation.cms.gov/initiatives/bundled-payments/. Accessed July 22, 2019.
3. Mechanic RE. When new Medicare payment systems collide. N Engl J Med. 2016;374(18):1706-1709. https://doi.org/10.1056/NEJMp1601464.
4. Ryan AM, Krinsky S, Adler-Milstein J, Damberg CL, Maurer KA, Hollingsworth JM. Association between hospitals’ engagement in value-based reforms and readmission reduction in the hospital readmission reduction program. JAMA Intern Med. 2017;177(6):863-868. https://doi.org/10.1001/jamainternmed.2017.0518.
5. Liao JM, Dykstra SE, Werner RM, Navathe AS. BPCI Advanced will further emphasize the need to address overlap between bundled payments and accountable care organizations. https://www.healthaffairs.org/do/10.1377/hblog20180409.159181/full/. Accessed May 14, 2019.
6. Census Bureau. United States Census Bureau. https://www.census.gov/. Accessed May 14, 2018.
7. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5.
8. Centers for Medicare and Medicaid Services. Next, Generation ACO Model. https://innovation.cms.gov/initiatives/next-generation-aco-model/. Accessed July 22, 2019.
9. Centers for Medicare and Medicaid Services. BPCI Advanced. https://innovation.cms.gov/initiatives/bpci-advanced. Accessed July 22, 2019.
10. Centers for Medicare and Medicaid Services. Direct Contracting. https://www.cms.gov/newsroom/fact-sheets/direct-contracting. Accessed July 22, 2019.
11. Centers for Medicare and Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed July 22, 2019.
1. Centers for Medicare and Medicaid Services. Shared Savings Program. https://www.cms.gov/Medicare/Medicare-Fee-For-Service-Payment/sharedsavingsprogram/index.html. Accessed July 22, 2019.
2. Centers for Medicare and Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative: General Information. https://innovation.cms.gov/initiatives/bundled-payments/. Accessed July 22, 2019.
3. Mechanic RE. When new Medicare payment systems collide. N Engl J Med. 2016;374(18):1706-1709. https://doi.org/10.1056/NEJMp1601464.
4. Ryan AM, Krinsky S, Adler-Milstein J, Damberg CL, Maurer KA, Hollingsworth JM. Association between hospitals’ engagement in value-based reforms and readmission reduction in the hospital readmission reduction program. JAMA Intern Med. 2017;177(6):863-868. https://doi.org/10.1001/jamainternmed.2017.0518.
5. Liao JM, Dykstra SE, Werner RM, Navathe AS. BPCI Advanced will further emphasize the need to address overlap between bundled payments and accountable care organizations. https://www.healthaffairs.org/do/10.1377/hblog20180409.159181/full/. Accessed May 14, 2019.
6. Census Bureau. United States Census Bureau. https://www.census.gov/. Accessed May 14, 2018.
7. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. https://doi.org/10.1097/MLR.0b013e31819432e5.
8. Centers for Medicare and Medicaid Services. Next, Generation ACO Model. https://innovation.cms.gov/initiatives/next-generation-aco-model/. Accessed July 22, 2019.
9. Centers for Medicare and Medicaid Services. BPCI Advanced. https://innovation.cms.gov/initiatives/bpci-advanced. Accessed July 22, 2019.
10. Centers for Medicare and Medicaid Services. Direct Contracting. https://www.cms.gov/newsroom/fact-sheets/direct-contracting. Accessed July 22, 2019.
11. Centers for Medicare and Medicaid Services. Comprehensive Care for Joint Replacement Model. https://innovation.cms.gov/initiatives/CJR. Accessed July 22, 2019.
© 2019 Society of Hospital Medicine
Policy in Clinical Practice: Medicare Advantage and Observation Hospitalizations
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
CLINICAL SCENARIO
A 73-year-old man presents to the emergency department with sepsis secondary to community-acquired pneumonia. The patient requires supplemental oxygen and is started on intravenous antibiotics. His admitting physician expects he will need more than two nights of hospital care and suggests that inpatient status, rather than outpatient (observation) status, would be appropriate under Medicare’s “Two-Midnight Rule.” The physician also suspects the patient may need a brief stay in a skilled nursing facility (SNF) following the mentioned hospitalization and notes that the patient has a Medicare Advantage plan (Table) and wonders if the Two-Midnight Rule applies. Further, she questions whether Medicare’s “Three-Midnight Rule” for SNF benefits will factor in the patient’s discharge planning.
BACKGROUND AND HISTORY
Since the 1970s, the Centers for Medicare & Medicaid Services (CMS) has allowed enrollees to receive their Medicare benefits from privately managed health plans through the so-called Medicare Advantage programs. CMS contracts with commercial insurers who, in exchange for a set payment per Medicare enrollee, “accept full responsibility (ie, risk) for the costs of their enrollees’ care.”1 Over the past 20 years the percent of Medicare Advantage enrollees has nearly doubled nationwide, from 18% to 34%, and is projected to grow even further to 42% by 2028.2,3 The reasons beneficiaries choose to enroll in Medicare Advantage over Traditional Medicare have yet to be thoroughly studied; ease of enrollment and plan administration, as well as lower deductibles, copays, and out-of-pocket maximums for in-network services, are thought to be some of the driving factors.
The federal government has asserted two goals for the development of Medicare Advantage: beneficiary choice and economic efficiency.1 Medicare Advantage plans must be actuarially equal to Traditional Medicare but do not have to cover services in precisely the same way. Medicare Advantage plans may achieve cost savings through narrower networks, strict control of access to SNF services and acute care inpatient rehabilitation, and prior authorization requirements, the latter of which has received recent congressional attention.4,5 On the other hand, many Medicare Advantage plans offer dental, fitness, optical, and caregiver benefits that are not included under Traditional Medicare. Beneficiaries can theoretically compare the coverage and costs of Traditional Medicare to Medicare Advantage programs and make informed choices based on their individualized needs. The second stated goal for the Medicare Advantage option assumes that privately managed plans provide care at lower costs compared with CMS; this assumption has yet to be confirmed with solid data. Indeed, a recent analysis comparing the overall costs of Medicare Advantage to those of Traditional Medicare concluded that Medicare Advantage costs CMS more than Traditional Medicare,6 perhaps in part due to risk adjustment practices.7
POLICY IN PRACTICE
There are a number of areas of uncertainty regarding the specifics of how Medicare Advantage plans work, including Medicare Advantage programs’ use of outpatient (observation) stays. CMS has tried to provide guidance to healthcare organizations and clinicians regarding the appropriate use of inpatient hospitalizations for patients with Traditional Medicare, including the implementation of the Two-Midnight Rule in 2013. According to the rule, clinicians should place inpatient admission orders when they reasonably expect a patient’s care to extend across two midnights.8 Such admission decisions are subject to review by Medicare contractors and Quality Improvement Organizations.
In contrast, Medicare Advantage plans which enter into contracts with specific healthcare systems are not required to abide by CMS’ guidelines for the Two-Midnight Rule.9 When Medicare Advantage firms negotiate contracts with individual hospitals and healthcare organizations, CMS has been clear that such contracts are not required to include the Two-Midnight Rule when it comes to making hospitalization status decisions.10 Instead, in these instances, Medicare Advantage plans often use proprietary decision tools containing clinical criteria, such as Milliman Care Guidelines or InterQual, and/or their own plan’s internal criteria as part of the decision-making process to grant inpatient or outpatient (observation) status. More importantly, CMS has stated that for hospitals and healthcare systems that do not contract with Medicare Advantage programs, the Two-Midnight Rule should apply when it comes to making hospitalization status decisions.10
Implications for Patients
Currently, there are no data available to compare between Medicare Advantage enrollees and traditional medicine beneficiaries in terms of the frequency of observation use and out-of-pocket cost for observation stays. As alluded to in the patient’s case, the use of outpatient (observation) status has implications for a patient’s posthospitalization SNF benefit. Under Traditional Medicare, patients must be hospitalized for three consecutive inpatient midnights in order to qualify for the SNF benefit. Time spent under outpatient (observation) status does not count toward this three-day requirement. Interestingly, some Medicare Advantage programs have demonstrated innovation in this area, waiving the three inpatient midnight requirement for their beneficiaries;11 there is evidence, however, that compared with their Traditional Medicare counterparts, Medicare Advantage beneficiaries are admitted to lower quality SNFs.12 The posthospitalization consequences of an inpatient versus outpatient (observation) status determination for a Medicare Advantage beneficiary is thus unclear, further complicating the decision-making process for patients when it comes to choosing a Medicare policy, and for providers when it comes to choosing an admission status.
Implications for Clinicians and Healthcare Systems
After performing an initial history and physical exam, if a healthcare provider determines that a patient requires hospitalization, an order is placed to classify the stay as inpatient or outpatient (observation). For beneficiaries with Traditional Medicare or a Medicare Advantage plan that has not contracted with the hospital, clinicians should follow the Two-Midnight Rule for making this determination. For contracted Medicare Advantage, the rules are variable. Under Medicare’s Conditions of Participation, hospitals and healthcare organizations are required to have utilization management (UM) programs to assist physicians in making appropriate admission decisions. UM reviews can happen at any point during or after a patient’s stay, however, and physicians may have to make decisions using their best judgment at the time of admission without real-time input from UM teams.
Outpatient (observation) care and the challenges surrounding appropriate status orders have complicated the admission decision. In one study of 2014 Traditional Medicare claims, almost half of outpatient (observation) stays contained a status change.13 Based on a recent survey of hospitalist physicians, about two-thirds of hospitalists report at least monthly requests from patients to change their status.14 Hospital medicine physicians report that these requests “can severely damage the therapeutic bond”14 between provider and patient because the provider must assign status based on CMS rules, not patient request.
COMMENTARY AND RECOMMENDATIONS
CMS could improve the current system in one of two ways. First, CMS could require that all Medicare Advantage plans follow the same polices as Traditional Medicare policies regarding the Two- and Three-Midnight Rules. This would eliminate the need for both hospitals and healthcare organizations to dedicate time and resources to negotiating with each Medicare Advantage program and to managing each Medicare Advantage patient admission based on a specific contract. Ideally, CMS could completely eliminate its outpatient (observation) policy so that all hospitalizations are treated exactly the same, classified under the same billing status and with beneficiaries having the same postacute benefit. This would be consistent with the sentiment behind the recent Office of Inspector General’s (OIG) report suggesting that CMS consider counting outpatient midnights toward the three-midnight requirement for postacute SNF care “so that beneficiaries receiving similar hospital care have similar access to these services.”15
WHAT SHOULD I TELL MY PATIENT?
The physician in the example above should tell their patient that they will be admitted as an inpatient given her expectation that the patient will need hospitalization for oxygen support, parenteral antibiotics, and evaluation by physical therapy to determine a medically appropriate discharge plan. The physician should document the medical necessity for the admission, specifically her expectation that the patient will require at least two midnights of medically necessary hospital care. If the patient has Traditional Medicare, this documentation, along with the inpatient status order, will fulfill the requirements for an inpatient stay. If the patient has a Medicare Advantage plan, the physician can advise the patient that the plan administrators will ultimately determine if an inpatient stay will be covered or denied.
CONCLUSIONS
In the proposed clinical scenario, the rules determining the patient’s hospitalization status depend on whether the hospital contracts with the patient’s Medicare Advantage plan, and if so, what the contracted criteria are in determining inpatient and outpatient (observation) status. The physician could consider real-time input from the hospital’s UM team, if available. Regardless of UM input, if the physician hospitalizes the patient as an inpatient, the Medicare Advantage plan administrators will make a determination regarding the appropriateness of the admission status, as well as whether the patient qualifies for posthospitalization Medicare SNF benefits (if requested) and, additionally, which SNFs will be covered. If denied, the hospitalist will have the option of a peer-to-peer discussion with the insurance company to overturn the denial. Given the confusion, complexity, and implications presented by this admission status decision-making process, standardization across Traditional Medicare and Medicare Advantage plans, or a budget-neutral plan to eliminate status distinction altogether, is certainly warranted.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
1.McGuire TG, Newhouse JP, Sinaiko AD. An economic history of Medicare Part C. Millbank Q. 2011;89(2):289-332. https://doi.org/10.1111/j.1468-0009.2011.00629.x.
2. Medicare Advantage. Available at: https://www.kff.org/medicare/fact-sheet/medicare-advantage/.
3. Neuman P, Jacobson G. Medicare Advantage checkup. N Engl J Med. 2018;379(22):2163-2172. https://doi.org/10.1056/NEJMhpr1804089.
4. HR 3107: improving seniors’ timely access to Care Act of 2019. Available at: https://www.congress.gov/bill/116th-congress/house-bill/3107/text?q=%7B%22search%22%3A%5B%22prior+authorization%22%5D%7D&r=1&s=1.
5. Gadbois EA, Tyler DA, Shield RR, et al. Medicare Advantage control of postacute costs: perspective from stakeholders. Am J Manag Care. 2018;24(12):e386-e392.
6. Rooke-Ley H, Broome T, Mostashari F, Cavanaugh S. Evaluating Medicare programs against saving taxpayer dollars. Health Affairs, August 16, 2019. Available at: https://www.healthaffairs.org/do/10.1377/hblog20190813.223707/full/.
7. Office of Inspector General. Billions in estimated Medicare Advantage payments from chart reviews raise concerns. December 2019. Available at: https://oig.hhs.gov/oei/reports/oei-03-17-00470.pdf. Accessed December 15, 2019.
8. Fact sheet: two-midnight rule. Available at: https://www.cms.gov/newsroom/fact-sheets/fact-sheet-two-midnight-rule-0.
9. Locke C, Hu E. Medicare’s two-midnight rule: what hospitalists must know. Available at: https://www.the-hospitalist.org/hospitalist/article/194971/medicares-two-midnight-rule.
10. Announcement of calendar year (CY) 2019 Medicare Advantage capitation rates and Medicare Advantage and part D payment policies and final call letter. Page 206. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Announcement2019.pdf. Accessed November 18, 2019.
11. Grebla R, Keohane L, Lee Y, Lipsitz L, Rahman M, Trivedi A. Waiving the three-day rule: admissions and length-of-stay at hospitals and skilled nursing facilities did not increase. Health Aff. 2015;34(8):1324-1330. https://doi.org/10.1377/hlthaff.2015.0054.
12. Meyers D, Mor V, Rahman M. Medicare Advantage enrollees more likely to enter lower-quality nursing homes compared to fee-for-service enrollees. Health Aff. 2018;37(1):78-85. https://doi.org/10.1377/hlthaff.2017.0714.
13. Sheehy A, Shi F, Kind AJH. Identifying observation stays in Medicare data: Policy implications of a definition. J Hosp Med. 2019;14(2):96-100. https://doi.org/10.12788/jhm.3038
14. The hospital observation care problem: perspectives and solutions from the Society of Hospital Medicine. Available at: https://www.hospitalmedicine.org/globalassets/policy-and-advocacy/advocacy-pdf/shms-observation-white-paper-2017. Accessed November 18, 2019.
15. U.S. Department of Health & Human Services, Office of Inspector General. Solutions to reduce fraud, waste and abuse in HHS programs: OIG’s top recommendations. Available at: https://oig.hhs.gov/reports-and-publications/compendium/. Accessed November 22, 2019.
© 2020 Society of Hospital Medicine
Next Steps in Improving Healthcare Value: Postacute Care Transitions: Developing a Skilled Nursing Facility Collaborative within an Academic Health System
Hospitals and health systems are under mounting financial pressure to shorten hospitalizations and reduce readmissions. These priorities have led to an ever-increasing focus on postacute care (PAC), and more specifically on improving transitions from the hospital.1,2 According to a 2013 Institute of Medicine report, PAC is the source of 73% of the variation in Medicare spending3 and readmissions during the postacute episode nearly double the average Medicare payment.4 Within the PAC landscape, discharges to skilled nursing facilities (SNFs) have received particular focus due to the high rates of readmission and associated care costs.5
Hospitals, hospital physicians, PAC providers, and payers need to improve SNF transitions in care. Hospitals are increasingly responsible for patient care beyond their walls through several mechanisms including rehospitalization penalties, value-based reimbursement strategies (eg, bundled payments), and risk-based contracting on the total cost of care through relationships with accountable care organizations (ACOs) and Medicare Advantage plans. Similarly, hospital-employed physicians and PAC providers are more engaged in achieving value-based goals through increased alignment of provider compensation models6,7 with risk-based contracting.
Current evidence suggests that rehospitalizations could be reduced by focusing on a concentrated referral network of preferred high-quality SNFs;8,9 however, less is known about how to develop and operate such linkages at the administrative or clinical levels.8 In this article, we propose a collaborative framework for the establishment of a preferred PAC network.
SKILLED NURSING FACILITY PREFERRED PROVIDER NETWORK
One mechanism employed to improve transitions to SNFs and reduce associated readmissions is to create a preferred provider network. Increasing the concentration of hospital discharges to higher performing facilities is associated with lower rehospitalization rates, particularly during the critical days following discharge.10
While the criteria applied for preferred provider networks vary, there are several emerging themes.10 Quality metrics are often applied, generally starting with Centers for Medicare and Medicaid Services (CMS) quality star ratings and Long-Term Care Minimum Data Set (MDS) metrics with additional criteria frequently layered upon those. Some examples include the extent of physician coverage,11 the extent of nursing coverage (eg, nursing ratios or 24/7 nursing care), geographic access, and flexible admission times (including weekends and nights).12 In addition, several outcome measures may be used such as 30-day readmission rates, patient/family satisfaction ratings, ED visits, primary care follow-up within seven days of PAC discharge, or impact on the total cost of care.
Beyond the specified criteria, some hospitals choose to build upon existing relationships when developing their preferred network. By selecting historically high-volume facilities, they are able to leverage the existing name recognition amongst patients and providers.13 This minimizes retraining of discharge planners, maintains institutional relationships, and aligns with the patients’ geographic preferences.2,13 While the high volume SNFs may not have the highest quality ratings, some hospitals find they can leverage the value of preferred partner status to push behavior change and improve performance.13
PROPOSED HEALTH SYSTEM FRAMEWORK FOR CREATING A SKILLED NURSING FACILITY COLLABORATIVE
Here we propose a framework for the establishment of a preferred provider network for a hospital or health system based on the early experience of establishing an SNF Collaborative within Johns Hopkins Medicine (JHM). JHM is a large integrated health care system, which includes five hospitals within the region, including two large academic hospitals and three community hospitals serving patients in Maryland and the District of Columbia.14
JHM identified a need for improved coordination with PAC providers and saw opportunities to build upon successful individual hospital efforts to create a system-level approach with a PAC partnership sharing the goals of improving care and reducing costs. Additional opportunities exist given the unique Maryland all-payer Global Budget Revenue system managed by the Health Services Cost Review Commission. This system imposes hospital-level penalties for readmissions or poor quality measure performance and is moving to a new phase that will place hospitals directly at risk for the total Part A and Part B Medicare expenditures for a cohort of attributed Medicare patients, inclusive of their PAC expenses. This state-wide program is one example of a shift in payment structures from volume to value that is occurring throughout the healthcare sector.
Developing a formal collaboration inclusive of the five local hospitals, Johns Hopkins HealthCare (JHHC)—the managed care division of JHM—and the JHM ACO (Johns Hopkins Medicine Alliance for Patients, JMAP), we established a JHM SNF Collaborative. This group was tasked with improving the continuum of care for our patients discharged to PAC facilities. Given the number and diversity of entities involved, we sought to draw on efforts already managed and piloted locally, while disseminating best practices and providing added services at the collaborative level. We propose a collaborative multistakeholder model (Figure) that we anticipate will be adaptable to other health systems.
At the outset, we established a Steering Committee and a broad Stakeholder Group (Figure). The Steering Committee is comprised of representatives from all participating JHM entities and serves as the collaborative governing body. This group initially identified 36 local SNF partners including a mixture of larger corporate chains and freestanding entities. In an effort to respect patient choice and acknowledge geographic preferences and capacity limitations, partner selection was based on a combination of publically available quality metrics, historic referral volumes, and recommendations of each JHM hospital. While we sought to align with high-performing SNFs, we also saw an opportunity to leverage collaboration to drive improvement in lower-performing facilities that continue to receive a high volume of referrals. The Stakeholder Group includes a broader representation from JHM, including subject matter experts from related medical specialties (eg, Physical Medicine and Rehabilitation, Internal Medicine, Emergency Medicine, and various surgical subspecialties); partner SNFs, and the local CMS-funded Quality Improvement Organization (QIO). Physician leadership was essential at all levels of the collaborative governing structure including the core Coordinating Team (Figure). Providers representing different hospitals were able to speak about variations in practice patterns and to assess the feasibility of suggested solutions on existing workflows.
After establishing the governance framework for the collaborative, it was determined that dedicated workgroups were needed to drive protocol-based initiatives, data, and analytics. For the former, we selected transitions of care as our initial focus area. All affiliated hospitals were working to address care transitions, but there were opportunities to develop a harmonized approach leveraging individual hospital input. The workgroup included representation from medical and administrative hospital leadership, JHHC, JMAP, our home care group, and SNF medical leadership. Initial priorities identified are reviewed in the Table. We anticipate new priorities for the collaborative over time and intend for the workgroup to evolve in line with shifting priorities.
We similarly established a multidisciplinary data and analytics workgroup to identify resources to develop the SNF, and a system-level dashboard to track our ongoing work. While incorporating data from five hospitals with varied patient populations, we felt that the risk-adjusted PAC data were critical to the collaborative establishment and goal setting. After exploring internal and external resources, we initially elected to engage an outside vendor offering risk-adjusted performance metrics. We have subsequently worked with the state health information exchange, CRISP,15 to develop a robust dashboard for Medicare fee-for-service beneficiaries that could provide similar data.
IMPLEMENTATION
In the process of establishing the SNF Collaborative at JHM, there were a number of early challenges faced and lessons learned:
- In a large integrated delivery system, there is a need to balance the benefits of central coordination with the support for ongoing local efforts to promote partner engagement at the hospital and SNF level. The forums created within the collaborative governance structure can facilitate sharing of the prior health system, hospital or SNF initiatives to grow upon successes and avoid prior pitfalls.
- Early identification of risk-adjusted PAC data sources is central to the collaborative establishment and goal setting. This requires assessment of internal analytic resources, budget, and desired timeline for implementation to determine the optimal arrangement. Similarly, identification of available data sources to drive the analytic efforts is essential and should include a health information exchange, claims, and MDS among others.
- Partnering with local QIOs provides support for facility-level quality improvement efforts. They have the staff and onsite expertise to facilitate process implementation within individual SNFs.
- Larger preferred provider networks require considerable administrative support to facilitate communication with the entities, coordinate completion of network agreements, and manage the dissemination of SNF- and hospital-specific performance data.
- Legal and contractual support related to data sharing and HIPAA compliance is needed due to the complexity of the health system and SNF legal structure. Multiple JHM legal entities were involved in this collaborative as were a mixture of freestanding SNFs and corporate chains. There was a significant effort required to execute both data-sharing agreements as well as charters to enable QIO participation.
- Physician leadership and insight are key to implementing meaningful and broad change. When devising system-wide solutions, incorporation and respect for local processes and needs are paramount for provider engagement and behavior change. This process will likely identify gaps in understanding the PAC patient’s experience and needs. It may also reveal practice variability and foster opportunities for provider education on the needs of PAC teams and how to best facilitate quality transitions.
CONCLUSION
We proposed a framework for establishing a collaborative partnership with a preferred network of SNF providers. Depending on organizational readiness, significant upfront investment of time and resources could be needed to establish a coordinated network of SNF providers. However, once established, such networks can be leveraged to support ongoing process improvement efforts within a hospital or delivery system and can be used strategically by such health systems as they implement value-based health strategies. Furthermore, the lessons learned from transitions to SNFs can be applied more broadly in the PAC landscape including transitions to home from both the hospital and SNF.
Acknowledgments
The authors wish to acknowledge all the members and participants in the Johns Hopkins Medicine Skilled Nursing Facility Collaborative and the executive sponsors and JHM hospital presidents for their support of this work.
Disclosures
Michele Bellantoni receives intramural salary support for being the medical director of the JHM SNF Collaborative. Damien Doyle is a part-time geriatrician at the Hebrew Home of Greater Washington, a skilled nursing facility. He received travel expense support for GAPNA, a local Advanced Practice Nurse Association meeting.The authors otherwise have no potential conflicts of interest to disclose.
Funding
The authors state that there were no external sponsors for this work.
1. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. doi:10.1016/j.jamda.2015.11.005. PubMed
2. Mchugh JP, Zinn J, Shield RR, et al. Strategy and risk sharing in hospital–post-acute care integration. Health Care Manage Rev. 2018:1. doi:10.1097/hmr.0000000000000204. PubMed
3. Institute of Medicine. Variation in Health Care Spending Assessing Geographic Variation.; 2013. http://nationalacademies.org/hmd/~/media/Files/Report Files/2013/Geographic-Variation2/geovariation_rb.pdf. Accessed January 4, 2018.
4. Dobson A, DaVanzo JE, Heath S, et al. Medicare Payment Bundling: Insights from Claims Data and Policy Implications Analyses of Episode-Based Payment. Washington, DC; 2012. http://www.aha.org/content/12/ahaaamcbundlingreport.pdf. Accessed January 4, 2018.
5. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. doi:10.1377/hlthaff.2009.0629. PubMed
6. Torchiana DF, Colton DG, Rao SK, Lenz SK, Meyer GS, Ferris TG. Massachusetts general physicians organization’s quality incentive program produces encouraging results. Health Aff. 2013;32(10):1748-1756. doi:10.1377/hlthaff.2013.0377. PubMed
7. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboembolism prophylaxis. J Hosp Med. 2014;10(3):172-178. doi:10.1002/jhm.2303. PubMed
8. Rahman M, Foster AD, Grabowski DC, Zinn JS, Mor V. Effect of hospital-SNF referral linkages on rehospitalization. Health Serv Res. 2013;48(6pt1):1898-1919. doi:10.1111/1475-6773.12112. PubMed
9. Huckfeldt PJ, Weissblum L, Escarce JJ, Karaca-Mandic P, Sood N. Do skilled nursing facilities selected to participate in preferred provider networks have higher quality and lower costs? Health Serv Res. 2018. doi:10.1111/1475-6773.13027. PubMed
10. American Hospital Association. The role of post-acute care in new care delivery models. TrendWatch. http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Published 2015. Accessed December 19, 2017.
11. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. doi:10.1111/jgs.13351. PubMed
12. Ouslander JG, Bonner A, Herndon L, Shutes J. The Interventions to Reduce Acute Care Transfers (INTERACT) quality improvement program: an overview for medical directors and primary care clinicians in long-term care. J Am Med Dir Assoc. 2014;15(3):162-170. doi:10.1016/j.jamda.2013.12.005. PubMed
13. McHugh JP, Foster A, Mor V, et al. Reducing hospital readmissions through preferred networks of skilled nursing facilities. Health Aff. 2017;36(9):1591-1598. doi:10.1377/hlthaff.2017.0211. PubMed
14. Fast Facts: Johns Hopkins Medicine. https://www.hopkinsmedicine.org/about/downloads/JHM-Fast-Facts.pdf. Accessed October 18, 2018.
15. CRISP – Chesapeake Regional Information System for our Patients. https://www.crisphealth.org/. Accessed October 17, 2018.
Hospitals and health systems are under mounting financial pressure to shorten hospitalizations and reduce readmissions. These priorities have led to an ever-increasing focus on postacute care (PAC), and more specifically on improving transitions from the hospital.1,2 According to a 2013 Institute of Medicine report, PAC is the source of 73% of the variation in Medicare spending3 and readmissions during the postacute episode nearly double the average Medicare payment.4 Within the PAC landscape, discharges to skilled nursing facilities (SNFs) have received particular focus due to the high rates of readmission and associated care costs.5
Hospitals, hospital physicians, PAC providers, and payers need to improve SNF transitions in care. Hospitals are increasingly responsible for patient care beyond their walls through several mechanisms including rehospitalization penalties, value-based reimbursement strategies (eg, bundled payments), and risk-based contracting on the total cost of care through relationships with accountable care organizations (ACOs) and Medicare Advantage plans. Similarly, hospital-employed physicians and PAC providers are more engaged in achieving value-based goals through increased alignment of provider compensation models6,7 with risk-based contracting.
Current evidence suggests that rehospitalizations could be reduced by focusing on a concentrated referral network of preferred high-quality SNFs;8,9 however, less is known about how to develop and operate such linkages at the administrative or clinical levels.8 In this article, we propose a collaborative framework for the establishment of a preferred PAC network.
SKILLED NURSING FACILITY PREFERRED PROVIDER NETWORK
One mechanism employed to improve transitions to SNFs and reduce associated readmissions is to create a preferred provider network. Increasing the concentration of hospital discharges to higher performing facilities is associated with lower rehospitalization rates, particularly during the critical days following discharge.10
While the criteria applied for preferred provider networks vary, there are several emerging themes.10 Quality metrics are often applied, generally starting with Centers for Medicare and Medicaid Services (CMS) quality star ratings and Long-Term Care Minimum Data Set (MDS) metrics with additional criteria frequently layered upon those. Some examples include the extent of physician coverage,11 the extent of nursing coverage (eg, nursing ratios or 24/7 nursing care), geographic access, and flexible admission times (including weekends and nights).12 In addition, several outcome measures may be used such as 30-day readmission rates, patient/family satisfaction ratings, ED visits, primary care follow-up within seven days of PAC discharge, or impact on the total cost of care.
Beyond the specified criteria, some hospitals choose to build upon existing relationships when developing their preferred network. By selecting historically high-volume facilities, they are able to leverage the existing name recognition amongst patients and providers.13 This minimizes retraining of discharge planners, maintains institutional relationships, and aligns with the patients’ geographic preferences.2,13 While the high volume SNFs may not have the highest quality ratings, some hospitals find they can leverage the value of preferred partner status to push behavior change and improve performance.13
PROPOSED HEALTH SYSTEM FRAMEWORK FOR CREATING A SKILLED NURSING FACILITY COLLABORATIVE
Here we propose a framework for the establishment of a preferred provider network for a hospital or health system based on the early experience of establishing an SNF Collaborative within Johns Hopkins Medicine (JHM). JHM is a large integrated health care system, which includes five hospitals within the region, including two large academic hospitals and three community hospitals serving patients in Maryland and the District of Columbia.14
JHM identified a need for improved coordination with PAC providers and saw opportunities to build upon successful individual hospital efforts to create a system-level approach with a PAC partnership sharing the goals of improving care and reducing costs. Additional opportunities exist given the unique Maryland all-payer Global Budget Revenue system managed by the Health Services Cost Review Commission. This system imposes hospital-level penalties for readmissions or poor quality measure performance and is moving to a new phase that will place hospitals directly at risk for the total Part A and Part B Medicare expenditures for a cohort of attributed Medicare patients, inclusive of their PAC expenses. This state-wide program is one example of a shift in payment structures from volume to value that is occurring throughout the healthcare sector.
Developing a formal collaboration inclusive of the five local hospitals, Johns Hopkins HealthCare (JHHC)—the managed care division of JHM—and the JHM ACO (Johns Hopkins Medicine Alliance for Patients, JMAP), we established a JHM SNF Collaborative. This group was tasked with improving the continuum of care for our patients discharged to PAC facilities. Given the number and diversity of entities involved, we sought to draw on efforts already managed and piloted locally, while disseminating best practices and providing added services at the collaborative level. We propose a collaborative multistakeholder model (Figure) that we anticipate will be adaptable to other health systems.
At the outset, we established a Steering Committee and a broad Stakeholder Group (Figure). The Steering Committee is comprised of representatives from all participating JHM entities and serves as the collaborative governing body. This group initially identified 36 local SNF partners including a mixture of larger corporate chains and freestanding entities. In an effort to respect patient choice and acknowledge geographic preferences and capacity limitations, partner selection was based on a combination of publically available quality metrics, historic referral volumes, and recommendations of each JHM hospital. While we sought to align with high-performing SNFs, we also saw an opportunity to leverage collaboration to drive improvement in lower-performing facilities that continue to receive a high volume of referrals. The Stakeholder Group includes a broader representation from JHM, including subject matter experts from related medical specialties (eg, Physical Medicine and Rehabilitation, Internal Medicine, Emergency Medicine, and various surgical subspecialties); partner SNFs, and the local CMS-funded Quality Improvement Organization (QIO). Physician leadership was essential at all levels of the collaborative governing structure including the core Coordinating Team (Figure). Providers representing different hospitals were able to speak about variations in practice patterns and to assess the feasibility of suggested solutions on existing workflows.
After establishing the governance framework for the collaborative, it was determined that dedicated workgroups were needed to drive protocol-based initiatives, data, and analytics. For the former, we selected transitions of care as our initial focus area. All affiliated hospitals were working to address care transitions, but there were opportunities to develop a harmonized approach leveraging individual hospital input. The workgroup included representation from medical and administrative hospital leadership, JHHC, JMAP, our home care group, and SNF medical leadership. Initial priorities identified are reviewed in the Table. We anticipate new priorities for the collaborative over time and intend for the workgroup to evolve in line with shifting priorities.
We similarly established a multidisciplinary data and analytics workgroup to identify resources to develop the SNF, and a system-level dashboard to track our ongoing work. While incorporating data from five hospitals with varied patient populations, we felt that the risk-adjusted PAC data were critical to the collaborative establishment and goal setting. After exploring internal and external resources, we initially elected to engage an outside vendor offering risk-adjusted performance metrics. We have subsequently worked with the state health information exchange, CRISP,15 to develop a robust dashboard for Medicare fee-for-service beneficiaries that could provide similar data.
IMPLEMENTATION
In the process of establishing the SNF Collaborative at JHM, there were a number of early challenges faced and lessons learned:
- In a large integrated delivery system, there is a need to balance the benefits of central coordination with the support for ongoing local efforts to promote partner engagement at the hospital and SNF level. The forums created within the collaborative governance structure can facilitate sharing of the prior health system, hospital or SNF initiatives to grow upon successes and avoid prior pitfalls.
- Early identification of risk-adjusted PAC data sources is central to the collaborative establishment and goal setting. This requires assessment of internal analytic resources, budget, and desired timeline for implementation to determine the optimal arrangement. Similarly, identification of available data sources to drive the analytic efforts is essential and should include a health information exchange, claims, and MDS among others.
- Partnering with local QIOs provides support for facility-level quality improvement efforts. They have the staff and onsite expertise to facilitate process implementation within individual SNFs.
- Larger preferred provider networks require considerable administrative support to facilitate communication with the entities, coordinate completion of network agreements, and manage the dissemination of SNF- and hospital-specific performance data.
- Legal and contractual support related to data sharing and HIPAA compliance is needed due to the complexity of the health system and SNF legal structure. Multiple JHM legal entities were involved in this collaborative as were a mixture of freestanding SNFs and corporate chains. There was a significant effort required to execute both data-sharing agreements as well as charters to enable QIO participation.
- Physician leadership and insight are key to implementing meaningful and broad change. When devising system-wide solutions, incorporation and respect for local processes and needs are paramount for provider engagement and behavior change. This process will likely identify gaps in understanding the PAC patient’s experience and needs. It may also reveal practice variability and foster opportunities for provider education on the needs of PAC teams and how to best facilitate quality transitions.
CONCLUSION
We proposed a framework for establishing a collaborative partnership with a preferred network of SNF providers. Depending on organizational readiness, significant upfront investment of time and resources could be needed to establish a coordinated network of SNF providers. However, once established, such networks can be leveraged to support ongoing process improvement efforts within a hospital or delivery system and can be used strategically by such health systems as they implement value-based health strategies. Furthermore, the lessons learned from transitions to SNFs can be applied more broadly in the PAC landscape including transitions to home from both the hospital and SNF.
Acknowledgments
The authors wish to acknowledge all the members and participants in the Johns Hopkins Medicine Skilled Nursing Facility Collaborative and the executive sponsors and JHM hospital presidents for their support of this work.
Disclosures
Michele Bellantoni receives intramural salary support for being the medical director of the JHM SNF Collaborative. Damien Doyle is a part-time geriatrician at the Hebrew Home of Greater Washington, a skilled nursing facility. He received travel expense support for GAPNA, a local Advanced Practice Nurse Association meeting.The authors otherwise have no potential conflicts of interest to disclose.
Funding
The authors state that there were no external sponsors for this work.
Hospitals and health systems are under mounting financial pressure to shorten hospitalizations and reduce readmissions. These priorities have led to an ever-increasing focus on postacute care (PAC), and more specifically on improving transitions from the hospital.1,2 According to a 2013 Institute of Medicine report, PAC is the source of 73% of the variation in Medicare spending3 and readmissions during the postacute episode nearly double the average Medicare payment.4 Within the PAC landscape, discharges to skilled nursing facilities (SNFs) have received particular focus due to the high rates of readmission and associated care costs.5
Hospitals, hospital physicians, PAC providers, and payers need to improve SNF transitions in care. Hospitals are increasingly responsible for patient care beyond their walls through several mechanisms including rehospitalization penalties, value-based reimbursement strategies (eg, bundled payments), and risk-based contracting on the total cost of care through relationships with accountable care organizations (ACOs) and Medicare Advantage plans. Similarly, hospital-employed physicians and PAC providers are more engaged in achieving value-based goals through increased alignment of provider compensation models6,7 with risk-based contracting.
Current evidence suggests that rehospitalizations could be reduced by focusing on a concentrated referral network of preferred high-quality SNFs;8,9 however, less is known about how to develop and operate such linkages at the administrative or clinical levels.8 In this article, we propose a collaborative framework for the establishment of a preferred PAC network.
SKILLED NURSING FACILITY PREFERRED PROVIDER NETWORK
One mechanism employed to improve transitions to SNFs and reduce associated readmissions is to create a preferred provider network. Increasing the concentration of hospital discharges to higher performing facilities is associated with lower rehospitalization rates, particularly during the critical days following discharge.10
While the criteria applied for preferred provider networks vary, there are several emerging themes.10 Quality metrics are often applied, generally starting with Centers for Medicare and Medicaid Services (CMS) quality star ratings and Long-Term Care Minimum Data Set (MDS) metrics with additional criteria frequently layered upon those. Some examples include the extent of physician coverage,11 the extent of nursing coverage (eg, nursing ratios or 24/7 nursing care), geographic access, and flexible admission times (including weekends and nights).12 In addition, several outcome measures may be used such as 30-day readmission rates, patient/family satisfaction ratings, ED visits, primary care follow-up within seven days of PAC discharge, or impact on the total cost of care.
Beyond the specified criteria, some hospitals choose to build upon existing relationships when developing their preferred network. By selecting historically high-volume facilities, they are able to leverage the existing name recognition amongst patients and providers.13 This minimizes retraining of discharge planners, maintains institutional relationships, and aligns with the patients’ geographic preferences.2,13 While the high volume SNFs may not have the highest quality ratings, some hospitals find they can leverage the value of preferred partner status to push behavior change and improve performance.13
PROPOSED HEALTH SYSTEM FRAMEWORK FOR CREATING A SKILLED NURSING FACILITY COLLABORATIVE
Here we propose a framework for the establishment of a preferred provider network for a hospital or health system based on the early experience of establishing an SNF Collaborative within Johns Hopkins Medicine (JHM). JHM is a large integrated health care system, which includes five hospitals within the region, including two large academic hospitals and three community hospitals serving patients in Maryland and the District of Columbia.14
JHM identified a need for improved coordination with PAC providers and saw opportunities to build upon successful individual hospital efforts to create a system-level approach with a PAC partnership sharing the goals of improving care and reducing costs. Additional opportunities exist given the unique Maryland all-payer Global Budget Revenue system managed by the Health Services Cost Review Commission. This system imposes hospital-level penalties for readmissions or poor quality measure performance and is moving to a new phase that will place hospitals directly at risk for the total Part A and Part B Medicare expenditures for a cohort of attributed Medicare patients, inclusive of their PAC expenses. This state-wide program is one example of a shift in payment structures from volume to value that is occurring throughout the healthcare sector.
Developing a formal collaboration inclusive of the five local hospitals, Johns Hopkins HealthCare (JHHC)—the managed care division of JHM—and the JHM ACO (Johns Hopkins Medicine Alliance for Patients, JMAP), we established a JHM SNF Collaborative. This group was tasked with improving the continuum of care for our patients discharged to PAC facilities. Given the number and diversity of entities involved, we sought to draw on efforts already managed and piloted locally, while disseminating best practices and providing added services at the collaborative level. We propose a collaborative multistakeholder model (Figure) that we anticipate will be adaptable to other health systems.
At the outset, we established a Steering Committee and a broad Stakeholder Group (Figure). The Steering Committee is comprised of representatives from all participating JHM entities and serves as the collaborative governing body. This group initially identified 36 local SNF partners including a mixture of larger corporate chains and freestanding entities. In an effort to respect patient choice and acknowledge geographic preferences and capacity limitations, partner selection was based on a combination of publically available quality metrics, historic referral volumes, and recommendations of each JHM hospital. While we sought to align with high-performing SNFs, we also saw an opportunity to leverage collaboration to drive improvement in lower-performing facilities that continue to receive a high volume of referrals. The Stakeholder Group includes a broader representation from JHM, including subject matter experts from related medical specialties (eg, Physical Medicine and Rehabilitation, Internal Medicine, Emergency Medicine, and various surgical subspecialties); partner SNFs, and the local CMS-funded Quality Improvement Organization (QIO). Physician leadership was essential at all levels of the collaborative governing structure including the core Coordinating Team (Figure). Providers representing different hospitals were able to speak about variations in practice patterns and to assess the feasibility of suggested solutions on existing workflows.
After establishing the governance framework for the collaborative, it was determined that dedicated workgroups were needed to drive protocol-based initiatives, data, and analytics. For the former, we selected transitions of care as our initial focus area. All affiliated hospitals were working to address care transitions, but there were opportunities to develop a harmonized approach leveraging individual hospital input. The workgroup included representation from medical and administrative hospital leadership, JHHC, JMAP, our home care group, and SNF medical leadership. Initial priorities identified are reviewed in the Table. We anticipate new priorities for the collaborative over time and intend for the workgroup to evolve in line with shifting priorities.
We similarly established a multidisciplinary data and analytics workgroup to identify resources to develop the SNF, and a system-level dashboard to track our ongoing work. While incorporating data from five hospitals with varied patient populations, we felt that the risk-adjusted PAC data were critical to the collaborative establishment and goal setting. After exploring internal and external resources, we initially elected to engage an outside vendor offering risk-adjusted performance metrics. We have subsequently worked with the state health information exchange, CRISP,15 to develop a robust dashboard for Medicare fee-for-service beneficiaries that could provide similar data.
IMPLEMENTATION
In the process of establishing the SNF Collaborative at JHM, there were a number of early challenges faced and lessons learned:
- In a large integrated delivery system, there is a need to balance the benefits of central coordination with the support for ongoing local efforts to promote partner engagement at the hospital and SNF level. The forums created within the collaborative governance structure can facilitate sharing of the prior health system, hospital or SNF initiatives to grow upon successes and avoid prior pitfalls.
- Early identification of risk-adjusted PAC data sources is central to the collaborative establishment and goal setting. This requires assessment of internal analytic resources, budget, and desired timeline for implementation to determine the optimal arrangement. Similarly, identification of available data sources to drive the analytic efforts is essential and should include a health information exchange, claims, and MDS among others.
- Partnering with local QIOs provides support for facility-level quality improvement efforts. They have the staff and onsite expertise to facilitate process implementation within individual SNFs.
- Larger preferred provider networks require considerable administrative support to facilitate communication with the entities, coordinate completion of network agreements, and manage the dissemination of SNF- and hospital-specific performance data.
- Legal and contractual support related to data sharing and HIPAA compliance is needed due to the complexity of the health system and SNF legal structure. Multiple JHM legal entities were involved in this collaborative as were a mixture of freestanding SNFs and corporate chains. There was a significant effort required to execute both data-sharing agreements as well as charters to enable QIO participation.
- Physician leadership and insight are key to implementing meaningful and broad change. When devising system-wide solutions, incorporation and respect for local processes and needs are paramount for provider engagement and behavior change. This process will likely identify gaps in understanding the PAC patient’s experience and needs. It may also reveal practice variability and foster opportunities for provider education on the needs of PAC teams and how to best facilitate quality transitions.
CONCLUSION
We proposed a framework for establishing a collaborative partnership with a preferred network of SNF providers. Depending on organizational readiness, significant upfront investment of time and resources could be needed to establish a coordinated network of SNF providers. However, once established, such networks can be leveraged to support ongoing process improvement efforts within a hospital or delivery system and can be used strategically by such health systems as they implement value-based health strategies. Furthermore, the lessons learned from transitions to SNFs can be applied more broadly in the PAC landscape including transitions to home from both the hospital and SNF.
Acknowledgments
The authors wish to acknowledge all the members and participants in the Johns Hopkins Medicine Skilled Nursing Facility Collaborative and the executive sponsors and JHM hospital presidents for their support of this work.
Disclosures
Michele Bellantoni receives intramural salary support for being the medical director of the JHM SNF Collaborative. Damien Doyle is a part-time geriatrician at the Hebrew Home of Greater Washington, a skilled nursing facility. He received travel expense support for GAPNA, a local Advanced Practice Nurse Association meeting.The authors otherwise have no potential conflicts of interest to disclose.
Funding
The authors state that there were no external sponsors for this work.
1. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. doi:10.1016/j.jamda.2015.11.005. PubMed
2. Mchugh JP, Zinn J, Shield RR, et al. Strategy and risk sharing in hospital–post-acute care integration. Health Care Manage Rev. 2018:1. doi:10.1097/hmr.0000000000000204. PubMed
3. Institute of Medicine. Variation in Health Care Spending Assessing Geographic Variation.; 2013. http://nationalacademies.org/hmd/~/media/Files/Report Files/2013/Geographic-Variation2/geovariation_rb.pdf. Accessed January 4, 2018.
4. Dobson A, DaVanzo JE, Heath S, et al. Medicare Payment Bundling: Insights from Claims Data and Policy Implications Analyses of Episode-Based Payment. Washington, DC; 2012. http://www.aha.org/content/12/ahaaamcbundlingreport.pdf. Accessed January 4, 2018.
5. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. doi:10.1377/hlthaff.2009.0629. PubMed
6. Torchiana DF, Colton DG, Rao SK, Lenz SK, Meyer GS, Ferris TG. Massachusetts general physicians organization’s quality incentive program produces encouraging results. Health Aff. 2013;32(10):1748-1756. doi:10.1377/hlthaff.2013.0377. PubMed
7. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboembolism prophylaxis. J Hosp Med. 2014;10(3):172-178. doi:10.1002/jhm.2303. PubMed
8. Rahman M, Foster AD, Grabowski DC, Zinn JS, Mor V. Effect of hospital-SNF referral linkages on rehospitalization. Health Serv Res. 2013;48(6pt1):1898-1919. doi:10.1111/1475-6773.12112. PubMed
9. Huckfeldt PJ, Weissblum L, Escarce JJ, Karaca-Mandic P, Sood N. Do skilled nursing facilities selected to participate in preferred provider networks have higher quality and lower costs? Health Serv Res. 2018. doi:10.1111/1475-6773.13027. PubMed
10. American Hospital Association. The role of post-acute care in new care delivery models. TrendWatch. http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Published 2015. Accessed December 19, 2017.
11. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. doi:10.1111/jgs.13351. PubMed
12. Ouslander JG, Bonner A, Herndon L, Shutes J. The Interventions to Reduce Acute Care Transfers (INTERACT) quality improvement program: an overview for medical directors and primary care clinicians in long-term care. J Am Med Dir Assoc. 2014;15(3):162-170. doi:10.1016/j.jamda.2013.12.005. PubMed
13. McHugh JP, Foster A, Mor V, et al. Reducing hospital readmissions through preferred networks of skilled nursing facilities. Health Aff. 2017;36(9):1591-1598. doi:10.1377/hlthaff.2017.0211. PubMed
14. Fast Facts: Johns Hopkins Medicine. https://www.hopkinsmedicine.org/about/downloads/JHM-Fast-Facts.pdf. Accessed October 18, 2018.
15. CRISP – Chesapeake Regional Information System for our Patients. https://www.crisphealth.org/. Accessed October 17, 2018.
1. Burke RE, Whitfield EA, Hittle D, et al. Hospital readmission from post-acute care facilities: risk factors, timing, and outcomes. J Am Med Dir Assoc. 2016;17(3):249-255. doi:10.1016/j.jamda.2015.11.005. PubMed
2. Mchugh JP, Zinn J, Shield RR, et al. Strategy and risk sharing in hospital–post-acute care integration. Health Care Manage Rev. 2018:1. doi:10.1097/hmr.0000000000000204. PubMed
3. Institute of Medicine. Variation in Health Care Spending Assessing Geographic Variation.; 2013. http://nationalacademies.org/hmd/~/media/Files/Report Files/2013/Geographic-Variation2/geovariation_rb.pdf. Accessed January 4, 2018.
4. Dobson A, DaVanzo JE, Heath S, et al. Medicare Payment Bundling: Insights from Claims Data and Policy Implications Analyses of Episode-Based Payment. Washington, DC; 2012. http://www.aha.org/content/12/ahaaamcbundlingreport.pdf. Accessed January 4, 2018.
5. Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff. 2010;29(1):57-64. doi:10.1377/hlthaff.2009.0629. PubMed
6. Torchiana DF, Colton DG, Rao SK, Lenz SK, Meyer GS, Ferris TG. Massachusetts general physicians organization’s quality incentive program produces encouraging results. Health Aff. 2013;32(10):1748-1756. doi:10.1377/hlthaff.2013.0377. PubMed
7. Michtalik HJ, Carolan HT, Haut ER, et al. Use of provider-level dashboards and pay-for-performance in venous thromboembolism prophylaxis. J Hosp Med. 2014;10(3):172-178. doi:10.1002/jhm.2303. PubMed
8. Rahman M, Foster AD, Grabowski DC, Zinn JS, Mor V. Effect of hospital-SNF referral linkages on rehospitalization. Health Serv Res. 2013;48(6pt1):1898-1919. doi:10.1111/1475-6773.12112. PubMed
9. Huckfeldt PJ, Weissblum L, Escarce JJ, Karaca-Mandic P, Sood N. Do skilled nursing facilities selected to participate in preferred provider networks have higher quality and lower costs? Health Serv Res. 2018. doi:10.1111/1475-6773.13027. PubMed
10. American Hospital Association. The role of post-acute care in new care delivery models. TrendWatch. http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Published 2015. Accessed December 19, 2017.
11. Lage DE, Rusinak D, Carr D, Grabowski DC, Ackerly DC. Creating a network of high-quality skilled nursing facilities: preliminary data on the postacute care quality improvement experiences of an accountable care organization. J Am Geriatr Soc. 2015;63(4):804-808. doi:10.1111/jgs.13351. PubMed
12. Ouslander JG, Bonner A, Herndon L, Shutes J. The Interventions to Reduce Acute Care Transfers (INTERACT) quality improvement program: an overview for medical directors and primary care clinicians in long-term care. J Am Med Dir Assoc. 2014;15(3):162-170. doi:10.1016/j.jamda.2013.12.005. PubMed
13. McHugh JP, Foster A, Mor V, et al. Reducing hospital readmissions through preferred networks of skilled nursing facilities. Health Aff. 2017;36(9):1591-1598. doi:10.1377/hlthaff.2017.0211. PubMed
14. Fast Facts: Johns Hopkins Medicine. https://www.hopkinsmedicine.org/about/downloads/JHM-Fast-Facts.pdf. Accessed October 18, 2018.
15. CRISP – Chesapeake Regional Information System for our Patients. https://www.crisphealth.org/. Accessed October 17, 2018.
© 2019 Society of Hospital Medicine
AHRQ Evidence-based Practice Center Program--Applying the Knowledge to Practice to Data Cycle to Strengthen the Value of Patient Care
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
Research evidence is critical for strengthening the value, quality, and safety of patient care. Learning healthcare systems (LHS) can support the delivery of evidence-based healthcare by establishing organizational processes that support three activities (Figure).1-3
- Knowledge: Identifying and synthesizing evidence to address clinical challenges
- Practice: Applying knowledge in the process of care delivery
- Data: Assessing performance and creating a feedback cycle for learning and improvement
The systematic implementation of evidence into practice continues to be a challenge for many healthcare organizations4-7 due to limited resources, expertise, and culture.5,8-12 Missing opportunities for translating knowledge into practice not only results in low-value care (ie, waste) but also in harm.1
The AHRQ (Agency for Healthcare Research and Quality) Evidence-based Practice Center (EPC) Program was established in 1997, with the goal of synthesizing research to inform evidence-based healthcare. The national impact of this program has been significant. Since the American Recovery and Reinvestment Act of 2009, EPC program reports have been used to inform over 95 clinical practice guidelines from societies such as the American College of Physicians, 16 health coverage decisions from payers such as the Centers for Medicare & Medicaid Services, and 24 government policies and program planning efforts, such as the National Institutes of Health Pathways to Prevention Program.13
The EPC program recognizes that evidence awareness is not sufficient to change practice and improve clinical outcomes. As such, the EPC program also embarked on initiatives to facilitate the translation of evidence into clinical practice and to measure and monitor how changes in practice impact health outcomes. AHRQ has historically worked with professional organizations to translate systematic reviews into clinical practice guidelines as well as federal agencies to inform payer decisions and program planning. Recently, the EPC program has increased collaborative efforts with hospitals and healthcare systems to understand how they use evidence and to partner with them to identify methods to improve the uptake of evidence into practice.9,12
In this perspective, we describe the AHRQ EPC Program’s work to address the three phases of the LHS cycle (knowledge, practice, and data) to support high-value care, using the topic of preventing and treating Clostridium difficile colitis as a relevant example to the hospital medicine field (Figure 2). By sharing this work, we hope it can serve as a model to illustrate how partnerships between organizations and AHRQ can lead to improvements in healthcare.
USING THE LEARNING HEALTHCARE SYSTEM CYCLE TO STRUCTURE AHRQ EPC WORK
Knowledge: Identifying and Synthesizing Evidence to Address Clinical Challenges
Systematic reviews use carefully formulated questions to summarize the literature results using specific and established methods.14 Given that individual studies can have disparate results, it is critical to summarize and synthesize findings across studies, so we know what the overall evidence suggests, and whether we can be confident in the findings. To date, the EPC program has developed more than 500 evidence synthesis reports. An example relevant to the field of hospital medicine is the 2016 review that examined the effects of interventions to prevent and treat Clostridium difficile colitis in adults.15
The review examined the best available evidence, including data from randomized controlled trials and observational studies, on diagnosing, preventing, and treating Clostridium difficile colitis. Major findings included the following: vancomycin is more effective than metronidazole for treating the first occurrence of Clostridium difficile colitis (high-strength evidence), fecal transplantation may have a significant benefit in the treatment of recurrent Clostridium difficile colitis (low-strength evidence), and institutional preventive interventions such as antibiotic stewardship practices, transmission interruption through terminal room cleaning, and handwashing campaigns reduce the incidence of Clostridium difficile colitis (low-strength evidence). The report results provided the most recent review of the evidence and were particularly important as they suggested a need for significant practice changes in the treatment of Clostridium difficile colitis based on the new evidence available. Previous to this report, the 2010 guidelines from the Infectious Diseases Society of America (IDSA) recommended metronidazole over vancomycin for the treatment of the first occurrence of Clostridium difficile colitis.16 Subsequently, the newly released 2018 IDSA guideline provides recommendations consistent with the findings in this AHRQ report.17
Practice: Applying Knowledge in the Process of Care Delivery
AHRQ recognizes there are many interim steps between having the results from a systematic review and changing practice and improving care. In 2017, the EPC program began piloting approaches to make it easier for healthcare systems and hospitals to use its reports to improve the delivery of patient care and clinical outcomes. A pilot project conducted by the ECRI Institute - Penn Medicine EPC evaluated the feasibility of using an existing clinical pathway development and dissemination framework18 to translate findings from the 2016 AHRQ EPC report on Clostridium difficile colitis into a pathway for Clostridium difficile colitis treatment in the acute care setting.
To develop a Clostridium difficile colitis treatment pathway, the ECRI-Penn EPC team recruited a representative stakeholder group from Penn Medicine to review the EPC report as well as existing society guidelines. The clinical pathway was subsequently developed and approved by the stakeholders and disseminated through the Penn Medicine cloud-based pathways repository beginning on April 16, 2018.19 Most recently, the pathway became available in the electronic health record (EHR; 2018 Epic Systems Corporation) to facilitate provider review during care. Specifically, hyperlinks to the pathway are embedded within the ordering screens for those antibiotics used to treat Clostridium difficile colitis (ie, oral and rectal vancomycin, fidaxomicin, and metronidazole). Upon clicking the link in the ordering screen, the pathway launches a floating internet explorer window. The pathway is now publicly available on the AHRQ’s Clinical Decision Support (CDS) Connect Project (https://cds.ahrq.gov/), which is a resource to share pathway artifacts for other healthcare systems to use.
Data: Assessing Performance and Creating a Feedback Cycle for Learning and Improvement
The last step in the LHS cycle is to identify the impact of interventions on practice change and clinical outcomes, to understand how local results compare to peer institutions, and to inform future research and knowledge.
For the ECRI Institute-Penn Medicine EPC pilot project, both qualitative and quantitative outcomes were assessed. The initial qualitative analysis focused on the feasibility of using the AHRQ report in an existing pathway development and dissemination framework.18 It was found that clinical stakeholders identified the EPC report as trustworthy and more current than the society guidelines available at the time of development, particularly regarding the finding that vancomycin was more effective than metronidazole for the first occurrence of Clostridium difficile colitis. Additional qualitative analysis will be conducted to understand provider satisfaction with the pathway and practice impact. The quantitative analysis focused on pathway use (clicks over time) and found that as of September 16, 2018, the pathway had been viewed by providers 403 times. Future analysis will evaluate the impact of the pathway on the use of oral vancomycin for the first occurrences of Clostridium difficile colitis.
Patient registries can also help clinicians and healthcare systems to complete the feedback cycle and evaluate outcomes. Patient registries collect data from clinical and other sources in a standardized way in order to evaluate specific outcomes for various populations.20 AHRQ has created a registry handbook, including best practices for how to create, operate, and evaluate registries.20 This handbook enables the development of high-quality registries with data that can be leveraged for both research and improvement.
In the example of the ECRI Institute-Penn Medicine EPC pilot project, one way that a learning healthcare system, such as Penn Medicine, might measure the impact of the clinical pathway is to develop a quality improvement registry, which might be developed with information from their electronic health record, to examine the impact on the use of vancomycin for first occurrences of Clostridium difficile colitis. This information could help drive improvement in the implementation of the clinical pathway.
Registries can also be used as a source for research data. The NIH-funded American Gastroenterological Association (AGA) Fecal Microbiota Transplantation National Registry is an example of a research registry that collects data on outcomes and adverse events associated with fecal transplants to fill gaps in existing research. The 2016 AHRQ EPC review found low-strength evidence on fecal transplant for treatment of recurrent Clostridium difficile colitis. When designing the protocol for this registry, the researchers used the AHRQ handbook to inform the design. Given that this is a research registry, it can be used by researchers to examine trends and outcomes of fecal transplant to treat Clostridium difficile colitis. Publications that use the registry as its source of data may be used in future systematic reviews, thus completing the cycle of learning.
ADDITIONAL RESOURCES
The EPC program recognizes that gaps remain in the evidence to practice translation process and that more support is needed. Some upcoming activities of the AHRQ EPC Program to address these gaps and make its evidence reports more actionable for healthcare systems include:
- Projects to Disseminate EPC Reports into Clinical Practice. In addition to the ECRI Institute - Penn Medicine EPC pilot dissemination project, other pilot projects are aimed at helping systems apply evidence to practice and include new ways to visualize evidence to make it more actionable and usable; creating other dissemination products, such as evidence summaries and presentations for decision makers; and other implementation tools, such as decision aids. These products and summary reports are available on the AHRQ Effective Health Care Program website at https://effectivehealthcare.ahrq.gov/topics/health-systems-use-evidence/overview.
- Healthcare Systems Stakeholder Panel. Starting in Fall 2018, the AHRQ EPC Program will be convening a panel of healthcare system leaders to help make its reports and products more useful and responsive to the needs of healthcare systems and promote the use of evidence in clinical practice.
- Rapid Evidence Products. AHRQ understands that healthcare systems need information rapidly and cannot wait a year or more for a traditional systematic review to be completed. Therefore, AHRQ is applying its methods work on rapid reviews21-24 to pilot new report types that systematically identify and summarize the evidence quickly for healthcare systems and quality improvement efforts.25
- Data Integration. Originally launched in 2012, the Systematic Review Data Repository (SRDR) is an AHRQ-supported online open-access repository of abstracted data from individual studies from systematic reviews. The goal is to enable more efficient updates of systematic reviews through data reuse. An updated version of the SRDR is scheduled to launch in 2020. With the new version, future sharing of summary data from systematic reviews digitally in a computable and portable format may allow integration into CDS tools and clinical practice guideline development and dissemination, facilitating the use of evidence in clinical practice.
CONCLUSIONS
The AHRQ EPC program supports initiatives to make evidence more actionable and provide resources and tools throughout all the phases of the learning healthcare system cycle. This case study on C. difficile is one example of how the EPC program is helping hospitals and healthcare systems improve clinical care delivery and its derivative value.
Disclosures
Dr. Umscheid reports grants from AHRQ, during the conduct of the study; serves on the Advisory Board of DynaMed, and founded and directed a hospital-based evidence-based practice center. All other authors have nothing to disclose.
Disclaimer
The findings and conclusions in this document are those of the author(s), who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
1. Committee on the Learning Health Care System in A, Institute of M. In: Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington (DC): National Academies Press (US); 2013. PubMed
2. Agency for Healthcare Research and Quality. Learning Health Systems. 2017; https://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. Accessed September 26, 2018.
3. Umscheid CA, Brennan PJ. Incentivizing “structures” over “outcomes” to bridge the knowing-doing gap. JAMA Intern Med. 2015;175(3):354-355. doi: 10.1001/jamainternmed.2014.5293. PubMed
4. Brownson RC, Colditz GA, Proctor EK. Dissemination and Implementation Research in Health: Translating Science to Practice. New York: Oxford University Press; 2012.
5. Marquez C, Johnson AM, Jassemi S, et al. Enhancing the uptake of systematic reviews of effects: what is the best format for health care managers and policy-makers? A mixed-methods study. Implement Sci. 2018;13(1):84. doi: 10.1186/s13012-018-0779-9. PubMed
6. Villa L, Warholak TL, Hines LE, et al. Health care decision makers’ use of comparative effectiveness research: report from a series of focus groups. J Manag Care Pharm. 2013;19(9):745-754. doi: 10.18553/jmcp.2013.19.9.745. PubMed
7. Guise JM, Savitz LA, Friedman CP. Mind the gap: putting evidence into practice in the era of learning health systems. J Gen Intern Med. 2018;33(12): 2237-2239. doi: 10.1007/s11606-018-4633-1. PubMed
8. Ako-Arrey DE, Brouwers MC, Lavis JN, Giacomini MK. Health systems guidance appraisal--a critical interpretive synthesis. Implement Sci. 2016;11(1):9. doi:10.1186/s13012-016-0373-y. PubMed
9. White CM, Butler M, Wang Z, et al. Understanding Health-Systems’ Use of and Need for Evidence To Inform Decisionmaking. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
10. Murthy L, Shepperd S, Clarke MJ, et al. Interventions to improve the use of systematic reviews in decision-making by health system managers, policy makers, and clinicians. Cochrane Database Syst Rev. 2012(9):Cd009401. doi: 10.1002/14651858.CD009401.pub2. PubMed
11. Bornstein S, Baker R, Navarro P, Mackey S, Speed D, Sullivan M. Putting research in place: an innovative approach to providing contextualized evidence synthesis for decision makers. Syst Rev. 2017;6(1):218. doi: 10.1186/s13643-017-0606-4. PubMed
12. Schoelles K, Umscheid CA, Lin JS, et al. A Framework for Conceptualizing Evidence Needs of Health Systems. Rockville, MD: Agency for Healthcare Research and Quality; 2017. PubMed
13. Chang S, Chang C, Borsky A. Putting the Evidence into Decision Making. Prevention Policy Matters Blog 2018; https://health.gov/news/blog/2018/04/putting-the-evidence-into-decision-making/. Accessed September 28, 2018.
14. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. In: Eden J, Levit L, Berg A, Morton S, eds. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. https://www.nihlibrary.nih.gov/sites/default/files/Finding_What_Works_in_Health_Care_Standards_for_Systematic_Reviews_IOM_2011.pdf. Accessed January 17, 2019.
15. Butler M, Olson A, Drekonja D, et al. AHRQ comparative effectiveness reviews. In: Early Diagnosis, Prevention, and Treatment of Clostridium difficile: Update. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. https://effectivehealthcare.ahrq.gov/topics/c-difficile-update/research. Accessed January 17, 2019.
16. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. doi: 10.1086/651706. PubMed
17. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7): e1-e48. doi: 10.1093/cid/cix1085. PubMed
18. Flores EJ, Mull NK, Lavenberg JG, et al. Utilizing a 10-step framework to support the implementation of an evidence-based clinical pathways. BMJ Qual Saf. 2018:bmjqs-2018. doi: 10.1136/bmjqs-2018-008454. PubMed
19. Flores E, Jue JJ, Girardi G, Schoelles K, Umscheid CA. Use of a Clinical Pathway to Facilitate the Translation and Utilization of AHRQ EPC Report Findings. Agency for Healthcare Research and Quality. Rockville, MD: Prepared by the ECRI Institute–Penn Medicine Evidence-based Practice Center; 2018. PubMed
20. AHRQ methods for effective health care. In: Gliklich RE, Dreyer NA, Leavy MB, eds. Registries for Evaluating Patient Outcomes: A User’s Guide. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014.
21. Hartling L, Guise JM, Kato E, et al. AHRQ comparative effectiveness reviews. In: EPC Methods: An Exploration of Methods and Context for the Production of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. PubMed
22. Hartling L, Guise JM, Kato E, et al. A taxonomy of rapid reviews links report types and methods to specific decision-making contexts. J Clin Epidemiol. 2015;68(12):1451-1462.e1453. doi: 10.1016/j.jclinepi.2015.05.036. PubMed
23. Hartling L, Guise JM, Hempel S, et al. AHRQ methods for effective health care. In: EPC Methods: AHRQ End-User Perspectives of Rapid Reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. PubMed
24. Hartling L, Guise JM, Hempel S, et al. Fit for purpose: perspectives on rapid reviews from end-user interviews. Syst Rev. 2017;6(1):32. doi: 10.1186/s13643-017-0425-7. PubMed
25. Agency for Healthcare Research and Quality. Synthesizing Evidence for Quality Improvement. 2018; https://effectivehealthcare.ahrq.gov/topics/health-systems/quality-improvement. Accessed September 26, 2018.
©2019 Society of Hospital Medicine