User login
All Hands on Deck
As hospitalist groups evolve, they seek ways to make the best use of support staff to improve patient care and efficiency.
A look at hospitalist groups around the country shows there is no one perfect formula for putting together the best support staff. Rather, the choices groups make are tailored to their specific needs and their relationship with their hospitals.
Support staff members can include secretaries, clerical workers, case managers, social workers, administrators and administrative assistants, office managers, nurses, nurse practitioners, and physician’s assistants.
New Approaches
One trend is the use of registered nurses in hybrid nursing/administrative roles that require medical knowledge and hospital savvy.
Brian Bossard, MD, created a nurse coordinator role in 2003 to provide support to the 18 hospitalists he directs at Inpatient Physician Associates, a group that provides care to patients at BryanLGH Medical Center in Lincoln, Neb.
The group’s three nurse coordinators serve as liaisons with patients and their families and with the hospital’s nursing staff and ancillary staff. The nurse coordinators expedite discharge management by initiating discharge orders, justifying medications, and fielding any questions or issues that need to be discussed with doctors. They keep track of the group’s 18 hospitalists and determine who is available to take on new admissions. It’s an often-complex process of knowing who’s where on rounds and whether they’re busy with difficult cases.
Before the nurse coordinator roles were established, physicians were in charge of figuring out who would take the next patient. “That physician would take all the information, but that may not be the physician available to take care of the patient,” says Dr. Bossard. “That physician would have to call another physician and give the same information—which occupied our doctors’ time. The nurse coordinators are really a time-saving feature.”
At the hospital medicine program at Temple University School of Medicine in Philadelphia, six clinical care coordinators, all trained RNs, play a similar role for the program’s 23 hospitalists.
William Ford, MD, medical director for Cogent Healthcare directing the program at Temple University, credits the clinical care coordinators, who help maintain communication with patients’ primary care physician during and after discharge, for cutting the hospital’s 30-day and 72-hour readmission rate in half within a year’s time. He says coordinators have played a significant role in boosting the group’s overall efficiency. “Our doctors can see three to five more patients a day because of the time the clinical care coordinators save them,” he says.
Some companies providing hospitalist services have relied mainly on office manager-type staff members to take care of clerical tasks and ensure the flow of information between hospitalists and primary care physicians. “Practice coordinators” play this role at the seven hospitalist groups run by The Schumacher Group’s Hospital Medicine Division of Lafayette, La.
David Grace, MD, area medical officer for Schumacher’s hospital medicine division, says practice coordinators are also in charge of collecting data on patients’ length of stay and level of satisfaction and ensuring accuracy in coding and documentation of diagnoses.
Practice coordinators are not required to have nursing degrees, as the job doesn’t include direct patient care. But he looks for applicants with a background in healthcare and an understanding of medical terminology. “Although practice coordinators don’t provide clinical care, the position improves the care delivered by the hospitalists,” he says.
Strike a Balance
One trap hospitalist groups fall into is hiring more support staff than they need, says John Nelson, MD, a principal in Nelson/Flores, a hospitalist management consulting firm, and the medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash.
In his consulting work, Dr. Nelson has seen secretaries file huge volumes of reports and spend their time creating charts and spreadsheets no one will look at again. “It’s very unusual for a hospitalist group to need any sort of medical records kept separately from the hospital,” he says. “So support staff may be doing busy work that doesn’t benefit the practice.”
His advice? “Think critically about whether adding that person is really likely to make the practice better. Challenge yourself to justify any support person you’re considering adding. Make sure every element of the job description contributes to the practice.”
The need for support staff often depends on the hospitalist group’s working relationship to the hospital. Julia Wright, MD, is director of hospital medicine at the University of Wisconsin Hospitals and Clinics (UWHC) in Madison, an academic hospital medicine group of 11 physicians and one advanced practice nurse practitioner. She says the group is assisted by support staff working within the Department of Medicine.
Her part-time program assistant, who tends to secretarial duties such as setting up meetings and assisting with policy development and scheduling, is employed by the primary care department. “We have a benevolent arrangement with the hospital,” she says. “It allows us to do more research and teaching. When we want to put forward an initiative, we usually have the person power and interest and the support to do it.”
Another trend in hiring among hospitalist groups is employing midlevel practitioners, such as nurse practitioners and physician’s assistants. Dr. Ford worked with a physician’s assistant when he was a hospitalist at Union Hospital, a 120-bed community hospital in Elkton, Md. He called midlevel practitioners, who make $70,000 to $90,000 a year —about half the average pay for a hospitalist—a “windfall.”
“They see less-acute patients,” he says. “Patients with stable pneumonia still generate the same billing code as a sicker patient who takes more time and expertise, so midlevels can be more efficient providers from that aspect.”
But this strategy can backfire, according to Dr. Nelson. “On paper, giving nurse practitioners patients who are less sick is logical,” he says. “But in practice, it’s hard to divide up the responsibilities efficiently every day, and there is often a lot of inefficient or unnecessary overlap in work done by the MD and the NP.”
At UWHC, Dr. Wright has found it useful to create a specific definition of the advanced practice nurse practitioner’s role, using feedback from the nurse practitioner and the group’s hospitalists. “We came up with a document that looked at patient complexity, diagnoses, patient volume, and the nondirect patient care issues she is able to help with,” Dr. Wright says. “It’s posted so everyone can remember what kinds of things she can do.”
Sometimes, hospitalist groups can’t make full use of nurse practitioners and other midlevel providers because of hospital regulations. Matthew Szvetecz, MD, head of the hospitalist program at Kadlec Medical Center in Richland, Wash., says he would like to hire nurse practitioners, but hospital bylaws prevent nurse practitioners from writing orders. “If we have to give that kind of direct level of supervision, we might as well take care of them ourselves,” he says.
Dr. Szvetecz’s program has 13 adult hospitalists, four intensivists, and four pediatric physicians. Support staff members include a coordinator in charge of secretarial and administrative assistant duties, a coder who helps with billing, a nurse coordinator, and an executive director. He says the support staff helps contain costs and prevents physicians from spending too much time on administrative duties.
But as a group, “it’s understood that we may not break even or generate positive cash flow,” he says. “Like most hospitalist groups, we have to be subsidized as far as the upfront cash flow, but there are benefits on the back end as far as reduced lengths of stay and better documentation.”
Caveats
Dr. Bossard cautions against letting support staff take over certain duties. The group has avoided letting anyone but physicians take calls from referring primary care physicians, or make calls to primary care physicians at the time of discharge.
“We want to market ourselves as service oriented and felt that placing an intermediary in the communications process isn’t a good thing to do,” he says. “We don’t think it’s good to have secretaries triage calls from physicians. It takes a lot of the physicians’ time, but that’s time well spent.”
Dr. Wright says efficiency and cost containment can improve according to how well the hospitalist group works with the hospital infrastructure and how invested hospitalists and support staff feel in the success of their program. “If they know they’re valued and they feel like they have a say in the work they do, they’ll be more invested in the work they do,” she says. “That usually leads to more efficiency, in my experience.”
Though it’s important for hospitalist groups to work closely with hospitals, Dr. Nelson warns hospitalists not to copy the administrative structures and systems they see in hospitals. “It’s too easy for practices to make mistakes based on what is going on in hospitals,” he says. “They need to critically think about what’s needed in their practice.” TH
Lisa Phillips is a medical writer based in New York.
As hospitalist groups evolve, they seek ways to make the best use of support staff to improve patient care and efficiency.
A look at hospitalist groups around the country shows there is no one perfect formula for putting together the best support staff. Rather, the choices groups make are tailored to their specific needs and their relationship with their hospitals.
Support staff members can include secretaries, clerical workers, case managers, social workers, administrators and administrative assistants, office managers, nurses, nurse practitioners, and physician’s assistants.
New Approaches
One trend is the use of registered nurses in hybrid nursing/administrative roles that require medical knowledge and hospital savvy.
Brian Bossard, MD, created a nurse coordinator role in 2003 to provide support to the 18 hospitalists he directs at Inpatient Physician Associates, a group that provides care to patients at BryanLGH Medical Center in Lincoln, Neb.
The group’s three nurse coordinators serve as liaisons with patients and their families and with the hospital’s nursing staff and ancillary staff. The nurse coordinators expedite discharge management by initiating discharge orders, justifying medications, and fielding any questions or issues that need to be discussed with doctors. They keep track of the group’s 18 hospitalists and determine who is available to take on new admissions. It’s an often-complex process of knowing who’s where on rounds and whether they’re busy with difficult cases.
Before the nurse coordinator roles were established, physicians were in charge of figuring out who would take the next patient. “That physician would take all the information, but that may not be the physician available to take care of the patient,” says Dr. Bossard. “That physician would have to call another physician and give the same information—which occupied our doctors’ time. The nurse coordinators are really a time-saving feature.”
At the hospital medicine program at Temple University School of Medicine in Philadelphia, six clinical care coordinators, all trained RNs, play a similar role for the program’s 23 hospitalists.
William Ford, MD, medical director for Cogent Healthcare directing the program at Temple University, credits the clinical care coordinators, who help maintain communication with patients’ primary care physician during and after discharge, for cutting the hospital’s 30-day and 72-hour readmission rate in half within a year’s time. He says coordinators have played a significant role in boosting the group’s overall efficiency. “Our doctors can see three to five more patients a day because of the time the clinical care coordinators save them,” he says.
Some companies providing hospitalist services have relied mainly on office manager-type staff members to take care of clerical tasks and ensure the flow of information between hospitalists and primary care physicians. “Practice coordinators” play this role at the seven hospitalist groups run by The Schumacher Group’s Hospital Medicine Division of Lafayette, La.
David Grace, MD, area medical officer for Schumacher’s hospital medicine division, says practice coordinators are also in charge of collecting data on patients’ length of stay and level of satisfaction and ensuring accuracy in coding and documentation of diagnoses.
Practice coordinators are not required to have nursing degrees, as the job doesn’t include direct patient care. But he looks for applicants with a background in healthcare and an understanding of medical terminology. “Although practice coordinators don’t provide clinical care, the position improves the care delivered by the hospitalists,” he says.
Strike a Balance
One trap hospitalist groups fall into is hiring more support staff than they need, says John Nelson, MD, a principal in Nelson/Flores, a hospitalist management consulting firm, and the medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash.
In his consulting work, Dr. Nelson has seen secretaries file huge volumes of reports and spend their time creating charts and spreadsheets no one will look at again. “It’s very unusual for a hospitalist group to need any sort of medical records kept separately from the hospital,” he says. “So support staff may be doing busy work that doesn’t benefit the practice.”
His advice? “Think critically about whether adding that person is really likely to make the practice better. Challenge yourself to justify any support person you’re considering adding. Make sure every element of the job description contributes to the practice.”
The need for support staff often depends on the hospitalist group’s working relationship to the hospital. Julia Wright, MD, is director of hospital medicine at the University of Wisconsin Hospitals and Clinics (UWHC) in Madison, an academic hospital medicine group of 11 physicians and one advanced practice nurse practitioner. She says the group is assisted by support staff working within the Department of Medicine.
Her part-time program assistant, who tends to secretarial duties such as setting up meetings and assisting with policy development and scheduling, is employed by the primary care department. “We have a benevolent arrangement with the hospital,” she says. “It allows us to do more research and teaching. When we want to put forward an initiative, we usually have the person power and interest and the support to do it.”
Another trend in hiring among hospitalist groups is employing midlevel practitioners, such as nurse practitioners and physician’s assistants. Dr. Ford worked with a physician’s assistant when he was a hospitalist at Union Hospital, a 120-bed community hospital in Elkton, Md. He called midlevel practitioners, who make $70,000 to $90,000 a year —about half the average pay for a hospitalist—a “windfall.”
“They see less-acute patients,” he says. “Patients with stable pneumonia still generate the same billing code as a sicker patient who takes more time and expertise, so midlevels can be more efficient providers from that aspect.”
But this strategy can backfire, according to Dr. Nelson. “On paper, giving nurse practitioners patients who are less sick is logical,” he says. “But in practice, it’s hard to divide up the responsibilities efficiently every day, and there is often a lot of inefficient or unnecessary overlap in work done by the MD and the NP.”
At UWHC, Dr. Wright has found it useful to create a specific definition of the advanced practice nurse practitioner’s role, using feedback from the nurse practitioner and the group’s hospitalists. “We came up with a document that looked at patient complexity, diagnoses, patient volume, and the nondirect patient care issues she is able to help with,” Dr. Wright says. “It’s posted so everyone can remember what kinds of things she can do.”
Sometimes, hospitalist groups can’t make full use of nurse practitioners and other midlevel providers because of hospital regulations. Matthew Szvetecz, MD, head of the hospitalist program at Kadlec Medical Center in Richland, Wash., says he would like to hire nurse practitioners, but hospital bylaws prevent nurse practitioners from writing orders. “If we have to give that kind of direct level of supervision, we might as well take care of them ourselves,” he says.
Dr. Szvetecz’s program has 13 adult hospitalists, four intensivists, and four pediatric physicians. Support staff members include a coordinator in charge of secretarial and administrative assistant duties, a coder who helps with billing, a nurse coordinator, and an executive director. He says the support staff helps contain costs and prevents physicians from spending too much time on administrative duties.
But as a group, “it’s understood that we may not break even or generate positive cash flow,” he says. “Like most hospitalist groups, we have to be subsidized as far as the upfront cash flow, but there are benefits on the back end as far as reduced lengths of stay and better documentation.”
Caveats
Dr. Bossard cautions against letting support staff take over certain duties. The group has avoided letting anyone but physicians take calls from referring primary care physicians, or make calls to primary care physicians at the time of discharge.
“We want to market ourselves as service oriented and felt that placing an intermediary in the communications process isn’t a good thing to do,” he says. “We don’t think it’s good to have secretaries triage calls from physicians. It takes a lot of the physicians’ time, but that’s time well spent.”
Dr. Wright says efficiency and cost containment can improve according to how well the hospitalist group works with the hospital infrastructure and how invested hospitalists and support staff feel in the success of their program. “If they know they’re valued and they feel like they have a say in the work they do, they’ll be more invested in the work they do,” she says. “That usually leads to more efficiency, in my experience.”
Though it’s important for hospitalist groups to work closely with hospitals, Dr. Nelson warns hospitalists not to copy the administrative structures and systems they see in hospitals. “It’s too easy for practices to make mistakes based on what is going on in hospitals,” he says. “They need to critically think about what’s needed in their practice.” TH
Lisa Phillips is a medical writer based in New York.
As hospitalist groups evolve, they seek ways to make the best use of support staff to improve patient care and efficiency.
A look at hospitalist groups around the country shows there is no one perfect formula for putting together the best support staff. Rather, the choices groups make are tailored to their specific needs and their relationship with their hospitals.
Support staff members can include secretaries, clerical workers, case managers, social workers, administrators and administrative assistants, office managers, nurses, nurse practitioners, and physician’s assistants.
New Approaches
One trend is the use of registered nurses in hybrid nursing/administrative roles that require medical knowledge and hospital savvy.
Brian Bossard, MD, created a nurse coordinator role in 2003 to provide support to the 18 hospitalists he directs at Inpatient Physician Associates, a group that provides care to patients at BryanLGH Medical Center in Lincoln, Neb.
The group’s three nurse coordinators serve as liaisons with patients and their families and with the hospital’s nursing staff and ancillary staff. The nurse coordinators expedite discharge management by initiating discharge orders, justifying medications, and fielding any questions or issues that need to be discussed with doctors. They keep track of the group’s 18 hospitalists and determine who is available to take on new admissions. It’s an often-complex process of knowing who’s where on rounds and whether they’re busy with difficult cases.
Before the nurse coordinator roles were established, physicians were in charge of figuring out who would take the next patient. “That physician would take all the information, but that may not be the physician available to take care of the patient,” says Dr. Bossard. “That physician would have to call another physician and give the same information—which occupied our doctors’ time. The nurse coordinators are really a time-saving feature.”
At the hospital medicine program at Temple University School of Medicine in Philadelphia, six clinical care coordinators, all trained RNs, play a similar role for the program’s 23 hospitalists.
William Ford, MD, medical director for Cogent Healthcare directing the program at Temple University, credits the clinical care coordinators, who help maintain communication with patients’ primary care physician during and after discharge, for cutting the hospital’s 30-day and 72-hour readmission rate in half within a year’s time. He says coordinators have played a significant role in boosting the group’s overall efficiency. “Our doctors can see three to five more patients a day because of the time the clinical care coordinators save them,” he says.
Some companies providing hospitalist services have relied mainly on office manager-type staff members to take care of clerical tasks and ensure the flow of information between hospitalists and primary care physicians. “Practice coordinators” play this role at the seven hospitalist groups run by The Schumacher Group’s Hospital Medicine Division of Lafayette, La.
David Grace, MD, area medical officer for Schumacher’s hospital medicine division, says practice coordinators are also in charge of collecting data on patients’ length of stay and level of satisfaction and ensuring accuracy in coding and documentation of diagnoses.
Practice coordinators are not required to have nursing degrees, as the job doesn’t include direct patient care. But he looks for applicants with a background in healthcare and an understanding of medical terminology. “Although practice coordinators don’t provide clinical care, the position improves the care delivered by the hospitalists,” he says.
Strike a Balance
One trap hospitalist groups fall into is hiring more support staff than they need, says John Nelson, MD, a principal in Nelson/Flores, a hospitalist management consulting firm, and the medical director of the hospitalist practice at Overlake Hospital Medical Center in Bellevue, Wash.
In his consulting work, Dr. Nelson has seen secretaries file huge volumes of reports and spend their time creating charts and spreadsheets no one will look at again. “It’s very unusual for a hospitalist group to need any sort of medical records kept separately from the hospital,” he says. “So support staff may be doing busy work that doesn’t benefit the practice.”
His advice? “Think critically about whether adding that person is really likely to make the practice better. Challenge yourself to justify any support person you’re considering adding. Make sure every element of the job description contributes to the practice.”
The need for support staff often depends on the hospitalist group’s working relationship to the hospital. Julia Wright, MD, is director of hospital medicine at the University of Wisconsin Hospitals and Clinics (UWHC) in Madison, an academic hospital medicine group of 11 physicians and one advanced practice nurse practitioner. She says the group is assisted by support staff working within the Department of Medicine.
Her part-time program assistant, who tends to secretarial duties such as setting up meetings and assisting with policy development and scheduling, is employed by the primary care department. “We have a benevolent arrangement with the hospital,” she says. “It allows us to do more research and teaching. When we want to put forward an initiative, we usually have the person power and interest and the support to do it.”
Another trend in hiring among hospitalist groups is employing midlevel practitioners, such as nurse practitioners and physician’s assistants. Dr. Ford worked with a physician’s assistant when he was a hospitalist at Union Hospital, a 120-bed community hospital in Elkton, Md. He called midlevel practitioners, who make $70,000 to $90,000 a year —about half the average pay for a hospitalist—a “windfall.”
“They see less-acute patients,” he says. “Patients with stable pneumonia still generate the same billing code as a sicker patient who takes more time and expertise, so midlevels can be more efficient providers from that aspect.”
But this strategy can backfire, according to Dr. Nelson. “On paper, giving nurse practitioners patients who are less sick is logical,” he says. “But in practice, it’s hard to divide up the responsibilities efficiently every day, and there is often a lot of inefficient or unnecessary overlap in work done by the MD and the NP.”
At UWHC, Dr. Wright has found it useful to create a specific definition of the advanced practice nurse practitioner’s role, using feedback from the nurse practitioner and the group’s hospitalists. “We came up with a document that looked at patient complexity, diagnoses, patient volume, and the nondirect patient care issues she is able to help with,” Dr. Wright says. “It’s posted so everyone can remember what kinds of things she can do.”
Sometimes, hospitalist groups can’t make full use of nurse practitioners and other midlevel providers because of hospital regulations. Matthew Szvetecz, MD, head of the hospitalist program at Kadlec Medical Center in Richland, Wash., says he would like to hire nurse practitioners, but hospital bylaws prevent nurse practitioners from writing orders. “If we have to give that kind of direct level of supervision, we might as well take care of them ourselves,” he says.
Dr. Szvetecz’s program has 13 adult hospitalists, four intensivists, and four pediatric physicians. Support staff members include a coordinator in charge of secretarial and administrative assistant duties, a coder who helps with billing, a nurse coordinator, and an executive director. He says the support staff helps contain costs and prevents physicians from spending too much time on administrative duties.
But as a group, “it’s understood that we may not break even or generate positive cash flow,” he says. “Like most hospitalist groups, we have to be subsidized as far as the upfront cash flow, but there are benefits on the back end as far as reduced lengths of stay and better documentation.”
Caveats
Dr. Bossard cautions against letting support staff take over certain duties. The group has avoided letting anyone but physicians take calls from referring primary care physicians, or make calls to primary care physicians at the time of discharge.
“We want to market ourselves as service oriented and felt that placing an intermediary in the communications process isn’t a good thing to do,” he says. “We don’t think it’s good to have secretaries triage calls from physicians. It takes a lot of the physicians’ time, but that’s time well spent.”
Dr. Wright says efficiency and cost containment can improve according to how well the hospitalist group works with the hospital infrastructure and how invested hospitalists and support staff feel in the success of their program. “If they know they’re valued and they feel like they have a say in the work they do, they’ll be more invested in the work they do,” she says. “That usually leads to more efficiency, in my experience.”
Though it’s important for hospitalist groups to work closely with hospitals, Dr. Nelson warns hospitalists not to copy the administrative structures and systems they see in hospitals. “It’s too easy for practices to make mistakes based on what is going on in hospitals,” he says. “They need to critically think about what’s needed in their practice.” TH
Lisa Phillips is a medical writer based in New York.
Watch and Earn
With recent changes in Medicare rules making reimbursement even trickier for patients who aren’t well enough to be sent home quickly but aren’t sick enough to move to an inpatient bed, hospitalists are increasingly being tapped to set up observation units at medical centers around the country.
These patients, experts say, are the ones hospitals are most likely to lose money on. That’s because the Centers for Medicare and Medicaid Services (CMS) won’t pay unless a patient meets stringent guidelines for admission to the hospital. And while recently rewritten rules allow payment for 24 hours of observation, they also can also lead to denial of claims when patients aren’t considered sick enough to have been admitted.
—Jason Napolitano, MD, medical director of the observation unit, University of California at Los Angeles Medical Center
When they’re well run, observation units can even help cover losses from emergency departments (ED) that have trouble collecting on bills because most of their patient population is uninsured or underinsured.
But the drive to create observation units isn’t just about money, says Frank W. Peacock, MD, vice chair of the emergency department at the Cleveland Clinic in Ohio. Studies have shown that death rates drop when hospitals add observation units, Dr. Peacock says.
Despite these clear benefits, experts estimate that a mere 20% of medical centers around the nation have observation units.
This may in part be because creating such a unit—also known as clinical decision unit—takes a lot of planning to start up, says William T. Ford, MD, medical director for Nashville, Tenn.-based Cogent Healthcare and chief of the section of hospital medicine at Temple University in Philadelphia. Without proper planning, observation units can fail to flourish—or just fail.
That’s what happened at Temple, Dr. Ford says. “The original observation unit got bogged down in its own infrastructure,” he explains. “It wasn’t cost effective.”
After that first attempt failed, Temple reached out to Cogent and Dr. Ford for help in developing an observation unit that would be financially viable.
Observation Origins
Classically, Dr. Ford says, observation units were developed and staffed by emergency department physicians. But these days, the units are increasingly being designed and run by hospitalists, he says, adding that this change makes a lot of sense.
“Emergency department physicians don’t have the time or the resources to monitor patients for long periods of time,” Dr. Ford says. “That’s why I think some of the early ones failed—they didn’t work as efficiently and were staffed by the wrong people.”
Hospitalist Jason Napolitano, MD, agrees with the choice to staff observation units with hospitalists. “We want our emergency department physicians to be able to focus on life-or-death issues and on the stabilization of very sick patients,” says Dr. Napolitano, medical director of the observation unit at the University of California at Los Angeles Medical Center. “These are things that ED physicians do spectacularly well. But when it gets down to management and reassessment of patients over time, we wanted a dedicated staff of hospitalists who were trained in internal medicine.”
It made sense that many of the early observation units were staffed by ED doctors, says Mark Flitcraft, a nurse and unit director of nursing at UCLA. That’s because the units were originally adjuncts to the ED. These early units were initially seen as a way to take the pressure off overcrowded, overworked EDs, Flitcraft says. “They were a way for hospitals to avoid [diverting patients] as the beds in the ED started filing up,” he adds.
Avoiding such diversions is still one of the main justifications for adding an observation unit, Dr. Ford says. “The observation unit helps increase throughput time.”
Still, he says, if you’re going to create an observation unit staffed by hospitalists, “you need to make sure that the emergency department buys in to the concept. They should be your best friends. Go over and meet with them. If they don’t buy into the idea, then you’re going to have problems.”
Time Is of the Essence
For an observation unit to work well, the staff needs to think about time in a different way, Flitcraft says.
“It’s more of an outpatient designation from a Medicare standpoint,” he explains. “The focus has to be hours rather than days. You really need to know that the clock is ticking and work on rapid turnaround.” Take discharge, for example, Flitcraft says. Normally a hospitalist would wait for morning to send a patient home. “But there are patients we might discharge at 10 p.m.,” he says. “When they are stable they go home.”
In the observation unit, staff members always have the end in sight, agrees Robin J. Trupp, a grad student at Ohio State University, expert on observation units, and president of The American Association of Heart Failure Nurses. “You know what your goal is,” she adds. “There’s a 24-hour clock and it’s always ticking. At the end of 24 hours you have to make a treatment decision: admit the patient or send him home.”
Because observation units are generally limited to treating a select group of medical conditions, they can be more efficient. Some observation units are limited to only one or two diagnoses (e.g., chest pain and heart failure). Others see a slightly broader spectrum of illnesses, including asthma, stomach pain, and pneumonia.
One byproduct of limiting the number of conditions treated in the unit is ending up with a staff that can become specialized in treating those ailments, experts say.
“In the observation unit you’re not looking at urinary tract infections or doing stitches,” Trupp says. “You’re just working on this population. You become an expert on how it’s treated and managed.”
And that offers another advantage: the possibility of doing more patient education.
She points to the example of a unit dedicated to treating heart failure patients.
“You can take advantage of the fact that at this moment, the patient can clearly see cause and effect and maybe you’ll have a chance at getting some behavior changes,” Trupp says. “It’s the case of having put their hand in the fire and feeling and having learned it’s hot; they’ll learn not to do it again. They might learn that the symptoms that landed them in the ED came from excess salt load due to eating Chinese food or chips and salsa.”
Ultimately, for certain conditions, observation units can provide better care. Studies have shown that in the three months following a visit to the hospital, heart failure patients are far less likely to return if they’ve been seen in the observation unit rather than being treated as inpatients.
And if that weren’t enough of an inducement to administrators to create observation units, Dr. Peacock offers one other: The units can do more than pay for themselves.
“We are in an urban environment, and our patient population is not well insured,” he says. “There are years when the ED loses money. The observation unit never loses money. In fact, it’s saved us a few times. That was a pleasant surprise.” TH
Linda Carroll is a medical writer based in New Jersey.
With recent changes in Medicare rules making reimbursement even trickier for patients who aren’t well enough to be sent home quickly but aren’t sick enough to move to an inpatient bed, hospitalists are increasingly being tapped to set up observation units at medical centers around the country.
These patients, experts say, are the ones hospitals are most likely to lose money on. That’s because the Centers for Medicare and Medicaid Services (CMS) won’t pay unless a patient meets stringent guidelines for admission to the hospital. And while recently rewritten rules allow payment for 24 hours of observation, they also can also lead to denial of claims when patients aren’t considered sick enough to have been admitted.
—Jason Napolitano, MD, medical director of the observation unit, University of California at Los Angeles Medical Center
When they’re well run, observation units can even help cover losses from emergency departments (ED) that have trouble collecting on bills because most of their patient population is uninsured or underinsured.
But the drive to create observation units isn’t just about money, says Frank W. Peacock, MD, vice chair of the emergency department at the Cleveland Clinic in Ohio. Studies have shown that death rates drop when hospitals add observation units, Dr. Peacock says.
Despite these clear benefits, experts estimate that a mere 20% of medical centers around the nation have observation units.
This may in part be because creating such a unit—also known as clinical decision unit—takes a lot of planning to start up, says William T. Ford, MD, medical director for Nashville, Tenn.-based Cogent Healthcare and chief of the section of hospital medicine at Temple University in Philadelphia. Without proper planning, observation units can fail to flourish—or just fail.
That’s what happened at Temple, Dr. Ford says. “The original observation unit got bogged down in its own infrastructure,” he explains. “It wasn’t cost effective.”
After that first attempt failed, Temple reached out to Cogent and Dr. Ford for help in developing an observation unit that would be financially viable.
Observation Origins
Classically, Dr. Ford says, observation units were developed and staffed by emergency department physicians. But these days, the units are increasingly being designed and run by hospitalists, he says, adding that this change makes a lot of sense.
“Emergency department physicians don’t have the time or the resources to monitor patients for long periods of time,” Dr. Ford says. “That’s why I think some of the early ones failed—they didn’t work as efficiently and were staffed by the wrong people.”
Hospitalist Jason Napolitano, MD, agrees with the choice to staff observation units with hospitalists. “We want our emergency department physicians to be able to focus on life-or-death issues and on the stabilization of very sick patients,” says Dr. Napolitano, medical director of the observation unit at the University of California at Los Angeles Medical Center. “These are things that ED physicians do spectacularly well. But when it gets down to management and reassessment of patients over time, we wanted a dedicated staff of hospitalists who were trained in internal medicine.”
It made sense that many of the early observation units were staffed by ED doctors, says Mark Flitcraft, a nurse and unit director of nursing at UCLA. That’s because the units were originally adjuncts to the ED. These early units were initially seen as a way to take the pressure off overcrowded, overworked EDs, Flitcraft says. “They were a way for hospitals to avoid [diverting patients] as the beds in the ED started filing up,” he adds.
Avoiding such diversions is still one of the main justifications for adding an observation unit, Dr. Ford says. “The observation unit helps increase throughput time.”
Still, he says, if you’re going to create an observation unit staffed by hospitalists, “you need to make sure that the emergency department buys in to the concept. They should be your best friends. Go over and meet with them. If they don’t buy into the idea, then you’re going to have problems.”
Time Is of the Essence
For an observation unit to work well, the staff needs to think about time in a different way, Flitcraft says.
“It’s more of an outpatient designation from a Medicare standpoint,” he explains. “The focus has to be hours rather than days. You really need to know that the clock is ticking and work on rapid turnaround.” Take discharge, for example, Flitcraft says. Normally a hospitalist would wait for morning to send a patient home. “But there are patients we might discharge at 10 p.m.,” he says. “When they are stable they go home.”
In the observation unit, staff members always have the end in sight, agrees Robin J. Trupp, a grad student at Ohio State University, expert on observation units, and president of The American Association of Heart Failure Nurses. “You know what your goal is,” she adds. “There’s a 24-hour clock and it’s always ticking. At the end of 24 hours you have to make a treatment decision: admit the patient or send him home.”
Because observation units are generally limited to treating a select group of medical conditions, they can be more efficient. Some observation units are limited to only one or two diagnoses (e.g., chest pain and heart failure). Others see a slightly broader spectrum of illnesses, including asthma, stomach pain, and pneumonia.
One byproduct of limiting the number of conditions treated in the unit is ending up with a staff that can become specialized in treating those ailments, experts say.
“In the observation unit you’re not looking at urinary tract infections or doing stitches,” Trupp says. “You’re just working on this population. You become an expert on how it’s treated and managed.”
And that offers another advantage: the possibility of doing more patient education.
She points to the example of a unit dedicated to treating heart failure patients.
“You can take advantage of the fact that at this moment, the patient can clearly see cause and effect and maybe you’ll have a chance at getting some behavior changes,” Trupp says. “It’s the case of having put their hand in the fire and feeling and having learned it’s hot; they’ll learn not to do it again. They might learn that the symptoms that landed them in the ED came from excess salt load due to eating Chinese food or chips and salsa.”
Ultimately, for certain conditions, observation units can provide better care. Studies have shown that in the three months following a visit to the hospital, heart failure patients are far less likely to return if they’ve been seen in the observation unit rather than being treated as inpatients.
And if that weren’t enough of an inducement to administrators to create observation units, Dr. Peacock offers one other: The units can do more than pay for themselves.
“We are in an urban environment, and our patient population is not well insured,” he says. “There are years when the ED loses money. The observation unit never loses money. In fact, it’s saved us a few times. That was a pleasant surprise.” TH
Linda Carroll is a medical writer based in New Jersey.
With recent changes in Medicare rules making reimbursement even trickier for patients who aren’t well enough to be sent home quickly but aren’t sick enough to move to an inpatient bed, hospitalists are increasingly being tapped to set up observation units at medical centers around the country.
These patients, experts say, are the ones hospitals are most likely to lose money on. That’s because the Centers for Medicare and Medicaid Services (CMS) won’t pay unless a patient meets stringent guidelines for admission to the hospital. And while recently rewritten rules allow payment for 24 hours of observation, they also can also lead to denial of claims when patients aren’t considered sick enough to have been admitted.
—Jason Napolitano, MD, medical director of the observation unit, University of California at Los Angeles Medical Center
When they’re well run, observation units can even help cover losses from emergency departments (ED) that have trouble collecting on bills because most of their patient population is uninsured or underinsured.
But the drive to create observation units isn’t just about money, says Frank W. Peacock, MD, vice chair of the emergency department at the Cleveland Clinic in Ohio. Studies have shown that death rates drop when hospitals add observation units, Dr. Peacock says.
Despite these clear benefits, experts estimate that a mere 20% of medical centers around the nation have observation units.
This may in part be because creating such a unit—also known as clinical decision unit—takes a lot of planning to start up, says William T. Ford, MD, medical director for Nashville, Tenn.-based Cogent Healthcare and chief of the section of hospital medicine at Temple University in Philadelphia. Without proper planning, observation units can fail to flourish—or just fail.
That’s what happened at Temple, Dr. Ford says. “The original observation unit got bogged down in its own infrastructure,” he explains. “It wasn’t cost effective.”
After that first attempt failed, Temple reached out to Cogent and Dr. Ford for help in developing an observation unit that would be financially viable.
Observation Origins
Classically, Dr. Ford says, observation units were developed and staffed by emergency department physicians. But these days, the units are increasingly being designed and run by hospitalists, he says, adding that this change makes a lot of sense.
“Emergency department physicians don’t have the time or the resources to monitor patients for long periods of time,” Dr. Ford says. “That’s why I think some of the early ones failed—they didn’t work as efficiently and were staffed by the wrong people.”
Hospitalist Jason Napolitano, MD, agrees with the choice to staff observation units with hospitalists. “We want our emergency department physicians to be able to focus on life-or-death issues and on the stabilization of very sick patients,” says Dr. Napolitano, medical director of the observation unit at the University of California at Los Angeles Medical Center. “These are things that ED physicians do spectacularly well. But when it gets down to management and reassessment of patients over time, we wanted a dedicated staff of hospitalists who were trained in internal medicine.”
It made sense that many of the early observation units were staffed by ED doctors, says Mark Flitcraft, a nurse and unit director of nursing at UCLA. That’s because the units were originally adjuncts to the ED. These early units were initially seen as a way to take the pressure off overcrowded, overworked EDs, Flitcraft says. “They were a way for hospitals to avoid [diverting patients] as the beds in the ED started filing up,” he adds.
Avoiding such diversions is still one of the main justifications for adding an observation unit, Dr. Ford says. “The observation unit helps increase throughput time.”
Still, he says, if you’re going to create an observation unit staffed by hospitalists, “you need to make sure that the emergency department buys in to the concept. They should be your best friends. Go over and meet with them. If they don’t buy into the idea, then you’re going to have problems.”
Time Is of the Essence
For an observation unit to work well, the staff needs to think about time in a different way, Flitcraft says.
“It’s more of an outpatient designation from a Medicare standpoint,” he explains. “The focus has to be hours rather than days. You really need to know that the clock is ticking and work on rapid turnaround.” Take discharge, for example, Flitcraft says. Normally a hospitalist would wait for morning to send a patient home. “But there are patients we might discharge at 10 p.m.,” he says. “When they are stable they go home.”
In the observation unit, staff members always have the end in sight, agrees Robin J. Trupp, a grad student at Ohio State University, expert on observation units, and president of The American Association of Heart Failure Nurses. “You know what your goal is,” she adds. “There’s a 24-hour clock and it’s always ticking. At the end of 24 hours you have to make a treatment decision: admit the patient or send him home.”
Because observation units are generally limited to treating a select group of medical conditions, they can be more efficient. Some observation units are limited to only one or two diagnoses (e.g., chest pain and heart failure). Others see a slightly broader spectrum of illnesses, including asthma, stomach pain, and pneumonia.
One byproduct of limiting the number of conditions treated in the unit is ending up with a staff that can become specialized in treating those ailments, experts say.
“In the observation unit you’re not looking at urinary tract infections or doing stitches,” Trupp says. “You’re just working on this population. You become an expert on how it’s treated and managed.”
And that offers another advantage: the possibility of doing more patient education.
She points to the example of a unit dedicated to treating heart failure patients.
“You can take advantage of the fact that at this moment, the patient can clearly see cause and effect and maybe you’ll have a chance at getting some behavior changes,” Trupp says. “It’s the case of having put their hand in the fire and feeling and having learned it’s hot; they’ll learn not to do it again. They might learn that the symptoms that landed them in the ED came from excess salt load due to eating Chinese food or chips and salsa.”
Ultimately, for certain conditions, observation units can provide better care. Studies have shown that in the three months following a visit to the hospital, heart failure patients are far less likely to return if they’ve been seen in the observation unit rather than being treated as inpatients.
And if that weren’t enough of an inducement to administrators to create observation units, Dr. Peacock offers one other: The units can do more than pay for themselves.
“We are in an urban environment, and our patient population is not well insured,” he says. “There are years when the ED loses money. The observation unit never loses money. In fact, it’s saved us a few times. That was a pleasant surprise.” TH
Linda Carroll is a medical writer based in New Jersey.
Preventing Venous Thromboembolism Throughout the Continuum of Care
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
Supplement Editor:
Amir K. Jaffer, MD
Contents
An overview of venous thromboembolism: Impact, risks, and issues in prophylaxis
A.K. Jaffer
Prevention of venous thromboembolism in the hospitalized medical patient
A.K. Jaffer, A.N. Amin, D.J. Brotman, S.B. Deitelzweig, S.C. McKean, A.C. Spyropoulos
Prevention of venous thromboembolism in the cancer surgery patient
A.C. Spyropoulos, D.J. Brotman, A.N. Amin, S.B. Deitelzweig, A.K. Jaffer, S.C. McKean
Prevention of venous thromboembolism in the orthopedic surgery patient
S.B. Deitelzweig, S.C. McKean, A.N. Amin, D.J. Brotman, A.K. Jaffer, A.C. Spyropoulos
Dropped gallstones disguised as a liver abscess
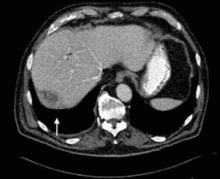
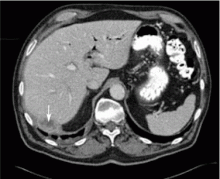
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
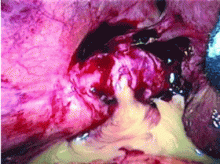
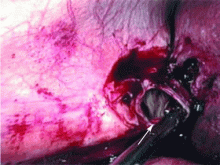
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
A 67-year-old retired man presents to his internist with a 3-month history of abdominal discomfort in the right upper quadrant on deep breathing. He has no other abdominal complaints, but he mentions that he underwent laparoscopic cholecystectomy 3 months ago for gallstone pancreatitis.
A biopsy specimen obtained with CT guidance shows chronic inflammation but is sterile on aerobic culture. There is no evidence of malignancy. Because of concern for underlying infection, the infectious disease staff recommends empirical treatment with a 4-week course of ampicillin-sulbactam (Unasyn). At completion of the antibiotic course, the patient’s symptoms have resolved.
LAPAROSCOPY’S DRAWBACKS
Complications of dropped stones, though rare, can include localized or systemic infection, inflammation, fibrosis, adhesion, cutaneous sinus formation, ileus, and abscess.1,6 Lohan et al1 estimated that dropped stones produce an intra-abdominal abscess in 0.6% to 2.9% of cases of dropped stones and bile spillage, based on reports by Rice et al4 and Morrin et al.7 Dropped stones should be recognized as a potential cause of intra-abdominal abscess in any cholecystectomy patient months or even years after the surgery. Also, these abscesses are not necessarily confined to the right upper quadrant: they can occur anywhere in the abdominal cavity.5,7
Given the ever-increasing popularity of laparoscopic cholecystectomy, the problem of intra-abdominal abscess due to dropped gallstones will only become a more common problem. Early diagnosis is the key to avoiding long and unnecessary treatment.
If dropped gallstones do become infected and eventually cause symptoms, they may require surgical or percutaneous removal in conjunction with antimicrobial therapy.8
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
- Lohan D, Walsh S, McLoughlin R, Murphy J. Imaging of the complications of laparoscopic cholecystectomy. Eur Radiol 2005; 15:904–912.
- Casillas S, Kittur DS. Late abscess formation after spilled gallstones masquerading as a liver mass. Surg Endosc 2003; 17:833.
- Tumer AR, Yuksek YN, Yasti AC, Gozalan U, Kama NA. Dropped gallstones during laparoscopic cholecystectomy: the consequences. World J Surg 2005; 29:437–440.
- Rice DC, Memon MA, Jamison RL, et al. Long-term consequences of intraoperative spillage of bile and gallstones during laparoscopic cholecystectomy. J Gastrointest Surg 1997; 1:85–91.
- Sathesh-Kumar T, Saklani AP, Vinayagam R, Blackett RL. Spilled gall stones during laparoscopic cholecystectomy: a review of the literature. Postgrad Med J 2004; 80:77–79.
- Horton M, Florence MG. Unusual abscess patterns following dropped gallstones during laparoscopic cholecystectomy. Am J Surg 1998; 175:375–379.
- Morrin MM, Kruskal JB, Hochman MG, Saldinger PF, Kane RA. Radiologic features of complications arising from dropped gallstones in laparoscopic cholecystectomy patients. AJR Am J Roentgenol 2000; 174:1441–1445.
- Akyar G, Aytac S, Yagci C, Akyar S. Abscess formation due to dropped gallstone after laparoscopic cholecystectomy. Eur J Radiol 1997; 25:242–245.
What role will ‘gliptins’ play in glycemic control?
The “gliptins”—the nickname for dipeptidyl peptidase 4 (DPP-4) inhibitors—are one of the newest classes of drugs for the treatment of type 2 diabetes mellitus.
These drugs work by prolonging the action of gut hormones called incretins, which boost insulin levels. The greatest advantage of the gliptins appears to be their ability to stimulate insulin production with little risk of corresponding hypoglycemia.
Sitagliptin (Januvia), the first commercially available DPP-4 inhibitor, has been approved by the US Food and Drug Administration (FDA) and is currently in clinical use, and vildagliptin (Galvus) awaits FDA approval at the time of this writing. Other drugs of this class are in development.
However, because these drugs are so new, a number of questions remain about their use. In this article, we discuss the rationale behind gliptin drugs, the evidence to date on their use alone or in combination with current oral hypoglycemic drugs (and even with insulin), and when and how to use them in daily practice.
THE NEED FOR MORE EFFECTIVE DIABETES TREATMENT
As the number of patients with type 2 diabetes continues its steep and steady rise,1,2 much work has gone into studying treatment goals and how to achieve them. Although experts generally agree on glycemic goals,3 we currently fail to achieve those goals in close to two-thirds of patients: only 37% have a hemo-globin A1c (HbA1c) value at or below the goal of 7%, and the same number have levels exceeding 8%.4
Part of the problem is that treatment regimens are not adjusted in a timely fashion. In a prescribing database of almost 4,000 patients with type 2 diabetes,5 the mean time from the first HbA1c reading above 8% to an actual change in therapy was about 15 months for those taking metformin (Glucophage) alone, and 21 months for those taking a sulfonylurea alone. Another part of the problem is that, on average, patients with an HbA1c of 8.0% to 8.9% can expect only a 0.6% lowering with the addition of one agent.6 Clearly, we need new pharmacologic approaches and new management paradigms. One new approach is the use of gliptins.
HOW GLIPTINS WORK
Incretins promote insulin secretion
We have known for more than 20 years that insulin levels rise considerably higher in response to an oral glucose load than to an intravenous glucose infusion, even though the plasma glucose concentrations may be similar.7 This phenomenon involves a myriad of neural and nutritional factors, but the gut hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) appear to be key.
These peptides—called incretins—have a high degree of homology, and both promote insulin secretion. However, GLP-1, produced by the L cells of the ileum and colon, inhibits glucagon secretion and slows gastric emptying, whereas GIP, secreted from the K cells of the duodenum, has no effect on glucagon and little effect on gastric emptying. Both peptides appear to promote pancreatic beta cell growth and survival,8,9 an effect that in theory might allow us to slow the progressive loss of insulin secretory capacity in type 2 diabetes.
Furthermore, the effect of GLP-1 on insulin secretion depends on the plasma glucose concentration, with a greater insulin secretory effect at higher glucose levels and minimal effect at euglycemic levels.10 This phenomenon suggests that drugs that boost GLP-1 activity should not cause the troublesome hypoglycemia typically seen in patients taking insulin, insulin secretagogues, sulfonyl-ureas, or the meglitinides repaglinide (Prandin) or nateglinide (Starlix). Studies of combination treatment with metformin and the GLP-1 receptor agonist exenatide (Byetta) have shown little risk of hypoglycemia,11 offering evidence favoring this conjecture.
Inhibition of DPP-4 boosts incretin action
The challenge for creating treatments that take advantage of the beneficial effects of GLP-1 and GIP is that they have very short physiologic half-lives, ie, less than 10 minutes. GLP-1 and GIP both have two N-terminal amino acids that are quickly cleaved by DPP-4,12 an enzyme present in the circulation13 and on endothelial cells.14
Currently, there are two classes of drugs based on incretins. One class, the incretin mimetics or GLP-1 receptor agonists, includes drugs that mimic the effect of GLP-1 but are not so quickly degraded by DPP-4. Examples of these drugs are exenatide, which is currently FDA-approved, and liraglutide, which is not yet approved.
On the other hand, by inhibiting the cleaving action of DPP-4, the gliptins can prolong the half-life of endogenous GLP-1, increasing its physiologic effects.
Studies comparing gliptins with GLP-1 receptor agonists are only at the preclinical phase. Liraglutide showed an antiglycemic effect similar to that of vildagliptin in an animal model of glucose intolerance.15 This and other16,17 preclinical studies have shown evidence of improved beta cell growth and survival with DPP-4 inhibitor treatment, to an extent similar to that reported with thiazo-lidinediones, whereas sulfonylureas show no evidence either of increase in beta cells or of improved intrinsic beta cell secretory function in these models. Of course, animal studies can only be cautiously extrapolated to potential effects in humans, and it is uncertain whether such benefits will occur with the therapeutic use of DPP-4 inhibitors.
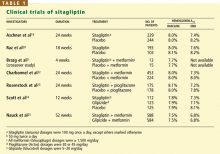
RANDOMIZED CLINICAL TRIALS OF SITAGLIPTIN
Sitagliptin is effective when used by itself,reducing a baseline HbA1c level of about 8% by 0.6% to 0.8%,19,20,24 and is similarly effective when combined with metformin21,22,25 or pioglitazone (Actos, a thiazolidinedione).23 It also decreases fasting blood glucose levels and improves other measures of glucose control.
A study comparing sitagliptin and the sul-fonylurea glipizide (Glucotrol) showed identical glucose-lowering over a 1-year period, with less hypoglycemia and weight gain with sitagliptin.25 Hypoglycemic episodes occurred in 32% of patients taking glipizide but in only 5% of those taking sitagliptin.
Studies noted several trends in laboratory values, though none was associated with clinical evidence of adverse outcome:
- White blood cell counts were noted to increase in three of the studies by 4.7% to 10%, owing to increases in neutro-phils19,20,22
- Alkaline phosphatase concentrations decreased in four studies19,20,22,23
- Uric acid levels increased in four studies.19,20,22,23
RENAL INSUFFICIENCY SLOWS SITAGLIPTIN CLEARANCE
Lower doses and periodic monitoring of renal function are recommended in patients taking sitagliptin who have some degree of renal insufficiency. Clearance of sitagliptin is delayed in patients with renal insufficiency (creatinine clearance < 50 mL/minute).
In a placebo-controlled study of sitagliptin safety, Scott et al26 found that the area under the sitagliptin concentration-time curve was 2.3 times greater in patients with moderate renal insufficiency (creatinine clearance rate 30–49.9 mL/minute), 3.8 times greater in those with severe renal insufficiency (15–29.9 mL/minute), and 4.5 times greater in those with end-stage renal disease (< 15 mL/minute).
The Januvia package insert27 recommends that the daily dose be decreased to 50 mg in patients with creatinine clearance rates of 30 to 49.9 mL/minute (serum creatinine > 1.7 mg/dL in men, > 1.5 mg/dL in women), and that the dose be decreased to 25 mg per day in those with creatinine clearance rates below 30 mL/minute (creatinine > 3.0/2.5 mg/dL).
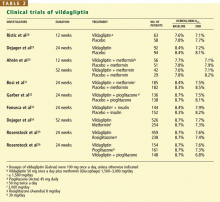
CLINICAL TRIALS OF VILDAGLIPTIN BEGIN
A study comparing vildagliptin against metformin34 showed less glucose-lowering over a 1-year period with vildagliptin, albeit with fewer gastrointestinal side effects, while comparisons with rosiglitazone (Avandia)35 and with pioglitazone36 showed similar glucose-lowering ability.
In a 24-week study,33 256 patients with type 2 diabetes who had a mean body mass index of 33 kg/m2 and who were taking more than 30 units of insulin daily (an average of 82 units) were randomized to additionally receive either vildagliptin 50 mg twice daily or placebo. The HbA1c decreased by 0.5% with vildagliptin and by 0.2% with placebo, from a baseline level of 8.5%. Of interest, 33 patients receiving vildagliptin had a hypo-glycemic episode (a total of 113 events), compared with 45 patients in the placebo group (185 events). None of the episodes in the vildagliptin group was classified as severe, whereas six episodes in the placebo group were classified as severe. This suggests that adding vildagliptin in patients taking insulin can improve glycemia without causing excessive hypoglycemia.
A weakness of the design of this study is that it did not include patients who were receiving an insulin sensitizer, an approach that is typically taken. Given this, it is understandable that overall glycemic control was relatively poor. More effort is needed to explore the use of gliptins with insulin.
WHAT ROLE FOR GLIPTINS?
The evidence from the studies reviewed in this article suggests that gliptins can play an important role in the treatment of type 2 diabetes. In certain patient groups such as the elderly, who cannot take either metformin or a thiazolidinedione and in whom concerns about hypoglycemia are greatest, thus precluding sulfonylurea therapy, gliptins may be the agents of choice. The trials reviewed here suggest that gliptins have glucose-lowering efficacy similar to that of these classes of agents. Gliptins are also effective when combined with metformin or a thiazolidinedione and, as discussed above, may prove to be useful in combination with insulin.
The eventual role of gliptins in the treatment of type 2 diabetes will depend on the answers to several questions. For example, do they preserve beta cell function and reverse the progression of diabetes? Do they affect insulin resistance? Do they have cardiovascular benefits beyond glucose-lowering? Also, since DPP-4 is widely distributed in the body, and since we do not yet know the effects of all the proteins cleaved by this enzyme, will this affect the long-term safety of these drugs?
For now, we can state with reasonable certainty that gliptins lower blood sugar levels to a degree similar to that of other oral hypo-glycemic therapies, with minimal risk of hypo-glycemia, with few immediate adverse effects, and without requiring dose titration. These characteristics suggest that gliptins should be considered useful agents in monotherapy and combination therapy for the treatment of type 2 diabetes.
- National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Last accessed February 28, 2008.
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29:2114–2116.
- American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007; 30 suppl 1:S4–S41.
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291:335–342.
- Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27:1535–1540.
- Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 2006; 29:2137–2139.
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003; 26:2929–2940.
- Bloomgarden ZT. Gut hormones and related concepts. Diabetes Care 2006; 29:2319–2324.
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyper-glycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-4 inhibitors. Diabetologia 2005; 48:612–615.
- Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140:5356–5363.
- Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 2007; 56:8–15.
- Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase IV with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006; 55:1695–1704.
- Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003; 52:741–750.
- Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91:4612–4619.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:186–193.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Nauck MA, Meininger JG, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Scott RS, Hartley P, Luo E, et al. Use of sitagliptin in patients with type 2 diabetes and renal insufficiency [abtract]. Diabetes 2006; 55 suppl 1:A462.
- Januvia prescribing information. www.merck.com/product/usa/pi_circulars/j/products_j.html. Last accessed February 28, 2008.
- Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab 2005; 7:692–698.
- Dejager S, Baron M, Razac S, Foley JE, Dickinson S, Schweizer S. Effect of vildagliptin on drug-naïve patients with type 2 diabetes. Diabetologia 2006; 49 suppl 1:479–480.
- Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase iv inhibitor laf237 in metformin-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2874–2880.
- Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007; 30:890–895.
- Garber A, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007; 50:1148–1155.
- Dejager S, LeBeaut A, Couturier A, Schweizer A. Sustained reduction in HbA1c during one-year treatment with vildagliptin in patients with type 2 diabetes (T2DM) [abstract]. Diabetes 2006; 55 suppl 1:A29.
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care 2007; 30:217–223.
- Rosenstock J, Baron MA, Camisasca R-P, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:175–185.
The “gliptins”—the nickname for dipeptidyl peptidase 4 (DPP-4) inhibitors—are one of the newest classes of drugs for the treatment of type 2 diabetes mellitus.
These drugs work by prolonging the action of gut hormones called incretins, which boost insulin levels. The greatest advantage of the gliptins appears to be their ability to stimulate insulin production with little risk of corresponding hypoglycemia.
Sitagliptin (Januvia), the first commercially available DPP-4 inhibitor, has been approved by the US Food and Drug Administration (FDA) and is currently in clinical use, and vildagliptin (Galvus) awaits FDA approval at the time of this writing. Other drugs of this class are in development.
However, because these drugs are so new, a number of questions remain about their use. In this article, we discuss the rationale behind gliptin drugs, the evidence to date on their use alone or in combination with current oral hypoglycemic drugs (and even with insulin), and when and how to use them in daily practice.
THE NEED FOR MORE EFFECTIVE DIABETES TREATMENT
As the number of patients with type 2 diabetes continues its steep and steady rise,1,2 much work has gone into studying treatment goals and how to achieve them. Although experts generally agree on glycemic goals,3 we currently fail to achieve those goals in close to two-thirds of patients: only 37% have a hemo-globin A1c (HbA1c) value at or below the goal of 7%, and the same number have levels exceeding 8%.4
Part of the problem is that treatment regimens are not adjusted in a timely fashion. In a prescribing database of almost 4,000 patients with type 2 diabetes,5 the mean time from the first HbA1c reading above 8% to an actual change in therapy was about 15 months for those taking metformin (Glucophage) alone, and 21 months for those taking a sulfonylurea alone. Another part of the problem is that, on average, patients with an HbA1c of 8.0% to 8.9% can expect only a 0.6% lowering with the addition of one agent.6 Clearly, we need new pharmacologic approaches and new management paradigms. One new approach is the use of gliptins.
HOW GLIPTINS WORK
Incretins promote insulin secretion
We have known for more than 20 years that insulin levels rise considerably higher in response to an oral glucose load than to an intravenous glucose infusion, even though the plasma glucose concentrations may be similar.7 This phenomenon involves a myriad of neural and nutritional factors, but the gut hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) appear to be key.
These peptides—called incretins—have a high degree of homology, and both promote insulin secretion. However, GLP-1, produced by the L cells of the ileum and colon, inhibits glucagon secretion and slows gastric emptying, whereas GIP, secreted from the K cells of the duodenum, has no effect on glucagon and little effect on gastric emptying. Both peptides appear to promote pancreatic beta cell growth and survival,8,9 an effect that in theory might allow us to slow the progressive loss of insulin secretory capacity in type 2 diabetes.
Furthermore, the effect of GLP-1 on insulin secretion depends on the plasma glucose concentration, with a greater insulin secretory effect at higher glucose levels and minimal effect at euglycemic levels.10 This phenomenon suggests that drugs that boost GLP-1 activity should not cause the troublesome hypoglycemia typically seen in patients taking insulin, insulin secretagogues, sulfonyl-ureas, or the meglitinides repaglinide (Prandin) or nateglinide (Starlix). Studies of combination treatment with metformin and the GLP-1 receptor agonist exenatide (Byetta) have shown little risk of hypoglycemia,11 offering evidence favoring this conjecture.
Inhibition of DPP-4 boosts incretin action
The challenge for creating treatments that take advantage of the beneficial effects of GLP-1 and GIP is that they have very short physiologic half-lives, ie, less than 10 minutes. GLP-1 and GIP both have two N-terminal amino acids that are quickly cleaved by DPP-4,12 an enzyme present in the circulation13 and on endothelial cells.14
Currently, there are two classes of drugs based on incretins. One class, the incretin mimetics or GLP-1 receptor agonists, includes drugs that mimic the effect of GLP-1 but are not so quickly degraded by DPP-4. Examples of these drugs are exenatide, which is currently FDA-approved, and liraglutide, which is not yet approved.
On the other hand, by inhibiting the cleaving action of DPP-4, the gliptins can prolong the half-life of endogenous GLP-1, increasing its physiologic effects.
Studies comparing gliptins with GLP-1 receptor agonists are only at the preclinical phase. Liraglutide showed an antiglycemic effect similar to that of vildagliptin in an animal model of glucose intolerance.15 This and other16,17 preclinical studies have shown evidence of improved beta cell growth and survival with DPP-4 inhibitor treatment, to an extent similar to that reported with thiazo-lidinediones, whereas sulfonylureas show no evidence either of increase in beta cells or of improved intrinsic beta cell secretory function in these models. Of course, animal studies can only be cautiously extrapolated to potential effects in humans, and it is uncertain whether such benefits will occur with the therapeutic use of DPP-4 inhibitors.
RANDOMIZED CLINICAL TRIALS OF SITAGLIPTIN
Sitagliptin is effective when used by itself,reducing a baseline HbA1c level of about 8% by 0.6% to 0.8%,19,20,24 and is similarly effective when combined with metformin21,22,25 or pioglitazone (Actos, a thiazolidinedione).23 It also decreases fasting blood glucose levels and improves other measures of glucose control.
A study comparing sitagliptin and the sul-fonylurea glipizide (Glucotrol) showed identical glucose-lowering over a 1-year period, with less hypoglycemia and weight gain with sitagliptin.25 Hypoglycemic episodes occurred in 32% of patients taking glipizide but in only 5% of those taking sitagliptin.
Studies noted several trends in laboratory values, though none was associated with clinical evidence of adverse outcome:
- White blood cell counts were noted to increase in three of the studies by 4.7% to 10%, owing to increases in neutro-phils19,20,22
- Alkaline phosphatase concentrations decreased in four studies19,20,22,23
- Uric acid levels increased in four studies.19,20,22,23
RENAL INSUFFICIENCY SLOWS SITAGLIPTIN CLEARANCE
Lower doses and periodic monitoring of renal function are recommended in patients taking sitagliptin who have some degree of renal insufficiency. Clearance of sitagliptin is delayed in patients with renal insufficiency (creatinine clearance < 50 mL/minute).
In a placebo-controlled study of sitagliptin safety, Scott et al26 found that the area under the sitagliptin concentration-time curve was 2.3 times greater in patients with moderate renal insufficiency (creatinine clearance rate 30–49.9 mL/minute), 3.8 times greater in those with severe renal insufficiency (15–29.9 mL/minute), and 4.5 times greater in those with end-stage renal disease (< 15 mL/minute).
The Januvia package insert27 recommends that the daily dose be decreased to 50 mg in patients with creatinine clearance rates of 30 to 49.9 mL/minute (serum creatinine > 1.7 mg/dL in men, > 1.5 mg/dL in women), and that the dose be decreased to 25 mg per day in those with creatinine clearance rates below 30 mL/minute (creatinine > 3.0/2.5 mg/dL).
CLINICAL TRIALS OF VILDAGLIPTIN BEGIN
A study comparing vildagliptin against metformin34 showed less glucose-lowering over a 1-year period with vildagliptin, albeit with fewer gastrointestinal side effects, while comparisons with rosiglitazone (Avandia)35 and with pioglitazone36 showed similar glucose-lowering ability.
In a 24-week study,33 256 patients with type 2 diabetes who had a mean body mass index of 33 kg/m2 and who were taking more than 30 units of insulin daily (an average of 82 units) were randomized to additionally receive either vildagliptin 50 mg twice daily or placebo. The HbA1c decreased by 0.5% with vildagliptin and by 0.2% with placebo, from a baseline level of 8.5%. Of interest, 33 patients receiving vildagliptin had a hypo-glycemic episode (a total of 113 events), compared with 45 patients in the placebo group (185 events). None of the episodes in the vildagliptin group was classified as severe, whereas six episodes in the placebo group were classified as severe. This suggests that adding vildagliptin in patients taking insulin can improve glycemia without causing excessive hypoglycemia.
A weakness of the design of this study is that it did not include patients who were receiving an insulin sensitizer, an approach that is typically taken. Given this, it is understandable that overall glycemic control was relatively poor. More effort is needed to explore the use of gliptins with insulin.
WHAT ROLE FOR GLIPTINS?
The evidence from the studies reviewed in this article suggests that gliptins can play an important role in the treatment of type 2 diabetes. In certain patient groups such as the elderly, who cannot take either metformin or a thiazolidinedione and in whom concerns about hypoglycemia are greatest, thus precluding sulfonylurea therapy, gliptins may be the agents of choice. The trials reviewed here suggest that gliptins have glucose-lowering efficacy similar to that of these classes of agents. Gliptins are also effective when combined with metformin or a thiazolidinedione and, as discussed above, may prove to be useful in combination with insulin.
The eventual role of gliptins in the treatment of type 2 diabetes will depend on the answers to several questions. For example, do they preserve beta cell function and reverse the progression of diabetes? Do they affect insulin resistance? Do they have cardiovascular benefits beyond glucose-lowering? Also, since DPP-4 is widely distributed in the body, and since we do not yet know the effects of all the proteins cleaved by this enzyme, will this affect the long-term safety of these drugs?
For now, we can state with reasonable certainty that gliptins lower blood sugar levels to a degree similar to that of other oral hypo-glycemic therapies, with minimal risk of hypo-glycemia, with few immediate adverse effects, and without requiring dose titration. These characteristics suggest that gliptins should be considered useful agents in monotherapy and combination therapy for the treatment of type 2 diabetes.
The “gliptins”—the nickname for dipeptidyl peptidase 4 (DPP-4) inhibitors—are one of the newest classes of drugs for the treatment of type 2 diabetes mellitus.
These drugs work by prolonging the action of gut hormones called incretins, which boost insulin levels. The greatest advantage of the gliptins appears to be their ability to stimulate insulin production with little risk of corresponding hypoglycemia.
Sitagliptin (Januvia), the first commercially available DPP-4 inhibitor, has been approved by the US Food and Drug Administration (FDA) and is currently in clinical use, and vildagliptin (Galvus) awaits FDA approval at the time of this writing. Other drugs of this class are in development.
However, because these drugs are so new, a number of questions remain about their use. In this article, we discuss the rationale behind gliptin drugs, the evidence to date on their use alone or in combination with current oral hypoglycemic drugs (and even with insulin), and when and how to use them in daily practice.
THE NEED FOR MORE EFFECTIVE DIABETES TREATMENT
As the number of patients with type 2 diabetes continues its steep and steady rise,1,2 much work has gone into studying treatment goals and how to achieve them. Although experts generally agree on glycemic goals,3 we currently fail to achieve those goals in close to two-thirds of patients: only 37% have a hemo-globin A1c (HbA1c) value at or below the goal of 7%, and the same number have levels exceeding 8%.4
Part of the problem is that treatment regimens are not adjusted in a timely fashion. In a prescribing database of almost 4,000 patients with type 2 diabetes,5 the mean time from the first HbA1c reading above 8% to an actual change in therapy was about 15 months for those taking metformin (Glucophage) alone, and 21 months for those taking a sulfonylurea alone. Another part of the problem is that, on average, patients with an HbA1c of 8.0% to 8.9% can expect only a 0.6% lowering with the addition of one agent.6 Clearly, we need new pharmacologic approaches and new management paradigms. One new approach is the use of gliptins.
HOW GLIPTINS WORK
Incretins promote insulin secretion
We have known for more than 20 years that insulin levels rise considerably higher in response to an oral glucose load than to an intravenous glucose infusion, even though the plasma glucose concentrations may be similar.7 This phenomenon involves a myriad of neural and nutritional factors, but the gut hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) appear to be key.
These peptides—called incretins—have a high degree of homology, and both promote insulin secretion. However, GLP-1, produced by the L cells of the ileum and colon, inhibits glucagon secretion and slows gastric emptying, whereas GIP, secreted from the K cells of the duodenum, has no effect on glucagon and little effect on gastric emptying. Both peptides appear to promote pancreatic beta cell growth and survival,8,9 an effect that in theory might allow us to slow the progressive loss of insulin secretory capacity in type 2 diabetes.
Furthermore, the effect of GLP-1 on insulin secretion depends on the plasma glucose concentration, with a greater insulin secretory effect at higher glucose levels and minimal effect at euglycemic levels.10 This phenomenon suggests that drugs that boost GLP-1 activity should not cause the troublesome hypoglycemia typically seen in patients taking insulin, insulin secretagogues, sulfonyl-ureas, or the meglitinides repaglinide (Prandin) or nateglinide (Starlix). Studies of combination treatment with metformin and the GLP-1 receptor agonist exenatide (Byetta) have shown little risk of hypoglycemia,11 offering evidence favoring this conjecture.
Inhibition of DPP-4 boosts incretin action
The challenge for creating treatments that take advantage of the beneficial effects of GLP-1 and GIP is that they have very short physiologic half-lives, ie, less than 10 minutes. GLP-1 and GIP both have two N-terminal amino acids that are quickly cleaved by DPP-4,12 an enzyme present in the circulation13 and on endothelial cells.14
Currently, there are two classes of drugs based on incretins. One class, the incretin mimetics or GLP-1 receptor agonists, includes drugs that mimic the effect of GLP-1 but are not so quickly degraded by DPP-4. Examples of these drugs are exenatide, which is currently FDA-approved, and liraglutide, which is not yet approved.
On the other hand, by inhibiting the cleaving action of DPP-4, the gliptins can prolong the half-life of endogenous GLP-1, increasing its physiologic effects.
Studies comparing gliptins with GLP-1 receptor agonists are only at the preclinical phase. Liraglutide showed an antiglycemic effect similar to that of vildagliptin in an animal model of glucose intolerance.15 This and other16,17 preclinical studies have shown evidence of improved beta cell growth and survival with DPP-4 inhibitor treatment, to an extent similar to that reported with thiazo-lidinediones, whereas sulfonylureas show no evidence either of increase in beta cells or of improved intrinsic beta cell secretory function in these models. Of course, animal studies can only be cautiously extrapolated to potential effects in humans, and it is uncertain whether such benefits will occur with the therapeutic use of DPP-4 inhibitors.
RANDOMIZED CLINICAL TRIALS OF SITAGLIPTIN
Sitagliptin is effective when used by itself,reducing a baseline HbA1c level of about 8% by 0.6% to 0.8%,19,20,24 and is similarly effective when combined with metformin21,22,25 or pioglitazone (Actos, a thiazolidinedione).23 It also decreases fasting blood glucose levels and improves other measures of glucose control.
A study comparing sitagliptin and the sul-fonylurea glipizide (Glucotrol) showed identical glucose-lowering over a 1-year period, with less hypoglycemia and weight gain with sitagliptin.25 Hypoglycemic episodes occurred in 32% of patients taking glipizide but in only 5% of those taking sitagliptin.
Studies noted several trends in laboratory values, though none was associated with clinical evidence of adverse outcome:
- White blood cell counts were noted to increase in three of the studies by 4.7% to 10%, owing to increases in neutro-phils19,20,22
- Alkaline phosphatase concentrations decreased in four studies19,20,22,23
- Uric acid levels increased in four studies.19,20,22,23
RENAL INSUFFICIENCY SLOWS SITAGLIPTIN CLEARANCE
Lower doses and periodic monitoring of renal function are recommended in patients taking sitagliptin who have some degree of renal insufficiency. Clearance of sitagliptin is delayed in patients with renal insufficiency (creatinine clearance < 50 mL/minute).
In a placebo-controlled study of sitagliptin safety, Scott et al26 found that the area under the sitagliptin concentration-time curve was 2.3 times greater in patients with moderate renal insufficiency (creatinine clearance rate 30–49.9 mL/minute), 3.8 times greater in those with severe renal insufficiency (15–29.9 mL/minute), and 4.5 times greater in those with end-stage renal disease (< 15 mL/minute).
The Januvia package insert27 recommends that the daily dose be decreased to 50 mg in patients with creatinine clearance rates of 30 to 49.9 mL/minute (serum creatinine > 1.7 mg/dL in men, > 1.5 mg/dL in women), and that the dose be decreased to 25 mg per day in those with creatinine clearance rates below 30 mL/minute (creatinine > 3.0/2.5 mg/dL).
CLINICAL TRIALS OF VILDAGLIPTIN BEGIN
A study comparing vildagliptin against metformin34 showed less glucose-lowering over a 1-year period with vildagliptin, albeit with fewer gastrointestinal side effects, while comparisons with rosiglitazone (Avandia)35 and with pioglitazone36 showed similar glucose-lowering ability.
In a 24-week study,33 256 patients with type 2 diabetes who had a mean body mass index of 33 kg/m2 and who were taking more than 30 units of insulin daily (an average of 82 units) were randomized to additionally receive either vildagliptin 50 mg twice daily or placebo. The HbA1c decreased by 0.5% with vildagliptin and by 0.2% with placebo, from a baseline level of 8.5%. Of interest, 33 patients receiving vildagliptin had a hypo-glycemic episode (a total of 113 events), compared with 45 patients in the placebo group (185 events). None of the episodes in the vildagliptin group was classified as severe, whereas six episodes in the placebo group were classified as severe. This suggests that adding vildagliptin in patients taking insulin can improve glycemia without causing excessive hypoglycemia.
A weakness of the design of this study is that it did not include patients who were receiving an insulin sensitizer, an approach that is typically taken. Given this, it is understandable that overall glycemic control was relatively poor. More effort is needed to explore the use of gliptins with insulin.
WHAT ROLE FOR GLIPTINS?
The evidence from the studies reviewed in this article suggests that gliptins can play an important role in the treatment of type 2 diabetes. In certain patient groups such as the elderly, who cannot take either metformin or a thiazolidinedione and in whom concerns about hypoglycemia are greatest, thus precluding sulfonylurea therapy, gliptins may be the agents of choice. The trials reviewed here suggest that gliptins have glucose-lowering efficacy similar to that of these classes of agents. Gliptins are also effective when combined with metformin or a thiazolidinedione and, as discussed above, may prove to be useful in combination with insulin.
The eventual role of gliptins in the treatment of type 2 diabetes will depend on the answers to several questions. For example, do they preserve beta cell function and reverse the progression of diabetes? Do they affect insulin resistance? Do they have cardiovascular benefits beyond glucose-lowering? Also, since DPP-4 is widely distributed in the body, and since we do not yet know the effects of all the proteins cleaved by this enzyme, will this affect the long-term safety of these drugs?
For now, we can state with reasonable certainty that gliptins lower blood sugar levels to a degree similar to that of other oral hypo-glycemic therapies, with minimal risk of hypo-glycemia, with few immediate adverse effects, and without requiring dose titration. These characteristics suggest that gliptins should be considered useful agents in monotherapy and combination therapy for the treatment of type 2 diabetes.
- National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Last accessed February 28, 2008.
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29:2114–2116.
- American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007; 30 suppl 1:S4–S41.
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291:335–342.
- Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27:1535–1540.
- Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 2006; 29:2137–2139.
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003; 26:2929–2940.
- Bloomgarden ZT. Gut hormones and related concepts. Diabetes Care 2006; 29:2319–2324.
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyper-glycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-4 inhibitors. Diabetologia 2005; 48:612–615.
- Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140:5356–5363.
- Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 2007; 56:8–15.
- Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase IV with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006; 55:1695–1704.
- Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003; 52:741–750.
- Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91:4612–4619.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:186–193.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Nauck MA, Meininger JG, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Scott RS, Hartley P, Luo E, et al. Use of sitagliptin in patients with type 2 diabetes and renal insufficiency [abtract]. Diabetes 2006; 55 suppl 1:A462.
- Januvia prescribing information. www.merck.com/product/usa/pi_circulars/j/products_j.html. Last accessed February 28, 2008.
- Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab 2005; 7:692–698.
- Dejager S, Baron M, Razac S, Foley JE, Dickinson S, Schweizer S. Effect of vildagliptin on drug-naïve patients with type 2 diabetes. Diabetologia 2006; 49 suppl 1:479–480.
- Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase iv inhibitor laf237 in metformin-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2874–2880.
- Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007; 30:890–895.
- Garber A, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007; 50:1148–1155.
- Dejager S, LeBeaut A, Couturier A, Schweizer A. Sustained reduction in HbA1c during one-year treatment with vildagliptin in patients with type 2 diabetes (T2DM) [abstract]. Diabetes 2006; 55 suppl 1:A29.
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care 2007; 30:217–223.
- Rosenstock J, Baron MA, Camisasca R-P, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:175–185.
- National Diabetes Surveillance System. www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Last accessed February 28, 2008.
- Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: US, 2005–2050. Diabetes Care 2006; 29:2114–2116.
- American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care 2007; 30 suppl 1:S4–S41.
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291:335–342.
- Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care 2004; 27:1535–1540.
- Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose-lowering efficacy: a meta-regression analysis. Diabetes Care 2006; 29:2137–2139.
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29:46–52.
- Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003; 26:2929–2940.
- Bloomgarden ZT. Gut hormones and related concepts. Diabetes Care 2006; 29:2319–2324.
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyper-glycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993; 36:741–744.
- DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005; 28:1092–1100.
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
- Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-4 inhibitors. Diabetologia 2005; 48:612–615.
- Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140:5356–5363.
- Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes 2007; 56:8–15.
- Mu J, Woods J, Zhou YP, et al. Chronic inhibition of dipeptidyl peptidase IV with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes 2006; 55:1695–1704.
- Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 2003; 52:741–750.
- Herman GA, Bergman A, Stevens C, et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J Clin Endocrinol Metab 2006; 91:4612–4619.
- Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006; 29:2632–2637.
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49:2564–2571.
- Brazg R, Xu L, Dalla Man C, Cobelli C, Thomas K, Stein PP. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:186–193.
- Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care 2006; 29:2638–2643.
- Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2006; 28:1556–1568.
- Scott R, Wu M, Sanchez M, Stein P. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract 2007; 61:171–180.
- Nauck MA, Meininger JG, Sheng D, Terranella L, Stein PP. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007; 9:194–205.
- Scott RS, Hartley P, Luo E, et al. Use of sitagliptin in patients with type 2 diabetes and renal insufficiency [abtract]. Diabetes 2006; 55 suppl 1:A462.
- Januvia prescribing information. www.merck.com/product/usa/pi_circulars/j/products_j.html. Last accessed February 28, 2008.
- Ristic S, Byiers S, Foley J, Holmes D. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab 2005; 7:692–698.
- Dejager S, Baron M, Razac S, Foley JE, Dickinson S, Schweizer S. Effect of vildagliptin on drug-naïve patients with type 2 diabetes. Diabetologia 2006; 49 suppl 1:479–480.
- Ahrén B, Gomis R, Standl E, Mills D, Schweizer A. Twelve- and 52-week efficacy of the dipeptidyl peptidase iv inhibitor laf237 in metformin-treated patients with type 2 diabetes. Diabetes Care 2004; 27:2874–2880.
- Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care 2007; 30:890–895.
- Garber A, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab 2007; 9:166–174.
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007; 50:1148–1155.
- Dejager S, LeBeaut A, Couturier A, Schweizer A. Sustained reduction in HbA1c during one-year treatment with vildagliptin in patients with type 2 diabetes (T2DM) [abstract]. Diabetes 2006; 55 suppl 1:A29.
- Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes. Diabetes Care 2007; 30:217–223.
- Rosenstock J, Baron MA, Camisasca R-P, Cressier F, Couturier A, Dejager S. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab 2007; 9:175–185.
KEY POINTS
- Sitagliptin (Januvia) is now available, and vildagliptin (Galvus) is awaiting approval. Other gliptins are under development.
- The gliptins effectively lower blood glucose levels, do not require titration, are unlikely to cause hypoglycemia, do not cause weight gain or loss, and are well tolerated.
- Gliptins can be used alone or in combination with metformin (Glucophage) or a thiazolidinedione. Preliminary studies also show evidence of benefit when they are used in combination with insulin.
- Comparative studies suggest that gliptins lower blood glucose levels by about the same amount as other oral hypoglycemic agents.
What is the role of dual antiplatelet therapy with clopidogrel and aspirin?
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
In patients at risk of myocardial infarction or stroke, two antiplatelet drugs are not always better than one. In a large recent trial,1,2 adding clopidogrel (Plavix) to aspirin therapy did not offer much benefit to a cohort of patients at risk of cardiovascular events, although a subgroup did appear to benefit: those at even higher risk because they already had a history of myocardial infarction, ischemic stroke, or peripheral arterial disease.
These were the principal findings in the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) study,1,2 in which one of us (D.L.B.) was principal investigator.
These findings further our understanding of who should receive dual antiplatelet therapy, and who would be better served with aspirin therapy alone. In this article, we discuss important studies that led up to the CHARISMA trial, review CHARISMA’s purpose and study design, and interpret its results.
PREVENTING ATHEROTHROMBOSIS BY BLOCKING PLATELETS
Platelets are key players in the atherothrom-botic process.3–5 The Antiplatelet Trialists’ Collaboration,6 in a meta-analysis of trials performed up to 1997, calculated that antiplatelet therapy (mostly with aspirin) reduced the vascular mortality rate by 15% in patients with acute or previous vascular disease or some other predisposing condition. Thus, aspirin has already been shown to be effective as primary prevention (ie, in patients at risk but without established vascular disease) and as secondary prevention (ie, in those with established disease).7,8
Yet many patients have significant vascular events in spite of taking aspirin.6 Aspirin failure is thought to be multifactorial, with causes that include weak platelet inhibition, noncompliance, discontinuation due to adverse effects (including severe bleeding), and drug interactions. In addition, aspirin resistance has been linked to worse prognosis and may prove to be another cause of aspirin failure.9–11
Clopidogrel, an adenosine diphosphate (ADP) receptor antagonist, has also been studied extensively as an antiplatelet agent.5,12 Several studies have indicated that clopidogrel and ticlopidine (Ticlid, a related drug) may be more potent than aspirin, both in the test tube and in real patients.13–15
KEY TRIALS LEADING TO CHARISMA
- Clopidogrel is more effective and slightly safer than aspirin as secondary prevention, as shown in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) trial.16–21
- The combination of clopidogrel plus aspirin is more beneficial than placebo plus aspirin in patients with acute coronary syndromes, as shown in the Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events (CURE) trial,22–24 the Clopidogrel as Adjunctive Reperfusion Therapy-Thrombolysis in Myo-car-dial Infarction (CLARITY-TIMI 28) trial,25 and the Clopidogrel and Metoprolol in Myocardial Infarction Trial (COMMIT).26
- The combination of clopidogrel plus aspirin is beneficial in patients undergoing percutaneous coronary interventions, with or without drug-eluting stent placement,27–30 as shown in the Clopidogrel for the Reduction of Events During Observation (CREDO) trial,28 the Effect of Clopidogrel Pretreatment Before Percutaneous Coronary Intervention in Patients With ST-Elevation Myocardial Infarction With Fibrinolytics (PCI-CLARITY) study,29 and the Effects of Pre-treatment With Clopidogrel and Aspirin Followed by Long-term Therapy in Patients Undergoing Percutaneous Coronary Intervention (PCI-CURE) study.30 In fact, most patients undergoing percutaneous interventions now receive a loading dose of clopidogrel before the procedure and continue to take it for up to 1 year afterward. However, the ideal long-term duration of clopidogrel treatment is still under debate.
In view of these previous studies, we wanted to test dual antiplatelet therapy in a broader population at high risk of atherothrombosis, ie, in patients with either established vascular disease or with multiple risk factors for it.
CHARISMA STUDY DESIGN
CHARISMA was a prospective, randomized, double-blind, placebo-controlled study of the efficacy and safety of clopidogrel plus aspirin vs placebo plus aspirin in patients at high risk of cardiovascular events.
A total of 15,603 patients, all older than 45 years, were randomly assigned to receive clopidogrel 75 mg/day plus aspirin 75 to 162 mg/day or placebo plus aspirin, in addition to standard therapy as directed by individual clinicians (eg, statins, beta-blockers). Patients were followed up at 1, 3, and 6 months and every 6 months thereafter until study completion, which occurred after 1,040 primary efficacy end points. The median duration of follow-up was 28 months.1
Patients had to have one of the following to be included: multiple atherothrombotic risk factors, documented coronary disease, documented cerebrovascular disease, or documented peripheral arterial disease (Table 2). Specific exclusion criteria included the use of oral antithrombotic or chronic nonsteroidal anti-inflammatory medications.1
End points
The primary end point was the combined incidence of the first episode of myocardial infarction or stroke, or death from cardiovascular causes.
The secondary end point was the combined incidence of myocardial infarction, stroke, death from cardiovascular causes, or hospitalization for unstable angina, a transient ischemic attack, or revascularization procedure.
The primary safety end point was severe bleeding, as defined in the Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO) study31 as intracranial hemorrhage, fatal bleeding, or bleeding leading to hemody-namic compromise. Moderate bleeding was defined as bleeding that required transfusion but did not meet the GUSTO definition of severe bleeding.
OVERALL, NO BENEFIT
The rates of the secondary end point were 16.7% vs 17.9% (absolute risk reduction 1.2%; relative risk reduction 8%; P = .04).
Possible benefit in symptomatic patients
In a prespecified analysis, patients were classified as being “symptomatic” (having documented cardiovascular disease, ie, coronary, cerebrovascular, or symptomatic peripheral arterial disease) or “asymptomatic” (having multiple risk factors without established cardiovascular disease).1
In the symptomatic group (n = 12,153), the primary end point was reached in 6.9% of patients treated with clopidogrel vs 7.9% with placebo (absolute risk reduction 1.0%; relative risk reduction 13%; P = .046). The 3,284 asymptomatic patients showed no benefit; the rate of the primary end point for the clopido-grel group was 6.6% vs 5.5% in the placebo group (P = .20).
In a post hoc analysis, we examined the data from 9,478 patients who were similar to those in the CAPRIE study (ie, with documented prior myocardial infarction, prior ischemic stroke, or symptomatic peripheral arterial disease). The rate of cardiovascular death, myocardial infarction, or stroke was 8.8% in the placebo-plus-aspirin group and 7.3% in the clopidogrel-plus-aspirin group (absolute risk reduction 1.5%; relative risk reduction 17%; P = .01; Figure 1).2
HOW SHOULD WE INTERPRET THESE FINDINGS?
CHARISMA was the first trial to evaluate whether adding clopidogrel to aspirin therapy would reduce the rates of vascular events and death from cardiovascular causes in stable patients at risk of ischemic events. As in other trials, the benefit of clopidogrel-plus-aspirin therapy was weighed against the risk of bleeding with this regimen. How are we to interpret the findings?
- In the group with multiple risk factors but without clearly documented cardiovascular disease, there was no benefit—and there was an increase in moderate bleeding. Given these findings, physicians should not prescribe dual antiplatelet therapy for primary prevention in patients without known vascular disease.
- A potential benefit was seen in a prespecified subgroup who had documented cardiovascular disease. Given the limitations of subgroup analysis, however, and given the increased risk of moderate bleeding, this positive result should be interpreted with some degree of caution.
- CHARISMA suggests that there may be benefit of protracted dual antiplatelet therapy in stable patients with documented prior ischemic events.
A possible reason for the observed lack of benefit in the overall cohort but the positive results in the subgroups with established vascular disease is that plaque rupture and thrombosis may be a precondition for dual antiplatelet therapy to work.
Another possibility is that, although we have been saying that diabetes mellitus (one of the possible entry criteria in CHARISMA) is a “coronary risk equivalent,” this may not be absolutely true. Although it had been demonstrated that patients with certain risk factors, such as diabetes, have an incidence of ischemic events similar to that in patients with prior MI and should be considered for antiplatelet therapy to prevent vascular events,32 more recent data have shown that patients with prior ischemic events are at much higher risk than patients without ischemic events, even if the latter have diabetes.33,34
- The observation in CHARISMA that the incremental bleeding risk of dual antiplatelet therapy vs aspirin does not persist beyond a year in patients who have tolerated therapy for a year without a bleeding event may affect the decision to continue clopidogrel beyond 1 year, such as in patients with acute coronary syndromes or patients who have received drug-eluting stents.35,36
- Another important consideration is cost-effectiveness. Several studies have analyzed the impact of cost and found clopidogrel to be cost-effective by preventing ischemic events and adding years of life.37,38 A recent analysis from CHARISMA also shows cost-effectiveness in the subgroup of patients enrolled with established cardiovascular disease.39 Once clopidogrel becomes generic, the cost-effectiveness will become even better.
Further studies should better define which stable patients with cardiovascular disease should be on more than aspirin alone.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
- Bhatt DL, Fox KA, Hacke W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 2006; 354:1706–1717.
- Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol 2007; 49:1982–1988.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med 2002; 8:1227–1234.
- Fuster V, Moreno PR, Fayad ZA, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: part I: evolving concepts. J Am Coll Cardiol 2005; 46:937–954.
- Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res 2007; 100:1261–1275.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Aspirin for primary prevention of coronary heart disease: safety and absolute benefit related to coronary risk derived from meta-analysis of randomised trials. Heart 2001; 85:265–271.
- Hayden M, Pignone M, Phillips C, Mulrow C. Aspirin for the primary prevention of cardiovascular events: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002; 136:161–172.
- Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke 1994; 25:2331–2336.
- Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke 1993; 24:345–350.
- Gum PA, Kottke-Marchant K, Poggio ED, et al. Profile and prevalence of aspirin resistance in patients with cardiovascular disease. Am J Cardiol 2001; 88:230–235.
- Coukell AJ, Markham A. Clopidogrel. Drugs 1997; 54:745–750.
- Humbert M, Nurden P, Bihour C, et al. Ultrastructural studies of platelet aggregates from human subjects receiving clopidogrel and from a patient with an inherited defect of an ADP-dependent pathway of platelet activation. Arterioscler Thromb Vasc Biol 1996; 16:1532–1543.
- Hass WK, Easton JD, Adams HP, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med 1989; 321:501–507.
- Savi P, Bernat A, Dumas A, Ait-Chek L, Herbert JM. Effect of aspirin and clopidogrel on platelet-dependent tissue factor expression in endothelial cells. Thromb Res 1994; 73:117–124.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopido-grel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348:1329–1339.
- Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–628.
- Bhatt DL, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Reduction in the need for hospitalization for recurrent ischemic events and bleeding with clopidogrel instead of aspirin. CAPRIE investigators. Am Heart J 2000; 140:67–73.
- Bhatt DL, Topol EJ. Antiplatelet and anticoagulant therapy in the secondary prevention of ischemic heart disease. Med Clin North Am 2000; 84 1:163–179.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events Investigators. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke 2004; 35:528–532.
- Bhatt DL, Chew DP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation 2001; 103:363–368.
- Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001; 345:494–502.
- Budaj A, Yusuf S, Mehta SR, et al. Benefit of clopidogrel in patients with acute coronary syndromes without ST-segment elevation in various risk groups. Circulation 2002; 106:1622–1626.
- Fox KA, Mehta SR, Peters R, et al. Benefits and risks of the combination of clopidogrel and aspirin in patients undergoing surgical revascularization for non–ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) Trial. Circulation 2004; 110:1202–1208.
- Sabatine MS, Cannon CP, Gibson CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005; 352:1179–1189.
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005; 366:1607–1621.
- Bhatt DL, Kapadia SR, Bajzer CT, et al. Dual antiplatelet therapy with clopidogrel and aspirin after carotid artery stenting. J Invasive Cardiol 2001; 13:767–771.
- Steinhubl SR, Berger PB, Mann JT, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA 2002; 288:2411–2420.
- Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pre-treatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA 2005; 294:1224–1232.
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001; 358:527–533.
- The GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med 1993; 329:673–682.
- Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339:229–234.
- Bhatt DL, Steg PG, Ohman EM, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA 2006; 295:180–189.
- Steg PG, Bhatt DL, Wilson PW, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA 2007; 297:1197–1206.
- Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med 2006; 119:1056–1061.
- Rabbat MG, Bavry AA, Bhatt DL, Ellis SG. Understanding and minimizing late thrombosis of drug-eluting stents. Cleve Clin J Med 2007; 74:129–136.
- Gaspoz JM, Coxson PG, Goldman PA, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med 2002; 346:1800–1806.
- Beinart SC, Kolm P, Veledar E, et al. Longterm cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention results: from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005; 46:761–769.
- Chen J, Bhatt DL, Schneider E, et al. Cost-effectiveness of clopidogrel + aspirin vs. aspirin alone for secondary prevention of cardiovascular events: results from the CHARISMA Trial Session; APS.96.1; Presentation 3855; American Heart Association Scientific Sessions; Nov 12–15, 2006; Chicago IL.
KEY POINTS
- Platelets are key players in atherothrombosis, and antiplatelet drugs such as aspirin and clopidogrel prevent events in patients at risk.
- In studies leading up to CHARISMA, the combination of clopidogrel and aspirin was found to be beneficial in patients with acute coronary syndromes and in those undergoing percutaneous coronary interventions.
- Clopidogrel should not be combined with aspirin as a primary preventive therapy (ie, for people without established vascular disease). How dual antiplatelet therapy should be used as secondary prevention in stable patients needs further study.
A 61-year-old with bipolar disorder and cognitive impairment: Dementia or polypharmacy?
A 61-year-old man presents for evaluation of new-onset cognitive impairment, which has developed over the past 6 to 8 months. He has bipolar disorder, for which he has been taking lithium carbonate (Eskalith) for the past 15 years. This therapy kept his mood stable until a relapse of depression and mania 1 year ago required hospitalization and an increase in the lithium dose, which was then lowered somewhat after he improved (see below). His cognitive symptoms appeared gradually within 2 months after his release from the hospital.
He now has difficulty concentrating, a tendency to substitute words incorrectly during conversation, and difficulty recalling names and “retrieving memories.” He also reports a worsening tremor in his dominant hand that compromises his ability to eat with a spoon or a fork. He complains of increasing daytime somnolence, which began when his lithium dose was increased and improved when the dose was decreased.
The patient is a mathematician and recently finished revising the curriculum for an undergraduate course in advanced mathematics that he teaches. He does not smoke cigarettes, and he drinks alcohol only socially. He has no other medical conditions and no known cardiovascular risk factors.
Current and recent medications
- Lithium carbonate 600 mg twice daily (before his hospitalization he had been taking 600 mg twice daily; this was increased to 1,500 mg/day during the hospitalization and then decreased to the current dose as maintenance therapy)
- Divalproex (Depakote) 250 mg every night
- Gabapentin (Neurontin) 400 mg every night (the dosages of divalproex and gabapentin have remained unchanged since before his hospitalization)
- A multivitamin daily
- Naproxen (Naprosyn, Aleve) 250 mg up to two times a week for arthritic knee pain
- Aripiprazole (Abilify). This antipsychotic drug was recently discontinued because of parkinsonian symptoms, which then gradually improved.
- Memantine (Namenda), which is indicated for the treatment of moderate to severe Alzheimer disease. The patient reports that he stopped taking it after 3 weeks because he did not perceive it to be helping.
THE INITIAL EVALUATION
Physical examination
Temperature 98.3°F (36.8°C), pulse 60 beats per minute, respirations 16 per minute, blood pressure 126/64 mm Hg sitting and 118/71 mm Hg standing.
The patient is well groomed, alert, and cooperative. His head, eyes, ears, nose, and throat are normal. His teeth are in good condition. His skin is normal. We note no thyromegaly, carotid bruits, or palpable lymphadenopathy. His lungs are clear to auscultation. Results of cardiac, abdominal, and musculoskeletal examinations are all normal.
His deep tendon reflexes, sensory and motor testing, and gait are normal. The cerebellar examination is normal, aside from a mild tremor in his right hand when it is outstretched, with no resting tremor or cogwheel rigidity.
On the Mini-Mental State Examination (MMSE) he scores a perfect 30/30 (normal 24–30). He can draw a clock normally. His score on the short-form Geriatric Depression Scale is 4/15 (a score of 6 or higher indicates depression).
Laboratory tests
- Serum lithium level 0.8 mmol/L (therapeutic range 0.5–1.5 mmol/L) (his previous values are not available)
- Thyroid-stimulating hormone level 1.61 μU/mL (normal 0.40–5.50)
- Complete blood cell count and comprehensive metabolic panel values are within normal limits.
Magnetic resonance imaging
Noncontrast magnetic resonance imaging of the head reveals two nonspecific punctate foci of high signal intensity on T2-weighted images in the left frontal white matter, but the results are otherwise normal.
DIFFERENTIAL DIAGNOSIS
1. On the basis of this information, which is the most likely cause of this patient’s cogitive impairment?
- Dementia with Lewy bodies
- Early-onset Alzheimer disease
- Stroke with vascular cognitive impairment
- Lithium neurotoxicity
Lithium neurotoxicity is the most likely cause of this patient’s symptoms, given the temporal relationship between the adjusting of his lithium dose and the onset of his symptoms. Lithium therapy causes subtle cognitive deficits. Its dosing in older patients requires careful monitoring because of age-related alterations in its pharmacology and its various drug interactions; both mechanisms played a role in precipitating lithium toxicity in this patient.
Although his lithium levels are in the broadly accepted therapeutic range, there is much debate about the best maintenance level for patients with bipolar disorder. A level in the range of 1 to 1.2 mmol/L may be best in acute mania, while a lower level of around 0.8 mmol/L is preferred in the depressive phase. Once the patient’s mood has stabilized, the best maintenance level may be in the range of 0.2 to 0.6 mmol/L.
Dementia with Lewy bodies, although suggested by the patient’s cognitive impairment, history of parkinsonian symptoms, and somnolence, is an unlikely cause because his motor symptoms resolved after the aripiprazole was discontinued, his somnolence improved after the dose of lithium was reduced, and his alertness did not fluctuate thereafter as would be expected in dementia with Lewy bodies.
Alzheimer disease usually manifests as gradually progressive cognitive deficits involving memory impairment with one or more of the following: aphasia, apraxia, agnosia, and disturbance in executive functioning. In contrast, this patient’s memory loss was fairly abrupt and not slowly progressive.
Stroke is also unlikely, as he has no history of stroke or focal neurologic deficits. Although a magnetic resonance scan of the brain showed some evidence of small-vessel ischemic changes, it showed no cortical infarcts.
MECHANISMS OF LITHIUM NEUROTOXICITY
2. What are the possible mechanisms of lithium neurotoxicity in this patient?
- The increased dose of lithium
- The interaction of nonsteroidal anti-inflammatory drugs (NSAIDs) and lithium
- The interaction of the other psychotropic medications with lithium
- All of the above
- None of the above
All of the above could be contributing.
Although lithium is thought to cause side effects in as many as 60% of patients of any age who take it, the rate of serious adverse effects is reportedly higher in older patients than in younger patients.1
That said, cognitive deficits are common in bipolar disorder irrespective of lithium use.
COGNITIVE IMPAIRMENT IN BIPOLAR DISORDER
3. If cognitive impairment in bipolar disorder is common, when does it occur?
- Only in the remission phase
- Only in the manic phase
- Only in the depression phase
- In all phases of the disease
Cognitive impairment occurs in all phases of bipolar disorder. Neuropsychological testing of bipolar patients in remission uncovers subtle, persistent cognitive impairment in executive function and in visuospatial memory without mood symptoms.3–5 Impaired executive functioning, predominantly frontal lobe dysfunction, interferes with one’s ability to initiate, plan, perform, and successfully complete a task and challenges one’s ability to function effectively in society and to comply with medical advice and instructions on taking medications.
RECOMMENDATIONS
4. What should we recommend to this patient?
- Decrease the current dose of lithium
- Stop all medications
- Undergo detailed neuropsychological testing
- Follow up with a psychiatrist, if needed
The patient’s lithium level was within the therapeutic range and his bipolar symptoms were well controlled. In older patients, however, the optimal serum level of lithium is often unclear, making it advisable to reduce the dose when an adverse effect is suspected.
His other medications should be reviewed. Gabapentin is not indicated for use as a mood stabilizer, and his divalproex dose (250 mg) is well below the usual therapeutic dose of 1,000 to 2,000 mg/day.6 The gabapentin could be discontinued, and the divalproex could be increased to a therapeutic dose.
NSAIDs can increase serum lithium levels, diminish renal lithium clearance, and possibly induce lithium toxicity, but the effect varies considerably among drugs and individuals.7 We would advise this patient to stop taking naproxen and switch to acetaminophen (Tylenol) for his arthritis pain, and we would inform him of the risk of lithium toxicity with continuous use of NSAIDs.
We would also recommend additional neuropsychological testing. The patient noticed subtle difficulties in his cognitive abilities that were not apparent on the MMSE. While the MMSE is an acceptable cognitive test, it is often not sensitive enough to detect milder forms of cognitive impairment, especially in well-educated patients at the usual cut-point of 24. A comprehensive neuropsychological examination is a more sensitive measure of cognition, involving the detailed testing of various cognitive domains. It can reveal a pattern of cognitive impairment that helps to differentiate between normal and mood disorders and also can detect subtle executive dysfunction.
However, detailed neuropsychological testing is time-consuming and may not be obtained rapidly enough to help in making clinical decisions quickly. In this patient’s case, immediate collaboration and follow-up with the patient’s psychiatrist would be the most expeditious way to reassess the patient’s medication regimen.
FOLLOW-UP COURSE
We informed the patient’s psychiatrist that we thought the patient had increased sensitivity to lithium (even at “therapeutic” levels), possibly related to a drug-drug interaction.
His dose of lithium was kept at 600 mg twice daily, as the lithium toxicity was most likely due to a drug-drug interaction.
We discontinued his memantine, since he did not have Alzheimer disease and since he wasn’t taking it anyway. He continued taking gabapentin and divalproex at the same doses, and he stopped taking naproxyn and substituted acetaminophen for his arthritis pain. We advised him about about health maintenance, including proper nutrition, mineral and vitamin supplements, and exercise.
The patient underwent neuropsychological testing to better characterize his cognitive impairment. The findings did not suggest dementia, but were consistent with minor cognitive deficits caused by lithium.
When seen at a follow-up visit 6 weeks later the patient was free of symptoms except for the tremor in his dominant hand. His mood was stable and his cognition was better. No further changes were required in his psychotropic drug regimen.
TAKE-HOME POINTS
When a bipolar patient develops acute changes in cognition, we should suspect adverse effects of lithium as the cause, because of its narrow therapeutic window and interactions with other prescribed drugs. The case presented here reminds us to consider adverse drug effects any time an older patient develops acute changes in cognition. One should also consider the potential for a drug-drug interaction when reviewing the patient’s medication list and be especially vigilant in monitoring patients taking lithium, since its safety and effectiveness are affected by aging and by the co-administration of drugs that influence its clearance.
Despite these caveats, lithium remains an effective treatment in elderly patients, provided we are aware of the risks and benefits of its use.
- Juurlink DN, Mamdani MM, Kopp A, Rochon PA, Shulman KI, Redelmeier DA. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc 2004; 52:794–798.
- Sproule BA, Hardy BG, Shulman KI. Differential pharma-cokinetics of lithium in elderly patients. Drugs Aging 2000; 16:165–177.
- Martinez-Aran A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord 2004; 6:224–232.
- Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270.
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med 2000; 30:1025–1036.
- Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005; 22:39–54.
- Ragheb M. The clinical significance of lithium-non-steroidal anti-inflammatory drug interactions. J Clin Psychopharmacol 1990; 10:350–354.
A 61-year-old man presents for evaluation of new-onset cognitive impairment, which has developed over the past 6 to 8 months. He has bipolar disorder, for which he has been taking lithium carbonate (Eskalith) for the past 15 years. This therapy kept his mood stable until a relapse of depression and mania 1 year ago required hospitalization and an increase in the lithium dose, which was then lowered somewhat after he improved (see below). His cognitive symptoms appeared gradually within 2 months after his release from the hospital.
He now has difficulty concentrating, a tendency to substitute words incorrectly during conversation, and difficulty recalling names and “retrieving memories.” He also reports a worsening tremor in his dominant hand that compromises his ability to eat with a spoon or a fork. He complains of increasing daytime somnolence, which began when his lithium dose was increased and improved when the dose was decreased.
The patient is a mathematician and recently finished revising the curriculum for an undergraduate course in advanced mathematics that he teaches. He does not smoke cigarettes, and he drinks alcohol only socially. He has no other medical conditions and no known cardiovascular risk factors.
Current and recent medications
- Lithium carbonate 600 mg twice daily (before his hospitalization he had been taking 600 mg twice daily; this was increased to 1,500 mg/day during the hospitalization and then decreased to the current dose as maintenance therapy)
- Divalproex (Depakote) 250 mg every night
- Gabapentin (Neurontin) 400 mg every night (the dosages of divalproex and gabapentin have remained unchanged since before his hospitalization)
- A multivitamin daily
- Naproxen (Naprosyn, Aleve) 250 mg up to two times a week for arthritic knee pain
- Aripiprazole (Abilify). This antipsychotic drug was recently discontinued because of parkinsonian symptoms, which then gradually improved.
- Memantine (Namenda), which is indicated for the treatment of moderate to severe Alzheimer disease. The patient reports that he stopped taking it after 3 weeks because he did not perceive it to be helping.
THE INITIAL EVALUATION
Physical examination
Temperature 98.3°F (36.8°C), pulse 60 beats per minute, respirations 16 per minute, blood pressure 126/64 mm Hg sitting and 118/71 mm Hg standing.
The patient is well groomed, alert, and cooperative. His head, eyes, ears, nose, and throat are normal. His teeth are in good condition. His skin is normal. We note no thyromegaly, carotid bruits, or palpable lymphadenopathy. His lungs are clear to auscultation. Results of cardiac, abdominal, and musculoskeletal examinations are all normal.
His deep tendon reflexes, sensory and motor testing, and gait are normal. The cerebellar examination is normal, aside from a mild tremor in his right hand when it is outstretched, with no resting tremor or cogwheel rigidity.
On the Mini-Mental State Examination (MMSE) he scores a perfect 30/30 (normal 24–30). He can draw a clock normally. His score on the short-form Geriatric Depression Scale is 4/15 (a score of 6 or higher indicates depression).
Laboratory tests
- Serum lithium level 0.8 mmol/L (therapeutic range 0.5–1.5 mmol/L) (his previous values are not available)
- Thyroid-stimulating hormone level 1.61 μU/mL (normal 0.40–5.50)
- Complete blood cell count and comprehensive metabolic panel values are within normal limits.
Magnetic resonance imaging
Noncontrast magnetic resonance imaging of the head reveals two nonspecific punctate foci of high signal intensity on T2-weighted images in the left frontal white matter, but the results are otherwise normal.
DIFFERENTIAL DIAGNOSIS
1. On the basis of this information, which is the most likely cause of this patient’s cogitive impairment?
- Dementia with Lewy bodies
- Early-onset Alzheimer disease
- Stroke with vascular cognitive impairment
- Lithium neurotoxicity
Lithium neurotoxicity is the most likely cause of this patient’s symptoms, given the temporal relationship between the adjusting of his lithium dose and the onset of his symptoms. Lithium therapy causes subtle cognitive deficits. Its dosing in older patients requires careful monitoring because of age-related alterations in its pharmacology and its various drug interactions; both mechanisms played a role in precipitating lithium toxicity in this patient.
Although his lithium levels are in the broadly accepted therapeutic range, there is much debate about the best maintenance level for patients with bipolar disorder. A level in the range of 1 to 1.2 mmol/L may be best in acute mania, while a lower level of around 0.8 mmol/L is preferred in the depressive phase. Once the patient’s mood has stabilized, the best maintenance level may be in the range of 0.2 to 0.6 mmol/L.
Dementia with Lewy bodies, although suggested by the patient’s cognitive impairment, history of parkinsonian symptoms, and somnolence, is an unlikely cause because his motor symptoms resolved after the aripiprazole was discontinued, his somnolence improved after the dose of lithium was reduced, and his alertness did not fluctuate thereafter as would be expected in dementia with Lewy bodies.
Alzheimer disease usually manifests as gradually progressive cognitive deficits involving memory impairment with one or more of the following: aphasia, apraxia, agnosia, and disturbance in executive functioning. In contrast, this patient’s memory loss was fairly abrupt and not slowly progressive.
Stroke is also unlikely, as he has no history of stroke or focal neurologic deficits. Although a magnetic resonance scan of the brain showed some evidence of small-vessel ischemic changes, it showed no cortical infarcts.
MECHANISMS OF LITHIUM NEUROTOXICITY
2. What are the possible mechanisms of lithium neurotoxicity in this patient?
- The increased dose of lithium
- The interaction of nonsteroidal anti-inflammatory drugs (NSAIDs) and lithium
- The interaction of the other psychotropic medications with lithium
- All of the above
- None of the above
All of the above could be contributing.
Although lithium is thought to cause side effects in as many as 60% of patients of any age who take it, the rate of serious adverse effects is reportedly higher in older patients than in younger patients.1
That said, cognitive deficits are common in bipolar disorder irrespective of lithium use.
COGNITIVE IMPAIRMENT IN BIPOLAR DISORDER
3. If cognitive impairment in bipolar disorder is common, when does it occur?
- Only in the remission phase
- Only in the manic phase
- Only in the depression phase
- In all phases of the disease
Cognitive impairment occurs in all phases of bipolar disorder. Neuropsychological testing of bipolar patients in remission uncovers subtle, persistent cognitive impairment in executive function and in visuospatial memory without mood symptoms.3–5 Impaired executive functioning, predominantly frontal lobe dysfunction, interferes with one’s ability to initiate, plan, perform, and successfully complete a task and challenges one’s ability to function effectively in society and to comply with medical advice and instructions on taking medications.
RECOMMENDATIONS
4. What should we recommend to this patient?
- Decrease the current dose of lithium
- Stop all medications
- Undergo detailed neuropsychological testing
- Follow up with a psychiatrist, if needed
The patient’s lithium level was within the therapeutic range and his bipolar symptoms were well controlled. In older patients, however, the optimal serum level of lithium is often unclear, making it advisable to reduce the dose when an adverse effect is suspected.
His other medications should be reviewed. Gabapentin is not indicated for use as a mood stabilizer, and his divalproex dose (250 mg) is well below the usual therapeutic dose of 1,000 to 2,000 mg/day.6 The gabapentin could be discontinued, and the divalproex could be increased to a therapeutic dose.
NSAIDs can increase serum lithium levels, diminish renal lithium clearance, and possibly induce lithium toxicity, but the effect varies considerably among drugs and individuals.7 We would advise this patient to stop taking naproxen and switch to acetaminophen (Tylenol) for his arthritis pain, and we would inform him of the risk of lithium toxicity with continuous use of NSAIDs.
We would also recommend additional neuropsychological testing. The patient noticed subtle difficulties in his cognitive abilities that were not apparent on the MMSE. While the MMSE is an acceptable cognitive test, it is often not sensitive enough to detect milder forms of cognitive impairment, especially in well-educated patients at the usual cut-point of 24. A comprehensive neuropsychological examination is a more sensitive measure of cognition, involving the detailed testing of various cognitive domains. It can reveal a pattern of cognitive impairment that helps to differentiate between normal and mood disorders and also can detect subtle executive dysfunction.
However, detailed neuropsychological testing is time-consuming and may not be obtained rapidly enough to help in making clinical decisions quickly. In this patient’s case, immediate collaboration and follow-up with the patient’s psychiatrist would be the most expeditious way to reassess the patient’s medication regimen.
FOLLOW-UP COURSE
We informed the patient’s psychiatrist that we thought the patient had increased sensitivity to lithium (even at “therapeutic” levels), possibly related to a drug-drug interaction.
His dose of lithium was kept at 600 mg twice daily, as the lithium toxicity was most likely due to a drug-drug interaction.
We discontinued his memantine, since he did not have Alzheimer disease and since he wasn’t taking it anyway. He continued taking gabapentin and divalproex at the same doses, and he stopped taking naproxyn and substituted acetaminophen for his arthritis pain. We advised him about about health maintenance, including proper nutrition, mineral and vitamin supplements, and exercise.
The patient underwent neuropsychological testing to better characterize his cognitive impairment. The findings did not suggest dementia, but were consistent with minor cognitive deficits caused by lithium.
When seen at a follow-up visit 6 weeks later the patient was free of symptoms except for the tremor in his dominant hand. His mood was stable and his cognition was better. No further changes were required in his psychotropic drug regimen.
TAKE-HOME POINTS
When a bipolar patient develops acute changes in cognition, we should suspect adverse effects of lithium as the cause, because of its narrow therapeutic window and interactions with other prescribed drugs. The case presented here reminds us to consider adverse drug effects any time an older patient develops acute changes in cognition. One should also consider the potential for a drug-drug interaction when reviewing the patient’s medication list and be especially vigilant in monitoring patients taking lithium, since its safety and effectiveness are affected by aging and by the co-administration of drugs that influence its clearance.
Despite these caveats, lithium remains an effective treatment in elderly patients, provided we are aware of the risks and benefits of its use.
A 61-year-old man presents for evaluation of new-onset cognitive impairment, which has developed over the past 6 to 8 months. He has bipolar disorder, for which he has been taking lithium carbonate (Eskalith) for the past 15 years. This therapy kept his mood stable until a relapse of depression and mania 1 year ago required hospitalization and an increase in the lithium dose, which was then lowered somewhat after he improved (see below). His cognitive symptoms appeared gradually within 2 months after his release from the hospital.
He now has difficulty concentrating, a tendency to substitute words incorrectly during conversation, and difficulty recalling names and “retrieving memories.” He also reports a worsening tremor in his dominant hand that compromises his ability to eat with a spoon or a fork. He complains of increasing daytime somnolence, which began when his lithium dose was increased and improved when the dose was decreased.
The patient is a mathematician and recently finished revising the curriculum for an undergraduate course in advanced mathematics that he teaches. He does not smoke cigarettes, and he drinks alcohol only socially. He has no other medical conditions and no known cardiovascular risk factors.
Current and recent medications
- Lithium carbonate 600 mg twice daily (before his hospitalization he had been taking 600 mg twice daily; this was increased to 1,500 mg/day during the hospitalization and then decreased to the current dose as maintenance therapy)
- Divalproex (Depakote) 250 mg every night
- Gabapentin (Neurontin) 400 mg every night (the dosages of divalproex and gabapentin have remained unchanged since before his hospitalization)
- A multivitamin daily
- Naproxen (Naprosyn, Aleve) 250 mg up to two times a week for arthritic knee pain
- Aripiprazole (Abilify). This antipsychotic drug was recently discontinued because of parkinsonian symptoms, which then gradually improved.
- Memantine (Namenda), which is indicated for the treatment of moderate to severe Alzheimer disease. The patient reports that he stopped taking it after 3 weeks because he did not perceive it to be helping.
THE INITIAL EVALUATION
Physical examination
Temperature 98.3°F (36.8°C), pulse 60 beats per minute, respirations 16 per minute, blood pressure 126/64 mm Hg sitting and 118/71 mm Hg standing.
The patient is well groomed, alert, and cooperative. His head, eyes, ears, nose, and throat are normal. His teeth are in good condition. His skin is normal. We note no thyromegaly, carotid bruits, or palpable lymphadenopathy. His lungs are clear to auscultation. Results of cardiac, abdominal, and musculoskeletal examinations are all normal.
His deep tendon reflexes, sensory and motor testing, and gait are normal. The cerebellar examination is normal, aside from a mild tremor in his right hand when it is outstretched, with no resting tremor or cogwheel rigidity.
On the Mini-Mental State Examination (MMSE) he scores a perfect 30/30 (normal 24–30). He can draw a clock normally. His score on the short-form Geriatric Depression Scale is 4/15 (a score of 6 or higher indicates depression).
Laboratory tests
- Serum lithium level 0.8 mmol/L (therapeutic range 0.5–1.5 mmol/L) (his previous values are not available)
- Thyroid-stimulating hormone level 1.61 μU/mL (normal 0.40–5.50)
- Complete blood cell count and comprehensive metabolic panel values are within normal limits.
Magnetic resonance imaging
Noncontrast magnetic resonance imaging of the head reveals two nonspecific punctate foci of high signal intensity on T2-weighted images in the left frontal white matter, but the results are otherwise normal.
DIFFERENTIAL DIAGNOSIS
1. On the basis of this information, which is the most likely cause of this patient’s cogitive impairment?
- Dementia with Lewy bodies
- Early-onset Alzheimer disease
- Stroke with vascular cognitive impairment
- Lithium neurotoxicity
Lithium neurotoxicity is the most likely cause of this patient’s symptoms, given the temporal relationship between the adjusting of his lithium dose and the onset of his symptoms. Lithium therapy causes subtle cognitive deficits. Its dosing in older patients requires careful monitoring because of age-related alterations in its pharmacology and its various drug interactions; both mechanisms played a role in precipitating lithium toxicity in this patient.
Although his lithium levels are in the broadly accepted therapeutic range, there is much debate about the best maintenance level for patients with bipolar disorder. A level in the range of 1 to 1.2 mmol/L may be best in acute mania, while a lower level of around 0.8 mmol/L is preferred in the depressive phase. Once the patient’s mood has stabilized, the best maintenance level may be in the range of 0.2 to 0.6 mmol/L.
Dementia with Lewy bodies, although suggested by the patient’s cognitive impairment, history of parkinsonian symptoms, and somnolence, is an unlikely cause because his motor symptoms resolved after the aripiprazole was discontinued, his somnolence improved after the dose of lithium was reduced, and his alertness did not fluctuate thereafter as would be expected in dementia with Lewy bodies.
Alzheimer disease usually manifests as gradually progressive cognitive deficits involving memory impairment with one or more of the following: aphasia, apraxia, agnosia, and disturbance in executive functioning. In contrast, this patient’s memory loss was fairly abrupt and not slowly progressive.
Stroke is also unlikely, as he has no history of stroke or focal neurologic deficits. Although a magnetic resonance scan of the brain showed some evidence of small-vessel ischemic changes, it showed no cortical infarcts.
MECHANISMS OF LITHIUM NEUROTOXICITY
2. What are the possible mechanisms of lithium neurotoxicity in this patient?
- The increased dose of lithium
- The interaction of nonsteroidal anti-inflammatory drugs (NSAIDs) and lithium
- The interaction of the other psychotropic medications with lithium
- All of the above
- None of the above
All of the above could be contributing.
Although lithium is thought to cause side effects in as many as 60% of patients of any age who take it, the rate of serious adverse effects is reportedly higher in older patients than in younger patients.1
That said, cognitive deficits are common in bipolar disorder irrespective of lithium use.
COGNITIVE IMPAIRMENT IN BIPOLAR DISORDER
3. If cognitive impairment in bipolar disorder is common, when does it occur?
- Only in the remission phase
- Only in the manic phase
- Only in the depression phase
- In all phases of the disease
Cognitive impairment occurs in all phases of bipolar disorder. Neuropsychological testing of bipolar patients in remission uncovers subtle, persistent cognitive impairment in executive function and in visuospatial memory without mood symptoms.3–5 Impaired executive functioning, predominantly frontal lobe dysfunction, interferes with one’s ability to initiate, plan, perform, and successfully complete a task and challenges one’s ability to function effectively in society and to comply with medical advice and instructions on taking medications.
RECOMMENDATIONS
4. What should we recommend to this patient?
- Decrease the current dose of lithium
- Stop all medications
- Undergo detailed neuropsychological testing
- Follow up with a psychiatrist, if needed
The patient’s lithium level was within the therapeutic range and his bipolar symptoms were well controlled. In older patients, however, the optimal serum level of lithium is often unclear, making it advisable to reduce the dose when an adverse effect is suspected.
His other medications should be reviewed. Gabapentin is not indicated for use as a mood stabilizer, and his divalproex dose (250 mg) is well below the usual therapeutic dose of 1,000 to 2,000 mg/day.6 The gabapentin could be discontinued, and the divalproex could be increased to a therapeutic dose.
NSAIDs can increase serum lithium levels, diminish renal lithium clearance, and possibly induce lithium toxicity, but the effect varies considerably among drugs and individuals.7 We would advise this patient to stop taking naproxen and switch to acetaminophen (Tylenol) for his arthritis pain, and we would inform him of the risk of lithium toxicity with continuous use of NSAIDs.
We would also recommend additional neuropsychological testing. The patient noticed subtle difficulties in his cognitive abilities that were not apparent on the MMSE. While the MMSE is an acceptable cognitive test, it is often not sensitive enough to detect milder forms of cognitive impairment, especially in well-educated patients at the usual cut-point of 24. A comprehensive neuropsychological examination is a more sensitive measure of cognition, involving the detailed testing of various cognitive domains. It can reveal a pattern of cognitive impairment that helps to differentiate between normal and mood disorders and also can detect subtle executive dysfunction.
However, detailed neuropsychological testing is time-consuming and may not be obtained rapidly enough to help in making clinical decisions quickly. In this patient’s case, immediate collaboration and follow-up with the patient’s psychiatrist would be the most expeditious way to reassess the patient’s medication regimen.
FOLLOW-UP COURSE
We informed the patient’s psychiatrist that we thought the patient had increased sensitivity to lithium (even at “therapeutic” levels), possibly related to a drug-drug interaction.
His dose of lithium was kept at 600 mg twice daily, as the lithium toxicity was most likely due to a drug-drug interaction.
We discontinued his memantine, since he did not have Alzheimer disease and since he wasn’t taking it anyway. He continued taking gabapentin and divalproex at the same doses, and he stopped taking naproxyn and substituted acetaminophen for his arthritis pain. We advised him about about health maintenance, including proper nutrition, mineral and vitamin supplements, and exercise.
The patient underwent neuropsychological testing to better characterize his cognitive impairment. The findings did not suggest dementia, but were consistent with minor cognitive deficits caused by lithium.
When seen at a follow-up visit 6 weeks later the patient was free of symptoms except for the tremor in his dominant hand. His mood was stable and his cognition was better. No further changes were required in his psychotropic drug regimen.
TAKE-HOME POINTS
When a bipolar patient develops acute changes in cognition, we should suspect adverse effects of lithium as the cause, because of its narrow therapeutic window and interactions with other prescribed drugs. The case presented here reminds us to consider adverse drug effects any time an older patient develops acute changes in cognition. One should also consider the potential for a drug-drug interaction when reviewing the patient’s medication list and be especially vigilant in monitoring patients taking lithium, since its safety and effectiveness are affected by aging and by the co-administration of drugs that influence its clearance.
Despite these caveats, lithium remains an effective treatment in elderly patients, provided we are aware of the risks and benefits of its use.
- Juurlink DN, Mamdani MM, Kopp A, Rochon PA, Shulman KI, Redelmeier DA. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc 2004; 52:794–798.
- Sproule BA, Hardy BG, Shulman KI. Differential pharma-cokinetics of lithium in elderly patients. Drugs Aging 2000; 16:165–177.
- Martinez-Aran A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord 2004; 6:224–232.
- Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270.
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med 2000; 30:1025–1036.
- Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005; 22:39–54.
- Ragheb M. The clinical significance of lithium-non-steroidal anti-inflammatory drug interactions. J Clin Psychopharmacol 1990; 10:350–354.
- Juurlink DN, Mamdani MM, Kopp A, Rochon PA, Shulman KI, Redelmeier DA. Drug-induced lithium toxicity in the elderly: a population-based study. J Am Geriatr Soc 2004; 52:794–798.
- Sproule BA, Hardy BG, Shulman KI. Differential pharma-cokinetics of lithium in elderly patients. Drugs Aging 2000; 16:165–177.
- Martinez-Aran A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord 2004; 6:224–232.
- Martinez-Aran A, Vieta E, Reinares M, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 2004; 161:262–270.
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med 2000; 30:1025–1036.
- Sajatovic M, Madhusoodanan S, Coconcea N. Managing bipolar disorder in the elderly: defining the role of the newer agents. Drugs Aging 2005; 22:39–54.
- Ragheb M. The clinical significance of lithium-non-steroidal anti-inflammatory drug interactions. J Clin Psychopharmacol 1990; 10:350–354.
Treating Patients With Autism and Anxiety
SAVANNAH, GA—Patients diagnosed with both anxiety disorder and autism spectrum disorder used almost tenfold more antipsychotic medications, and fewer SSRIs, than those diagnosed with anxiety disorder alone, reported Alya Reeve, MD, at the 19th Annual Meeting of the American Neuropsychiatric Association.
Dr. Reeve’s group determined the effect of autism spectrum disorder on medications prescribed for anxiety disorders. A retrospective review of 218 charts for nine years found that 98 patients (45%) had anxiety disorder; of these, 31 (32%) also had a diagnosis of autism spectrum disorder.
Additional comorbid psychiatric conditions included mood, impulse control, and attention disorders, as well as psychosis. Thirteen percent of those with anxiety disorder and autism spectrum disorder had mood disorder, compared with 51% of those without autism spectrum disorder. Rates for other psychiatric conditions were higher in the autism spectrum disorder group than in the non–autism spectrum disorder group for impulse control disorders (60% vs 46%, respectively) and attention disorders (6% vs 4%, respectively) and were the same for psychosis (13%). Thirty-five percent of those with anxiety disorder and autism spectrum disorder had hypothyroidism versus 23% of those without autism spectrum disorder; and 26% of those with anxiety disorder and autism spectrum disorder had seizures versus 33% of those without autism spectrum disorder. For patients with GERD, the rates were 16% versus 18%, respectively.
“Psychotropic medications and their indication for usage were derived from chart notes and forms,” said Dr. Reeve, an Associate Professor in the Department of Psychiatry at the University of New Mexico Health Sciences Center in Albuquerque. Psychotropic medications used for anxiety included SSRIs, antipsychotics, tricyclic antidepressants, and heterocyclics. Each medication was classified as “current use,” “used > 50% duration of service,” or “ever used.”
Despite similar psychotropic medication prescribing rates, 48% of patients with anxiety disorder and autism spectrum disorder were currently using an SSRI, compared with 70% of those without autism spectrum disorder. Conversely, 1.5% of patients without autism spectrum disorder were using an antipsychotic, compared with 13% of those with autism spectrum disorder.
Psychotropic medications prescribed but not used for anxiety included antipsychotics for psychotic symptoms such as impulse control, aggression, agitation, sleep, or self-injurious behaviors. These medications were used by 84% of patients with anxiety disorder and autism spectrum disorder, compared with 69% of those without autism spectrum disorder. Other psychotropics, such as antiepileptics, anxiolytics, antidepressants, sedatives, and antihypertensives prescribed for impulse control, sleep attention, agitation, aggression, or self-injurious behaviors, were used by more patients with anxiety disorder and autism spectrum disorder than those without (87% vs 63%, respectively). Medications used as needed (eg, for anxiety prior to a dentist visit) or for nonanxiety symptoms (eg, trazodone for sleep) were excluded.
“Patients with autism spectrum disorder used SSRIs less successfully, and antipsychotics more successfully, than those without autism spectrum disorder,” Dr. Reeve concluded. “This may reflect a population with higher behavior challenges compounding anxiety disorder.”
—Debra Hughes
SAVANNAH, GA—Patients diagnosed with both anxiety disorder and autism spectrum disorder used almost tenfold more antipsychotic medications, and fewer SSRIs, than those diagnosed with anxiety disorder alone, reported Alya Reeve, MD, at the 19th Annual Meeting of the American Neuropsychiatric Association.
Dr. Reeve’s group determined the effect of autism spectrum disorder on medications prescribed for anxiety disorders. A retrospective review of 218 charts for nine years found that 98 patients (45%) had anxiety disorder; of these, 31 (32%) also had a diagnosis of autism spectrum disorder.
Additional comorbid psychiatric conditions included mood, impulse control, and attention disorders, as well as psychosis. Thirteen percent of those with anxiety disorder and autism spectrum disorder had mood disorder, compared with 51% of those without autism spectrum disorder. Rates for other psychiatric conditions were higher in the autism spectrum disorder group than in the non–autism spectrum disorder group for impulse control disorders (60% vs 46%, respectively) and attention disorders (6% vs 4%, respectively) and were the same for psychosis (13%). Thirty-five percent of those with anxiety disorder and autism spectrum disorder had hypothyroidism versus 23% of those without autism spectrum disorder; and 26% of those with anxiety disorder and autism spectrum disorder had seizures versus 33% of those without autism spectrum disorder. For patients with GERD, the rates were 16% versus 18%, respectively.
“Psychotropic medications and their indication for usage were derived from chart notes and forms,” said Dr. Reeve, an Associate Professor in the Department of Psychiatry at the University of New Mexico Health Sciences Center in Albuquerque. Psychotropic medications used for anxiety included SSRIs, antipsychotics, tricyclic antidepressants, and heterocyclics. Each medication was classified as “current use,” “used > 50% duration of service,” or “ever used.”
Despite similar psychotropic medication prescribing rates, 48% of patients with anxiety disorder and autism spectrum disorder were currently using an SSRI, compared with 70% of those without autism spectrum disorder. Conversely, 1.5% of patients without autism spectrum disorder were using an antipsychotic, compared with 13% of those with autism spectrum disorder.
Psychotropic medications prescribed but not used for anxiety included antipsychotics for psychotic symptoms such as impulse control, aggression, agitation, sleep, or self-injurious behaviors. These medications were used by 84% of patients with anxiety disorder and autism spectrum disorder, compared with 69% of those without autism spectrum disorder. Other psychotropics, such as antiepileptics, anxiolytics, antidepressants, sedatives, and antihypertensives prescribed for impulse control, sleep attention, agitation, aggression, or self-injurious behaviors, were used by more patients with anxiety disorder and autism spectrum disorder than those without (87% vs 63%, respectively). Medications used as needed (eg, for anxiety prior to a dentist visit) or for nonanxiety symptoms (eg, trazodone for sleep) were excluded.
“Patients with autism spectrum disorder used SSRIs less successfully, and antipsychotics more successfully, than those without autism spectrum disorder,” Dr. Reeve concluded. “This may reflect a population with higher behavior challenges compounding anxiety disorder.”
—Debra Hughes
SAVANNAH, GA—Patients diagnosed with both anxiety disorder and autism spectrum disorder used almost tenfold more antipsychotic medications, and fewer SSRIs, than those diagnosed with anxiety disorder alone, reported Alya Reeve, MD, at the 19th Annual Meeting of the American Neuropsychiatric Association.
Dr. Reeve’s group determined the effect of autism spectrum disorder on medications prescribed for anxiety disorders. A retrospective review of 218 charts for nine years found that 98 patients (45%) had anxiety disorder; of these, 31 (32%) also had a diagnosis of autism spectrum disorder.
Additional comorbid psychiatric conditions included mood, impulse control, and attention disorders, as well as psychosis. Thirteen percent of those with anxiety disorder and autism spectrum disorder had mood disorder, compared with 51% of those without autism spectrum disorder. Rates for other psychiatric conditions were higher in the autism spectrum disorder group than in the non–autism spectrum disorder group for impulse control disorders (60% vs 46%, respectively) and attention disorders (6% vs 4%, respectively) and were the same for psychosis (13%). Thirty-five percent of those with anxiety disorder and autism spectrum disorder had hypothyroidism versus 23% of those without autism spectrum disorder; and 26% of those with anxiety disorder and autism spectrum disorder had seizures versus 33% of those without autism spectrum disorder. For patients with GERD, the rates were 16% versus 18%, respectively.
“Psychotropic medications and their indication for usage were derived from chart notes and forms,” said Dr. Reeve, an Associate Professor in the Department of Psychiatry at the University of New Mexico Health Sciences Center in Albuquerque. Psychotropic medications used for anxiety included SSRIs, antipsychotics, tricyclic antidepressants, and heterocyclics. Each medication was classified as “current use,” “used > 50% duration of service,” or “ever used.”
Despite similar psychotropic medication prescribing rates, 48% of patients with anxiety disorder and autism spectrum disorder were currently using an SSRI, compared with 70% of those without autism spectrum disorder. Conversely, 1.5% of patients without autism spectrum disorder were using an antipsychotic, compared with 13% of those with autism spectrum disorder.
Psychotropic medications prescribed but not used for anxiety included antipsychotics for psychotic symptoms such as impulse control, aggression, agitation, sleep, or self-injurious behaviors. These medications were used by 84% of patients with anxiety disorder and autism spectrum disorder, compared with 69% of those without autism spectrum disorder. Other psychotropics, such as antiepileptics, anxiolytics, antidepressants, sedatives, and antihypertensives prescribed for impulse control, sleep attention, agitation, aggression, or self-injurious behaviors, were used by more patients with anxiety disorder and autism spectrum disorder than those without (87% vs 63%, respectively). Medications used as needed (eg, for anxiety prior to a dentist visit) or for nonanxiety symptoms (eg, trazodone for sleep) were excluded.
“Patients with autism spectrum disorder used SSRIs less successfully, and antipsychotics more successfully, than those without autism spectrum disorder,” Dr. Reeve concluded. “This may reflect a population with higher behavior challenges compounding anxiety disorder.”
—Debra Hughes