User login
40-year-old woman • fever • rash • arthralgia • Dx?
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8
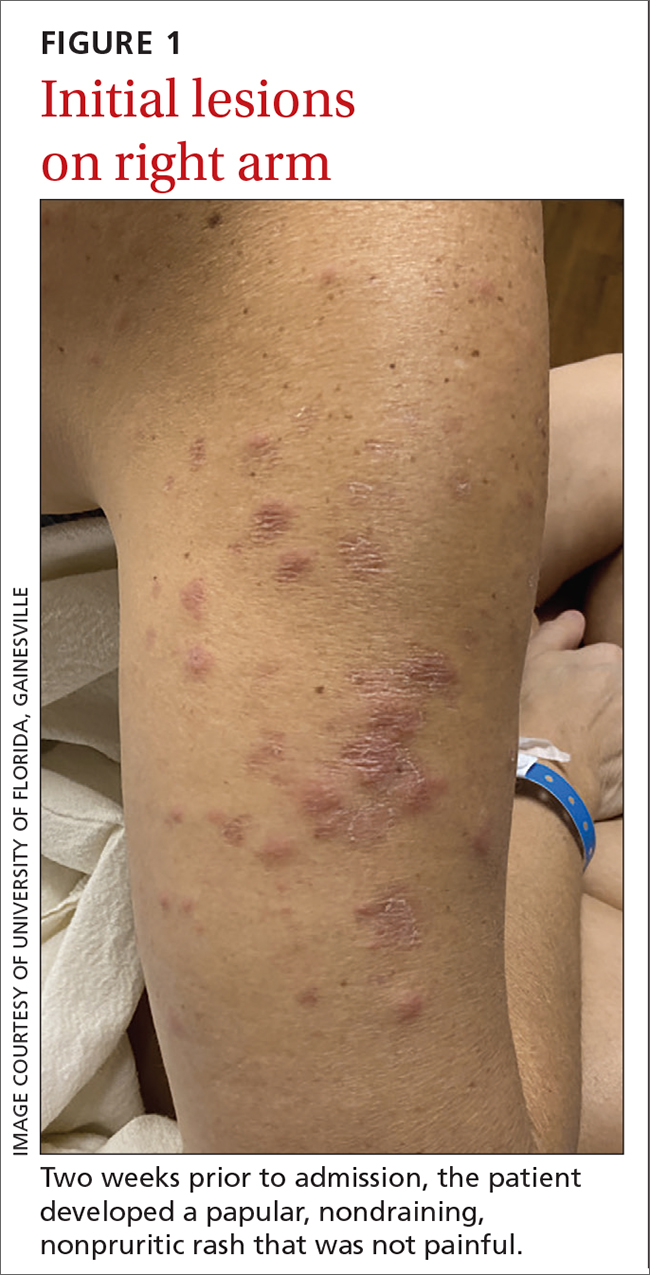
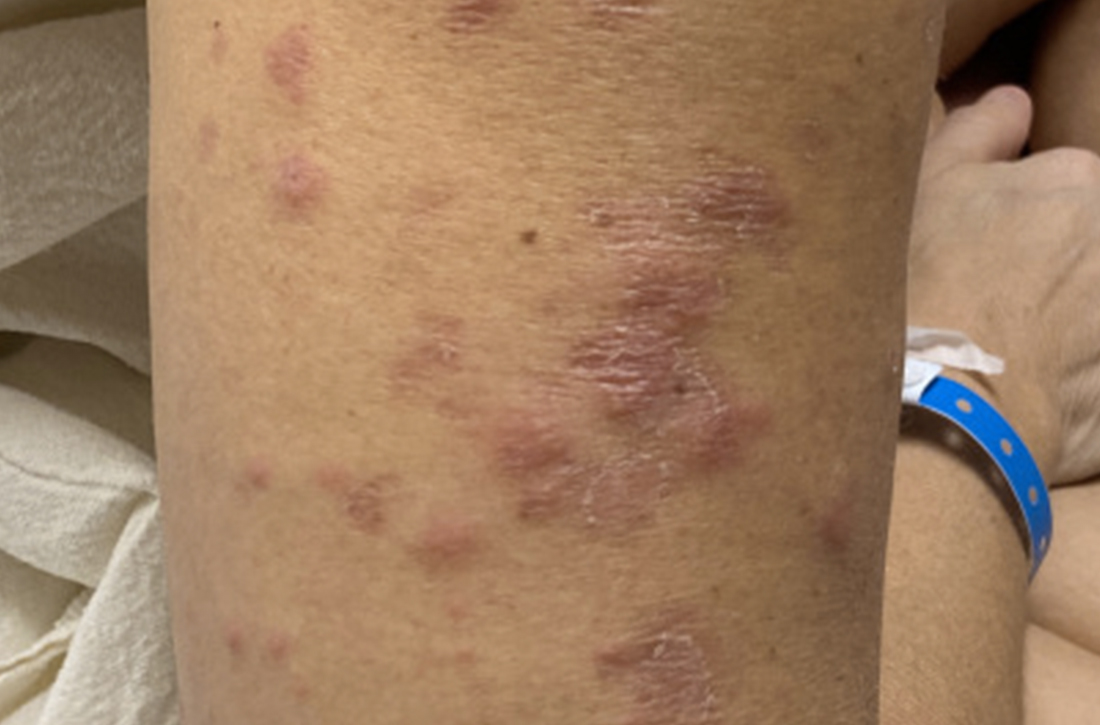
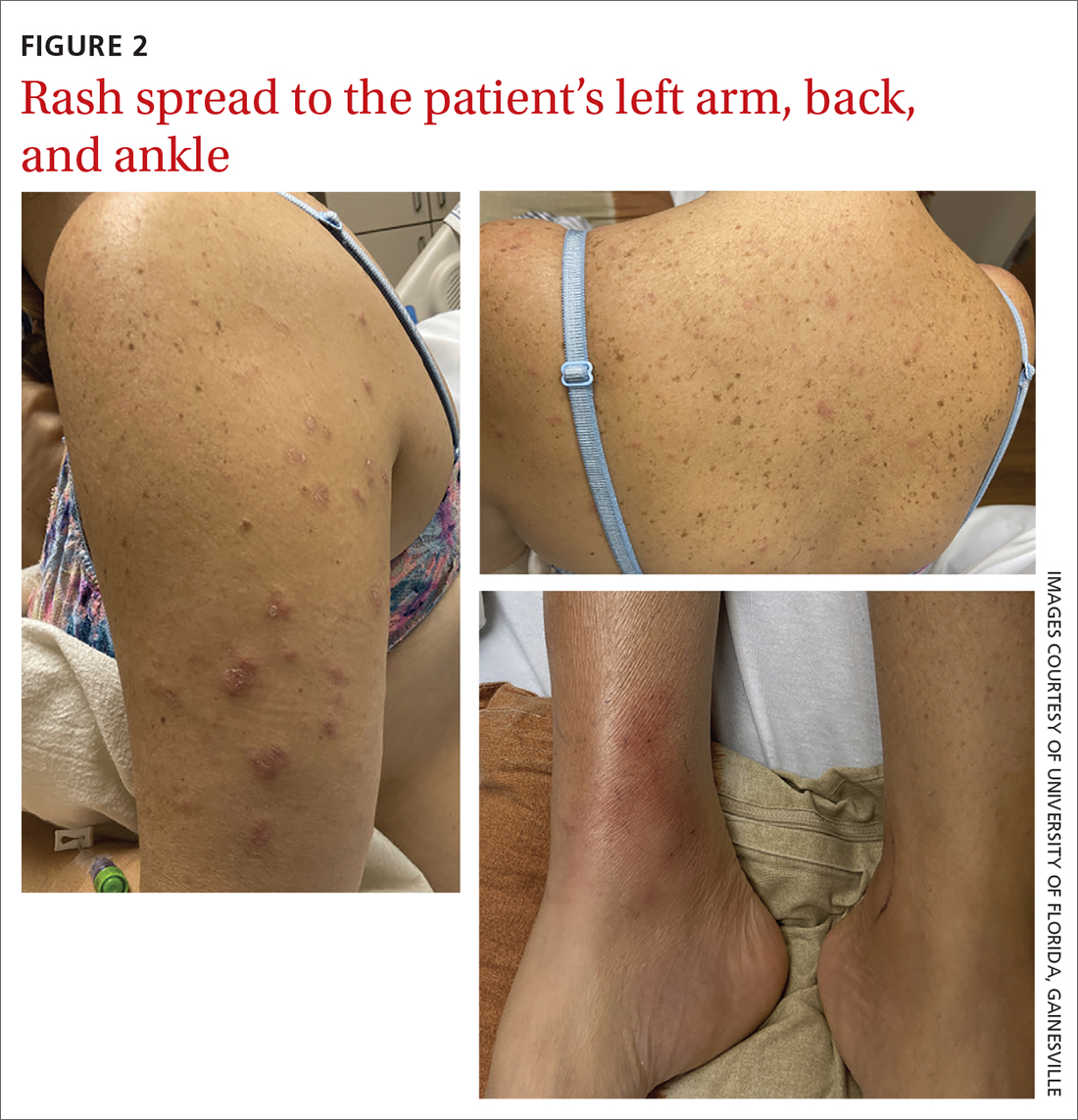
The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.
At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome.Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; farahleclercq@ufl.edu
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome.Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; farahleclercq@ufl.edu
THE CASE
A 40-year-old woman with no significant medical history sought care at the emergency department for a fever, rash, and arthralgia. On admission, she had worsening bilateral ankle pain and was having difficulty walking. During the previous 3 months, she’d had 3 episodes of tonsillitis, all of which were presumed to be caused by Streptococcus, although no swabs were obtained. Her primary care physician treated her with antibiotics each time: 1 round of amoxicillin 500 mg twice daily for 10 days and 2 rounds of amoxicillin/clavulanate 875 mg twice daily for 7 to 10 days. During the previous month, she’d experienced intermittent fevers ranging from 100.2 °F to 100.8

The patient said that 2 weeks prior to her admission to the hospital, she’d developed a rash on her right arm, which was papular, nondraining, nonpruritic, and not painful (FIGURE 1). Six days later, the rash spread to her left arm, chest, and back, with a few lesions on her legs (FIGURE 2). A few days later, she developed arthralgias in her hips, knees, and ankles. These were associated with the appearance of large, flat, erythematous lesions on her anterior lower extremities (FIGURE 2). About 5 days before she was admitted to our hospital, the patient was seen at another hospital and treated for possible cellulitis with cephalexin (500 mg 4 times daily for 5-7 days), but her symptoms persisted.

At this point, she sought care at our hospital for her worsening lower extremity arthralgia, difficulty walking, and the persistent rash. An initial lab report showed a white blood cell (WBC) count of 12.6 × 103/µL (normal range, 4.0-10.0 × 103/µL) with an absolute neutrophil count of 9.7 × 103/µL (normal, 1.7-7.0 × 103/µL). Her C-reactive protein (CRP) level was elevated (194.7 mg/L; normal, 0.0-5.0 mg/L), as was her erythrocyte sedimentation rate (ESR) (102.0 mm/h; normal, 0.0-20.0 mm/h). A rapid pharyngeal strep test was negative. Her anti-streptolysin O (ASO) titer was elevated (2092.0 IU/mL; normal, < 250.0 IU/mL), and her rheumatic factor was mildly elevated (19.0 IU/mL; normal, 0.0-14.0 IU/mL). An antinuclear antibody panel was positive at 1:80. Further testing was performed, and the patient was found to be negative for Sjögren syndrome A, Sjögren syndrome B, anti-Smith, scleroderma-70, double-stranded DNA, and chromatin AB—making an autoimmune disease unlikely.
THE DIAGNOSIS
The patient met the American Heart Association’s revised Jones criteria for the diagnosis of rheumatic fever: She had a positive ASO titer; polyarthritis and subcutaneous nodules (2 major criteria); and ESR > 60 mm/h and CRP > 3 mg/L (1 minor criterion).1 She started taking naproxen 500 mg twice per day and was given a penicillin G 1.5-million-unit injection. A transthoracic echocardiogram also was performed during her admission to rule out endocarditis; no abnormalities were found.
A few days after starting treatment for rheumatic fever, the patient’s WBC count returned to within normal limits and her joint swelling and pain improved; however, her rash did not go away, leading us to wonder if there was a second disease at work. Dermatology was consulted, and a punch biopsy was obtained. The results showed acute febrile neutrophilic dermatosis, or Sweet syndrome.
DISCUSSION
Sweet syndrome is considered rare, and incidence numbers are elusive.2 It has a worldwide distribution and no racial bias.3 Sweet syndrome usually occurs in women ages 30 to 50 years, although it may also occur in younger adults and children.
Three subtypes have been defined based on etiology: (1) classical (or idiopathic) Sweet syndrome; (2) malignancy-associated Sweet syndrome, which is most often related to acute myelogenous leukemia; and (3) drug-induced Sweet syndrome, which is usually associated with granulocyte colony–stimulating factor treatment.4 Our patient had the most common subtype: classical Sweet syndrome.
Continue to: What you'll see
What you’ll see.
Corticosteroid therapy is the gold standard for treatment of classical Sweet syndrome.Dosing usually starts with prednisone 1 mg/kg/d, which can be tapered to 10 mg/d within 4 to 6 weeks.5 If steroid treatment is contraindicated in the patient, alternative treatments are colchicine 0.5 mg 3 times daily for 10 to 21 days or enteric-coated potassium iodide 300 mg 3 times daily until the rash subsides.5 Without treatment, symptoms may resolve within weeks to months; with treatment, the rash usually resolves within 2 to 5 days. Some resistant forms may require 2 to 3 months of treatment.
There is a risk of recurrence in approximately one-third of patients after successful treatment of classical Sweet syndrome.5 Recurrence can be caused by another inciting factor (ie, irritable bowel disease, upper respiratory tract infection, malignancy, or a new medication), making a new investigation necessary. However, treatment would entail the same medications.5
The patient was placed on penicillin V 250 mg twice daily for 5 years due to the significant risk of carditis in the setting of rheumatic fever. She started an oral steroid regimen of a prednisone weekly taper, starting with 60 mg/d, for 4 to 6 weeks. Her papular rash improved soon after initiation of steroid therapy.
THE TAKEAWAY
On presentation, this patient’s symptoms met the Jones criteria for rheumatic fever, but she did not respond to treatment. This led us to revisit her case, order additional tests, and identify a second diagnosis—Sweet syndrome—that responded positively to treatment. This case is a reminder that sometimes the signs and symptoms we are looking at are the result of 2 underlying illnesses, with 1 possibly triggering the other. That was likely what occurred in this case.
Farah Leclercq, DO, Department of Family Medicine, University of Florida, 12041 Southwest 1 Lane, Gainesville, FL 32607; farahleclercq@ufl.edu
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
1. Gewitz MH, Baltimore SR, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of doppler echocardiography: a scientific statement from the American Heart Association. Circulation. 2015;131:1806-1818. doi: 10.1161/CIR.0000000000000205
2. Joshi TP, Friske SK, Hsiou DA, Duvic M. New practical aspects of Sweet syndrome. Am J Clin Dermatol. 2022;23:301-318. doi: 10.1007/s40257-022-00673-4
3. Cohen PR, Kurzrock R. Sweets syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778. doi: 10.1046/j.1365-4362.2003.01891.x
4. Merola JF. Sweet syndrome (acute febrile neutrophilic dermatosis): pathogenesis, clinical manifestations, and diagnosis. UpToDate. August 9, 2020. Accessed October 27, 2022. www.uptodate.com/contents/sweet-syndrome-acute-febrile-neutrophilic-dermatosis-pathogenesis-clinical-manifestations-and-diagnosis
5. Cohen PR. Sweets syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi: 10.1186/1750-1172-2-34
Patient With Severe Headache After IV Immunoglobulin
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
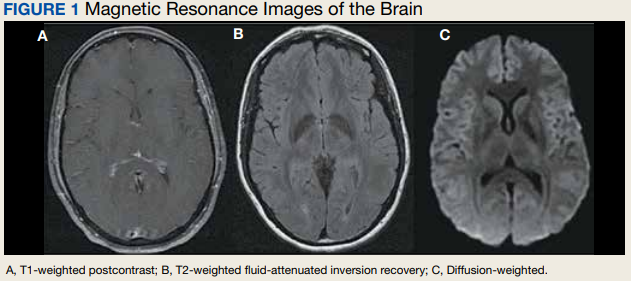
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?
Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
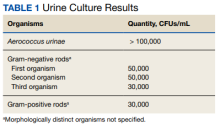
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
A 35-year-old woman with a history of hypothyroidism and idiopathic small fiber autonomic and sensory neuropathy presented to the emergency department (ED) 48 hours after IV immunoglobulin (IG) infusion with a severe headache, nausea, neck stiffness, photophobia, and episodes of intense positional eye pressure. The patient reported previous episodes of headaches post-IVIG infusion but not nearly as severe. On ED arrival, the patient was afebrile with vital signs within normal limits. Initial laboratory results were notable for levels within reference range parameters: 5.9 × 109/L white blood cell (WBC) count, 13.3 g/dL hemoglobin, 38.7% hematocrit, and 279 × 109/L platelet count; there were no abnormal urinalysis findings, and she was negative for human chorionic gonadotropin.
Due to the patient’s symptoms concerning for an acute intracranial process, a brain computed tomography (CT) without contrast was ordered. The CT demonstrated no intracranial abnormalities, but the patient’s symptoms continued to worsen. The patient was started on IV fluids and 1 g IV acetaminophen and underwent a lumbar puncture (LP). Her opening pressure was elevated at 29 cm H2O (reference range, 6-20 cm), and the fluid was notably clear. During the LP, 25 mL of cerebrospinal fluid (CSF) was collected for laboratory analysis to include a polymerase chain reaction (PCR) panel and cultures, and a closing pressure of 12 cm H2O was recorded at the end of the procedure with the patient reporting some relief of pressure. The patient was admitted to the medicine ward for further workup and observations.The patient’s meningitis/encephalitis PCR panel detected no pathogens in the CSF, but her WBC count was 84 × 109/L (reference range, 4-11) with 30 segmented neutrophils (reference range, 0-6) and red blood cell count of 24 (reference range, 0-1); her normal glucose at 60 mg/dL (reference range, 40-70) and protein of 33 mg/dL (reference range, 15-45) were within normal parameters. Brain magnetic resonance images with and without contrast was inconsistent with any acute intracranial pathology to include subarachnoid hemorrhage or central nervous system neoplasm (Figure 1). Bacterial and fungal cultures were negative.
- What is your diagnosis?
- How would you treat this patient?

Discussion
Aseptic meningitis presents with a typical clinical picture of meningitis to include headache, stiffened neck, and photophobia. In the event of negative CSF bacterial and fungal cultures and negative viral PCR, a diagnosis of aseptic meningitis is considered.1 Though the differential for aseptic meningitis is broad, in the immunocompetent patient, the most common etiology of aseptic meningitis in the United States is by far viral, and specifically, enterovirus (50.9%). It is less commonly caused by herpes simplex virus (8.3%), varicella zoster virus, and finally, the mosquito-borne St. Louis encephalitis and West Nile viruses typically acquired in the summer or early fall months. Other infectious agents that can present with aseptic meningitis are spirochetes (Lyme disease and syphilis), tuberculous meningitis, fungal infections (cryptococcal meningitis), and other bacterial infections that have a negative culture.
The patient’s history, physical examination, vital signs, imaging, and lumbar puncture findings were most concerning for drug-induced aseptic meningitis (DIAM) secondary to her recent IVIG infusion. An algorithm can be used to work through the diagnostic approach (Figure 2).3,4
Immediate and delayed adverse reactions to IVIG are known risks for IVIG therapy. About 1% to 15% of patients who receive IVIG will experience mild immediate reactions to the infusion.6 These immediate reactions include fever (78.6%), acrocyanosis (71.4%), rash (64.3%), headache (57.1%), shortness of breath (42.8%), hypotension (35.7%), and chest pain (21.4%).
IVIG is an increasingly used biologic pharmacologic agent used for a variety of medical conditions. This can be attributed to its multifaceted properties and ability to fight infection when given as replacement therapy and provide immunomodulation in conjunction with its more well-known anti-inflammatory properties.8 The number of conditions that can potentially benefit from IVIG is so vast that the American Academy of Allergy, Asthma and Immunology had to divide the indication for IVIG therapy into definitely beneficial, probably beneficial, may provide benefit, and unlikely to provide benefit categories.8
Conclusions
We encourage heightened clinical suspicion of DIAM in patients who have recently undergone IVIG infusion and present with meningeal signs (stiff neck, headache, photophobia, and ear/eye pressure) without any evidence of infection on physical examination or laboratory results. With such, we hope to improve clinician suspicion, detection, as well as patient education and outcomes in cases of DIAM.
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
1. Kareva L, Mironska K, Stavric K, Hasani A. Adverse reactions to intravenous immunoglobulins—our experience. Open Access Maced J Med Sci. 2018;6(12):2359-2362. doi:10.3889/oamjms.2018.513
2. Mount HR, Boyle SD. Aseptic and bacterial meningitis: evaluation, treatment, and prevention. Am Fam Physician. 2017;96(5):314-322.
3. Seehusen DA, Reeves MM, Fomin DA. Cerebrospinal fluid analysis. Am Fam Physician. 2003;68(6):1103-1108.
4. Connolly KJ, Hammer SM. The acute aseptic meningitis syndrome. Infect Dis Clin North Am. 1990;4(4):599-622.
5. Jolles S, Sewell WA, Leighton C. Drug-induced aseptic meningitis: diagnosis and management. Drug Saf. 2000;22(3):215-226. doi:10.2165/00002018-200022030-00005
6. Yelehe-Okouma M, Czmil-Garon J, Pape E, Petitpain N, Gillet P. Drug-induced aseptic meningitis: a mini-review. Fundam Clin Pharmacol. 2018;32(3):252-260. doi:10.1111/fcp.12349
7. Kepa L, Oczko-Grzesik B, Stolarz W, Sobala-Szczygiel B. Drug-induced aseptic meningitis in suspected central nervous system infections. J Clin Neurosci. 2005;12(5):562-564. doi:10.1016/j.jocn.2004.08.024
8. Perez EE, Orange JS, Bonilla F, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1-S46. doi:10.1016/j.jaci.2016.09.023
9. Kaarthigeyan K, Burli VV. Aseptic meningitis following intravenous immunoglobulin therapy of common variable immunodeficiency. J Pediatr Neurosci. 2011;6(2):160-161. doi:10.4103/1817-1745.92858
Oral Therapy for Aerococcus urinae Bacteremia and Thoracic Spondylodiscitis of Presumed Urinary Origin
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
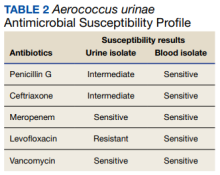
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
Aerococcus urinae (A urinae), a gram-positive coccus readily mistaken for a Staphylococcus species, was first identified in 1992.1-3 It now reportedly accounts for 0.2% to 0.8% of clinical urine isolates.4-6A urinae bacteriuria is typically asymptomatic and mainly occurs in women.7-9 Symptomatic A urinae urinary tract infection (UTI) occurs predominantly in older men with underlying urologic abnormalities.4-10
Serious A urinae infections are rare. The first 2 reported cases involved men with A urinae endocarditis, one of whom died.11,12 To date, only 8 cases of spondylodiscitis due to A urinae have been reported.13-20 Optimal treatment for invasive A urinae infection is undefined; however, the reported cases were treated successfully with diverse antibiotic regimen combinations; all including a β-lactam and beginning with at least 2 weeks of IV antibiotics.13-20 We describe a man with A urinae bacteremia and spondylodiscitis, presumably arising from a urinary source in the setting of bladder outlet obstruction, who was treated successfully.
Case Presentation
A 74-year-old man with morbid obesity, type 2 diabetes mellitus, stage 2 chronic kidney disease, and tobacco use presented to the emergency department after 2 weeks of progressive, nonradiating, midthoracic back pain, lower extremity weakness, gait imbalance, fatigue, anorexia, rigors, and subjective fevers. On presentation, he was afebrile and hemodynamically stable. A physical examination revealed point tenderness of the midthoracic vertebrae, nontender costovertebral angles, diffusely decreased strength, nonsustained clonus in both lower extremities, inguinal intertrigo, and a buried penis with purulent meatal discharge.
Laboratory results indicated a white blood cell (WBC) count of 13.5 K/μL (reference range, 4.0-11.0), absolute neutrophil count of 11.48 K/μL (reference range, 2.0-7.7), C-reactive protein (CRP) level of 225.3 mg/L (reference range, ≤ 5.0), erythrocyte sedimentation rate of 85 mm/h (reference range, 5-15), serum blood urea nitrogen of 76 mg/dL (reference range, 8-26), and serum creatinine (SCr) of 1.9 mg/dL (reference range, 1.1-1.4). A urinalysis showed positive leukocyte esterase, WBC clumps, and little bacteria. Abdominal/pelvic computed tomography showed spondylodiscitis-like changes at T7-T8, bilateral perinephric fat stranding, bladder distension, and bladder wall thickening.
The patient was presumed to have discitis secondary to a UTI, with possible pyelonephritis, and was given empiric vancomycin and ceftriaxone. Spinal magnetic resonance imaging with contrast supported spondylodiscitis at T7-T8, extending to T8-T9. Preliminary results from the admission blood and urine cultures showed gram-positive cocci in clusters, which were presumed initially to be Staphylococcus aureus (S aureus).
The final urine culture report listed multiple organisms, predominantly A urinae (Table 1);
On hospital day 6, the patient’s back pain had resolved, micturition was normal, appetite had normalized, and SCr was minimally above baseline (1.4 mg/dL). He insisted on completing antibiotic treatment at home and had no other medical indication for continued hospitalization. Thus, antibiotic therapy was changed to an all-oral regimen of amoxicillin 1 g 3 times daily for 10 days and levofloxacin 750 mg daily for 6 weeks, and the patient was discharged to home.
The patient returned 5 days postdischarge due to anuria. Investigation showed severe acute kidney injury (SCr, 6.8 mg/dL) and bladder outlet obstruction due to phimosis and urethral meatal stenosis. Urinalysis was unremarkable. His CRP had declined from 225 mg/L (initial admission) to 154 mg/L. A urinae culture and 2 sets of blood cultures were finalized as no growth. He was diagnosed with postrenal acute kidney injury and underwent meatal dilation and Foley catheterization but declined surgical correction. When seen in the clinic 2 months postantimicrobial therapy, the patient had normal micturition, no symptoms or signs of infection, and steadily down-trending inflammatory markers.
Discussion
A urinae, historically considered a rare pathogen, has been identified with increasing frequency in urine cultures due to improved microbiologic diagnostic techniques. However, there are only 8 reported cases of A urinae spondylodiscitis. Urinary pathology is an accepted risk factor for A urinae infections; consequently, we suspect that our patient’s urinary outflow obstruction and poor genitourinary hygiene were related to his invasive A urinae infection.10,21,22 We surmise that he had a chronic urinary outflow obstruction contributing to his infection, as evidenced by imaging findings, while the phimosis and urethral meatal stenosis were most likely infectious sequelae considering his anuria and acute kidney injury 5 days postdischarge. Indeed, the correlation between A urinae and urinary tract pathology may justify an evaluation for urinary pathology in any man with A urinae infection, regardless of the presence of symptoms.
By contrast, the implications of A urinae bacteriuria remain unclear. From a public health perspective, A urinae bacteriuria is rare, but the infectious mechanism remains undetermined with a case report suggesting the possibility of sexual transmission.4-6,23 In our case, the patient was not sexually active and had no clear origin of infection. Considering the potential severity of infection, more studies are needed to determine the infectious mechanism of A urinae.
In terms of infectious morbidity, the results seem to vary by sex. In a retrospective study of about 30,000 clinical urine samples, 62 (58 from women, 4 from men) yielded A urinae. The 62 corresponding patients lacked systemic infectious complications, leading the authors to conclude that A urinae is a relatively avirulent organism.24 Although possibly true in women, we are wary of drawing conclusions, especially regarding men, from a study that included only 62 urine samples were A urinae–positive, with only 4 from men. More evidence is needed to define the prognostic implications of A urinae bacteriuria in men.
As illustrated by the present case and previous reports, severe A urinae infections can occur, and the contributory factors deserve consideration. In our patient, the actual mechanism for bacteremia remains unclear. The initial concern for acute pyelonephritis was prompted by a computed tomography finding of bilateral perinephric fat stranding. This finding was questioned because it is common in older patients without infection, hence, is highly nonspecific. A correlation with urinary outflow obstruction may be an important clue in cases like this one.25,26
Furthermore, whether the urinary tract truly was the source of the patient’s bacteremia is clouded by the differing antimicrobial susceptibility patterns of the A urinae blood and urine isolates. The simplest explanation for this discordance may be that all the isolates shared a common initial origin but adapted to different environments in the host (perhaps over time) or laboratory, producing phenotypic variation. Alternatively, the infection could have been polyclonal from the onset, with sampling error leading to the differing detected susceptibility patterns, or the blood and urine isolates may have represented independent acquisition events, involving distinct A urinae strains. Unfortunately (from an academic perspective), given patient preferences and recommendations from the infectious disease consultant, no bone biopsy was done for histology and culture to confirm infection and to allow comparative strain identification if A urinae was isolated.
Optimal treatment for A urinae spondylodiscitis has yet to be established. β-lactams have shown good clinical efficacy despite being bacteriostatic in vitro.27 Early in vitro studies showed synergistic bactericidal synergistic activity with penicillin plus aminoglycoside combination therapies.27-30 Cases of endocarditis have been successfully treated mainly with the combination of a β-lactam plus aminoglycoside combination therapy.30,31 Previous cases of spondylodiscitis have been treated successfully with diverse antimicrobial agents, including clindamycin, β-lactams, cephalosporins, fluoroquinolones, and aminoglycosides.14
Our patient improved rapidly while receiving empiric therapy with vancomycin and ceftriaxone and tolerated a rapid transition to oral amoxicillin and levofloxacin. This is the shortest IV treatment course for A urinae spondylodiscitis reported to date. We suspect that such rapid IV-to-oral transitions will suffice in most stable patients with A urinae spondylodiscitis or other invasive A urinae infections in line with the results of the OVIVA and POET trials.32,33
Conclusions
We believe A urinae UTI in the absence of obvious predisposing factors should prompt evaluation for urinary outflow obstruction. Despite improved laboratory diagnostic techniques, spondylodiscitis related to A urinae remains a rare entity and thus definitive treatment recommendations are difficult to make. However, we suspect that in many cases it is reasonable to extrapolate from the results of the POET and OVIVA trials and rapidly transition therapy of A urinae spondylodiscitis from IV to oral antibiotics. We suspect a review of the US Department of Veterans Affairs population might uncover a higher incidence of A urinae infection than previously estimated due to the population demographics and the epidemiology of A urinae.
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
1. Christensen JJ, Korner B, Kjaergaard H. Aerococcus-like organism—an unnoticed urinary tract pathogen. APMIS. 1989;97(6):539-546. doi:10.1111/j.1699-0463.1989.tb00828.x
2. Aguirre M, Collins MD. Phylogenetic analysis of some Aerococcus-like organisms from urinary tract infections: description of Aerococcus urinae sp. nov. J Gen Microbiol. 1992;138(2):401-405. doi:10.1099/00221287-138-2-401
3. Williams RE, Hirch A, Cowan ST. Aerococcus, a new bacterial genus. J Gen Microbiol. 1953;8(3):475-480. doi:10.1099/00221287-8-3-475
4. Kline KA, Lewis AL. Gram-positive uropathogens, polymicrobial urinary tract infection, and the emerging microbiota of the urinary tract. Microbiol Spectr. 2016;4(2). doi:10.1128/microbiolspec.UTI-0012-2012
5. Schuur PM, Kasteren ME, Sabbe L, Vos MC, Janssens MM, Buiting AG. Urinary tract infections with Aerococcus urinae in the south of The Netherlands. Eur J Clin Microbiol Infect Dis. 1997;16(12):871-875. doi:10.1007/BF01700552
6. Grude N, Tveten Y. Aerococcus urinae og urinveisinfeksjon [Aerococcus urinae and urinary tract infection]. Tidsskr Nor Laegeforen. 2002;122(2):174-175.
7. Narayanasamy S, King K, Dennison A, Spelman DW, Aung AK. Clinical characteristics and laboratory identification of Aerococcus infections: an Australian tertiary centre perspective. Int J Microbiol. 2017;2017. doi:10.1155/2017/5684614
8. Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2014;52(3):871-876. doi:10.1128/JCM.02876-13
9. Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. mBio. 2014;5(4):e01283-14. doi:10.1128/mBio.01283-14
10. Sahu KK, Lal A, Mishra AK, Abraham GM. Aerococcus-related infections and their significance: a 9-year retrospective study. J Microsc Ultrastruct. 2021;9(1):18-25. doi:10.4103/JMAU.JMAU_61_19
11. Skov RL, Klarlund M, Thorsen S. Fatal endocarditis due to Aerococcus urinae. Diagn Microbiol Infect Dis. 1995;21(4):219-221. doi:10.1016/0732-8893(95)00037-b
12. Kristensen B, Nielsen G. Endocarditis caused by Aerococcus urinae, a newly recognized pathogen. Eur J Clin Microbiol Infect Dis. 1995;14(1):49-51. doi:10.1007/BF02112619
13. Astudillo L, Sailler L, Porte L, Lefevre JC, Massip P, Arlet-Suau E. Spondylodiscitis due to Aerococcus urinae: a first report. Scand J Infect Dis. 2003;35(11-12):890-891. doi:10.1080/00365540310016664
14. Lyagoubi A, Souffi C, Baroiller V, Vallee E. Spondylodiscitis: an increasingly described localization. EJIFCC. 2020;31(2):169-173.
15. Jerome M, Slim J, Sison R, Marton R. A case of Aerococcus urinae vertebral osteomyelitis. J Glob Infect Dis. 2015;7(2):85-86. doi:10.4103/0974-777X.157246
16. Tekin A, Tekin G, Turunç T, Demiroğlu Z, Kizilkiliç O. Infective endocarditis and spondylodiscitis in a patient due to Aerococcus urinae: first report. Int J Cardiol. 2007;115(3):402-403. doi:10.1016/j.ijcard.2006.01.046
17. Rougier E, Braud A, Argemi X, et al. Spondylodiscitis due to Aerococcus urinae and literature review. Infection. 2018;46(3):419-421. doi:10.1007/s15010-017-1106-0
18. Degroote E, Yildiz H, Lecouvet F, Verroken A, Belkhir L. Aerococcus urinae: an underestimated cause of spine infection? Case report and review of the literature. Acta Clin Belg. 2018;73(6):444-447. doi:10.1080/17843286.2018.1443003
19. Torres-Martos E, Pérez-Cortés S, Sánchez-Calvo JM, López-Prieto MD. Spondylodiscitis due to Aerococcus urinae infection in an elderly immunocompetent patient. Enferm Infecc Microbiol Clin. 2017;35(10):682-684. doi:10.1016/j.eimc.2017.02.005
20. Senneby E, Petersson AC, Rasmussen M. Clinical and microbiological features of bacteraemia with Aerococcus urinae. Clin Microbiol Infect. 2012;18(6):546-550. doi:10.1111/j.1469-0691.2011.03609.x
21. Sunnerhagen T, Nilson B, Olaison L, Rasmussen M. Clinical and microbiological features of infective endocarditis caused by aerococci. Infection. 2016;44(2):167-173. doi:10.1007/s15010-015-0812-8
22. de Jong MF, Soetekouw R, ten Kate RW, Veenendaal D. Aerococcus urinae: severe and fatal bloodstream infections and endocarditis. J Clin Microbiol. 2010;48(9):3445-3447. doi:10.1128/JCM.00835-10
23. Babaeer AA, Nader C, Iacoviello V, Tomera K. Necrotizing urethritis due to Aerococcus urinae. Case Rep Urol. 2015;2015:136147. doi:10.1155/2015/136147
24. Sierra-Hoffman M, Watkins K, Jinadatha C, Fader R, Carpenter JL. Clinical significance of Aerococcus urinae: a retrospective review. Diagn Microbiol Infect Dis. 2005;53(4):289-292. doi:10.1016/j.diagmicrobio.2005.06.021
25. Fukami H, Takeuchi Y, Kagaya S, et al. Perirenal fat stranding is not a powerful diagnostic tool for acute pyelonephritis. Int J Gen Med. 2017;10:137-144. doi:10.2147/IJGM.S133685
26. Han NY, Sung DJ, Kim MJ, Park BJ, Sim KC, Cho SB. Perirenal fat stranding on CT: is there an association with bladder outlet obstruction? Br J Radiol. 2016;89(1063):20160195. doi:10.1259/bjr.20160195
27. Hirzel C, Hirzberger L, Furrer H, Endimiani A. Bactericidal activity of penicillin, ceftriaxone, gentamicin and daptomycin alone and in combination against Aerococcus urinae. Int J Antimicrob Agents. 2016;48(3):271-276. doi:10.1016/j.ijantimicag.2016.05.007
28. Zbinden R, Santanam P, Hunziker L, Leuzinger B, von Graevenitz A. Endocarditis due to Aerococcus urinae: diagnostic tests, fatty acid composition and killing kinetics. Infection. 1999;27(2):122-124. doi:10.1007/BF02560511
29. Skov R, Christensen JJ, Korner B, Frimodt-Møller N, Espersen F. In vitro antimicrobial susceptibility of Aerococcus urinae to 14 antibiotics, and time-kill curves for penicillin, gentamicin and vancomycin. J Antimicrob Chemother. 2001;48(5):653-658. doi:10.1093/jac/48.5.653
30. Ebnöther C, Altwegg M, Gottschalk J, Seebach JD, Kronenberg A. Aerococcus urinae endocarditis: case report and review of the literature. Infection. 2002;30(5):310-313. doi:10.1007/s15010-002-3106-x
31. Tai DBG, Go JR, Fida M, Saleh OA. Management and treatment of Aerococcus bacteremia and endocarditis. Int J Infect Dis. 2021;102:584-589. doi:10.1016/j.ijid.2020.10.096
32. Li H-K, Rombach I, Zambellas R, et al; OVIVA Trial Collaborators. Oral versus intravenous antibiotics for bone and joint infection. N Engl J Med. 2019;380(5):425-436. doi:10.1056/NEJMoa1710926
33. Iversen K, Ihlemann N, Gill SU, et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N Engl J Med. 2019;380(5):415-424. doi:10.1056/NEJMoa1808312
26-year-old woman • nausea and vomiting • currently breastfeeding • ketogenic diet • Dx?
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; cwfergus@gmail.com
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; cwfergus@gmail.com
THE CASE
A 26-year-old woman presented to the emergency department (ED) with a history of nausea and vomiting for more than 24 hours. The vomiting began when she awoke to breastfeed her 3-month-old infant. She had been unable to eat or drink anything for about 16 hours.
She’d seen her primary care provider earlier in the day. Antiemetics were prescribed, but they did not provide relief. So 10 hours later, when her symptoms worsened, she presented to the ED.
Her medical history was notable for a body mass index of 26. The patient also reported positional back pain, but the review of systems was otherwise negative. The patient indicated that she’d been on a ketogenic diet for about 1 month, but she denied use of supplements.
Upon presentation to the ED, the patient was found to have a metabolic acidosis with a pH of 7.02 and an anion gap of 25. Her glucose level was 132 mg/dL, and she had a positive serum acetone and a beta-hydroxybutyrate level of 75 mg/dL (reference range, 0-2.8 mg/dL). Her salicylate testing was negative, and her lactate level was 1.4 mmol/L (reference range, 0.4-2.0 mmol/L).
THE DIAGNOSIS
This patient, with severe acidosis and an elevated anion gap, received a diagnosis of starvation ketoacidosis—specifically, lactation ketoacidosis. Other causes of elevated anion gap metabolic acidosis were ruled out, including salicylate overdose, lactic acidosis, diabetic ketoacidosis, and other ingestions. The elevated acetone and beta-hydroxybutyrate levels confirmed the diagnosis. The patient was treated with a bolus of 1 L normal saline with 5% dextrose (D5NS) in the ED and admitted.
DISCUSSION
Lactation ketoacidosis is a relatively uncommon condition, but reports have increased with the growing popularity of low-carbohydrate diets. The treatment approach has differed in previous reports in regard to insulin and bicarbonate use.1-9
The use of bicarbonate is controversial in diabetic ketoacidosis and unlikely to be helpful in lactation ketoacidosis, but it is something to consider when the patient’s pH is < 6.9. Insulin use is likely unnecessary for lactation ketoacidosis, as metabolic derangements have been corrected without intervention.
Continue to: With an increasing prevalence of cases...
With an increasing prevalence of cases, we suggest a conservative approach for treatment based on this case presentation and review of other presentations. Our patient responded rapidly to conservative treatment with intravenous (IV) fluids (D5NS), a liberalized diet, and electrolyte repletion (described in detail later).
Suggested management
Once other causes of a patient’s signs and symptoms are excluded and the diagnosis of lactation ketoacidosis is made, you’ll want to follow the initial set of lab work with the following: a venous blood gas, basic metabolic panel, and testing of magnesium and phosphorous levels every 8 hours after initial presentation, with repletion as indicated. Some patients may require more frequent monitoring based on repletion of electrolytes.
The patient will initially require IV fluid resuscitation; the initial fluid of choice would be D5NS. Patients will likely need no more than 2 L, but this will depend on the degree of hypovolemia.
Diet should be advanced as tolerated and include no restriction of carbohydrates.
Previous reports have varied regarding continuation of breastfeeding and pumping. In this case, the patient continued to breastfeed without any adverse effects. Continuation of breastfeeding is unlikely to cause harm in these circumstances, but severity of symptoms (pain, nausea, vomiting) or unresolved acidosis may require discontinuation.
Continue to: Discharge should be determined...
Discharge should be determined by resolution of symptoms and correction of metabolic derangements. In previous reports, discharge time varied from 48 hours up to 144 hours, with most patients discharged on Day 2 or 3. Pending clinical factors, discharge is likely appropriate between 36 to 72 hours from time of admission.
Our patient received an additional 1 L of D5NS for continued signs of dehydration during admission. Her pH and electrolyte levels were monitored every 8 hours, with repletion of electrolytes as needed. Her acidosis, nausea, vomiting, and pain resolved within 36 hours. The patient continued to breastfeed her infant throughout her stay. With resolution of symptoms and metabolic derangements, the patient was discharged about 36 hours after admission. She was advised to follow up with her primary care provider within 1 week after discharge.
THE TAKEAWAY
As the popularity of low-carbohydrate diets increases, patients should be educated about the warning signs of clinically significant ketoacidosis. This information is especially important for those who are lactating, as this metabolic state increases predilection to ketoacidosis. When cases do present, conservative management with IV fluids and a liberalized diet is likely to be an appropriate course of care for most patients.
CORRESPONDENCE
C.W. Ferguson, DO, Navy Medicine Readiness and Training Command, Camp Lejeune Family Medicine Residency, 100 Brewster Boulevard, Camp Lejeune, NC 28547; cwfergus@gmail.com
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
1. Al Alawi AM, Falhammar H. Lactation ketoacidosis: case presentation and literature review. BMJ Case Rep. 2018;2018:bcr2017223494. doi:10.1136/bcr-2017-223494
2. von Geijer L, Ekelund M. Ketoacidosis associated with low-carbohydrate diet in a non-diabetic lactating woman: a case report. J Med Case Rep. 2015;9:224. doi:10.1186/s13256-015-0709-2
3. Hudak SK, Overkamp D, Wagner R, et al. Ketoacidosis in a non-diabetic woman who was fasting during lactation. Nutr J. 2015;14:117. doi:10.1186/s12937-015-0076-2
4. Azzam O, Prentice D. Lactation ketoacidosis: an easily missed diagnosis. Intern Med J. 2019;49:256‐259. doi:10.1111/imj.14207
5. Sandhu HS, Michelis MF, DeVita MV. A case of bovine ketoacidosis in a lactating woman. NDT Plus. 2009;2:278‐279. doi:10.1093/ndtplus/sfp052
6. Heffner AC, Johnson DP. A case of lactation “bovine” ketoacidosis. J Emerg Med. 2008;35:385‐387. doi:10.1016/j.jemermed.2007.04.013
7. Szulewski A, Howes D, Morton AR. A severe case of iatrogenic lactation ketoacidosis. BMJ Case Rep. 2012;2012:bcr1220115409. doi:10.1136/bcr.12.2011.5409
8. Nnodum BN, Oduah E, Albert D, et al. Ketogenic diet-induced severe ketoacidosis in a lactating woman: a case report and review of the literature. Case Rep Nephrol. 2019;2019:1214208. doi:10.1155/2019/1214208
9. Gleeson S, Mulroy E, Clarke DE. Lactation ketoacidosis: an unusual entity and a review of the literature. Perm J. 2016;20:71‐73. doi:10.7812/TPP/15-097
34-year-old man • chronic lower back pain • peripheral neuropathy • leg spasms with increasing weakness • Dx?
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; charles.webb@lsuhs.edu
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; charles.webb@lsuhs.edu
THE CASE
A 34-year-old man was referred to the sports medicine clinic for evaluation of lumbar radiculopathy. He had a 2-year history of chronic lower back pain that started while he was working on power line towers in Puerto Rico. The back pain was achy, burning, shooting, and stabbing in nature. He had been treated with anti-inflammatories by a company health care provider while in Puerto Rico, but he did not have any imaging done.
At that time, he had tingling and burning that radiated down his left leg to his ankle. The patient also had leg spasms—in his left leg more than his right—and needed a cane when walking. His symptoms did not worsen at any particular time of day or with activity. He had no history of eating exotic foods or sustaining any venomous bites/stings. Ultimately, the back pain and leg spasms forced him to leave his job and return home to Louisiana.
Upon presentation to the sports medicine clinic, he explained that things had worsened since his return home. The pain and burning in his left leg had increased and were now present in his right leg, as well (bilateral paresthesias). In addition, he said he was feeling anxious (and described symptoms of forgetfulness, confusion, and agitation), was sleeping less, and was experiencing worsening fatigue.
Work-ups over the course of the previous 2 years had shed little light on the cause of his symptoms. X-rays of his lumbar spine revealed moderate degenerative changes at L5-S1. A lab work-up was negative and included a complete blood count, testing for HIV and herpes, a hepatitis panel, an antinuclear antibody screen, a C-reactive protein test, and a comprehensive metabolic panel. Thyroid-stimulating hormone, creatine kinase, rapid plasma reagin, and human leukocyte antigen B27 tests were also normal.
Magnetic resonance imaging (MRI) revealed a cystic lesion in the right ilium near the sacroiliac joint. A more recent follow-up MRI and computed tomography scan of the pelvis found the cyst to be stable and well marginalized, with no cortical erosion. Attempts at physical therapy had been unsuccessful because of the pain and decreasing muscle strength in his lower extremities. The patient’s primary care provider was treating him with meloxicam 15 mg/d and duloxetine 60 mg/d, but that had not provided any relief.
Our physical examination revealed a patient who was in mild distress and had limited lumbar spine range of motion (secondary to pain in all planes) and significant paraspinal spasms on the right side in both the lumbar and thoracic regions. The patient had reduced vibratory sensation on his left side vs the right, with a 256-Hz tuning fork at the great toe, as well as reduced sensation to fine touch with a cotton swab and a positive Babinski sign bilaterally. Lower extremity reflexes were hyperreflexic on the left compared with the right. He had no pronator drift; Trendelenburg, straight leg raise, Hoover sign, and slump tests were all negative. His gait was antalgic with a cane, as he described bilateral paresthesias.
THE DIAGNOSIS
The differential diagnosis for low back pain is quite extensive and includes simple mechanical low back pain, lumbar radiculopathy, facet arthritis, spinal stenosis, spondylolysis/spondylolisthesis, and referred pain from the hip, knee, or upper back. It can also be caused by referred pain from visceral organs such as the liver, colon, or kidneys. Low back pain can also signal primary or metastatic disease. However, most of these potential diagnoses had been ruled out with imaging and lab tests.
Two things caught our attention. First: Mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain. Our patient had weakness in gait and pain and burning in both of his legs. Second: Our patient described decreased sleep and feeling anxious, with symptoms of forgetfulness, confusion, and agitation. These factors prompted us to look beyond the normal differential and consider a potential toxicity. A heavy metal screen was ordered, and the results were positive for arsenic toxicity.
DISCUSSION
Arsenic toxicity is a global health problem that affects millions of people.1,2 Arsenic has been used for centuries in depilatories, cosmetics, poisons, and therapeutic agents. Today it is used as a treatment for leukemia and in several ayurvedic and homeopathic remedies.3-7 It is a common earth element found in ground water and a waste product from mining and the manufacturing of glass, computer chips, wood preservatives, and various pesticides.2,3,7,8
A great masquerader. Once in the body, arsenic can cause many serious ailments ranging from urinary tract, liver, and skin cancers to various peripheral and central nervous system disorders.2 Arsenic can cause symmetrical peripheral neuropathy characterized by sensory nerves being more sensitive than motor nerves.2,3,5,6 Clinically, it causes numbness and paresthesias of the distal extremities, with the lower extremities more severely affected.3,6 Symptoms can develop within 2 hours to 2 years of exposure, with vomiting, diarrhea, or both preceding the onset of the neuropathy.2,3,5,6 Arsenic is linked to forgetfulness, confusion, visual distortion, sleep disturbances, decreased concentration, disorientation, severe agitation, paranoid ideation, emotional lability, and decreases in locomotor activity.3,5,6
Testing and treatment. Arsenic levels in the body are measured by blood and urine testing. Blood arsenic levels are typically detectable immediately after exposure and with continued exposure, but quickly normalize as the metal integrates into the nonvascular tissues. Urine arsenic levels can be detected for weeks. Normal levels for arsenic in both urine and blood are ≤ 12 µg/L.3 Anything greater than 12 µg/L is considered high; critically high values are those above 50 µg/L.3,5 Our patient’s blood arsenic level was 13 µg/L.
Treatment involves removing the source of the arsenic. Chelation therapy should be pursued when urine arsenic levels are greater than 50 µg/L or when removing the source of the arsenic fails to reduce arsenic levels. Chelation therapy should be continued until urine arsenic levels are below 20 µg/L.5,6
Continue to: After discussing potential sources of exposure
After discussing potential sources of exposure, our patient decided to move out of the house he shared with his ex-wife. He started to recover soon after moving out. Two weeks after his clinic visit, he no longer needed a cane to walk, and his blood arsenic level had dropped to 6 µg/L. Two months after his clinic visit, the patient’s blood arsenic level was undetectable. The patient’s peripheral neuropathy symptoms continued to improve.
The source of this patient’s arsenic exposure was never confirmed. The exposure could have occurred in Puerto Rico or in Louisiana. Even though no one else in the Louisiana home became ill, the patient was instructed to contact the local health department and water department to have the water tested. However, when he returned to the clinic for follow-up, he had not followed through.
THE TAKEAWAY
When evaluating causes of peripheral neuropathy, consider the possibility of heavy metal toxicity, which can be easily overlooked by the busy clinician. In this case, the patient initially experienced asymmetric paresthesia that gradually increased to burning pain and weakness, with reduced motor control bilaterally. This was significant because mechanical low back pain and the associated discogenic radiculopathy would be unilateral, manifesting with asymmetric paresthesias and pain.
Our patient’s leg symptoms, the constellation of forgetfulness, confusion, and agitation, and his sleep issues prompted us to look outside our normal differential. Fortunately, once arsenic exposure ceases, patients will gradually improve because arsenic is rapidly cleared from the bloodstream.3,6
CORRESPONDENCE
Charles W. Webb, DO, CAQSM, FAMSSM, FAAFP, Department of Family Medicine, 1501 Kings Highway, PO Box 33932, Shreveport, LA 71130-3932; charles.webb@lsuhs.edu
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
1. Ahmad SA, Khan MH, Haque M. Arsenic contamination in groundwater in Bangladesh: implications and challenges for healthcare policy. Risk Manag Health Policy. 2018;11:251-261. doi: 10.2147/RMHP.S153188
2. Roh T, Steinmaus C, Marshall G, et al. Age at exposure to arsenic in water and mortality 30-40 years after exposure cessation. Am J Epidemiol. 2018;187:2297-2305. doi: 10.1093/aje/kwy159
3. Baker BA, Cassano VA, Murray C, ACOEM Task Force on Arsenic Exposure. Arsenic exposure, assessment, toxicity, diagnosis, and management. J Occup Environ Med. 2018;60:634-639. doi: 10.1097/JOM.0000000000001485
4. Lasky T, Sun W, Kadry A, Hoffman MK. Mean total arsenic concentrations in chicken 1989-2000 and estimated exposures for consumers of chicken. Environ Health Perspect. 2004;112:18-21. doi: 10.1289/ehp.6407
5. Lindenmeyer G, Hoggett K, Burrow J, et al. A sickening tale. N Engl J Med. 2018;379:75-80. doi: 10.1056/NEJMcps1716775
6. Rodríguez VM, Jímenez-Capdevill ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicol Lett. 2003;145: 1-18. doi: 10.1016/s0378-4274(03)00262-5
7. Saper RB, Phillips RS, Sehgal A, et al. Lead, mercury, and arsenic in US- and Indian- manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915-923. doi: 10.1001/jama.300.8.915
8. Rose M, Lewis J, Langford N, et al. Arsenic in seaweed—forms, concentration and dietary exposure. Food Chem Toxicol. 2007;45:1263-1267. doi: 10.1016/j.fct.2007.01.007
Leukocytoclastic Vasculitis Masquerading as Chronic ITP
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
Idiopathic thrombocytopenic purpura (ITP) is an immune-mediated acquired condition affecting both adults and children.1 Acute ITP is the most common form, which happens in the presence of a precipitant, leading to a drop in platelet counts. However, chronic ITP can occur when all the causes that might precipitate thrombocytopenia have been ruled out, and it is persistent for ≥ 12 months.2 Its presence can mask other diseases that exhibit somewhat similar signs and symptoms. We present a case of a patient presenting with chronic ITP with diffuse rash and was later diagnosed with idiopathic leukocytoclastic vasculitis (LCV).
Case Presentation
A 79-year-old presented to the hospital with 2-day history of a rash. The rash was purpureal and petechial and located on the trunk and bilateral upper and lower extremities. The rash was associated with itchiness and pain in the wrists, ankles, and small joints of the hands. The patient reported no changes in medication or diet,
The patient mentioned that at the time of diagnosis the platelet count was about 90,000 but had been fluctuating between 50 and 60,000 recently. The patient also reported no history of gum bleeding, nosebleeds, hemoptysis, hematemesis, or any miscarriages. She also had difficulty voiding for 2 to 3 days but no dysuria, frequency, urgency, or incontinence.
Laboratory results were significant for 57,000/µL platelet count (normal range, 150,000-450,000), elevated d-dimer (6.07), < 6 mg/dL C4 (normal range, 88-201). Hemoglobin level, coagulation panel, hemolytic panel, and fibrinogen level results were unremarkable. The hepatitis panel, Lyme disease, and HIV test were negative. The peripheral blood smear showed moderate thrombocytopenia, mild monocytosis, and borderline normochromic normocytic anemia without schistocytes. The autoimmune panel to evaluate thrombocytopenia showed platelet antibody against glycoprotein (GP) IIb/IIIa, GP Ib/Ix, GP Ia/IIa, suggestive toward a diagnosis of chronic idiopathic ITP. However, the skin biopsy of the rash was indicative of LCV.
An autoimmune panel for vasculitis, including antinuclear antibody and antidouble-stranded DNA, was negative. While in the hospital, the patient completed the course of ciprofloxacin for the UTI, the rash started to fade without any intervention, and the platelet count improved to 69,000/µL. The patient was discharged after 3 days with the recommendation to follow up with her hematologist.
Discussion
LCV is a small vessel vasculitis of the dermal capillaries and venules. Histologically, LCV is characterized by fibrinoid necrosis of the vessel wall with frequent neutrophils, nuclear dust, and extravasated erythrocytes.3
Although a thorough evaluation is recommended to determine etiology, about 50% of cases are idiopathic. The most common precipitants are acute infection or a new medication. Postinfectious LCV is most commonly seen after streptococcal upper respiratory tract infection. Among other infectious triggers, Mycobacterium, Staphylococcus aureus, chlamydia, Neisseria, HIV, hepatitis B, hepatitis C, and syphilis are noteworthy. Foods, autoimmune disease, collagen vascular disease, and malignancy are also associated with LCV.4
In our patient we could not find any specific identifiable triggers. However, the presence of a UTI as a precipitating factor cannot be ruled out.5 Moreover, the patient received ciprofloxacin and there have been several case reports of LCV associated with use of a fluroquinolone.6 Nevertheless, in the presence of chronic ITP, which also is an auto-immune condition, an idiopathic cause seemed a reasonable explanation for the patient’s etiopathogenesis.
The cutaneous manifestations of LCV may appear about 1 to 3 weeks after the triggering event if present. The major clinical findings include palpable purpura and/or petechiae that are nonblanching. These findings can easily be confused with other diagnoses especially in the presence of a similar preexisting diagnosis. For example, our patient already had chronic ITP, and in such circumstances, a diagnosis of superimposed LCV can be easily missed without a thorough investigation. Extracutaneous manifestations with LCV are less common. Systemic symptoms may include low-grade fevers, malaise, weight loss, myalgia, and arthralgia. These findings have been noted in about 30% of affected patients, with arthralgia the most common manifestation.7 Our patient also presented with pain involving multiple joints.
The mainstay of diagnosis for LCV is a skin biopsy with direct immunofluorescence. However, a workup for an underlying condition should be considered based on clinical suspicion. If a secondary cause is found, management should target treating the underlying cause, including withdrawal of the offending drug, treatment or control of the underlying infection, malignancy, or connective tissue disease. Most cases of idiopathic cutaneous LCV resolve with supportive measures, including leg elevation, rest, compression stockings, and antihistamines. In resistant cases, a 4- to 6-week tapering dose of corticosteroids and immunosuppressive steroid-sparing agents may be needed.8
Conclusions
Although most cases of LCV are mild and resolve without intervention, many cases go undiagnosed due to a delay in performing a biopsy. However, we should always look for the root cause of a patient’s condition to rule out underlying contributing conditions. Differentiating LCV from any other preexisting condition presenting similarly is important.
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
1. Gaurav K, Keith RM. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013;27(3): 495-520. doi:10.1016/j.hoc.2013.03.001
2. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393.
3. James WD, Berger TG, Elston DM. Andrews’ Diseases of the Skin: Clinical Dermatology. 11th ed. Saunders/Elsevier; 2011.
4. Einhorn J, Levis JT. Dermatologic diagnosis: leukocytoclastic vasculitis. Perm J. 2015;19(3):77-78. doi:10.7812/TPP/15-001
5. The role of infectious agents in the pathogenesis of vasculitis. Nicolò P, Carlo S. Best Pract Res Clin Rheumatol. 2008;22(5):897-911. doi:10.7812/TPP/15-001
6. Maunz G, Conzett T, Zimmerli W. Cutaneous vasculitis associated with fluoroquinolones. Infection. 2009;37(5):466-468. doi:10.1007/s15010-009-8437-4
7. Baigrie D, Goyal A, Crane J.C. Leukocytoclastic vasculitis. StatPearls [internet]. Updated May 8, 2022. Accessed October 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK482159
8. Micheletti RG, Pagnoux C. Management of cutaneous vasculitis. Presse Med. 2020; 49(3):104033. doi:10.1016/j.lpm.2020.104033
Challenges and Considerations in Treating Negative and Cognitive Symptoms of Schizophrenia Spectrum Disorders
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
Schizophrenia spectrum disorders (SSDs) represent some of the most debilitating mental health disorders.1 While these disorders have myriad presentations, the prototypical patient with SSD is often thought to possess positive symptoms. More recently, clinicians and researchers are raising awareness of another presentation of SSD: predominantly negative and cognitive symptoms. This symptom profile is not a novel phenomenon; for many years this presentation was recognized as a “deficit” presentation, referring to negative symptoms as the prominent feature.2,3 However, it presents unique diagnostic and treatment considerations that are often underappreciated in clinical settings.
Negative symptoms (blunted/flat affect, avolition, alogia, anhedonia, asociality) have long been identified as key features of SSD and are widely recognized as predictive of poor prognostic outcomes for patients with SSDs.1 In many patients, negative symptoms may precede the development of positive symptoms and emerge as a more robust predictor of functional outcomes than positive symptoms.1 Negative symptoms also appear to be inextricably linked to cognitive symptoms. Specifically, patients with primary negative symptoms seem to perform poorly on measures of global cognitive functioning.1 Similar to negative symptoms, cognitive symptoms of SSDs are a primary source of functional impairment and persistent disability.1 Despite this, little attention is given in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) to the neurocognitive and social cognitive deficits seen in patients with SSDs. Previous research highlights broad deficits in a range of neurocognitive abilities, including attention, working memory, processing speed, executive functioning, learning and memory, and receptive and expressive language.4 Similarly, patients also display deficits in domains of social cognition, such as emotion processing, identifying and utilizing social cues, evaluating attributions of others, and perspective-taking.5
A predominantly negative and cognitive symptom presentation can present diagnostic and treatment challenges. We present a case of a patient with such a presentation and the unique considerations given to diagnostic clarification and her treatment.
Case Presentation
A 33-year-old female veteran presented to the emergency department (ED) at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, in 2020. She was brought to the ED by local police following an attempted assault of her neighbor. Per collateral information from the police, the veteran stated she “had the urge to hurt someone” but was unable to provide any other information about this event. The veteran demonstrated diminished speech output, providing 2- to 3-word responses before refusing to speak entirely. She also presented with markedly blunted affect and tangential speech. She was not oriented to situation, stating confusion as to how she was brought to the hospital, and appeared to be responding to internal stimuli. She was subsequently admitted to the inpatient mental health unit due to unspecified psychosis.
The veteran presented as an unreliable historian, and much of her medical history was obtained via a review of US Department of Defense (DoD) records and collateral interview with her parents. Before her hospitalization, the veteran had been diagnosed with major depressive disorder (MDD) and adjustment disorder while serving in the Navy. Her psychiatric history before her military career was otherwise unremarkable. At that time, she began a trial of sertraline 50 mg and completed 10 sessions of psychotherapy. After approximately 1 year, she elected to stop taking sertraline due to improved mental health. However, shortly after this she began experiencing significant depressive symptoms and was ultimately released early from the Navy due to her mental health concerns.
The veteran’s parents provided interim history between her discharge and establishing care at MEDVAMC as the veteran was reluctant to discuss this period of her life. According to her parents the veteran had prior diagnoses of borderline personality disorder and MDD and had difficulty adhering to her current medications (bupropion and duloxetine) for about 1 month before her hospitalization. During the previous month, her parents observed her staying in her room around the clock and “[going] mute.”
The veteran remained hospitalized for about 1 month, during which she was diagnosed with schizoaffective disorder and stabilized on injections of long-acting olanzapine 210 mg (administered every 2 weeks). She was referred for outpatient psychotherapy in a specialty clinic for veterans with SSDs. However, she did not attend her initial intake assessment.
About 2 weeks after discharge from the hospital, the veteran presented for her injection appointment. At this time, she was noted to be disorganized in her thinking and behavior, displaying thought blocking and catatonic behavior. Her parents also described concerning behavior since her discharge. They stated she went to a hotel after her discharge and spent all her available money. She then returned to her parents’ home, where she did not sleep or bathe for several days. She was observed wandering around the house aimlessly and in a confused manner and had become verbally aggressive and threatening toward her parents. The veteran was again psychiatrically admitted due to psychosis and concerns for her safety. She was discharged about 2 weeks later and continued olanzapine injections. She was also referred for outpatient psychotherapy; although she did not initially engage in psychotherapy, she was referred again about 5 months after discharge and began psychotherapy at that time.
The veteran began a course of weekly outpatient psychotherapy employing cognitive behavior therapy for psychosis (CBTp).6 During this time, she described her primary concerns as anxiety and feeling disconnected from others. She reported a history of depression but not of schizoaffective disorder. When asked about this, the veteran stated that she did not feel this diagnosis was accurate and instead believed she had severe depression. When asked why she was prescribed olanzapine, the veteran stated that this medication was for depression. As with her inpatient stays, the veteran demonstrated several negative symptoms during her course of psychotherapy. She presented with noticeably blunted affect, evidenced by lack of facial expression and monotonic speech. She also routinely displayed alogia (ie, lack of speech), often stating that she “did not feel like talking much.” She described difficulty finding motivation to initiate tasks (avolition) as well as a tendency toward social isolation (asociality).
The veteran also described concerns related to neurocognitive and social cognitive symptoms. She reported difficulties in processing speed, cognitive set-shifting (mentally switching between tasks), and inhibition, describing how these concerns interfered with her occupational functioning. She noted difficulty maintaining the expected pace of work at her previous positions, stating that she felt it took her longer to complete tasks compared with others. In addition, she displayed some difficulties with attention and memory. On more than one occasion, she seemed to have forgotten the previous day’s conversations with clinicians. Regarding social cognitive symptoms, she noted difficulties in emotion processing, indicating that it was difficult for her to identify and manage her emotions. This was especially prominent during times of depressed mood.
She also displayed a hostile attribution bias, or tendency to overattribute hostile intent to others’ ambiguous actions. For example, she described an instance where a family member sat too close to her on the couch, stating that she felt this behavior indicated the family member did not care about her. Relatedly, the veteran demonstrated difficulty with perspective taking, which became evident during cognitive restructuring regarding interpretations of her family’s behavior. Finally, the veteran displayed some deficits in social perception, or the ability to identify social context and rules based on nonverbal communication, verbal cues, and vocal intonation. She stated that she often felt conversing with others was difficult for her and indicated that she was “not good at conversations.” This may have in part been due to deficits in social perception.
During the first 2 months of psychotherapy, the veteran regularly attended sessions (conducted over telephone due to the COVID-19 pandemic) and was adherent to twice-weekly olanzapine injections. Despite this, she began experiencing an increase in depressive symptoms accompanied by a noticeable worsening of her blunted affect, alogia, and avolition. After about 2 months of psychotherapy, she described active suicidal ideation and requested to be voluntarily hospitalized. During this hospitalization, the veteran was consulted about the use of clozapine in treatment-refractory conditions and began a trial of clozapine 400 mg. She demonstrated marked improvement in her depressed mood after taking the medication and was discharged about 2 weeks after admission. The veteran completed 10 sessions of CBTp before electing to terminate due to an upcoming move. She was adherent to weekly blood draws per the requirements of clozapine and described intentions to engage in mental health care after her move. The patient’s mother contacted the clinic to inform the treatment team that the patient and her family had moved to a different city and the patient had started receiving care at the VAMC in that city.
Discussion
As the veteran’s case highlights, a predominantly negative and cognitive symptom presentation may present diagnostic challenges. Since this presentation may not be viewed as representative of SSDs, patients with this presentation may be misdiagnosed. This was evident in the current case, not only in the veteran’s prodromal phase of illness while in the Navy, but also in her reported previous diagnoses of borderline personality disorder and MDD. More than one clinician at the MEDVAMC provisionally considered a diagnosis of MDD before collecting collateral information from the veteran’s family regarding her clear psychotic symptoms. Unfortunately, such misdiagnoses may have prevented early intervention of the veteran’s schizoaffective disorder, which is found to be instrumental in reducing impairment and disability among patients with SSDs.7,8
These misdiagnoses are understandable given the considerable symptom overlap between SSDs and other mental health disorders. For instance, anhedonia and avolition are 2 key symptoms seen in depressive episodes. Both anhedonia and lack of positive emotion are often seen in posttraumatic stress disorder. Additionally, anxiety disorders may induce a lack of positive emotion, loss of interest in previously enjoyed activities, and lack of motivation secondary to primary symptoms of anxiety. Furthermore, schizoaffective disorder requires the presence of a major mood episode. In the absence of apparent positive symptoms (as is the case for patients with a predominantly negative symptom presentation), schizoaffective disorder may be easily misdiagnosed as a mood disorder.
Patients with predominantly negative or cognitive symptoms may also be less accepting of a diagnosis of SSD. A wealth of research points to the clear stigma of SSDs, with many suggesting that these disorders are among the most stigmatized mental health disorders.9 Therefore, patients with predominantly negative and cognitive symptoms may be more likely to attribute their symptoms to another, less stigmatized mental health disorder. This was seen in the current case, as the veteran repeatedly denied a diagnosis of schizoaffective disorder and instead claimed to have severe depression. This reluctance to accept a diagnosis of an SSD, coupled with the diagnostic ambiguity of negative symptoms, is likely to make it challenging for clinicians to accurately identify patients with a predominantly negative and cognitive symptom presentation of SSDs.
Clinicians working within a team-based setting may be less likely to misdiagnose patients as they can consult others. Diagnostic clarity in the current case was undoubtedly facilitated by the multidisciplinary team involved in the veteran’s care; clinicians involved in her care were able to consult with one another to determine that her symptoms were indicative of an SSD rather than a mood disorder. Mental health professionals in private practice are unlikely to have access to such multidisciplinary specialty services and may be particularly vulnerable to misdiagnoses.
Treatment Considerations
This case also highlights several psychotherapy and psychopharmacology treatment considerations for patients with a predominantly negative and cognitive symptom presentation. The veteran was initially difficult to engage in psychotherapy. Although patients with SSDs often have difficulty engaging in treatment, patients with a predominant negative and cognitive symptom profile may experience more difficulty doing so.10 Previous research suggests that both negative symptoms and cognitive symptoms are inversely related to treatment engagement.11,12
By their very nature, negative symptoms may make it difficult to fully engage in psychotherapy. First, avolition and amotivation likely make it difficult for patients to attend psychotherapy appointments. Furthermore, negative symptoms may make it difficult to emotionally engage with the content of psychotherapy, thus limiting the potential benefits. Cognitive symptoms may also make it more difficult for patients to fully reap the benefits of psychotherapy. Deficits in attention, memory, and abstract reasoning seen in other mental health and medical conditions are associated with poorer treatment outcomes in psychotherapy.13,14 Thus, it may be especially difficult to engage patients with primarily negative and cognitive symptoms of SSDs in psychotherapy. However, given the link between these symptoms and functional impairment, it is even more important to evaluate and address such barriers to treatment.
This case highlights the utility of clozapine in the treatment of SSDs. Many commonly prescribed antipsychotic medications have questionable efficacy in treating negative symptoms, and none of the currently available antipsychotics are approved for this indication.15 In our case, the veteran saw a limited reduction of her negative or cognitive symptoms from her use of olanzapine. However, case reports, naturalistic follow-up, and open-label studies suggest that clozapine may be efficacious in targeting negative symptoms of SSDs.16-19 Previous research also suggests clozapine is more effective than other antipsychotic medications, including olanzapine, quetiapine, and risperidone, in decreasing overall SSD symptoms.20,21 Additionally, there is initial evidence of the efficacy of clozapine in treating cognitive symptoms, suggesting that some areas of cognition may improve in response to this medication.22-24 On the other hand, a recent case study suggests high doses of clozapine may be associated with cognitive impairment, although cognitive impairment was still greater without medication than at this higher dose.25 Thus, further research is needed to refine our understanding of the impact of clozapine on cognitive symptoms in SSDs.
Despite the promising research behind clozapine, it remains widely underprescribed, likely due to concerns regarding the potential adverse effects.26,27 Clozapine has been associated with many adverse effects, the most concerning being neutropenia, which can lead to serious infection and death. Thus, one concern among clinicians may be the potential lethality of clozapine. However, a wealth of research indicates clozapine can be safely administered under medical supervision.26,28 In fact, clozapine has been linked to lower all-cause mortality rates and lower mortality rates by suicide compared with other antipsychotic medications.29-31 It may therefore be argued that clozapine lowers the overall risk of mortality. Prescribers may also be weary of adherence to regular blood tests that patients must undergo to monitor their risk for neutropenia. This is the most frequently cited anticipated barrier to beginning a trial of clozapine.27 These concerns may not be unfounded; indeed, if avolition and amotivation make it difficult to attend psychotherapy sessions, these factors may logically make it difficult to attend blood draw appointments. In response to such barriers, several solutions have been suggested regarding potential blood draw nonadherence, including the use of in-home treatment teams and point-of-care monitoring.32,33
Conclusions
Predominant negative and cognitive symptom presentations of SSDs require unique considerations to accurately identify and provide optimal treatment for patients with such presentations. As our case highlights, patients with such presentations may often be misdiagnosed, as negative and cognitive symptoms may be attributed to other disorders. Additionally, patients with this presentation may experience difficulty engaging in psychotherapy and may not see the same benefits from common antipsychotic medications as patients with predominantly positive symptoms. Clozapine emerges as a promising treatment for addressing negative and cognitive symptoms, although it remains widely underutilized. In cases where clinicians encounter patients with predominantly negative and cognitive symptoms, we strongly recommend consultation and referral to psychiatric care for medication management.
The current case highlights the need for individually tailored treatment plans for individuals seeking mental health care. Clinicians of patients with any mental disorder, but especially those with SSDs of predominantly negative and cognitive symptoms, should carefully formulate a treatment plan based on relevant case history, presentation, and current empirical literature. A singular, one-size-fits-all approach should not be universally implemented for such patients. Our case demonstrates how careful multidisciplinary evaluations, review of medical records, collateral information from patients’ family members, and other diagnostic and treatment considerations in patients with predominant negative and cognitive symptoms of SSDs can refine and enhance the clinical care offered to such patients.
Acknowledgments
A.K. is supported by the US Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Central Texas Veterans Affairs Health Care System, and the VISN 17 Center of Excellence for Research on Returning War Veterans.
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415
1. Kantrowitz JT. Managing negative symptoms of schizophrenia: how far have we come? CNS Drugs. 2017;31(5):373-388. doi:10.1007/s40263-017-0428-x
2. Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151(3):351-356. doi:10.1176/ajp.151.3.351
3. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58(2):165. doi:10.1001/archpsyc.58.2.165
4. Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373-390. doi:10.1007/7854_2010_42
5. Green MF, Horan WP. Social cognition in schizophrenia. Curr Dir Psychol Sci. 2010;19(4):243-248. doi:10.1177/0963721410377600
6. Kingdon DG, Turkington D. Cognitive Therapy of Schizophrenia. Guilford Press; 2008.
7. Correll CU, Galling B, Pawar A, et al. Comparison of early intervention services vs treatment as usual for early-phase psychosis: a systematic review, meta-analysis, and meta-regression. JAMA Psychiatry. 2018;75(6):555. doi:10.1001/jamapsychiatry.2018.0623
8. McGorry PD. Early intervention in psychosis: obvious, effective, overdue. J Nerv Ment Dis. 2015;203(5):310-318. doi:10.1097/NMD.0000000000000284
9. Crisp AH, Gelder MG, Rix S, Meltzer HI, Rowlands OJ. Stigmatisation of people with mental illnesses. Br J Psychiatry. 2000;177(1):4-7. doi:10.1192/bjp.177.1.4
10. Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13-20. doi:10.1002/wps.20306
11. Kukla M, Davis LW, Lysaker PH. Cognitive behavioral therapy and work outcomes: correlates of treatment engagement and full and partial success in schizophrenia. Behav Cogn Psychother. 2014;42(5):577-592. doi:10.1017/S1352465813000428
12. Johansen R, Hestad K, Iversen VC, et al. Cognitive and clinical factors are associated with service engagement in early-phase schizophrenia spectrum disorders. J Nerv Ment Dis. 2011;199(3):176-182. doi:10.1097/NMD.0b013e31820bc2f9
13. Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, Nunes EV. Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug Alcohol Depend. 2006;81(3):313-322. doi:10.1016/j.drugalcdep.2005.08.003
14. Aarsland D, Taylor JP, Weintraub D. Psychiatric issues in cognitive impairment. Mov Disord. 2014;29(5):651-662. doi:10.1002/mds.25873
15. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. doi:10.1016/S0140-6736(13)60733-3
16. Khan AH, Zaidi S. Clozapine: Improvement of Negative Symptoms of Schizophrenia. Cureus. 2017;9(12):e1973. Published 2017 Dec 20. doi:10.7759/cureus.1973
17. Brar JS, Chengappa KN, Parepally H, et al. The effects of clozapine on negative symptoms in patients with schizophrenia with minimal positive symptoms. Ann Clin Psychiatry. 1997;9(4):227-234. doi:10.1023/a:1022352326334
18. Llorca PM, Lancon C, Farisse J, Scotto JC. Clozapine and negative symptoms. An open study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24(3):373-384. doi:10.1016/s0278-5846(99)00105-0
19. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385-392. doi:10.1192/bjp.bp.115.177261
20. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. doi:10.1176/appi.ajp.163.4.600
21. Stroup TS, Gerhard T, Crystal S, Huang C, Olfson M. Comparative Effectiveness of Clozapine and Standard Antipsychotic Treatment in Adults With Schizophrenia. Am J Psychiatry. 2016;173(2):166-173. doi:10.1176/appi.ajp.2015.15030332
22. Lee MA, Thompson PA, Meltzer HY. Effects of clozapine in cognitive function in schizophrenia. J Clin Psychiatry. 1994;55(suppl B):82-87.
23. Sharma T, Hughes C, Soni W, Kumari V. Cognitive effects of olanzapine and clozapine treatment in chronic schizophrenia. Psychopharmacology (Berl). 2003;169(3-4):398-403. doi:10.1007/s00213-003-1506-y
24. Spagna A, Dong Y, Mackie MA, et al. Clozapine improves the orienting of attention in schizophrenia. Schizophr Res. 2015;168(1-2):285-291. doi:10.1016/j.schres.2015.08.009
25. Savulich G, Mezquida G, Atkinson S, Bernardo M, Fernandez-Egea E. A case study of clozapine and cognition: friend or foe? J Clin Psychopharmacol. 2018;38(2):152-153. doi:10.1097/JCP.0000000000000847
26. Bogers JPAM, Schulte PFJ, Van Dijk D, Bakker B, Cohen D. Clozapine underutilization in the treatment of schizophrenia: how can clozapine prescription rates be improved? J Clin Psychopharmacol. 2016;36(2):109-111. doi:10.1097/JCP.0000000000000478
27. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing Barriers to Clozapine Underutilization: A National Effort. Psychiatr Serv. 2018;69(2):224-227. doi:10.1176/appi.ps.201700162
28. Honigfeld G, Arellano F, Sethi J, Bianchini A, Schein J. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3-7.
29. Cho J, Hayes RD, Jewell A, et al. Clozapine and all-cause mortality in treatment-resistant schizophrenia: a historical cohort study. Acta Psychiatr Scand. 2019;139(3):237-247. doi:10.1111/acps.12989
30. Kane JM. Clozapine Reduces All-Cause Mortality. Am J Psychiatry. 2017;174(10):920-921. doi:10.1176/appi.ajp.2017.17070770
31. Taipale H, Lähteenvuo M, Tanskanen A, Mittendorfer-Rutz E, Tiihonen J. Comparative Effectiveness of Antipsychotics for Risk of Attempted or Completed Suicide Among Persons With Schizophrenia. Schizophr Bull. 2021;47(1):23-30. doi:10.1093/schbul/sbaa111
32. Love RC, Kelly DL, Freudenreich O, Sayer MA. Clozapine underutilization: addressing the barriers. National Association of State Mental Health Program Directors; 2016. Accessed October 6, 2022. https://www.nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
33. Kelly DL, Ben-Yoav H, Payne GF, et al. Blood draw barriers for treatment with clozapine and development of a point-of-care monitoring device. Clin Schizophr Relat Psychoses. 2018;12(1):23-30. doi:10.3371/CSRP.KEBE.070415