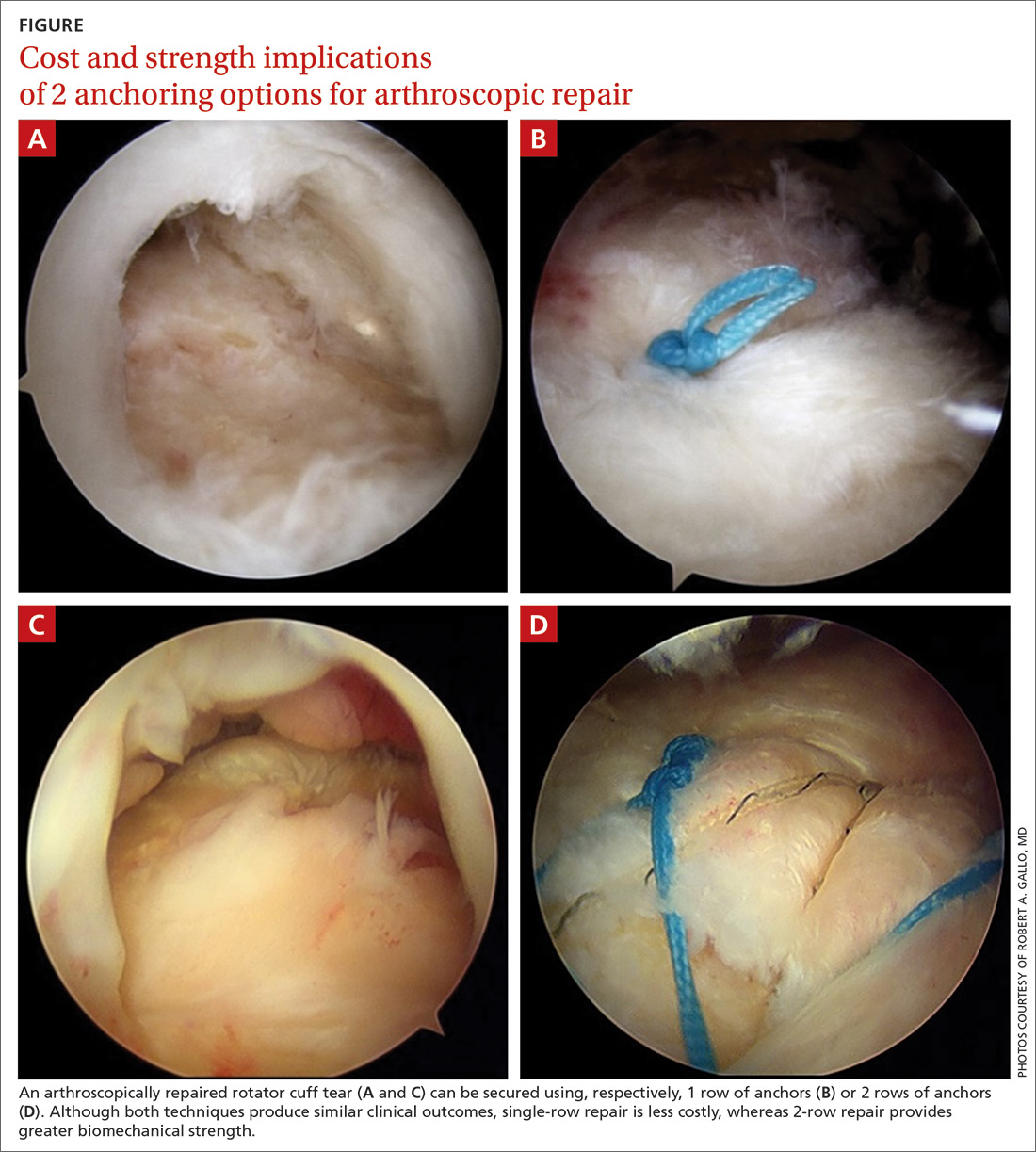
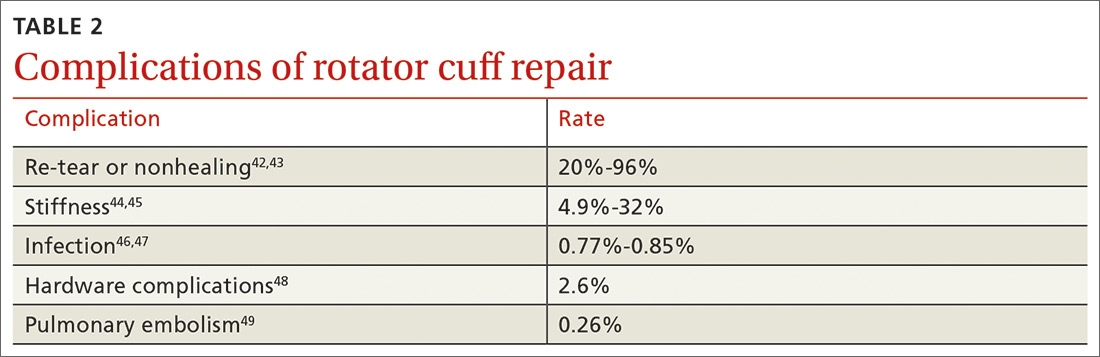
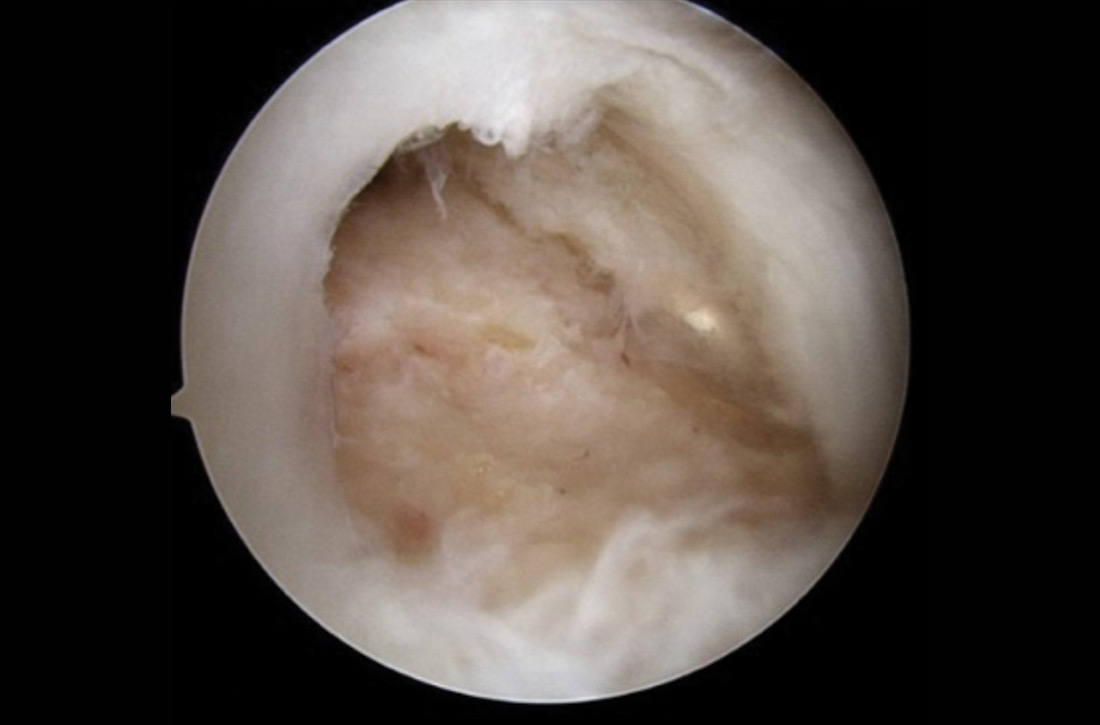
User login
Hypertriglyceridemia: A strategic approach
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
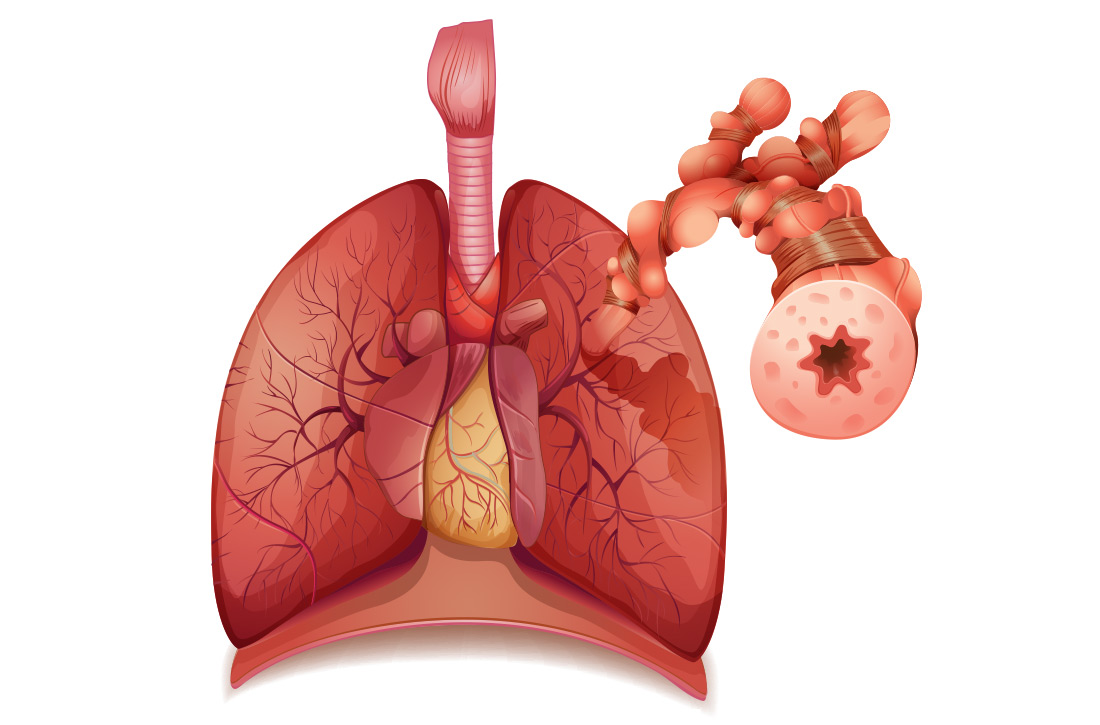
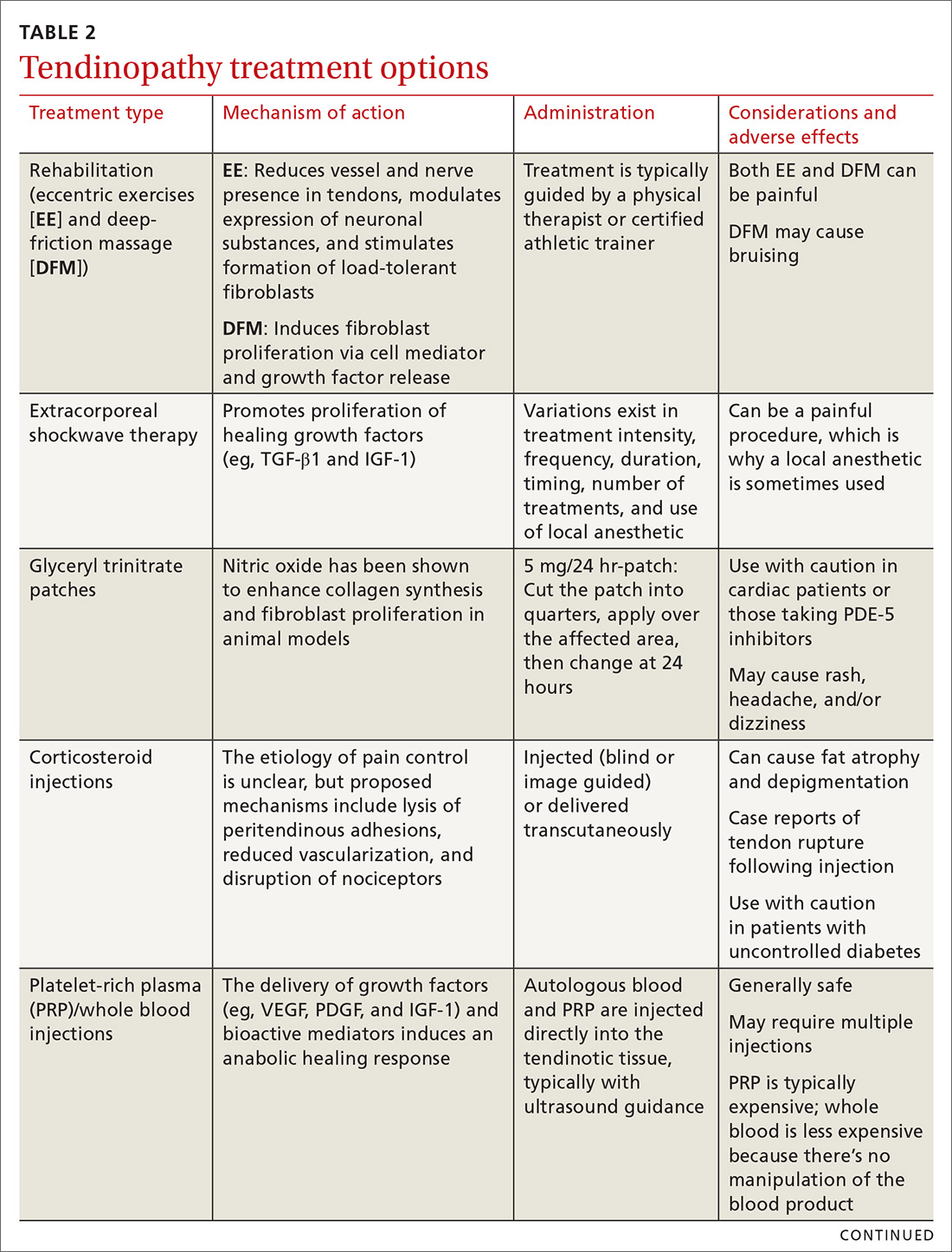
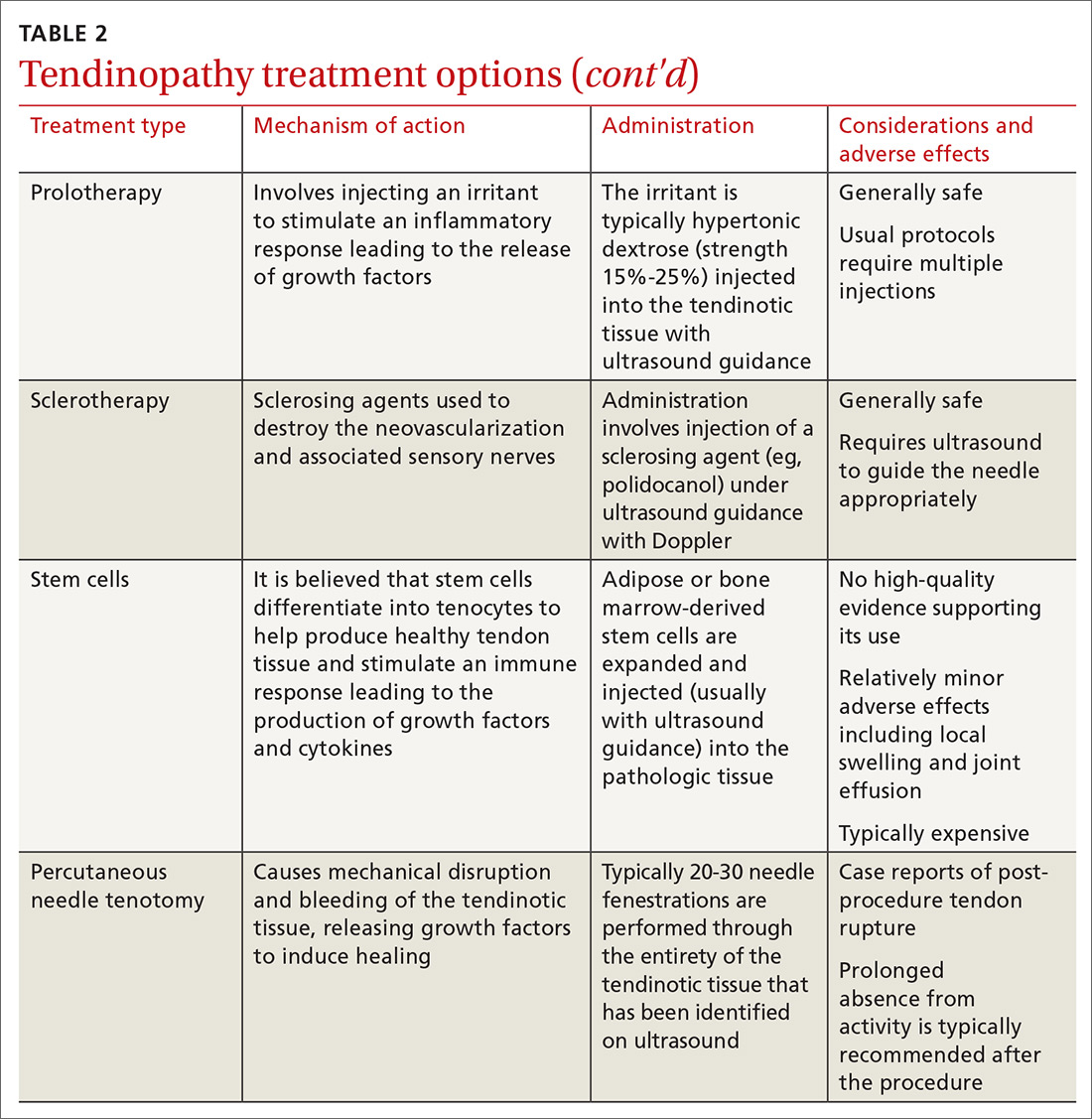
How HTG is defined: Causes, cutoffs, signs
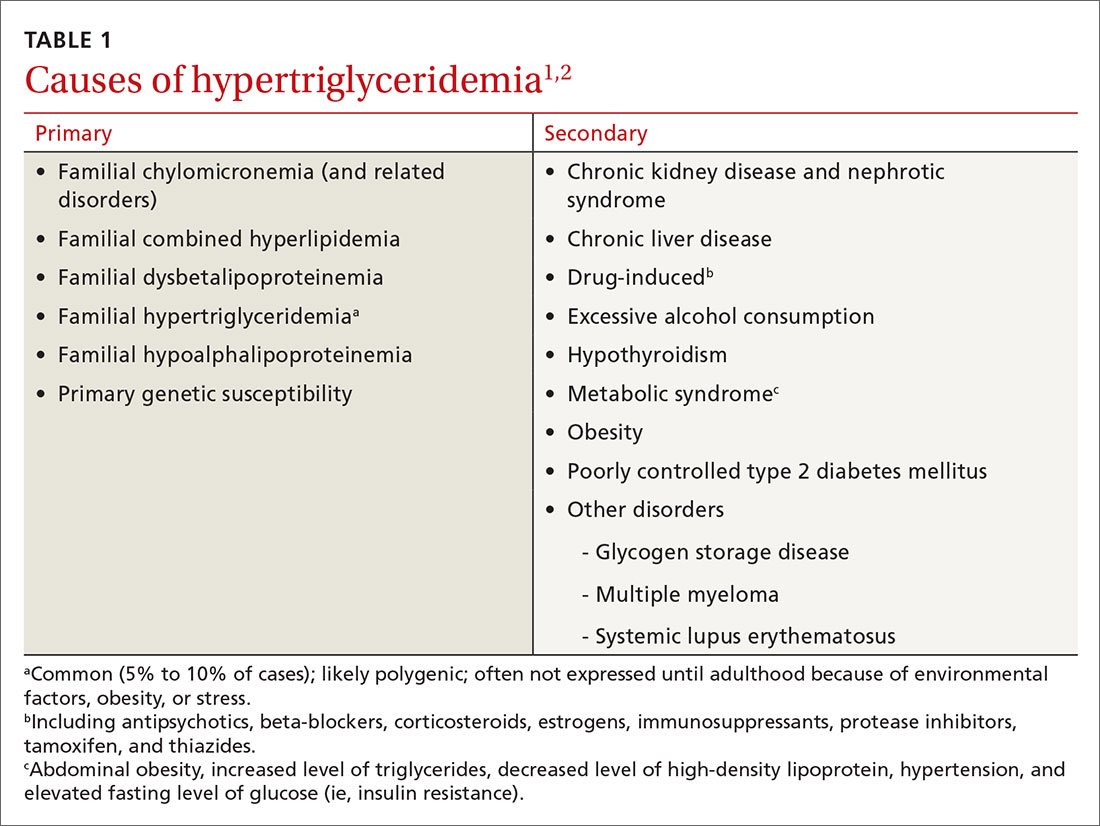
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
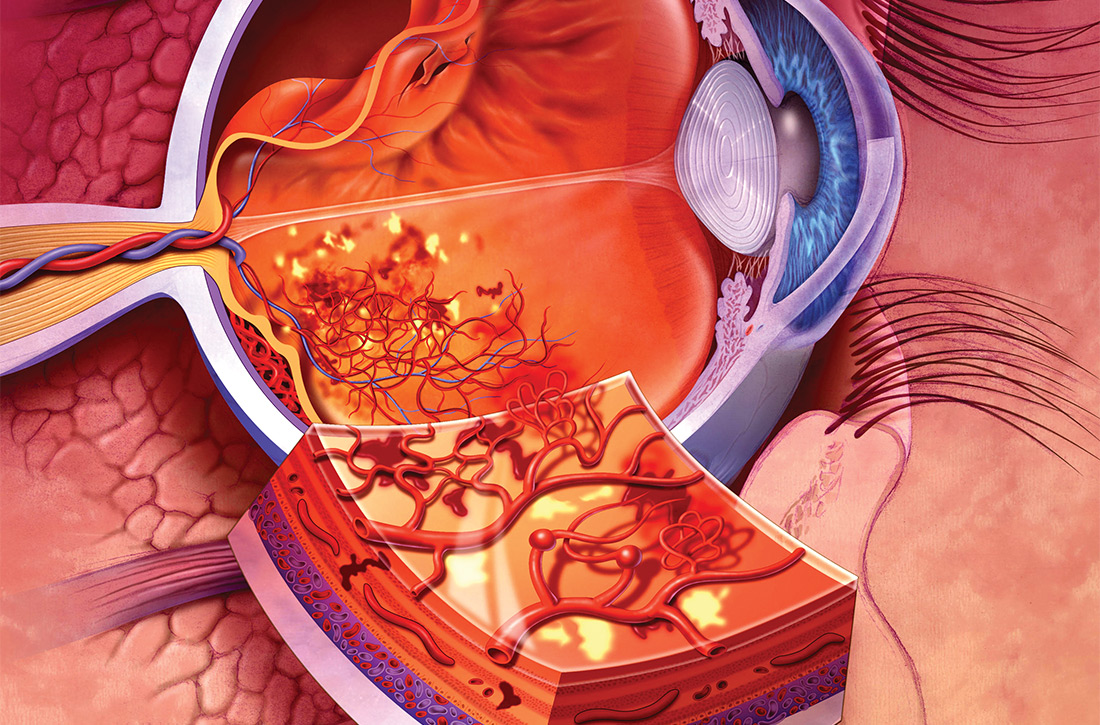
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
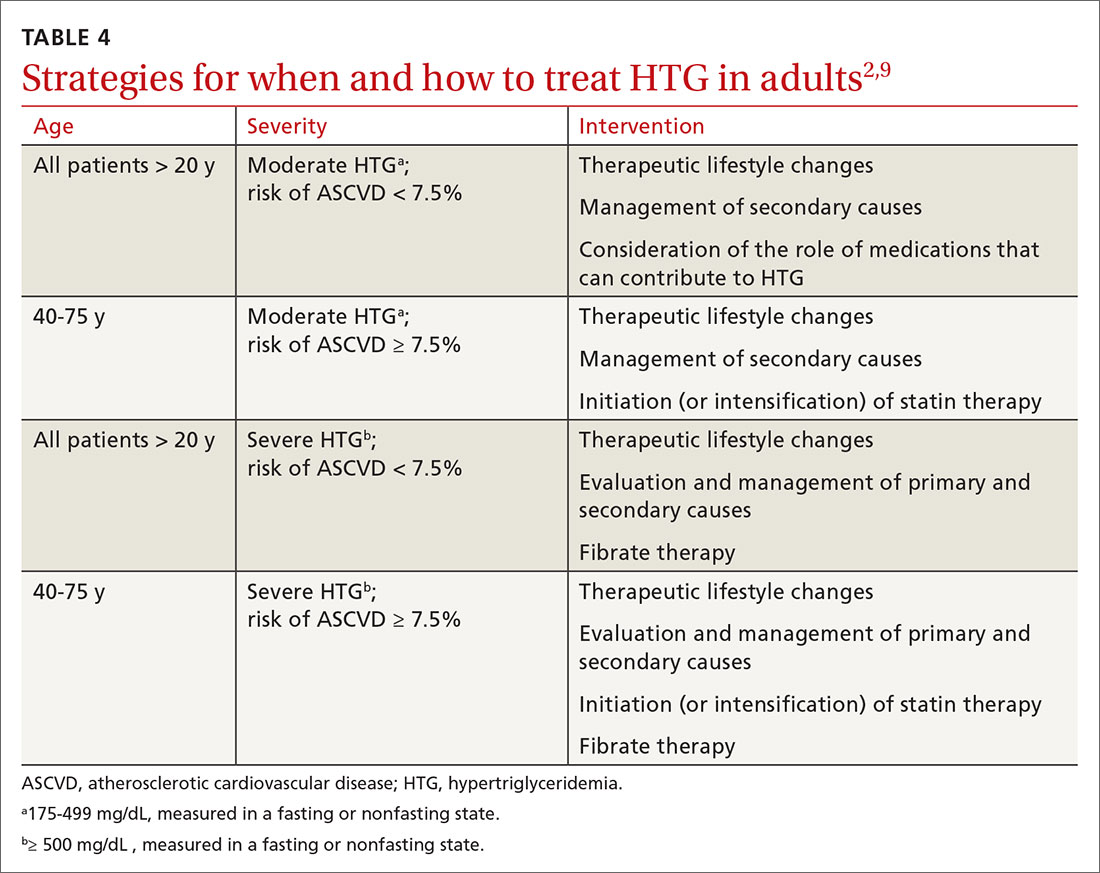
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
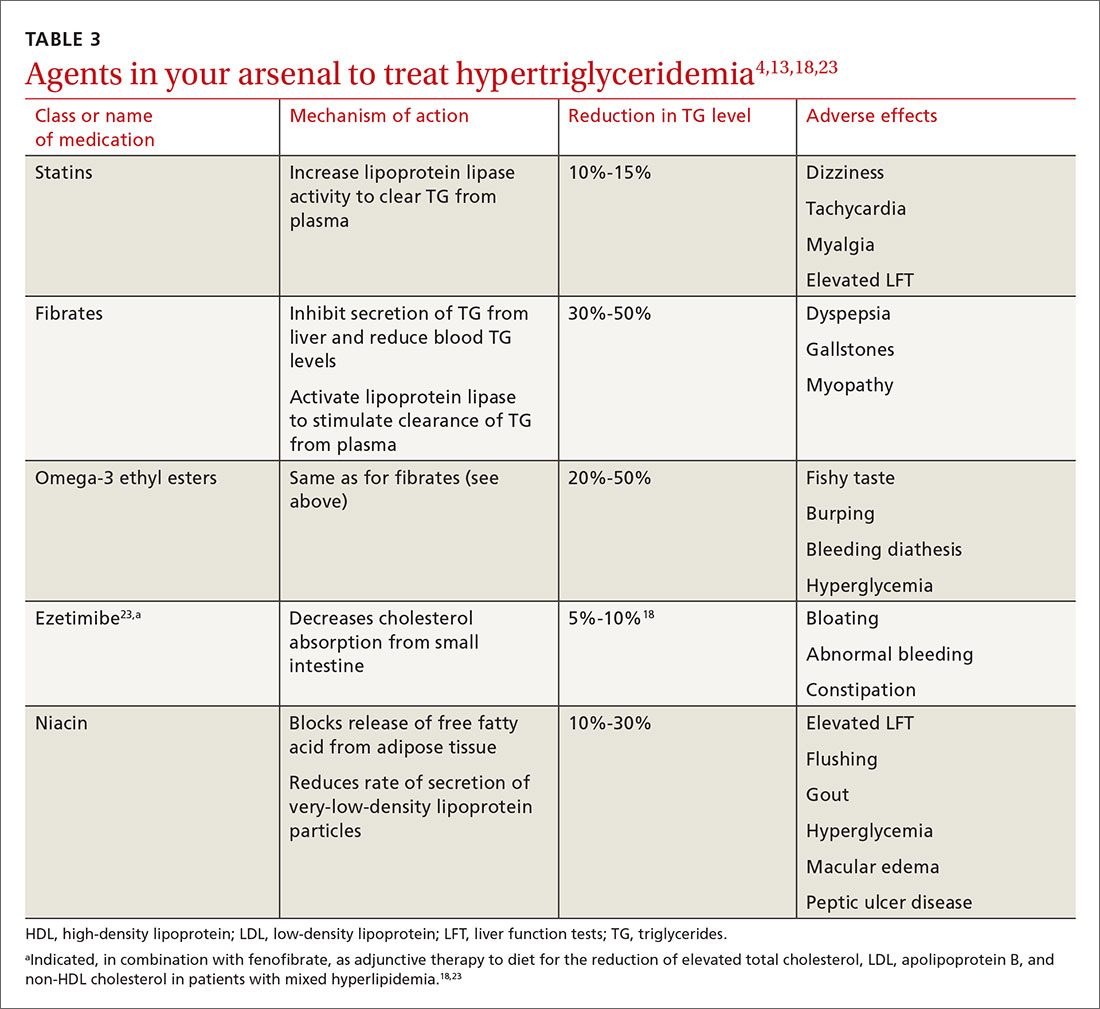
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
CASE 1
Tyler M, age 40, otherwise healthy, and with a body mass index (BMI) of 30, presents to your office for his annual physical examination. He does not have a history of alcohol or tobacco use.
Mr. M’s obesity raises concern about metabolic syndrome, which warrants evaluation for hypertriglyceridemia (HTG). You offer him lipid testing to estimate his risk of atherosclerotic cardiovascular disease (ASCVD).
The only abnormal value on the lipid panel is a triglyceride (TG) level of 264 mg/dL (normal, < 175 mg/dL). Mr. M’s 10-yr ASCVD risk is determined to be < 5%.
What, if any, intervention would be triggered by the finding of moderate HTG?
CASE 2
Alicia F, age 30, with a BMI of 28 and ASCVD risk < 7.5%, comes to the clinic for evaluation of anxiety and insomnia. She reports eating a high-carbohydrate diet and drinking 3 to 5 alcoholic beverages nightly to help her sleep.
Ms. F’s daily alcohol use prompts evaluation for HTG. Results show a TG level of 1300 mg/dL and a high-density lipoprotein (HDL) level of 25 mg/dL (healthy HDL levels: adult females, ≥ 50 mg/dL; adult males, ≥ 40 mg/dL). Other test results are normal, except for elevated transaminase levels (just under twice normal).
What, if any, action would be prompted by the patient’s severe HTG and below-normal HDL level?
Continue to: How HTG is defined
How HTG is defined: Causes, cutoffs, signs
HTG is most commonly caused by obesity and a sedentary lifestyle; certain associated comorbid medical conditions can also be a precipitant (Table 11,2). Because the condition is a result of polygenic phenotypic expression, even a genetically low-risk patient can present with HTG when exposed to certain medical conditions and environmental causes.
Primary HTG (genetic or familial) is rare. Genetic testing may be considered for patients with TG > 1000 mg/dL (severely elevated TG = 500 to 1999 mg/dL, measured in fasting state*) or a family history of early ASCVD (TABLE 11,2).2,3
Typically, HTG is asymptomatic. Xanthelasmas, xanthomas, and lipemia retinalis are found in hereditary disorders of elevated TGs. Occasionally, HTG manifests as chylomicronemia syndrome, characterized by recurrent abdominal pain, nausea, vomiting, and, in severe HTG, pancreatitis.3
Fine points of TG measurement
Triglycerides are a component of a complete lipid profile, which also includes total cholesterol, calculated low-density lipoprotein (LDL-C), and HDL.4 As in both case vignettes, detection of HTG is often incidental, when a lipid profile is ordered to evaluate the risk of ASCVD. (Of note, for people older than 20 years, the US Preventive Services Task Force no longer addresses the question, “Which population should be screened for dyslipidemia?” Instead, current recommendations answer the question, “For which population should statin therapy be prescribed?”5)
Effect on ASCVD risk assessment. TG levels are known to vary, depending on fasting or nonfasting status, with lower levels reported when fasting. An elevated TG level can lead to inaccurate calculation of LDL when using the Friedewald formula6:
LDL = total cholesterol – (triglycerides/5) – HDL
Continue to: The purpose of testing...
The purpose of testing lipids in a fasting state (> 9 hours) is to minimize the effects of an elevated TG level on the calculated LDL. In severe HTG, beta-quantitation by ultracentrifugation and electrophoresis can be performed to determine the LDL level directly.
Advantage of nonfasting measurement. When LDL-C is not a concern, there is, in fact, value in measuring TGs in the nonfasting state. Why? Because a nonfasting TG level is a better indicator of a patient’s average TG status: Studies have found a higher ASCVD risk in the setting of an elevated postprandial TG level accompanied by a low HDL level.7
The Copenhagen City Heart Study identified postprandial HTG as an independent risk factor for atherogenicity, even in the setting of a normal fasting TG level.8 American Association of Clinical Endocrinologists and American College of Endocrinology guidelines endorse testing the nonfasting TG level when the fasting TG level is elevated in a lipid profile; if the nonfasting TG level is > 500 mg/dL, evaluation for secondary causes is warranted9,10 (Table 11,2).
In a practical sense, therefore, offering patients nonfasting lipid testing allows more people to obtain access to timely care.
Pancreatitis. Acute pancreatitis commonly prompts an evaluation for HTG. The risk of acute pancreatitis in the general population is 0.04%, but that risk increases to 8% to 31% for a person with HTG.11 Incidence when the TG level is > 500 mg/dL is thought to be increased because chylomicrons, acting as a TG carrier in the bloodstream, are responsible for pancreatitis.3 Treating HTG can reduce both the risk and recurrence of pancreatitis12,13; given that the postprandial TG level can change rapidly from severe to very severe (> 2000 mg/dL), multiple guidelines recommend pharmacotherapy to a TG goal of < 500-1000 mg/dL.1,9,13,14
Continue to: An ASCVD risk-HTG connection?
An ASCVD risk–HTG connection? In the population already at higher risk of ASCVD (> 7.5%), HTG is recognized as a risk-enhancing factor because of its atherogenic potential (Table 22); however, there is insufficient evidence that TGs have a role as an independent risk factor for ASCVD. In a population-based study of 58,000 people, 40 to 65 years of age, conducted at Copenhagen [Denmark] University Hospital, investigators found that those who did not meet criteria for statin treatment and who had a TG level > 264 mg/dL had a 10-year risk of a major adverse cardiovascular event similar to that of people who did meet criteria for statin therapy.15
The FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) and AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes) studies, among others, have failed to show a significant reduction in coronary events by treating HTG.10
That said, it’s worth considering the findings of other trials:
- In the PROVE IT-TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, an overall 28% reduction in endpoint events (myocardial infarction, acute coronary syndrome) was seen with high-intensity statin therapy, compared to moderate-intensity therapy.10 However, there was a sizeable residual risk identified that was theorized by investigators to be associated with high non-HDL lipoproteins, including TGs.
- A 2016 study in Israel, in which 22 years of data on 15,355 patients with established ASCVD were studied, revealed that elevated TGs are associated with an increased long-term mortality risk that is independent of the HDL level.16
- A cross-sectional study, nested in the prospective Copenhagen City Heart Study, demonstrated that HTG is associated with an increase in ischemic stroke events.17
Treatment
Therapeutic lifestyle changes
Changes in lifestyle are the foundation of management of, and recommended first-line treatment for, all patients with HTG. Patients with a moderately elevated TG level (175-499 mg/dL, measured in a fasting or nonfasting state) can be treated with therapeutic lifestyle changes alone1,2; a trial of 3 to 6 months (see specific interventions below) is recommended before considering adding medications.10
Weight loss. There is a strong association between BMI > 30 and HTG. Visceral adiposity is a much more significant risk than subcutaneous adipose tissue. Although weight loss to an ideal range is recommended, even a 10% to 15% reduction in an obese patient can reduce the TG level by 20%. A combination of moderate-intensity exercise and healthy eating habits appears to assist best with this intervention.18
Continue to: Exercise
Exercise. Thirty minutes a day of moderate-intensity exercise is associated with a significant drop in postprandial TG. This benefit can last as long as 3 days, suggesting a goal of at least 3 days a week of an active lifestyle. Such a program can include intermittent aerobics or mild resistance exercise.19
Healthy eating habits. The difference between a low-fat, high-carbohydrate diet and a high-fat, low-carbohydrate diet is less important than the overall benefit of weight loss from either of these diets. Complex carbohydrates are recommended over simple carbohydrates. A low-carbohydrate diet in a patient with diabetes has been demonstrated to improve the TG level, irrespective of weight change.
A Mediterranean diet can reduce the TG level by 10% to 15%, and is recommended over a low-fat diet.14 (This diet generally includes a high intake of extra virgin olive oil; leafy green vegetables, fruits, cereals, nuts, and legumes; moderate intake of fish and other meat, dairy products, and red wine; and low intake of eggs and sugars.) The American Heart Association recommends 2 servings of fatty fish a week for its omega-3 oil benefit of reducing ASCVD risk. Working with a registered dietician to assist with lipid lowering can produce better results than physician-only instruction on healthy eating.9
Alcohol consumption. Complete cessation or moderation of alcohol consumption (1 drink a day/women and 2 drinks a day/men*) is recommended to improve HTG. Among secondary factors, alcohol is commonly the cause of an unusually high elevation of the TG level.14
Smoking cessation. Smoking increases the postprandial TG level.10 Complete cessation for just 1 year can reduce a person’s ASCVD risk by approximately 50%. However, in a clinical trial,22 smoking cessation did not significantly decrease the TG level—possibly because of the counterbalancing effect of weight gain following cessation.
Continue to: Medical therapy
Medical therapy
In addition to lifestyle modification, medications are recommended to reduce atherogenic potential in patients with moderate or severe HTG and an ASCVD risk > 7.5% (Table 34,13,18,23 and Table 42,9). Before initiating medical therapy, we recommend that you engage in shared decision-making with patients to (1) delineate treatment goals and (2) describe the risks and benefits of medications for HTG.2
Statins. These agents are recommended first-line therapy for reducing ASCVD risk.2 If the TG level remains elevated (> 500 mg/dL) after statin therapy is maximized, an additional agent can be added—ie, a fibrate or fish oil (see below).
Fibrates. If a fibrate is used as an add-on to a statin, fenofibrate is preferred over gemfibrozil because it presents less risk of the severe myopathy that can develop when taken with a statin.13 Despite the effectiveness of fibrates in reducing the TG level, these drugs have not been shown to reduce overall mortality.24 The evidence on improved cardiovascular outcomes is subgroup-specific (ie, prevention of a second myocardial infarction in the setting of optimal statin use and elevated non-HDL lipoproteins).12 A study demonstrated that gemfibrozil reduced the incidence of transient ischemic attack and stroke in a subgroup of male US veterans who had coronary artery disease and a low HDL level.25
Fish oil. The omega-3 ethyl esters eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), available as EPA alone or in combination with DHA, do not interact with statins and are tolerated well. They reduce hypertriglyceridemia by 20% to 50%.13
Eicosapentaenoic acid, EPA plus DHA, and icosapent ethyl, an ethyl ester product containing EPA without DHA, are approved by the US Food and Drug Administration for HTG > 500 mg/dL, at a dosage of 2000 mg twice daily. In the REDUCE-IT trial, adding icosapent ethyl, 2 g twice daily, to a statin in patients with HTG was associated with fewer ischemic events, compared to placebo.23,26
Continue to: Fish oil formulations...
Fish oil formulations can inhibit platelet aggregation and increase bleeding time in otherwise healthy people; however, such episodes are minor and nonfatal. Patients on anticoagulation or an antiplatelet medication should be monitored periodically for bleeding events, although recommendations on how to monitor aren’t specified in a recent advisory by the American Heart Association.23
DHA was thought to increase the LDL-C levels and, by doing so, potentially counterbalance benefit,23,27 but most studies have failed to reproduce this effect.28 Instead, studies have shown minimal elevation of LDL-C when DHA is used to treat HTG.23,27
Niacin. At a dosage of 500-2000 mg/dL, niacin lowers the TG level by 10% to 30%. It also increases HDL by 10% to 40% and lowers LDL by 5% to 20%.13
Considerations in pancreatitis. For management of recurrent pancreatitis in patients with HTG, lifestyle modification remains the mainstay of treatment. When medication is considered for persistent severe HTG, fibrates have evidence of primary and secondary prevention of pancreatitis.
CASE 1
Recommendation for Mr. M: Therapeutic lifestyle changes to address moderate HTG.
Continue to: Because Mr. M's...
Because Mr. M’s 10-yr ASCVD risk is < 5%, statin therapy is not indicated for risk reduction. With a fasting TG value < 500 mg/dL, he is not considered at increased risk of pancreatitis.
CASE 2
Recommendations for Ms. F:
- Therapeutic lifestyle changes to address severe HTG. Ms. F agrees to wean off alcohol; add relaxation exercises before bedtime; do aerobic exercise 30 minutes a day, 3 times a week; decrease dietary carbohydrates daily by cutting portion size in half; and increase intake of fresh vegetables and lean protein.
- Treatment with fenofibrate to reduce the risk of pancreatitis. Ms. F begins a trial. Six months into treatment, she has reduced her BMI to 24 and the TG level has fallen to < 500 mg/dL. Ms. F also reports that she is sleeping well, believes that she is able to manage her infrequent anxiety, and is now in a routine that feels sustainable.
You congratulate Ms. F on her success and support her decision to undertake a trial of discontinuing fenofibrate, after shared decision-making about the risks and potential benefits of doing so.
Summing up: Management of HTG
Keep these treatment strategy highlights in mind:
- Lifestyle modification with a low-fat, low-carbohydrate diet, avoidance of alcohol, and moderate-intensity exercise is the mainstay of HTG management.
- The latest evidence supports that (1) HTG is a risk-enhancing factor for ASCVD and (2) statin therapy is recommended for patients who have HTG and an ASCVD risk > 7.5%.
- When the TG level remains elevated despite statin therapy and lifestyle changes, an omega-3 ethyl ester can be used as an adjunct for additional atherogenic risk reduction.
- For severe HTG, a regimen of therapeutic lifestyle changes plus a fibrate is recommended to reduce the risk and recurrence of pancreatitis.1,24
* In comparison, a normal level of triglycerides is < 175 mg/dL; a moderately elevated level, measured in a fasting or nonfasting state, 175-499 mg/dL; and a very severely elevated level, ≥ 2000 mg/dL.2
CORRESPONDENCE
Ashwini Kamath Mulki, MD, Family Health Center, 1730 Chew Street, Allentown, PA 18104; Ashwini.KamathMulki@lvhn.org.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
1. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2969-2989.
2. Grundy SM, Stone NJ, Bailey AL, et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285-e350.
3. Brahm A, Hegele RA. Hypertriglyceridemia. Nutrients. 2013;5:981-1001.
4. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486-2497.
5. US Preventive Services Task Force. Final recommendation statement. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. November 13, 2016. www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/statin-use-in-adults-preventive-medication. Accessed April 24, 2020.
6. Fukuyama N, Homma K, Wakana N, et al. Validation of the Friedewald equation for evaluation of plasma LDL-cholesterol. J Clin Biochem Nutr. 2007;43:1-5.
7. Scherer DJ, Nicholls SJ. Lowering triglycerides to modify cardiovascular risk: Will icosapent deliver? Vasc Health Risk Manag. 2015;11:203.
8. Nordestgaard BG, Benn M, Schnohr P, et al. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299-308.
9. Jellinger PS. American Association of Clinical Endocrinologists/American College of Endocrinology Management of Dyslipidemia and Prevention of Cardiovascular Disease Clinical Practice Guidelines. Diabetes Spectr. 2018;31:234-245.
10. Malhotra G, Sethi A, Arora R. Hypertriglyceridemia and cardiovascular outcomes. Am J Therapeut. 2016;23:e862-e870.
11. Carr RA, Rejowski BJ, Cote GA, et al. Systematic review of hypertriglyceridemia-induced acute pancreatitis: a more virulent etiology? Pancreatology. 2016;16:469-476.
12. Charlesworth A, Steger A, Crook MA. Acute pancreatitis associated with severe hypertriglyceridemia; a retrospective cohort study. Int J Surg. 2015;23(pt A):23-27.
13. Berglund L, Brunzell JD, Goldberg AC, et al. Treatment options for hypertriglyceridemia: from risk reduction to pancreatitis. Best Pract Res Clin Endocrinol Metab. 2014;28:423-437.
14. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935-2959. [Erratum. J Am Coll Cardiol. 2014;63:3026.]
15. Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Euro Heart J. 2017;39:610-619.
16. Klempfner R, Erez A, Sagit B-Z, et al. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease: twenty-two-year follow-up of the Bezafibrate Infarction Prevention Study and Registry. Circ Cardiovasc Qual Outcomes. 2016;9:100-108.
17. Freiberg JJ, Tybjaerg-Hansen A, Jensen JS, et al. Nonfasting triglycerides and risk of ischemic stroke in the general population. JAMA. 2008;300:2142-2152.
18. Miller M, Stone NJ, Ballantyne C, et al; ; ; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease. Triglycerides and cardiovascular disease. Circulation. 2011;123:2292-2333.
19. Graham TE. Exercise, postprandial triacylglyceridemia, and cardiovascular disease risk. Can J Appl Physiol. 2004;29:781-799.
20. Meng Y, Bai H, Wang S, et al. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017;131:124-131.
21. What is a standard drink? National Institute on Alcohol Abuse and Alcoholism Web site. www.niaaa.nih.gov/what-standard-drink. Accessed April 24, 2020.
22. Gepner AD, Piper ME, Johnson HM, et al. Effects of smoking and smoking cessation on lipids and lipoproteins: outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
23. Skulas-Ray AC, Wilson PWF, Harris WS, et al; American Heart Association Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140:e673-e691.
24. Jakob T, Nordmann AJ, Schandelmaier S, et al. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst Rev. 2016;11:CD009753.
25. Lisak M, Demarin V, Trkanjec Z, et al. Hypertriglyceridemia as a possible independent risk factor for stroke. Acta Clin Croat. 2013;52:458-463.
26. Bhatt DL, Steg PG, Miller M, et al; REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11-22.
27. Barter P, Ginsberg HN. Effectiveness of combined statin plus omega-3 fatty acid therapy for mixed dyslipidemia. Am J Cardiol. 2008;102:1040-1045.
28. Bays H, Ballantyne C, Kastelein J, et al. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] Trial). Am J Cardiol. 2011;108:682-690.
PRACTICE RECOMMENDATIONS
› Evaluate patients for hypertriglyceridemia when they have a comorbid condition (eg, type 2 diabetes, obesity, hypothyroidism, metabolic syndrome, alcoholism). B
› Do not require fasting status when evaluating triglycerides in a lipid panel. B
› Make therapeutic lifestyle changes first-line treatment for hypertriglyceridemia. C
› Prescribe fibrates for severe hypertriglyceridemia to reduce the risk and recurrence of pancreatitis. A
› Prescribe a statin and an omega-3 fatty acid (fish oil) to lower the triglyceride level and thus reduce resulting atherogenicity when the risk of atherosclerotic cardiovascular disease is > 7.5%. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics as a Tx resource in primary care
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
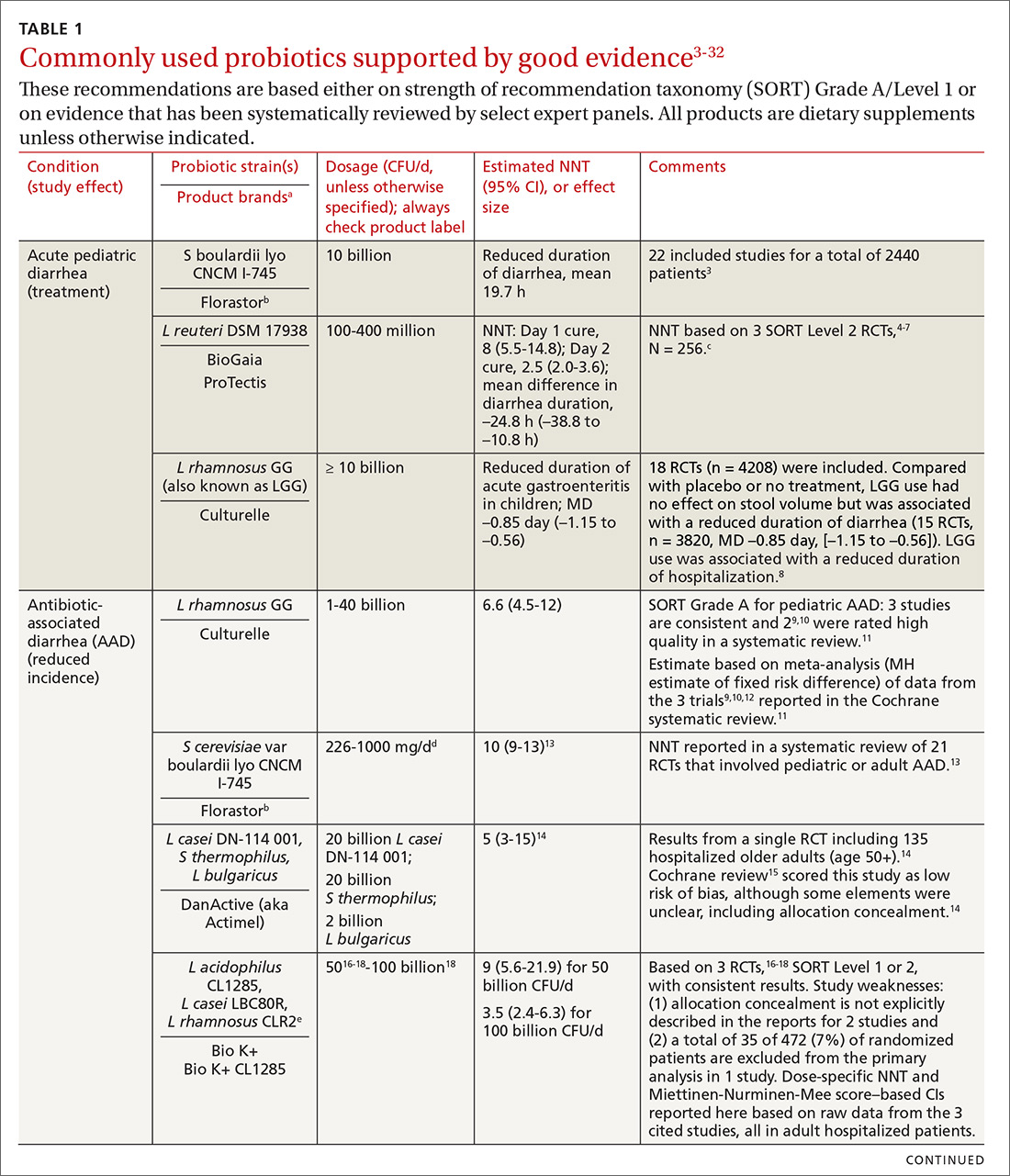
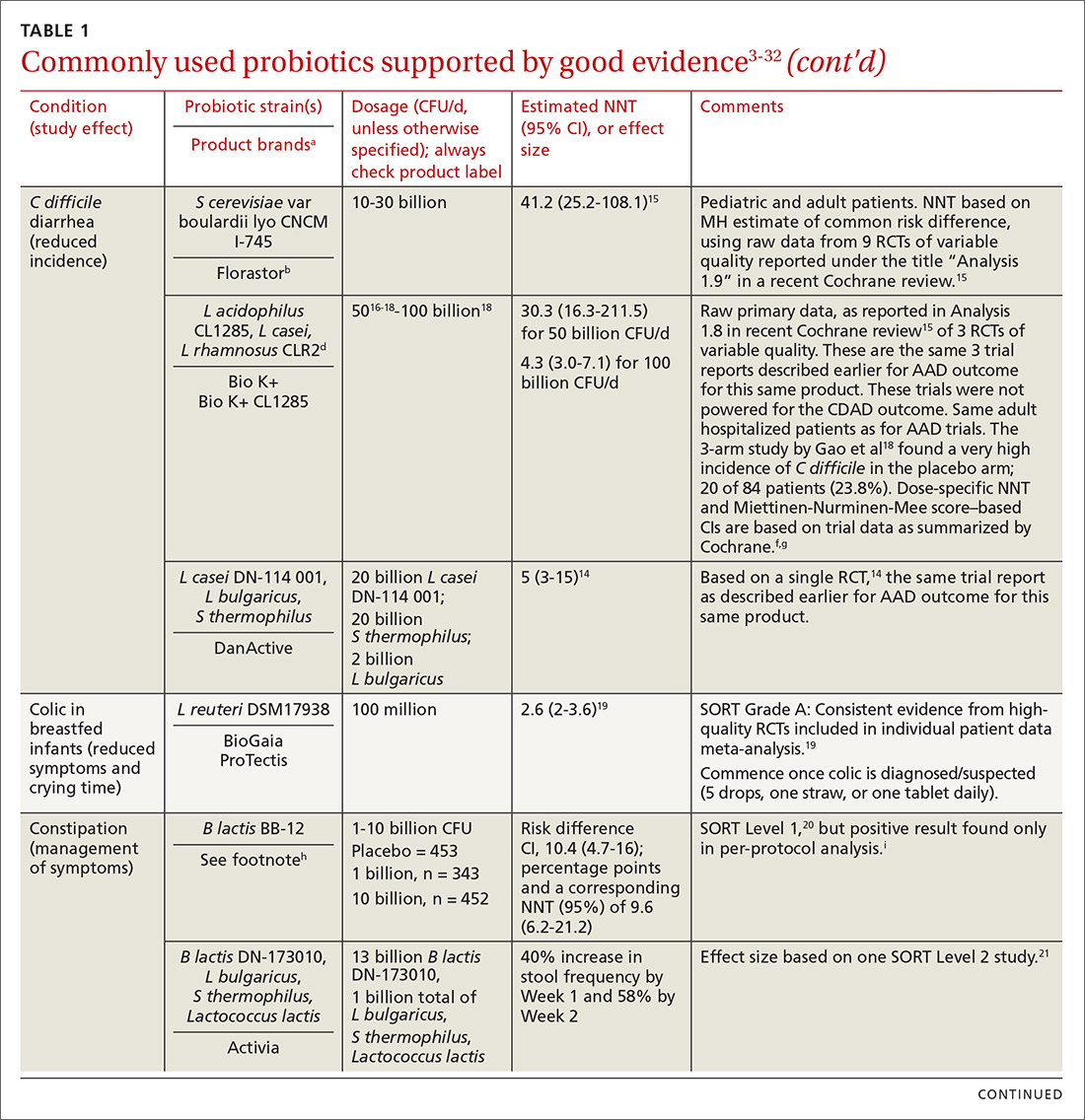
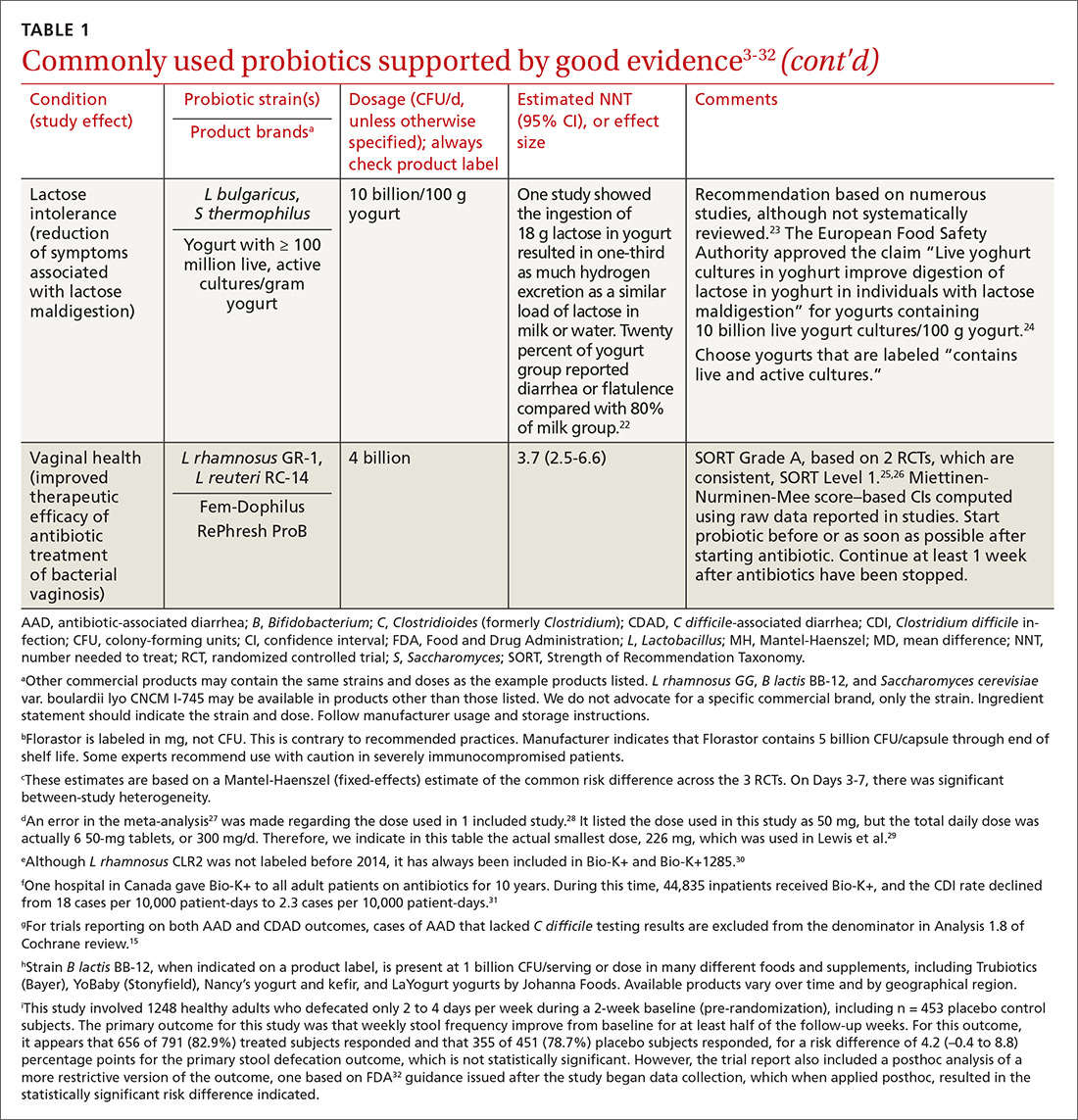
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
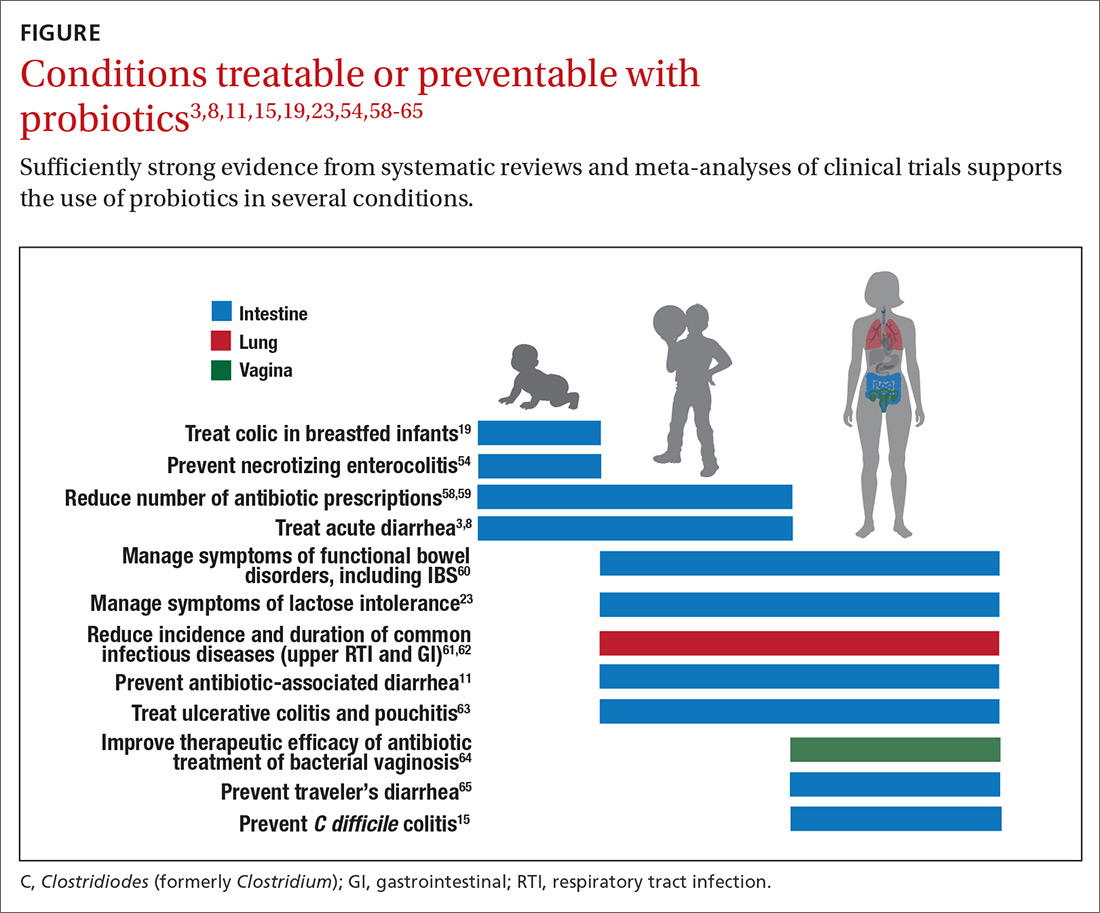
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
We are in the age of the microbiome. Both lay and scientific press proliferate messages about the importance of the microbiome to our health even while they often remain unclear on how to correct microbiota patterns associated with different diseases or suboptimal health states. Probiotics are defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.”1
Certain probiotics have been shown to prevent and treat specific diseases or conditions, inside or outside the gut. But the level and quality of evidence varies greatly. In addition, the health claims allowed by government regulators depend on making discrete distinctions (food vs drug, maintaining health vs treating disease, and emerging evidence vs significant scientific agreement) along dimensions that are increasingly recognized as continuous and complex.2 This leads to confusion among doctors and patients about whether to trust claims on product labels and what to make of the absence of such claims.
Find out which probiotic is effective for a patient’s condition. Simply recommending that a patient “take probiotics” is not particularly helpful when the individual wants a product that will aid a specific condition. While probiotics, to date, have not been marketed as drugs in the United States, clinicians can still approach recommending them in an evidence-based manner.
In this article, we review diseases/conditions for which probiotic products have good efficacy data. We discuss probiotic efficacy and safety, offer relevant information on regulatory categories of probiotics, and give direction for proper usage based on the current evidence base. Although this review is meant to be an easy-to-use resource for clinicians, it is not a comprehensive or detailed review of the numerous probiotic products and studies currently available.
Regulatory and commercial variances with probiotics
In the United States, probiotics have been marketed as dietary supplements, medical foods, or conventional foods, all of which require different levels of evidence and types of oversight than drugs. The efficacy of some probiotics in treating or preventing certain diseases and conditions is similar to, if not better than, effects observed with traditional drug interventions (TABLE 13-32). However, unlike drugs, which are subject to premarket oversight, the probiotic marketplace contains products with uneven levels of evidence, from well substantiated to greatly limited. Currently, no probiotics are sold in the United States as over-the-counter or prescription drugs, although probiotic drugs will likely enter the US market eventually.
What to consider when recommending a product. When considering probiotics, remember that strain, dosage, and indication are all important. Just as we know that not all antibiotics are equally effective for all infections, so, too, effectiveness among probiotics can—and often does—vary for any given condition. Effectiveness also may vary from patient to patient. Most recommendations made in this review are tied to specific probiotic strains and doses. In some cases, more than one probiotic may be efficacious, likely due to the same or similar underlying mechanism of action. For example, most probiotics produce short-chain fatty acids in the colon, providing a common mechanism supporting digestive health.33-35
Contrary to the blanket recommendation preferring higher dosages or a greater number of strains,36 our recommendations are based on levels shown to be effective in clinical trials, which in some contexts can be as low as 100 million colony-forming units (CFU) per day.37,38 Indeed, a survey we conducted previously of retail dietary supplement products indicated that products with lower CFUs or fewer strains could more readily be linked to evidence of efficacy than multistrain, high-CFU products.39
Continue to: Understanding probiotic product labels is a good start
Understanding probiotic product labels is a good start. Information shown on the label of a probiotic dietary supplement in the United States should include the genus, species, and strains contained in the product, the dose delivered in CFU (the most common measure of the number of live microbes in a probiotic product) through the end of shelf life, and expected benefits. (For help in deciphering these labels, see the label schematic developed by the International Scientific Association for Probiotics and Prebiotics40 at https://isappscience.org/infographics/probiotic-labelling/.)
Per guidelines from the Food and Agricultural Organization of the United Nations and the World Health Organization, all probiotic products should have this type of information clearly displayed on the product packaging.41 However, some probiotic foods display less information; for example, they may not specify the product’s strains or recommended dosage levels. Product Web sites may or may not disclose details missing from the food label. The absence of such information makes it impossible to make evidence-based recommendations about those products.
Probiotics are generally safe, with caveats
The overall safety of typical probiotics (Lactobacillus species, Bifidobacterium species, and Saccharomyces cerevisiae var. boulardii) has been well documented.42,43 Many probiotic strains have been granted Generally Recognized as Safe status for use in foods in the United States.44,45 Many traditional probiotic species have been evaluated by the European Food Safety Authority (similar to FDA, except jurisdiction is only over foods, not drugs) and are considered safe for use in food in the European Union.
Be aware that probiotics delivered in dietary supplements and foods are intended for the general population and not for patient populations. Manufacturers therefore are not required to assure safety in vulnerable populations. Nevertheless, probiotics are often stocked in hospital formularies.46,47 Probiotic usage in vulnerable patient groups has been considered by an expert working group from the standpoint of quality assurance for microbiologic products used to treat and prevent disease, with the experts recommending that health care professionals (including pharmacists and physicians) seek quality information from manufacturers and that manufacturers participate in programs providing third-party (eg, United States Pharmacopeia [USP] or Underwriters Laboratories [UL]) verification of probiotic products to assure products meet applicable purity standards.48,49
Published case studies have reported that probiotics may be a rare cause of sepsis.43 Recently, Lactobacillus rhamnosus GG was linked to bacteremia in 6 critically ill patients, but all cases resolved without complications.50 Further, the death of a premature infant was linked to administration of a probiotic contaminated with an opportunistic pathogenic mold.51 A randomized controlled trial (RCT) of a multispecies probiotic product in critically ill pancreatitis patients showed higher mortality in the group given the multispecies probiotic.52 However, additional examination of the data suggests that the observed higher mortality was due to problems with randomization for disease severity and other concerns, and not to the probiotic.53 Much more frequently, probiotics have been administered orally in at-risk patient groups, including premature infants, cancer patients, and critically ill patients, with no significant increases in adverse events.54-56
Continue to: Taken together...
Taken together, clinical trials have reported more adverse events in the placebo than probiotic group.42 Infection data collected in these trials have been used in subsequent analyses to demonstrate that in some settings, certain probiotics actually reduce the risk of infections. One notable example was a meta-analysis of 37 RCTs that showed that probiotics reduce the incidence of late-onset neonatal sepsis in premature infants.57
At the present time, risk of probiotic use is low but still demands awareness, especially in unusual circumstances such as use in particularly vulnerable patients not yet studied or use of a product with limited available safety data. Any recommended product should be manufactured in compliance with applicable regulatory standards and preferably assured through voluntary quality audits.49
Evidence of effectiveness is strong for many conditions
Probiotics have been studied for clinical benefit in numerous conditions (FIGURE3,8,11,15,19,23,54,58-65), and systematic reviews of the clinical trials have found the overall results to be sufficiently strong to warrant recommendations, even though some individual trials were of low quality.66 Some evidence may require confirmatory studies to clarify which specific product should be recommended.
Admittedly some of the indications are for diseases that most family physicians do not typically manage. For example, the evidence for probiotics for preventing necrotizing enterocolitis in premature infants was reviewed in a Cochrane analysis, which gave an estimated number needed to treat (NNT) of 41 and concluded, “our updated review of available evidence strongly supports a change in practice.”54 A recent study of > 4500 infants in India found a probiotic/prebiotic supplement resulted in a 40% reduction in clinical sepsis compared with placebo.67 Another common use of probiotics is as adjunctive therapy for mild to moderately active ulcerative colitis, where the current estimated NNT is 4.63 Probiotics may also address gut and non-gut conditions and serve different functions throughout the lifespan.
Probiotic applications most relevant to primary care
We summarize in TABLE 13-32 probiotic uses supported by good evidence for indications of general interest in primary care medicine. This table includes endpoints with actionable evidence (including many strength of recommendation taxonomy [SORT] Level 1 studies) that allow us to make strong recommendations. Not all evidence is SORT Grade A, but we agree with the expert groups that deem evidence to be sufficient to warrant recommendations.
Continue to: The granular data...
The granular data we provide can help shape recommendations of a product for a specific indication. Numerous probiotics have been tested on suboptimal gastrointestinal health, including managing functional bowel symptoms ranging from occasional gas, bloating, or constipation through diagnosed irritable bowel syndrome (IBS). Supplements such as Bifidobacterium infantis subsp. longum 35624 (the probiotic in Align), Lactobacillus plantarum 299V (the probiotic in NatureMade Digestive Probiotic Daily Balance), and foods such as Activia yogurt, Yakult cultured milk, or Good Belly juice can be recommended for digestive symptoms.
For patients experiencing gut symptoms unrelated to diagnosed disease, it may be reasonable for them to try a well-documented strain for 3 to 4 weeks. Currently it is difficult to predict success a priori; this may change as we learn more about how an individual’s microbiome, diet, and genetics affect response to specific probiotics. TABLE 268-71 presents sample recommendations from international expert panels for select contexts.
The popular press today commonly recommends consuming more fermented foods. Although we agree in general with this recommendation, physicians should be clear that fermented foods may be a source of live cultures, but not all fermented foods retain live microbes. Further, many fermented foods lack evidence documenting health effects, and therefore are not a source of probiotics. If the patient’s goal is to support regular diet with live microbes, any number of probiotic products or fermented foods that retain viable cultures may suffice. However, when patients request probiotics for specific needs, recommendations should be based on available evidence for specific studied products. (See also, “Questions patients frequently ask about probiotics.”)
SIDEBAR
9 questions patients frequently ask about probiotics
Q. Is a higher dose and greater number of strains better?
A. Not necessarily. The best approach is to recommend products that have been tested in human studies with positive outcomes. Sometimes these products are single strain and have doses lower than other commercial products. If your patient’s goal is to simply add live, potentially beneficial microbes to a diet, and he or she is not presenting with any specific health complaints, then fermented foods or any probiotic supplement should be sufficient.
Q. Is yogurt a good choice for managing antibiotic-associated diarrhea (AAD)?
A. In patients at high risk, recommend a probiotic from TABLE 1. 3-32 Simply recommending “yogurt” is not a strong recommendation, since few yogurts contain specific probiotics that are known to help with AAD. Yogurt usually contains live cultures, but the only cultures required in yogurt (Lactobacillus bulgaricus and Streptococcus thermophilus) do not survive intestinal transit and, with the exception of improving lactose digestion, are not likely to promote digestive health. Yogurts stipulating the strain and dose of added microbes are more likely to be supported by evidence.
Q. Does the sugar in probiotic yogurts negate the benefits of probiotic yogurt?
A. Most studies testing the health benefits of yogurt have been conducted on sweetened yogurts. Therefore, the sugar present in these products does not negate the probiotic effects. However, sweetened yogurts should be consumed as part of a balanced diet.
Q. Are probiotics beneficial for healthy people?
A. Studies have shown that probiotics can modestly decrease the incidence and duration of some common infectious symptoms such as those occurring in the gastrointestinal and upper respiratory tracts. These studies have been conducted on healthy subjects. But like multivitamins, improving health in healthy people is difficult to demonstrate.
Q. Are probiotic products unregulated?
A. Most probiotic products in the United States are marketed as foods or dietary supplements. These products are regulated by the US Food and Drug Administration (FDA), but not in the same way drugs are regulated. The FDA does not conduct premarket review of data on safety or health benefits. However, the FDA requires that these products are manufactured under current Good Manufacturing Procedures. Further, products are required to be labeled in a truthful (and not misleading) fashion. Enforcement of these standards requires action by the FDA, and limited resources within the agency result in products on the market that may not comply with standards.
Q. Are refrigerated products better than nonrefrigerated?
A. The stability of the live microbes in a probiotic product depends on product formulation and conditions of storage. Some products may require refrigeration, but others do not. Responsible product manufacturers make certain that their probiotic is able to meet the label claim through the end of shelf life if stored as recommended.
Q. Is it better to take probiotics as supplements or foods?
A. It is important to take the product tested for the specific effect, whether it is in food or supplement format. If products with equivalent efficacy are available in different formats, then have patients take the product that best fits with his or her diet and lifestyle.
Q. What is the difference between probiotics and prebiotics?
A. Probiotics are live microorganisms beneficial to one’s health. Prebiotics are not live microbes, but are substances that are used by beneficial, resident microorganisms. Simply put, prebiotics are food for the beneficial bacteria in your gut. Most prebiotics are a type of fiber.
Q. The body already has so many bacteria, how can we expect the comparatively small number of live microbes in a probiotic product to have any benefits?
A. Our bodies are home to trillions of microbes. But remember that we are not uniformly colonized, even throughout the digestive tract. Orally consumed probiotics travel through some sparsely colonized regions of the upper digestive tract, and may become dominant in those segments. But even as minor components of the lower digestive tract, probiotics can impact the gut environment and clinical outcomes.
Continue to: What to look for in the future
What to look for in the future
Basic research, human trials, and market development in the field of probiotics are progressing rapidly. Probiotics at this time are primarily from the genera Lactobacillus, Bifidobacterium, and Saccharomyces. But the potential of probiotics has spurred research into previously untapped microbial members of the healthy human microbiota. Microbes such as Akkermansia, Faecalibacterium, and Rosburia may comprise “next-generation probiotics” that will likely be developed as drugs.72
Active areas of research holding some promise involve microbiome-driven components of intractable problems such as metabolic syndrome (obesity,73 diabetes, and lipid dysregulation) and brain dysfunction74 (depression, anxiety, cognition, autism). A guide to the clinical use of probiotic products available in the United States, updated yearly, may be a useful reference (but the reader may want to examine the referenced studies as their level of evidence is different than the SORT method).75 Science-based videos, infographics, and other resources are available from the International Scientific Association for Probiotics and Prebiotics, (mentioned earlier; www.isappscience.org/).
It appears that probiotics will continue to be widely used and hopefully in a more evidence-based manner. As we learn more about individual microbiome variations, recommendations will likely be more patient specific. Probiotics that have robust evidence represent the strongest recommendations. Even so, since the risks of using traditional probiotics (such as Lactobacillus, Bifidobacterium and Saccharomyces strains) are low, trial and error may be warranted at times.
CORRESPONDENCE
Daniel J. Merenstein, MD, 4000 Reservoir Road NW, Building D 240, Washington, DC 20007; djm23@georgetown.edu.
ACKNOWLEDGMENT
We thank Alexandra Mannerings, PhD, for preparing the FIGURE.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
1. Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506-514.
2. Sanders ME, Heimbach JT, Pot B, et al. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2:127-133.
3. Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134:e176-e191.
4. Francavilla R, Lionetti E, Castellaneta S, et al. Randomised clinical trial: Lactobacillus reuteri DSM 17938 vs. placebo in children with acute diarrhoea—a double-blind study. Aliment Pharmacol Ther. 2012;36:363-369.
5. Dinleyici EC, Dalgic N, Guven S, et al. Lactobacillus reuteri DSM 17938 shortens acute infectious diarrhea in a pediatric outpatient setting. J Pediatr (Rio J). 2015;91:392-396.
6. Dinleyici EC, Group PS, Vandenplas Y. Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children. Acta Paediatr. 2014;103:e300-e305.
7. Urbanska M, Gieruszczak-Bialek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther. 2016;43:1025-1034.
8. Szajewska H, Kołodziej M, Gieruszczak-Białek D, et al. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—a 2019 update. Aliment Pharmacol Ther. 2019;49:1376-1384.
9. Vanderhoof JA, Whitney DB, Antonson DL, et al. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr. 1999;135:564-568.
10. Szajewska H, Albrecht P, Topczewska-Cabanek A. Randomized, double-blind, placebo-controlled trial: effect of Lactobacillus GG supplementation on Helicobacter pylori eradication rates and side effects during treatment in children. J Pediatr Gastroenterol Nutr. 2009;48:431-436.
11. Guo Q, Goldenberg JZ, Humphrey C, et al. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev. 2019;4:CD004827.
12. Arvola T, Laiho K, Torkkeli S, et al. Prophylactic Lactobacillus GG reduces antibiotic-associated diarrhea in children with respiratory infections: a randomized study. Pediatrics. 1999;104:e64.
13. Szajewska H, Kolodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2015;42:793-801.
14. Hickson M, D’Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ. 2007;335:80.
15. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;(12):CD006095.
16. Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol. 2007;21:732-736.
17. Sampalis J, Psaradellis E, Rampakakis E. Efficacy of BIO K+ CL1285 in the reduction of antibiotic-associated diarrhea— a placebo controlled double-blind randomized, multi-center study. Arch Med Sci. 2010;6:56-64.
18. Gao XW, Mubasher M, Fang CY, et al. Dose-response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol. 2010;105:1636-1641.
19. Sung V, D’Amico F, Cabana MD, et al. Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 2018;141. pii: e20171811.
20. Eskesen D, Jespersen L, Michelsen B, et al. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double-blind, placebo-controlled, parallel-group trial. Br J Nutr. 2015;114:1638-1646.
21. Yang YX, He M, Hu G, et al. Effect of a fermented milk containing Bifidobacterium lactis DN-173010 on Chinese constipated women. World J Gastroenterol. 2008;14:6237-6243.
22. Kolars JC, Levitt MD, Aouji M, et al. Yogurt—an autodigesting source of lactose. N Engl J Med. 1984;310:1-3.
23. Savaiano DA. Lactose digestion from yogurt: mechanism and relevance. Am J Clin Nutr. 2014;99(5 suppl):1251S-1255S.
24. EFSA Panel on Dietetic Products Nutrition and Allergy. Scientific Opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion (ID 1143, 2976) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2010;8(10):1763.
25. Martinez RC, Franceschini SA, Patta MC, et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can J Microbiol. 2009;55:133-138.
26. Anukam K, Osazuwa E, Ahonkhai I, et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 2006;8:1450-1454.
27. Szajewska H, Ruszczynski M, Radzikowski A. Probiotics in the prevention of antibiotic-associated diarrhea in children: a meta-analysis of randomized controlled trials. J Pediatr. 2006;149:367-372.
28. Kyriakos N, Papamichael K, Roussos A, et al. A lyophilized form of Saccharomyces boulardii enhances the Helicobacter pylori eradication rates of omeprazole-triple therapy in patients with peptic ulcer disease or functional dyspepsia. Hospital Chronicles. 2013;8:127-133.
29. Lewis SJ, Potts LF, Barry RE. The lack of therapeutic effect of Saccharomyces boulardii in the prevention of antibiotic-related diarrhoea in elderly patients. J Infect. 1998;36:171-174.
30. Auclair J, Frappier M, Millette M. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+): characterization, manufacture, mechanisms of action, and quality control of a specific probiotic combination for primary prevention of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S135-S143.
31. Maziade PJ, Pereira P, Goldstein EJ. A decade of experience in primary prevention of Clostridium difficile infection at a community hospital using the probiotic combination Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R, and Lactobacillus rhamnosus CLR2 (Bio-K+). Clin Infect Dis. 2015;60(Suppl 2):S144-S147.
32. FDA. Guidance for industry on irritable bowel syndrome-clinical evaluation of drugs for treatment. 2012. www.federalregister.gov/documents/2012/05/31/2012-13143/guidance-for-industry-on-irritable-bowel-syndrome-clinical-evaluation-of-drugs-for-treatment. Accessed March 25, 2020.
33. Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol. 2010;72:297-313.
34. Kim HK, Rutten NB, Besseling-van der Vaart I, et al. Probiotic supplementation influences faecal short chain fatty acids in infants at high risk for eczema. Benef Microbes. 2015;6:783-790.
35. Surendran Nair M, Amalaradjou MA, Venkitanarayanan K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv Appl Microbiol. 2017;98:1-29.
36. Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician. 2017;96:170-178.
37. Urbanska M, Szajewska H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr. 2014;173:1327-1337.
38. Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590.
39. Merenstein D, Guzzi J, Sanders ME. More information needed on probiotic supplement product labels. J Gen Intern Med. 2019;34:2735-2737.
40. International Scientific Association for Probiotics and Prebiotics. Deciphering a probiotic label. https://isappscience.org/infographics/probiotic-labelling/. Accessed March 25, 2020.
41. Food and Agricultural Organization of the United Nations and World Health Organization. Guidelines for the evaluation of probiotics in food. 2002. www.who.int/foodsafety/fs_management/en/probiotic_guidelines.pdf. Accessed March 25, 2020.
42. Agency for Healthcare Research and Quality. Safety of probiotics to reduce risk and prevent or treat disease. AHRQ Publication No. 11-E007. 2011. www.ahrq.gov/downloads/pub/evidence/pdf/probiotics/probiotics.pdf. Accessed March 25, 2020.
43. Sanders ME, Akkermans LM, Haller D, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164-185.
44. European Food Safety Authority. Statement on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA. 2: Suitability of taxonomic units notified to EFSA until March 2015. EFSA J. 2015;12:4138.
45. U.S. Food and Drug Administration. Generally Recognized as Safe (GRAS) Notification Program. 2020. http://www.fda.gov/animalveterinary/products/animalfoodfeeds/generallyrecognizedassafegrasnotifications/default.htm. Accessed March 25, 2020.
46. Yi SH, Jernigan JA, McDonald LC. Prevalence of probiotic use among inpatients: a descriptive study of 145 U.S. hospitals. Am J Infect Control. 2016;44:548-553.
47. Abe AM, Gregory PJ, Hein DJ, et al. Survey and systematic literature review of probiotics stocked in academic medical centers within the United States. Hosp Pharm. 2013;48:834-847.
48. Sanders ME, Merenstein DJ, Ouwehand AC, et al. Probiotic use in at-risk populations. J Am Pharm Assoc. 2016;56:680-686.
49. Jackson SA, Shoeni JL, Vegge C, et al. Improving end-user trust in the quality of commercial probiotic products. Front Microbiol. 2019;10:739.
50. Yelin I, Flett KB, Merakou C, et al. Genomic and epidemiological evidence of bacterial transmission from probiotic capsule to blood in ICU patients. Nat Med. 2019;25:1728-1732.
51. Vallabhaneni S, Walker TA, Lockhart SR, et al. Notes from the field: fatal gastrointestinal mucormycosis in a premature infant associated with a contaminated dietary supplement—Connecticut, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:155-156.
52. Besselink MG, van Santvoort HC, Buskens E, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371:651-659.
53. van den Nieuwboer M, Claassen E. Dealing with the remaining controversies of probiotic safety. Benef Microbes. 2019;27:1-12.
54. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;(4):CD005496.
55. Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Ann Oncol. 2014;25:1919-1929.
56. Liu KX, Zhu YG, Zhang J, et al. Probiotics’ effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care. 2012;16:R109.
57. Rao SC, Athalye-Jape GK, Deshpande GC, et al. Probiotic supplementation and late-onset sepsis in preterm infants: a meta-analysis. Pediatrics. 2016;137:e20153684.
58. King S, Tancredi D, Lenoir-Wijnkoop I, et al. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur J Public Health. 2019;29:494-499.
59. Scott AM, Clark J, Julien B, et al. Probiotics for preventing acute otitis media in children. Cochrane Database Syst Rev. 2019;(6):CD012941.
60. Niu HL, Xiao JY. The efficacy and safety of probiotics in patients with irritable bowel syndrome: evidence based on 35 randomized controlled trials. Int J Surg. 2020;75:116-127.
61. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112:41-54.
62. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895.
63. Mardini HE, Grigorian AY. Probiotic mix VSL#3 is effective adjunctive therapy for mild to moderately active ulcerative colitis: a meta-analysis. Inflamm Bowel Dis. 2014;20:1562-1567.
64. Senok AC, Verstraelen H, Temmerman M, et al. Probiotics for the treatment of bacterial vaginosis. Cochrane Database Syst Rev. 2009;(4):CD006289.
65. McFarland LV, Goh S. Are probiotics and prebiotics effective in the prevention of travellers’ diarrhea: a systematic review and meta-analysis. Travel Med Infect Dis. 2019;27:11-19.
66. Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Phys. 2004;69:548-556.
67. Panigrahi P, Parida S, Nanda NC, et al. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature. 2017;548:407-412.
68. Howick J, Chalmers I, Glasziou P, et al. Oxford Centre for Evidence-based Medicine Levels of Evidence. www.cebm.net/2016/05/ocebm-levels-of-evidence/2011. Accessed March 25, 2020.
69. World Gastroenterology Organisation. WGO practice guideline—probiotics and prebiotics. 2017. www.worldgastroenterology.org/guidelines/global-guidelines/probiotics-and-prebiotics. Accessed March 25, 2020.
70. Szajewska H, Canani RB, Guarino A, et al. Probiotics for the prevention of antibiotic-associated diarrhea in children. J Pediatr Gastroenterol Nutr. 2016;62:495-506.
71. Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr. 2014;58:531-539.
72. O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: the spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057.
73. John GK, Wang L, Nanavati J, et al. Dietary alteration of the gut microbiome and its impact on weight and fat mass: a systematic review and meta-analysis. Genes (Basel). 2018;9. pii:E167.
74. Sherwin E, Dinan TG, Cryan JF. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann N Y Acad Sci. 2018;1420:5-25.
75. Skokovic-Sunjic D. Clinical guide to probiotic products available in USA. 2020. www.usprobioticguide.com. Accessed March 25, 2020.
PRACTICE RECOMMENDATIONS
› Consider specific probiotics to prevent antibioticassociated diarrhea, reduce crying time in colicky infants, and improve therapeutic effectiveness of antibiotics for bacterial vaginosis. A
› Consider specific probiotics to reduce the risk for Clostridioides (formerly Clostridium) difficile infections, to treat acute pediatric diarrhea, and to manage symptoms of constipation. B
› Check a product’s label to ensure that it includes the probiotic’s genus, species, and strains; the dose delivered in colony-forming units through the end of shelf life; and expected benefits C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Asthma: Newer Tx options mean more targeted therapy
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; mrali@northwell.edu
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
PRACTICE RECOMMENDATIONS
› Consider inhaled corticosteroids (ICS) as your first choice for a long-term control agent to treat asthma; add a long-acting beta agonist (LABA) when needed. A
› Use long-acting muscarinic antagonists (LAMA) as add-on therapy for patients whose asthma is uncontrolled despite the use of low-dose ICS-LABA, or as an alternative to high-dose ICS-LABA. A
› Consider biological therapies for patients with asthma exacerbations that require steroids at least twice a year. B
› Use azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Getting tendinopathy treatment (and terminology) right
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5
Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5
Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.
First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14
Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; kvg3355@ksu.edu.
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5
Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5
Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.
First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14
Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; kvg3355@ksu.edu.
The vast majority of patients with tendon problems are successfully treated nonoperatively. But which treatments should you try (and when), and which are not quite ready for prime time? This review presents the evidence for the treatment options available to you. But first, it’s important to get our terminology right.
Tendinitis vs tendinosis vs paratenonitis: Words matter
The term “tendinopathy” encompasses many issues related to tendon pathology including tendinitis, tendinosis, and paratenonitis.1,2 The clinical syndrome consists of pain, swelling, and functional impairment associated with activities of daily living or athletic performance.3 Tendinopathy may be acute or chronic, but most cases result from overuse.1
In healthy tendons, the collagen fibers are packed tightly and organized in a linear pattern (FIGURE 1A). However, tendons that are chronically overused develop cumulative microtrauma that leads to a degenerative process within the tendon that is slow (typically measured in months) to heal. This is due to the relative lack of vasculature and the slow rate of tissue turnover in tendons.2,4,5
Sports and manual labor are the most common causes of tendinopathy, but medical conditions including obesity, high blood pressure, diabetes, and high cholesterol are associated risk factors. Medications, particularly fluoroquinolones and statins, can cause tendon problems, and steroids, particularly those injected intratendinously, have been implicated in tendon rupture.4,6
The term “tendinitis” has long been used for all tendon disorders although it is best reserved for acute inflammatory conditions. For most tendon conditions resulting from overuse, the term “tendinosis” is now more widely recognized and preferred.7,8 Family physicians (FPs) should recognize that tendinitis and tendinosis differ greatly in pathophysiology and treatment.3
Tendinitis: Not as common as you think
Tendinitis is an acute inflammatory condition that accounts for only about 3% of all tendon disorders.3 Patients presenting with tendinitis usually have acute onset of pain and swelling typically either from a new activity or one to which they are unaccustomed (eg, lateral elbow pain after painting a house) or from an acute injury. Partial tearing of the affected tendon is likely, especially following injury.2,3
Tendinosis: A degenerative condition
In contrast to the acute inflammation of tendinitis, tendinosis is a degenerative condition induced by chronic overuse. It is typically encountered in athletes and laborers.2,5,8,9 Tendinotic tissue is generally regarded as noninflammatory, but recent research supports inflammation playing at least a small role, especially in closely associated tissues such as bursae and the paratenon tissue.10
Continue to: Histologically, tendinosis shows...
Histologically, tendinosis shows loss of the typical linear collagen fiber organization, increased mucoid ground substance, hypercellularity, and increased growth of nerves and vessels (FIGURE 1B).
Tendinosis is not always symptomatic.5,11 When pain is present, experts have proposed that it is neurogenically derived rather than from local chemical inflammation. This is supported by evidence of increases in the excitatory neurotransmitter glutamate and its receptor N-methyl-D-aspartate in tendinotic tissue with nerve ingrowth. Tendinotic tissue also contains substance P and calcitonin gene-related peptide, neuropeptides that are associated with pain and nociceptive nerve endings.2,3,6,10
Patients with tendinosis typically present with an insidious onset of a painful, thickened tendon.11 The most common tendons affected include the Achilles, the patellar, the supraspinatus, and the common extensor tendon of the lateral elbow.2 Lower extremity tendinosis is common in athletes, while upper extremity tendinopathies are more often work-related.3
Paratenonitis: Inflammation surrounding the tendon
Occasionally, tendinosis may be associated with paratenonitis, which is inflammation of the paratenon (tissue surrounding some tendons).2,5,10 Paratendinous tissue contains a higher concentration of sensory nerves than the tendon itself and may generate significant discomfort.10,11
The clinical presentation of paratenonitis includes a swollen and erythematous tendon.5 The classic example—de Quervain disease—involves the first dorsal wrist compartment, in which the abductor pollicis longus and extensor pollicis brevis tendons are encased in a synovial sheath. The term tenosynovitis is commonly used to indicate inflammation of both the paratenon and synovial sheath (TABLE 12,3,5,6,9-11).5
Continue to: Treatment demands time and patience
Treatment demands time and patience
Treating tendon conditions is challenging for both the patient and the clinician. Improvement takes time and several different treatment strategies may be required for success. Given the large number of available treatment options and the often weak or limited supporting evidence of their efficacy, designing a treatment plan can be difficult. TABLE 2 summarizes the information detailed below about specific treatment options.
First-line treatments. The vast majority of patients with tendon problems are successfully treated nonoperatively. Reasonable first-line treatments, especially for inflammatory conditions like tendinitis, tenosynovitis, and paratenonitis, include relative rest, activity modification, cryotherapy, and bracing.12-14
Nonsteroidal anti-inflammatory drugs (NSAIDs) for pain control are somewhat controversial. At best, they provide pain relief in the short term (7-14 days); at worst, some studies suggest potential detrimental effects to the tendon.14 If considered, NSAIDs should be used for no longer than 2 weeks. They are ideally reserved for pain control in patients with acute injuries when an inflammatory condition is likely. An alternative for pain control in inflammatory cases is a short course of oral steroids, but the adverse effects of these medications may be challenging for some patients.
Other options. If these more conservative treatments fail, or the patient is experiencing significant and debilitating pain, FPs may consider a corticosteroid injection. If this fails, or the condition is clearly past an inflammatory stage, then physical therapy should be considered. More advanced treatments, such as platelet-rich plasma injections and percutaneous needle tenotomies, are typically reserved for chronic, recalcitrant cases of tendinosis. Various other treatment options are detailed below and can be used on a case-by-case basis. Surgical management should be considered only as a last resort.
Realize that certain barriers may exist to some of these treatments. With extracorporeal shockwave therapy, for example, access to a machine can be challenging, as they are typically only found in major metropolitan areas. Polidocanol, used during sclerotherapy, can be difficult to obtain in the United States. Another challenge is cost. Not all of these procedures are covered by insurance, and they can be expensive when paying out of pocket.
Continue to: Rehabilitation...
Rehabilitation: Eccentric exercises and deep-friction massage
Studies show that eccentric exercises (EEs) help to decrease vascularity and nerve presence in affected tendons, modulate expression of neuronal substances, and may stimulate formation of load-tolerant fibroblasts.2,3
For Achilles tendinosis, EE is a well-established treatment supported by multiple randomized controlled trials (RCTs). Improvements in patient satisfaction and pain range from 60% to 90%; evidence suggests greater success in midsubstance vs insertional Achilles tendinosis.15 The addition of deep-friction massage (DFM), which we’ll discuss in a moment, to EE appears to improve outcomes even more than EE alone.16
EE is also a beneficial treatment for patellar tendinosis,3,14 and it appears to benefit rotator cuff tendinosis,3 but research has shown EE for lateral epicondylosis to be no more effective than stretching alone.17
DFM is for treating tendinosis—not inflammatory conditions. Mechanical stimulation of the tissue being massaged releases cell mediators and growth factors that activate fibroblasts. It is typically performed with plastic or metal tools.16 DFM appears to be a reliable treatment option for the lateral elbow.18
Extracorporeal shockwave therapy appears promising; evidence is limited
Research has shown that extracorporeal shockwave therapy (ESWT) promotes the production of TGF-β1 and IGF-1 in rat models,2 and it is believed to be able to disintegrate calcium deposits and stimulate tissue repair.14 Research is generally supportive of its effectiveness in treating tendinosis; however, evidence is limited by great variability in studies in terms of treatment intensity, frequency, duration, timing, number of treatments, and use of a local anesthetic.14 ESWT appears to be useful in augmenting treatment with EE, particularly with regard to the rotator cuff.19
Continue to: A review of 10 RCTs...
A review of 10 RCTs demonstrated the effectiveness of ESWT for tennis elbow.2 ESWT for greater trochanteric pain syndrome (GTPS, formerly known as trochanteric bursitis) appears to be more effective than corticosteroids and home exercises for outcomes at 4 months and equivalent to home exercises at 15 months.20 In patellar tendinosis, ESWT has been shown to be an effective treatment, especially under ultrasound guidance.12 Studies involving the use of ESWT for Achilles tendinosis have had mixed results for midsubstance tendinosis, and more positive results for insertional tendinosis.15 For a video on how the therapy is administered, see https://www.youtube.com/watch?v=Fq5yqiWByX4.
Glyceryl trinitrate patches: Mixed results
Basic science studies have shown that nitric oxide modulates tendon healing by enhancing fibroblast proliferation and collagen synthesis,2,14 but that it should be used with caution in cardiac patients and in those who take PDE-5 inhibitors. Common adverse effects include rash, headache, and dizziness.
In clinical studies, glyceryl trinitrate (GTN) patches show mixed results. For the upper extremity, GTN appears to be helpful for pain in the short term when combined with physical therapy, but long-term positive outcomes have been absent.21 In one Level 1 study for patellar tendinosis comparing GTN patches with EE to a placebo patch with EE, no significant difference was noted at 24 weeks.22 Benefit for Achilles tendinosis also appears to be lacking.3,23
Corticosteroid injections: Mechanism unknown
The mechanism for the beneficial effects of corticosteroid injections (CSIs) for tendinosis remains controversial. Proposed mechanisms include lysis of peritendinous adhesions, disruption of the nociceptors in the region of the injection, and decreased vascularization.10,15 Given tendinosis is generally regarded as a noninflammatory condition, and the fact that these medications have demonstrated potential negative effects on tendon healing, exercise caution when considering CSIs.2,24
Although steroids can effectively reduce pain in the short term, intermediate- and long-term studies generally show no difference or worse outcomes when they are compared to no treatment, placebo, or other treatment modalities. In fact, strong evidence exists for negative effects of steroids on lateral epicondylosis in both the intermediate (6 months) and long (1 year) term.24 Particular care is required when administering a CSI for medial epicondylosis, as the ulnar nerve is immediately posterior to the medial epicondyle.25
Continue to: In contrast...
In contrast, CSIs appear to be a reliable treatment option for de Quervain disease.26 Landmark-guided injections for GTPS can improve pain in the short term (< 1 month), but are inferior to either home exercises or ESWT beyond a few months. Thus, CSIs are a reasonable option for pain control in GTPS, but should not be the sole treatment modality.20
Studies regarding corticosteroid use for Achilles and patellar tendinosis have had mixed results. Patients can hope for mild improvement in pain at best, and the risk for relapse and tendon rupture is ever present.27 This is especially concerning given the significant load-bearing of the patellar and Achilles tendons.14,15 If you are considering a CSI for these purposes, use imaging guidance to ensure the injection is not placed intratendinously.
Platelet-rich plasma and whole blood: Inducing an anabolic healing response
Platelet-rich plasma (PRP) and whole blood injections both aim to deliver autologous growth factors (eg, VEGF, PDGF, and IGF-1) and bioactive mediators to the site of tendinosis to induce an anabolic healing response. PRP therapy differs from whole blood therapy in that it is withdrawn and then concentrated in a centrifuge before being injected. Patients are typically injected under ultrasound guidance. The great variation in PRP preparation, platelet concentration, use of adjunctive treatments, leukocyte concentration, and number and technique of injections makes it difficult to determine the optimal PRP treatment protocol.10,28,29
In 1 prospective RCT comparing subacromial PRP injections to CSI for the shoulder, the PRP group had better outcomes at 3 months, but similar outcomes at 6 months. The suggestion was made that PRP therapy could be an alternative treatment for individuals with a contraindication to CSIs.30
PRP therapy appears to be an effective treatment option for patellar tendinopathy.28,31 A Level 1 study comparing dry needle tenotomy and EE to dry needle tenotomy with both PRP therapy and EE found faster recovery in the PRP group.32 In another patellar tendon study comparing ESWT to PRP therapy, both were found to be effective, but PRP performed better in terms of pain, function, and satisfaction at 6 and 12 months.12 For Achilles tendons, however, the evidence is mixed; case series have had generally positive outcomes, but the only double-blind RCT found no benefit.28,31
Continue to: In lateral epicondylosis...
In lateral epicondylosis, the use of auto-logous whole blood or PRP injections appears to help both pain and function, with several studies failing to demonstrate superiority of 1 modality over the other.24,25,28,33 This raises the issue of whether PRP therapy is any more effective than whole blood for the treatment of other tendinopathies. Unfortunately, there is a paucity of studies comparing the effectiveness of 1 modality to the other, apart from those for lateral epicondylosis.
Prolotherapy: An option for these 3 conditions
Prolotherapy involves the injection of hypertonic dextrose and local anesthetic, which is believed to lead to an upregulation of inflammatory mediators and growth factors. This treatment usually involves several injections spaced 2 to 6 weeks apart over several months. High-quality studies are not available to clarify the optimal dextrose concentration or number of injections required. The few high-quality studies available support prolotherapy for lateral epicondylosis, rotator cuff tendinopathy, and Osgood Schlatter disease. Lesser-quality studies support its use for refractory pain of the Achilles, hip adductors, and plantar fascia.24,34
Sclerotherapy: Not just for veins
As discussed earlier, tendinotic tissue can have neovascularization that is easily detected on Doppler ultrasound. Sensory nerves typically grow alongside the new vessels. Sclerosing agents, such as polidocanol, can be injected with ultrasound guidance into areas of neovascularization, with the intention of causing denervation and pain relief.15 Neovascularization does not always correlate with pathology, so careful patient selection is necessary.35
Studies of sclerotherapy for patellar tendinopathy are generally favorable. One comparing sclerotherapy to arthroscopic debridement showed improvement in pain from both treatments at 6 and 12 months, but the arthroscopy group had less pain, better satisfaction scores, and a faster return to sport.14 Sclerotherapy is also effective for Achilles tendinosis.15
Stem cells: Not at this time
Stem cell use for tendinosis is based on the theory that these cells possess the capability to differentiate into tenocytes to produce new, healthy tendon tissue. Additionally, stem cell injections are believed to create a local immune response, recruiting local growth factors and cytokines to aide in tendon repair. A recent systematic review failed to identify any high-quality studies (Level 4 data at best) supporting the use of stem cells in tendinopathy, and the researchers did not recommend stem cell use outside of clinical trials at this time.36
Continue to: Percutaneous needle tenotomy...
Percutaneous needle tenotomy: Consider it for difficult cases
Percutaneous needle tenotomy is thought to benefit tendinosis by disrupting the tendinotic tissue via needling, while simultaneously causing bleeding and the release of growth factors to aid in healing. Unlike surgical tenotomy, the procedure is typically performed with ultrasound guidance in the office or other ambulatory setting. After local anesthesia is administered, a needle is passed multiple times through the entire region of abnormality noted on ultrasound. Generally, around 20 to 30 needle fenestrations are performed.37,38
In one retrospective study evaluating 47 patellar tendons, 81% had excellent or good results.38 In a retrospective study for lateral epicondylosis, 80% had good to excellent results.39
CORRESPONDENCE
Kyle Goerl, MD, CAQSM, Lafene Health Center, 1105 Sunset Avenue, Manhattan, KS, 66502-3761; kvg3355@ksu.edu.
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
1. Andres BM, Murrell GAC. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466:1539-1554.
2. Kaeding C, Best TM. Tendinosis: pathophysiology and nonoperative treatment. Sports Health. 2009;1:284-292.
3. Ackermann PW, Renstrom P. Tendinopathy in sport. Sports Health. 2012;4:193-201.
4. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393-408.
5. Maffulli N, Wong J, Almekinders LC. Types and epidemiology of tendinopathy. Clin Sports Med. 2003;22:675-692.
6. Scott A, Backman LJ, Speed C. Tendinopathy: update on pathophysiology. J Orthop Sport Phys Ther. 2015;45:833-841.
7. Puddu G, Ippolito E, Postacchini F. A classification of achilles tendon disease. Am J Sports Med. 1976;4:145-150.
8. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14:840-843.
9. Kraushaar B, Nirschl R. Current concepts review: tendinosis of the elbow (tennis elbow). J Bone Jt Surg. 1999;81:259-278.
10. Rees JD, Stride M, Scott A. Tendons—time to revisit inflammation. Br J Sports Med. 2014;48:1553-1557.
11. Scott A, Docking S, Vicenzino B, et al. Sports and exercise-related tendinopathies: a review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br J Sports Med. 2013;47:536-544.
12. Smith J, Sellon J. Comparing PRP injections with ESWT for athletes with chronic patellar tendinopathy. Clin J Sport Med. 2014;24:88-89.
13. Mallow M, Nazarian LN. Greater trochanteric pain syndrome diagnosis and treatment. Phys Med Rehabil Clin N Am. 2014;25:279-289.
14. Schwartz A, Watson JN, Hutchinson MR. Patellar tendinopathy. Sports Health. 2015;7:415-420.
15. Magnussen RA, Dunn WR, Thomson AB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med. 2009;19:54-64.
16. Mccormack JR, Underwood FB, Slaven EJ, et al. Eccentric exercise versus eccentric exercise and soft tissue treatment (Astym) in the management of insertional Achilles tendinopathy: a randomized controlled trial. Sports Health. 2016;8:230-237.
17. Wen DY, Schultz BJ, Schaal B, et al. Eccentric strengthening for chronic lateral epicondylosis: a prospective randomized study. Sports Health. 2011;3:500-503.
18. Yi R, Bratchenko WW, Tan V. Deep friction massage versus steroid injection in the treatment of lateral epicondylitis. Hand (N Y). 2018;13:56-59.
19. Su X, Li Z, Liu Z, et al. Effects of high- and low-energy radial shock waves therapy combined with physiotherapy in the treatment of rotator cuff tendinopathy: a retrospective study. Disabil Rehabil. 2018;40:2488-2494.
20. Barratt PA, Brookes N, Newson A. Conservative treatments for greater trochanteric pain syndrome: a systematic review. Br J Sports Med. 2017;51:97-104.
21. Nguyen L, Kelsberg G, Beecher D, et al. Clinical inquiries: are topical nitrates safe and effective for upper extremity tendinopathies? J Fam Pract. 2014;63:469-470.
22. Steunebrink M, Zwerver J, Brandsema R, et al. Topical glyceryl trinitrate treatment of chronic patellar tendinopathy: a randomised, double-blind, placebo-controlled clinical trial. Br J Sports Med. 2013;47:34-39.
23. Kane TPC, Ismail M, Calder JDF. Topical glyceryl trinitrate and noninsertional Achilles tendinopathy. Am J Sports Med. 2008;36:1160-1163.
24. Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet. 2010;376:1751-1767.
25. Taylor SA, Hannafin JA. Evaluation and management of elbow tendinopathy. Sports Health. 2012;4:384-393.
26. Sawaizumi T, Nanno M, Ito H. De Quervain’s disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 2007;31:265-268.
27. Chen SK, Lu CC, Chou PH, et al. Patellar tendon ruptures in weight lifters after local steroid injections. Arch Orthop Trauma Surg. 2009;129:369-372.
28. Filardo G, Di Matteo B, Kon E, et al. Platelet-rich plasma in tendon-related disorders: results and indications. Knee Surg Sports Traumatol Arthrosc. 2018;26:1984-1999.
29. Cong GT, Carballo C, Camp CL, et al. Platelet-rich plasma in treating patellar tendinopathy. Oper Tech Orthop. 2016;26:110-116.
30. Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol. 2016;26:837-842.
31. DiMatteo B, Filardo G, Kon E, et al. Platelet-rich plasma: evidence for the treatment of patellar and Achilles tendinopathy — a systematic review. Musculoskelet Surg. 2015;99:1-9.
32. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy. Am J Sports Med. 2014;42:610-618.
33. Ellenbecker TS, Nirschl R, Renstrom P. Current concepts in examination and treatment of elbow tendon injury. Sports Health. 2013;5:186-194.
34. Rabago D, Nourani B. Prolotherapy for osteoarthritis and tendinopathy: a descriptive review. Curr Rheumatol Rep. 2017;19:34.
35. Kardouni JR, Seitz AL, Walsworth MK, et al. Neovascularization prevalence in the supraspinatus of patients with rotator cuff tendinopathy. Clin J Sport Med. 2013;23:444-449.
36. Pas HIMFL, Moen MH, Haisma HJ, et al. No evidence for the use of stem cell therapy for tendon disorders: a systematic review. Br J Sports Med. 2017;51:996-1002.
37. Housner JA, Jacobson JA, Misko R. Sonographically guided percutaneous needle tenotomy for the treatment of chronic tendinosis. J Ultrasound Med. 2009;28:1187-1192.
38. Housner JA, Jacobson JA, Morag Y, et al. Should ultrasound-guided needle fenestration be considered as a treatment option for recalcitrant patellar tendinopathy? A retrospective study of 47 cases. Clin J Sport Med. 2010;20:488-490.
39. McShane JM, Nazarian LN, Harwood MI. Sonographically guided percutaneous needle tenotomy for treatment of common extensor tendinosis in the elbow. J Ultrasound Med. 2006;25:1281-1289.
PRACTICE RECOMMENDATIONS
› Recommend eccentric exercises to treat patients with tendinosis; research has consistently shown them to be an effective and safe treatment for many types of this disorder. A
› Use corticosteroid injections with caution for tendinosis; pain relief is typically short lived, and good evidence exists for long-term relapse and worse outcomes including post-injection tendon rupture, especially in the lower extremity. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetic retinopathy: The FP’s role in preserving vision
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
As of 2015, an estimated 30.2 million adults in the United States—12.2% of the population— had diabetes mellitus (DM). During that year, approximately 1.5 million new cases (6.7 cases for every 1000 people) were diagnosed in adults (≥ 18 years of age).1
As the number of people with DM increases, so will the number of cases of diabetic retinopathy, the main cause of new cases of blindness in adults in the United States2 and the leading cause of blindness among US working-age (20 to 74 years) adults.3 It is estimated that 4.1 million Americans have diabetic retinopathy3; it is projected that prevalence will reach 6 million this year.4
Blindness related to DM costs the United States approximately $500 million each year,5 including health care utilization: physician office visits, diagnostic testing, medication and other treatments, and hospitalization.6 Impairment of vision also results in social isolation, dependence on others to perform daily functions, and a decline in physical activity.
Several professional organizations, including the American Diabetes Association and the American Academy of Ophthalmology, have developed practice guidelines for diabetic retinopathy screening. Guidelines notwithstanding, only about 55% of people with DM in the United States receive the recommended dilated eye examination at established intervals.2,3 In addition to screening by an ophthalmologist or optometrist, adherence to clinical guidelines for risk assessment, prevention, and early referral helps reduce the incidence and severity of retinopathy.5
This article describes how to assess the risk of diabetic retinopathy in your patients, details the crucial role that you, the primary care physician, can play in prevention, and emphasizes the importance of referral to an eye specialist for screening, evaluation, treatment (when indicated), and follow-up.
Pathophysiology and classification
Diabetic retinopathy, the result of progressive blood vessel damage to the retina, has 2 major forms: nonproliferative and proliferative. Those forms are distinguished by the absence or presence of new growth of blood vessels (retinal neovascularization).3,7 To improve communication and coordination among physicians who care for patients with DM worldwide, the International Clinical Diabetic Retinopathy Disease Severity Scale for diabetic retinopathy was developed,8-10 comprising 5 levels of severity that are based on findings on dilated ophthalmoscopy (Table 18-10):
- Level 1. No apparent retinopathy. Funduscopic abnormalities are absent.
- Level 2. Mild nonproliferative diabetic retinopathy (NPDR). Only a few microaneurysms are seen.
- Level 3: Moderate NPDR. Characterized by microaneurysms and by intraretinal hemorrhage and venous beading, but less severe than what is seen in Level 4.
- Level 4. Severe NPDR. More than 20 intraretinal hemorrhages in each quadrant of the retina, definite venous beading in > 2 quadrants, intraretinal microvascular abnormalities in > 1 quadrant, or any combination of these findings.
- Level 5. Proliferative diabetic retinopathy. Characterized by neovascularization of the disc, retina, iris, or angle; vitreous hemorrhage; retinal detachment; or any combination of these findings. Further classified as “mild,” “moderate,” or “severe” if macular edema is present; severity is dependent on the distance of thickening and exudates from the center of the macula.9
Be attentive to risk factors
There are several risk factors for diabetic retinopathy, including duration of disease, type 1 DM, male gender, black race (non-Hispanic), elevated hemoglobin A1C(HbA1C) level, elevated systolic and diastolic blood pressure (BP), and insulin therapy. 4,5,11,12
Continue to: Time since diagnosis
Time since diagnosis. The Wisconsin Epidemiologic Study of Diabetic Retinopathy found that the prevalence of diabetic retinopathy varied from 28.8% in people who had DM for < 5 years to 77.8% in people who had DM for ≥ 15 years. The rate of proliferative diabetic retinopathy was 2% in people who had DM for < 5 years and 15.5% in those who had DM for ≥ 15 years.11
Demographic variables. The prevalence of diabetic retinopathy is higher in men, non-Hispanic blacks (38.8%), and people with type 1 DM.4,5,11-13 The Veterans Affairs Diabetes Trial found a higher prevalence of moderate-to-severe diabetic retinopathy in Hispanics (36%) and African Americans (29%) than in non-Hispanic whites (22%).14
Among people with DM who have diabetic retinopathy, systolic and diastolic BP and the HbA1C level tend to be higher. They are more likely to use insulin to control disease.4,5,13 In a recent cross-sectional analysis, the prevalence of vision-threatening retinopathy was higher among people ≥ 65 years of age (1%; 95% confidence interval [CI], 0.7%-1.5%) than among people 40 to 64 years of age (0.4%; 95% CI, 0.3%-0.7%) (P = .009).5
Does pregnancy exacerbate retinopathy? Controversy surrounds the role of pregnancy in the development and progression of diabetic retinopathy. The Diabetes Control and Complications Trial found a short-term increase in the level of retinopathy during pregnancy that persisted into the first postpartum year. A 1.63-fold greater risk of any deterioration of retinopathy was observed in women who received intensive DM treatment from before to during pregnancy (P < .05); pregnant women who received conventional treatment had a 2.48-fold greater risk than nonpregnant women with DM who received conventional treatment (P < .001).
Deterioration of retinopathy during pregnancy had no long-term consequences, however, regardless of type of treatment.15 More importantly, in most cases, changes in the level of retinopathy revert to the pre-pregnancy level after 1 year or longer, and pregnancy does not appear to affect long-term progression of retinopathy.15
Continue to: Proven primary prevention strategies
Proven primary prevention strategies
Glycemic control. Optimal glycemic control is an essential component of prevention of diabetic retinopathy. From 1983 to 1993, the Diabetes Control and Complications Trial randomized 1441 patients with type 1 DM to receive intensive therapy (median HbA1C level, 7.2%) or conventional therapy (median HbA1C level, 9.1%). During a mean of 6 years of follow-up, intensive therapy reduced the adjusted mean risk of retinopathy by 76% (95% CI, 62%-85%).16,17 A 2007 systematic review of 44 studies of the treatment of diabetic retinopathy found that strict glycemic control was beneficial in reducing the incidence and progression of retinopathy.17
The American Diabetes Association’s Standards of Medical Care in Diabetes—2019 Abridged for Primary Care Providers recommends that most nonpregnant adults maintain an HbA1Clevel < 7%. For patients with a history of hypoglycemia, limited life expectancy, advanced microvascular or macrovascular disease, other significant comorbid conditions, or longstanding DM in which it is difficult to achieve the optimal goal, a higher HbA1clevel (< 8%) might be appropriate.18
Control of BP. Strict control of BP is a major modifier of the incidence and progression of diabetic retinopathy.17,19 In the United Kingdom Prospective Diabetes Study, 1148 patients with type 2 DM and a mean BP of 160/94 mm Hg at the onset of the study were randomly assigned to either (1) a “tight” blood pressure group (< 150/85 mm Hg) or (2) a “less-tight” group (< 180/105 mm Hg). The primary therapy for controlling BP was captopril or atenolol. After 9 years of follow-up, the tight-control group had a 34% mean reduction in risk in the percentage of patients with deterioration of retinopathy (99% CI, 11%-50%; P = .0004) and a 47% reduction in risk (99% CI, 7%-70%; P = .004) of deterioration in visual acuity.20
Most patients with DM and hypertension should be treated to maintain a BP < 140/90 mm Hg. Although there is insufficient evidence to recommend a specific antihypertensive agent for preventing diabetic retinopathy, therapy should include agents from drug classes that have a demonstrated reduction in cardiovascular events in patients with DM. These include angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, thiazide diuretics, and dihydropyridine calcium channel blockers.18
Lipid management. The benefit of targeted therapy for lowering lipids for the prevention of diabetic retinopathy is not well established.17 In the Collaborative Atorvastatin Diabetes Study, 2838 patients with type 2 DM were randomized to atorvastatin (10 mg) or placebo; microvascular endpoint analysis demonstrated that patients taking atorvastatin needed less laser therapy (P = .14); however, progression of diabetic retinopathy was not reduced.21 Similarly, in the Action to Control Cardiovascular Risk in Diabetes Eye Study, slowing of progression to retinopathy was observed in patients with type 2 DM who were treated with fenofibrate (ie, progression in 6.5%, compared with progression in 10.2% of untreated subjects [odds ratio = 0.60 (95% CI, 0.42-0.87); P = .0056]).22
Continue to: Despite limited data on...
Despite limited data on the impact of lipid-lowering agents on patients with diabetic retinopathy, those with type 2 DM (especially) and those who have, or are at risk of, atherosclerotic cardiovascular disease should receive statin therapy.18
Aspirin therapy. Aspirin has not been found to be beneficial for slowing progression of diabetic retinopathy. However, aspirin did not cause further deterioration of disease, specifically in patients with vitreous hemorrhages4; patients with diabetic retinopathy who require aspirin therapy for other medical reasons can therefore continue to take it without increasing the risk of damage to the retina.4,18
When should you refer patients for screening?
Screening for diabetic retinopathy is important because affected patients can be asymptomatic but have significant disease. Early detection also helps determine which patients need treatment when it is most beneficial: early in its course.4
Type 1 DM. Retinopathy can become apparent as early as 6 or 7 years after the onset of disease, and is rare in children prior to puberty.4,11 As a result, patients with type 1 DM should first be screened with a comprehensive eye examination by an ophthalmologist or optometrist within 5 years of DM onset.4,18
Type 2 DM. Because of the insidious onset of type 2 DM, patients who are given a diagnosis of DM after 30 years of age might already have high-risk features of retinopathy.9 In patients with type 2 DM, therefore, initial screening for diabetic retinopathy should begin at the time of diagnosis and include a comprehensive eye examination by an ophthalmologist or optometrist.4,18,23
Continue to: Components of the exam
Components of the exam. Initial evaluation by the ophthalmologist or optometrist should include a detailed history and comprehensive eye exam with pupil dilation. Table 24 lists elements of the initial physical exam, which should assess for features that often lead to visual impairment. These features include macular edema, retinal hemorrhage, venous beading, neovascularization, and vitreous hemorrhage.4
Frequency of follow-up. The interval between subsequent examinations should be individualized, based on the findings of the initial assessment. Consider that:
- Screening should occur every 1 or 2 years in patients without evidence of retinopathy and with adequate glycemic control.4,18,23
- Screening every 1 or 2 years appears to be cost-effective in patients who have had 1 or more normal eye exams.
- A 3-year screening interval does not appear to present a risk in well-controlled patients with type 2 DM.24
- Women with type 1 or type 2 DM who are planning pregnancy or who are pregnant should have an eye exam prior to pregnancy or early in the first trimester.4,18,23 They should then be monitored each trimester and at the end of the first postpartum year, depending on the severity of retinopathy.18
Alternative screening modalities
Seven-field stereoscopic fundus photography is an alternative screening tool that compares favorably to ophthalmoscopy when performed by an experienced ophthalmologist, optometrist, or ophthalmologic technician.25 Nonmydriatic digital stereoscopic retinal imaging has been shown to be a cost-effective method of screening patients for diabetic retinopathy.26 In a study that compared digital imaging with dilated funduscopic examination, investigators reported that, of 311 eyes evaluated, there was agreement between the methods in 86% of cases. Disagreement was mostly related to the greater frequency of finding mild-to-moderate NPDR when using digital imaging.27
Screening in primary care
Programs that use telemedicine-based fundus photography to screen for diabetic retinopathy during primary care visits, followed by remote interpretation by an ophthalmologist, have been shown to increase the rate of retinal screening by offering an option other than direct referral to an ophthalmologist or optometrist.28 However, telemedicine-based retinal photography can be successful as a screening tool for retinopathy only if timely referral to an eye specialist is arranged when indicated by findings.18
SIDEBAR
Key points in the progression of diabetic retinopathy care
Duration of diabetes, poor glycemic control, and uncontrolled hypertension are major risk factors for diabetic retinopathy.
To reduce the risk of diabetic retinopathy, patients with diabetes mellitus should:
- sustain good glycemic control (hemoglobin A1C level, < 7%)
- maintain blood pressure < 140/90 mm Hg
- undergo periodic routine screening eye examination.
Early detection of diabetic retinopathy by dilated eye examination or fundus photography can lead to early therapeutic intervention, which can reduce the risk of visual impairment and vision loss.
Treatment is based on severity of disease and can include anti-vascular-endothelial growth factor therapy, photocoagulation, or surgery.
What therapy will your referred patients receive?
Patients found to have signs of diabetic retinopathy should be referred to an ophthalmologist who is knowledgeable and experienced in the management of diabetic retinopathy. Care will be managed according to the severity of the patient’s diabetic retinopathy.
Continue to: Patients with mild-to-moderate NPDR but without macular edema
Patients with mild-to-moderate NPDR but without macular edema. Treatment is generally not recommended. Patients should be reevaluated every 6 to 12 months because they have an increased risk of progression.5
Patients with mild-to-moderate NPDR and clinically significant macular edema (CSME). It is important for the eye specialist to assess for edema at the center of the macula because the risk of vision loss and need for treatment is greater when the center is involved. Vascular–endothelial growth factor (VEGF) is an important mediator of neovascularization and macular edema in diabetic retinopathy. For patients with center-involving CSME, intravitreous injection of an anti-VEGF agent provides significant benefit and is first-line treatment in these cases.4,29
The Early Treatment for Diabetic Retinopathy Study evaluated the efficacy of focal photocoagulation, a painless laser therapy, for CSME and demonstrated that this modality reduces the risk of moderate visual loss; increases the likelihood of improvement in vision; and decreases the frequency of persistent macular edema.30 Focal photocoagulation has been found effective in both non-center-involving CSME and center-involving CSME.5
Patients with severe NPDR. The recommendation is to initiate full panretinal photocoagulation prior to progression to proliferative diabetic retinopathy PDR. Researchers noted a 50% reduction in vision loss and vitrectomy when patients with type 2 DM were treated with panretinal photocoagulation early, compared with those in whom treatment was deferred until PDR developed.4,31 The role of anti-VEGF treatment of severe NPDR is under investigation.4
Patients with high-risk and severe PDR. Panretinal photocoagulation is the recommended treatment for patients with high-risk and severe PDR, and usually induces regression of retinal neovascularization. In patients with CSME and high-risk PDR, the combination of anti-VEGF therapy and panretinal photocoagulation should be considered. Vitrectomy should be considered for patients who have failed panretinal photocoagulation or are not amenable to photocoagulation.4
CORRESPONDENCE
Bryan Farford, DO, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224; Farford.Bryan@mayo.edu.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
1. National Diabetes Statistic Report 2020: Estimates of Diabetes and Its Burden in the United States. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed March 20, 2020.
2. Fitch K, Weisman T, Engel T, et al. Longitudinal commercial claims-based cost analysis of diabetic retinopathy screening patterns. Am Health Drug Benefits. 2015;8:300-308.
3. Centers for Disease Control and Prevention. Common eye disorders. September 29, 2015. www.cdc.gov/visionhealth/basics/ced/index.html. Accessed March 20, 2020.
4. American Academy of Ophthalmology PPP Retina/Vitreous Committee, Hoskins Center for Quality Eye Care. Diabetic Retinopathy PPP 2019. San Francisco, CA: American Academy of Ophthalmology. October 2019. https://www.aao.org/preferred-practice-pattern/diabetic-retinopathy-ppp. Accessed March 20, 2020.
5. Zhang X, Saaddine JB, Chou C-F, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA. 2010;304:649-656.
6. Stewart MW. Socioeconomic cost of diabetic retinopathy and therapy. In: Diabetic Retinopathy. Singapore: Adis; 2017:257-268.
7. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
8. Wilkinson CP, Ferris FL 3rd, Klein RE, et al. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110:1677-1682.
9. Wu L, Fernandez-Loaiza P, Sauma J, et al. Classification of diabetic retinopathy and diabetic macular edema. World J Diabetes. 2013;4:290-294.
10. American Academy of Ophthalmology. International Clinical Diabetic Retinopathy Disease Severity Scale detailed table. October 2002. http://www.icoph.org/downloads/Diabetic-Retinopathy-Detail.pdf. Accessed March 20, 2020.
11. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1984;102:527-532.
12. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217-1228.
13. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXII. The twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868.
14. Emanuele N, Sacks J, Klein R, et al. Ethnicity, race, and baseline retinopathy correlates in the Veterans Affairs Diabetes Trial. Diabetes Care. 2005;28:1954-1958.
15. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. The Diabetes Control and Complications Trial Research Group. Diabetes Care. 2000;23:1084-1091.
16. , , The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986.
17. Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902-916.
18. American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes. 2019;37:11-34.
19. Do DV, Wang X, Vedula SS, et al. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2015;(1):CD006127.
20. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713.
21. Colhoun HM, Betteridge DJ, Durrington PN; CARDS Investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685-696.
22. Chew EY, Davis MD, Danis RP, et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology. 2014;121:2443-2451.
23. Fong DS, Aiello L, Gardner TW, et al American Diabetes Association. Retinopathy in diabetes. Diabetes Care. 2004;27(suppl 1):S84-S87.
24. 11. Microvascular complications and foot care: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S124-S138.
25. Moss SE, Klein R, Kessler SD, et al. Comparison between ophthalmoscopy and fundus photography in determining severity of diabetic retinopathy. Ophthalmology. 1985;92:62-67.
26. Kirkizlar E, Serban N, Sisson JA, et al. Evaluation of telemedicine for screening of diabetic Retinopathy in the Veterans Health Administration. Ophthalmology. 2013;120:2604-2610.
27. Ahmed J, Ward TP, Bursell S-E, et al. The sensitivity and specificity of nonmydriatic digital stereoscopic retinal imaging in detecting diabetic retinopathy. Diabetes Care. 2006;29:2205-2209.
28. Taylor CR, Merin LM, Salunga AM, et al. Improving diabetic retinopathy screening ratios using telemedicine-based digital retinal imaging technology: the Vine Hill study. Diabetes Care. 2007;30:574.
29. , , , Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193-1203.
30. Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):766-785.
31. Ferris F. Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophthalmol Soc. 1996;94:505-537.
PRACTICE RECOMMENDATIONS
› Refer patients with type 1 diabetes mellitus (DM) to an ophthalmologist or optometrist for a dilated and comprehensive eye examination within 5 years of disease onset. B
› Refer patients with type 2 DM to an ophthalmologist or optometrist for an initial dilated and comprehensive eye examination at time of diagnosis. B
› Control blood pressure—ideally, < 140/90 mm Hg—in patients with DM to reduce the risk of diabetic retinopathy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Refining your approach to hypothyroidism treatment
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
CASE
A 38-year-old woman presents for a routine physical. Other than urgent care visits for 1 episode of influenza and 2 upper respiratory illnesses, she has not seen a physician for a physical in 5 years. She denies any significant medical history. She takes naproxen occasionally for chronic right knee pain. She does not use tobacco or alcohol. Recently, she has started using a meal replacement shake at lunchtime for weight management. She performs aerobic exercise 30 to 40 minutes per day, 5 days per week. Her family history is significant for type 2 diabetes mellitus, arthritis, heart disease, and hyperlipidemia on her mother’s side. She is single, is not currently sexually active, works as a pharmacy technician, and has no children. A high-risk human papillomavirus test was normal 4 years ago.
A review of systems is notable for a 20-pound weight gain over the past year, worsening heartburn over the past 2 weeks, and chronic knee pain, which is greater in the right knee than the left. She denies weakness, fatigue, nausea, diarrhea, constipation, or abdominal pain. Vital signs reveal a blood pressure of 146/88 mm Hg, a heart rate of 63 bpm, a temperature of 98°F (36.7°C), a respiratory rate of 16, a height of 5’7’’ (1.7 m), a weight of 217 lbs (98.4 kg), and a peripheral capillary oxygen saturation (SpO2) of 99% on room air. The physical exam reveals a body mass index (BMI) of 34, warm dry skin, and coarse brittle hair.
Lab results reveal a thyroid-stimulating hormone (TSH) level of 11.17 mIU/L (reference range, 0.45-4.5 mIU/L) and a free thyroxine (T4) of 0.58 ng/dL (reference range, 0.8-2.8 ng/dL). A basic metabolic panel and hemoglobin A1C level are normal.
What would you recommend?
In the United States, the prevalence of overt hypothyroidism (defined as a TSH level > 4.5 mIU/L and a low free T4) among people ≥ 12 years of age was estimated at 0.3% based on National Health and Nutrition Examination Survey (NHANES) data from 1999-2002.1 Subclinical hypothyroidism (TSH level > 4.5 mIU/L but < 10 mIU/L and a normal T4 level) is even more common, with an estimated prevalence of 3.4%.1 Hypothyroidism is more common in females and occurs more frequently in Caucasian Americans and Mexican Americans than in African Americans.1
The most common etiologies of hypothyroidism include autoimmune thyroiditis (eg, Hashimoto thyroiditis, atrophic autoimmune thyroiditis) and iatrogenic causes (eg, after radioactive iodine ablation or thyroidectomy) (TABLE 1).2-4
Initiating thyroid hormone replacement
Factors to consider when starting a patient on thyroid hormone replacement include age, weight, symptom severity, TSH level, goal TSH value, adverse effects from thyroid supplements, history of cardiac disease, and, for women of child-bearing age, the desire for pregnancy vs the use of contraceptives. Most adult patients < 50 years with overt hypothyroidism can begin a weight-based dose of levothyroxine: ~1.6 mcg/kg/d (based on ideal body weight).3
Continue to: For adults with cardiac disease...
For adults with cardiac disease, the risk of over-replacement limits initial dosing to 25 to 50 mcg/d for patients < 50 years (12.5-25 mcg/d; ≥ 50 years).3 For adults with subclinical hypothyroidism, it is reasonable to begin therapy at a lower daily dose (eg, 25-75 mcg/d) depending on baseline TSH level, symptoms (the patient may be asymptomatic), and the presence of cardiac disease (TABLE 23,4). Consider treatment in patients with subclinical hypothyroidism particularly when patients have a goiter or dyslipidemia and in women contemplating pregnancy in the near future. Elderly patients may require a dose 20% to 25% lower than younger adults because of decreased body mass.3
Levothyroxine is considered first-line therapy for hypothyroidism because of its low cost, dose consistency, low risk of allergic reactions, and potential to cause fewer cardiac adverse effects than triiodothyronine (T3) products such as desiccated thyroid extract.5 Although data have not shown an absolute increase in cardiovascular adverse effects, T3 products have a higher T3 vs T4 ratio, giving them a theoretically increased risk.5,6 Desiccated thyroid extract also has been associated with allergic reactions.5
Use of liothyronine alone or in combination with levothyroxine lacks evidence and guideline support.4 Furthermore, it is dosed twice daily, which makes it less convenient, and concerns still exist that there may be an increase in cardiovascular adverse effects.4,6 See TABLE 37 for a summary of available products and their equivalent doses.
Maintaining patients on therapy
The maintenance phase begins once hypothyroidism is diagnosed and treatment is initiated. This phase includes regular monitoring with laboratory studies, office visits, and as-needed adjustments in hormone replacement dosing. The frequency at which all of these occur is variable and based on a number of factors including the patient’s other medical conditions, use of other medications including over-the-counter agents, the patient’s age, weight changes, and pregnancy status.3,4,8 In general, dosage adjustments of 12.5 to 25 mcg can be made at 6- to 8-week intervals based on repeat TSH measurements, patient symptoms, and comorbidities.3
Once a patient is symptomatically stable and laboratory values have normalized, the recommended frequency of laboratory evaluation and office visits is every 12 months, barring significant changes in any of the factors mentioned above. At each visit, physicians should perform medication (including supplements) reconciliation and discuss any health condition updates. Changes to the therapy plan, including frequency or timing of laboratory tests, may be necessary if patients begin taking medications that alter the absorption or function of levothyroxine (eg, steroids).
Continue to: To maximize absorption...
To maximize absorption, providers should review with patients the optimal way to take thyroid hormones. Levothyroxine is approximately 70% to 80% absorbed under ideal conditions, which means taking it in the morning at least 30 to 60 minutes before eating or 3 to 4 hours after the last meal of the day.3,9-13 Of note, TSH levels may increase slightly in patients taking proton pump inhibitors, but this does not usually require a dose increase of thyroid hormone.11 Given that some supplements, particularly iron and calcium, can interfere with absorption, it is recommended to maintain a 3- to 4-hour gap between taking those supplements and taking levothyroxine.12-14 For those patients unable or unwilling to adhere to these recommendations, an increase in levothyroxine dose may be required in order to compensate for the decreased absorption.
Don’t adjust hormone therapy based on clinical presentation alone. While clinical symptoms are important, it is not recommended to adjust hormone therapy based solely on clinical presentation. Common hypothyroid symptoms of dry skin, edema, weight gain, and fatigue may be caused by other medical conditions. While indices including Achilles reflex time and basal metabolic rate have shown some correlation to thyroid dysfunction, there has been limited evidence to show that longitudinal index changes reflect subtle changes in thyroid hormone levels.3
The most recent guidelines from the American Thyroid Association recommend that, “Symptoms should be followed, but considered in the context of serum thyrotropin values, relevant comorbidities, and other potential causes.”3
Special populations/circumstances to keep in mind
Malabsorption conditions. When a higher than expected weight-based dose of levothyroxine is required, physicians should review administration timing, adherence, and comorbid medical conditions that can affect absorption.
Several studies, for example, have demonstrated the impact of Helicobacter pylori gastritis on levothyroxine absorption and subsequent TSH levels.15-17 In one nonrandomized prospective study, patients with H pylori and hypothyroidism who were previously thought to be unresponsive to levothyroxine therapy had a decrease in average TSH level from 30.5 mIU/L to 4.2 mIU/L after H pylori was eradicated.15 Autoimmune atrophic gastritis and celiac disease, both of which are more common in those with other autoimmune diseases, are also associated with the need for higher than expected levothyroxine doses.17,18
Continue to: A history of gastric bypass surgery...
A history of gastric bypass surgery alone is not considered a risk factor for poor absorption of thyroid hormone, given that the majority of levothyroxine absorption occurs in the ileum.19,20 However, advancing age (> 70 years) and extreme obesity (BMI > 40) are independent risk factors for decreased levothyroxine absorption.20,21
Women of reproductive age and pregnant women. Overt untreated or undertreated hypothyroidism can be associated with increased risk of maternal and fetal complications including decreased fertility, miscarriage, preterm delivery, lower birth rates, and infant cognitive deficits.3,22 Therefore, the main focus should be optimization of thyroid hormone levels prior to and during pregnancy.3,4,8,22 Thyroid hormone replacement needs to be increased during pregnancy in approximately 50% to 85% of women using thyroid replacement prior to pregnancy, but the dose requirements vary based on the underlying etiology of thyroid dysfunction.
One initial option for patients on a stable dose before pregnancy is to increase their daily dose by a half tablet (1.5 × daily dose) immediately after home confirmation of pregnancy, until finer dose adjustments (usually increases of 25%-60% ) can be made by a physician. Experts recommend that a TSH level be obtained every 4 weeks until mid-gestation and then at least once around 30 weeks’ gestation to ensure specific targets are being met with dose adjustments.22 Optimal thyrotropin reference ranges during conception and pregnancy can be found in the literature.23
Patients who have positive antibodies and normal thyroid function tests. Patients who are screened for thyroid disorders may demonstrate normal thyroid function (ie, euthyroid) with TSH, free T4, and, if checked, free T3, all within normal ranges. Despite these normal lab results, patients may have additional test results that demonstrate positive thyroid autoantibodies including thyroglobulin antibodies and/or thyroid peroxidase antibodies. Thyroid autoimmunity itself has been associated with a range of other autoimmune conditions as well as an increased risk of thyroid cancer in those with Hashimoto thyroiditis.24 Two studies showed that prophylactic treatment of euthyroid patients with levothyroxine led to a reduction in antibody levels and a lower TSH level.25,26 However, no studies have focused on patient-oriented outcomes such as hospitalizations, quality of life, or symptoms. If the patient remains asymptomatic, we recommend no treatment, but that the patient’s TSH levels be monitored every 12 months.27
Elderly patients. Population data have shown that TSH increases normally with age, with a TSH level of 7.5 mIU/L being the upper limit of normal for a population of healthy adults > 80 years of age.28,29 Overall, studies have failed to show any benefit in treating elderly patients with subclinical hypothyroidism unless their TSH level exceeds 10 mIU/L.6,21 The one exception is elderly patients with heart failure in whom untreated subclinical hypothyroidism has been shown to be associated with higher mortality.30
Continue to: Elderly patients are at higher risk...
Elderly patients are at higher risk for adverse effects of thyroid over-replacement, including atrial fibrillation and osteoporosis. While there have been no randomized trials examining target TSH levels in this population, a reasonable recommendation is a goal TSH level of 4 to 6 mIU/L for elderly patients ≥ 70 years.4
CASE
As a result of the patient’s elevated TSH level and symptoms of hypothyroidism, you start levothyroxine 150 mcg/d by mouth, counsel her on potential adverse effects, and schedule a follow-up visit with another TSH check in 6 weeks.
Follow-up laboratory studies 6 weeks later reveal a TSH level of 5.86 mIU/L (reference range, 0.45-4.5 mIU/L) and a free T4 level of 0.74 ng/dL (reference range, 0.8-2.8 ng/dL). Based on those results, you increase the dose of levothyroxine to 175 mcg/d.
At her follow-up visit 12 weeks after initial presentation, her TSH level is 3.85 mIU/L. She reports feeling better overall with less fatigue, and she has lost 5 pounds since her last visit. You recommend she continue levothyroxine 175 mcg/d after reviewing medication compliance with the patient and ensuring she is indeed taking it in the morning, at least 30 minutes prior to eating. With improved but not resolved symptoms, she agrees to follow-up with repeat TSH laboratory studies in 6 weeks to determine whether further dose adjustments are necessary. Given that she is of reproductive age and her TSH level is suboptimal for pregnancy, you caution her about heightened pregnancy/fetal risks with a suboptimal TSH and recommend that she use reliable contraception.
CORRESPONDENCE
Christopher Bunt, MD, FAAFP, 5 Charleston Center Drive, Suite 263, MSC 192,Charleston, SC 29425; buntc@musc.edu
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
1. Aoki Y, Belin RM, Clickner R, et al. Serum TSH and total T4 in the United States population and their association with participant characteristics: National Health and Nutrition Examination Survey (NHANES 1999-2002). Thyroid. 2007;17:1211-1223.
2. Vaidya B, Pearce SH. Management of hypothyroidism in adults. BMJ. 2008;337:a801.
3. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
4. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751.
5. Toft AD. Thyroxine therapy. N Engl J Med. 1994;331:174-180.
6. Floriani C, Gencer B, Collet TH, et al. Subclinical thyroid dysfunction and cardiovascular diseases: 2016 update. Eur Heart J. 2018;39:503-507.
7. Lexi-Comp, Inc. (Lexi-Drugs®). https://online.lexi.com/lco/action/login. Accessed July 7, 2017.
8. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British Thyroid Association Executive Committee. Clin Endocrinol (Oxf). 2016;84:799-808.
9. Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med. 1987;316:764-770.
10. John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption. Thyroid. 2007;17:763-765.
11. Sachmechi I, Reich DM, Aninyei M, et al. Effect of proton pump inhibitors on serum thyroid-stimulating hormone level in euthyroid patients treated with levothyroxine for hypothyroidism. Endocr Pract. 2007;13:345-349.
12. Sperber AD, Liel Y. Evidence for interference with the intestinal absorption of levothyroxine sodium by aluminum hydroxide. Arch Intern Med. 1992;152:183-184.
13. Zamfirescu I, Carlson HE. Absorption of levothyroxine when coadministered with various calcium formulations. Thyroid. 2011;21:483-486.
14. Campbell NR, Hasinoff BB, Stalts H, et al. Ferrous sulfate reduces thyroxine efficacy in patients with hypothyroidism. Ann Intern Med. 1992;117:1010-1013.
15. Bugdaci MS, Zuhur SS, Sokmen M, et al. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130.
16. Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med. 2006;354:1787-1795.
17. Centanni M, Marignani M, Gargano L, et al. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730.
18. Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125:278-282.
19. Azizi F, Belur R, Albano J. Malabsorption of thyroid hormones after jejunoileal bypass for obesity. Ann Intern Med. 1979;90:941-942.
20. Gkotsina M, Michalaki M, Mamali I, et al. Improved levothyroxine pharmacokinetics after bariatric surgery. Thyroid. 2013;23:414-419.
21. Hennessey JV, Espaillat R. Diagnosis and management of subclinical hypothyroidism in elderly adults: a review of the literature. J Am Geriatr Soc. 2015;63:1663-1673.
22. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315-389.
23. Carney LA, Quinlan JD, West JM. Thyroid disease in pregnancy. Am Fam Physician. 2014;89:273-278.
24. Fröhlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521.
25. Aksoy DY, Kerimoglu U, Okur H, et al. Effects of prophylactic thyroid hormone replacement in euthyroid Hashimoto’s thyroiditis. Endocr J. 2005;52:337-343.
26. Padberg S, Heller K, Usadel KH, et al. One-year prophylactic treatment of euthyroid Hashimoto’s thyroiditis patients with levothyroxine: is there a benefit? Thyroid. 2001;11:249-255.
27. Rugge B, Balshem H, Sehgal R, et al. Screening and Treatment of Subclinical Hypothyroidism or Hyperthyroidism [Internet]. Comparative Effectiveness Reviews, No. 24. Rockville, MD: Agency for Healthcare Research and Quality; October 2011. www.ncbi.nlm.nih.gov/books/NBK83492/. Accessed February 21, 2020.
28. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489-499.
29. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575-4582.
30. Pasqualetti G, Tognini S, Polini A, et al. Is subclinical hypothyroidism a cardiovascular risk factor in the elderly? J Clin Endocrinol Metab. 2013;98:2256-2266.
PRACTICE RECOMMENDATIONS
› Prescribe levothyroxine 1.6 mcg/kg/d for healthy adult patients < 50 years of age with overt hypothyroidism. B
› Consider lower initial doses of levothyroxine in patients with cardiac disease (12.5-50 mcg/d) or subclinical hypothyroidism (25-75 mcg/d). B
› Titrate levothyroxine by 12.5 to 25 mcg/d at 6- to 8-week intervals based on thyroid-stimulating hormone measurements, comorbidities, and symptoms. C
› Closely monitor and provide thyroid supplementation to female patients who are pregnant or of reproductive age with concomitant hypothyroidism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Avoiding missteps in BP measurement
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
Blood pressure (BP) measurement is an essential component of the physical examination. The information gleaned through this simple but vitally important assessment provides a basis for critical decisions about diagnosis, prognosis, and therapy in a variety of health care settings. In the emergency department, it helps guide resuscitation efforts; in the intensive care unit, it helps to identify the deteriorating patient and guide vasopressor drug titration; in the ambulatory office setting, it helps to identify hypertension and the need for antihypertensive therapy.
In the office setting, inaccurate BP measurement can have profound effects. An overestimation by only 5 mm Hg would result in an erroneous diagnosis and unnecessary treatment of hypertension for about 27 million patients—entailing medication costs, potential adverse effects, and psychologic issues associated with this diagnosis. Conversely, underestimation by 5 mm Hg would miss about 21 million patients who actually have hypertension.1
Why accurate BP measurement matters so much
About 75 million adults in the United States have high BP,2 which costs the nation $46 billion annually in health care services, antihypertensive medications, and missed days of work.3 Among US adults ages 20 or older, the age-adjusted prevalence of hypertension is estimated to be 34%, equivalent to 85.7 million adults.4
Defining hypertension. For the general population, the Eighth Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defines hypertension as a BP of 140/90 mm Hg or higher in adults younger than 60 and a BP of 150/90 mm Hg or higher in adults ages 60 or older. For patients with comorbid hypertension and diabetes, JNC-8 recommends pharmacologic treatment when BP is 140/90 mm Hg or higher, regardless of age.5
Accurate measurement of BP provides the rational basis for the management of hypertension, which in turn may decrease the risk for stroke, congestive heart failure, and other cardiovascular diseases. Several investigators6-8 have observed that differences in interarm systolic BP are associated with an increased risk for peripheral vascular disease, stroke, and other cardiovascular problems.
Multiple factors impact accuracy; some might surprise you
A number of factors may influence the accuracy of BP measurement in the office; these are generally classified as related to the patient, the observer, the technique or procedure, or the equipment used. A recent systematic review by Kallioinen et al9 empirically evaluated 29 potential sources of inaccuracy in the measurement of adult resting BP. Among them were
Patient-related: Recent meal or alcohol intake; recent caffeine or nicotine use; full bladder distention; cold exposure; white-coat effect. Given the simplicity of assessing for these influences, it is worthwhile for office staff to ask patients, prior to the recommended 3 to 5 minutes of rest before BP measurement, if they were rushing to make their appointment, need to void their bladder, or have consumed food or drink or used tobacco within the past 30 minutes.
Continue to: Observer-related...
Observer-related: Hearing deficit; terminal digit bias (ie, preference for rounding BP reading to a specific end digit, eg, 0); measurement of diastolic BP at Korotkoff phase IV rather than phase V.
Procedure-related: Patient’s body position (eg, standing vs supine; legs crossed at knee; unsupported back or arm; arm lower than heart level); incorrect size or placement of cuff; talking during measurement (the content of conversation may influence results); and reliance on a single BP measurement.
Equipment-related: Device model bias; device calibration error.
As reported by Kallioinen et al9, the magnitude of these potential errors ranges from small to large in both the positive and negative direction for both systolic and diastolic BP, and several sources of error are potentially bidirectional. For example, talking during BP measurement may result in an increase in systolic BP of 4 to 19 mm Hg and in diastolic BP of 5 to 14.3 mm Hg; measurement of diastolic BP at Korotkoff phase IV rather than phase V significantly increases diastolic BP by 12.5 mm Hg; and recent alcohol intake can affect systolic BP by –23.6 to +24 mm Hg and diastolic BP by –14 to +16 mm Hg. Overall, the researchers found significant directional effects for 27 of the 29 potential sources of error, ranging from a mean –24 mm Hg to +33 mm Hg error for estimating systolic BP and a mean –14 mm Hg to +23 mm Hg for estimating diastolic BP.9
Careful adherence to guidelines ensures accurate BP measurement
Adequate training and standardized procedures can target and mitigate many of the identified sources of error; accordingly, all clinical staff responsible for obtaining a patient’s BP measurement should be trained not only in the correct method for accurate measurement but also in the identification of factors that may introduce errors.
Continue to: The American Heart Association...
The American Heart Association (AHA) recommends that BP be measured in both arms at the initial evaluation, with the higher measurement used for monitoring BP. The AHA also recommends obtaining at least 2 readings at least 1 minute apart and averaging them as the patient’s BP.10 Other research recommends using a fully automated sphygmomanometer to take multiple readings with the patient resting quietly alone in either the exam room or the waiting room11 as an effective and efficient method for accurate BP averaging.
The 2 principal noninvasive methods of BP measurement are the manual auscultatory technique and the oscillatory technique. Because of its simplicity and relative degree of accuracy (when correctly performed), the auscultatory measurement remains common in everyday medical practice. Remarkably, it is one of only a few techniques for clinical examination of patients that has remained relatively unchanged since it was introduced by the Russian physician and scientist Nikolai Sergeevich Korotkoff in 1905.12 However, accurate performance of the auscultatory method requires adequate training and experience.
In contrast, automated oscillometric BP measurement is easily performed and requires minimal training. However, it is important to note that any condition altering oscillation amplitude or regularity (eg, arterial wall stiffness or cardiac arrhythmia) will produce erroneous results, and the reading must be confirmed by auscultatory measurement.13, 14
Auscultatory methods of BP measurement
The mainstay of clinical BP measurement has been auscultatory methods to detect the Korotkoff sounds, using a stethoscope and either mercury, aneroid, or “hybrid” sphygmomanometers. Traditionally, the mercury device was the “gold standard,” but the widespread ban of mercury in health care settings has now all but eliminated its use.
Aneroid gauge sphygmomanometers have a metallic spring and a metal membrane that flexes elastically to translate pressure signals from the cuff and operate a needle in the gauge. Owing to their complexity, these devices require regular recalibration, since inaccurate results may occur anytime the needle does not rest on 0 before use.
Contine to: The newer hybrid sphygmomanometers...
The newer hybrid sphygmomanometers have an electronic transducer in place of a mercury column; BP measurement is performed in the same fashion as with a mercury device, using a stethoscope and auscultation for the Korotkoff sounds.
Variations in technique for BP measurement can result in significantly different readings. In 2005, the AHA published recommendations for BP monitoring to increase the accuracy of in-clinic measurements.10 Recommendations for accurate BP measurement include:
Patient preparation. The patient should be seated in a chair with his or her back supported, legs uncrossed, and feet flat on the floor. The patient’s bare arm should be supported such that the midpoint of the upper arm is at heart level. An appropriately sized cuff (ie, bladder encircles 80% of the arm for an adult or 100% of the arm for a child younger than 13 years) should be secured around the bare upper arm and the bladder centered over the brachial artery, with the lower edge of the cuff about 2 cm above the antecubital fossa.10
Technique. The cuff is inflated while palpating the radial artery to the approximate systolic pressure (ie, the point at which the radial pulse is no longer palpated). The bell of the stethoscope is placed just proximal and medial to the antecubital fossa and the cuff is inflated another 20 to 30 mm Hg above the point at which the radial pulse is no longer felt. The cuff is deflated at a rate of about 2 mm Hg per second.10
BP recording. The systolic BP is recorded at the appearance of the Korotkoff sounds (phase I) for an auscultatory measurement. The diastolic BP is recorded at the disappearance of the Korotkoff sounds (phase V) in adults and at the muffling of sounds (phase IV) in children for an auscultatory measurement.10
Continue to: Oscillometric methods of BP measurement
Oscillometric methods of BP measurement
The auscultatory methods of BP measurement are gradually being replaced by oscillometric techniques that are better suited to automated methods of measurement. When oscillations of pressure in the gradually deflating bladder cuff are sensed and recorded, the point of maximal oscillation corresponds to the mean intra-arterial pressure.15 The oscillations sensed are vibrations in the arterial wall that are detected and transduced to an electric signal, producing a digital readout, and correspond approximately to the systolic pressure and continue below the diastolic pressure. The actual systolic and diastolic pressures are indirectly estimated according to a proprietary, empirically derived algorithm that differs from 1 manufacturer to another.
Validated oscillometric techniques have been successfully used in ambulatory BP monitors, which record pressure at regular intervals (typically 20 to 30 minutes) over a 24-hour period while patients perform normal daily activities, including sleep. The US Preventive Services Task Force16, the UK’s National Institute for Health and Clinical Excellence17, the European Society of Hypertension18, and the Canadian Hypertension Education Program19 collectively endorse ambulatory BP monitoring as the optimal method for BP measurement.
The oscillometric method has also been used for automated office BP measurement, which averages multiple BP readings recorded with a fully automated device while the patient rests alone in a quiet room in clinic. Compared with conventional auscultatory office BP measurement, this method has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect.20-22
There are some limitations to oscillometric methods. The amplitude of oscillations is influenced by factors other than BP, notably, arterial wall stiffness. Therefore, in older patients13 or those with diabetes14 who have reduced arterial wall elasticity, oscillometric BP measurements overestimate systolic pressure and underestimate diastolic pressure. In contrast, acutely ill patients, particularly those with hypovolemia and more compliant arterial walls, may have significant underestimation of BP by oscillometric techniques.23 In patients with peripheral arterial disease, calcified leg vessels can affect the diagnostic accuracy of oscillometric measurement of the ankle-brachial index (ABI).24 A meta-analysis reported that in patients with atrial fibrillation, oscillometric measurement accurately assesses systolic BP but not diastolic BP, and therefore it may be inappropriate for office measurement of BP in these patients.25 Other studies have reported that atrial fibrillation does not significantly affect the accuracy of oscillometric BP measurement if 3 repeated measurements are performed.26,27
Moreover, the algorithms used in these devices are proprietary trade secrets that can be modified by the manufacturer at any time without notice. Therefore, different devices—and even different models from the same manufacturer—may function differently. Only devices calibrated using a validated protocol should be used.10,28 There are currently 4 unique protocols for validation of BP devices, although an international collaborative group recently published recommendations for a universal protocol for validation of BP measurement devices.29
Continue to: The takeaway
The takeaway
Accurate office BP measurement is essential for patient evaluation and provides the basis for critical decisions about diagnosis, prognosis, and treatment of hypertensive disease. It is imperative to control for factors that may introduce error in BP determination by using a standard protocol and calibrated BP measurement equipment.
Both manual auscultatory and oscillometric methods of measurement are appropriate for office assessment, but oscillometric evaluation is inappropriate for patients with severe atherosclerotic disease, peripheral arterial disease (for ABI), or small arm circumference. If oscillometric BP measurement is performed in patients with atrial fibrillation, at least 3 repeated measurements should be done to improve accuracy. Automated oscillometric BP assessment that records multiple measurements in the quietly resting patient has been promoted to provide a more standardized BP measurement by reducing observer error and the “white coat” effect. Ambulatory oscillometric BP monitoring has been widely endorsed as the optimal method for BP measurement.
CORRESPONDENCE
Darrell R. Over, MD, MSc, FAAFP, 1601 West 40th Street, Pine Bluff, AR 71603; OverDarrellR@uams.edu
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.
1. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
2. Meral R, Rakotz M, Bausch P, et al. CDC Grand Rounds: a public health approach to detect and control hypertension. Morb Mortal Wkly Rep. 2016;18:65:1261-1264.
3. Mozzafarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131:e29-e322.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146-e603.
5. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
6. Weinberg I, Gona P, O’Donnell CJ, et al. The systolic blood pressure difference between arms and cardiovascular disease in the Framingham study. Am J Med. 2014;127:209-215.
7. Lane D, Beevers M, Barnes N, et al. Interarm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089-1095.
8. Clark CE, Taylor RS, Shore AC, et al. The difference in blood pressure readings between arms and survival: primary cohort study. BMJ. 2012;344:e1327. [Erratum in BMJ. 2012;344:e2714.]
9. Kallioinen N, Hill A, Horswill MS, et al. Sources of inaccuracy in measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hyertens. 2017;35:421-441.
10. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142-161.
11. Armstrong D, Matangi M, Brouillard D, et al. Automated office blood pressure—being alone and not location is what matters most. Blood Pressure Monit. 2015;20:204-208.
12. Shevchenko YL, Tsitlik JE. 90th anniversary of the development by Nikolai S. Korotkoff of the auscultatory method of measuring blood pressure. Circulation. 1996;94:116-118.
13. Van Montfrans GA. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287-290.
14. Van Popele NM, Bos WJ, de Beer NA, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484-488.
15. Mauck GW, Smith CR, Geddes LA, et al. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure—part ii. J Biomech Eng. 1980;102:28-33.
16. Siu AL; US Preventive Services Task Force. Screening for high blood pressure in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
17. National Institute for Health and Clinical Excellence (NICE). Hypertension: the clinical management of hypertension in adults. London: Royal College of Physicians (UK); 2011.
18. O’Brien E, Parati G, Stergiou G, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731-1768.
19. Leung AA, Nerenberg K, Daskalopoulou SS, et al; CHEP Guidelines Task Force. Hypertension Canada’s 2016 Canadian Hypertension Education Program guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol. 2016;32:569-588.
20. Myers MG. Eliminating the human factor in office blood pressure measurement. J Clin Hypertens. 2014;16:83-86.
21. Myers MG, Godwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. J Clin Hypertens. 2010;55:195-200.
22. Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27:280-286.
23. Bur A, Herkner H, Vlcek M, et al. Factors influencing the accuracy of oscillometric blood pressure measurements in critically ill patients. Crit Care Med. 2003;31:793-799.
24. Herrálz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. Diagnostic accuracy of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One. 2016;11:e0167408.
25. Stergiou GS, Kollias A, Destounis A, et al. Automated blood pressure measurement in atrial fibrillation: a systematic review and meta-analysis. J Hypertens. 2012;30:2074-2082.
26. Pagonas N, Schmidt S, Eysel J, et al. Impact of atrial fibrillation on the accuracy of oscillometric blood pressure monitoring. Hypertension. 2013;62:579-584.
27. Myers MG, Stergiou GS. Should oscillometric blood pressure monitors be used in patients with atrial fibrillation? J Clin Hypertens. 2015;17:565-566.
28. Munter P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans. a scientific statement from the American Heart Association. Hypertension. 2019;73:e35-e66.
29. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71:368-374.
Conservative care or surgery for rotator cuff tears?
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
Rotator cuff disease accounts for as many as 65% of shoulder-related visits to physicians’ offices,1 yet the natural course of rotator cuff tears is still not well understood.2 Treatment options are controversial because both conservative and surgical management have been successful. Physical therapy is a durable and reliable treatment option, but there are concerns about long-term progression of the tear.3 Surgical arthroscopic techniques, which result in less morbidity than open surgery, have improved overall surgical care; as such, the rate of rotator cuff procedures has increased significantly.4
Our goal in this article is to provide clinical guidance to the primary care provider. We review management options for rotator cuff injury; summarize considerations for proceeding with conservative or surgical management; and discuss surgical risks and complications.
Conservative management: Who is most likely to benefit?
The choice of treatment for rotator cuff injury depends on a host of variables, including shoulder dominance, duration of symptoms, type of tear (partial or full), age, demands (activity level, occupation, sport), and comorbidities (diabetes, tobacco use). Treatment goals include resolution of pain, normalized range of motion and strength, and restored arm and shoulder function.5
Initial nonoperative management is indicated in patients who
- have a partial-thickness tear (a notable exception is young patients with traumatic injury),6
- have lower functional demands and moderate symptoms, or
- refuse surgery.7
Patients who respond to nonoperative management will, typically, do so within 6 to 12 weeks.5,8
Few randomized, controlled trials have compared conservative and surgical management of rotator cuff tears; furthermore, the findings of these studies have been mixed. Nonoperative management has been shown to be the favored initial treatment for isolated, symptomatic, nontraumatic, supraspinatus tears in older patients.9 In a recent study,10 5-year outcomes were examined in a prospective cohort enrolled in a rotator cuff treatment program: Approximately 75% of patients remained successfully treated with nonoperative management, and clinical outcomes of the operative and nonoperative groups were not significantly different at 5-year follow-up. Investigators concluded that nonoperative treatment is effective for many patients who have a chronic, full-thickness rotator cuff tear.
In a study investigating the treatment of degenerative rotator cuff tear, patients were randomly treated using an operative or nonoperative protocol. No differences in functional outcomes were observed at 1 year after treatment; however, surgical treatment significantly improved subjective parameters of pain and disability.11 A similar study suggested statistically significant improvement in outcomes for patients managed operatively, compared with those treated nonoperatively, but differences in shoulder outcome and the visual analog pain score were small and failed to meet thresholds considered clinically significant. Larger studies, with longer follow-up, are required to determine whether clinical differences between these types of treatment become more evident over time.12
Continue to: A look at nonoperative options and outcomes
A look at nonoperative options and outcomes
Surveillance. Rotator cuff disease of the supraspinatus tendon often results from a degenerative process that progresses to partial and, eventually, full-thickness tearing.8 Once a tear develops, progression is difficult to predict. Many rotator cuff tears grow larger over time; this progression is commonly associated with new or increased pain and weakness, or both. Although asymptomatic progression of a tear is uncommon, many patients—and physicians—are apprehensive about proceeding with nonoperative treatment for a full-thickness tear.8
To diminish such fears, surveillance can include regular assessment of shoulder motion and strength, with consideration of repeat imaging until surgery is performed or the patient is no longer a surgical candidate or interested in surgical treatment.7 Patients and providers need to remain vigilant because tears that are initially graded as repairable can become irreparable if the tendon retracts or there is fatty infiltration of the muscle belly. Results of secondary surgical repair following failed prolonged nonoperative treatment tend to be inferior to results seen in patients who undergo primary tendon repair.7
Analgesics. Simple analgesics, such as acetaminophen, are a low-risk first-line option for pain relief; however, there are limited data on the efficacy of acetaminophen in rotator cuff disease. A topical or oral nonsteroidal anti-inflammatory drug (NSAID), or both, can be considered, but potential contraindications, such as gastrointestinal, renal, and cardiovascular risks, should be monitored.13 Avoid opioids, given the potential for abuse, except during the immediate postoperative period.5
Glucocorticoid injection. Injection of a glucocorticoid drug into the subacromial space should be considered in patients whose pain interferes with sleep, limits activities of daily living, or hinders the ability to participate in physical therapy.5 A recent systematic review demonstrated that NSAIDs and glucocorticoids brought similar pain relief and active abduction at 4 to 6 weeks, but that glucocorticoids were significantly better at achieving remission of symptoms.14 There are no data comparing glucocorticoid preparations (ie, different glucocorticoids or anesthetics, dosages, volumes), and ultrasound guidance does not appear to be necessary for short-term pain relief.15 Note: Repeated injection has been shown to decrease the durability of surgically repaired tendons16; if a patient is a candidate for surgery, repeat injections should be carefully considered—and avoided if possible.
Physical therapy. The goals of physical therapy are activity modification, stretching the shoulder capsule, and strengthening the surrounding musculature (periscapular, rotator cuff, and deltoid). Patients advance through 3 phases of recovery: shoulder mobility, strengthening, and function (ie, joint reactivation to improve shoulder proprioception and coordination).
Continue to: A recent meta-analysis...
A recent meta-analysis17 found comparative evidence on treating rotator cuff tears with physical therapy to be inconclusive. At 1-year follow-up, there was no clinically significant difference between surgery and active physical therapy in either improving the Constant Shoulder Score (an assessment of function) or reducing pain caused by a rotator cuff tear. Therefore, the authors proposed, given the low risk of harm, a conservative approach should be the initial treatment modality for a tear.
A Cochrane review18 examined 60 eligible trials, in which the mean age of patients was 51 years and the mean duration of symptoms, 11 months. Overall, the review concluded that the effects of manual therapy and exercise might be similar to those of glucocorticoid injection and arthroscopic subacromial decompression. The authors noted that this conclusion is based on low-quality evidence, with only 1 study in the review that compared the combination of manual therapy and exercise to placebo.
Other conservative options. Ultrasound, topical nitroglycerin, topical lidocaine, glucocorticoid iontophoresis, transcutaneous electrical nerve stimulation, massage, acupuncture, extracorporeal shockwave therapy, hyaluronic acid, and platelet-rich plasma have been used to treat rotator cuff disease. These modalities require further study, however, to determine their effectiveness for this indication.7,19
Who is a candidate for surgical management?
Although nonoperative treatment is preferred for rotator cuff tendinitis or tendinosis and partial-thickness tears, appropriate management of full-thickness tears is debatable.20 Some surgeons advocate early operative intervention of repairable full-thickness tears to prevent further progression and reduce the risk of long-term dysfunction.
The decision to pursue operative repair depends on
- patient characteristics (age, activity level, comorbidities),
- patient function (amount of disability caused by the tear),
- characteristics of the tear (length, depth, retraction), and
- chronicity of the tear (acuity).
Continue to: TABLE 1...
TABLE 121,22 highlights variables that influence the decision to proceed, or not to proceed, with operative intervention. Because enlargement of a tear usually exacerbates symptoms,23 patients with a tear who are successfully managed nonoperatively should be counseled on the potential of the tear to progress.
What are the surgical options?
Little clinical evidence favors one exposure technique over another. This equivalency has been demonstrated by a systematic review of randomized controlled trials comparing arthroscopic and mini-open rotator cuff repair, which showed no difference in function, pain, or range of motion.24 That conclusion notwithstanding, arthroscopic repair is increasingly popular because it results in less pain, initially, and faster return to work.20
There is controversy among surgeons regarding the choice of fixation technique: Tendons can be secured using 1 or 2 rows of anchors (FIGURE). Advocates of single-row repair cite shorter surgical time, decreased cost, and equivalent outcomes; surgeons who favor double-row, or so-called transosseous-equivalent, repair claim that it provides better restoration of normal anatomy and biomechanical superiority.25,26
Regardless of technique, most patients are immobilized for 4 to 6 weeks postoperatively.27 Physical therapy usually commences within the first week or 2 postop, limited to passive motion for 6 to 12 weeks. Active motion and strengthening of rotator-cuff muscles often is initiated by 3 months postop, although this phase is sometimes delayed because of concern over slow tendon healing. Typically, patients make a full return to sports and manual work at 6 months postop. Patients experience most symptomatic improvement during the first 6 months following surgery, although functional gains can be realized for as long as 2 years after surgery.28
Most torn rotator cuffs can be fixed back to the greater tuberosity, but some chronic, massive, retracted tears lack the mobility to be repaired, or re-tear shortly after repair. Over time, the humeral head in a rotator cuff–deficient shoulder can migrate superiorly to abut the undersurface of the acromion, which can lead to significant glenohumeral osteoarthritis. To prevent or remedy elevation of the humeral head, salvage procedures—debridement, partial repair, spanning graft, tendon transfer, superior capsule reconstruction, balloon arthroplasty, reverse total shoulder replacement—can be used to alleviate pain and restore function. These procedures have significant limitations, however, and usually provide less favorable outcomes than standard repair.29-35
Continue to: Surgical outcomes
Surgical outcomes
Pain, function, and patient satisfaction outcomes following rotator cuff repair are generally favorable: 90% of patients are “happy” 6 months postop.28 Younger populations often have traumatic rotator cuff tears; they generally are interested in returning to sporting activities following their injury. Nearly 85% of younger patients who undergo rotator cuff repair return to sports, and 65.9% return to an equivalent level of play.36
Variables associated with an unfavorable outcome include increasing age, smoking, increased size of the tear, poor tendon quality, hyperlipidemia, workers’ compensation status, fatty infiltration of muscle, obesity, diabetes, and additional procedures to the biceps tendon and acromioclavicular joint performed at the time of rotator cuff repair.37-39 Interestingly, a study concluded that, if a patient expects a good surgical outcome, they are more likely to go on to report a favorable outcome—suggesting that a patient’s expectations might influence their actual outcome.40
Risks and complications
Although rotator cuff surgery has much lower morbidity than other orthopedic surgeries, it is not without risk of complications. If re-tears are excluded, postop complications have been reported in approximately 10% of patients.41 Common complications and their anticipated rate of occurrence are listed in TABLE 2.42-49
Re-tear of the surgically repaired tendon is the most common postop complication. Published re-tear rates range from 20% to 96%42,43 and generally correlate with initial tear size: A small tear is twice as likely to heal as a massive tear.50 That large range—a span of 76%—results from using a variety of methods to measure re-tear and might not have clinical meaning. A meta-analysis that examined more than 8000 shoulder surgeries reported an overall re-tear rate of 26.6%; however, both patients whose tendons healed and those who re-tore demonstrated clinical improvement.51 In a separate study, patients reported improvement in pain, function, range of motion, and satisfaction regardless of the integrity of the tendon; however, significant improvement in strength was seen only in those whose repair had healed.52
Postop stiffness is more common with arthroscopic repair than with open surgery, and with smaller rather than larger tears.53 Patient variables associated with an increased risk of postop adhesive capsulitis include workers’ compensation insurance, age < 50 years, and preoperative calcific tendonitis or adhesive capsulitis.53 Stiffness generally responds to physical therapy and rarely requires surgical lysis of adhesions or capsular release.
Continue to: Significant injury...
Significant injury to the deltoid muscle has become increasingly uncommon with the advancement of arthroscopic surgery. In traditional open surgery, detachment of the deltoid (and subsequent repair) is required to improve visualization; however, doing so can lead to atrophy and muscle rupture and dehiscence. Deltoid damage occurs in ≤ 60% of open surgeries but is negligible in arthroscopic and mini-open repairs, which involve splitting deltoid fibers to gain exposure of the underlying rotator cuff.54
SIDEBAR
Key takeaways in the management of rotator cuff injury
- Chronic, nontraumatic, and partial-thickness tears respond well to conservative management as first-line treatment. Poor surgical candidates should also be offered a trial of conservative therapy.
- Consider referral for surgical consultation if the patient does not respond to conservative therapy in 6 to 12 weeks; also, patients who have a full-thickness tear and young patients with traumatic injury should be referred for surgical consultation.
- Arthroscopy has become the preferred approach to rotator cuff repair because it is associated with less pain, fewer complications, and faster recovery.
- Patients should be counseled that recovery from surgical repair of a torn rotator cuff takes, on average, 6 months. Some massive or retracted rotator cuff injuries require more extensive procedures that increase healing time.
- Overall, patients are “happy” with rotator cuff repair at 6 months; clinical complications are uncommon, making surgery a suitable option in appropriately selected patients.
CORRESPONDENCE
Cayce Onks, DO, MS, ATC, Penn State Health Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; conks@pennstathealth.psu.edu.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
1. Vecchio P, Kavanagh R, Hazleman BL, et al. Shoulder pain in a community-based rheumatology clinic. Br J Rheumatol. 1995;34:440-442.
2. Eljabu W, Klinger HM, von Knoch M. The natural history of rotator cuff tears: a systematic review. Arch Orthop Trauma Surg. 2015;135:1055-1061.
3. Dunn WR, Kuhn JE, Sanders R, et al; MOON Shoulder Group. 2013 Neer Award: predictors of failure of nonoperative treatment of chronic, symptomatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2016;25:1303-1311.
4. Colvin AC, Egorova N, Harrison AK, et al. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94:227-233.
5. Whittle S, Buchbinder R. In the clinic. Rotator cuff disease. Ann Intern Med. 2015;162:ITC1-ITC15.
6. Lazarides AL, Alentorn-Geli E, Choi JHJ, et al. Rotator cuff tears in young patients: a different disease than rotator cuff tears in elderly patients. J Shoulder Elbow Surg. 2015;24:1834-1843.
7. Petri M, Ettinger M, Brand S, et al. Non-operative management of rotator cuff tears. Open Orthop J. 2016;10:349-356.
8. Schmidt CC, Jarrett CD, Brown BT. Management of rotator cuff tears. J Hand Surg Am. 2015;40:399-408.
9. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of nontraumatic rotator cuff tears: a randomized controlled trial with two years of clinical and imaging follow-up. J Bone Joint Surg Am. 2015;97:1729-1737.
10. Boorman RS, More KD, Hollinshead RM, et al. What happens to patients when we do not repair their cuff tears? Five-year rotator cuff quality-of-life index outcomes following nonoperative treatment of patients with full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2018;27:444-448.
11. Lambers Heerspink FO, van Raay JJ, Koorevaar RCT, et al. Comparing surgical repair with conservative treatment for degenerative rotator cuff tears: a randomized controlled trial. J Shoulder Elbow Surg. 2015;24:1274-1281.
12. Piper CC, Hughes AJ, Ma Y, et al. Operative versus nonoperative treatment for the management of full-thickness rotator cuff tears: a systematic review and meta-analysis. J Shoulder Elbow Surg. 2018;27:572-576.
13. Boudreault J, Desmeules F, Roy J-S, et al. The efficacy of oral non-steroidal anti-inflammatory drugs for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 2014;46:294-306.
14. Zheng X-Q, Li K, Wei Y-D, et al. Nonsteroidal anti-inflammatory drugs versus corticosteroid for treatment of shoulder pain: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2014;95:1824-1831.
15. Bloom JE, Rischin A, Johnston RV, et al. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev. 2012;(8):CD009147.
16. Wiggins ME, Fadale PD, Ehrlich MG, et al. Effects of local injection of corticosteroids on the healing of ligaments. A follow-up report. J Bone Joint Surg Am. 1995;77:1682-1691.
17. Ryösä A, Laimi K, Äärimaa V, et al. Surgery or conservative treatment for rotator cuff tear: a meta-analysis. Disabil Rehabil. 2017;39:1357-1363.
18. Page MJ, Green S, McBain B, et al. Manual therapy and exercise for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012224.
19. Page MJ, Green S, Mrocki MA, et al. Electrotherapy modalities for rotator cuff disease. Cochrane Database Syst Rev. 2016;(6):CD012225.
20. Acevedo DC, Paxton ES, Williams GR, et al. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96:e123.
21. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94:163-167.
22. Thorpe A, Hurworth M, O’Sullivan P, et al. Rotator cuff disease: opinion regarding surgical criteria and likely outcome. ANZ J Surg. 2017;87:291-295.
23. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92:2623-2633.
24. Ji X, Bi C, Wang F, et al. Arthroscopic versus mini-open rotator cuff repair: an up-to-date meta-analysis of randomized controlled trials. Arthroscopy. 2015;31:118-124.
25. Duquin TR, Buyea C, Bisson LJ. Which method of rotator cuff repair leads to the highest rate of structural healing? A systematic review. Am J Sports Med. 2010;38:835-841.
26. Choi S, Kim MK, Kim GM, et al. Factors associated with clinical and structural outcomes after arthroscopic rotator cuff repair with a suture bridge technique in medium, large, and massive tears. J Shoulder Elbow Surg. 2014;23:1675-1681.
27. Shen C, Tang Z-H, Hu J-Z, et al. Does immobilization after arthroscopic rotator cuff repair increase tendon healing? A systematic review and meta-analysis. Arch Orthop Trauma Surg. 2014;134:1279-1285.
28. Gulotta LV, Nho SJ, Dodson CC, et al; . Prospective evaluation of arthroscopic rotator cuff repairs at 5 years: part I. Functional outcomes and radiographic healing rates. J Shoulder Elbow Surg. 2011;20:934-940.
29. Liem D, Lengers N, Dedy N, et al. Arthroscopic debridement of massive irreparable rotator cuff tears. Arthroscopy. 2008;24:743-748.
30. Weber SC. Partial rotator cuff repair in massive rotator cuff tears: long-term follow-up. J Shoulder Elbow Surg. 2017;26:e171.
31. Lewington MR, Ferguson DP, Smith TD, et al. Graft utilization in the bridging reconstruction of irreparable rotator cuff tears: a systematic review. Am J Sports Med. 2017;45:3149-3157.
32. Longo UG, Franceschetti E, Petrillo S, et al. Latissimus dorsi tendon transfer for massive irreparable rotator cuff tears: a systematic review. Sports Med Arthrosc Rev. 2011;19:428-437.
33. Noyes MP, Denard PJ. Arthroscopic superior capsular reconstruction: indications and outcomes. Oper Tech Sports Med. 2018;26:29-34.
34. Piekaar RSM, Bouman ICE, van Kampen PM, et al. Early promising outcome following arthroscopic implantation of the subacromial balloon spacer for treating massive rotator cuff tear. Musculoskeletal Surg. 2018;102:247-255.
35. Ek ETH, Neukom L, Catanzaro S, et al. Reverse total shoulder arthroplasty for massive irreparable rotator cuff tears in patients younger than 65 years old: results after five to fifteen years. J Shoulder Elbow Surg. 2013;22:1199-1208.
36. Klouche S, Lefevre N, Herman S, et al. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44:1877-1887.
37. Garcia GH, Liu JN, Wong A, et al. Hyperlipidemia increases the risk of retear after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2017;26:2086-2090.
38. Khair MM, Lehman J, Tsouris N, et al. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J. 2016;12:170-176.
39. Lambers Heerspink FO, Dorrestijn O, van Raay JJAM, et al. Specific patient-related prognostic factors for rotator cuff repair: a systematic review. J Shoulder Elbow Surg. 2014;23:1073-1080.
40. Henn RF 3rd, Kang L, Tashjian RZ, et al. Patients’ preoperative expectations predict the outcome of rotator cuff repair. J Bone Joint Surg Am. 2007;89:1913-1919.
41. Mansat P, Cofield RH, Kersten TE, et al. Complications of rotator cuff repair. Orthop Clin North Am. 1997;28:205-213.
42. Boileau P, Brassart N, Watkinson DJ, et al. Arthroscopic repair of full-thickness tears of the supraspinatus: does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229-1240.
43. Galatz LM, Ball CM, Teefey SA, et al. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219-224.
44. Aydin N, Kocaoglu B, Guven O. Single-row versus double-row arthroscopic rotator cuff repair in small- to medium-sized tears. J Shoulder Elbow Surg. 2010;19:722-725.
45. Peltz CD, Dourte LM, Kuntz AF, et al. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am. 2009;91:2421-2429.
46. Vopat BG, Lee BJ, DeStefano S, et al. Risk factors for infection after rotator cuff repair. Arthroscopy. 2016;32:428-434.
47. Pauzenberger L, Grieb A, Hexel M, et al. Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc. 2017;25:595-601.
48. Randelli P, Spennacchio P, Ragone V, et al. Complications associated with arthroscopic rotator cuff repair: a literature review. Musculoskelet Surg. 2012;96:9-16.
49. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism following rotator cuff repair. Int J Shoulder Surg. 2008;2:49-51.
50. Wu XL, Briggs L, Murrell GAC. Intraoperative determinants of rotator cuff repair integrity: an analysis of 500 consecutive repairs. Am J Sports Med. 2012;40:2771-2776.
51. McElvany MD, McGoldrick E, Gee AO, et al. Rotator cuff repair: published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med. 2015;43:491-500.
52. Yoo JH, Cho NS, Rhee YG. Effect of postoperative repair integrity on health-related quality of life after rotator cuff repair: healed versus retear group. Am J Sports Med. 2013;41;2637-2644.
53. Huberty DP, Schoolfield JD, Brady PC, et al. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. 2009;25:880-890.
54. Cho NS, Cha SW, Rhee YG. Alterations of the deltoid muscle after open versus arthroscopic rotator cuff repair. Am J Sports Med. 2015;43:2927-2934.
PRACTICE RECOMMENDATIONS
› Offer a trial of conservative management to patients with chronic, nontraumatic, or partial-thickness rotator cuff injury and to those who are poor surgical candidates. B
› Counsel patients that the rate of surgical complications is low and outcomes are favorable in properly selected patients for operative repair of rotator cuff tear. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series