User login
Transcatheter aortic valve-in-ring for mitral disease a winner
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
DENVER – Transseptal mitral valve implantation of an off-the-shelf, commercially available TAVR valve in high-surgical-risk patients with a failing surgically implanted mitral ring prosthesis has become a reasonable treatment strategy in light of the interim findings of the ground-breaking MITRAL trial, Mayra E. Guerrero, MD, said at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Her presentation of the preliminary results of the MITRAL (Mitral Implantation of Transcatheter Valves) trial showed this valve-in-ring (ViR) treatment strategy using the Sapien 3 valve was associated with low 30-day morbidity and mortality rates and impressive symptomatic improvement.
In contrast, another arm of the MITRAL trial showed that placement of the Sapien 3 TAVR valve in high-surgical-risk patients with severe mitral stenosis due to mitral annular calcification (MAC) of their native valve is a treatment strategy that’s not yet ready for prime time, she added at the meeting, which was sponsored by the Cardiovascular Research Foundation.
“Transcatheter mitral valve replacement in MAC is a challenging procedure associated with complications,” Dr. Guerrero observed. “It may become a reasonable alternative for high-surgical-risk patients with favorable anatomy, but techniques require further refinement.”
The ViR arm of the observational multicenter prospective MITRAL trial included 30 patients with extremely high surgical risk and either severe mitral stenosis as defined by a mitral valve area of 1.5 cm2 or less or moderate mitral stenosis plus severe mitral regurgitation. The most common type of failing ring was the Edwards Physio, in nine patients. Access for transcatheter mitral valve replacement (TMVR) was transseptal in 100% of patients.
The technical success rate at exit from the catheterization lab was 70%. The procedural success rate at 30 days was 62%.
Six patients required a second valve. This was mainly because of malpositioning of the first valve with resultant mitral regurgitation; however, this problem became a nonissue as operator experience grew. All six affected patients were alive at 30 days, and four of the six were New York Heart Association (NYHA) functional class I or II.
In-hospital and 30-day mortality rates were low. There was a single cardiovascular death and one noncardiac death in hospital, with no additional deaths through 30 days. No cases of stroke, acute MI, or valve embolization or thrombosis occurred. The mean mitral valve area at 30 days was 2.1 cm2, although three patients still had a mitral valve area of less 1.5 cm2. Three patients experienced acute renal failure requiring hemodialysis. Seventy-five percent of patients had no or trace mitral regurgitation by echocardiography; the rest had mild regurgitation.
Although at baseline more than 60% of the patients were New York Heart Association class III, 10% were class IV, and the rest were class II, at 30 days more than 30% were New York Heart Association class I, 40% were class II, and the rest were class III.
The 30-day all-cause mortality rate of 6.8% in the MITRAL study is roughly half that reported for ViR patients in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Dr. Guerrero attributed this to refined procedural techniques and improved patient selection through the use of CT imaging and echocardiography.
Heart valve design changes, such as a longer inner skirt, might further improve the technical success rate for ViR, according to Dr. Guerrero, an interventional cardiologist at NorthShore University Health System in Evanston, Ill.
Picking the right ring
Given that studies show one-third of recipients of a surgical mitral ring or surgical mitral valve will require a repeat intervention within 10 years, she made a plea to surgeons: “If we are going to be treating patients with valve-in-ring TMVR, that means when surgeons do a repair they should pick a ring that is amenable to a ViR procedure. So don’t use flexible incomplete bands or very rigid rings because those are really difficult to treat later on. We should pick a ring thinking of the future. That ring is going to fail at some point, and when it fails it’s going to make our lives much easier if we’d picked the right ring.”
MAC TMVR needs more work
In the MAC arm of the MITRAL trial, 96 patients were screened so the researchers could find 30 candidates for TMVR. The 61 rejections were for high risk of left ventricular outflow tract obstruction (LVOTO), embolization, or both.
Fourteen patients underwent transseptal TMVR, and one with anatomy unsuitable for a transseptal procedure had a transapical approach. The other 15 patients had a transatrial surgical approach, which allows resection of the anterior leaflet to reduce the risk of LVOTO and placement of sutures to reduce the embolization risk. However, this came at the cost of increased mortality risk: Three of the five in-hospital deaths were in the transatrial TMVR group.
The technical success rate at exit from the cath lab in the MAC patients was 73%, with a 30-day procedural success rate of 46% and a 19% 30-day mortality. Three patients developed severe LVOTO with hemodynamic compromise.
One transseptal and one transapical TMVR were complicated by LVOTO, both treated by bailout alcohol septal ablation. This led Dr. Guerrero and her coinvestigators to the concept of preemptive alcohol septal ablation, which they used in seven patients deemed at high risk for LVOTO an average of 6 weeks prior to transseptal TMVR as a successful risk reduction strategy.
Survival climbing with operator experience
“In the early days of the TMVR MAC registry, the 30-day mortality rate was 37%. It came down to 22% in the middle third of the registry, then about 18% in the final third. Now we’ve got it down in MITRAL to 16.7%, but when you separate the rate in the transseptal versus the transatrial patients, it’s 13% versus 20%. The difference is not statistically significant, but it’s promising, and I think we are making great progress,” Dr. Guerrero said.
Safety and efficacy endpoints in MITRAL will be reported again at 1 year of follow-up.
The MITRAL trial was partially supported by Edwards Lifesciences. Dr. Guerrero reported receiving a research grant from that company and serving as a consultant to Tendyne Holdings/Abbott and on a speakers bureau for Abiomed.
SOURCE: Guerrero M. No abstract.
REPORTING FROM TCT 2017
Key clinical point: .
Major finding: Thirty-day all-cause mortality following a transcatheter valve-in-ring procedure in unacceptably high surgical-risk patients with severe mitral valve disease due to a failing annuloplasty ring was 6.8%.
Study details: This prospective observational study included 60 patients who underwent transcatheter mitral valve replacement for severe mitral valve disease, half due to a failed annuloplasty ring and half secondary to mitral annular calcification.
Disclosures: The MITRAL trial was partially supported by Edwards Lifesciences. The study presenter reported receiving a research grant from the company.
Source: Guerrero M. No abstract.
New frontier in TAVR is bicuspid disease
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.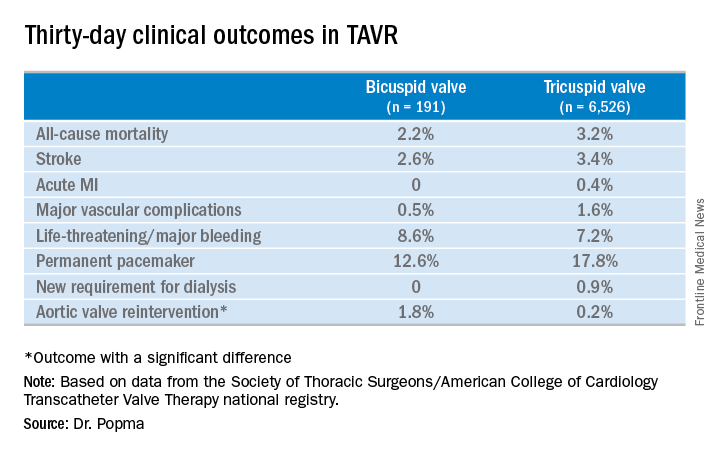
No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.
No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
DENVER – Thirty-day transcatheter aortic valve replacement (TAVR) outcomes in real-world clinical practice using the Evolut R self-expanding valve were as good in patients treated for bicuspid disease as for tricuspid disease, according to a retrospective analysis of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy (STS/ACC TVT) national registry.
“I’ve always been insecure about whether we have the right technology to be able to treat bicuspid disease. This registry data is reassuring to me that we might. I think it may be time to do a prospective registry for low-surgical-risk patients with bicuspid disease and see if we can emulate these kinds of results,” said Dr. Popma, the director of interventional cardiology at Beth Israel Deaconess Medical Center and a professor of medicine at Harvard Medical School, both in Boston.
“I think that the one limitation to recruitment in our low-risk TAVR trial is patients with bicuspid disease. Probably 25%-30% of low-risk patients are bicuspid, so we can’t include them right now in our low-risk trial,” he added at the meeting sponsored by the Cardiovascular Research Foundation.
Even though TAVR for patients with bicuspid disease is off-label, operators do perform the procedure. All of these cases are captured in the STS/ACC TVT registry. Dr. Popma reported on 6,717 patients who underwent TAVR with placement of the Evolut R valve at 305 U.S. centers during 2014-2016. The purpose of this retrospective study was to compare 30-day outcomes in the 191 TAVR patients with native valve bicuspid disease with the outcomes in the 6,526 with tricuspid disease.
The two groups were evenly matched in terms of key baseline characteristics, including aortic valve mean gradient, severity of aortic, mitral, and tricuspid regurgitation, and comorbid conditions – with the exception of coronary artery disease, which was present in 48% of the bicuspid group versus 65% of those with tricuspid disease. Also, the bicuspid disease group was younger by an average of nearly 9 years, and their mean baseline left ventricular ejection fraction of 52.5% was lower than the LVEF of 55.5% seen in the tricuspid group.
Procedure time averaged 126 minutes in the bicuspid group and 116 in the tricuspid group. Femoral access was utilized in 87% of the bicuspid patients and in 92% of tricuspid patients. The device was implanted successfully in 97% of the bicuspid group and in 99% of the tricuspid group. More than one valve was required in 3.7% of the bicuspid disease group, a rate similar to that in the tricuspid group. Total hospital length of stay was roughly 6 days in both groups.
Rates of symptomatic improvement at 30 days were closely similar in the two groups. Preprocedurally, two-thirds of patients in both groups had a New York Heart Association class III; at 30 days, however, that was true for a mere 2.4% of the bicuspid patients and 10.3% of the tricuspid patients. By day 30, 52% of the bicuspid group and 48% of the tricuspid group were NYHA class I.
No or only trace aortic regurgitation was present at 30 days in 62% of the bicuspid group and in 61% of the tricuspid group, while mild aortic regurgitation was noted in 31% and 33%, respectively.
Thirty-day mean aortic valve gradient improved to a similar extent in the two groups: from a baseline of 47.2 mm Hg to 9.4 mm Hg in the bicuspid group and from 42.9 mm Hg to 7.5 mm Hg in the tricuspid group.
Dr. Popma noted that an earlier analysis he carried out comparing outcomes of TAVR using the earlier-generation CoreValve in bicuspid versus tricuspid disease showed suboptimal rates of paravalvular regurgitation and an increased need for multiple valves in the bicuspid group.
“The lesson is ‘Thank God we’ve got new technology!’ because the new technology has made a big difference for us,” the cardiologist observed. “We think that the advancement in the technique and the advancement in the valves is going to give us fairly comparable outcomes with Evolut in bicuspid and tricuspid patients.”
Discussant Hasan Jilaihawi, MD, a codirector of transcatheter valve therapy at New York University, pronounced the short-term outcomes in patients with bicuspid aortic valve disease “better than I would have expected,” adding that he, too, thinks it’s time for a prospective registry study of the Evolut valve in such patients.
Dr. Popma’s study was supported by Medtronic. He reported having received research grants from Medtronic and other medical device companies.
SOURCE: Popma JJ. TCT 2017.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: Thirty-day clinical outcomes and symptomatic improvement were reassuringly similar both in TAVR patients who received the Evolut R valve for tricuspid disease and off-label for bicuspid disease.
Study details: This was a retrospective U.S. national registry study comparing 30-day outcomes in 191 TAVR patients with native valve bicuspid disease and 6,526 with tricuspid disease, all of whom underwent TAVR with placement of the Evolut R valve.
Disclosures: The study presenter reported having received research grants from Medtronic, the study sponsor, as well as other medical device companies.
Source: Popma JJ. TCT 2017.
DAPT duration: How low can you go?
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The composite endpoint of death, MI, stroke, revascularization, and major bleeding 24 months after primary PCI with a second-generation DES was 6.6% in patients who got the standard 12 months of DAPT and 4.8% in those who got 6.
Study details: A prospective randomized international study that enrolled 1,100 STEMI patients who underwent primary PCI.
Disclosures: The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. The presenter reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
Source: Kedhi, E. No abstract.
VAMPIRE 3: Embolic filter for primary PCI reduces MACE
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
DENVER – Distal embolic protection using a filter device during primary PCI for acute MI significantly reduced the incidence of the no-reflow phenomenon and serious adverse events in the randomized VAMPIRE 3 trial.
The reason VAMPIRE 3 (Vacuum Aspiration Thrombus Removal) was a positive trial when other studies of distal embolic protection during coronary intervention have failed was that VAMPIRE 3 targeted a high-risk, high-benefit population: the subset of acute MI patients with attenuated, high-risk coronary plaque identified by intravascular ultrasound, Kiyoshi Hibi, MD, explained at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
Also, the filter device used in the trial was easily deployed and problem free, which doesn’t appear to be true of distal protection devices used in some earlier trials.
Discussants agreed with his conclusion, but predicted distal protection during primary PCI for acute coronary syndrome is unlikely to catch on with U.S. interventional cardiologists anytime soon. That’s because, for the most part, they don’t perform advanced imaging in the setting of primary PCI for ACS.
VAMPIRE 3 was a multicenter Japanese study involving 200 patients undergoing primary PCI for acute MI who displayed intravascular ultrasound evidence of attenuated plaque at least 5 mm in length as defined by images showing backward signal attenuation of 180 degrees or more behind a noncalcified plaque. Participants were randomized to placement of the Filtrap distal embolic protection device manufactured by Nipro of Tokyo or to no filter during their procedure, which in all cases entailed the use of a thrombus aspiration catheter.
The primary endpoint was the incidence of the no-reflow phenomenon, an adverse event previously shown by German investigators to be associated with a 66% increase in 5-year mortality (J Am Coll Cardiol. 2010 May 25;55[21]:2383-9). In VAMPIRE 3, the no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally. The secondary endpoint – the corrected TIMI frame count – was 23.0 in the distal protection group, significantly better than the 30.5 in control patients, the cardiologist reported at the meeting, which was sponsored by the Cardiovascular Research Foundation.
The rate of in-hospital adverse events – a composite of death, cardiac arrest, cardiogenic shock, and ischemic stroke adjudicated by an independent committee – was 1% in the distal protection group and significantly higher at 8.3% in conventionally managed controls. All five cases of cardiac arrest/cardiogenic shock occurred in patients who didn’t receive the prophylactic filter, he continued.
In a previous study, Dr. Hibi and his coworkers showed that the incidence of the no-reflow phenomenon in a series of 179 acute MI patients was 18%. More importantly, the incidence was 71% among patients with attenuated plaque of 5 mm or more, compared with 10%-11% among those with less or no attenuated plaque (JACC Cardiovasc Interv. 2010 May;3[5]:540-9).
“I am convinced by these results,” discussant Jonathan M. Hill, MD, said about VAMPIRE 3, “but the concern is the dependency on imaging. All of these cases were entirely dependent on an imaging diagnosis.
“Certainly in the acute setting, I think Japanese practice is the extreme, with greater than 80% penetration for intravascular imaging, compared to less than 10% in the United States. So to implement your protocol would be difficult unless there was acceptance of the requirement for intravascular imaging,” added Dr. Hill, an interventional cardiologist at King’s College London.
The prevailing philosophy among American cardiologists is that time is heart muscle. The priority is to open the blocked coronary vessel as quickly as possible.
“We don’t use that much advanced imaging,“ agreed David J. Cohen, MD, director of cardiovascular research at Saint Luke’s Mid America Heart Institute in Kansas City, Mo.
“It seems like the difference between this trial and previous ones that have not used advanced imaging was the use of intravascular imaging to identify the optimal group of patients that might benefit from this. So I think until that practice changes in the United States, I suspect this is going to get very limited use. I don’t see these devices ramping up quickly for coronary applications,” he predicted.
VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. Dr. Hibi reported serving as a consultant to Nipro and Boston Scientific.
SOURCE: Hibi K. TCT 2017
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The no-reflow phenomenon occurred in 26.5% of patients randomized to distal protection and 41.7% of those managed conventionally.
Study details: This randomized multicenter trial included 200 acute MI patients.
Disclosures: VAMPIRE 3 was sponsored by Yokohama City University Medical Center and Teikyo University. The presenter reported serving as a consultant to Nipro and Boston Scientific.
Watchman device PREVAILs for stroke prevention
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
DENVER – Left atrial appendage closure using the Watchman device is as effective as warfarin in preventing strokes in patients with atrial fibrillation, but the strokes in Watchman recipients are 55% less likely to be disabling, according to a meta-analysis of 5-year outcomes in the PREVAIL and PROTECT AF randomized trials.
The device therapy showed additional advantages over warfarin: significantly reduced risks of mortality, non–procedure-related major bleeding, and hemorrhagic stroke, Saibal Kar, MD, reported in presenting the results of the meta-analysis at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“We have prevailed,” he declared, referring to device safety concerns that arose early on and have since been laid to rest.
The patient-level meta-analysis of 5-year outcomes included 1,114 patients with atrial fibrillation who were randomized 2:1 to the Watchman device or warfarin, with 4,343 patient-years of follow-up. This was a fairly high–stroke risk population, with CHA2DS2-VASc scores in the 3.6-3.9 range, and 40% of patients aged 75 years or more. At baseline, 23% of subjects had a history of stroke or transient ischemic attack.
At 5 years’ follow-up, the composite endpoint of all stroke or systemic embolism was the same in the two study arms. However, the rate of hemorrhagic stroke was 80% lower in the Watchman group, the risk of disabling or fatal stroke was reduced by 55%, the rate of cardiovascular or unexplained death was 41% lower, all-cause mortality was reduced by 27%, and postprocedure major bleeding was 52% less frequent in the device therapy group. All of these differences achieved statistical significance, the cardiologist reported at the meeting sponsored by the Cardiovascular Research Foundation.
On the downside, the rate of ischemic stroke trended higher in the Watchman group, although the 71% increase in relative risk didn’t achieve statistical significance (P = .08). Dr. Kar asserted that this unhappy trend was a statistical fluke resulting from a low number of events and an implausibly low ischemic stroke rate of 0.73% per year in the warfarin group.
“This is the lowest rate of ischemic stroke in any study of warfarin. In fact, if this was the ischemic stroke rate in any of the NOAC [novel oral anticoagulant] studies, none of those drugs would actually have been approved. Why did we get such an implausibly low ischemic stroke rate? It’s a function of lower numbers and larger confidence intervals,” he said.
Gregg W. Stone, MD, who moderated the discussion panel at the late-breaking clinical trial session, advised Dr. Kar to be less defensive about the ischemic stroke findings.
“I think we have to be a little less apologetic for the great outcomes in the warfarin arm in PREVAIL. We do these randomized trials, and we get what we get,” said Dr. Stone, professor of medicine at Columbia University in New York.
Stephen G. Ellis, MD, said he was particularly impressed with the reduced rate of disabling stroke in the Watchman group.
“Severity of stroke is important. I hadn’t seen that data before,” commented Dr. Ellis, professor of medicine and director of the cardiac catheterization laboratory at the Cleveland Clinic. “The overall message, I think, is that for the patients who would have been candidates to be enrolled in these trials, the device seems to be quite worthwhile. I take note of the overall benefit in terms of cardiovascular death and all-cause death.”
Robert J. Sommer, MD, said that in patients with high CHA2DS2-VASc scores and previous bleeding on oral anticoagulants, the new data show that the Watchman “is a no-brainer. The patients all want it, the physicians all want it. It’s a very easy decision to make.”
“But you get into other groups who may potentially be interested in the device – particularly the younger patients who are very active and don’t want to be on anticoagulation – I think the ischemic stroke rate in the device arm trending to be higher is a problem for them. And it’s certainly going to be a problem for their physicians. But as we go on, I think with further studies we’ll see expanded indications. Patients with CAD who potentially would need triple therapy – that’s a nice population to study in this area. We’ll also be seeing data on other devices that may have different ischemic stroke rates,” said Dr. Sommer, director of invasive adult congenital heart disease at Columbia University Medical Center.
“I find this data extraordinarily helpful as I think about my conversations with patients about stroke prevention,” said Brian K. Whisenant, MD, medical director of structural heart disease at the Intermountain Medical Center Heart Institute in Salt Lake City.
“I tell them we think of oral anticoagulants as first-line therapy based on a stroke rate of 1% per year or less in most datasets. The data for the Watchman device has been very consistent in that we have a stroke rate that’s a little bit higher, at 1.3%-1.8% per year. And we have extensive data for predicting the stroke rate in the absence of oral anticoagulation: In most of our patients, that rate is in excess of 5% per year. So while the Watchman device may not provide the absolute reduction in ischemic stroke rate that oral anticoagulants do, a stroke rate of less than 2% is a whole lot better than no therapy for many of these patients,” the cardiologist said.
Martin B. Leon, MD, opined that the ischemic stroke data cannot be explained away. But he added that the totality of the meta-analysis data gives him confidence that this is the appropriate treatment in these patients.
“It does leave open the question of whether we can do better with ischemic strokes. Some people are suggesting that maybe adjunctive pharmacotherapy – perhaps a low-dose NOAC – may be a reasonable option in some patients to get even better results. That’s something I believe is open for discussion,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York.
Dr. Stone summed up: “There’s uniformity among the panel that there may be a slightly lower ischemic stroke rate with oral anticoagulation, and the NOACs probably provide some additional benefit, with an additional 50% reduction in hemorrhagic stroke, compared to warfarin. But that being said, I believe that left atrial appendage closure with the Watchman is the viable and now clearly safe approach for patients with any sort of contraindication or strong desire to avoid oral anticoagulation.”
The PREVAIL and PROTECT AF trials and meta-analysis were sponsored by Boston Scientific. Dr. Kar reported receiving research grants from and serving as a consultant to that company as well as Abbott Vascular.
Simultaneously with Dr. Kar’s presentation at TCT 2017, the findings were published online in the Journal of the American College of Cardiology.
SOURCE: Reddy VY et al. TCT 2017; J Am Coll Cardiol. 2017 Nov 4. pii:S0735-1097(17)41187-9. doi: 10.1016/j.jacc.2017.10.021.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: All-cause mortality was reduced by 27% in patients randomized to left atrial appendage closure with the Watchman device, compared with those assigned to warfarin.
Study details: This patient-level meta-analysis included 5-year follow-up data on 1,114 patients with atrial fibrillation at increased stroke risk who were randomized 2:1 to the Watchman device or warfarin.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to study sponsor Boston Scientific.
FFR-guided PCI in stable CAD beats medical management
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
DENVER – Percutaneous coronary intervention plus optimal medical therapy in stable coronary artery disease (CAD) patients with at least one coronary lesion having an abnormal fractional flow reserve measurement resulted in superior clinical outcomes, better quality of life, and virtually identical cost, compared with optimal medical management alone over 3 years of follow-up in the FAME 2 trial.
“These results reinforce the point that the greater the burden of ischemia, the greater the benefit of revascularization with PCI [percutaneous coronary intervention],” William F. Fearon, MD, said while presenting the FAME 2 findings at the Transcatheter Cardiovascular Therapeutics annual meeting.
FAME 2 was a randomized, multicenter trial designed to help bring clarity regarding the optimal treatment strategy for patients with stable angina and CAD. This is an issue surrounded by considerable controversy. The fog descended a decade ago, when the COURAGE trial created a stir with its conclusion that optimal medical therapy (OMT) alone was as good as PCI plus OMT in terms of clinical outcome and quality of life – and was considerably less expensive as well. And the British ORBITA trial, presented earlier in the same session at TCT 2017 as Dr. Fearon’s report on FAME 2, caused an uproar with its finding that PCI plus OMT was no more effective than sham PCI plus OMT in the setting of stable CAD.
However, FAME 2 (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) differed from those studies in a crucial aspect: randomization in FAME 2 was restricted to patients with physiologically significant cardiac ischemia as evidenced by a fractional flow reserve (FFR) measurement of 0.80 or less.
In contrast, COURAGE and ORBITA randomized patients without FFR guidance. As a result, in those trials PCI was performed in a substantial proportion of patients who actually should not have undergone the intervention because they didn’t have physiologic evidence of clinically important ischemia. The non–physiologically based approach to PCI utilized in COURAGE and ORBITA – disappointingly commonplace in daily clinical practice – diluted any true benefit of the procedure when applied appropriately, explained Dr. Fearon, professor of medicine and director of interventional cardiology at Stanford (Calif.) University.
FAME 2 randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus OMT or an initial strategy of OMT alone at 28 European and North American sites. The primary outcome was the rate of major adverse cardiac events – death, MI, and urgent revascularization – at 3 years. The rate was 10.1% in the PCI group, compared with 22% in the medically managed cohort, Dr. Fearon reported at the meeting sponsored by the Cardiovascular Research Foundation.
Death or MI occurred in 8.3% of the PCI group versus 10.4% in the OMT group, a trend that didn’t reach significance. Of note, however, fully 44% of patients in the OMT group crossed over to PCI during the 3-year study. In the prespecified intent-to-treat analysis they were counted in the OMT group, whereas an as-treated analysis might well have shown statistically significant reductions in death and MI in the PCI group.
The proportion of patients with class II-IV angina was significantly lower in the PCI plus medical therapy group at every time point, including 5.9% versus 15.2% for OMT alone at 1 year, 5.9% versus 12% at 2 years, and 5.2% versus 9.7% at 3 years. This was the case even though the OMT group received significantly more antianginal therapy in an effort to control symptoms.
FAME 2 featured a first-of-its-kind comprehensive cost-effectiveness analysis of OMT vs. PCI over a 3-year period. It showed that, while mean initial costs were as expected higher in the PCI group ($9,944 versus $4,440), by 3 years the cumulative costs were near identical at $16,792 in the PCI group and $16,737 in the initial OMT group. The incremental cost-effectiveness ratio for PCI, compared with OMT at 3 years was attractive at $1,600 per quality-adjusted life-year gained.
The question on TCT attendees’ minds following presentation of the bombshell ORBITA findings was, what would have happened had FAME 2 featured a sham PCI arm? Could the advantageous outcomes for the initial PCI strategy seen in FAME 2 possibly have been due to a placebo effect?
Extremely unlikely, according to Dr. Fearon. For one thing, when he and his coinvestigators broke down the FFR values in the OMT group into quintiles, they saw a clear dose-response effect: The clinical event rate rose further with worsening quintile of FFR. Also, the study endpoints were death, MI, and urgent revascularization – triggered by ACS in half of cases – which are less susceptible to a placebo effect than, say, treadmill exercise time, the primary endpoint in ORBITA.
Moreover, as noted by Gary S. Mintz, MD, who moderated a press conference highlighting the ORBITA and FAME 2 results, placebo effects don’t last for years.
“Most people would say the placebo effect wanes over time. That’s why these 3-year data, analyzed by intent-to-treat, which allow for crossovers to still be analyzed in the medical therapy arm, are pretty compelling to me,” commented Dr. Mintz, chief medical officer for the Cardiovascular Research Foundation in New York.
FAME 2 was supported by St. Jude Medical. Dr. Fearon reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The rate of major adverse cardiac events at 3 years was 10.1% in the PCI group and 22% in the medically managed cohort.
Data source: The FAME 2 trial randomized 888 patients with stable single- or multivessel CAD and an FFR of 0.80 or less to PCI plus optimal medical therapy or an initial strategy of optimal medical management alone.
Disclosures: The trial was supported by St. Jude Medical. The presenter reported receiving institutional research support from Medtronic, Abbott Vascular, ACIST Medical, CathWorks, and Edwards LifeSciences.
EXCEL: Quality of life better after PCI than CABG
DENVER – Several key validated measures of health status were significantly more favorable a full year after percutaneous coronary intervention using an everolimus-eluting stent than with coronary artery bypass surgery in patients with unprotected left main CAD in the prespecified quality-of-life analysis of the landmark EXCEL trial.
By 3 years of follow-up, there was no longer a difference between the PCI and CABG groups in terms of the various quality-of-life measures, Suzanne J. Baron, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting. Nor as previously reported was there any significant difference in the primary composite endpoint comprised of all-cause mortality, stroke, or MI.
“My take away from this is that these results provide an ideal opportunity to give patients a choice about which choice they would want: An earlier recovery with angioplasty versus really similar outcomes long-term with either procedure. For me, these EXCEL results make me feel that angioplasty for less complex coronary disease is really probably the preferred option,” said John A. Spertus, MD, director of health outcomes research at Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri-Kansas City.
“The faster recovery with PCI, the similar angina relief after 3 years, and also the less depression with PCI, which is an important finding, I think – all this goes in favor of PCI for left main disease,” commented Evald H. Christiansen, MD, a cardiologist at Aarhus (Denmark) University and senior investigator in the NOBLE trial (Lancet. 2016 Dec 3;388[10061]:2743-52), which randomized patients to CABG or a stent that’s no longer marketed.
The previously published clinical outcomes of EXCEL (N Engl J Med. 2016 Dec 8;375[23]:2223-35) were based upon a median 3 years of follow-up. Dr. Baron presented updated outcomes in which all study participants had completed the full 3 years of follow-up. The results were little changed: The primary composite endpoint of all-cause mortality, stroke, or MI occurred in 15.2% of the group treated with the everolimus-eluting Xience stent and was closely similar at 14.7% of the CABG patients, while the 12.5% repeat revascularization rate in the PCI arm was significantly greater than the 7.4% rate with CABG.
But the prespecified EXCEL quality-of-life substudy with assessments at baseline, 1 month, 1 year, and 3 years in 1,788 participants is all new information. Among the highlights: The proportion of patients with clinically significant depression as defined by a Patient Health Questionnaire 8 (PHQ-8) score of 10 or more was 21% at baseline in both groups; 8% in the PCI group, compared with 19% in the CABG arm at 1 month; and 8% in the PCI arm versus 12% with CABG at 12 months of follow-up, with the differences at both time points being significant. By 3 years, the rate was 8%-9% in both groups, reported Dr. Baron of Saint Luke’s Mid America Health Institute and the University of Missouri-Kansas City.
“We now have a new treatment for depression: PCI,” quipped session moderator Gregg W. Stone, MD, professor of medicine at Columbia University in New York, who was lead investigator in EXCEL.
Also, scores on the SF-12 physical summary scale improved sharply from a baseline of 39 points in the PCI group during the first month of follow-up while worsening in the CABG group, such that at 1 month the between-group difference averaged 8.2 points. The gap narrowed over the next 11 months, and by 1 year the CABG patients had caught up.
Scores on the Seattle Angina Questionnaire and the Rose Dyspnea Scale were significantly better in the PCI group than the CABG arm at 1 month, but at 12 and 36 months the two groups were indistinguishable in these domains.
Dr. Spertus, who is credited with inventing both the Seattle Angina Questionnaire and the Kansas City Cardiomyopathy Questionnaire, said that in the context of EXCEL he puts more stock in the SF-12 and PHQ-8 results than the angina and dyspnea measures.
Jonathan Hill, MD, an interventional cardiologist at King’s College London, said the EXCEL quality-of-life substudy provides a valuable picture of the real-life impact of sternotomy.
“We mustn’t underplay that, the months and even up to a year of your life for recovery from the revascularization procedure, compared with days of recovery time with PCI. Patients want the option of PCI if it’s available. This data really vindicates that decision making,” he said.
“I think we minimize the recovery period from CABG. People talk a lot about outcomes at 3 years and 5 years, but look at this prolonged recovery. I think that’s very, very important,” said Dr. Grines, chair of cardiology at Hofstra University, Hempstead, N.Y.
The updated full 3-year data show a trend, albeit not statistically significant, for higher all-cause mortality in the PCI group, by a margin of 8% versus 5.8% with CABG. When asked about it, Dr. Baron said she and her coinvestigators took a closer look and determined that the cardiovascular death rate was virtually identical in the two groups.
“You have to wonder if this was just a random signal regarding the non-cardiovascular-associated deaths,” she added.
The EXCEL trial was supported by Abbott Vascular. Dr. Baron reported serving as a consultant to Edwards Lifesciences and St. Jude Medical.
DENVER – Several key validated measures of health status were significantly more favorable a full year after percutaneous coronary intervention using an everolimus-eluting stent than with coronary artery bypass surgery in patients with unprotected left main CAD in the prespecified quality-of-life analysis of the landmark EXCEL trial.
By 3 years of follow-up, there was no longer a difference between the PCI and CABG groups in terms of the various quality-of-life measures, Suzanne J. Baron, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting. Nor as previously reported was there any significant difference in the primary composite endpoint comprised of all-cause mortality, stroke, or MI.
“My take away from this is that these results provide an ideal opportunity to give patients a choice about which choice they would want: An earlier recovery with angioplasty versus really similar outcomes long-term with either procedure. For me, these EXCEL results make me feel that angioplasty for less complex coronary disease is really probably the preferred option,” said John A. Spertus, MD, director of health outcomes research at Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri-Kansas City.
“The faster recovery with PCI, the similar angina relief after 3 years, and also the less depression with PCI, which is an important finding, I think – all this goes in favor of PCI for left main disease,” commented Evald H. Christiansen, MD, a cardiologist at Aarhus (Denmark) University and senior investigator in the NOBLE trial (Lancet. 2016 Dec 3;388[10061]:2743-52), which randomized patients to CABG or a stent that’s no longer marketed.
The previously published clinical outcomes of EXCEL (N Engl J Med. 2016 Dec 8;375[23]:2223-35) were based upon a median 3 years of follow-up. Dr. Baron presented updated outcomes in which all study participants had completed the full 3 years of follow-up. The results were little changed: The primary composite endpoint of all-cause mortality, stroke, or MI occurred in 15.2% of the group treated with the everolimus-eluting Xience stent and was closely similar at 14.7% of the CABG patients, while the 12.5% repeat revascularization rate in the PCI arm was significantly greater than the 7.4% rate with CABG.
But the prespecified EXCEL quality-of-life substudy with assessments at baseline, 1 month, 1 year, and 3 years in 1,788 participants is all new information. Among the highlights: The proportion of patients with clinically significant depression as defined by a Patient Health Questionnaire 8 (PHQ-8) score of 10 or more was 21% at baseline in both groups; 8% in the PCI group, compared with 19% in the CABG arm at 1 month; and 8% in the PCI arm versus 12% with CABG at 12 months of follow-up, with the differences at both time points being significant. By 3 years, the rate was 8%-9% in both groups, reported Dr. Baron of Saint Luke’s Mid America Health Institute and the University of Missouri-Kansas City.
“We now have a new treatment for depression: PCI,” quipped session moderator Gregg W. Stone, MD, professor of medicine at Columbia University in New York, who was lead investigator in EXCEL.
Also, scores on the SF-12 physical summary scale improved sharply from a baseline of 39 points in the PCI group during the first month of follow-up while worsening in the CABG group, such that at 1 month the between-group difference averaged 8.2 points. The gap narrowed over the next 11 months, and by 1 year the CABG patients had caught up.
Scores on the Seattle Angina Questionnaire and the Rose Dyspnea Scale were significantly better in the PCI group than the CABG arm at 1 month, but at 12 and 36 months the two groups were indistinguishable in these domains.
Dr. Spertus, who is credited with inventing both the Seattle Angina Questionnaire and the Kansas City Cardiomyopathy Questionnaire, said that in the context of EXCEL he puts more stock in the SF-12 and PHQ-8 results than the angina and dyspnea measures.
Jonathan Hill, MD, an interventional cardiologist at King’s College London, said the EXCEL quality-of-life substudy provides a valuable picture of the real-life impact of sternotomy.
“We mustn’t underplay that, the months and even up to a year of your life for recovery from the revascularization procedure, compared with days of recovery time with PCI. Patients want the option of PCI if it’s available. This data really vindicates that decision making,” he said.
“I think we minimize the recovery period from CABG. People talk a lot about outcomes at 3 years and 5 years, but look at this prolonged recovery. I think that’s very, very important,” said Dr. Grines, chair of cardiology at Hofstra University, Hempstead, N.Y.
The updated full 3-year data show a trend, albeit not statistically significant, for higher all-cause mortality in the PCI group, by a margin of 8% versus 5.8% with CABG. When asked about it, Dr. Baron said she and her coinvestigators took a closer look and determined that the cardiovascular death rate was virtually identical in the two groups.
“You have to wonder if this was just a random signal regarding the non-cardiovascular-associated deaths,” she added.
The EXCEL trial was supported by Abbott Vascular. Dr. Baron reported serving as a consultant to Edwards Lifesciences and St. Jude Medical.
DENVER – Several key validated measures of health status were significantly more favorable a full year after percutaneous coronary intervention using an everolimus-eluting stent than with coronary artery bypass surgery in patients with unprotected left main CAD in the prespecified quality-of-life analysis of the landmark EXCEL trial.
By 3 years of follow-up, there was no longer a difference between the PCI and CABG groups in terms of the various quality-of-life measures, Suzanne J. Baron, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting. Nor as previously reported was there any significant difference in the primary composite endpoint comprised of all-cause mortality, stroke, or MI.
“My take away from this is that these results provide an ideal opportunity to give patients a choice about which choice they would want: An earlier recovery with angioplasty versus really similar outcomes long-term with either procedure. For me, these EXCEL results make me feel that angioplasty for less complex coronary disease is really probably the preferred option,” said John A. Spertus, MD, director of health outcomes research at Saint Luke’s Mid America Heart Institute and professor of medicine at the University of Missouri-Kansas City.
“The faster recovery with PCI, the similar angina relief after 3 years, and also the less depression with PCI, which is an important finding, I think – all this goes in favor of PCI for left main disease,” commented Evald H. Christiansen, MD, a cardiologist at Aarhus (Denmark) University and senior investigator in the NOBLE trial (Lancet. 2016 Dec 3;388[10061]:2743-52), which randomized patients to CABG or a stent that’s no longer marketed.
The previously published clinical outcomes of EXCEL (N Engl J Med. 2016 Dec 8;375[23]:2223-35) were based upon a median 3 years of follow-up. Dr. Baron presented updated outcomes in which all study participants had completed the full 3 years of follow-up. The results were little changed: The primary composite endpoint of all-cause mortality, stroke, or MI occurred in 15.2% of the group treated with the everolimus-eluting Xience stent and was closely similar at 14.7% of the CABG patients, while the 12.5% repeat revascularization rate in the PCI arm was significantly greater than the 7.4% rate with CABG.
But the prespecified EXCEL quality-of-life substudy with assessments at baseline, 1 month, 1 year, and 3 years in 1,788 participants is all new information. Among the highlights: The proportion of patients with clinically significant depression as defined by a Patient Health Questionnaire 8 (PHQ-8) score of 10 or more was 21% at baseline in both groups; 8% in the PCI group, compared with 19% in the CABG arm at 1 month; and 8% in the PCI arm versus 12% with CABG at 12 months of follow-up, with the differences at both time points being significant. By 3 years, the rate was 8%-9% in both groups, reported Dr. Baron of Saint Luke’s Mid America Health Institute and the University of Missouri-Kansas City.
“We now have a new treatment for depression: PCI,” quipped session moderator Gregg W. Stone, MD, professor of medicine at Columbia University in New York, who was lead investigator in EXCEL.
Also, scores on the SF-12 physical summary scale improved sharply from a baseline of 39 points in the PCI group during the first month of follow-up while worsening in the CABG group, such that at 1 month the between-group difference averaged 8.2 points. The gap narrowed over the next 11 months, and by 1 year the CABG patients had caught up.
Scores on the Seattle Angina Questionnaire and the Rose Dyspnea Scale were significantly better in the PCI group than the CABG arm at 1 month, but at 12 and 36 months the two groups were indistinguishable in these domains.
Dr. Spertus, who is credited with inventing both the Seattle Angina Questionnaire and the Kansas City Cardiomyopathy Questionnaire, said that in the context of EXCEL he puts more stock in the SF-12 and PHQ-8 results than the angina and dyspnea measures.
Jonathan Hill, MD, an interventional cardiologist at King’s College London, said the EXCEL quality-of-life substudy provides a valuable picture of the real-life impact of sternotomy.
“We mustn’t underplay that, the months and even up to a year of your life for recovery from the revascularization procedure, compared with days of recovery time with PCI. Patients want the option of PCI if it’s available. This data really vindicates that decision making,” he said.
“I think we minimize the recovery period from CABG. People talk a lot about outcomes at 3 years and 5 years, but look at this prolonged recovery. I think that’s very, very important,” said Dr. Grines, chair of cardiology at Hofstra University, Hempstead, N.Y.
The updated full 3-year data show a trend, albeit not statistically significant, for higher all-cause mortality in the PCI group, by a margin of 8% versus 5.8% with CABG. When asked about it, Dr. Baron said she and her coinvestigators took a closer look and determined that the cardiovascular death rate was virtually identical in the two groups.
“You have to wonder if this was just a random signal regarding the non-cardiovascular-associated deaths,” she added.
The EXCEL trial was supported by Abbott Vascular. Dr. Baron reported serving as a consultant to Edwards Lifesciences and St. Jude Medical.
AT TCT 2017
Key clinical point:
Major finding: The rate of clinically significant depression 1 year after revascularization of unprotected left main CAD via PCI using an everolimus-eluting stent was 8%, significantly lower than the 12% rate in CABG patients.
Data source: This was a prespecified prospective quality-of-life substudy featuring 3 years of follow-up in 1,788 patients randomized to PCI or CABG.
Disclosures: The EXCEL trial was supported by Abbott Vascular. The presenter reported serving as a consultant to Edwards Lifesciences and St. Jude Medical.
Bare metal stents: Rest in peace
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
DENVER – Percutaneous coronary intervention using a contemporary drug-eluting stent on a shortened regimen of dual antiplatelet therapy proved significantly more effective and equally safe as a bare metal stent in elderly patients in the randomized, multicenter SENIOR trial.
“I think BMS [bare metal stents] should no longer be used as a strategy to reduce DAPT [dual antiplatelet therapy] duration in the elderly,” Olivier Varenne, MD, concluded in presenting the SENIOR findings at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
“This trial adds to what I think is now a very large body of evidence showing that DES [drug-eluting stents] are the way to go, pretty much across the board, putting aside the economic factors that might come into play in certain regions of the world,” Deepak L. Bhatt, MD, commented at the meeting sponsored by the Cardiovascular Research Foundation.
“I think in general there’s really no good reason to use a BMS. I’d use a DES. I think DES is a winning strategy, whether it’s for a thrombotic lesion or an older patient,” added Dr. Bhatt, professor of medicine at Harvard Medical School in Boston and executive director of international cardiovascular programs at Brigham and Women’s Hospital.
Martin B. Leon, MD, concurred.
“I cannot think of any indication for using a BMS anymore, other than cost considerations. I think this study is yet another nail in the coffin of BMS. I think they should be relegated to the past because they really have very little role in environments where DES – which are becoming much more cost-efficient – can be used,” said Dr. Leon, professor of medicine at Columbia University and director of the Center for Interventional Vascular Therapy at New York-Presbyterian/Columbia University Medical Center, New York. Yet in contemporary practice many cardiologists turn to BMS for percutaneous coronary intervention (PCI) in elderly patients with coronary artery disease (CAD), reasoning that the shorter DAPT duration recommended for BMS in the practice guidelines is attractive as a means of minimizing the risk of bleeding complications, which is typically high in the elderly.
The SENIOR trial demonstrates that using a modern DES in combination with the shortened 1- or 6-month DAPT duration typically reserved for BMS recipients results in fewer major adverse cardiac and cerebrovascular events with no increase in bleeding, compared with BMS. This is a finding of high clinical relevance because so many elderly patients undergo PCI; indeed, today one in four PCI patients in the United States is over age 75, explained Dr. Varenne of Cochin Hospital in Paris.
SENIOR was a single-blind, randomized trial of 1,200 PCI patients age 75 and older at 44 centers in nine countries. Their mean age was 81.4 years. This was essentially an all-comers trial: 45% of participants had an acute coronary syndrome, 55% had stable or silent CAD. If they had stable CAD, they were slated for 1 month of DAPT. If they had ACS, they got 6 months of DAPT. All participants were then randomized to PCI with either the thin-strut everolimus-eluting bioabsorbable polymer Synergy DES or the thin-strut Omega or Rebel BMS.
The primary composite efficacy endpoint was the 1-year rate of all-cause mortality, acute MI, stroke, or ischemia-driven target lesion revascularization. The rate was 11.6% in the DES group and 16.4% in the BMS group, for a 29% reduction in favor of the DES strategy and a favorably low number-needed-to-treat of 21. The difference in outcome was driven mainly by a higher ischemia-driven target lesion revascularization rate in the BMS group.
The 1-year rate of bleeding complications was 5% in each group. Probable or definite stent thrombosis occurred in 0.5% of the DES group and 1.4% of the BMS group at 1 year. Ten of the 11 cases of stent thrombosis in the study occurred during the first 30 days after PCI while the patients were on DAPT; the other case occurred on day 31, the day after DAPT was stopped.
Discussant Eric D. Peterson, MD, applauded Dr. Varenne and his coinvestigators for conducting a study focused on elderly individuals, an understudied population largely excluded from the landmark clinical trials in interventional cardiology.
He found the study convincing: “My takeaway is DES is better than BMS. As it is in young people, it continues in old.”
But the study leaves two important questions unanswered: Is the Synergy stent the best DES in the elderly, and what is the best duration of DAPT therapy? Ideally, the SENIOR trial would have included a study arm with the standard 6- and 12-month DAPT durations recommended in the American guidelines for DES recipients with stable and unstable CAD, respectively, observed Dr. Peterson, professor of medicine at Duke University in Durham, N.C., and director of the Duke Clinical Research Institute.
Dr. Varenne replied that SENIOR wasn’t designed as a DAPT duration trial. DAPT duration wasn’t randomized. But other planned and ongoing studies are attempting to define the best DAPT durations in various patient subsets.
Dr. Bhatt said he believes the superiority of contemporary DES over modern BMS is a class effect.
The SENIOR trial was funded by Boston Scientific. Dr. Varenne reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
AT TCT 2017
Key clinical point:
Major finding: The number of elderly patients with CAD who would need to be treated with a contemporary drug-eluting stent backed by a shortened DAPT regimen instead of a modern-era bare metal stent in order to avoid one additional major adverse cardiac and cerebrovascular event over the course of a year is 21.
Data source: This randomized, prospective, single-blind trial included 1,200 patients age 75 or older who underwent PCI at 44 centers in nine countries.
Disclosures: The SENIOR trial was funded by Boston Scientific. The presenter reported receiving lecture fees from that company as well as Abbott Vascular, AstraZeneca, and Servier within the past year.
ORBITA: PCI no better than meds for stable angina
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
DENVER – The first-ever blinded, sham-controlled randomized trial of percutaneous coronary intervention for stable angina failed to show a significant improvement in exercise time for PCI, compared with placebo PCI, Rasha Al-Lamee, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The blockbuster results of the ORBITA trial, published online in the Lancet simultaneously with Dr. Al-Lamee’s presentation in Denver, quickly went viral, with a story splashed across the front page of the New York Times under the headline “‘Unbelievable’: Heart Stents Fail to Ease Chest Pain.” Interventional cardiology thought leaders at TCT said the newspaper piece, and a Lancet editorial commentary entitled “Last nail in the coffin for PCI in stable angina?” that accompanied publication of ORBITA, failed to convey the study’s major limitations, drawbacks that Dr. Al-Lamee readily acknowledged.
What ORBITA did
ORBITA (Objective Randomized Blinded Investigation with Optimal Medical Therapy of Angioplasty in Stable Angina) included 200 patients referred to five U.K. cardiac catheterization labs for diagnostic angiography. Participants had to have stable angina, single-vessel disease, and at least one 70% or greater stenosis; in fact, their stenotic severity averaged 84.4% by quantitative coronary angiography.
The patients received 6 weeks of intensive medical therapy during which they were uptitrated to an average of three antianginal medications. They then underwent either real or sham PCI followed by 6 weeks of recovery, during which both the patients and care team remained blinded. Then the same assessments done before randomization were repeated, including exercise treadmill testing, the Seattle Angina Questionnaire, and dobutamine stress echocardiography, explained Dr. Al-Lamee of Imperial College London.
The primary outcome was achievement of at least a 30-second greater improvement in total exercise time following PCI, compared with sham PCI, an effect size chosen based on placebo-controlled studies of antianginal drugs. The PCI group improved by a mean of 28.4 seconds, the controls by 11.8 seconds, and the resultant 16.6-second difference made for a negative result (Lancet. 2017 Nov 2;doi: 10.1016/S0140-6736[17]32714-9).
PCI did, however, result in significant improvement in the secondary endpoint of ischemia reduction as assessed by blinded evaluation of dobutamine stress echocardiography results. The PCI group’s mean peak stress wall motion index score improved from 1.11 prerandomization to 1.03 – that is, normal – at follow-up 6 weeks post procedure while remaining unchanged in the sham PCI group, Dr. Al-Lamee noted at the meeting, sponsored by the Cardiovascular Research Foundation.
What the results mean
Dr. Al-Lamee said the ORBITA results should enable cardiologists to sit with patients similar to those in the trial and have a more informed, patient-centered discussion in which intensive medical management can be offered as an initial first-line option with an understanding that it will likely improve their symptoms to the same degree as angioplasty.
“There will be those patients who would rather avoid having to take high doses of antianginal medications with the side effects they involve, who may well prefer to have an upfront procedure with a small risk in order to reduce their pill count, and who also would rather have improved blood flow to the heart, which may have prognostic implications,” Dr. Al-Lamee said.
Carl L. Tommaso, MD, part of the panel of discussants at the late-breaking clinical trials session in which Dr. Al-Lamee presented the ORBITA findings, applauded the investigators for their ingenious study design, which included elaborate blinding techniques involving music played through headphones throughout the procedure, heavy sedation, separate angioplasty and clinical care teams, the same postprocedural instructions and discharge letter, and dual-antiplatelet therapy in both study arms.
“This is a great study. I don’t think any of us could get this study past an institutional review board in the United States,” commented Dr. Tommaso, director of the cardiac catheterization laboratory at Skokie (Ill.) Hospital.
He added, however, that he wouldn’t have performed PCI on the basis of angiographic findings alone in stable angina patients with a 9-minute treadmill exercise time.
Where OPTIMA fell short
Angiography vs. functional testing
“Twenty-nine percent of patients, we’d all agree, should not have had angioplasty because they had no ischemia,” said Dr. Stone, professor of medicine at Columbia University, New York, and director of the TCT conference.
All subjects in ORBITA did indeed undergo measurement of both FFR and instant Wave-Free Ratio (iFR) while on the table immediately before and after their real or sham PCI. The mean stenosis severity was 0.69 by FFR and 0.76 by iFR, readings indicative of significantly impaired flow. However, the operators were blinded as to those results. The rationale for withholding that information was that, even though it has been shown to be clinically useful, studies show that 80% of angioplasties are done based upon angiography alone, and the ORBITA investigators wanted the study to reflect routine clinical practice, Dr. Al-Lamee explained.
“I think one of the many lessons coming out of this trial is to see the discrepancy between the angiogram and functional testing. We cannot guide our therapy solely by the angiogram. We have to get physiologic data and consider that together with symptoms in the patient’s clinical context,” said panelist Allen Jeremias, MD, director of interventional cardiology research at St. Francis Hospital in Rosyln, N.Y.
Commentary goes too far
The “last-nail-in-the-coffin” Lancet commentary (2017 Nov 2. doi: 10.1016/S0140-6736[17]32757-5) penned by David L. Brown, MD, of Washington University in St. Louis and Rita F. Redberg, MD, of the University of California, San Francisco, emphatically declared that the ORBITA results mean all cardiology guidelines should be revised to downgrade the recommendation for PCI in patients with angina despite medical therapy. Dr. Al-Lamee was one of many at TCT 2017 who took strong exception to that.
“This is the first trial of its kind. I think it would be very easy to take the results of this trial and overextrapolate. To downgrade the guideline recommendations based on this study would be an incredibly large overreach,” she said.
Ajay J. Kirtane, MD, who chaired a press conference in which Dr. Al-Lamee presented the ORBITA results, had a further criticism of the editorial.
“Some of the risks of PCI as described in the editorial are just factually inaccurate. An MI rate of 15%, an acute kidney injury rate of 13% – those are simply factually incorrect,” said Dr. Kirtane, director of the cardiac catheterization laboratories at New York-Presbyterian/Columbia University Medical Center.
The ORBITA trial was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. Dr. Al-Lamee reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
AT TCT 2017
Key clinical point:
Major finding: PCI on top of intensive antianginal medications was not significantly more effective at improving exercise tolerance than sham PCI.
Data source: ORBITA, a randomized, multicenter, blinded, sham-controlled study of 200 patients with mild angina and single-vessel CAD.
Disclosures: ORBITA was sponsored by Imperial College London and funded by grants from the National Institute of Health Research Imperial Biomedical Research Center and charity organizations. The presenter reported serving as a paid consultant to Philips Volcano, which supplied the coronary pressure wires for physiologic testing.
TAVR wallops SAVR in cost-effectiveness for intermediate-risk patients
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
DENVER – A formal cost-effectiveness analysis indicates that transcatheter aortic valve replacement (TAVR) is substantially more cost effective than surgical valve replacement in patients at intermediate surgical risk similar to those enrolled in the landmark PARTNER 2 trial.
The analysis demonstrated that over a 1- and 2-year follow-up period, as well as with projected lifetime follow-up, TAVR entails both lower long-term costs and greater quality-adjusted life expectancy, David J. Cohen, MD, reported at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
His two-part, patient-level economic analysis examined data from nearly 2,000 participants in the PARTNER 2A randomized trial comparing TAVR, using the Sapien XT valve, with surgical aortic valve replacement (SAVR), as well as the experience with the current-generation Sapien 3 TAVR valve in 1,077 intermediate–surgical risk TAVR patients in the S3i registry. The analysis utilized Medicare claims data on the costs of the index hospitalization and follow-up care.
In PARTNER 2A, the average total cost of the index hospitalization for valve replacement was $61,433 with TAVR. That was just $2,888 more than the SAVR hospitalization, despite the far higher acquisition cost of the Sapien 3 valve, which was roughly $32,500, compared with $5,000 for the surgical valve. Most of this additional cost of the TAVR valve was counterbalanced by TAVR’s 2-hour shorter procedural duration, the 6.4-day average length of stay, compared with 10.9 days for SAVR, and the fact that TAVR patients spent only 2.4 days in intensive care while SAVR patients averaged 4.6 days, Dr. Cohen explained at the meeting sponsored by the Cardiovascular Research Foundation.
During 24 months of postdischarge follow-up in the PARTNER 2A trial, SAVR patients racked up an average of $9,303 more in costs than TAVR patients. This was mainly because of their much higher rates of rehospitalization and time spent in skilled nursing facilities and rehabilitation centers, mainly during months 2-6 post discharge. The result was that 2-year total costs including the index hospitalization averaged $107,716 per TAVR patient and $114,132 per SAVR patient.
“One of the really remarkable findings of this study was what happened during follow-up,” the cardiologist observed.
Extrapolating to projected remaining lifetime years, TAVR using the Sapien XT valve resulted in a cost savings of $7,949 per patient and a 0.15-year increase in quality-adjusted life expectancy compared with SAVR.
But since the time of PARTNER 2A, the Sapien XT valve has been replaced by the updated Sapien 3 valve. The analysis of the S3i registry showed that the economic dominance of TAVR over SAVR was even greater owing to improved valve technology and contemporary care patterns. For this analysis, because there has been no randomized trial of TAVR with the Sapien 3 valve versus SAVR, patients in the SAVR of arm of PARTNER 2A served as the comparison group.
The cost of the index hospitalization was more than $4,000 less with TAVR in the S3i registry than with SAVR. The total cost of TAVR through 1 year of follow-up averaged $80,977, which was $15,511 less than the $96,489 for SAVR. The cost post discharge out to 1 year was more than $11,000 less per TAVR patient, driven by sharply lower rates of both cardiovascular and noncardiovascular hospitalizations as well as a greater than 50% reduction in days spent in rehab centers and skilled nursing facilities, compared with SAVR patients.
Projected over estimated remaining years of life, TAVR with the Sapien 3 valve yielded a cost savings of $9,692 per patient compared with SAVR, as well as a 0.27-year gain in quality-adjusted life-years.
Eighty-eight percent of patients in the S3i registry received their Sapien 3 valve via a transfemoral approach. When Dr. Cohen and his coinvestigators compared their costs and clinical outcomes to the subset of PARTNER 2A TAVR patients who got the Sapien XT valve transfemorally, the outcomes were “virtually identical,” he said.
“These findings are reassuring with regard to the S3i results and also suggest that the primary mechanism of benefit of the Sapien 3 valve over the XT valve is its lower profile, which allows roughly 90% of patients to be treated via a transfemoral approach,” according to Dr. Cohen.
He predicted the new cost-effectiveness findings will not substantially increase patient demand for TAVR, which is already high.
“By far what’s driving patients to TAVR today are the quality of life advantages. They love the idea of recovering quickly,” he said.
Michael Mack, MD, commented that this analysis probably underestimates the true cost advantage of TAVR by a fair amount, since the average hospital length of stay for TAVR patients in PARTNER 2A was 6.4 days.
“We now know that half of U.S. TAVR patients in many centers go home the day after the procedure, so you would expect that TAVR would look even more favorable based on current practice,” said Dr. Mack, medical director of cardiovascular surgery for the Baylor Health Care System and chairman of the Heart Hospital Baylor Plano (Tex.) Research Center.
Session moderator Patrick W. Serruys, MD, of Imperial College, London, observed that the cost differential between TAVR and SAVR will grow even larger once the sky-high cost of TAVR valves comes down. He predicted that’s likely to happen as a result of increased competition once a third valve receives marketing approval, just as occurred after a third drug-eluting stent hit the market.
Several physicians grumbled about the unfairness of current reimbursement for TAVR, which in effect penalizes hospitals. Dr. Cohen said that situation will change.
“I think the future of health care financing in the U.S. is bundled payment and accountable care organizations. In the setting of bundled payment for a 6-month period or even for 90 days, TAVR would look fantastic to a hospital or an health maintenance organization due to avoidance of rehospitalizations and rehabilitation and skilled nursing facility stays,” the cardiologist said.
The PARTNER 2A trial, the S3i registry, and the cost-effectiveness analysis were funded by Edwards Lifesciences. Dr. Cohen reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.
AT TCT 2017
Key clinical point:
Major finding: The total cost of TAVR with the Sapien 3 valve in intermediate-risk patients, including the index hospitalization and costs incurred during the first year after, averaged $80,977, compared with $96,489 per SAVR patient.
Data source: This patient-level formal cost-effectiveness analysis included nearly 2,000 patients in the PARTNER 2A trial and more than 1,700 in a registry of recipients of the Sapien 3 TAVR valve.
Disclosures: The cost-effectiveness analysis was funded by Edwards Lifesciences. The presenter reported receiving research funding from and serving as a consultant to Edwards Lifesciences and other device companies.

























