User login
It all just clicks: Development of an inpatient e-consult program
Electronic consultation (e-consult) in the outpatient setting allows subspecialists to provide assessment and recommendations for patients without in-person visits.1 An e-consult is an asynchronous communication that uses the electronic medical record (EMR) and typically involves an electronic order from a requesting provider and an electronic note from a consulting provider. The initial motivation for developing this consultation modality was to improve access to subspecialty care for patients in the primary care setting, and findings of studies at several sites support this claim.1-4 In addition, e-consult may also reduce cost because converting unnecessary face-to-face encounters into e-consults reduces patients’ travel costs and healthcare organizations’ expensive subspecialty clinic time.3,5 Moreover, instead of addressing less complex clinical questions in informal, undocumented face-to-face or telephone “curbside” consultations with specialists, providers can instead ask for e-consults and thereby ensure thorough chart review and proper documentation.6
Use of e-consults in the inpatient setting is relatively novel.7 In addition to having the advantages already mentioned, e-consults are faster than in-person bedside consultations and may be beneficial in the fast-moving inpatient care setting. Finally, healthcare systems with multiple hospital sites may not have the capacity to physically locate subspecialists at each site, which makes e-consults attractive for avoiding unnecessary travel time.
In this article, we describe how we developed an inpatient e-consult protocol for a new, remote hospital within our healthcare system and explore data on safety and physician attitudes after e-consult implementation.
METHODS
The Institutional Review Board of the University of California San Francisco (UCSF) approved this study.
Setting
In February 2015, UCSF opened a new hospital in the Mission Bay neighborhood of San Francisco, 4 miles from the existing hospital. The new hospital is home to several adult inpatient services: urology, otolaryngology, colorectal surgery, obstetrics, and gynecologic surgery. A hospitalist is on-site 24 hours a day to provide consultation for these services around issues that relate to internal medicine. A hospitalist who requires subspecialty expertise to answer a clinical question can request a consultation by in-person visit, video telemedicine, or e-consult, each of which is available 24/7. Almost all of the medicine subspecialists work on the existing campus, not in Mission Bay.
Protocol Development and Implementation
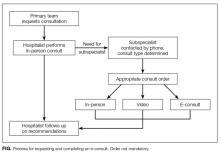
The protocol for the e-consult program was developed over several months by an interdisciplinary group that included 3 hospitalists, 1 obstetrician, 1 project manager, and 1 informaticist. The group outlined the process for requesting and completing an e-consult (Figure), designed a note template for consultants to use for EMR documentation, conducted outreach with subspecialty groups to discuss the protocol, and developed an EMR report to track e-consult use and content over time. As our medical center does not bill payers for inpatient e-consults, e-consult note tracking is used to provide reimbursement internally, from the medical center to the respective departments of the consultants. Reimbursement is made at a set rate per e-consult note, with the rate set to approximate the reimbursement of a low-acuity in-person consult on the main campus.
The workflow of an e-consult is as follows: (1) Whe
Evaluation
Each month, we tracked e-consult use using an EMR report built as part of the implementation of the program. For the first four months of implementation, every patient who received an e-consult also had a manual chart review of the period around the e-consult, performed by a hospitalist, in order to audit for any potential safety issues. These issues included, for example, an e-consult performed for a patient whose complexity or severity of illness was felt to be too great to defer an in-person visit, or a patient who received e-consult recommendations that were significantly retracted in a follow-up in-person note.
Eight months after the program started, we assessed experience by electronically surveying the 9 hospitalists and 11 consultants who had requested or performed at least 2 e-consults.8 Survey items were measured on a 5-point Likert scale: strongly disagree to strongly agree. The items, which related to ease of calling for a consultation, quality of e-consults, impact on clinical care, safety concerns, and satisfaction, were inspired by themes identified in a systematic review of the literature on e-consults in the outpatient setting.2 We sent 2 reminders to responders. Data were summarized using descriptive statistics. Analysis was performed in SPSS version 22.0 (IBM).
RESULTS
There were 143 initial subspecialty consultations by e-consult between program launch in February 2015 and manuscript preparation in February 2016, an average of 11 e-consults per month. There were 313 total e-consult notes (these included both initial and follow-up e-consult notes). By comparison, 240 initial in-person consultations occurred during the same period, and there were 435 total in-person consultation notes (46% new or initial notes, 54% follow-up notes). The top 5 subspecialties by volume of e-consults were infectious disease (35%), hematology (20%), endocrinology (14%), nephrology (13%), and cardiology (8%). For reference, e-consults are also available from psychiatry, neurology, oncology, gastroenterology, pulmonology, and rheumatology. Percentage of consultations performed during daytime hours (defined as 8 a.m. to 5 p.m.) was 92% for e-consults and 96% for in-person consultations.
There were no e-consult–related patient safety issues reported through the medical center’s incident reporting system during the study period. There were also no patient safety issues identified in the manual audits of 80 charts during the first 4 months of the program.
Seven (78%) of 9 hospitalists and 7 (64%) of 11 consultants completed the survey. Both groups agreed that e-consults were easy to use and efficient (Table). All hospitalists were satisfied with the quality of e-consult recommendations, but only 3 (43%) of the 7 consultants agreed they could provide high-quality consultation by e-consult. In their comments, 2 consultants expressed concerns. One concern was about missing crucial information by performing only a chart review, and the other was about being tempted to perform an e-consult simply because it is expedient.
DISCUSSION
Although use of e-consults in the outpatient setting is relatively commonplace, our program represents a novel use of e-consults in safely and efficiently providing subspecialty consultation to inpatients at a remote hospital.
For hospitalists, an e-consult system offers numerous benefits. Clinical questions beyond an internists’ scope of practice come up often, and simple questions might traditionally result in an informal curbside consult. While a curbside consult provides answers faster than an in-person visit, it creates risks for the requesting hospitalists: the consultants only know what they are told, whether the information is incomplete or erroneous; their opinions are given without documentation or compensation, which reduces a sense of accountability; and the lack of documentation does not allow their advice to persist in the chart as a reference for future providers.9 Our e-consult program solves these problems by requiring that consultants perform chart review and provide documentation as well as obligating the medical center to pay a small compensation to consultants for their time. We hope this lowers the bar to requesting consultation for remote sites, where the alternative would be burdensome travel time to do an in-person visit.
In our study, hospitalists were universally pleased with the quality of e-consult recommendations, but only 43% of consultants agreed. These findings correlate with the literature on e-consults in the outpatient setting.2 Unfortunately, our survey comments did not shed further light on this sentiment. In the outpatient literature, consultants were most concerned with having a clear clinical question, facing the liability of providing recommendations without performing an examination, and receiving appropriate compensation for answering e-consults.
The generalizability of our program findings is limited most significantly by the particular arrangement of our clinical services: Our remote site is home to a select group of adult inpatient services, a hospitalist is available on-site for these services 24 hours a day, and the distance to the remote site can be overcome with modest effort should a patient require an in-person visit in the initial or follow-up period. The generalizability of our safety findings is limited by the use of a single reviewer for chart auditing.
Given the rise of accountable care organizations and the prevalence of hospital mergers in the healthcare landscape, we believe that healthcare systems that operate remote sites under constrained budgets could look to e-consults to more cost-effectively extend subspecialty expertise across the inpatient enterprise. With improvements in health information exchange, it may also become feasible for consultants to offer e-consults to hospitals outside a medical center’s network. Our study showed that inpatient e-consult programs can be developed and implemented, that they appear not to pose any significant safety issues, and that they can facilitate delivery of timely clinical care.
Acknowledgment
The authors thank Raphaela Levy-Moore for creating and implementing the e-consult note template for our electronic medical record.
Disclosure
Nothing to report.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453. PubMed
2. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. PubMed
3. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654. PubMed
4. Bergman J, Neuhausen K, Chamie K, et al. Building a medical neighborhood in the safety net: an innovative technology improves hematuria workups. Urology. 2013;82(6):1277-1282. PubMed
5. Wasfy JH, Rao SK, Chittle MD, Gallen KM, Isselbacher EM, Ferris TG. Initial results of a cardiac e-consult pilot program. J Am Coll Cardiol. 2014;64(24):2706-2707. PubMed
6. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144. PubMed
7. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs health care system. JMIR Med Inform. 2016;4(1):e6. PubMed
8. REDCap. Vanderbilt University website. http://www.project-redcap.org. 2015. Accessed March 3, 2016.
9. Burden M, Sarcone E, Keniston A, et al. Prospective comparison of curbside versus formal consultations. J Hosp Med. 2013;8(1):31-35. PubMed
Electronic consultation (e-consult) in the outpatient setting allows subspecialists to provide assessment and recommendations for patients without in-person visits.1 An e-consult is an asynchronous communication that uses the electronic medical record (EMR) and typically involves an electronic order from a requesting provider and an electronic note from a consulting provider. The initial motivation for developing this consultation modality was to improve access to subspecialty care for patients in the primary care setting, and findings of studies at several sites support this claim.1-4 In addition, e-consult may also reduce cost because converting unnecessary face-to-face encounters into e-consults reduces patients’ travel costs and healthcare organizations’ expensive subspecialty clinic time.3,5 Moreover, instead of addressing less complex clinical questions in informal, undocumented face-to-face or telephone “curbside” consultations with specialists, providers can instead ask for e-consults and thereby ensure thorough chart review and proper documentation.6
Use of e-consults in the inpatient setting is relatively novel.7 In addition to having the advantages already mentioned, e-consults are faster than in-person bedside consultations and may be beneficial in the fast-moving inpatient care setting. Finally, healthcare systems with multiple hospital sites may not have the capacity to physically locate subspecialists at each site, which makes e-consults attractive for avoiding unnecessary travel time.
In this article, we describe how we developed an inpatient e-consult protocol for a new, remote hospital within our healthcare system and explore data on safety and physician attitudes after e-consult implementation.
METHODS
The Institutional Review Board of the University of California San Francisco (UCSF) approved this study.
Setting
In February 2015, UCSF opened a new hospital in the Mission Bay neighborhood of San Francisco, 4 miles from the existing hospital. The new hospital is home to several adult inpatient services: urology, otolaryngology, colorectal surgery, obstetrics, and gynecologic surgery. A hospitalist is on-site 24 hours a day to provide consultation for these services around issues that relate to internal medicine. A hospitalist who requires subspecialty expertise to answer a clinical question can request a consultation by in-person visit, video telemedicine, or e-consult, each of which is available 24/7. Almost all of the medicine subspecialists work on the existing campus, not in Mission Bay.
Protocol Development and Implementation
The protocol for the e-consult program was developed over several months by an interdisciplinary group that included 3 hospitalists, 1 obstetrician, 1 project manager, and 1 informaticist. The group outlined the process for requesting and completing an e-consult (Figure), designed a note template for consultants to use for EMR documentation, conducted outreach with subspecialty groups to discuss the protocol, and developed an EMR report to track e-consult use and content over time. As our medical center does not bill payers for inpatient e-consults, e-consult note tracking is used to provide reimbursement internally, from the medical center to the respective departments of the consultants. Reimbursement is made at a set rate per e-consult note, with the rate set to approximate the reimbursement of a low-acuity in-person consult on the main campus.
The workflow of an e-consult is as follows: (1) Whe
Evaluation
Each month, we tracked e-consult use using an EMR report built as part of the implementation of the program. For the first four months of implementation, every patient who received an e-consult also had a manual chart review of the period around the e-consult, performed by a hospitalist, in order to audit for any potential safety issues. These issues included, for example, an e-consult performed for a patient whose complexity or severity of illness was felt to be too great to defer an in-person visit, or a patient who received e-consult recommendations that were significantly retracted in a follow-up in-person note.
Eight months after the program started, we assessed experience by electronically surveying the 9 hospitalists and 11 consultants who had requested or performed at least 2 e-consults.8 Survey items were measured on a 5-point Likert scale: strongly disagree to strongly agree. The items, which related to ease of calling for a consultation, quality of e-consults, impact on clinical care, safety concerns, and satisfaction, were inspired by themes identified in a systematic review of the literature on e-consults in the outpatient setting.2 We sent 2 reminders to responders. Data were summarized using descriptive statistics. Analysis was performed in SPSS version 22.0 (IBM).
RESULTS
There were 143 initial subspecialty consultations by e-consult between program launch in February 2015 and manuscript preparation in February 2016, an average of 11 e-consults per month. There were 313 total e-consult notes (these included both initial and follow-up e-consult notes). By comparison, 240 initial in-person consultations occurred during the same period, and there were 435 total in-person consultation notes (46% new or initial notes, 54% follow-up notes). The top 5 subspecialties by volume of e-consults were infectious disease (35%), hematology (20%), endocrinology (14%), nephrology (13%), and cardiology (8%). For reference, e-consults are also available from psychiatry, neurology, oncology, gastroenterology, pulmonology, and rheumatology. Percentage of consultations performed during daytime hours (defined as 8 a.m. to 5 p.m.) was 92% for e-consults and 96% for in-person consultations.
There were no e-consult–related patient safety issues reported through the medical center’s incident reporting system during the study period. There were also no patient safety issues identified in the manual audits of 80 charts during the first 4 months of the program.
Seven (78%) of 9 hospitalists and 7 (64%) of 11 consultants completed the survey. Both groups agreed that e-consults were easy to use and efficient (Table). All hospitalists were satisfied with the quality of e-consult recommendations, but only 3 (43%) of the 7 consultants agreed they could provide high-quality consultation by e-consult. In their comments, 2 consultants expressed concerns. One concern was about missing crucial information by performing only a chart review, and the other was about being tempted to perform an e-consult simply because it is expedient.
DISCUSSION
Although use of e-consults in the outpatient setting is relatively commonplace, our program represents a novel use of e-consults in safely and efficiently providing subspecialty consultation to inpatients at a remote hospital.
For hospitalists, an e-consult system offers numerous benefits. Clinical questions beyond an internists’ scope of practice come up often, and simple questions might traditionally result in an informal curbside consult. While a curbside consult provides answers faster than an in-person visit, it creates risks for the requesting hospitalists: the consultants only know what they are told, whether the information is incomplete or erroneous; their opinions are given without documentation or compensation, which reduces a sense of accountability; and the lack of documentation does not allow their advice to persist in the chart as a reference for future providers.9 Our e-consult program solves these problems by requiring that consultants perform chart review and provide documentation as well as obligating the medical center to pay a small compensation to consultants for their time. We hope this lowers the bar to requesting consultation for remote sites, where the alternative would be burdensome travel time to do an in-person visit.
In our study, hospitalists were universally pleased with the quality of e-consult recommendations, but only 43% of consultants agreed. These findings correlate with the literature on e-consults in the outpatient setting.2 Unfortunately, our survey comments did not shed further light on this sentiment. In the outpatient literature, consultants were most concerned with having a clear clinical question, facing the liability of providing recommendations without performing an examination, and receiving appropriate compensation for answering e-consults.
The generalizability of our program findings is limited most significantly by the particular arrangement of our clinical services: Our remote site is home to a select group of adult inpatient services, a hospitalist is available on-site for these services 24 hours a day, and the distance to the remote site can be overcome with modest effort should a patient require an in-person visit in the initial or follow-up period. The generalizability of our safety findings is limited by the use of a single reviewer for chart auditing.
Given the rise of accountable care organizations and the prevalence of hospital mergers in the healthcare landscape, we believe that healthcare systems that operate remote sites under constrained budgets could look to e-consults to more cost-effectively extend subspecialty expertise across the inpatient enterprise. With improvements in health information exchange, it may also become feasible for consultants to offer e-consults to hospitals outside a medical center’s network. Our study showed that inpatient e-consult programs can be developed and implemented, that they appear not to pose any significant safety issues, and that they can facilitate delivery of timely clinical care.
Acknowledgment
The authors thank Raphaela Levy-Moore for creating and implementing the e-consult note template for our electronic medical record.
Disclosure
Nothing to report.
Electronic consultation (e-consult) in the outpatient setting allows subspecialists to provide assessment and recommendations for patients without in-person visits.1 An e-consult is an asynchronous communication that uses the electronic medical record (EMR) and typically involves an electronic order from a requesting provider and an electronic note from a consulting provider. The initial motivation for developing this consultation modality was to improve access to subspecialty care for patients in the primary care setting, and findings of studies at several sites support this claim.1-4 In addition, e-consult may also reduce cost because converting unnecessary face-to-face encounters into e-consults reduces patients’ travel costs and healthcare organizations’ expensive subspecialty clinic time.3,5 Moreover, instead of addressing less complex clinical questions in informal, undocumented face-to-face or telephone “curbside” consultations with specialists, providers can instead ask for e-consults and thereby ensure thorough chart review and proper documentation.6
Use of e-consults in the inpatient setting is relatively novel.7 In addition to having the advantages already mentioned, e-consults are faster than in-person bedside consultations and may be beneficial in the fast-moving inpatient care setting. Finally, healthcare systems with multiple hospital sites may not have the capacity to physically locate subspecialists at each site, which makes e-consults attractive for avoiding unnecessary travel time.
In this article, we describe how we developed an inpatient e-consult protocol for a new, remote hospital within our healthcare system and explore data on safety and physician attitudes after e-consult implementation.
METHODS
The Institutional Review Board of the University of California San Francisco (UCSF) approved this study.
Setting
In February 2015, UCSF opened a new hospital in the Mission Bay neighborhood of San Francisco, 4 miles from the existing hospital. The new hospital is home to several adult inpatient services: urology, otolaryngology, colorectal surgery, obstetrics, and gynecologic surgery. A hospitalist is on-site 24 hours a day to provide consultation for these services around issues that relate to internal medicine. A hospitalist who requires subspecialty expertise to answer a clinical question can request a consultation by in-person visit, video telemedicine, or e-consult, each of which is available 24/7. Almost all of the medicine subspecialists work on the existing campus, not in Mission Bay.
Protocol Development and Implementation
The protocol for the e-consult program was developed over several months by an interdisciplinary group that included 3 hospitalists, 1 obstetrician, 1 project manager, and 1 informaticist. The group outlined the process for requesting and completing an e-consult (Figure), designed a note template for consultants to use for EMR documentation, conducted outreach with subspecialty groups to discuss the protocol, and developed an EMR report to track e-consult use and content over time. As our medical center does not bill payers for inpatient e-consults, e-consult note tracking is used to provide reimbursement internally, from the medical center to the respective departments of the consultants. Reimbursement is made at a set rate per e-consult note, with the rate set to approximate the reimbursement of a low-acuity in-person consult on the main campus.
The workflow of an e-consult is as follows: (1) Whe
Evaluation
Each month, we tracked e-consult use using an EMR report built as part of the implementation of the program. For the first four months of implementation, every patient who received an e-consult also had a manual chart review of the period around the e-consult, performed by a hospitalist, in order to audit for any potential safety issues. These issues included, for example, an e-consult performed for a patient whose complexity or severity of illness was felt to be too great to defer an in-person visit, or a patient who received e-consult recommendations that were significantly retracted in a follow-up in-person note.
Eight months after the program started, we assessed experience by electronically surveying the 9 hospitalists and 11 consultants who had requested or performed at least 2 e-consults.8 Survey items were measured on a 5-point Likert scale: strongly disagree to strongly agree. The items, which related to ease of calling for a consultation, quality of e-consults, impact on clinical care, safety concerns, and satisfaction, were inspired by themes identified in a systematic review of the literature on e-consults in the outpatient setting.2 We sent 2 reminders to responders. Data were summarized using descriptive statistics. Analysis was performed in SPSS version 22.0 (IBM).
RESULTS
There were 143 initial subspecialty consultations by e-consult between program launch in February 2015 and manuscript preparation in February 2016, an average of 11 e-consults per month. There were 313 total e-consult notes (these included both initial and follow-up e-consult notes). By comparison, 240 initial in-person consultations occurred during the same period, and there were 435 total in-person consultation notes (46% new or initial notes, 54% follow-up notes). The top 5 subspecialties by volume of e-consults were infectious disease (35%), hematology (20%), endocrinology (14%), nephrology (13%), and cardiology (8%). For reference, e-consults are also available from psychiatry, neurology, oncology, gastroenterology, pulmonology, and rheumatology. Percentage of consultations performed during daytime hours (defined as 8 a.m. to 5 p.m.) was 92% for e-consults and 96% for in-person consultations.
There were no e-consult–related patient safety issues reported through the medical center’s incident reporting system during the study period. There were also no patient safety issues identified in the manual audits of 80 charts during the first 4 months of the program.
Seven (78%) of 9 hospitalists and 7 (64%) of 11 consultants completed the survey. Both groups agreed that e-consults were easy to use and efficient (Table). All hospitalists were satisfied with the quality of e-consult recommendations, but only 3 (43%) of the 7 consultants agreed they could provide high-quality consultation by e-consult. In their comments, 2 consultants expressed concerns. One concern was about missing crucial information by performing only a chart review, and the other was about being tempted to perform an e-consult simply because it is expedient.
DISCUSSION
Although use of e-consults in the outpatient setting is relatively commonplace, our program represents a novel use of e-consults in safely and efficiently providing subspecialty consultation to inpatients at a remote hospital.
For hospitalists, an e-consult system offers numerous benefits. Clinical questions beyond an internists’ scope of practice come up often, and simple questions might traditionally result in an informal curbside consult. While a curbside consult provides answers faster than an in-person visit, it creates risks for the requesting hospitalists: the consultants only know what they are told, whether the information is incomplete or erroneous; their opinions are given without documentation or compensation, which reduces a sense of accountability; and the lack of documentation does not allow their advice to persist in the chart as a reference for future providers.9 Our e-consult program solves these problems by requiring that consultants perform chart review and provide documentation as well as obligating the medical center to pay a small compensation to consultants for their time. We hope this lowers the bar to requesting consultation for remote sites, where the alternative would be burdensome travel time to do an in-person visit.
In our study, hospitalists were universally pleased with the quality of e-consult recommendations, but only 43% of consultants agreed. These findings correlate with the literature on e-consults in the outpatient setting.2 Unfortunately, our survey comments did not shed further light on this sentiment. In the outpatient literature, consultants were most concerned with having a clear clinical question, facing the liability of providing recommendations without performing an examination, and receiving appropriate compensation for answering e-consults.
The generalizability of our program findings is limited most significantly by the particular arrangement of our clinical services: Our remote site is home to a select group of adult inpatient services, a hospitalist is available on-site for these services 24 hours a day, and the distance to the remote site can be overcome with modest effort should a patient require an in-person visit in the initial or follow-up period. The generalizability of our safety findings is limited by the use of a single reviewer for chart auditing.
Given the rise of accountable care organizations and the prevalence of hospital mergers in the healthcare landscape, we believe that healthcare systems that operate remote sites under constrained budgets could look to e-consults to more cost-effectively extend subspecialty expertise across the inpatient enterprise. With improvements in health information exchange, it may also become feasible for consultants to offer e-consults to hospitals outside a medical center’s network. Our study showed that inpatient e-consult programs can be developed and implemented, that they appear not to pose any significant safety issues, and that they can facilitate delivery of timely clinical care.
Acknowledgment
The authors thank Raphaela Levy-Moore for creating and implementing the e-consult note template for our electronic medical record.
Disclosure
Nothing to report.
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453. PubMed
2. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. PubMed
3. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654. PubMed
4. Bergman J, Neuhausen K, Chamie K, et al. Building a medical neighborhood in the safety net: an innovative technology improves hematuria workups. Urology. 2013;82(6):1277-1282. PubMed
5. Wasfy JH, Rao SK, Chittle MD, Gallen KM, Isselbacher EM, Ferris TG. Initial results of a cardiac e-consult pilot program. J Am Coll Cardiol. 2014;64(24):2706-2707. PubMed
6. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144. PubMed
7. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs health care system. JMIR Med Inform. 2016;4(1):e6. PubMed
8. REDCap. Vanderbilt University website. http://www.project-redcap.org. 2015. Accessed March 3, 2016.
9. Burden M, Sarcone E, Keniston A, et al. Prospective comparison of curbside versus formal consultations. J Hosp Med. 2013;8(1):31-35. PubMed
1. Chen AH, Murphy EJ, Yee HF Jr. eReferral—a new model for integrated care. N Engl J Med. 2013;368(26):2450-2453. PubMed
2. Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330. PubMed
3. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654. PubMed
4. Bergman J, Neuhausen K, Chamie K, et al. Building a medical neighborhood in the safety net: an innovative technology improves hematuria workups. Urology. 2013;82(6):1277-1282. PubMed
5. Wasfy JH, Rao SK, Chittle MD, Gallen KM, Isselbacher EM, Ferris TG. Initial results of a cardiac e-consult pilot program. J Am Coll Cardiol. 2014;64(24):2706-2707. PubMed
6. Perley CM. Physician use of the curbside consultation to address information needs: report on a collective case study. J Med Libr Assoc. 2006;94(2):137-144. PubMed
7. Gupte G, Vimalananda V, Simon SR, DeVito K, Clark J, Orlander JD. Disruptive innovation: implementation of electronic consultations in a Veterans Affairs health care system. JMIR Med Inform. 2016;4(1):e6. PubMed
8. REDCap. Vanderbilt University website. http://www.project-redcap.org. 2015. Accessed March 3, 2016.
9. Burden M, Sarcone E, Keniston A, et al. Prospective comparison of curbside versus formal consultations. J Hosp Med. 2013;8(1):31-35. PubMed
© 2017 Society of Hospital Medicine
Clinical utility of routine CBC testing in patients with community-acquired pneumonia
Avoiding repeated complete blood count (CBC) tests in the face of clinical and lab stability is a focus of the Choosing Wisely® initiatives launched by the American Board of Internal Medicine Foundation1 and endorsed by the Society of Hospital Medicine.2 However, specific scenarios in which daily morning labs can be safely avoided have not been identified. The goal of this study was to identify situations in which routine CBC testing can be avoided in patients with community-acquired pneumonia (CAP), one of the most common reasons for hospital admission.3
METHODS
This was a retrospective study of 50 patients with CAP discharged from our hospital between February 1, 2015 and May 1, 2015. We performed chart abstractions collecting daily vital signs, lab results, provider notes including assessments and plans (A&Ps), and order entry logs, as well as documentation indicating whether a lab result or clinical finding appeared to affect clinical management (eg, a new order or documentation of changing plans). Both escalations and de-escalations were included as management changes. For example, if the note stated “Persistent leukocytosis, add vancomycin,” then the clinical action of expanded antibiotic coverage would be attributed to the CBC.
We defined clinical stability based on Definition B of the Pneumonia Patient Outcomes Research Team (PORT) study criteria.4 We used descriptive statistics and likelihood ratios to characterize the utility of CBC testing in terms of producing clinical management changes. Likelihood ratios were calculated with the “test” representing a CBC being ordered or not ordered and the outcome being any change in management independent of whether it was due to the CBC.
RESULTS
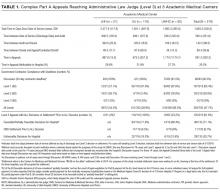
Of 50 patients, 33 (66%) were female, the mean age was 75 years, the mean length of stay was 2.8 days, and the median CURB-65 score,5 an estimate of mortality in CAP used for decision-making about inpatient versus outpatient treatment, was 1 (25th to 75th interquartile range: 1, 2); no patients had a CURB score greater than 3 (Table 1). Forty-one (82%) patients met PORT clinical stability criteria prior to discharge, and 30 (75% of stable patients) had CBCs obtained.
On days after admission, 94 subsequent CBCs were obtained. Of these CBCs, 6 (6.4%) were associated with management changes indicated in documentation or orders (Table 2). In 2 of the 6 patients, management changes were likely relevant to pneumonia. In the first case, the patient had a white blood cell count (WBC) of 15.4 on the planned day of discharge but no accompanying clinical changes. Her discharge was potentially delayed pending a repeat CBC which again showed a WBC 14.7; the patient was then discharged without any additional changes in plan. In the second case, the patient experienced new-onset altered mental status on hospital day 3 and increasing O2 requirement with a rising WBC noted on hospital day 4. Repeat chest x-ray, repeat blood cultures, and an ultrasound for parapneumonic effusion were obtained, and the patient’s symptoms and signs resolved over a period of days without changes in treatment. In the 4 other cases, available documentation suggested the hemoglobin abnormalities found represented chronic or incidental illnesses, specifically iron deficiency anemia, iatrogenic anemia due to fluid resuscitation and hemodilution, previously known chronic lymphocytic leukemia, and thrombocytopenia due to acute infection. In all 6 instances, CBC values improved without treatment intervention.
Among all patients, the positive likelihood ratio of CBCs obtained after admission in terms of being followed by a change in clinical management was very poor (1.12, 95% confidence interval [CI], 0.86-1.44). For clinically unstable patients, there were 64 CBCs ordered, and the likelihood ratio was similar at 0.98 (95% CI, 0.75-1.29). The positive likelihood ratio among clinically stable patients, who had 30 CBCs ordered, was still quite weak, though confidence intervals were wider (1.23, 95% CI, 0.66-2.29).
DISCUSSION
Though small, our initial study suggests the potential opportunity for savings if Choosing Wisely® recommendations for CBC testing were implemented in patients with community-acquired pneumonia.
Our study has several limitations. Note-writing practices and ordering patterns likely varied between providers, and documentation bias may play a role in our results. However, we defined whether a CBC was associated with changes in clinical decision-making or management by incorporating a number of mutually reinforcing elements of the medical record. We recognize, however, that our approach may not capture undocumented clinical issues or other cognitive (eg, reassurance of clinical resolution) reasons why CBCs were obtained.
Even with these limitations, the likelihood of a CBC value meaningfully changing clinical management among patients with CAP appears to be quite low as evidenced by the case descriptions, particularly when obtained in stable patients by PORT criteria and on the day of discharge. Whether clinical stability as measured by PORT score can be used to target patients in whom CBC testing is unnecessary is difficult to discern from our data, as the overall utility of CBCs obtained after admission was quite low and the rate of changes in management was also low. However, even if CBCs are not particularly costly, unnecessary testing may produce harm in the form of prolonged length of stay, making even one unnecessary CBC potentially extremely expensive. More research involving larger-scale studies are needed to determine the “number needed to screen” for the daily CBC in CAP to determine if the cost savings from overtesting and treatment outweigh the potential benefit of a single CBC that changes management.
Disclosure
Nothing to report.
1. Choosing Wisely. Promoting conversations between providers and patients. Choosing Wisely. http://www.choosingwisely.org/. Accessed March 28, 2016.
2. Beresford L. The Society of Hospital Medicine’s “Choosing Wisely” Recommendations for Hospitalists. 2013. http://www.the-hospitalist.org/article/the-society-of-hospital-medicines-choosing-wisely-recommendations-for-hospitalists/. Accessed March 28, 2016.
3. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. PubMed
4. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: Implications for practice guidelines. JAMA. 1998;279(18):1452-1457. PubMed
5. Lim W, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382. PubMed
Avoiding repeated complete blood count (CBC) tests in the face of clinical and lab stability is a focus of the Choosing Wisely® initiatives launched by the American Board of Internal Medicine Foundation1 and endorsed by the Society of Hospital Medicine.2 However, specific scenarios in which daily morning labs can be safely avoided have not been identified. The goal of this study was to identify situations in which routine CBC testing can be avoided in patients with community-acquired pneumonia (CAP), one of the most common reasons for hospital admission.3
METHODS
This was a retrospective study of 50 patients with CAP discharged from our hospital between February 1, 2015 and May 1, 2015. We performed chart abstractions collecting daily vital signs, lab results, provider notes including assessments and plans (A&Ps), and order entry logs, as well as documentation indicating whether a lab result or clinical finding appeared to affect clinical management (eg, a new order or documentation of changing plans). Both escalations and de-escalations were included as management changes. For example, if the note stated “Persistent leukocytosis, add vancomycin,” then the clinical action of expanded antibiotic coverage would be attributed to the CBC.
We defined clinical stability based on Definition B of the Pneumonia Patient Outcomes Research Team (PORT) study criteria.4 We used descriptive statistics and likelihood ratios to characterize the utility of CBC testing in terms of producing clinical management changes. Likelihood ratios were calculated with the “test” representing a CBC being ordered or not ordered and the outcome being any change in management independent of whether it was due to the CBC.
RESULTS
Of 50 patients, 33 (66%) were female, the mean age was 75 years, the mean length of stay was 2.8 days, and the median CURB-65 score,5 an estimate of mortality in CAP used for decision-making about inpatient versus outpatient treatment, was 1 (25th to 75th interquartile range: 1, 2); no patients had a CURB score greater than 3 (Table 1). Forty-one (82%) patients met PORT clinical stability criteria prior to discharge, and 30 (75% of stable patients) had CBCs obtained.
On days after admission, 94 subsequent CBCs were obtained. Of these CBCs, 6 (6.4%) were associated with management changes indicated in documentation or orders (Table 2). In 2 of the 6 patients, management changes were likely relevant to pneumonia. In the first case, the patient had a white blood cell count (WBC) of 15.4 on the planned day of discharge but no accompanying clinical changes. Her discharge was potentially delayed pending a repeat CBC which again showed a WBC 14.7; the patient was then discharged without any additional changes in plan. In the second case, the patient experienced new-onset altered mental status on hospital day 3 and increasing O2 requirement with a rising WBC noted on hospital day 4. Repeat chest x-ray, repeat blood cultures, and an ultrasound for parapneumonic effusion were obtained, and the patient’s symptoms and signs resolved over a period of days without changes in treatment. In the 4 other cases, available documentation suggested the hemoglobin abnormalities found represented chronic or incidental illnesses, specifically iron deficiency anemia, iatrogenic anemia due to fluid resuscitation and hemodilution, previously known chronic lymphocytic leukemia, and thrombocytopenia due to acute infection. In all 6 instances, CBC values improved without treatment intervention.
Among all patients, the positive likelihood ratio of CBCs obtained after admission in terms of being followed by a change in clinical management was very poor (1.12, 95% confidence interval [CI], 0.86-1.44). For clinically unstable patients, there were 64 CBCs ordered, and the likelihood ratio was similar at 0.98 (95% CI, 0.75-1.29). The positive likelihood ratio among clinically stable patients, who had 30 CBCs ordered, was still quite weak, though confidence intervals were wider (1.23, 95% CI, 0.66-2.29).
DISCUSSION
Though small, our initial study suggests the potential opportunity for savings if Choosing Wisely® recommendations for CBC testing were implemented in patients with community-acquired pneumonia.
Our study has several limitations. Note-writing practices and ordering patterns likely varied between providers, and documentation bias may play a role in our results. However, we defined whether a CBC was associated with changes in clinical decision-making or management by incorporating a number of mutually reinforcing elements of the medical record. We recognize, however, that our approach may not capture undocumented clinical issues or other cognitive (eg, reassurance of clinical resolution) reasons why CBCs were obtained.
Even with these limitations, the likelihood of a CBC value meaningfully changing clinical management among patients with CAP appears to be quite low as evidenced by the case descriptions, particularly when obtained in stable patients by PORT criteria and on the day of discharge. Whether clinical stability as measured by PORT score can be used to target patients in whom CBC testing is unnecessary is difficult to discern from our data, as the overall utility of CBCs obtained after admission was quite low and the rate of changes in management was also low. However, even if CBCs are not particularly costly, unnecessary testing may produce harm in the form of prolonged length of stay, making even one unnecessary CBC potentially extremely expensive. More research involving larger-scale studies are needed to determine the “number needed to screen” for the daily CBC in CAP to determine if the cost savings from overtesting and treatment outweigh the potential benefit of a single CBC that changes management.
Disclosure
Nothing to report.
Avoiding repeated complete blood count (CBC) tests in the face of clinical and lab stability is a focus of the Choosing Wisely® initiatives launched by the American Board of Internal Medicine Foundation1 and endorsed by the Society of Hospital Medicine.2 However, specific scenarios in which daily morning labs can be safely avoided have not been identified. The goal of this study was to identify situations in which routine CBC testing can be avoided in patients with community-acquired pneumonia (CAP), one of the most common reasons for hospital admission.3
METHODS
This was a retrospective study of 50 patients with CAP discharged from our hospital between February 1, 2015 and May 1, 2015. We performed chart abstractions collecting daily vital signs, lab results, provider notes including assessments and plans (A&Ps), and order entry logs, as well as documentation indicating whether a lab result or clinical finding appeared to affect clinical management (eg, a new order or documentation of changing plans). Both escalations and de-escalations were included as management changes. For example, if the note stated “Persistent leukocytosis, add vancomycin,” then the clinical action of expanded antibiotic coverage would be attributed to the CBC.
We defined clinical stability based on Definition B of the Pneumonia Patient Outcomes Research Team (PORT) study criteria.4 We used descriptive statistics and likelihood ratios to characterize the utility of CBC testing in terms of producing clinical management changes. Likelihood ratios were calculated with the “test” representing a CBC being ordered or not ordered and the outcome being any change in management independent of whether it was due to the CBC.
RESULTS
Of 50 patients, 33 (66%) were female, the mean age was 75 years, the mean length of stay was 2.8 days, and the median CURB-65 score,5 an estimate of mortality in CAP used for decision-making about inpatient versus outpatient treatment, was 1 (25th to 75th interquartile range: 1, 2); no patients had a CURB score greater than 3 (Table 1). Forty-one (82%) patients met PORT clinical stability criteria prior to discharge, and 30 (75% of stable patients) had CBCs obtained.
On days after admission, 94 subsequent CBCs were obtained. Of these CBCs, 6 (6.4%) were associated with management changes indicated in documentation or orders (Table 2). In 2 of the 6 patients, management changes were likely relevant to pneumonia. In the first case, the patient had a white blood cell count (WBC) of 15.4 on the planned day of discharge but no accompanying clinical changes. Her discharge was potentially delayed pending a repeat CBC which again showed a WBC 14.7; the patient was then discharged without any additional changes in plan. In the second case, the patient experienced new-onset altered mental status on hospital day 3 and increasing O2 requirement with a rising WBC noted on hospital day 4. Repeat chest x-ray, repeat blood cultures, and an ultrasound for parapneumonic effusion were obtained, and the patient’s symptoms and signs resolved over a period of days without changes in treatment. In the 4 other cases, available documentation suggested the hemoglobin abnormalities found represented chronic or incidental illnesses, specifically iron deficiency anemia, iatrogenic anemia due to fluid resuscitation and hemodilution, previously known chronic lymphocytic leukemia, and thrombocytopenia due to acute infection. In all 6 instances, CBC values improved without treatment intervention.
Among all patients, the positive likelihood ratio of CBCs obtained after admission in terms of being followed by a change in clinical management was very poor (1.12, 95% confidence interval [CI], 0.86-1.44). For clinically unstable patients, there were 64 CBCs ordered, and the likelihood ratio was similar at 0.98 (95% CI, 0.75-1.29). The positive likelihood ratio among clinically stable patients, who had 30 CBCs ordered, was still quite weak, though confidence intervals were wider (1.23, 95% CI, 0.66-2.29).
DISCUSSION
Though small, our initial study suggests the potential opportunity for savings if Choosing Wisely® recommendations for CBC testing were implemented in patients with community-acquired pneumonia.
Our study has several limitations. Note-writing practices and ordering patterns likely varied between providers, and documentation bias may play a role in our results. However, we defined whether a CBC was associated with changes in clinical decision-making or management by incorporating a number of mutually reinforcing elements of the medical record. We recognize, however, that our approach may not capture undocumented clinical issues or other cognitive (eg, reassurance of clinical resolution) reasons why CBCs were obtained.
Even with these limitations, the likelihood of a CBC value meaningfully changing clinical management among patients with CAP appears to be quite low as evidenced by the case descriptions, particularly when obtained in stable patients by PORT criteria and on the day of discharge. Whether clinical stability as measured by PORT score can be used to target patients in whom CBC testing is unnecessary is difficult to discern from our data, as the overall utility of CBCs obtained after admission was quite low and the rate of changes in management was also low. However, even if CBCs are not particularly costly, unnecessary testing may produce harm in the form of prolonged length of stay, making even one unnecessary CBC potentially extremely expensive. More research involving larger-scale studies are needed to determine the “number needed to screen” for the daily CBC in CAP to determine if the cost savings from overtesting and treatment outweigh the potential benefit of a single CBC that changes management.
Disclosure
Nothing to report.
1. Choosing Wisely. Promoting conversations between providers and patients. Choosing Wisely. http://www.choosingwisely.org/. Accessed March 28, 2016.
2. Beresford L. The Society of Hospital Medicine’s “Choosing Wisely” Recommendations for Hospitalists. 2013. http://www.the-hospitalist.org/article/the-society-of-hospital-medicines-choosing-wisely-recommendations-for-hospitalists/. Accessed March 28, 2016.
3. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. PubMed
4. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: Implications for practice guidelines. JAMA. 1998;279(18):1452-1457. PubMed
5. Lim W, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382. PubMed
1. Choosing Wisely. Promoting conversations between providers and patients. Choosing Wisely. http://www.choosingwisely.org/. Accessed March 28, 2016.
2. Beresford L. The Society of Hospital Medicine’s “Choosing Wisely” Recommendations for Hospitalists. 2013. http://www.the-hospitalist.org/article/the-society-of-hospital-medicines-choosing-wisely-recommendations-for-hospitalists/. Accessed March 28, 2016.
3. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med. 2010;122(2):130-141. PubMed
4. Halm EA, Fine MJ, Marrie TJ, et al. Time to clinical stability in patients hospitalized with community-acquired pneumonia: Implications for practice guidelines. JAMA. 1998;279(18):1452-1457. PubMed
5. Lim W, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377-382. PubMed
Planning and designing the Improving Addiction Care Team (IMPACT) for hospitalized adults with substance use disorder
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
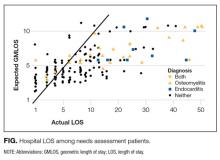
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
Addiction is a national epidemic that represents both a pressing need and a significant burden to the healthcare system.1 Hospitals are increasingly filled with people admitted for medical complications of substance use disorders (SUD).2People with SUD have longer lengths of stay (LOS) and high readmission rates.3 Hospitalization often does not address the root cause—the SUD. For example, many hospitals replace heart valves and deliver prolonged courses of intravenous (IV) antibiotics for endocarditis from injection drug use but do not offer addiction medicine consultation, medication for addiction treatment (MAT), or linkage to posthospital SUD treatment.4,5
Hospitalization can provide reachable moments for initiating addiction care.6 Medications for opioid7 and alcohol use disorders8 can be started during hospitalization, promoting engagement in outpatient SUD care7 and increased uptake of MAT,7-9 and reducing readmissions.8,10 Yet, medications for SUD are underprescribed,11,12 and most hospitals lack inpatient addiction medicine services and pathways to timely SUD care after discharge. Furthermore, traditional SUD treatment programs are often not equipped to manage medically complex patients or they have long waitlists.13 Most behavioral-physical health integration occurs in ambulatory settings. This fails to engage patients who do not access primary care. There is an urgent need for models that can improve care for hospitalized patients with SUD.
Here, we describe our experience using patient needs assessment to engage stakeholders and drive local systems change. We also describe the resulting care model, the Improving Addiction Care Team (IMPACT). Our experience provides a potentially useful example to other hospitals and communities seeking to address the national SUD epidemic.
METHODS
Setting
In 2012, Oregon transformed its Medicaid system by establishing 16 regional “coordinated care organizations” (CCOs) to improve outcomes and slow healthcare spending.14 In a CCO environment, hospitals assume increased financial risk, yet reforms have focused on the outpatient setting. Therefore, executive leadership at Oregon Health & Science University (OHSU), an urban academic medical center, asked clinician-leaders to design point-of-care improvements for Medicaid-funded adults and build on existing models to improve care for socioeconomically vulnerable adults.15,16 One priority that emerged was to make improvements for hospitalized adults with SUD. Of the adult inpatients at OHSU, 30% have Medicaid and 15% have SUD by administrative data alone. Before we started our work, OHSU lacked inpatient addiction medicine services.
Local Needs Assessment
To understand local needs and opportunities, we surveyed hospitalized adults with SUD. We used the electronic health record to generate a list of inpatients flagged by nurses for risky alcohol or drug use. A research assistant screened consecutive adults (≥18 years old) and invited those who screened positive for alcohol use (Alcohol Use Disorders Identification Test–Consumption [AUDIT-C])17 or drug use (single-item screener)18 to participate. We excluded non-English speakers, incarcerated adults, people using only marijuana or tobacco, psychiatry inpatients, and people unable to consent. Surveys assessed social and demographic factors, healthcare utilization, substance use severity, and treatment experience. Participants who reported high-risk illicit drug or alcohol use19 were asked to indicate their readiness to change on a 3-point scale developed for this study. Response range included: no interest, interest in cutting back, or interest in quitting. A subset of participants completed in-depth qualitative interviews exploring patient perceptions of substance use treatment needs.20 We obtained hospital administrative data from hospital financial services.
Partner Engagement
We identified community partners with which we had an individual or organizational relationship and a common interest and potential for collaboration. All invited partners agreed to attend initial meetings. We convened leadership and frontline staff across partners. OHSU staff included hospital nursing and social work leaders; infectious disease, hospitalist, and addiction physicians; and health services researchers. Community organizations included Central City Concern (CCC), a community organization serving people facing homelessness and addiction; CODA, Inc., a nonprofit SUD treatment agency; and Coram/CVS infusion pharmacy.
Collectively, we reviewed needs assessment findings and examples from the literature7-9 to develop strategies to address patient and system needs. We used patient narratives to foster alignment and prioritized areas in which integration could improve quality and costs. We assumed we would petition OHSU and/or Medicaid CCOs to finance efforts and saved potentially challenging budget discussions for later, when partnerships would be more developed. Our task force attended more than 3 large-group meetings and numerous small-group meetings to develop IMPACT.
RESULTS
Needs Assessment
Between September 2014 and April 2015, a research assistant approached 326 patients. Of these, 235 (72%) met study inclusion criteria, and 185 (78%) agreed to participate (Table 1). Of people who reported any substance use within the preceding 3 months, 58% of alcohol users and 67% of drug users said they were interested in cutting back or quitting. Fifty-four percent of participants with moderate- to high-risk opioid use and 16% with moderate- to high-risk alcohol use reported strong interest in MAT. In qualitative interviews, participants described inadequately treated withdrawal, the importance of trust and choice, and long wait times as a barriers to entering treatment after hospital discharge.20
Administrative data revealed high rates of hospital readmissions and longer than expected LOS (Figure). Mean LOS was 10.26 days—4 days more than medicine patients’. Mean LOS was high among participants who required long-term IV antibiotics, particularly those with endocarditis or osteomyelitis (21.75 days; range, 1.00-51.00 days). We excluded one outlier with a 116-day hospitalization.
Intervention Design
Mapping needs to intervention components. We mapped needs assessment findings to 3 main IMPACT components: inpatient addiction medicine consultation service, pathways to posthospital SUD treatment, and medically enhanced residential treatment (MERT) (Table 2).
Inpatient addiction medicine consultation service. We developed this service to address patients’ report of high readiness to change and interest in starting MAT in the hospital. Community partners highlighted the need for peers to increase engagement and trust. Therefore, we included a physician, a social worker, and two peers on our team. The inpatient service engages patients, advises on withdrawal and pain, performs SUD assessments, initiates MAT, and provides counseling and treatment.
Pathways to posthospital SUD treatment. As pathways from hospital to community SUD treatment were lacking, and long administrative wait times limited access to community treatment, we employed “in-reach” liaisons—community SUD treatment staff who perform in-hospital assessments to triage and coordinate care across systems. Given that patients value having treatment choices, we linked pathways to an array of MAT and abstinence-based treatments, including office-based, intensive outpatient and residential levels of care. For patients who live outside the Portland area, we developed relationships with rural stakeholders and engaged the help of the Oregon State Opioid Authority in introducing our program to SUD treatment providers around the state.
Medically Enhanced Residential Treatment (MERT). In many cases where patients required prolonged courses of IV antibiotics, hospital stays were longer for two reasons: At-home central-line self-administration of antibiotics was deemed unsafe, and patients were denied admission to a skilled nursing facility due to history of substance use. These long LOS create an opportunity to initiate and engage patients in treatment, and to render savings by shifting care to a residential addiction treatment setting that can accommodate IV antibiotic administration and MAT. We increased residential staffing and collaborated with a home infusion pharmacy to administer daily infusions on site.
Funding the Intervention
We used administrative data to estimate potential savings and tailored a business case to CCO and hospital payers. The CCO business case centered on hospitalization as an opportunity to engage out-of-treatment adults and potentially reduce high-cost readmissions by managing physical and behavioral health needs. Working within budgeting time lines, we used data from the first 165 participants. These participants had 137 readmissions over a mean observation period of 4.5 months. Mean charge per readmission was $31,157 (range, $699-$206,596) and was highest for people with endocarditis (mean, $55,493; range, $23,204-$145,066) and osteomyelitis (mean, $68,774; range, $29,359-$124,481). We estimated that a 10% reduction in 6-month readmissions could avoid $674,863 in charges.
For the hospital, the primary financial incentive was reduced LOS. Given the possibility of shortening hospitalization through MERT, we estimated a 20% mean LOS reduction; for budgeting, we estimated a conservative 10% reduction. A 10% mean LOS reduction would free 205 bed-days (10% × 10.26 days mean LOS × 200 patients) and create space for another 32 inpatient admissions in year 1, assuming no change from medical patients’ 6.26 days mean LOS. The future of bundled payments further bolstered our business case, as did the potential to improve care quality, reduce nonproductive staff time, and increase institutional learning about SUD. Overall program costs approximated projected savings, and the hospital and a local CCO agreed to equally share the costs of the intervention (Table 2).
DISCUSSION
We have described an innovative approach to developing an SUD intervention for hospitalized adults. Using a process of broad stakeholder engagement, data-driven understanding of population needs, and analysis of financial incentives, we built consensus and secured funding for a multicomponent intervention across hospital and post–acute care settings. Other studies have demonstrated the feasibility and efficacy of starting a single medication for a specific indication7-9 (eg, methadone for opioid use disorder), yet strategies for expanding SUD services in hospitals and facilitating posthospital treatment linkages remain scarce.21 Our model addresses a widespread need and could be adapted to other hospitals, SUD treatment organizations, and Medicaid payers.
Our experience has several limitations. First, it took place at a single academic medical center in Oregon, a Medicaid expansion state. Second, our needs assessment involved a convenience sample of limited racial/ethnic diversity. Third, almost all patients had insurance, which could limit generalizability. Fourth, to secure funding, it was essential we had a clinical champion who was persuasive with hospital and CCO leadership; though increasing disease burden and skyrocketing costs2 may drive administrators’ increased demand for ways to address SUD in hospitalized adults.
Our experience has several key implications. First, diverse partners were vital at all stages of program design, suggesting hospitals should look beyond traditional healthcare partners to address the SUD epidemic. Second, an interprofessional team that includes physicians, social workers, and peers may better engage patients and address complex system needs. Finally, a planned IMPACT evaluation will assess effects on substance use, healthcare use, and costs.
The United States faces a burgeoning SUD epidemic. Our experience describes an innovative care model and supports the idea that hospitals may play a leading role in convening partners, providing treatment, and driving population health improvements for adults with SUD.
Acknowledgment
The authors would like to acknowledge Peter Rapp and Thomas Yackel for leadership support; Tara Williams for administrative data support; Sarann Bielavitz and Naomi Wright for project management support, and Lynn Smith-Stott and Maria Michalczyk for help with model design. This work was presented at the American Society of Addiction Medicine national conference in Baltimore, MD in April 2016.
Disclosure
This work was funded by Oregon Health & Science University and CareOregon. The authors have no conflicts of interest to disclose.
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
1. Volkow N, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N Engl J Med. 2014; 370:2063-2066. PubMed
2. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002-12. Health Aff (Millwood). 2016;35(5):832-837. PubMed
3. Walley AY, Paasche-Orlow M, Lee EC, et al. Acute care hospital utilization among medical inpatients discharged with a substance use disorder diagnosis. J Addict Med. 2012;6(1):50-56. PubMed
4. Rosenthal ES, Karchmer AW, Thiesen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. PubMed
5. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;379(9):811-813. PubMed
6. Pollini RA, O’Toole TP, Ford D, Bigelow G. Does this patient really want treatment? Factors associated with baseline and evolving readiness for change among hospitalized substance using adults interested in treatment. Addict Behav. 2006;31(10):1904-1918. PubMed
7. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
8. Wei J, Defries T, Lozada M, Young N, Huen W, Tulsky J. An inpatient treatment and discharge planning protocol for alcohol and dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365-370. PubMed
9. Shanahan CW, Beers D, Alford DP, Brigandi E, Samet JH. A transitional opioid program to engage hospitalized drug users. J Gen Intern Med. 2010;25(8):803-808. PubMed
10. Pecoraro A, Horton T, Ewen E, et al. Early data from Project Engage: a program to identify and transition medically hospitalized patients into addictions treatment. Addict Sci Clin Pract. 2012;7:20. PubMed
11. National Center on Addiction and Substance Abuse; Addiction Medicine: Closing the Gap between Science and Practice. June 2012. http://www.centeronaddiction.org/addiction-research/reports/addiction-medicine-closing-gap-between-science-and-practice. Accessed May 2, 2016.
12. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Department of Health and Human Services. Results From the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, US Dept of Health and Human Services; 2011. NSDUH series H-41, HHS publication SMA 11-4658. https://www.samhsa.gov/data/sites/default/files/NSDUHNationalFindingsResults2010-web/2k10ResultsRev/NSDUHresultsRev2010.pdf. Published September 2011. Accessed March 31, 2017.
13. Vestal C. Few doctors are willing, able to prescribe powerful anti-addiction drugs. http://www.pewtrusts.org/en/research-and-analysis/blogs/stateline/2016/01/15/few-doctors-are-willing-able-to-prescribe-powerful-anti-addiction-drugs. Published January 15, 2016. Accessed May 2, 2016.
14. McConnell KJ. Oregon’s Medicaid coordinated care organizations. JAMA. 2016;315(9):869-870. PubMed
15. Englander H, Kansagara D. Planning and designing the Care Transitions Innovation (C-TraIn) for uninsured and Medicaid patients. J Hosp Med. 2012;7(7):524-529. PubMed
16. Englander H, Michaels L, Chan B, Kansagara D. The Care Transitions Innovation (C-TraIn) for socioeconomically disadvantaged adults: results of a cluster randomized controlled trial. J Gen Intern Med. 2014;29(11):1460-1467. PubMed
17. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. PubMed
18. Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. A single-question screening test for drug use in primary care. Arch Intern Med. 2010;170(13):1155-1160. PubMed
19. Humeniuk R, Ali R, Babor TF, et al. Validation of the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST). Addiction. 2008;103(6):1039-1047. PubMed
20. Velez CM, Nicolaidis C, Korthuis PT, Englander H. “It’s been an experience, a life learning experience”: a qualitative study of hospitalized patients with substance use disorders. J Gen Intern Med. 2017;32(3):296-303. PubMed
21. Gryczynski J, Schwartz RP, O’Grady KE, Restivo L, Mitchell SG, Jaffe JH. Understanding patterns of high-cost health care use across different substance user groups. Health Aff (Millwood). 2016.;35(1):12-19. PubMed
© 2017 Society of Hospital Medicine
Hospitalizations with observation services and the Medicare Part A complex appeals process at three academic medical centers
Hospitalists and other inpatient providers are familiar with hospitalizations classified observation. The Centers for Medicare & Medicaid Services (CMS) uses the “2-midnight rule” to distinguish between outpatient services (which include all observation stays) and inpatient services for most hospitalizations. The rule states that “inpatient admissions will generally be payable … if the admitting practitioner expected the patient to require a hospital stay that crossed two midnights and the medical record supports that reasonable expectation.”1
Hospitalization under inpatient versus outpatient status is a billing distinction that can have significant financial consequences for patients, providers, and hospitals. The inpatient or outpatient observation orders written by hospitalists and other hospital-based providers direct billing based on CMS and other third-party regulation. However, providers may have variable expertise writing such orders. To audit the correct use of the visit-status orders by hospital providers, CMS uses recovery auditors (RAs), also referred to as recovery audit contractors.2,3
Historically, RAs had up to 3 years from date of service (DOS) to perform an audit, which involves asking a hospital for a medical record for a particular stay. The audit timeline includes 45 days for hospitals to produce such documentation, and 60 days for the RA either to agree with the hospital’s billing or to make an “overpayment determination” that the hospital should have billed Medicare Part B (outpatient) instead of Part A (inpatient).3,4 The hospital may either accept the RA decision, or contest it by using the pre-appeals discussion period or by directly entering the 5-level Medicare administrative appeals process.3,4 Level 1 and Level 2 appeals are heard by a government contractor, Level 3 by an administrative law judge (ALJ), Level 4 by a Medicare appeals council, and Level 5 by a federal district court. These different appeal types have different deadlines (Appendix 1). The deadlines for hospitals and government responses beyond Level 1 are set by Congress and enforced by CMS,3,4 and CMS sets discussion period timelines. Hospitals that miss an appeals deadline automatically default their appeals request, but there are no penalties for missed government deadlines.
Recently, there has been increased scrutiny of the audit-and-appeals process of outpatient and inpatient status determinations.5 Despite the 2-midnight rule, the Medicare Benefit Policy Manual (MBPM) retains the passage: “Physicians should use a 24-hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis.”6 Auditors often cite “medical necessity” in their decisions, which is not well defined in the MBPM and can be open to different interpretation. This lack of clarity likely contributed to the large number of status determination discrepancies between providers and RAs, thereby creating a federal appeals backlog that caused the Office of Medicare Hearings and Appeals to halt hospital appeals assignments7 and prompted an ongoing lawsuit against CMS regarding the lengthy appeals process.4 To address these problems and clear the appeals backlog, CMS proposed a “$0.68 settlement offer.”4 The settlement “offered an administrative agreement to any hospital willing to withdraw their pending appeals in exchange for timely partial payment (68% of the net allowable amount)”8 and paid out almost $1.5 billion to the third of eligible hospitals that accepted the offer.9 CMS also made programmatic improvements to the RA program.10
Despite these efforts, problems remain. On June 9, 2016, the U.S. Government Accountability Office (GAO) published Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process, citing an approximate 2000% increase in hospital inpatient appeals during the period 2010–2014 and the concern that appeals requests will continue to exceed adjudication capabilities.11 On July 5, 2016, CMS issued its proposed rule for appeals reform that allows the Medicare Appeals Council (Level 4) to set precedents which would be binding at lower levels and allows senior attorneys to handle some cases and effectively increase manpower at the Level 3 (ALJ). In addition, CMS proposes to revise the method for calculating dollars at risk needed to schedule an ALJ hearing, and develop methods to better adjudicate similar claims, and other process improvements aimed at decreasing the more than 750,000 current claims awaiting ALJ decisions.12
We conducted a study to better understand the Medicare appeals process in the context of the proposed CMS reforms by investigating all appeals reaching Level 3 at Johns Hopkins Hospital (JHH), University of Wisconsin Hospitals and Clinics (UWHC), and University of Utah Hospital (UU). Because relatively few cases nationally are appealed beyond Level 3, the study focused on most-relevant data.3 We examined time spent at each appeal Level and whether it met federally mandated deadlines, as well as the percentage accountable to hospitals versus government contractors or ALJs. We also recorded the overturn rate at Level 3 and evaluated standardized text in de-identified decision letters to determine criteria cited by contractors in their decisions to deny hospital appeal requests.
METHODS
The JHH, UWHC, and UU Institutional Review Boards did not require a review. The study included all complex Part A appeals involving DOS before October 1, 2013 and reaching Level 3 (ALJ) as of May 1, 2016.
Our general methods were described previously.2 Briefly, the 3 academic medical centers are geographically diverse. JHH is in region A, UWHC in region B, and UU in region D (3 of the 4 RA regions are represented). The hospitals had different Medicare administrative contractors but the same qualified independent contractor until March 1, 2015 (Appendix 2).
For this paper, time spent in the discussion period, if applicable, is included in appeals time, except as specified (Table 1). The term partially favorable is used for UU cases only, based on the O’Connor Hospital decision13 (Table 1). Reflecting ambiguity in the MBPM, for time-based encounter length of stay (LOS) statements, JHH and UU used time between admission order and discharge order, whereas UWHC used time between decision to admit (for emergency department patients) or time care began (direct admissions) and time patient stopped receiving care (Table 2). Although CMS now defines when a hospital encounter begins under the 2-midnight rule,14 there was no standard definition when the cases in this study were audited.
We reviewed de-identified standardized text in Level 1 and Level 2 decision letters. Each hospital designated an analyst to search letters for Medicare Benefit Policy Manual chapter 1, which references the 24-hour benchmark, or the MBPM statement regarding use of the 24-hour period as a benchmark to guide inpatient admission orders.6 Associated paragraphs that included these terms were coded and reviewed by Drs. Sheehy, Engel, and Locke to confirm that the 24-hour time-based benchmark was mentioned, as per the MBPM statement (Table 2, Appendix 3).
Descriptive statistics are used to describe the data, and representative de-identified standardized text is included.
RESULTS
Of 219 Level 3 cases, 135 (61.6%) concluded at Level 3. Of these 135 cases, 96 (71.1%) were decided in favor of the hospital, 11 (8.1%) were settled in the CMS $0.68 settlement offer, and 28 (20.7%) were unfavorable to the hospital (Table 1).
Mean total days since DOS was 1,663.3 (536.8) (mean [SD]), with median 1708 days. This included 560.4 (351.6) days between DOS and audit (median 556 days) and 891.3 (320.3) days in appeal (median 979 days). The hospitals were responsible for 29.3% of that time (260.7 [68.2] days) while government contractors were responsible for 70.7% (630.6 [277.2] days). Government contractors and ALJs met deadlines 47.7% of the time, meeting appeals deadlines 92.5% of the time for Discussion, 85.4% for Level 1, 38.8% for Level 2, and 0% for Level 3 (Table 1).
All “redetermination” (level 1 appeals letters) received at UU and UWHC, and all “reconsideration” (level 2 appeals letters) received by UU, UWHC, and JHH contained standardized time-based 24–hour benchmark text directly or referencing the MBPM containing such text, to describe criteria for inpatient status (Table 2 and Appendix 3).6 In total, 417 of 438 (95.2%) of Level 1 and Level 2 appeals results letters contained time-based 24-hour benchmark criteria for inpatient status despite 154 of 219 (70.3%) of denied cases exceeding a 24-hour LOS.
DISCUSSION
This study demonstrated process and timeliness concerns in the Medicare RA program for Level 3 cases at 3 academic medical centers. Although hospitals forfeit any appeal for which they miss a filing deadline, government contractors and ALJs met their deadlines less than half the time without default or penalty. Average time from the rendering of services to the conclusion of the audit-and-appeals process exceeded 4.5 years, which included an average 560 days between hospital stay and initial RA audit, and almost 900 days in appeals, with more than 70% of that time attributable to government contractors and ALJs.
Objective time-based 24-hour inpatient status criteria were referenced in 95% of decision letters, even though LOS exceeded 24 hours in more than 70% of these cases, suggesting that objective LOS data played only a small role in contractor decisions, or that contractors did not actually audit for LOS when reviewing cases. Unclear criteria likely contributed to payment denials and improper payments, despite admitting providers’ best efforts to comply with Medicare rules when writing visit-status orders. There was also a significant cost to hospitals; our prior study found that navigating the appeals process required 5 full-time equivalents per institution.2
At the 2 study hospitals with Level 3 decisions, more than two thirds of the decisions favored the hospital, suggesting the hospitals were justified in appealing RA Level 1 and Level 2 determinations. This proportion is consistent with the 43% ALJ overturn rate (including RA- and non-RA-derived appeals) cited in the recent U.S. Court of Appeals for the DC Circuit decision.9
This study potentially was limited by contractor and hospital use of the nonstandardized LOS calculation during the study period. That the majority of JHH and UU cases cited the 24-hour benchmark in their letters but nevertheless exceeded 24-hour LOS (using the most conservative definition of LOS) suggests contractors did not audit for or consider LOS in their decisions.
Our results support recent steps taken by CMS to reform the appeals process, including shortening the RA “look-back period” from 3 years to 6 months,10 which will markedly shorten the 560-day lag between DOS and audit found in this study. In addition, CMS has replaced RAs with beneficiary and family-centered care quality improvement organizations (BFCC-QIOs)1,8 for initial status determination audits. Although it is too soon to tell, the hope is that BFCC-QIOs will decrease the volume of audits and denials that have overwhelmed the system and most probably contributed to process delays and the appeals backlog.
However, our data demonstrate several areas of concern not addressed in the recent GAO report11 or in the rule proposed by CMS.12 Most important, CMS could consider an appeals deadline missed by a government contractor as a decision for the hospital, in the same way a hospital’s missed deadline defaults its appeal. Such equity would ensure due process and prevent another appeals backlog. In addition, the large number of Level 3 decisions favoring hospitals suggests a need for process improvement at the Medicare administrative contractor and qualified independent contractor Level of appeals—such as mandatory review of Level 1 and Level 2 decision letters for appeals overturned at Level 3, accountability for Level 1 and Level 2 contractors with high rates of Level 3 overturn, and clarification of criteria used to judge determinations.
Medicare fraud cannot be tolerated, and a robust auditing process is essential to the integrity of the Medicare program. CMS’s current and proposed reforms may not be enough to eliminate the appeals backlog and restore a timely and fair appeals process. As CMS explores bundled payments and other reimbursement reforms, perhaps the need to distinguish observation hospital care will be eliminated. Short of that, additional actions must be taken so that a just and efficient Medicare appeals system can be realized for observation hospitalizations.
Acknowledgments
For invaluable assistance in data preparation and presentation, the authors thank Becky Borchert, RN, MS, MBA, Program Manager for Medicare/Medicaid Utilization Review, University of Wisconsin Hospital and Clinics; Carol Duhaney, Calvin Young, and Joan Kratz, RN, Johns Hopkins Hospital; and Morgan Walker and Lisa Whittaker, RN, University of Utah.
Disclosure
Nothing to report.
1. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Fact sheet: 2-midnight rule. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-07-01-2.html. Published July 1, 2015. Accessed August 9, 2016.
2. Sheehy AM, Locke C, Engel JZ, et al. Recovery Audit Contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. PubMed
3. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery auditing in Medicare for fiscal year 2014. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Recovery-Audit-Program/Downloads/RAC-RTC-FY2014.pdf. Accessed August 9, 2016.
4. American Hospital Association vs Burwell. No 15-5015. Circuit court decision. https://www.cadc.uscourts.gov/internet/opinions.nsf/CDFE9734F0D36C2185257F540052A39D/$file/15-5015-1597907.pdf. Decided February 9, 2016. Accessed August 9, 2016
5. AMA news: Payment recovery audit program needs overhaul: Doctors to CMS. https://wire.ama-assn.org/ama-news/payment-recovery-audit-program-needs-overhaul-doctors-cms. Accessed March 17, 2017.
6. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital services covered under Part A. In: Medicare Benefit Policy Manual. Chapter 1. Publication 100-02. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c01.pdf. Accessed August 9, 2016.
7. Griswold NJ; Office of Medicare Hearings and Appeals, US Dept of Health and Human Services. Memorandum to OMHA Medicare appellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed August 9, 2016.
8. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital reviews. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Medical-Review/InpatientHospitalReviews.html. Accessed August 9, 2016.
9. Galewitz P. CMS identifies hospitals paid nearly $1.5B in 2015 Medicare billing settlement. Kaiser Health News. http://khn.org/news/cms-identifies-hospitals-paid-nearly-1-5b-in-2015-medicare-billing-settlement/. Published August 23, 2016. Accessed October 14, 2016.
10. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery audit program improvements. https://www.cms.gov/research-statistics-data-and-systems/monitoring-programs/medicare-ffs-compliance-programs/recovery-audit-program/downloads/RAC-program-improvements.pdf. Accessed August 9, 2016.
11. US Government Accountability Office. Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process. http://www.gao.gov/assets/680/677034.pdf. Publication GAO-16-366. Published May 10, 2016. Accessed August 9, 2016.
12. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Changes to the Medicare Claims and Entitlement, Medicare Advantage Organization Determination, and Medicare Prescription Drug Coverage Determination Appeals Procedures. https://www.gpo.gov/fdsys/pkg/FR-2016-07-05/pdf/2016-15192.pdf. Accessed August 9, 2016.
13. Departmental Appeals Board, US Dept of Health and Human Services. Action and Order of Medicare Appeals Council: in the case of O’Connor Hospital. http://www.hhs.gov/dab/divisions/medicareoperations/macdecisions/oconnorhospital.pdf. Accessed August 9, 2016.
14. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Frequently asked questions: 2 midnight inpatient admission guidance & patient status reviews for admissions on or after October 1, 2013. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medical-Review/Downloads/QAsforWebsitePosting_110413-v2-CLEAN.pdf. Accessed August 9, 2016.
Hospitalists and other inpatient providers are familiar with hospitalizations classified observation. The Centers for Medicare & Medicaid Services (CMS) uses the “2-midnight rule” to distinguish between outpatient services (which include all observation stays) and inpatient services for most hospitalizations. The rule states that “inpatient admissions will generally be payable … if the admitting practitioner expected the patient to require a hospital stay that crossed two midnights and the medical record supports that reasonable expectation.”1
Hospitalization under inpatient versus outpatient status is a billing distinction that can have significant financial consequences for patients, providers, and hospitals. The inpatient or outpatient observation orders written by hospitalists and other hospital-based providers direct billing based on CMS and other third-party regulation. However, providers may have variable expertise writing such orders. To audit the correct use of the visit-status orders by hospital providers, CMS uses recovery auditors (RAs), also referred to as recovery audit contractors.2,3
Historically, RAs had up to 3 years from date of service (DOS) to perform an audit, which involves asking a hospital for a medical record for a particular stay. The audit timeline includes 45 days for hospitals to produce such documentation, and 60 days for the RA either to agree with the hospital’s billing or to make an “overpayment determination” that the hospital should have billed Medicare Part B (outpatient) instead of Part A (inpatient).3,4 The hospital may either accept the RA decision, or contest it by using the pre-appeals discussion period or by directly entering the 5-level Medicare administrative appeals process.3,4 Level 1 and Level 2 appeals are heard by a government contractor, Level 3 by an administrative law judge (ALJ), Level 4 by a Medicare appeals council, and Level 5 by a federal district court. These different appeal types have different deadlines (Appendix 1). The deadlines for hospitals and government responses beyond Level 1 are set by Congress and enforced by CMS,3,4 and CMS sets discussion period timelines. Hospitals that miss an appeals deadline automatically default their appeals request, but there are no penalties for missed government deadlines.
Recently, there has been increased scrutiny of the audit-and-appeals process of outpatient and inpatient status determinations.5 Despite the 2-midnight rule, the Medicare Benefit Policy Manual (MBPM) retains the passage: “Physicians should use a 24-hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis.”6 Auditors often cite “medical necessity” in their decisions, which is not well defined in the MBPM and can be open to different interpretation. This lack of clarity likely contributed to the large number of status determination discrepancies between providers and RAs, thereby creating a federal appeals backlog that caused the Office of Medicare Hearings and Appeals to halt hospital appeals assignments7 and prompted an ongoing lawsuit against CMS regarding the lengthy appeals process.4 To address these problems and clear the appeals backlog, CMS proposed a “$0.68 settlement offer.”4 The settlement “offered an administrative agreement to any hospital willing to withdraw their pending appeals in exchange for timely partial payment (68% of the net allowable amount)”8 and paid out almost $1.5 billion to the third of eligible hospitals that accepted the offer.9 CMS also made programmatic improvements to the RA program.10
Despite these efforts, problems remain. On June 9, 2016, the U.S. Government Accountability Office (GAO) published Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process, citing an approximate 2000% increase in hospital inpatient appeals during the period 2010–2014 and the concern that appeals requests will continue to exceed adjudication capabilities.11 On July 5, 2016, CMS issued its proposed rule for appeals reform that allows the Medicare Appeals Council (Level 4) to set precedents which would be binding at lower levels and allows senior attorneys to handle some cases and effectively increase manpower at the Level 3 (ALJ). In addition, CMS proposes to revise the method for calculating dollars at risk needed to schedule an ALJ hearing, and develop methods to better adjudicate similar claims, and other process improvements aimed at decreasing the more than 750,000 current claims awaiting ALJ decisions.12
We conducted a study to better understand the Medicare appeals process in the context of the proposed CMS reforms by investigating all appeals reaching Level 3 at Johns Hopkins Hospital (JHH), University of Wisconsin Hospitals and Clinics (UWHC), and University of Utah Hospital (UU). Because relatively few cases nationally are appealed beyond Level 3, the study focused on most-relevant data.3 We examined time spent at each appeal Level and whether it met federally mandated deadlines, as well as the percentage accountable to hospitals versus government contractors or ALJs. We also recorded the overturn rate at Level 3 and evaluated standardized text in de-identified decision letters to determine criteria cited by contractors in their decisions to deny hospital appeal requests.
METHODS
The JHH, UWHC, and UU Institutional Review Boards did not require a review. The study included all complex Part A appeals involving DOS before October 1, 2013 and reaching Level 3 (ALJ) as of May 1, 2016.
Our general methods were described previously.2 Briefly, the 3 academic medical centers are geographically diverse. JHH is in region A, UWHC in region B, and UU in region D (3 of the 4 RA regions are represented). The hospitals had different Medicare administrative contractors but the same qualified independent contractor until March 1, 2015 (Appendix 2).
For this paper, time spent in the discussion period, if applicable, is included in appeals time, except as specified (Table 1). The term partially favorable is used for UU cases only, based on the O’Connor Hospital decision13 (Table 1). Reflecting ambiguity in the MBPM, for time-based encounter length of stay (LOS) statements, JHH and UU used time between admission order and discharge order, whereas UWHC used time between decision to admit (for emergency department patients) or time care began (direct admissions) and time patient stopped receiving care (Table 2). Although CMS now defines when a hospital encounter begins under the 2-midnight rule,14 there was no standard definition when the cases in this study were audited.
We reviewed de-identified standardized text in Level 1 and Level 2 decision letters. Each hospital designated an analyst to search letters for Medicare Benefit Policy Manual chapter 1, which references the 24-hour benchmark, or the MBPM statement regarding use of the 24-hour period as a benchmark to guide inpatient admission orders.6 Associated paragraphs that included these terms were coded and reviewed by Drs. Sheehy, Engel, and Locke to confirm that the 24-hour time-based benchmark was mentioned, as per the MBPM statement (Table 2, Appendix 3).
Descriptive statistics are used to describe the data, and representative de-identified standardized text is included.
RESULTS
Of 219 Level 3 cases, 135 (61.6%) concluded at Level 3. Of these 135 cases, 96 (71.1%) were decided in favor of the hospital, 11 (8.1%) were settled in the CMS $0.68 settlement offer, and 28 (20.7%) were unfavorable to the hospital (Table 1).
Mean total days since DOS was 1,663.3 (536.8) (mean [SD]), with median 1708 days. This included 560.4 (351.6) days between DOS and audit (median 556 days) and 891.3 (320.3) days in appeal (median 979 days). The hospitals were responsible for 29.3% of that time (260.7 [68.2] days) while government contractors were responsible for 70.7% (630.6 [277.2] days). Government contractors and ALJs met deadlines 47.7% of the time, meeting appeals deadlines 92.5% of the time for Discussion, 85.4% for Level 1, 38.8% for Level 2, and 0% for Level 3 (Table 1).
All “redetermination” (level 1 appeals letters) received at UU and UWHC, and all “reconsideration” (level 2 appeals letters) received by UU, UWHC, and JHH contained standardized time-based 24–hour benchmark text directly or referencing the MBPM containing such text, to describe criteria for inpatient status (Table 2 and Appendix 3).6 In total, 417 of 438 (95.2%) of Level 1 and Level 2 appeals results letters contained time-based 24-hour benchmark criteria for inpatient status despite 154 of 219 (70.3%) of denied cases exceeding a 24-hour LOS.
DISCUSSION
This study demonstrated process and timeliness concerns in the Medicare RA program for Level 3 cases at 3 academic medical centers. Although hospitals forfeit any appeal for which they miss a filing deadline, government contractors and ALJs met their deadlines less than half the time without default or penalty. Average time from the rendering of services to the conclusion of the audit-and-appeals process exceeded 4.5 years, which included an average 560 days between hospital stay and initial RA audit, and almost 900 days in appeals, with more than 70% of that time attributable to government contractors and ALJs.
Objective time-based 24-hour inpatient status criteria were referenced in 95% of decision letters, even though LOS exceeded 24 hours in more than 70% of these cases, suggesting that objective LOS data played only a small role in contractor decisions, or that contractors did not actually audit for LOS when reviewing cases. Unclear criteria likely contributed to payment denials and improper payments, despite admitting providers’ best efforts to comply with Medicare rules when writing visit-status orders. There was also a significant cost to hospitals; our prior study found that navigating the appeals process required 5 full-time equivalents per institution.2
At the 2 study hospitals with Level 3 decisions, more than two thirds of the decisions favored the hospital, suggesting the hospitals were justified in appealing RA Level 1 and Level 2 determinations. This proportion is consistent with the 43% ALJ overturn rate (including RA- and non-RA-derived appeals) cited in the recent U.S. Court of Appeals for the DC Circuit decision.9
This study potentially was limited by contractor and hospital use of the nonstandardized LOS calculation during the study period. That the majority of JHH and UU cases cited the 24-hour benchmark in their letters but nevertheless exceeded 24-hour LOS (using the most conservative definition of LOS) suggests contractors did not audit for or consider LOS in their decisions.
Our results support recent steps taken by CMS to reform the appeals process, including shortening the RA “look-back period” from 3 years to 6 months,10 which will markedly shorten the 560-day lag between DOS and audit found in this study. In addition, CMS has replaced RAs with beneficiary and family-centered care quality improvement organizations (BFCC-QIOs)1,8 for initial status determination audits. Although it is too soon to tell, the hope is that BFCC-QIOs will decrease the volume of audits and denials that have overwhelmed the system and most probably contributed to process delays and the appeals backlog.
However, our data demonstrate several areas of concern not addressed in the recent GAO report11 or in the rule proposed by CMS.12 Most important, CMS could consider an appeals deadline missed by a government contractor as a decision for the hospital, in the same way a hospital’s missed deadline defaults its appeal. Such equity would ensure due process and prevent another appeals backlog. In addition, the large number of Level 3 decisions favoring hospitals suggests a need for process improvement at the Medicare administrative contractor and qualified independent contractor Level of appeals—such as mandatory review of Level 1 and Level 2 decision letters for appeals overturned at Level 3, accountability for Level 1 and Level 2 contractors with high rates of Level 3 overturn, and clarification of criteria used to judge determinations.
Medicare fraud cannot be tolerated, and a robust auditing process is essential to the integrity of the Medicare program. CMS’s current and proposed reforms may not be enough to eliminate the appeals backlog and restore a timely and fair appeals process. As CMS explores bundled payments and other reimbursement reforms, perhaps the need to distinguish observation hospital care will be eliminated. Short of that, additional actions must be taken so that a just and efficient Medicare appeals system can be realized for observation hospitalizations.
Acknowledgments
For invaluable assistance in data preparation and presentation, the authors thank Becky Borchert, RN, MS, MBA, Program Manager for Medicare/Medicaid Utilization Review, University of Wisconsin Hospital and Clinics; Carol Duhaney, Calvin Young, and Joan Kratz, RN, Johns Hopkins Hospital; and Morgan Walker and Lisa Whittaker, RN, University of Utah.
Disclosure
Nothing to report.
Hospitalists and other inpatient providers are familiar with hospitalizations classified observation. The Centers for Medicare & Medicaid Services (CMS) uses the “2-midnight rule” to distinguish between outpatient services (which include all observation stays) and inpatient services for most hospitalizations. The rule states that “inpatient admissions will generally be payable … if the admitting practitioner expected the patient to require a hospital stay that crossed two midnights and the medical record supports that reasonable expectation.”1
Hospitalization under inpatient versus outpatient status is a billing distinction that can have significant financial consequences for patients, providers, and hospitals. The inpatient or outpatient observation orders written by hospitalists and other hospital-based providers direct billing based on CMS and other third-party regulation. However, providers may have variable expertise writing such orders. To audit the correct use of the visit-status orders by hospital providers, CMS uses recovery auditors (RAs), also referred to as recovery audit contractors.2,3
Historically, RAs had up to 3 years from date of service (DOS) to perform an audit, which involves asking a hospital for a medical record for a particular stay. The audit timeline includes 45 days for hospitals to produce such documentation, and 60 days for the RA either to agree with the hospital’s billing or to make an “overpayment determination” that the hospital should have billed Medicare Part B (outpatient) instead of Part A (inpatient).3,4 The hospital may either accept the RA decision, or contest it by using the pre-appeals discussion period or by directly entering the 5-level Medicare administrative appeals process.3,4 Level 1 and Level 2 appeals are heard by a government contractor, Level 3 by an administrative law judge (ALJ), Level 4 by a Medicare appeals council, and Level 5 by a federal district court. These different appeal types have different deadlines (Appendix 1). The deadlines for hospitals and government responses beyond Level 1 are set by Congress and enforced by CMS,3,4 and CMS sets discussion period timelines. Hospitals that miss an appeals deadline automatically default their appeals request, but there are no penalties for missed government deadlines.
Recently, there has been increased scrutiny of the audit-and-appeals process of outpatient and inpatient status determinations.5 Despite the 2-midnight rule, the Medicare Benefit Policy Manual (MBPM) retains the passage: “Physicians should use a 24-hour period as a benchmark, i.e., they should order admission for patients who are expected to need hospital care for 24 hours or more, and treat other patients on an outpatient basis.”6 Auditors often cite “medical necessity” in their decisions, which is not well defined in the MBPM and can be open to different interpretation. This lack of clarity likely contributed to the large number of status determination discrepancies between providers and RAs, thereby creating a federal appeals backlog that caused the Office of Medicare Hearings and Appeals to halt hospital appeals assignments7 and prompted an ongoing lawsuit against CMS regarding the lengthy appeals process.4 To address these problems and clear the appeals backlog, CMS proposed a “$0.68 settlement offer.”4 The settlement “offered an administrative agreement to any hospital willing to withdraw their pending appeals in exchange for timely partial payment (68% of the net allowable amount)”8 and paid out almost $1.5 billion to the third of eligible hospitals that accepted the offer.9 CMS also made programmatic improvements to the RA program.10
Despite these efforts, problems remain. On June 9, 2016, the U.S. Government Accountability Office (GAO) published Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process, citing an approximate 2000% increase in hospital inpatient appeals during the period 2010–2014 and the concern that appeals requests will continue to exceed adjudication capabilities.11 On July 5, 2016, CMS issued its proposed rule for appeals reform that allows the Medicare Appeals Council (Level 4) to set precedents which would be binding at lower levels and allows senior attorneys to handle some cases and effectively increase manpower at the Level 3 (ALJ). In addition, CMS proposes to revise the method for calculating dollars at risk needed to schedule an ALJ hearing, and develop methods to better adjudicate similar claims, and other process improvements aimed at decreasing the more than 750,000 current claims awaiting ALJ decisions.12
We conducted a study to better understand the Medicare appeals process in the context of the proposed CMS reforms by investigating all appeals reaching Level 3 at Johns Hopkins Hospital (JHH), University of Wisconsin Hospitals and Clinics (UWHC), and University of Utah Hospital (UU). Because relatively few cases nationally are appealed beyond Level 3, the study focused on most-relevant data.3 We examined time spent at each appeal Level and whether it met federally mandated deadlines, as well as the percentage accountable to hospitals versus government contractors or ALJs. We also recorded the overturn rate at Level 3 and evaluated standardized text in de-identified decision letters to determine criteria cited by contractors in their decisions to deny hospital appeal requests.
METHODS
The JHH, UWHC, and UU Institutional Review Boards did not require a review. The study included all complex Part A appeals involving DOS before October 1, 2013 and reaching Level 3 (ALJ) as of May 1, 2016.
Our general methods were described previously.2 Briefly, the 3 academic medical centers are geographically diverse. JHH is in region A, UWHC in region B, and UU in region D (3 of the 4 RA regions are represented). The hospitals had different Medicare administrative contractors but the same qualified independent contractor until March 1, 2015 (Appendix 2).
For this paper, time spent in the discussion period, if applicable, is included in appeals time, except as specified (Table 1). The term partially favorable is used for UU cases only, based on the O’Connor Hospital decision13 (Table 1). Reflecting ambiguity in the MBPM, for time-based encounter length of stay (LOS) statements, JHH and UU used time between admission order and discharge order, whereas UWHC used time between decision to admit (for emergency department patients) or time care began (direct admissions) and time patient stopped receiving care (Table 2). Although CMS now defines when a hospital encounter begins under the 2-midnight rule,14 there was no standard definition when the cases in this study were audited.
We reviewed de-identified standardized text in Level 1 and Level 2 decision letters. Each hospital designated an analyst to search letters for Medicare Benefit Policy Manual chapter 1, which references the 24-hour benchmark, or the MBPM statement regarding use of the 24-hour period as a benchmark to guide inpatient admission orders.6 Associated paragraphs that included these terms were coded and reviewed by Drs. Sheehy, Engel, and Locke to confirm that the 24-hour time-based benchmark was mentioned, as per the MBPM statement (Table 2, Appendix 3).
Descriptive statistics are used to describe the data, and representative de-identified standardized text is included.
RESULTS
Of 219 Level 3 cases, 135 (61.6%) concluded at Level 3. Of these 135 cases, 96 (71.1%) were decided in favor of the hospital, 11 (8.1%) were settled in the CMS $0.68 settlement offer, and 28 (20.7%) were unfavorable to the hospital (Table 1).
Mean total days since DOS was 1,663.3 (536.8) (mean [SD]), with median 1708 days. This included 560.4 (351.6) days between DOS and audit (median 556 days) and 891.3 (320.3) days in appeal (median 979 days). The hospitals were responsible for 29.3% of that time (260.7 [68.2] days) while government contractors were responsible for 70.7% (630.6 [277.2] days). Government contractors and ALJs met deadlines 47.7% of the time, meeting appeals deadlines 92.5% of the time for Discussion, 85.4% for Level 1, 38.8% for Level 2, and 0% for Level 3 (Table 1).
All “redetermination” (level 1 appeals letters) received at UU and UWHC, and all “reconsideration” (level 2 appeals letters) received by UU, UWHC, and JHH contained standardized time-based 24–hour benchmark text directly or referencing the MBPM containing such text, to describe criteria for inpatient status (Table 2 and Appendix 3).6 In total, 417 of 438 (95.2%) of Level 1 and Level 2 appeals results letters contained time-based 24-hour benchmark criteria for inpatient status despite 154 of 219 (70.3%) of denied cases exceeding a 24-hour LOS.
DISCUSSION
This study demonstrated process and timeliness concerns in the Medicare RA program for Level 3 cases at 3 academic medical centers. Although hospitals forfeit any appeal for which they miss a filing deadline, government contractors and ALJs met their deadlines less than half the time without default or penalty. Average time from the rendering of services to the conclusion of the audit-and-appeals process exceeded 4.5 years, which included an average 560 days between hospital stay and initial RA audit, and almost 900 days in appeals, with more than 70% of that time attributable to government contractors and ALJs.
Objective time-based 24-hour inpatient status criteria were referenced in 95% of decision letters, even though LOS exceeded 24 hours in more than 70% of these cases, suggesting that objective LOS data played only a small role in contractor decisions, or that contractors did not actually audit for LOS when reviewing cases. Unclear criteria likely contributed to payment denials and improper payments, despite admitting providers’ best efforts to comply with Medicare rules when writing visit-status orders. There was also a significant cost to hospitals; our prior study found that navigating the appeals process required 5 full-time equivalents per institution.2
At the 2 study hospitals with Level 3 decisions, more than two thirds of the decisions favored the hospital, suggesting the hospitals were justified in appealing RA Level 1 and Level 2 determinations. This proportion is consistent with the 43% ALJ overturn rate (including RA- and non-RA-derived appeals) cited in the recent U.S. Court of Appeals for the DC Circuit decision.9
This study potentially was limited by contractor and hospital use of the nonstandardized LOS calculation during the study period. That the majority of JHH and UU cases cited the 24-hour benchmark in their letters but nevertheless exceeded 24-hour LOS (using the most conservative definition of LOS) suggests contractors did not audit for or consider LOS in their decisions.
Our results support recent steps taken by CMS to reform the appeals process, including shortening the RA “look-back period” from 3 years to 6 months,10 which will markedly shorten the 560-day lag between DOS and audit found in this study. In addition, CMS has replaced RAs with beneficiary and family-centered care quality improvement organizations (BFCC-QIOs)1,8 for initial status determination audits. Although it is too soon to tell, the hope is that BFCC-QIOs will decrease the volume of audits and denials that have overwhelmed the system and most probably contributed to process delays and the appeals backlog.
However, our data demonstrate several areas of concern not addressed in the recent GAO report11 or in the rule proposed by CMS.12 Most important, CMS could consider an appeals deadline missed by a government contractor as a decision for the hospital, in the same way a hospital’s missed deadline defaults its appeal. Such equity would ensure due process and prevent another appeals backlog. In addition, the large number of Level 3 decisions favoring hospitals suggests a need for process improvement at the Medicare administrative contractor and qualified independent contractor Level of appeals—such as mandatory review of Level 1 and Level 2 decision letters for appeals overturned at Level 3, accountability for Level 1 and Level 2 contractors with high rates of Level 3 overturn, and clarification of criteria used to judge determinations.
Medicare fraud cannot be tolerated, and a robust auditing process is essential to the integrity of the Medicare program. CMS’s current and proposed reforms may not be enough to eliminate the appeals backlog and restore a timely and fair appeals process. As CMS explores bundled payments and other reimbursement reforms, perhaps the need to distinguish observation hospital care will be eliminated. Short of that, additional actions must be taken so that a just and efficient Medicare appeals system can be realized for observation hospitalizations.
Acknowledgments
For invaluable assistance in data preparation and presentation, the authors thank Becky Borchert, RN, MS, MBA, Program Manager for Medicare/Medicaid Utilization Review, University of Wisconsin Hospital and Clinics; Carol Duhaney, Calvin Young, and Joan Kratz, RN, Johns Hopkins Hospital; and Morgan Walker and Lisa Whittaker, RN, University of Utah.
Disclosure
Nothing to report.
1. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Fact sheet: 2-midnight rule. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-07-01-2.html. Published July 1, 2015. Accessed August 9, 2016.
2. Sheehy AM, Locke C, Engel JZ, et al. Recovery Audit Contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. PubMed
3. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery auditing in Medicare for fiscal year 2014. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Recovery-Audit-Program/Downloads/RAC-RTC-FY2014.pdf. Accessed August 9, 2016.
4. American Hospital Association vs Burwell. No 15-5015. Circuit court decision. https://www.cadc.uscourts.gov/internet/opinions.nsf/CDFE9734F0D36C2185257F540052A39D/$file/15-5015-1597907.pdf. Decided February 9, 2016. Accessed August 9, 2016
5. AMA news: Payment recovery audit program needs overhaul: Doctors to CMS. https://wire.ama-assn.org/ama-news/payment-recovery-audit-program-needs-overhaul-doctors-cms. Accessed March 17, 2017.
6. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital services covered under Part A. In: Medicare Benefit Policy Manual. Chapter 1. Publication 100-02. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c01.pdf. Accessed August 9, 2016.
7. Griswold NJ; Office of Medicare Hearings and Appeals, US Dept of Health and Human Services. Memorandum to OMHA Medicare appellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed August 9, 2016.
8. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital reviews. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Medical-Review/InpatientHospitalReviews.html. Accessed August 9, 2016.
9. Galewitz P. CMS identifies hospitals paid nearly $1.5B in 2015 Medicare billing settlement. Kaiser Health News. http://khn.org/news/cms-identifies-hospitals-paid-nearly-1-5b-in-2015-medicare-billing-settlement/. Published August 23, 2016. Accessed October 14, 2016.
10. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery audit program improvements. https://www.cms.gov/research-statistics-data-and-systems/monitoring-programs/medicare-ffs-compliance-programs/recovery-audit-program/downloads/RAC-program-improvements.pdf. Accessed August 9, 2016.
11. US Government Accountability Office. Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process. http://www.gao.gov/assets/680/677034.pdf. Publication GAO-16-366. Published May 10, 2016. Accessed August 9, 2016.
12. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Changes to the Medicare Claims and Entitlement, Medicare Advantage Organization Determination, and Medicare Prescription Drug Coverage Determination Appeals Procedures. https://www.gpo.gov/fdsys/pkg/FR-2016-07-05/pdf/2016-15192.pdf. Accessed August 9, 2016.
13. Departmental Appeals Board, US Dept of Health and Human Services. Action and Order of Medicare Appeals Council: in the case of O’Connor Hospital. http://www.hhs.gov/dab/divisions/medicareoperations/macdecisions/oconnorhospital.pdf. Accessed August 9, 2016.
14. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Frequently asked questions: 2 midnight inpatient admission guidance & patient status reviews for admissions on or after October 1, 2013. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medical-Review/Downloads/QAsforWebsitePosting_110413-v2-CLEAN.pdf. Accessed August 9, 2016.
1. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Fact sheet: 2-midnight rule. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-07-01-2.html. Published July 1, 2015. Accessed August 9, 2016.
2. Sheehy AM, Locke C, Engel JZ, et al. Recovery Audit Contractor audits and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. PubMed
3. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery auditing in Medicare for fiscal year 2014. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Recovery-Audit-Program/Downloads/RAC-RTC-FY2014.pdf. Accessed August 9, 2016.
4. American Hospital Association vs Burwell. No 15-5015. Circuit court decision. https://www.cadc.uscourts.gov/internet/opinions.nsf/CDFE9734F0D36C2185257F540052A39D/$file/15-5015-1597907.pdf. Decided February 9, 2016. Accessed August 9, 2016
5. AMA news: Payment recovery audit program needs overhaul: Doctors to CMS. https://wire.ama-assn.org/ama-news/payment-recovery-audit-program-needs-overhaul-doctors-cms. Accessed March 17, 2017.
6. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital services covered under Part A. In: Medicare Benefit Policy Manual. Chapter 1. Publication 100-02. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/bp102c01.pdf. Accessed August 9, 2016.
7. Griswold NJ; Office of Medicare Hearings and Appeals, US Dept of Health and Human Services. Memorandum to OMHA Medicare appellants. http://www.modernhealthcare.com/assets/pdf/CH92573110.pdf. Accessed August 9, 2016.
8. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Inpatient hospital reviews. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medicare-FFS-Compliance-Programs/Medical-Review/InpatientHospitalReviews.html. Accessed August 9, 2016.
9. Galewitz P. CMS identifies hospitals paid nearly $1.5B in 2015 Medicare billing settlement. Kaiser Health News. http://khn.org/news/cms-identifies-hospitals-paid-nearly-1-5b-in-2015-medicare-billing-settlement/. Published August 23, 2016. Accessed October 14, 2016.
10. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Recovery audit program improvements. https://www.cms.gov/research-statistics-data-and-systems/monitoring-programs/medicare-ffs-compliance-programs/recovery-audit-program/downloads/RAC-program-improvements.pdf. Accessed August 9, 2016.
11. US Government Accountability Office. Medicare Fee-for-Service: Opportunities Remain to Improve Appeals Process. http://www.gao.gov/assets/680/677034.pdf. Publication GAO-16-366. Published May 10, 2016. Accessed August 9, 2016.
12. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Changes to the Medicare Claims and Entitlement, Medicare Advantage Organization Determination, and Medicare Prescription Drug Coverage Determination Appeals Procedures. https://www.gpo.gov/fdsys/pkg/FR-2016-07-05/pdf/2016-15192.pdf. Accessed August 9, 2016.
13. Departmental Appeals Board, US Dept of Health and Human Services. Action and Order of Medicare Appeals Council: in the case of O’Connor Hospital. http://www.hhs.gov/dab/divisions/medicareoperations/macdecisions/oconnorhospital.pdf. Accessed August 9, 2016.
14. Centers for Medicare & Medicaid Services, US Dept of Health and Human Services. Frequently asked questions: 2 midnight inpatient admission guidance & patient status reviews for admissions on or after October 1, 2013. https://www.cms.gov/Research-Statistics-Data-and-Systems/Monitoring-Programs/Medical-Review/Downloads/QAsforWebsitePosting_110413-v2-CLEAN.pdf. Accessed August 9, 2016.
© 2017 Society of Hospital Medicine
Detecting sepsis: Are two opinions better than one?
Sepsis is a leading cause of hospital mortality in the United States, contributing to up to half of all deaths.1 If the infection is identified and treated early, however, its associated morbidity and mortality can be significantly reduced.2 The 2001 sepsis guidelines define sepsis as the suspicion of infection plus meeting 2 or more systemic inflammatory response syndrome (SIRS) criteria.3 Although the utility of SIRS criteria has been extensively debated, providers’ accuracy and agreement regarding suspicion of infection are not yet fully characterized. This is very important, as the source of infection is often not identified in patients with severe sepsis or septic shock.4
Although much attention recently has been given to ideal objective criteria for accurately identifying sepsis, less is known about what constitutes ideal subjective criteria and who can best make that assessment.5-7 We conducted a study to measure providers’ agreement regarding this subjective assessment and the impact of that agreement on patient outcomes.
METHODS
We performed a secondary analysis of prospectively collected data on consecutive adults hospitalized on a general medicine ward at an academic medical center between April 1, 2014 and March 31, 2015. This study was approved by the University of Chicago Institutional Review Board with a waiver of consent.
A sepsis screening tool was developed locally as part of the Surviving Sepsis Campaign Quality Improvement Learning Collaborative8 (Supplemental Figure). This tool was completed by bedside nurses for each patient during each shift. Bedside registered nurse (RN) suspicion of infection was deemed positive if the nurse answered yes to question 2: “Does the patient have evidence of an active infection?” We compared RN assessment with assessment by the ordering provider, a medical doctor or advanced practice professionals (MD/APP), using an existing order for antibiotics or a new order for either blood or urine cultures placed within 12 hours before nursing screen time to indicate MD/APP suspicion of infection.
All nursing screens were transcribed into an electronic database, excluding screens not performed, or missing RN suspicion of infection. For quality purposes, screening data were merged with electronic health record data to verify SIRS criteria at the time of the screens as well as the presence of culture and/or antibiotic orders preceding the screens. Outcome data were obtained from an administrative database and confirmed by chart review using the 2001 sepsis definitions.6 Data were de-identified and time-shifted before this analysis. SIRS-positive criteria were defined as meeting 2 or more of the following: temperature higher than 38°C or lower than 36°C; heart rate higher than 90 beats per minute; respiratory rate more than 20 breaths per minute; and white blood cell count more than 2,000/mm3 or less than 4,000/mm3.The primary clinical outcome was progression to severe sepsis or septic shock. Secondary outcomes included transfer to intensive care unit (ICU) and in-hospital mortality. Given that RN and MD/APP suspicion of infection can vary over time, only the initial screen for each patient was used in assessing progression to severe sepsis or septic shock and in-hospital mortality. All available screens were used to investigate the association between each provider’s suspicion of infection over time and ICU transfer.
Demographic characteristics were compared using the χ2 test and analysis of variance, as appropriate. Provider agreement was evaluated with a weighted κ statistic. Fisher exact tests were used to compare proportions of mortality and severe sepsis/septic shock, and the McNemar test was used to compare proportions of ICU transfers. The association of outcomes based on provider agreement was evaluated with a nonparametric test for trend.
RESULTS
During the study period, 1386 distinct patients had 13,223 screening opportunities, with a 95.4% compliance rate. A total of 1127 screens were excluded for missing nursing documentation of suspicion of infection, leaving 1192 first screens and 11,489 total screens for analysis. Of the completed screens, 3744 (32.6%) met SIRS criteria; suspicion of infection was noted by both RN and MD/APP in 5.8% of cases, by RN only in 22.2%, by MD/APP only in 7.2%, and by neither provider in 64.7% (Figure 1). Overall agreement rate was 80.7% for suspicion of infection (κ = 0.11, P < 0.001). Demographics by subgroup are shown in the Supplemental Table. Progression to severe sepsis or shock was highest when both providers suspected infection in a SIRS-positive patient (17.7%), was substantially reduced with single-provider suspicion (6.0%), and was lowest when neither provider suspected infection (1.5%) (P < 0.001). A similar trend was found for in-hospital mortality (both providers, 6.3%; single provider, 2.7%; neither provider, 2.5%; P = 0.01). Compared with MD/APP-only suspicion, SIRS-positive patients in whom only RNs suspected infection had similar frequency of progression to severe sepsis or septic shock (6.5% vs 5.6%; P = 0.52) and higher mortality (5.0% vs 1.1%; P = 0.32), though these findings were not statistically significant.
For the 121 patients (10.2%) transferred to ICU, RNs were more likely than MD/APPs to suspect infection at all time points (Figure 2). The difference was small (P = 0.29) 48 hours before transfer (RN, 12.5%; MD/APP, 5.6%) but became more pronounced (P = 0.06) by 3 hours before transfer (RN, 46.3%; MD/APP, 33.1%). Nursing assessments were not available after transfer, but 3 hours after transfer the proportion of patients who met MD/APP suspicion-of-infection criteria (44.6%) was similar (P = 0.90) to that of the RNs 3 hours before transfer (46.3%).
DISCUSSION
Our findings reveal that bedside nurses and ordering providers routinely have discordant assessments regarding presence of infection. Specifically, when RNs are asked to screen patients on the wards, they are suspicious of infection more often than MD/APPs are, and they suspect infection earlier in ICU transfer patients. These findings have significant implications for patient care, compliance with the new national SEP-1 Centers for Medicare & Medicaid Services quality measure, and identification of appropriate patients for enrollment in sepsis-related clinical trials.
To our knowledge, this is the first study to explore agreement between bedside RN and MD/APP suspicion of infection in sepsis screening and its association with patient outcomes. Studies on nurse and physician concordance in other domains have had mixed findings.9-11 The high discordance rate found in our study points to the highly subjective nature of suspicion of infection.
Our finding that RNs suspect infection earlier in patients transferred to ICU suggests nursing suspicion has value above and beyond current practice. A possible explanation for the higher rate of RN suspicion, and earlier RN suspicion, is that bedside nurses spend substantially more time with their patients and are more attuned to subtle changes that often occur before any objective signs of deterioration. This phenomenon is well documented and accounts for why rapid response calling criteria often include “nurse worry or concern.”12,13 Thus, nurse intuition may be an important signal for early identification of patients at high risk for sepsis.
That about one third of all screens met SIRS criteria and that almost two thirds of those screens were not thought by RN or MD/APP to be caused by infection add to the literature demonstrating the limited value of SIRS as a screening tool for sepsis.14 To address this issue, the 2016 sepsis definitions propose using the quick Sepsis-Related Organ Failure Assessment (qSOFA) to identify patients at high risk for clinical deterioration; however, the Surviving Sepsis Campaign continues to encourage sepsis screening using the SIRS criteria.15
Limitations of this study include its lack of generalizability, as it was conducted with general medical patients at a single center. Second, we did not specifically ask the MD/APPs whether they suspected infection; instead, we relied on their ordering practices. Third, RN and MD/APP assessments were not independent, as RNs had access to MD/APP orders before making their own assessments, which could bias our results.
Discordance in provider suspicion of infection is common, with RNs documenting suspicion more often than MD/APPs, and earlier in patients transferred to ICU. Suspicion by either provider alone is associated with higher risk for sepsis progression and in-hospital mortality than is the case when neither provider suspects infection. Thus, a collaborative method that includes both RNs and MD/APPs may improve the accuracy and timing of sepsis detection on the wards.
Acknowledgments
The authors thank the members of the Surviving Sepsis Campaign (SSC) Quality Improvement Learning Collaborative at the University of Chicago for their help in data collection and review, especially Meredith Borak, Rita Lanier, Mary Ann Francisco, and Bill Marsack. The authors also thank Thomas Best and Mary-Kate Springman for their assistance in data entry and Nicole Twu for administrative support. Data from this study were provided by the Clinical Research Data Warehouse (CRDW) maintained by the Center for Research Informatics (CRI) at the University of Chicago. CRI is funded by the Biological Sciences Division of the Institute for Translational Medicine/Clinical and Translational Science Award (CTSA) (National Institutes of Health UL1 TR000430) at the University of Chicago.
Disclosures
Dr. Bhattacharjee is supported by postdoctoral training grant 4T32HS000078 from the Agency for Healthcare Research and Quality. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by career development award K08 HL121080 from the National Heart, Lung, and Blood Institute. Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), American Heart Association (Dallas, Texas), and Laerdal Medical (Stavanger, Norway) and has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. The other authors report no conflicts of interest.
1. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. PubMed
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. PubMed
3. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256. PubMed
4. Vincent JL, Sakr Y, Sprung CL, et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353. PubMed
5. Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629-1638. PubMed
6. Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381(9868):774-775. PubMed
7. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. PubMed
8. Surviving Sepsis Campaign (SSC) Sepsis on the Floors Quality Improvement Learning Collaborative. Frequently asked questions (FAQs). Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/About-Collaboratives.pdf. Published October 8, 2013.
9. Fiesseler F, Szucs P, Kec R, Richman PB. Can nurses appropriately interpret the Ottawa ankle rule? Am J Emerg Med. 2004;22(3):145-148. PubMed
10. Blomberg H, Lundström E, Toss H, Gedeborg R, Johansson J. Agreement between ambulance nurses and physicians in assessing stroke patients. Acta Neurol Scand. 2014;129(1):4955. PubMed
11. Neville TH, Wiley JF, Yamamoto MC, et al. Concordance of nurses and physicians on whether critical care patients are receiving futile treatment. Am J Crit Care. 2015;24(5):403410. PubMed
12. Odell M, Victor C, Oliver D. Nurses’ role in detecting deterioration in ward patients: systematic literature review. J Adv Nurs. 2009;65(10):1992-2006. PubMed
13. Howell MD, Ngo L, Folcarelli P, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012;40(9):2562-2568. PubMed
14. Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192(8):958-964. PubMed
15. Antonelli M, DeBacker D, Dorman T, Kleinpell R, Levy M, Rhodes A; Surviving Sepsis Campaign Executive Committee. Surviving Sepsis Campaign responds to Sepsis-3. Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/SSC-Statements-Sepsis-Definitions-3-2016.pdf. Published March 1, 2016. Accessed May 11, 2016.
Sepsis is a leading cause of hospital mortality in the United States, contributing to up to half of all deaths.1 If the infection is identified and treated early, however, its associated morbidity and mortality can be significantly reduced.2 The 2001 sepsis guidelines define sepsis as the suspicion of infection plus meeting 2 or more systemic inflammatory response syndrome (SIRS) criteria.3 Although the utility of SIRS criteria has been extensively debated, providers’ accuracy and agreement regarding suspicion of infection are not yet fully characterized. This is very important, as the source of infection is often not identified in patients with severe sepsis or septic shock.4
Although much attention recently has been given to ideal objective criteria for accurately identifying sepsis, less is known about what constitutes ideal subjective criteria and who can best make that assessment.5-7 We conducted a study to measure providers’ agreement regarding this subjective assessment and the impact of that agreement on patient outcomes.
METHODS
We performed a secondary analysis of prospectively collected data on consecutive adults hospitalized on a general medicine ward at an academic medical center between April 1, 2014 and March 31, 2015. This study was approved by the University of Chicago Institutional Review Board with a waiver of consent.
A sepsis screening tool was developed locally as part of the Surviving Sepsis Campaign Quality Improvement Learning Collaborative8 (Supplemental Figure). This tool was completed by bedside nurses for each patient during each shift. Bedside registered nurse (RN) suspicion of infection was deemed positive if the nurse answered yes to question 2: “Does the patient have evidence of an active infection?” We compared RN assessment with assessment by the ordering provider, a medical doctor or advanced practice professionals (MD/APP), using an existing order for antibiotics or a new order for either blood or urine cultures placed within 12 hours before nursing screen time to indicate MD/APP suspicion of infection.
All nursing screens were transcribed into an electronic database, excluding screens not performed, or missing RN suspicion of infection. For quality purposes, screening data were merged with electronic health record data to verify SIRS criteria at the time of the screens as well as the presence of culture and/or antibiotic orders preceding the screens. Outcome data were obtained from an administrative database and confirmed by chart review using the 2001 sepsis definitions.6 Data were de-identified and time-shifted before this analysis. SIRS-positive criteria were defined as meeting 2 or more of the following: temperature higher than 38°C or lower than 36°C; heart rate higher than 90 beats per minute; respiratory rate more than 20 breaths per minute; and white blood cell count more than 2,000/mm3 or less than 4,000/mm3.The primary clinical outcome was progression to severe sepsis or septic shock. Secondary outcomes included transfer to intensive care unit (ICU) and in-hospital mortality. Given that RN and MD/APP suspicion of infection can vary over time, only the initial screen for each patient was used in assessing progression to severe sepsis or septic shock and in-hospital mortality. All available screens were used to investigate the association between each provider’s suspicion of infection over time and ICU transfer.
Demographic characteristics were compared using the χ2 test and analysis of variance, as appropriate. Provider agreement was evaluated with a weighted κ statistic. Fisher exact tests were used to compare proportions of mortality and severe sepsis/septic shock, and the McNemar test was used to compare proportions of ICU transfers. The association of outcomes based on provider agreement was evaluated with a nonparametric test for trend.
RESULTS
During the study period, 1386 distinct patients had 13,223 screening opportunities, with a 95.4% compliance rate. A total of 1127 screens were excluded for missing nursing documentation of suspicion of infection, leaving 1192 first screens and 11,489 total screens for analysis. Of the completed screens, 3744 (32.6%) met SIRS criteria; suspicion of infection was noted by both RN and MD/APP in 5.8% of cases, by RN only in 22.2%, by MD/APP only in 7.2%, and by neither provider in 64.7% (Figure 1). Overall agreement rate was 80.7% for suspicion of infection (κ = 0.11, P < 0.001). Demographics by subgroup are shown in the Supplemental Table. Progression to severe sepsis or shock was highest when both providers suspected infection in a SIRS-positive patient (17.7%), was substantially reduced with single-provider suspicion (6.0%), and was lowest when neither provider suspected infection (1.5%) (P < 0.001). A similar trend was found for in-hospital mortality (both providers, 6.3%; single provider, 2.7%; neither provider, 2.5%; P = 0.01). Compared with MD/APP-only suspicion, SIRS-positive patients in whom only RNs suspected infection had similar frequency of progression to severe sepsis or septic shock (6.5% vs 5.6%; P = 0.52) and higher mortality (5.0% vs 1.1%; P = 0.32), though these findings were not statistically significant.
For the 121 patients (10.2%) transferred to ICU, RNs were more likely than MD/APPs to suspect infection at all time points (Figure 2). The difference was small (P = 0.29) 48 hours before transfer (RN, 12.5%; MD/APP, 5.6%) but became more pronounced (P = 0.06) by 3 hours before transfer (RN, 46.3%; MD/APP, 33.1%). Nursing assessments were not available after transfer, but 3 hours after transfer the proportion of patients who met MD/APP suspicion-of-infection criteria (44.6%) was similar (P = 0.90) to that of the RNs 3 hours before transfer (46.3%).
DISCUSSION
Our findings reveal that bedside nurses and ordering providers routinely have discordant assessments regarding presence of infection. Specifically, when RNs are asked to screen patients on the wards, they are suspicious of infection more often than MD/APPs are, and they suspect infection earlier in ICU transfer patients. These findings have significant implications for patient care, compliance with the new national SEP-1 Centers for Medicare & Medicaid Services quality measure, and identification of appropriate patients for enrollment in sepsis-related clinical trials.
To our knowledge, this is the first study to explore agreement between bedside RN and MD/APP suspicion of infection in sepsis screening and its association with patient outcomes. Studies on nurse and physician concordance in other domains have had mixed findings.9-11 The high discordance rate found in our study points to the highly subjective nature of suspicion of infection.
Our finding that RNs suspect infection earlier in patients transferred to ICU suggests nursing suspicion has value above and beyond current practice. A possible explanation for the higher rate of RN suspicion, and earlier RN suspicion, is that bedside nurses spend substantially more time with their patients and are more attuned to subtle changes that often occur before any objective signs of deterioration. This phenomenon is well documented and accounts for why rapid response calling criteria often include “nurse worry or concern.”12,13 Thus, nurse intuition may be an important signal for early identification of patients at high risk for sepsis.
That about one third of all screens met SIRS criteria and that almost two thirds of those screens were not thought by RN or MD/APP to be caused by infection add to the literature demonstrating the limited value of SIRS as a screening tool for sepsis.14 To address this issue, the 2016 sepsis definitions propose using the quick Sepsis-Related Organ Failure Assessment (qSOFA) to identify patients at high risk for clinical deterioration; however, the Surviving Sepsis Campaign continues to encourage sepsis screening using the SIRS criteria.15
Limitations of this study include its lack of generalizability, as it was conducted with general medical patients at a single center. Second, we did not specifically ask the MD/APPs whether they suspected infection; instead, we relied on their ordering practices. Third, RN and MD/APP assessments were not independent, as RNs had access to MD/APP orders before making their own assessments, which could bias our results.
Discordance in provider suspicion of infection is common, with RNs documenting suspicion more often than MD/APPs, and earlier in patients transferred to ICU. Suspicion by either provider alone is associated with higher risk for sepsis progression and in-hospital mortality than is the case when neither provider suspects infection. Thus, a collaborative method that includes both RNs and MD/APPs may improve the accuracy and timing of sepsis detection on the wards.
Acknowledgments
The authors thank the members of the Surviving Sepsis Campaign (SSC) Quality Improvement Learning Collaborative at the University of Chicago for their help in data collection and review, especially Meredith Borak, Rita Lanier, Mary Ann Francisco, and Bill Marsack. The authors also thank Thomas Best and Mary-Kate Springman for their assistance in data entry and Nicole Twu for administrative support. Data from this study were provided by the Clinical Research Data Warehouse (CRDW) maintained by the Center for Research Informatics (CRI) at the University of Chicago. CRI is funded by the Biological Sciences Division of the Institute for Translational Medicine/Clinical and Translational Science Award (CTSA) (National Institutes of Health UL1 TR000430) at the University of Chicago.
Disclosures
Dr. Bhattacharjee is supported by postdoctoral training grant 4T32HS000078 from the Agency for Healthcare Research and Quality. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by career development award K08 HL121080 from the National Heart, Lung, and Blood Institute. Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), American Heart Association (Dallas, Texas), and Laerdal Medical (Stavanger, Norway) and has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. The other authors report no conflicts of interest.
Sepsis is a leading cause of hospital mortality in the United States, contributing to up to half of all deaths.1 If the infection is identified and treated early, however, its associated morbidity and mortality can be significantly reduced.2 The 2001 sepsis guidelines define sepsis as the suspicion of infection plus meeting 2 or more systemic inflammatory response syndrome (SIRS) criteria.3 Although the utility of SIRS criteria has been extensively debated, providers’ accuracy and agreement regarding suspicion of infection are not yet fully characterized. This is very important, as the source of infection is often not identified in patients with severe sepsis or septic shock.4
Although much attention recently has been given to ideal objective criteria for accurately identifying sepsis, less is known about what constitutes ideal subjective criteria and who can best make that assessment.5-7 We conducted a study to measure providers’ agreement regarding this subjective assessment and the impact of that agreement on patient outcomes.
METHODS
We performed a secondary analysis of prospectively collected data on consecutive adults hospitalized on a general medicine ward at an academic medical center between April 1, 2014 and March 31, 2015. This study was approved by the University of Chicago Institutional Review Board with a waiver of consent.
A sepsis screening tool was developed locally as part of the Surviving Sepsis Campaign Quality Improvement Learning Collaborative8 (Supplemental Figure). This tool was completed by bedside nurses for each patient during each shift. Bedside registered nurse (RN) suspicion of infection was deemed positive if the nurse answered yes to question 2: “Does the patient have evidence of an active infection?” We compared RN assessment with assessment by the ordering provider, a medical doctor or advanced practice professionals (MD/APP), using an existing order for antibiotics or a new order for either blood or urine cultures placed within 12 hours before nursing screen time to indicate MD/APP suspicion of infection.
All nursing screens were transcribed into an electronic database, excluding screens not performed, or missing RN suspicion of infection. For quality purposes, screening data were merged with electronic health record data to verify SIRS criteria at the time of the screens as well as the presence of culture and/or antibiotic orders preceding the screens. Outcome data were obtained from an administrative database and confirmed by chart review using the 2001 sepsis definitions.6 Data were de-identified and time-shifted before this analysis. SIRS-positive criteria were defined as meeting 2 or more of the following: temperature higher than 38°C or lower than 36°C; heart rate higher than 90 beats per minute; respiratory rate more than 20 breaths per minute; and white blood cell count more than 2,000/mm3 or less than 4,000/mm3.The primary clinical outcome was progression to severe sepsis or septic shock. Secondary outcomes included transfer to intensive care unit (ICU) and in-hospital mortality. Given that RN and MD/APP suspicion of infection can vary over time, only the initial screen for each patient was used in assessing progression to severe sepsis or septic shock and in-hospital mortality. All available screens were used to investigate the association between each provider’s suspicion of infection over time and ICU transfer.
Demographic characteristics were compared using the χ2 test and analysis of variance, as appropriate. Provider agreement was evaluated with a weighted κ statistic. Fisher exact tests were used to compare proportions of mortality and severe sepsis/septic shock, and the McNemar test was used to compare proportions of ICU transfers. The association of outcomes based on provider agreement was evaluated with a nonparametric test for trend.
RESULTS
During the study period, 1386 distinct patients had 13,223 screening opportunities, with a 95.4% compliance rate. A total of 1127 screens were excluded for missing nursing documentation of suspicion of infection, leaving 1192 first screens and 11,489 total screens for analysis. Of the completed screens, 3744 (32.6%) met SIRS criteria; suspicion of infection was noted by both RN and MD/APP in 5.8% of cases, by RN only in 22.2%, by MD/APP only in 7.2%, and by neither provider in 64.7% (Figure 1). Overall agreement rate was 80.7% for suspicion of infection (κ = 0.11, P < 0.001). Demographics by subgroup are shown in the Supplemental Table. Progression to severe sepsis or shock was highest when both providers suspected infection in a SIRS-positive patient (17.7%), was substantially reduced with single-provider suspicion (6.0%), and was lowest when neither provider suspected infection (1.5%) (P < 0.001). A similar trend was found for in-hospital mortality (both providers, 6.3%; single provider, 2.7%; neither provider, 2.5%; P = 0.01). Compared with MD/APP-only suspicion, SIRS-positive patients in whom only RNs suspected infection had similar frequency of progression to severe sepsis or septic shock (6.5% vs 5.6%; P = 0.52) and higher mortality (5.0% vs 1.1%; P = 0.32), though these findings were not statistically significant.
For the 121 patients (10.2%) transferred to ICU, RNs were more likely than MD/APPs to suspect infection at all time points (Figure 2). The difference was small (P = 0.29) 48 hours before transfer (RN, 12.5%; MD/APP, 5.6%) but became more pronounced (P = 0.06) by 3 hours before transfer (RN, 46.3%; MD/APP, 33.1%). Nursing assessments were not available after transfer, but 3 hours after transfer the proportion of patients who met MD/APP suspicion-of-infection criteria (44.6%) was similar (P = 0.90) to that of the RNs 3 hours before transfer (46.3%).
DISCUSSION
Our findings reveal that bedside nurses and ordering providers routinely have discordant assessments regarding presence of infection. Specifically, when RNs are asked to screen patients on the wards, they are suspicious of infection more often than MD/APPs are, and they suspect infection earlier in ICU transfer patients. These findings have significant implications for patient care, compliance with the new national SEP-1 Centers for Medicare & Medicaid Services quality measure, and identification of appropriate patients for enrollment in sepsis-related clinical trials.
To our knowledge, this is the first study to explore agreement between bedside RN and MD/APP suspicion of infection in sepsis screening and its association with patient outcomes. Studies on nurse and physician concordance in other domains have had mixed findings.9-11 The high discordance rate found in our study points to the highly subjective nature of suspicion of infection.
Our finding that RNs suspect infection earlier in patients transferred to ICU suggests nursing suspicion has value above and beyond current practice. A possible explanation for the higher rate of RN suspicion, and earlier RN suspicion, is that bedside nurses spend substantially more time with their patients and are more attuned to subtle changes that often occur before any objective signs of deterioration. This phenomenon is well documented and accounts for why rapid response calling criteria often include “nurse worry or concern.”12,13 Thus, nurse intuition may be an important signal for early identification of patients at high risk for sepsis.
That about one third of all screens met SIRS criteria and that almost two thirds of those screens were not thought by RN or MD/APP to be caused by infection add to the literature demonstrating the limited value of SIRS as a screening tool for sepsis.14 To address this issue, the 2016 sepsis definitions propose using the quick Sepsis-Related Organ Failure Assessment (qSOFA) to identify patients at high risk for clinical deterioration; however, the Surviving Sepsis Campaign continues to encourage sepsis screening using the SIRS criteria.15
Limitations of this study include its lack of generalizability, as it was conducted with general medical patients at a single center. Second, we did not specifically ask the MD/APPs whether they suspected infection; instead, we relied on their ordering practices. Third, RN and MD/APP assessments were not independent, as RNs had access to MD/APP orders before making their own assessments, which could bias our results.
Discordance in provider suspicion of infection is common, with RNs documenting suspicion more often than MD/APPs, and earlier in patients transferred to ICU. Suspicion by either provider alone is associated with higher risk for sepsis progression and in-hospital mortality than is the case when neither provider suspects infection. Thus, a collaborative method that includes both RNs and MD/APPs may improve the accuracy and timing of sepsis detection on the wards.
Acknowledgments
The authors thank the members of the Surviving Sepsis Campaign (SSC) Quality Improvement Learning Collaborative at the University of Chicago for their help in data collection and review, especially Meredith Borak, Rita Lanier, Mary Ann Francisco, and Bill Marsack. The authors also thank Thomas Best and Mary-Kate Springman for their assistance in data entry and Nicole Twu for administrative support. Data from this study were provided by the Clinical Research Data Warehouse (CRDW) maintained by the Center for Research Informatics (CRI) at the University of Chicago. CRI is funded by the Biological Sciences Division of the Institute for Translational Medicine/Clinical and Translational Science Award (CTSA) (National Institutes of Health UL1 TR000430) at the University of Chicago.
Disclosures
Dr. Bhattacharjee is supported by postdoctoral training grant 4T32HS000078 from the Agency for Healthcare Research and Quality. Drs. Churpek and Edelson have a patent pending (ARCD.P0535US.P2) for risk stratification algorithms for hospitalized patients. Dr. Churpek is supported by career development award K08 HL121080 from the National Heart, Lung, and Blood Institute. Dr. Edelson has received research support from Philips Healthcare (Andover, Massachusetts), American Heart Association (Dallas, Texas), and Laerdal Medical (Stavanger, Norway) and has ownership interest in Quant HC (Chicago, Illinois), which is developing products for risk stratification of hospitalized patients. The other authors report no conflicts of interest.
1. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. PubMed
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. PubMed
3. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256. PubMed
4. Vincent JL, Sakr Y, Sprung CL, et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353. PubMed
5. Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629-1638. PubMed
6. Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381(9868):774-775. PubMed
7. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. PubMed
8. Surviving Sepsis Campaign (SSC) Sepsis on the Floors Quality Improvement Learning Collaborative. Frequently asked questions (FAQs). Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/About-Collaboratives.pdf. Published October 8, 2013.
9. Fiesseler F, Szucs P, Kec R, Richman PB. Can nurses appropriately interpret the Ottawa ankle rule? Am J Emerg Med. 2004;22(3):145-148. PubMed
10. Blomberg H, Lundström E, Toss H, Gedeborg R, Johansson J. Agreement between ambulance nurses and physicians in assessing stroke patients. Acta Neurol Scand. 2014;129(1):4955. PubMed
11. Neville TH, Wiley JF, Yamamoto MC, et al. Concordance of nurses and physicians on whether critical care patients are receiving futile treatment. Am J Crit Care. 2015;24(5):403410. PubMed
12. Odell M, Victor C, Oliver D. Nurses’ role in detecting deterioration in ward patients: systematic literature review. J Adv Nurs. 2009;65(10):1992-2006. PubMed
13. Howell MD, Ngo L, Folcarelli P, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012;40(9):2562-2568. PubMed
14. Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192(8):958-964. PubMed
15. Antonelli M, DeBacker D, Dorman T, Kleinpell R, Levy M, Rhodes A; Surviving Sepsis Campaign Executive Committee. Surviving Sepsis Campaign responds to Sepsis-3. Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/SSC-Statements-Sepsis-Definitions-3-2016.pdf. Published March 1, 2016. Accessed May 11, 2016.
1. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90-92. PubMed
2. Rivers E, Nguyen B, Havstad S, et al; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377. PubMed
3. Levy MM, Fink MP, Marshall JC, et al; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256. PubMed
4. Vincent JL, Sakr Y, Sprung CL, et al; Sepsis Occurrence in Acutely Ill Patients Investigators. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34(2):344-353. PubMed
5. Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629-1638. PubMed
6. Vincent JL, Opal SM, Marshall JC, Tracey KJ. Sepsis definitions: time for change. Lancet. 2013;381(9868):774-775. PubMed
7. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. PubMed
8. Surviving Sepsis Campaign (SSC) Sepsis on the Floors Quality Improvement Learning Collaborative. Frequently asked questions (FAQs). Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/About-Collaboratives.pdf. Published October 8, 2013.
9. Fiesseler F, Szucs P, Kec R, Richman PB. Can nurses appropriately interpret the Ottawa ankle rule? Am J Emerg Med. 2004;22(3):145-148. PubMed
10. Blomberg H, Lundström E, Toss H, Gedeborg R, Johansson J. Agreement between ambulance nurses and physicians in assessing stroke patients. Acta Neurol Scand. 2014;129(1):4955. PubMed
11. Neville TH, Wiley JF, Yamamoto MC, et al. Concordance of nurses and physicians on whether critical care patients are receiving futile treatment. Am J Crit Care. 2015;24(5):403410. PubMed
12. Odell M, Victor C, Oliver D. Nurses’ role in detecting deterioration in ward patients: systematic literature review. J Adv Nurs. 2009;65(10):1992-2006. PubMed
13. Howell MD, Ngo L, Folcarelli P, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012;40(9):2562-2568. PubMed
14. Churpek MM, Zadravecz FJ, Winslow C, Howell MD, Edelson DP. Incidence and prognostic value of the systemic inflammatory response syndrome and organ dysfunctions in ward patients. Am J Respir Crit Care Med. 2015;192(8):958-964. PubMed
15. Antonelli M, DeBacker D, Dorman T, Kleinpell R, Levy M, Rhodes A; Surviving Sepsis Campaign Executive Committee. Surviving Sepsis Campaign responds to Sepsis-3. Society of Critical Care Medicine website. http://www.survivingsepsis.org/SiteCollectionDocuments/SSC-Statements-Sepsis-Definitions-3-2016.pdf. Published March 1, 2016. Accessed May 11, 2016.
© 2017 Society of Hospital Medicine
Perceived safety and value of inpatient “very important person” services
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
Recent publications in the medical literature and lay press have stirred controversy regarding the use of inpatient ‘very important person’ (VIP) services.1-3 The term “VIP services” often refers to select conveniences offered in addition to the assumed basic level of care and services provided by a hospital. Examples include additional space, enhanced facilities, specific comforts, or personal support. In some instances, these amenities may only be provided to patients who have close financial, social, or professional relationships with the hospital.
How VIP patients interact with their health system to obtain VIP services has raised unique concerns. Some have speculated that the presence of a VIP patient may be disruptive to the care of non-VIP patients, while others have cautioned physicians about potential dangers to the VIP patients themselves.4-6 Despite much being written on the topics of VIP patients and services in both the lay and academic press, our literature review identified only 1 study on the topic, which cataloged the preferential treatment of VIP patients in the emergency department.6 We are unaware of any investigations of VIP-service use in the inpatient setting. Through a multisite survey of hospital medicine physicians, we assessed physician viewpoints and behavior regarding VIP services.
METHODS
The Hospital Medicine Reengineering Network (HOMERuN) is a nation-wide learning organization focused on measuring and improving the outcomes of hospitalized patients.7 We surveyed hospitalists from 8 HOMERuN hospitals (Appendix 1). The survey instrument contained 4 sections: nonidentifying respondent demographics, local use of VIP services, reported physician perceptions of VIP services, and case-based assessments (Appendix 2). Survey questions and individual cases were developed by study authors and based on real scenarios and concerns provided by front-line clinical providers. Content, length, and reliability of physician understanding were assessed by a 5-person focus group consisting of physicians not included in the survey population.
Subjects were identified via administrative rosters from each HOMERuN site. Surveys were administered via SurveyMonkey, and results were analyzed descriptively. Populations were compared via the Fisher exact test. “VIP services” were defined as conveniences provided in addition to the assumed basic level of care and services (eg, private or luxury-style rooms, access to a special menu, better views, dedicated personal care attendants, hospital liaisons). VIP patients were defined as those patients receiving VIP services. A hospital was identified as providing VIP services if 50% or more of respondents from that site reported the presence of VIP services.
RESULTS
Of 366 hospitalists contacted, 160 completed the survey (44%). Respondent characteristics and reported prevalence of VIP services are demonstrated in Table 1. In total, 78 respondents (45%) reported the presence of VIP services at their hospital. Of the 8 sites surveyed, a majority of physicians at 4 sites (50%) reported presence of VIP services.
Of respondents reporting the presence of VIP services at their hospital, a majority felt that, from a patient safety perspective, the care received by VIP patients was the same as care received by non-VIP patients (Table 2). A majority reported they had felt pressured by a VIP patient or a family member to order additional tests or treatments that the physician believed were medically unnecessary and that they would be more likely to comply with VIP patient’s requests for tests or treatments they felt were unnecessary. More than one-third (36%) felt pressured by other hospital employees or representatives to comply with VIP services patient’s requests for additional tests or treatments that the physicians believed were medically unnecessary.
When presented the case of a VIP patient with community-acquired pneumonia who is clinically stable for discharge but expressing concerns about leaving the hospital, 61 (38%) respondents reported they would not discharge this patient home: 39 of 70 (55.7%) who reported the presence of VIP services at their hospital, and 22 of 91 (24.2%) who reported the absence of VIP services (P < 0.001). Of those who reported they would not discharge this patient home, 37 (61%) reported the reason for this related to the patient’s connection to the Board of Trustees; 48 (79%) reported the reason for this related to the patient’s concerns; 9 (15%) reported the reason for this related to their own concerns regarding medical details of the patient’s case (respondents could select more than 1 reason).
When presented the case of a VIP patient with acute pulmonary embolism who is medically ready for discharge with primary care physician-approved anticoagulation and discharge plans but for whom their family requests additional consultations and inpatient hypercoagulable workup, 33 (21%) respondents reported they would order additional testing and specialist consultation: 17 of 69 (24.6%) who reported the presence of VIP services their hospital, and 16 of 91 (17.6%) who reported the absence of VIP services (P = 0.33). Of those who reported they would order additional testing and specialist consultation, 14 (42%) reported the reason for this related to the family’s financial connections to the hospital; 30 (91%) reported the reason for this related to the family’s concerns; 3 (9%) reported the reason for this related to their own concerns about the medical details of the patient’s case (respondents could select more than 1 reason).
DISCUSSION
In our study, a majority of physicians who reported the presence of VIP services at their hospital felt pressured by VIP patients or their family members to perform unnecessary testing or treatment. While this study was not designed to quantify the burden of unnecessary care for VIP patients, our results have implications for individual patients and public health, including potential effects on resource availability, the identification of clinically irrelevant incidental findings, and short- and long-term medical complications of procedures, testing and radiation exposure.
Prior publications have advocated that physicians and hospitals should not allow VIP status to influence management decisions.3,5 We found that more than one-third of physicians who reported the presence of VIP services at their hospital also reported receiving pressure from hospital representatives to provide care to VIP patients that was not medically indicated. These findings highlight an example of the tension faced by physicians who are caught between patient requests and the delivery of value-based care. This potential conflict may be amplified particularly for those patients with close financial, social, or professional ties to the hospitals (and physicians) providing their care. These results suggest the need for physicians, administrators, and patients to work together to address the potential blurring of ethical boundaries created by VIP relationships. Prevention of harm and avoidance of placing physicians in morally distressing situations are common goals for all involved parties.
Efforts to reduce unnecessary care have predominantly focused on structural and knowledge-based drivers.4,8,9 Our results highlight the presence of additional forces. A majority of physician respondents who reported the presence of VIP services at their hospital also reported that they would be more likely to comply with requests for unnecessary care for a VIP patient as compared to a non-VIP patient. Furthermore, in case-based questions about the requests of a VIP patient and their family for additional unnecessary care, a significant portion of physicians who reported they would comply with these requests listed the VIP status of the patient or family as a factor underlying this decision. Only a minority of physicians reported their decision to provide additional care was the result of their own medically-based concerns. Because these cases were hypothetical and we did not include comparator cases involving non-VIP patients, it remains uncertain whether the observed perceptions accurately reflect real-world differences in the care of VIP and non-VIP patients. Nonetheless, our findings emphasize the importance of better understanding the social drivers of overuse and physician communication strategies related to medically inappropriate tests.10,11
Demand for unnecessary testing may be driven by the mentality that “more is better.”12 Contrary to this belief, provision of unnecessary care can increase the risk of patient harm.13 Despite physician respondents reporting that VIP patients requested and/or received additional unnecessary care, a majority of respondents felt that patient safety for VIP patients was equivalent to that for non-VIP patients. As we assessed only physician perceptions of safety, which may not necessarily correlate with actual safety, further research in this area is needed.
Our study was limited by several factors. While our study population included hospitalists from 8 geographically broad hospitals, including university, safety net, and community hospitals, study responses may not be reflective of nationwide trends. Our response rate may limit our ability to generalize conclusions beyond respondents. Second, our study captured physician perceptions of behavior and safety rather than actually measuring practice and outcomes. Studies comparing physician practice patterns and outcomes between VIP and non-VIP patients would be informative. Additionally, despite our inclusive survey design process, our survey was not validated, and it is possible that our questions were not interpreted as intended. Lastly, despite the anonymous nature of our survey, physicians may have felt compelled to respond in a particular way due to conflicting professional, financial, or social factors.
Our findings provide initial insight into how care for the VIP patient may present unique challenges for physicians, hospitals, and society by systematizing care inequities, as well as potentially incentivizing low-value care practices. Whether these imbalances produce clinical harms or benefits remains worthy of future studies.
Disclosure
Nothing to report.
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
1. Bernstein N. Chefs, butlers, marble baths: Hospitals vie for the affluent. New York Times. January 21, 2012. http://www.nytimes.com/2012/01/22/nyregion/chefs-butlers-and-marble-baths-not-your-average-hospital-room.html. Accessed February 1, 2017.
2. Kennedy DW, Kagan SH, Abramson KB, Boberick C, Kaiser LR. Academic medicine amenities unit: developing a model to integrate academic medical care with luxury hotel services. Acad Med. 2009;84(2):185-191. PubMed
3. Alfandre D, Clever S, Farber NJ, Hughes MT, Redstone P, Lehmann LS. Caring for ‘very important patients’--ethical dilemmas and suggestions for practical management. Am J Med. 2016;129(2):143-147. PubMed
4. Bulger J, Nickel W, Messler J, et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486-492. PubMed
5. Martin A, Bostic JQ, Pruett K. The V.I.P.: hazard and promise in treating “special” patients. J Am Acad Child Adolesc Psychiatry. 2004;43(3):366-369. PubMed
6. Smally AJ, Carroll B, Carius M, Tilden F, Werdmann M. Treatment of VIPs. Ann Emerg Med. 2011;58(4):397-398. PubMed
7. Auerbach AD, Patel MS, Metlay JP, et al. The hospital medicine reengineering network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420. PubMed
8. Caverly TJ, Combs BP, Moriates C, Shah N, Grady D. Too much medicine happens too often: the teachable moment and a call for manuscripts from clinical trainees. JAMA Intern Med. 2014;174(1):8-9. PubMed
9. Schwartz AL, Chernew ME, Landon BE, McWilliams JM. Changes in low-value services in year 1 of the Medicare pioneer accountable care organization program. JAMA Intern Med. 2015;175(11):1815-1825. PubMed
10. Paterniti DA, Fancher TL, Cipri CS, Timmermans S, Heritage J, Kravitz RL. Getting to “no”: strategies primary care physicians use to deny patient requests. Arch Intern Med. 2010;170(4):381-388. PubMed
11. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff (Millwood). 2013;32(2):285-293. PubMed
12. Korenstein D. Patient perception of benefits and harms: the Achilles heel of high-value care. JAMA Intern Med. 2015;175(2):287-288. PubMed
13. Moynihan R, Doust J, Henry D. Preventing overdiagnosis: how to stop harming the healthy. BMJ. 2012;344:e3502. PubMed
© 2017 Society of Hospital Medicine
A time and motion study of pharmacists and pharmacy technicians obtaining admission medication histories
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
Using pharmacists to obtain admission medication histories (AMHs) reduces medication errors by 70% to 83% and resultant adverse drug events (ADEs) by 15%.1-3 Dissemination of this practice has been limited by several factors, including clinician practice models, staff availability, confusion in provider roles and accountability, and absence of standardized best practices.4-5 This paper assesses one of these barriers: the high cost of utilizing pharmacists. Third-person observer time and motion analysis shows that pharmacists require 46 and 92 minutes to obtain AMHs from medical and geriatric patients,6 respectively, resulting in pharmacist costs of $44 to $88 per patient, based on 2015 US Bureau of Labor Statistics (BLS) hourly wage data for pharmacists ($57.34).7
Ph
METHODS
This study originated as part of a randomized, controlled trial conducted during January-February 2014 at Cedars-Sinai Medical Center (CSMC), an 896-bed, university-affiliated, not-for-profit hospital.9 Pharmacy staff included pharmacists, PGY-1 pharmacy residents, and pharmacy technicians, each of whom received standardized didactic and experiential training (Appendix 1).
The pharmacists’ AMH and general pharmacy experience ranged from <1 to 3 years and <1 to 5 years, respectively. For PSPTs, AMH and general pharmacy experience ranged from <1 to 2 years and 1 to 17 years, respectively. Three additional pharmacists were involved in supervising PSPTs, and their experience fell within the aforementioned ranges, except for one pharmacist with general pharmacy experience of 16 years. The CSMC Institutional Review Board approved this study with oral consent from pharmacy staff.
For the trial, pharmacists and PSPTs obtained AMHs from 185 patients identified as high-risk for ADEs in the CSMC Emergency Department (ED). Patients were randomized into each arm using RANDI2 software11 if they met one of the trial inclusion criteria, accessed via electronic health record (EHR) (Appendix 2). For several days during this trial, a trained research nurse shadowed pharmacists and PSPTs to record tasks performed, as well as the actual time, including start and end times, dedicated to each task.
After excluding AMHs with incomplete data, we calculated mean AMH times and component task times (Table). We compared mean times for pharmacists and PSPTs using two sample t tests (Table). We calculated mean times of tasks across only AMHs that required the task, mean times of tasks across all AMHs studied, regardless of whether the AMH required the task or not (assigning 0 minutes for the task if it was not required), and percent mean time of task per patient for providers combined (Table).
We calculated Pearson product-moment correlation estimates between AMH time and these continuous variables: patient age; total number of EHR medications; number of chronic EHR medications; years of provider AMH experience; and years of provider general pharmacy experience. Using two sample t tests, we also checked for associations between AMH time and the following categorical variables: sex; presence of a patient-provided medication list; caregiver availability; and altered mental status, as determined by review of the ED physician’s note. Caregiver availability was defined as the availability of a family member, caregiver, or medication administration record (MAR) for patients residing at a skilled nursing facility (SNF). The rationale for combining these variables is that SNF nurses are the primary caregivers responsible for administering medications, and the MAR is reflective of their actions.
After reviewing our initial data, we decided to increase our sample size from 20 to 30 complete AMHs. Because the trial had concluded, we selected 10 additional patients who met trial criteria and who would already have an AMH obtained by pharmacy staff for operational reasons. The only difference with the second set of patients (n = 10) is that we did not randomize patients into each arm, but chose to focus on AMHs obtained by PSPTs, as there is a greater need in the literature to study PSPTs. After finalizing data collection, the aforementioned analyses were conducted on the complete data set.
Lastly, we estimated the mean labor cost for pharmacists and PSPTs to obtain an AMH by using 2015 US BLS hourly wage data for pharmacists ($57.34) and pharmacy technicians ($15.23).7 The cost for a pharmacist-obtained AMH was calculated by multiplying the measured mean time a pharmacist needed to obtain an AMH by $57.34 per hour. The cost for a PSPT-obtained AMH was the sum of the PSPT’s measured mean time to obtain an AMH multiplied by $15.23 per hour and the measured mean pharmacist supervisory time multiplied by $57.34 per hour.
RESULTS
Of the 37 observed AMHs, 30 had complete data. Seven AMHs were excluded because not all task times were recorded, due to the schedule restraints of the research nurse. Pharmacists and PSPTs obtained 12 and 18 AMHs, respectively. Mean patient ages were 83.3 (95% confidence interval [CI], 77.3-89.2) and 79.8 (95% CI, 71.5-88.0), for pharmacists and PSPTs, respectively (P = 0.55). Patient’s EHRs contained a mean of 14.3 (95% CI, 11.2-17.5) and 16.3 (95% CI, 13.2-19.5) medications, prior to pharmacists and PSPTs obtaining an AMH, respectively (P = 0.41).
The mean time pharmacists and PSPTs needed to obtain an AMH was 58.5 (95% CI, 46.9-70.1) and 79.4 (95% CI, 59.1-99.8) minutes, respectively (P = 0.14). Summary time data per provider is reported in the Figure. The mean time for pharmacist supervision of technicians was 26 (95% CI, 14.9-37.1) minutes. Mean times of tasks and comparisons of these means times between providers are reported in the Table. The percent mean time for each task per patient for providers combined is also reported in the Table, in which utilizing the EHR was associated with the greatest percentage of time spent at 42.8% (95% CI, 37.4-48.2).
In the 18 cases for which a caregiver (or SNF medication list) was available, providers needed only 58.1 (95% CI, 44.1-72.1) minutes to obtain an AMH, as compared with 90.5 (95% CI, 67.9-113.1) minutes for the 12 cases lacking these resources (P = 0.02). We also found that among PSPTs, years of AMH experience were positively correlated with AMH time (coefficient of correlation 0.49, P = 0.04). No other studied variables were correlated with or associated with differential AMH times.
We estimated mean labor costs for pharmacists and PSPTs to obtain AMHs as $55.91 (95% CI, 44.9-67.0) and $45.00 (95% CI, 29.7-60.4) per patient, respectively (P = 0.32). In the latter case, $24.85 (95% CI, 14.3-35.4) of the $45.00 would be needed for pharmacist supervisory time. The labor cost for a PSPT-obtained AMH ($45.00) was the sum of the PSPT’s mean time (79.4 minutes) multiplied by technician wage data ($15.23/hour) and supervising pharmacist’s mean time (26.0 minutes) multiplied by pharmacist wage data ($57.34/hour).
DISCUSSION
Although limited by sample size, we observed no difference in time or costs of obtaining AMHs between pharmacists and PSPTs. Several prior studies reported that pharmacists and technicians needed less time to obtain AMHs (20-40 minutes), as compared with our findings.12-14 However, most prior studies used younger, healthier patients. Additionally, they used clinician self-reporting instead of third-person observer time and motion methodology. Indeed, the pharmacist times we observed in this study were consistent with prior findings6 that used accepted third-person observer time and motion methodology.10
We observed more variation in time to obtain AMHs among PSPTs than among pharmacists. While variation may be at least in part to the greater number of technicians studied, variation also points to the need for training and oversight of PSPTs. Selection of PSPTs with prior experience interacting with patients and functioning with higher levels of autonomy, standardized training of PSPTs, and consistent dedication of trained PSPTs to AMH functions to maintain their skills, may help to minimize such variation.
Limitations include the use of a single center and a small sample size. As such, the study may be underpowered to demonstrate statistically significant differences between providers. Furthermore, 7 AMHs (19%) had to be excluded because complete task times were missing. This was exclusively because the workday of the research nurse ended before the AMH had been completed. Another limitation was that the tasks observed could have been dissected further to identify even more specific factors that could be targeted to decrease AMH times. We recommend that future studies be larger, investigate in more depth various factors associated with time needed to obtain AMHs, consider which patients would most likely benefit from PSPTs, and use a measure of value (eg, number of history errors prevented/dollar spent).
In summary, we found that PSPTs can obtain AMHs for similar cost to pharmacists. It will be especially important to know whether PSPTs maintain the accuracy documented in prior studies.8-9 If that continues to be the case, we expect our findings to allow many hospitals to implement programs using PSPTs to obtain accurate AMHs.
Acknowledgment
The authors thank Katherine M. Abdel-Razek for her role in data collection.
Disclosure
This research was supported by NIH/National Center for Advancing Translational Science UCLA CTSI Grant Number KL2TR000122 and National Institute on Aging Grant Number K23 AG049181-01 (Pevnick). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The investigators retained full independence in the conduct of this research.
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
1. Mergenhagen KA, Blum SS, Kugler A, et al. Pharmacist- versus physician-initiated admission medication reconciliation: impact on adverse drug events. Am J Geriatr Pharmacother. 2012;10(4):242-250. PubMed
2. Mills PR, McGuffie AC. Formal medication reconciliation within the emergency department reduces the medication error rates for emergency admissions. Emerg Med J. 2010;27(12):911-915. PubMed
3. Boockvar KS, LaCorte HC, Giambanco V, Fridman B, Siu A. Medication reconciliation for reducing drug-discrepancy adverse events. Am J Geriatr Pharmacother. 2006;4(3):236-243. PubMed
4. Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057-1069. PubMed
5. Lee KP, Hartridge C, Corbett K, Vittinghoff E, Auerbach AD. “Whose job is it, really?” Physicians’, nurses’, and pharmacists’ perspectives on completing inpatient medication reconciliation. J Hosp Med. 2015;10(3):184-186. PubMed
6. Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13:485. PubMed
7. Bureau of Labor Statistics, US Department of Labor, Occupational Employment Statistics, May 2015. Pharmacists and Pharmacy Technicians. http://www.bls.gov/oes/. Accessed July 15, 2016.
8. Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359-365. PubMed
9. Pevnick JM, Nguyen CB, Jackevicius CA, et al. Minimizing medication histories errors for patients admitted to the hospital through the emergency department: a three-arm pragmatic randomized controlled trial of adding admission medication history interviews by pharmacists or pharmacist-supervised pharmacy technicians to usual care. J Patient Cent Res Rev. 2015;2:93.
10. Zheng K, Guo MH, Hanauer DA. Using the time and motion method to study clinical work processes and workflow: methodological inconsistencies and a call for standardized research. J Am Med Inform Assoc. 2011;18(5):704-710. PubMed
11. Schrimpf D, Plotnicki L, Pilz LR. Web-based open source application for the randomization process in clinical trials: RANDI2. Int J Clin Pharmacol Ther. 2010;48(7):465-467. PubMed
12. American Society of Health-System Pharmacists and the American Pharmacists Association. ASHP-APhA medication management in care transitions best practices. http://media.pharmacist.com/practice/ASHP_APhA_MedicationManagementinCareTransitionsBestPracticesReport2_2013.pdf. Accessed January 15, 2016.
13. Kent AJ, Harrington L, Skinner J. Medication reconciliation by a pharmacist in the emergency department: a pilot project. Can J Hosp Pharm. 2009;62(3):238-242. PubMed
14. Sen S, Siemianowski L, Murphy M, McAllister SC. Implementation of a pharmacy technician-centered medication reconciliation program at an urban teaching medical center. Am J Health Syst Pharm. 2014;71(1):51-56. PubMed
© 2017 Society of Hospital Medicine
Patient-level exclusions from mHealth in a safety-net health system
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
Interest in mHealth—the use of mobile communication devices for clinical and public health—has exploded among clinicians and researchers for its potential to efficiently improve patient health. Recent studies have used mHealth’s asynchronous receptive and expressive communication functions in interventions targeted to managing care transitions and hospital readmissions.1-3 We also recently published on improved readmission risk assessments using postdischarge measures of patient reported outcomes, which could be collected through mobile devices. 4 But persistent disparities in access to5 and engagement with6 smartphones may threaten validity and equity when mHealth strategies do not fully address its own limitations.
Disparities introduced by uneven access to technology are well known, but the rapid, albeit belated, adoption of mobile devices by racial minority groups in the United States has allowed authors of recent thoughtful publications to recast mHealth as itself offering solutions to the disparities’ problem.7,8 Others have cautioned the emergence of disparities along domains other than race, such as low literacy and limited English proficiency (LEP).9 In this paper, we assessed the impact of inadequate reading health literacy (IRHL) and LEP on factors related to access and engagement with mHealth. We conducted our study among urban low-income adults in whom IRHL and LEP are common.
METHODS
We surveyed patients in a large public safety-net health system serving 132 municipalities, including the city of Chicago, in northeastern Illinois. In 2015, nearly 90% of patients were racial-ethnic minorities with more than one-third insured by Medicaid and another one-third uninsured. We sampled adult inpatients and outpatients separately by nonselectively approaching patients in November 2015 to complete an in-person questionnaire in a 464-bed hospital and in 2 primary-care clinics. All inpatients occupied a nonisolation room in a general medical-surgical ward that had been sampled for data collection for that day in 9-day cycles with 8 other similar units. All outpatients in the clinic waiting areas were approached on consecutive days until a predetermined recruitment target was met. Each participant was surveyed once in his/her preferred language (English or Spanish), was 18 years and older, consented verbally, and received no compensation. Sample size provided 80% power to detect a device ownership rate of 50% in an evenly allocated low literacy population compared to a reference rate of 66% assuming a 2-sided α of 0.05 using the Fisher exact test.
The 18-item questionnaire was informed by constructs addressed in the 2015 Pew Research Center smartphone survey.10 However, in addition to device ownership, we inquired about device capabilities, service-plan details, service interruptions due to difficulty paying bills in the previous year, home-Internet access, an active e-mail account, and self-assigned demographics. Self-reported reading health literacy,11 more directly measured than e-health literacy, was screened using a parsimonious instrument validated as a dichotomized measure.12 Instruments in English and Spanish were tested for appropriate and comprehensible word choices and syntax through pilot testing. We inferred LEP among patients preferring to complete the survey in Spanish based on our familiarity with the population. We defined any Internet access as having a mobile data-service plan or having home-Internet access. In addition, we inquired about primary insurance provider and offered Medicaid patients an informational brochure about the federal Lifeline Program (https://www.fcc.gov/lifeline) that subsidizes text-messaging-enabled cellular telephone service for low-income patients. Notably, we assessed engagement by asking about the extent of patients’ interest in “new ways of communicating with your doctor, clinic, or pharmacy using” text, e-mail, or mobile apps with a 5-level response scale ranging from “not at all interested” to “very interested”.
Participant characteristics were confirmed to be similar to the Cook County Health and Hospitals System patient population in 2015 with regards to age, gender, and race/ethnicity. We calculated unadjusted and adjusted odds ratios for IRHL and LEP’s association with each dependent measure of access (to smartphone, Internet, or e-mail) and engagement (using text messaging, e-mail, or mobile apps) controlling for age, gender, primary payer, recruitment location, IRHL, and LEP. Because we oversampled inpatients, we estimated sampling-weight-adjusted proportions and 95% confidence intervals (CI) of the entire CCHHS patient population with access to smartphone, data/text plan, non-prepaid plan, and service interruptions using STATA v13 (StataCorp LP, College Station Texas). The project received a waiver upon review by the local Institutional Review Board.
RESULTS
Participation rate was 65% (302/464). Differences in patients by site are shown in Table 1. IRHL was more frequent and LEP less frequent among hospitalized patients. As shown in Table 2, patients with IRHL were less likely to have any Internet access, to have an active e-mail account, and to be interested in using e-mail for healthcare communications. Patients with LEP were less likely than English speakers to be interested in using mobile apps. Inpatients were less likely than outpatients to be interested in text messaging for healthcare communications.
The estimated proportion (95% CI) of the health system’s patients owning a text-enabled mobile device was 87% (75%-94%) and an Internet-enabled mobile device was 64% (47%-78%). The proportion with no data service interruptions in the previous year was 40% (31%-50%).
DISCUSSION
In this cross-section of urban low-income adult patients, IRHL and LEP were factors associated with potential disparities introduced by mHealth. Even as access to smartphones becomes ubiquitous, lagging access to Internet and e-mail among low literacy patients, and low levels of technology engagement for healthcare communications among patients with IRHL or LEP, underscore concerns about equity in health systems’ adoption of mHealth strategies. Hospitalized patients were found to have diminished engagement with mHealth independent of IRHL and LEP.
Regarding engagement, significantly fewer patients with IRHL or LEP were interested in using technology for healthcare communications. Our finding suggests that health disparities already associated with these conditions13 may not be reduced by mobile device outreach alone and may even be worsened by it. Touch screens, audio-enabled questionnaires, and language translation engines are innovations that may be helpful to mitigate IRHL and LEP, but evidence is scarce. Privacy and security concerns, and lack of experience with technology, may also lower engagement. A contemporaneous study found lower apps’ usage among Latinos, also suggesting that language concordance between apps, their source, and targeted users is important.14 Low-tech solutions involving mobile telephone or even lower tech in-person communications targeted to the estimated 26% of the US population with low literacy15 and 20% with LEP16 may be practical stopgap measures. Even as disparities in access to technology across race-ethnicity are diminishing,10 equity across poverty levels, low levels of education, cultural norms, and disabilities may be more challenging to overcome. Our assessment indicates that large exclusions of a safety-net population in 2015 are a legitimate concern in communication strategies that rely too heavily on mHealth. These findings underscore the CONSORT-EHEALTH recommendation that investigators report web-based recruitment strategies and data-collection methods comprehensively.17
Regarding access, our estimates suggest that historical disparities in smartphone ownership are diminishing, but access to Internet capabilities may still be lower among the urban poor compared to the nation as a whole. The Pew Research Center found that 64% of Americans owned a smartphone in 2015 (respondents defined smartphone).10 In comparison, 87% (95% CI, 75%, 94%) of our study participants owned a text-enabled mobile device and 64% (47%, 78%) owned an Internet-enabled mobile device. However, the 40% (31%, 50%) of our safety-net population with an uninterrupted data plan over the previous year may be lower than the 50% of Americans reporting uninterrupted data plans over their lifetime.10 The impact of expense-related data plan interruptions is magnified by the 40% of our study population—compared to 15% of Americans—who are dependent on mobile devices for Internet access.10 The association between Internet connectivity and literacy evokes multiple bidirectional pathways yet to be elucidated. But if mHealth can reduce health disparities, closing the gap in device ownership is only a partial accomplishment, and future work also needs to expand Internet connectivity to allow literacy-enhancing and literacy-naïve technologies to flourish.
This study has limitations. Our study population was a consecutive sample and participation rate was less than 100%. However, we recruited participants into the study the way we may also have approached patients to introduce mHealth options in our clinical settings. Our sampling method proved adequate for our primary goal to explain differences in technology access and engagement using regression analysis. Although our patient population may not directly generalize to many healthcare systems, including other safety-net systems serving regions with variable technology uptake,18 our findings reflect the capacities and the preferences of the most disadvantaged segments of urban populations. We systematically excluded LEP non-Spanish speakers, but they consisted of less than 5% of inpatients and no outpatients. We did not assess current technology use. Finally, as discussed earlier, access and use of new technologies change rapidly and frequent updates are necessary.
mHealth is a promising tool because it may increase healthcare access, improve care quality, and promote research. All these potential benefits will be obtained with accompanying efforts to reduce healthcare disparities, especially where some technologies themselves are exclusionary.
Disclosures
The authors report no financial conflicts of interest.
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
1. Khosravi P, Ghapanchi AH. Investigating the effectiveness of technologies applied to assist seniors: a systematic literature review. Int J Med Inform. 2016;85:17-26. PubMed
2. Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774-784. PubMed
3. Prieto-Centurion V, Gussin HA, Rolle AJ, Krishnan JA. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10:680-684. PubMed
4. Hinami K, Smith J, Deamant CD, BuBeshter K, Trick WE. When do patient-reported outcome measures inform readmission risk? J Hosp Med. 2015;10:294-300. PubMed
5. Gibbons MC. A historical overview of health disparities and the potential of eHealth solutions. J Med Internet Res. 2005;7:e50. PubMed
6. Nelson LA, Mulvaney SA, Gebretsadik T, Ho YX, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. PubMed
7. Martin T. Assessing mHealth: opportunities and barriers to patient engagement. J Health Care Poor Underserved. 2012;23:935-941. PubMed
8. Horn IB, Mendoza FS. Reframing the disparities agenda: a time to rethink, a time to focus. Acad Pediatr. 2013;14:115-116. PubMed
9. Viswanath K, Nagler RH, Bigman-Galimore CA, McCauley MP, Jung M, Ramanadhan S. The communications revolution and health inequalities in the 21st century: implications for cancer control. Cancer Epidemiol, BiomarkersPrev. 2012;21:1701-1708. PubMed
10. Pew Research Center. The Smartphone Difference. Pew Research Center; April 2015. Available at: http://www.pewinternet.org/2015/04/01/us-smartphone-use-in-2015/. Accessed January 12, 2017.
11. Baker DW. The meaning and measure of health literacy. J Gen Intern Med. 2011;21:878-883. PubMed
12. Morris NS, MacLean CD, Chew LD, Littenberg B. The single item literacy screener: evalution of a brief instrument to identify limited reading ability. BMC Fam Pract. 2006;7:21. PubMed
13. Sentell T, Braun K. Low health literacy, limited English proficiency, and health status in Asians, Latinos, and other racial/ethnic groups in California. J Health Commun. 2012;17:82-99. PubMed
14. Arora S, Ford K, Terp S, et al. Describing the evolution of mobile technology usage for Latino patients and comparing findings to national mHealth estimates. J Am Med Inform Assoc. 2016;23:979-983. PubMed
15. Paasche-Orlow MK, Parker RM, Gazmararian JA, Nielsen-Bohlman LT, Rudd RR. The prevalence of limited health literacy. J Gen Intern Med. 2005;20:175-184. PubMed
16. US Census Bureau. Detailed languages spoken at home and ability to speak English for the population 5 years and over: 2009-2013. Published 2015. Available at: http://www.census.gov/data/tables/2013/demo/2009-2013-lang-tables.html. Accessed March 31, 2016.
17. Eysenbach G, CONSORT-EHEALTH Group. CONSORT-EHEALTH: improving and standardizing evaluation reports of Web-based and mobile health intervention. J Med Internet Res. 2011;13:e126. PubMed
18. Schickedanz A, Huang D, Lopex A, et al. Access, interest, and attitudes toward electronic communication for health care among patients in the medical safety net. J Gen Intern Med. 2013;28:914-920. PubMed
© 2017 Society of Hospital Medicine
Medical and economic burden of heparin-induced thrombocytopenia: A retrospective nationwide inpatient sample (NIS) study
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
Each year, approximately one-third of all hospitalized medical and surgical patients in the United States (about 12 million patients) are exposed to heparin products for the prevention or treatment of thromboembolism.1 Although generally safe, heparin can trigger an immune response in which platelet factor 4–heparin complexes set off an antibody-mediated cascade that can result in heparin-induced thrombocytopenia (HIT) and paradoxical arterial and venous thromboses, or heparin-induced thrombocytopenia with thrombosis (HITT). The incidence of HIT appears to be significantly higher with the more immunogenic unfractionated heparin (UFH) (2%-3% if treated for ≥5 days) than with low-molecular-weight heparin (LMWH) (0.2%-0.6%)2 and is significantly higher in postoperative patients (1%-5%) than in medical patients.3 Older patients and female patients, especially those who undergo surgery, are thought to be at higher risk.4 Progression from HIT to HITT can occur in up to 50% of surgical patients,5 and HITT can significantly increase mortality.4
In the United States, LMWH use has increased 5-fold since 2000—an increase attributed to the 2010 release of generic enoxaparin.6 As US hospitals switch from UFH to LMWH with its significantly lower risk of HIT, up-to-date HIT incidence data may help physicians and payers better understand the impact of the disorder on mortality and hospital length of stay (LOS) for medical patients and subsets of surgical patients and subsequently direct screening efforts to those at highest risk. Therefore, in the present study, we used national data to determine the latest incidence and economic implications of HIT overall and for high-risk surgical groups.
METHODS
In this study, we analyzed data from the Nationwide Inpatient Sample (NIS) database, part of the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). The period studied was 2009-2011. We used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 289.84, introduced in 2009, to identify patients who were at least 18 years old and had a primary or secondary diagnosis of HIT. Validated Clinical Classifications Software (CCS) was used to identify those who underwent cardiac, vascular, or orthopedic surgery, and ICD-9-CM codes for various thromboses were used to identify those with HITT (Supplemental Figure, Supplemental Table 1). Baseline patient and hospital characteristics were compared using the Pearson’s Chi-square test for categorical variables and the Student t test for continuous variables (2-sided P < 0.05 for statistical significance) (Table 1). We calculated the incidence of HIT overall and for the 3 surgical subgroups and compared the cohorts on their mean hospital LOS, mean hospital charge, and in-hospital mortality (Table 2).
Statistical analysis was performed with Stata Version 13.1 (Stata Corp, College Station, TX). Survey commands were used to account for the complex survey design in NIS. Reading Health System’s Institutional Review Board determined that our study protocol was exempt.
RESULTS
Of 98,636,364 hospitalizations, 72,515 (0.07%) involved HIT. There were no significant differences in the annual incidence of HIT during the study period (0.06% in 2009, 0.05% in 2010, 0.06% in 2011).
Regarding HIT, the death rate was 4-fold higher for patients with the disorder (9.63%) than for those without it (2.19%); hospital LOS and costs were significantly higher, too (Table 2). In addition, in-hospital mortality was higher (P < 0.001) for patients with HITT (12.28%) than for patients with HIT without thrombosis (8.24%); HITT patients’ hospital LOS and costs were higher as well. In patients who had cardiac, vascular, or orthopedic surgery, development of HIT was also associated with significantly higher in-hospital mortality, mean hospital LOS, and mean hospital charge. In patients with HITT, deep vein thrombosis (DVT) and pulmonary embolism represented the majority of reported cases (Supplemental Table 2). However, in patients who had cardiac surgery, acute arterial thromboses of coronary and cerebral vessels were more common.
DISCUSSION
In this national database survey, the overall incidence of HIT during the study period 2009-2011 was 0.07%, or 1 in 1350 hospitalized patients. Although earlier studies reported rates as high as 5% for high-risk subgroups of surgical patients,7 our data are more in line with more recently reported rates: about 0.02% for hospital admissions8 and from less than 0.1% to 0.4% for patients who received heparin.9 Older studies, which predominantly involved postoperative patients and were conducted when UFH often was the first-line heparin product used, may account for higher rates relative to ours. Of the 3 types of surgeries we evaluated, cardiac surgery had the highest HIT rate (0.5%), consistent with other studies.4 The higher HIT/HITT rates found for larger urban hospitals in our study might be attributable to increased awareness and testing, availability of hematology consultation, and higher risk of heparin use in this setting, where patients are sicker and cases and procedures more complicated.
Age was an important determinant of HIT risk in our study and in similar large-database series.4 Whether increased UFH use in the elderly (because of age or kidney disease) was a causative factor in this finding is unknown. In our study, although men and women had a nearly equal incidence of HIT, women had a significantly higher risk of HIT after both cardiac surgery and vascular surgery. Immune-mediated mechanisms that are more common in females may play a causative role in these settings.10
Our study results showed HIT associated with increased hospital LOS and an almost 4-fold increase in inpatient mortality and costs. The increased economic burden in HIT cases may be driven by the diagnostic work-up cost and expensive alternative anticoagulation.11,12 Similarly, compared with HIT without thrombosis, HITT was associated with significantly increased hospital LOS (3.7 days), total hospital charge ($64,279) and mortality (49% increase, to 12.2% from 8.2%), consistent with prior studies.13 In addition, 34.1% (24,704) of our HIT patients developed at least 1 thrombotic complication, with venous thromboses more common than arterial thromboses, as previously reported.13 Lower extremity DVT was the most common thrombosis in orthopedic and vascular surgery. However, in cardiac surgery, acute coronary occlusion was the most common thrombotic complication. We postulate that the difference stems from the increased propensity of HIT-related thrombosis to occur in areas of vascular injury.14
The strengths of our study include its large size, which increases the generalizability of its results and avoids the biases inherent in small, single-center studies. As with any administrative dataset, the NIS may include coding errors related to underdiagnosis and overdiagnosis (eg, a HIT/HITT diagnosis carried forward from prior episodes). In our study, we inferred the HITT diagnosis in HIT cases with a vascular complication, but we could have missed HIT cases that had not been coded for vascular complications, and we could have overassociated vascular complications that had predated HIT and been treated with heparin. Although HIT and HITT were associated with worse clinical outcomes and increased hospital LOS, it is possible patients who were hospitalized longer had more opportunities for heparin use, and this exposure led to HIT or HITT. The lack of details regarding prior heparin use, including type of heparin (UFH or LMWH), prevented us from inferring the actual risks of individual heparin products.
In conclusion, in cardiac, vascular, and orthopedic surgery, HIT and especially HITT can significantly increase hospital LOS, inpatient costs, and mortality. Lower extremity DVT and acute coronary artery occlusion are the most common thrombotic complications in these cases. HIT screening strategies that incorporate platelet counts are recommended only in patients at highest risk (>1%), according to the most recent American College of Chest Physicians guidelines, but this recommendation was made on the basis of the high cost of alternative anticoagulants. Given our more recent data regarding the very high costs of HIT and especially HITT, screening strategies with platelet counts may prove more cost-effective. Recent genome-wide studies that found higher rates of HIT in patients with T-cell death–associated gene 8 (TDAG8) may help explain sex differences in postoperative patients and identify patients at highest risk so alternative anticoagulants can be used.15
Disclosures
This study was funded by the Reading Health System (grant RHS0010). Dr. Bhatt is supported by the Physician-Scientist Training Program (grant 2015-2016), College of Medicine, University of Nebraska Medical Center. The other authors report no financial conflicts of interest.
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
1. Fahey VA. Heparin-induced thrombocytopenia. J Vasc Nurs. 1995;13(4):112-116. PubMed
2. TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(6):2121-2131.
3. Junqueira DR, Perini E, Penholati RR, Carvalho MG. Unfractionated heparin versus low molecular weight heparin for avoiding heparin-induced thrombocytopenia in postoperative patients. Cochrane Database Syst Rev. 2012;(9):CD007557. PubMed
4. Seigerman M, Cavallaro P, Itagaki S, Chung I, Chikwe J. Incidence and outcomes of heparin-induced thrombocytopenia in patients undergoing cardiac surgery in North America: an analysis of the Nationwide Inpatient Sample. J Cardiothorac Vasc Anesth. 2014;28(1):98-102. PubMed
5. Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(3):252-261. PubMed
6. Grabowski HG, Guha R, Salgado M. Regulatory and cost barriers are likely to limit biosimilar development and expected savings in the near future. Health Aff (Millwood). 2014;33(6):1048-1057. PubMed
7. Prandoni P, Siragusa S, Girolami B, Fabris F; BELZONI Investigators Group. The incidence of heparin-induced thrombocytopenia in medical patients treated with low-molecular-weight heparin: a prospective cohort study. Blood. 2005;106(9):3049-3054. PubMed
8. Jenkins I, Helmons PJ, Martin-Armstrong LM, Montazeri ME, Renvall M. High rates of venous thromboembolism prophylaxis did not increase the incidence of heparin-induced thrombocytopenia. Jt Comm J Qual Patient Saf. 2011;37(4):163-169. PubMed
9. Zhou A, Winkler A, Emamifar A, et al. Is the incidence of heparin-induced thrombocytopenia affected by the increased use of heparin for VTE prophylaxis? Chest. 2012;142(5):1175-1178. PubMed
10. Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108(9):2937-2941. PubMed
11. Baroletti S, Piovella C, Fanikos J, Labreche M, Lin J, Goldhaber SZ. Heparin-induced thrombocytopenia (HIT): clinical and economic outcomes. Thromb Haemost. 2008;100(6):1130-1135. PubMed
12. Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568-573. PubMed
13. Nand S, Wong W, Yuen B, Yetter A, Schmulbach E, Gross Fisher S. Heparin-induced thrombocytopenia with thrombosis: Incidence, analysis of risk factors, and clinical outcomes in 108 consecutive patients treated at a single institution. Am J Hematol. 1997;56(1):12-16. PubMed
14. Hong AP, Cook DJ, Sigouin CS, Warkentin TE. Central venous catheters and upper-extremity deep-vein thrombosis complicating immune heparin-induced thrombocytopenia. Blood. 2003;101(8):3049-3051. PubMed
15. Karnes JH, Cronin RM, Rollin J, et al. A genome-wide association study of heparin-induced thrombocytopenia using an electronic medical record. Thromb Haemost. 2015;113(4):772-781. PubMed
© 2017 Society of Hospital Medicine