User login
PCI bests medical therapy for FFR grey zone stable angina
PARIS – Patients with stable angina and a fractional flow reserve (FFR) value in the grey zone of 0.75-0.81 experienced a significant reduction in myocardial ischemia and substantially greater quality of life improvement if they were randomized to percutaneous coronary intervention (PCI) plus optimal medical therapy than to optimal medical therapy alone in the Scottish Grey-zone FFR Study.
The Grey-zone FFR Study was a single-center, prospective, unblinded, randomized trial that included 100 patients with stable angina, single-vessel disease, and a fractional flow reserve in the grey zone of 0.75-0.81. While broad consensus exists that an FFR below 0.75 constitutes evidence of a hemodynamically significant coronary lesion warranting revascularization and an FFR greater than 0.80 indicates a lesion isn’t functionally significant and therefore PCI can safely be deferred, there has been uncertainty on what to do about lesions in the grey zone, which are frequently encountered in the cardiac catheterization laboratory.
“In my clinical practice, I tend to go ahead with PCI for patients in the grey zone if I felt it was clinically feasible and safe to do so, particularly if I was worried about their lesion morphology,” Barry Hennigan, MD, said in response to questions after presenting the results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. “If it’s a proximal LAD [left anterior descending artery] lesion and it’s a grey zone patient, particularly if it’s a lesion morphology that you’re not comfortable with, I think you need to be very careful before you defer a case.”
The twin purposes of the Scottish study were to define the prevalence of major ischemia by stress MRI and invasive flow assessment via a pressure wire in grey zone patients – something which hadn’t been done before – and to determine if PCI deferral in such patients is appropriate in terms of symptom control. The primary outcome was change in angina severity at 3 months follow-up using the Seattle Angina Questionnaire (SAQ).
Scores on two of the five domains of the SAQ – anginal frequency and quality of life – were significantly improved in the PCI group. Anginal frequency scores improved by a mean of 20.58 points in the PCI plus optimal medical therapy (OMT) group, compared with a 9.39-point improvement with OMT alone. Quality of life scores improved by 24.04 points in the PCI group versus 9.39 points in controls, said Dr. Hennigan, an interventional cardiologist at the University of Glasgow and Golden Jubilee National Hospital. Scores in the other three SAQ domains – physical limitations, anginal stability, and treatment satisfaction – didn’t differ significantly between the two study arms, although consistently greater improvements were seen in the PCI group.
Baseline stress perfusion MRI as assessed by two blinded observers demonstrated that 17.4% of patients with stable angina and a grey zone FFR had major ischemia, while any ischemia – major or minor – was present in 24.4%. Follow-up scans at 3 months showed a roughly 50% reduction in the prevalence of ischemia in the PCI group, with 7.3% of treated patients still having major ischemia and 12.2% having any ischemia.
Also, 28% of participants had evidence of ischemia at baseline based upon their coronary flow reserve measurements and 8% had a hyperemic stenosis resistance measurement indicative of ischemia. So the FFR grey zone encompasses a range of cardiovascular risks.
In the PCI plus OMT group, 89% of patients (eight of nine) with baseline ischemia on stress MRI had a greater than 10-point improvement in quality of life scores on the SAQ at follow-up in contrast to 53% of patients without ischemia, which made for a statistically significant difference. An improvement of that magnitude is generally considered clinically meaningful. In contrast, in the OMT-only group, 9 of 14 patients with baseline ischemia (64.2%) had a greater than 10-point quality of life improvement, which wasn’t significantly different from the 45.5% improvement rate in patients with no ischemia.
The lessons? Grey zone patients who benefit most from prompt revascularization are those with demonstrable ischemia. In addition, roughly half of grey zone patients with stable angina will improve their quality of life scores by more than 10 points with OMT alone regardless of the presence of myocardial ischemia or not.
Dr. Hennigan was repeatedly asked how he reconciles the results of the grey zone study with those of the much-discussed ORBITA trial, the first and only randomized trial of real versus sham PCI in patients with stable angina. ORBITA didn’t find a significant quality of life advantage for real PCI over sham PCI.
“It is quite possible that a lot of the effect that we saw in our PCI group was placebo related,” he conceded. “However, we do have objective evidence that we reduced ischemia on MRI. Also, 29% of ORBITA patients had an FFR above 0.8, whereas nearly all our patients were below that threshold. So we perhaps had more prevalent ischemia than the ORBITA cohort.”
Also informative is a comparison of SAQ scores at follow-up in the sham PCI ORBITA control group versus the grey zone Scottish PCI group, Dr. Hennigan continued. The Scottish PCI group had a mean 20.6-point improvement in anginal frequency scores while on an average of 1.3 antianginal medications, compared with a 9.6-point improvement in ORBITA patients on 2.9 drugs. The grey zone group who got PCI plus OMT also had a mean 16.1-point improvement in the SAQ physical limitations domain, versus a 5.0-point improvement in the ORBITA controls.
The Grey-zone FFR Study was supported by the British Heart Foundation. Dr. Hennigan reported having no financial conflicts of interest.
PARIS – Patients with stable angina and a fractional flow reserve (FFR) value in the grey zone of 0.75-0.81 experienced a significant reduction in myocardial ischemia and substantially greater quality of life improvement if they were randomized to percutaneous coronary intervention (PCI) plus optimal medical therapy than to optimal medical therapy alone in the Scottish Grey-zone FFR Study.
The Grey-zone FFR Study was a single-center, prospective, unblinded, randomized trial that included 100 patients with stable angina, single-vessel disease, and a fractional flow reserve in the grey zone of 0.75-0.81. While broad consensus exists that an FFR below 0.75 constitutes evidence of a hemodynamically significant coronary lesion warranting revascularization and an FFR greater than 0.80 indicates a lesion isn’t functionally significant and therefore PCI can safely be deferred, there has been uncertainty on what to do about lesions in the grey zone, which are frequently encountered in the cardiac catheterization laboratory.
“In my clinical practice, I tend to go ahead with PCI for patients in the grey zone if I felt it was clinically feasible and safe to do so, particularly if I was worried about their lesion morphology,” Barry Hennigan, MD, said in response to questions after presenting the results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. “If it’s a proximal LAD [left anterior descending artery] lesion and it’s a grey zone patient, particularly if it’s a lesion morphology that you’re not comfortable with, I think you need to be very careful before you defer a case.”
The twin purposes of the Scottish study were to define the prevalence of major ischemia by stress MRI and invasive flow assessment via a pressure wire in grey zone patients – something which hadn’t been done before – and to determine if PCI deferral in such patients is appropriate in terms of symptom control. The primary outcome was change in angina severity at 3 months follow-up using the Seattle Angina Questionnaire (SAQ).
Scores on two of the five domains of the SAQ – anginal frequency and quality of life – were significantly improved in the PCI group. Anginal frequency scores improved by a mean of 20.58 points in the PCI plus optimal medical therapy (OMT) group, compared with a 9.39-point improvement with OMT alone. Quality of life scores improved by 24.04 points in the PCI group versus 9.39 points in controls, said Dr. Hennigan, an interventional cardiologist at the University of Glasgow and Golden Jubilee National Hospital. Scores in the other three SAQ domains – physical limitations, anginal stability, and treatment satisfaction – didn’t differ significantly between the two study arms, although consistently greater improvements were seen in the PCI group.
Baseline stress perfusion MRI as assessed by two blinded observers demonstrated that 17.4% of patients with stable angina and a grey zone FFR had major ischemia, while any ischemia – major or minor – was present in 24.4%. Follow-up scans at 3 months showed a roughly 50% reduction in the prevalence of ischemia in the PCI group, with 7.3% of treated patients still having major ischemia and 12.2% having any ischemia.
Also, 28% of participants had evidence of ischemia at baseline based upon their coronary flow reserve measurements and 8% had a hyperemic stenosis resistance measurement indicative of ischemia. So the FFR grey zone encompasses a range of cardiovascular risks.
In the PCI plus OMT group, 89% of patients (eight of nine) with baseline ischemia on stress MRI had a greater than 10-point improvement in quality of life scores on the SAQ at follow-up in contrast to 53% of patients without ischemia, which made for a statistically significant difference. An improvement of that magnitude is generally considered clinically meaningful. In contrast, in the OMT-only group, 9 of 14 patients with baseline ischemia (64.2%) had a greater than 10-point quality of life improvement, which wasn’t significantly different from the 45.5% improvement rate in patients with no ischemia.
The lessons? Grey zone patients who benefit most from prompt revascularization are those with demonstrable ischemia. In addition, roughly half of grey zone patients with stable angina will improve their quality of life scores by more than 10 points with OMT alone regardless of the presence of myocardial ischemia or not.
Dr. Hennigan was repeatedly asked how he reconciles the results of the grey zone study with those of the much-discussed ORBITA trial, the first and only randomized trial of real versus sham PCI in patients with stable angina. ORBITA didn’t find a significant quality of life advantage for real PCI over sham PCI.
“It is quite possible that a lot of the effect that we saw in our PCI group was placebo related,” he conceded. “However, we do have objective evidence that we reduced ischemia on MRI. Also, 29% of ORBITA patients had an FFR above 0.8, whereas nearly all our patients were below that threshold. So we perhaps had more prevalent ischemia than the ORBITA cohort.”
Also informative is a comparison of SAQ scores at follow-up in the sham PCI ORBITA control group versus the grey zone Scottish PCI group, Dr. Hennigan continued. The Scottish PCI group had a mean 20.6-point improvement in anginal frequency scores while on an average of 1.3 antianginal medications, compared with a 9.6-point improvement in ORBITA patients on 2.9 drugs. The grey zone group who got PCI plus OMT also had a mean 16.1-point improvement in the SAQ physical limitations domain, versus a 5.0-point improvement in the ORBITA controls.
The Grey-zone FFR Study was supported by the British Heart Foundation. Dr. Hennigan reported having no financial conflicts of interest.
PARIS – Patients with stable angina and a fractional flow reserve (FFR) value in the grey zone of 0.75-0.81 experienced a significant reduction in myocardial ischemia and substantially greater quality of life improvement if they were randomized to percutaneous coronary intervention (PCI) plus optimal medical therapy than to optimal medical therapy alone in the Scottish Grey-zone FFR Study.
The Grey-zone FFR Study was a single-center, prospective, unblinded, randomized trial that included 100 patients with stable angina, single-vessel disease, and a fractional flow reserve in the grey zone of 0.75-0.81. While broad consensus exists that an FFR below 0.75 constitutes evidence of a hemodynamically significant coronary lesion warranting revascularization and an FFR greater than 0.80 indicates a lesion isn’t functionally significant and therefore PCI can safely be deferred, there has been uncertainty on what to do about lesions in the grey zone, which are frequently encountered in the cardiac catheterization laboratory.
“In my clinical practice, I tend to go ahead with PCI for patients in the grey zone if I felt it was clinically feasible and safe to do so, particularly if I was worried about their lesion morphology,” Barry Hennigan, MD, said in response to questions after presenting the results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions. “If it’s a proximal LAD [left anterior descending artery] lesion and it’s a grey zone patient, particularly if it’s a lesion morphology that you’re not comfortable with, I think you need to be very careful before you defer a case.”
The twin purposes of the Scottish study were to define the prevalence of major ischemia by stress MRI and invasive flow assessment via a pressure wire in grey zone patients – something which hadn’t been done before – and to determine if PCI deferral in such patients is appropriate in terms of symptom control. The primary outcome was change in angina severity at 3 months follow-up using the Seattle Angina Questionnaire (SAQ).
Scores on two of the five domains of the SAQ – anginal frequency and quality of life – were significantly improved in the PCI group. Anginal frequency scores improved by a mean of 20.58 points in the PCI plus optimal medical therapy (OMT) group, compared with a 9.39-point improvement with OMT alone. Quality of life scores improved by 24.04 points in the PCI group versus 9.39 points in controls, said Dr. Hennigan, an interventional cardiologist at the University of Glasgow and Golden Jubilee National Hospital. Scores in the other three SAQ domains – physical limitations, anginal stability, and treatment satisfaction – didn’t differ significantly between the two study arms, although consistently greater improvements were seen in the PCI group.
Baseline stress perfusion MRI as assessed by two blinded observers demonstrated that 17.4% of patients with stable angina and a grey zone FFR had major ischemia, while any ischemia – major or minor – was present in 24.4%. Follow-up scans at 3 months showed a roughly 50% reduction in the prevalence of ischemia in the PCI group, with 7.3% of treated patients still having major ischemia and 12.2% having any ischemia.
Also, 28% of participants had evidence of ischemia at baseline based upon their coronary flow reserve measurements and 8% had a hyperemic stenosis resistance measurement indicative of ischemia. So the FFR grey zone encompasses a range of cardiovascular risks.
In the PCI plus OMT group, 89% of patients (eight of nine) with baseline ischemia on stress MRI had a greater than 10-point improvement in quality of life scores on the SAQ at follow-up in contrast to 53% of patients without ischemia, which made for a statistically significant difference. An improvement of that magnitude is generally considered clinically meaningful. In contrast, in the OMT-only group, 9 of 14 patients with baseline ischemia (64.2%) had a greater than 10-point quality of life improvement, which wasn’t significantly different from the 45.5% improvement rate in patients with no ischemia.
The lessons? Grey zone patients who benefit most from prompt revascularization are those with demonstrable ischemia. In addition, roughly half of grey zone patients with stable angina will improve their quality of life scores by more than 10 points with OMT alone regardless of the presence of myocardial ischemia or not.
Dr. Hennigan was repeatedly asked how he reconciles the results of the grey zone study with those of the much-discussed ORBITA trial, the first and only randomized trial of real versus sham PCI in patients with stable angina. ORBITA didn’t find a significant quality of life advantage for real PCI over sham PCI.
“It is quite possible that a lot of the effect that we saw in our PCI group was placebo related,” he conceded. “However, we do have objective evidence that we reduced ischemia on MRI. Also, 29% of ORBITA patients had an FFR above 0.8, whereas nearly all our patients were below that threshold. So we perhaps had more prevalent ischemia than the ORBITA cohort.”
Also informative is a comparison of SAQ scores at follow-up in the sham PCI ORBITA control group versus the grey zone Scottish PCI group, Dr. Hennigan continued. The Scottish PCI group had a mean 20.6-point improvement in anginal frequency scores while on an average of 1.3 antianginal medications, compared with a 9.6-point improvement in ORBITA patients on 2.9 drugs. The grey zone group who got PCI plus OMT also had a mean 16.1-point improvement in the SAQ physical limitations domain, versus a 5.0-point improvement in the ORBITA controls.
The Grey-zone FFR Study was supported by the British Heart Foundation. Dr. Hennigan reported having no financial conflicts of interest.
REPORTING FROM EUROPCR 2018
Key clinical point:
Major finding: Patients with stable angina and a fractional flow reserve in the grey zone of 0.75-0.81 experienced a 50% reduction in objectively defined myocardial ischemia if they received percutaneous coronary intervention plus medical therapy, compared with medical therapy alone.
Study details: This single-center, prospective, open-label trial randomized 100 stable angina patients with a grey zone fractional flow reserve of 0.75-0.81 to percutaneous coronary intervention plus optimal medical therapy or optimal medical therapy alone.
Disclosures: The study was supported by the British Heart Foundation. The presenter reported having no financial conflicts.
Think DEB, not BMS, with high bleeding risk
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
PARIS – Treatment with a drug-eluting balloon rather than bare-metal stent provided superior outcomes in patients at high bleeding risk with large-vessel coronary lesions, according to the results of the randomized DEBUT study.
“PCI with a drug-eluting balloon, with the possibility of bailout stenting if needed, is a safe and efficient novel option in patients with high bleeding risk,” Tuomas T. Rissanen, MD, PhD, said in presenting the results of the trial at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The major advantage of the drug-eluting balloon–only strategy is that DAPT [dual-antiplatelet therapy] duration is short – usually 1 month – and positive remodeling of the treated vessel may occur because there is no metallic material present,” added Dr. Rissanen, head of the Heart Center at the University of Eastern Finland in Joensuu.
DEBUT (Drug-Eluting Balloon in Stable and Unstable Angina in a Randomized Controlled Noninferiority Trial) was a five-center, single-blind Finnish study in which patients at elevated bleeding risk – most often because they required oral anticoagulation and were over age 80 – were randomized to a paclitaxel-coated drug-eluting balloon (DEB) applied for a minimum of 30 seconds or a bare-metal stent (BMS). They were placed on DAPT for 1 month if they had stable coronary artery disease and 6 months after an acute coronary syndrome.
Participants had to have a target vessel diameter amenable for PCI with a DEB: that is, 2.5-4.0 mm. Patients with in-stent restenosis, an unprotected left main lesion, ST-elevation MI, chronic total occlusion, a dissection sufficient to reduce flow, greater than 30% recoil after predilation, or a bifurcation lesion requiring side branch stenting were excluded.
The impetus for the DEBUT trial was a recognition that, while the use of DEBs is recommended for treatment of in-stent restenosis by European Society of Cardiology guidelines, until DEBUT there were no high-quality randomized trial data regarding the use of such devices in de novo coronary lesions, the cardiologist noted.
The study results were unequivocal. Indeed, DEBUT, planned for 530 patients, was halted after enrollment of only 208 because an interim analysis showed clear superiority for the DEB strategy.
To wit, the primary endpoint – a composite of cardiovascular death, nonfatal MI, or target lesion revascularization at 9 months post PCI – occurred in 1.9% of the DEB group, compared with 12.4% of BMS recipients. This absolute 10.5% difference in risk translated to an 85% relative risk reduction.
Target lesion revascularization, a major secondary outcome, occurred in none of the DEB group and 4.8% of the BMS group. Bleeding Academic Research Consortium (BARC) type 2 bleeding rates were similar at 11%-12% in the two groups.
Four percent of the DEB group required bailout stenting.
“Importantly, at 9 months, there were two definite stent thrombosis cases in the BMS group and no vessel closures in the DEB group,” Dr. Rissanen observed.
Discussant Antonio Colombo, MD, said, “I think a strategy with a drug-eluting balloon makes sense.”
Even though the 2-year results of the LEADERS FREE trial have shown that the BioFreedom polymer-free drug-coated stent proved safer and more effective than a BMS in high–bleeding risk patients with 1 month of DAPT (J Am Coll Cardiol. 2017 Jan 17;69[2]:162-71), not all PCI centers have access to the BioFreedom stent.
“Why do you need to place a stent in everyone? If you have a good result with the DEB, there is no reason to. Maybe you should use fractional flow reserve [FFR] to give reassurance that the result is really good, but I am in favor of this strategy. I think if you find a small dissection, and the residual lumen is large, it’s okay. It will usually heal. I think a dissection is problematic when the residual lumen is not large,” said Dr. Colombo, chief of invasive cardiology at San Raffaele Hospital in Milan.
There is a practical problem with the DEB-only strategy, however: “Many operators are uncomfortable in not using a stent in a large vessel, even when they have a good result,” he noted.
His fellow discussant Marc Bosiers, MD, said interventional cardiologists need to get over that hangup, which isn’t evidence based.
“We have the same experience in the periphery: We leave arteries as is after DEB therapy with only small Type A, B, and even C dissections, and we have fantastic results. We have total vessel remodeling. In many cases we see the patients back after 6 months or a year and do follow-up angiography, and you’ll be surprised at what you see with DEB alone,” according to Dr. Bosiers, head of the department of vascular surgery at St. Blasius Hospital in Dendermonde, Belgium.
Dr. Rissanen said that, for their next research project, he and his coinvestigators plan to mount a multicenter randomized trial of DEB versus a drug-eluting stent rather than a BMS in high–bleeding risk patients with de novo coronary lesions. And they’re considering ditching the 1 month of DAPT in the DEB patients.
“What is this 1-month DAPT for DEB based on, anyway? I don’t think we need it at all. We could use single-antiplatelet therapy or only the loading dose of the second agent,” he asserted.
But, as one of the discussants responded, that may well be true, and perhaps in the future a course of post-DEB therapy with a single antiplatelet agent or a direct-acting oral anticoagulant will be the routine strategy, but before clinical practice is revised such novel proposals will need to be well-grounded in proof of safety and efficacy. Dr. Rissanen reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
REPORTING FROM EUROPCR 2018
Key clinical point:
Major finding: The 9-month MACE rate was 1.9% in the drug-eluting balloon group versus 12.4% with a bare-metal stent.
Study details: This prospective, multicenter, single-blind trial randomized 208 high–bleeding risk patients with de novo lesions in large coronary vessels to PCI with a drug-eluting balloon-only or a bare-metal stent.
Disclosures: The presenter reported having no financial conflicts regarding the DEBUT study, conducted free of commercial support.
BIO-RESORT trial: Synergy, Orsiro, Resolution stents go head to head to head
PARIS – Three contemporary yet very different drug-eluting stents – two very-thin-strut biodegradable devices and one that leaves behind a durable polymer – achieved similarly low target vessel failure rates through 2 years of prospective follow-up in the large, all-comers, randomized BIO-RESORT trial, Marlies M. Kok, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
That being said, the study provided an intriguing signal that the Orsiro sirolimus-eluting stent might reduce the risk of revascularization after the first year of follow-up, compared with the Synergy everolimus-eluting stent and Resolute Integrity zotarolimus-eluting stent.
“This is a signal of great interest,” noted Dr. Kok, a cardiologist at Thoraxcentrum Twente in Enschede, the Netherlands.
The BIO-RESORT trial was a multicenter, three-arm, patient- and assessor-blinded Dutch study in which 3,514 patients undergoing percutaneous coronary intervention (PCI) were randomized to the Resolute Integrity, Synergy, or Orsiro stent. This was an all-comers trial in which everyone who needed PCI with a drug-eluting stent was included. Most (70%) patients had an acute coronary syndrome; 31% presented with ST-segment elevation MI. At 1 year of follow-up, 86% of patients were on dual-antiplatelet therapy. The 1-year safety and efficacy outcomes, previously reported, were reassuringly similar in the three groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
This was the first randomized trial to examine the safety and efficacy of the Synergy stent in all comers. Dr. Kok presented the new 2-year results, featuring 99% patient follow-up. It’s important to look at outcomes well beyond 1 year, she said, because the three stents are so different.
The Synergy and Orsiro stents feature very-thin, flexible struts with an uncoated thickness of 74 mm and 60 mm, respectively, compared with 91 mm for the Resolute Integrity stent. While the Synergy and Orsiro stents both utilize biodegradable polymer frames, the two devices utilize different drugs with different drug-eluting and polymer-resorption times. The polymer coating of the Synergy stent is resorbed within 4 months, while the Orsiro stent’s polymer degrades after 12 months and is resorbed by 24 months. Everolimus is distributed abluminally in the Synergy stent, while the other two stents feature circumferential drug placement. The Synergy frame is platinum and chromium, while other two utilize cobalt-chromium frames.
The thinking has been that treatment of coronary artery disease with drug-eluting stents, such as the Resolute Integrity, might delay arterial healing as a consequence of the life-long presence of the polymer in the vessel. Very-thin-strut biodegradable polymer stents were developed as a means of getting around this problem. But, at 1 year, the primary endpoint of target vessel failure – a safety and efficacy composite comprising cardiac death, target vessel-related MI, or clinically driven target vessel revascularization – was 5% in all three groups.
Dr. Kok reported that, at 2 years, the target vessel failure rate remained nonsignificantly different between the three study arms: 8.3% with the zotarolimus-eluting Resolute Integrity stent, 6.8% with the everolimus-eluting Synergy stent, and 6.6% with the sirolimus-eluting Orsiro stent. Nor were there any significant differences in the individual components of the primary endpoint. However, the secondary endpoint of target lesion failure – comprising cardiac death, any MI, or clinically driven target lesion revascularization – was a different story. At 1 year, there were no significant between-group differences in this composite outcome. But, a landmark analysis showed that between years 1 and 2, the incidence was 2.4% in the Resolute Integrity group, 1.6% with the Synergy group, and 1.1% in patients randomized to the Orsiro stent. The difference in target lesion failure between the Resolute Integrity and Orsiro groups was statistically significant. This difference was driven in part by the rates of target lesion revascularization: 1.5% with the Resolute Integrity, 0.9% with the Synergy, and 0.6% with the Orsiro stent.
The 2-year rates of definite or probable stent thrombosis were low and similar in the three groups: 1.0% with the Synergy stent, 0.8% with the Resolute Integrity, and 0.6% with the Orsiro, she continued.
Longer-term follow-up is planned.
The latest BIO-RESORT results received a skeptical reception from the session’s discussion panel. Nick E. J. West, MD, of Royal Papworth Hospital in Cambridge, England, praised the study as large and well run, yet, “notwithstanding some post hoc statistical nitty-gritty, there are essentially no differences. Have we come as far as we’re going to go with metallic stents in terms of the devices, and is it now a bit more about implantation, about accuracy, about dual-antiplatelet therapy?” he asked.
Mirvat Al Alasnag, MD, an interventional cardiologist at King Fahd Armed Forces Hospital in Jeddah, Saudi Arabia, said, “An all-comers trial design does not necessarily tell you that something is good for everyone. We try and make everything simple, and it is not that simple. I do believe that stent choice decisions should be individualized.”
The BIO-RESORT trial was funded by institutional research grants from Biotronik, Boston Scientific, and Medtronic.
PARIS – Three contemporary yet very different drug-eluting stents – two very-thin-strut biodegradable devices and one that leaves behind a durable polymer – achieved similarly low target vessel failure rates through 2 years of prospective follow-up in the large, all-comers, randomized BIO-RESORT trial, Marlies M. Kok, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
That being said, the study provided an intriguing signal that the Orsiro sirolimus-eluting stent might reduce the risk of revascularization after the first year of follow-up, compared with the Synergy everolimus-eluting stent and Resolute Integrity zotarolimus-eluting stent.
“This is a signal of great interest,” noted Dr. Kok, a cardiologist at Thoraxcentrum Twente in Enschede, the Netherlands.
The BIO-RESORT trial was a multicenter, three-arm, patient- and assessor-blinded Dutch study in which 3,514 patients undergoing percutaneous coronary intervention (PCI) were randomized to the Resolute Integrity, Synergy, or Orsiro stent. This was an all-comers trial in which everyone who needed PCI with a drug-eluting stent was included. Most (70%) patients had an acute coronary syndrome; 31% presented with ST-segment elevation MI. At 1 year of follow-up, 86% of patients were on dual-antiplatelet therapy. The 1-year safety and efficacy outcomes, previously reported, were reassuringly similar in the three groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
This was the first randomized trial to examine the safety and efficacy of the Synergy stent in all comers. Dr. Kok presented the new 2-year results, featuring 99% patient follow-up. It’s important to look at outcomes well beyond 1 year, she said, because the three stents are so different.
The Synergy and Orsiro stents feature very-thin, flexible struts with an uncoated thickness of 74 mm and 60 mm, respectively, compared with 91 mm for the Resolute Integrity stent. While the Synergy and Orsiro stents both utilize biodegradable polymer frames, the two devices utilize different drugs with different drug-eluting and polymer-resorption times. The polymer coating of the Synergy stent is resorbed within 4 months, while the Orsiro stent’s polymer degrades after 12 months and is resorbed by 24 months. Everolimus is distributed abluminally in the Synergy stent, while the other two stents feature circumferential drug placement. The Synergy frame is platinum and chromium, while other two utilize cobalt-chromium frames.
The thinking has been that treatment of coronary artery disease with drug-eluting stents, such as the Resolute Integrity, might delay arterial healing as a consequence of the life-long presence of the polymer in the vessel. Very-thin-strut biodegradable polymer stents were developed as a means of getting around this problem. But, at 1 year, the primary endpoint of target vessel failure – a safety and efficacy composite comprising cardiac death, target vessel-related MI, or clinically driven target vessel revascularization – was 5% in all three groups.
Dr. Kok reported that, at 2 years, the target vessel failure rate remained nonsignificantly different between the three study arms: 8.3% with the zotarolimus-eluting Resolute Integrity stent, 6.8% with the everolimus-eluting Synergy stent, and 6.6% with the sirolimus-eluting Orsiro stent. Nor were there any significant differences in the individual components of the primary endpoint. However, the secondary endpoint of target lesion failure – comprising cardiac death, any MI, or clinically driven target lesion revascularization – was a different story. At 1 year, there were no significant between-group differences in this composite outcome. But, a landmark analysis showed that between years 1 and 2, the incidence was 2.4% in the Resolute Integrity group, 1.6% with the Synergy group, and 1.1% in patients randomized to the Orsiro stent. The difference in target lesion failure between the Resolute Integrity and Orsiro groups was statistically significant. This difference was driven in part by the rates of target lesion revascularization: 1.5% with the Resolute Integrity, 0.9% with the Synergy, and 0.6% with the Orsiro stent.
The 2-year rates of definite or probable stent thrombosis were low and similar in the three groups: 1.0% with the Synergy stent, 0.8% with the Resolute Integrity, and 0.6% with the Orsiro, she continued.
Longer-term follow-up is planned.
The latest BIO-RESORT results received a skeptical reception from the session’s discussion panel. Nick E. J. West, MD, of Royal Papworth Hospital in Cambridge, England, praised the study as large and well run, yet, “notwithstanding some post hoc statistical nitty-gritty, there are essentially no differences. Have we come as far as we’re going to go with metallic stents in terms of the devices, and is it now a bit more about implantation, about accuracy, about dual-antiplatelet therapy?” he asked.
Mirvat Al Alasnag, MD, an interventional cardiologist at King Fahd Armed Forces Hospital in Jeddah, Saudi Arabia, said, “An all-comers trial design does not necessarily tell you that something is good for everyone. We try and make everything simple, and it is not that simple. I do believe that stent choice decisions should be individualized.”
The BIO-RESORT trial was funded by institutional research grants from Biotronik, Boston Scientific, and Medtronic.
PARIS – Three contemporary yet very different drug-eluting stents – two very-thin-strut biodegradable devices and one that leaves behind a durable polymer – achieved similarly low target vessel failure rates through 2 years of prospective follow-up in the large, all-comers, randomized BIO-RESORT trial, Marlies M. Kok, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
That being said, the study provided an intriguing signal that the Orsiro sirolimus-eluting stent might reduce the risk of revascularization after the first year of follow-up, compared with the Synergy everolimus-eluting stent and Resolute Integrity zotarolimus-eluting stent.
“This is a signal of great interest,” noted Dr. Kok, a cardiologist at Thoraxcentrum Twente in Enschede, the Netherlands.
The BIO-RESORT trial was a multicenter, three-arm, patient- and assessor-blinded Dutch study in which 3,514 patients undergoing percutaneous coronary intervention (PCI) were randomized to the Resolute Integrity, Synergy, or Orsiro stent. This was an all-comers trial in which everyone who needed PCI with a drug-eluting stent was included. Most (70%) patients had an acute coronary syndrome; 31% presented with ST-segment elevation MI. At 1 year of follow-up, 86% of patients were on dual-antiplatelet therapy. The 1-year safety and efficacy outcomes, previously reported, were reassuringly similar in the three groups (Lancet. 2016 Nov 26;388[10060]:2607-17).
This was the first randomized trial to examine the safety and efficacy of the Synergy stent in all comers. Dr. Kok presented the new 2-year results, featuring 99% patient follow-up. It’s important to look at outcomes well beyond 1 year, she said, because the three stents are so different.
The Synergy and Orsiro stents feature very-thin, flexible struts with an uncoated thickness of 74 mm and 60 mm, respectively, compared with 91 mm for the Resolute Integrity stent. While the Synergy and Orsiro stents both utilize biodegradable polymer frames, the two devices utilize different drugs with different drug-eluting and polymer-resorption times. The polymer coating of the Synergy stent is resorbed within 4 months, while the Orsiro stent’s polymer degrades after 12 months and is resorbed by 24 months. Everolimus is distributed abluminally in the Synergy stent, while the other two stents feature circumferential drug placement. The Synergy frame is platinum and chromium, while other two utilize cobalt-chromium frames.
The thinking has been that treatment of coronary artery disease with drug-eluting stents, such as the Resolute Integrity, might delay arterial healing as a consequence of the life-long presence of the polymer in the vessel. Very-thin-strut biodegradable polymer stents were developed as a means of getting around this problem. But, at 1 year, the primary endpoint of target vessel failure – a safety and efficacy composite comprising cardiac death, target vessel-related MI, or clinically driven target vessel revascularization – was 5% in all three groups.
Dr. Kok reported that, at 2 years, the target vessel failure rate remained nonsignificantly different between the three study arms: 8.3% with the zotarolimus-eluting Resolute Integrity stent, 6.8% with the everolimus-eluting Synergy stent, and 6.6% with the sirolimus-eluting Orsiro stent. Nor were there any significant differences in the individual components of the primary endpoint. However, the secondary endpoint of target lesion failure – comprising cardiac death, any MI, or clinically driven target lesion revascularization – was a different story. At 1 year, there were no significant between-group differences in this composite outcome. But, a landmark analysis showed that between years 1 and 2, the incidence was 2.4% in the Resolute Integrity group, 1.6% with the Synergy group, and 1.1% in patients randomized to the Orsiro stent. The difference in target lesion failure between the Resolute Integrity and Orsiro groups was statistically significant. This difference was driven in part by the rates of target lesion revascularization: 1.5% with the Resolute Integrity, 0.9% with the Synergy, and 0.6% with the Orsiro stent.
The 2-year rates of definite or probable stent thrombosis were low and similar in the three groups: 1.0% with the Synergy stent, 0.8% with the Resolute Integrity, and 0.6% with the Orsiro, she continued.
Longer-term follow-up is planned.
The latest BIO-RESORT results received a skeptical reception from the session’s discussion panel. Nick E. J. West, MD, of Royal Papworth Hospital in Cambridge, England, praised the study as large and well run, yet, “notwithstanding some post hoc statistical nitty-gritty, there are essentially no differences. Have we come as far as we’re going to go with metallic stents in terms of the devices, and is it now a bit more about implantation, about accuracy, about dual-antiplatelet therapy?” he asked.
Mirvat Al Alasnag, MD, an interventional cardiologist at King Fahd Armed Forces Hospital in Jeddah, Saudi Arabia, said, “An all-comers trial design does not necessarily tell you that something is good for everyone. We try and make everything simple, and it is not that simple. I do believe that stent choice decisions should be individualized.”
The BIO-RESORT trial was funded by institutional research grants from Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM EUROPCR 2018
Key clinical point: Three contemporary, very dissimilar drug-eluting stents show impressively low 2-year target vessel failure rates.
Major finding:
Study details: This was a multicenter, randomized trial with 99% 2-year follow-up of 3,514 patients, deemed to need PCI, who were randomized to one of three contemporary drug-eluting stents.
Disclosures: The study was funded by institutional research grants from Biotronik, Boston Scientific, and Medtronic.
Hybrid PCI strategy rules for complex CTO
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
PARIS – The so-called hybrid strategy is now the preferred approach to percutaneous coronary intervention (PCI) for chronic total occlusions (CTO), 12-month outcomes of the multicenter observational CONSISTENT CTO trial suggest, according to Simon J. Walsh, MD, an interventional cardiologist at Belfast Health and Social Care Trust.
With use of the hybrid strategy, a CTO’s anatomic complexity is no longer a barrier to performing PCI with a success rate that by former standards would be considered astronomical, he said in presenting the CONSISTENT CTO results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Indeed, the key take-home messages from CONSISTENT CTO were that technical success rates were dramatic at 98.6%, the target vessel failure rate at 12 months was impressively low at 5.24%, and quality of life scores showed clinically meaningful gains maintained through 1 year.
“Opening a CTO makes people better, reduces their symptom burden, and is something worth doing,” Dr. Walsh concluded.
That hasn’t always been the prevailing view. Historically, most interventional cardiologists had a pessimistic attitude regarding PCI for CTOs. The procedures had a low technical success rate, frequent complications, and lousy durability. Moreover, these subpar results came at a considerable price in terms of extensive radiation exposure and catheterization laboratory time. These various shortcomings became particularly prominent when traditional wire-based PCI strategies were applied to long, complex occlusions.
All that has changed as a result of improved stent technologies and procedural techniques, along with the development of the hybrid PCI algorithm by U.S. cardiologists. Using these tools, an interventionalist skilled in the hybrid strategy now selects cases on the basis of clinical indications, such as disabling angina, rather than on anatomic considerations. This point was emphatically brought home in CONSISTENT CTO (Conventional antegrade vs. sub-intimal synergy stenting in chronic total occlusions).
“This is the most complex set of treated CTO lesions ever investigated in this rigorous way,” according to Dr. Walsh.
This assertion was bolstered by the fact that the average Japan CTO (JCTO) score in the 210 study participants was 2.4, climbing to 2.9 in the 101 patients with dissection present. Further adding to the lesion complexity was the fact that roughly one in five subjects had a degenerated coronary artery graft in their target vessel. The average lesion length as measured at the study core lab was 29.1 mm. The impetus for the CONSISTENT CTO Study was a recognition that, even though a growing number of skilled operators are embracing the hybrid PCI strategy with heretofore unprecedented favorable results, there remains an evidence gap, with little in the way of long-term studies featuring rigorous follow-up. Participants in CONSISTENT CTO therefore underwent baseline formal quality of life assessment, repeated at 12 months of follow-up together with angiography and/or optical coherence tomography. Further follow-up is planned at 2, 3, and 5 years.
Of the 231 patients who consented to participate in the study, 210, or 90%, were able to have their CTO opened with a Synergy everolimus-eluting stent. Those 210 constituted the study population.
The hybrid algorithm-based PCI strategy utilizes an individual’s CTO anatomy to dictate the initial choice of approach. The goal is to pick the strategy that is most likely to achieve successful outcome efficiently, with minimal contrast and radiation doses for that particular type of CTO. The hybrid algorithm begins with dual-catheter injection and intravascular ultrasound aimed at determining whether four key anatomic features are present: an ambiguous proximal cap, a poor distal target, good interventional collaterals, and a major side branch at the distal cap.
If those four features are absent and the CTO lesion is less than 20-mm long, the algorithm dictates that the initial approach is antegrade wire escalation; if the lesion is 20 mm or more, the first approach is antegrade dissection reentry. However, if those anatomic features are present, the initial strategy is retrograde wire escalation if the lesion is less than 20 mm, and retrograde dissection reentry for longer lesions. When the initial approach fails, structured protocols dictate the selection of second and third approaches (JACC Cardiovasc Interv. 2012 Apr;5[4]:367-79).
The right coronary artery was the site of the CTO in 70% of study participants. Dual-catheter access was utilized in 79% of patients; only 10% had exclusively radial access. An average of 2.8 Synergy stents were deployed, with a whopping mean total stent length of 96.6 mm in the 48% of patients with dissection and 75.4 mm in those without. Mean procedure time was 122 minutes, with a fluoroscopy time of 44.6 minutes.
The primary efficacy endpoint was the rate of target vessel failure (TVF) at 12 months, defined as cardiac death, MI related to the target vessel, or any ischemia-driven revascularization of the target vessel. The rate was 5.24%.
“We were pleasantly surprised by that,” Dr. Walsh admitted.
Indeed, based upon studies of PCI for CTO published by other investigators, he and his colleagues had initially projected a 13% TVF rate, then bumped it up to 15% because of the high degree of lesion complexity.
Diabetes was the main predictor of target vessel revascularization.
At the time of the index PCI, 19% of patients were scheduled to return for an early optimization procedure within 3 months. This was arranged when operators anticipated significant positive remodeling would occur as the target vessel readjusted to blood flow or distal disease of borderline severity was noted beyond the CTO segment. These were not counted as adverse events. Half of the returnees underwent angiography and no further action was undertaken. The others underwent postdilation of their Synergy stents or new stenting of distal disease.
Complete revascularization of the target territory was achieved in 98.6% of patients. Key complications consisted of 5 perforations, 10 hematomas at vascular access sites, 2 cases of pericardiocentesis, and 4 instances of bleeding requiring transfusion.
The final successful CTO revascularization strategy was antegrade dissection reentry in 18% of patients, retrograde dissection reentry in 30%, antegrade wire escalation in 34%, and retrograde wire escalation in 18%. A switch from the initial algorithm-based strategy to a second strategy occurred in 41% of patients, and a third strategy was employed in 9%.
“I think the key message is you need to have more than one option to treat complicated disease. Half of patients had a switch in strategy,” Dr. Walsh observed.
Intravascular ultrasound (IVUS) adjudication in the central core lab showed a humbling rate of discordance between the operators’ impression of how their procedures were proceeding and what was really going on.
“There’s a bit of a mantra [that says] if you’re wiring stuff you’re always in the lumen, and if you’re using dissection techniques you’re always not in the lumen. In fact, that’s nonsense. You get it wrong one in six times. When you use IVUS adjudication to see if you’re outside the lumen or not, with wire-based retrograde escalation you’re out of the lumen and in the subintimal space 27% of the time. And with dissection strategies you’re wrong in about 15%,” according to the cardiologist.
He described the study participants as “extremely limited” at baseline as evidenced by their scores on the Seattle Angina Questionnaire Physical Limitation, Angina Stability, and Angina Frequency domains. At 12 months of follow-up, patients averaged 20- to 40-point improvements across all three domains.
One member of the discussion panel expressed a wish that the study had included a sham PCI arm. He raised the possibility that PCI had exerted an enormous placebo effect that could conceivably account for the substantial quality of life benefits documented in the study. But another panelist scoffed at this notion. This wasn’t a modest improvement in quality of life, nor was it measured after a mere 6 weeks, as was the case in the sham-controlled ORBITA trial.
“It’s really difficult to imagine a sham effect that persists out to a year,” he argued.
Dr. Walsh reported receiving research grants from and serving as a consultant to Boston Scientific, which funded the CONSISTENT CTO Study.
REPORTING FROM EUROPCR 2018
Key clinical point: The hybrid PCI strategy is now the preferred approach to treatment of CTOs.
Major finding: The 1-year target vessel failure rate following PCI for complex CTOs was 5.24%, with durable major quality of life improvements.
Study details: This prospective multicenter study included 210 patients with highly complex CTOs treated using Synergy stents according to the hybrid algorithm.
Disclosures: The presenter reported receiving research grants from and serving as a consultant to Boston Scientific, which sponsored the CONSISTENT CTO Study.
Sapien 3 performs well (mostly) in bicuspid aortic stenosis
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.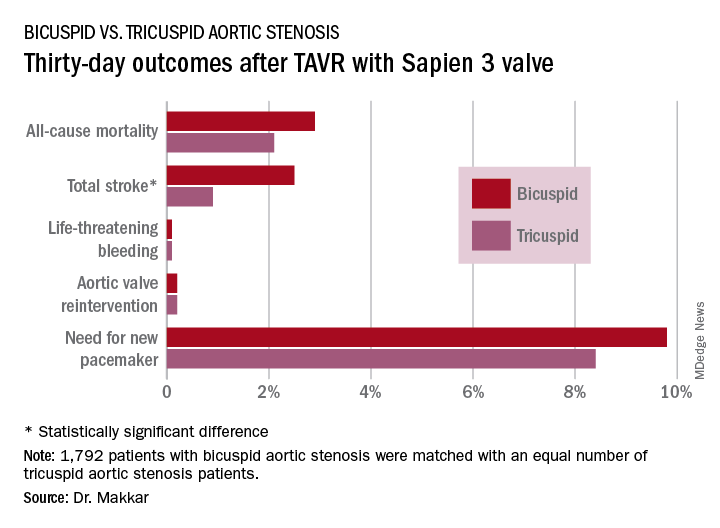
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
PARIS – Use of the Sapien 3 transcatheter heart valve led to similarly favorable short-term and 1-year outcomes in a propensity-matched comparison of patients with bicuspid versus tricuspid aortic stenosis, with
But the higher stroke rate isn’t necessarily a deal breaker for efforts to develop transcatheter aortic valve replacement (TAVR) as an option for patients with bicuspid aortic stenosis, according to Rajendra Makkar, MD, who presented the study results at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
Dr. Makkar noted that “75% of the strokes in the bicuspid aortic stenosis group occurred in the periprocedural time period, and these are all heavily calcified valves.” “So I would make the argument that, in young bicuspid patients where you decide to treat using TAVR, the safety gain from using an embolic protection device may be even more [than in most tricuspid patients]. I say that should be the way to do it. I think carefully selected patients with bicuspid aortic stenosis can be managed with TAVR with an embolic protection device very safely.”
He presented the results of this comparison of TAVR outcomes using the Sapien 3 valve in patients with native bicuspid versus tricuspid valves; all patients had enrolled in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry between June 2015 and February 2018. The initial analysis included 1,792 Sapien 3 recipients with severely symptomatic bicuspid aortic stenosis and 55,023 with severely symptomatic tricuspid aortic stenosis.
As TAVR increasingly becomes an option for younger and healthier patients with symptomatic aortic stenosis, operators will encounter more patients with congenital bicuspid valves. Outcomes using early-generation TAVR valves in such patients were poor, so pivotal randomized trials of the Sapien 3 and other contemporary TAVR valves – including the ongoing trials of TAVR versus surgery in patients with low surgical risk – have excluded those with bicuspid aortic stenosis.
As a result, there has been little clinical data to guide interventionalists, so there was an impetus for a study like this one, explained Dr. Makkar, director of interventional cardiology and the cardiac catheterization laboratory at Cedars-Sinai Medical Center in Los Angeles.
In the registry analysis, the unadjusted 1-year all-cause mortality rate was 10.4% in the bicuspid patients and 15% in the tricuspid patients, for a significant 22% relative risk reduction. The 1-year total stroke rates were nearly identical at 3.4% in the bicuspid patients and 3.3% in the tricuspid patients. However, the two groups differed in many key ways. The bicuspid patients were on average 8 years younger, and their mean Society of Thoracic Surgeons risk score was 5.1 versus 6.7 in the tricuspid patients. The bicuspid patients also had less atrial fibrillation, peripheral artery disease, and prior revascularization.
Because of these differences, Dr. Makkar and his coinvestigators carefully propensity-matched the 1,792 bicuspid aortic stenosis who received the Sapien 3 valve at 386 U.S. sites with an equal number of tricuspid aortic stenosis patients treated at 424 sites. This yielded two populations that were virtually identical in terms of age, Society of Thoracic Surgeons score, and 22 other baseline characteristics. Of the patients in both groups, 93%had transfemoral access, 38% had conscious sedation, and the device success rate was in 97%.
Thirty-day outcomes in the two groups didn’t differ significantly except for the total stroke rate: 2.5% in the bicuspid group versus 0.9% in the tricuspid group (see graphic). The 1-year mortality rates didn’t differ significantly: 10.4% in the bicuspid group and 10.8% in the patients with tricuspid disease. However, the 1-year total stroke rate remained significantly higher in the bicuspid group by a margin of 3.4%-2.7%.
The reduction in aortic valve mean gradient and increase in aortic valve area were similar in both groups through 1 year of follow-up, as was the increase in left ventricular ejection fraction. Rates of significant paravalvular leak were similarly low in both groups.
Quality of life as measured by the Kansas City Cardiomyopathy Questionnaire showed what Dr. Makkar called “remarkable” improvement in both groups: There was an average 30-point improvement from baseline at 30 days after TAVR that was sustained through 1 year, at which point the average gain over baseline was 32 points.
Dr. Makkar drew attention to the impressively low rates of major procedural complications in both groups: Conversion to open-heart surgery took place in 0.9% of the bicuspid and 0.4% of the tricuspid group; annulus rupture occurred in 0.3% of bicuspid TAVR patients and none of the tricuspid group; the aortic dissection rates were 0.3% and 0.1%, respectively; coronary obstruction occurred in 0.4% and 0.1%; and a second valve was needed in 0.6% of the bicuspid group and 0.1% of the tricuspid group. The fact that each of those adverse events happened in fewer than 1% of the bicuspid recipients of the Sapien 3 valve stands in striking contrast to the far higher rates when earlier-generation devices were used in TAVR for bicuspid aortic valves.
“I think our data suggest that in patients with bicuspid aortic stenosis who are at high or intermediate surgical risk, it is really reasonable to actually use TAVR as one of the treatment modalities. And I would make the argument that based on these data it is very reasonable to enroll carefully selected low–surgical risk bicuspid patients in ongoing TAVR versus surgery clinical trials,” the cardiologist said.
Session cochair Alain Cribier, MD, was put off by the higher total stroke rate in the bicuspid group.
“I think, really, that in young patients with a true congenital calcific bicuspid aortic valve, these patients should remain in the hands of the surgeons. In the future, this will be one of the remaining indications for surgery if TAVR works in low-risk patients,” predicted Dr. Cribier, professor of medicine at the University of Rouen (France) and a TAVR pioneer.
Dr. Makkar reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
REPORTING FROM EUROPCR 2018
Key clinical point: Overall, outcomes were similarly favorable between patients with bicuspid aortic stenosis and those with tricuspid aortic stenosis.
Major finding: The 1-year all-cause mortality and total stroke rates in 1,792 TAVR patients who got the Sapien 3 valve for bicuspid aortic stenosis were 10.4% and 3.4%.
Study details: This was a propensity-matched comparison of TAVR outcomes using the Sapien 3 valve in 1,792 patients with bicuspid aortic stenosis and in an equal number with tricuspid aortic stenosis in the STS/ACC TVT Registry.
Disclosures: The study presenter reported receiving research grants from and serving as a consultant to Edwards Lifesciences, which sponsored the study, as well as from Abbott Laboratories, Pfizer, Medtronic, and Claret Medical.
Synergy DES shines in acute MI
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
PARIS – The Synergy bioabsorbable polymer everolimus-eluting stent performed equally well for treatment of acute MI, compared with other newer-generation drug-eluting stents, through 2 years of follow-up in a massive observational study of all patients undergoing percutaneous coronary intervention in Sweden during a recent multiyear period.
This report from the prospective Swedish Coronary Angiography and Angioplasty Registry (SCAAR) was undertaken because, even though the Synergy stent has demonstrated outstanding clinical results in randomized trials and observational studies, the stent’s performance specifically in the setting of acute MI had not previously been investigated, Sergio Buccheri, MD, noted at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
SCAAR, which documents every PCI performed in Sweden, provided the capability to fill that important knowledge gap in an unselected real-world population of acute MI patients. Dr. Buccheri, of Uppsala (Sweden) University, reported on 36,292 consecutive patients who underwent PCI with a newer-generation drug-eluting stent (DES) in Sweden from March 2013 to September 2016. Forty percent of them had ST-elevation MI. The Synergy stent was used in 4,889 patients. Among the most commonly used newer DES in the other 31,000-plus patients were the Xience Xpedition, the Resolute Integrity and Resolute Onyx, the Orsiro, BioMatrix, and Promus Element Plus and Promus Premier.
The coprimary endpoints in this analysis were the rates of definite stent thrombosis and clinically relevant restenosis at 2 years of follow-up. Stent thrombosis occurred in 0.69% of the Synergy patients and 0.81% of those who received other newer-generation DES, a nonsignificant difference. Similarly, no significant difference was found in the rate of clinically relevant restenosis: 1.48% and 1.25%, respectively.
“,” Dr. Buccheri noted. “These findings may be useful to support a more informed and evidence-based stent selection process in daily clinical practice.”
The key secondary outcomes were all-cause mortality and recurrent MI. Again, there were no significant between-group differences. The cumulative all-cause mortality at 2 years was 10.1% in the Synergy group and 9.1% in the others. Recurrent MI occurred in 6.49% of the Synergy group and 6.32% with other DES.
Patients who received the Synergy stent were on average older, had a higher burden of cardiovascular risk factors, and presented more often with left main, triple-vessel disease or vein graft lesions. For that reason, Dr. Buccheri and his coinvestigators developed a propensity score using an array of covariates to adjust for these differences. Plugging those scores into multivariate Cox regression models, there remained no significant differences between the two groups in the adjusted risk of any of the endpoints.
Operators were advised to use dual antiplatelet therapy for 12 months in all patients. However, SCAAR does not include data on adherence to DAPT, which is a study limitation, Dr. Buccheri noted.
The Synergy stent is made up of a thin strut chromium-platinum platform with a bioabsorbable polymer that releases everolimus. The polymer is completely reabsorbed within 4 months, leaving behind a bare metal stent. In animal models, this has been associated with lower levels of inflammation, compared with permanent polymer DES. And inflammation is thought to be one of the main mechanisms underlying stent failure in the late and very late phases after PCI.
The discussion panel was clearly impressed with – and envious of – the sheer size of the SCAAR study population. As one panelist noted, real-life data of this magnitude can really only be obtained in Sweden. Another panelist confessed: “We’re shy of presenting our own studies when we see these numbers.”
Simultaneously with Dr. Buccheri’s presentation, the SCAAR report was published online (EuroIntervention. 2018 May 24. pii: EIJ-D-18-00392. doi: 10.4244/EIJ-D-18-00392).
SCAAR is funded solely by the Swedish government. This study was supported by a grant from Boston Scientific. Dr. Buccheri reported having no financial conflicts of interest.
REPORTING FROM EUROPCR 2018
Key clinical point: Two years post PCI for acute MI, stent thrombosis and restenosis rates in Synergy stent recipients were as low as with other newer-generation drug eluting stents.
Major finding: The 2-year rate of definite stent thrombosis was 0.69% in the Synergy stent group and 0.81% in recipients of other contemporary drug-eluting stents.
Study details: This was an observational study of 36,292 consecutive Swedish patients with acute MI who received the Synergy stent or other newer-generation drug-eluting stents.
Disclosures: The study was funded by a grant from Boston Scientific. The presenter reported having no financial conflicts of interest.
Culotte stenting impresses in CELTIC Bifurcation Study
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
PARIS – Technical success rates were high and major adverse events impressively low with a two-stent culotte strategy using contemporary drug-eluting stents for coronary bifurcation lesions in the randomized CELTIC Bifurcation Study.
“We initiated this study because of a conviction that the story isn’t finished with bifurcation stenting. We’re very much under the impression that the accepted wisdom of a conservative approach is, we think, not correct, and the issue needs to be kept open,” said Dr. Foley, an interventional cardiologist at Beaumont Hospital in Dublin.
The widely accepted provisional single-stent strategy is based on early-days randomized trial evidence using first-generation drug-eluting stents and older techniques that are no longer relevant in contemporary practice. Moreover, this conservative single-stent approach doesn’t address the important issue of ischemia arising from large side branches, he asserted.
“I’ve always been fond of culotte stenting myself because I think it’s a very elegant, simple, repeatable strategy, and with modern stents it becomes easier for modest-volume operators to carry it out well. We’ve kept on trying to convert new colleagues and older colleagues who are set in their ways,” Dr. Foley said.
The CELTIC Bifurcation Study was an investigator-initiated trial in which 177 patients at nine centers in Ireland and the United Kingdom were randomized to culotte stenting using either two-connector, third-generation Synergy everolimus-eluting stents or the three-connector, second-generation Xience everolimus-eluting stents. All participants had Medina 1,1,1 coronary bifurcation lesions, which were left anterior descending/diagonal lesions in more than 80% of cases. A radial approach was used in more than 95% of the procedures. The indication for percutaneous coronary intervention was stable angina in more than 60% of cases. The rate of technical procedural success with final kissing balloon inflation exceeded 96%. The primary outcome – a MACCE (major adverse cardiovascular and cerebrovascular events) composite of death, MI, cerebrovascular accident, and target vessel revascularization over the course of 9 months – occurred in 5.9% of patients: 8.6% of the Synergy group and 3.7% with Xience stents, a nonsignificant difference. This MACCE rate was considerably lower than the 10% figure that the investigators had expected on the basis of published studies of PCI in these complex bifurcation lesions.
“The results were better than expected,” the cardiologist said. “We don’t get excited that easily, to be honest, but nonetheless we’re a little bit excited that the overall MACCE rate in this complex lesion presentation was 5.9%.”
Discussant Volker Schächinger, MD, director of cardiology at Fulda (Germany) Hospital, observed: “It’s always good to reassess what are believed to be answered questions when there are new devices available.” But why not compare culotte stenting to the provisional single-stent strategy? he asked.
“We think provisional versus culotte stenting has been thrashed to death already. And you’d need a bigger trial than we had funding for,” Dr. Foley replied.
“Many of us use the DK [double kissing] crush technique,” another panelist said. “It’s very popular. But if you look at bench testing, perhaps culotte is a better approach by many parameters. So I think it was important for you to highlight the value of culotte and how it can be done properly.”
Discussant James Nolan, MD, a cardiologist at the University Hospital of North Staffordshire (England), said, “The most critical thing with these bifurcation procedures is the operators and how they do it. So you have to do the culotte to the standard done in this trial. If you do a sloppy culotte, it’s not going to be great. It’s probably more important to deliver an excellently performed procedure, whatever it is. You’ll get a better result if you’re good at what you’re doing rather than selecting one procedure or another.”
Dr. Foley agreed, adding: “In some of the DK crush versus culotte randomized trials, I’m not convinced that culotte was done the way I would suggest it should be done.”
Operators in the CELTIC Bifurcation Study were asked to follow a standardized culotte procedure: predilate both limbs of the bifurcation, keep both wires in place, deploy the first stent in the side branch unless the main branch was awkwardly angulated, then cross by going from distally into the optimized first stent, and placing the second stent proximal to the first stent so that the two stents overlap in the proximal main vessel.
“We call that ‘nailing it down,’ ” he explained.
The procedure is completed by sequential high-pressure kissing balloon dilatation of both branches, with intravascular ultrasound or optical coherence tomography recommended but not required.
Simultaneously with this presentation, the study results were published online (EuroIntervention 2018 Jun 8;14[3]:e318-24).
The CELTIC Bifurcation Study was funded by an unrestricted grant from Boston Scientific. Dr. Foley reported having no financial conflicts of interest regarding the study.
REPORTING FROM EUROPCR 2018
Key clinical point: .
Major finding: The 9-month MACCE rate following culotte stenting for bifurcation lesions was 5.9%, with no significant difference between patients randomized to the Xience or Synergy stents.
Study details: This multicenter randomized trial comprised 177 patients with coronary bifurcation lesions who underwent culotte stenting with either Xience or Synergy everolimus-eluting stents.
Disclosures: The presenter reported having no financial conflicts regarding the study, funded by an unrestricted grant from Boston Scientific.
Firehawk: A new ‘workhorse’ DES
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
PARIS – The Xience everolimus-eluting coronary stent is widely considered the current standard treatment, implanted by interventional cardiologists far more often than any other drug-eluting stent (DES). But judging from the results of the TARGET All Comers trial, some serious competition may be headed Xience’s way in the form of the new Firehawk rapamycin-eluting, thin-strut stent featuring a biodegradable polymer drug delivery system.
What’s more, the Firehawk offers a theoretical advantage in that the rapamycin is delivered via a polymer that’s fully absorbed by 9 months, leaving behind a bare metal stent made of cobalt chromium. This structure is believed to be less proinflammatory, atherogenic, and thrombogenic over the long haul, compared with a permanent durable polymer, such as that employed in the Xience stent. This should translate into less late restenosis and in-stent thrombosis.
Also, the Firehawk features thin, 86-mcm struts and the rapamycin, also known as sirolimus, is contained in abluminal grooves directed specifically to the vessel wall. As a result, this DES exposes patients to only one-third as much active drug as other DESs. Ninety percent of the rapamycin is released within 90 days after implantation, according to Dr. Baumbach, director of interventional research at Barts Heart Centre in London and president of the European Association of Percutaneous Cardiovascular Interventions.
The TARGET All Comers trial is a prospective, open-label, noninferiority trial comparing the safety and efficacy of the Firehawk with those of Xience stents in 1,656 DES-eligible patients with symptomatic coronary artery disease randomized at 21 centers.
The primary endpoint was the 12-month composite of target lesion failure, comprising rates of cardiac death, target vessel MI, or ischemia-driven target lesion revascularization. In an intention-to-treat analysis, the rate was 6.1% in the Firehawk patients and 5.9% in the Xience recipients. Results for each of the three components of the composite endpoint were similar in the two groups as well.
A secondary endpoint was in-stent late loss as measured by quantitative coronary angiography at 13 months in a 137-patient subgroup. The rate was 0.17 mm in the Firehawk recipients and similar at 0.11 mm in those receiving the Xience stent, again, which provided solid evidence of noninferiority.
The rate of definite stent thrombosis at 1 year was 1.2% in both study arms.
Discussant Giulio Guagliumi, MD, an interventional cardiologist at Pope Giovanni XXIII Hospital in Bergamo, Italy, pronounced the results “quite reassuring.” But where, he asked, is the evidence of late benefit for the completely biodegradable polymer utilized in the Firehawk?
“We would expect to see such an effect later on, after the stent in question becomes a simple bare metal stent as opposed to a stent with a durable polymer. But we don’t have the ultimate answer yet. In this trial we will have an extended follow-up out to 5 years to see whether there is any translation of these differences into clinical benefit,” Dr. Baumbach replied.
Discussion panelist Julinda Mehilli, MD, inquired how this new stent, which has been approved for the European market, will fit into everyday clinical practice.
“We have many biodegradable polymer DES already. We have the Ultimaster, we have Synergy – and now, the Firehawk. What kind of special features does it have? Is it for use in routine practice or in special populations?” asked Dr. Mehilli, director of interventional cardiology at the German Heart Center at the University of Munich.
“That’s of course the question: What’s the unique point of this stent? I think that the unique point is that there is really no unique point. This is a classic workhorse stent. This is a stent with good radial force and all the other features for everyday use,” according to Dr. Baumbach.
Indeed, he and his Barts colleagues did more than 100 cases in TARGET All Comers and found one of the Firehawk’s strengths was its versatility. It performed well in challenging cases, including left main interventions, as well as in more straightforward cases in this all comers trial.
The Firehawk was developed by MicroPort in China, where its safety and efficacy was established in clinical trials totaling more than 1,000 patients. It then moved to Europe, where it has earned regulatory approval. A pivotal U.S. trial is being planned with the Food and Drug Administration, which has indicated that the European TARGET All Comers data can be incorporated in the study.
Dr. Baumbach reported receiving research grants from Abbott and consultation fees from Keystone Heart, MicroPort, Sinomed, and Stentys.
REPORTING FROM EUROPCR 2018
Key clinical point: The Firehawk has established itself as a versatile workhorse coronary stent.
Major finding:
Study details: This open-label international study randomized 1,656 patients with symptomatic CAD to one of two drug-eluting stents.
Disclosures: The TARGET All Comers trial was sponsored by MicroPort. The presenter reported serving as a consultant to MicroPort and other companies.
TAVR-related stroke risk unrelated to anatomy
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
PARIS – The most appropriate stroke prevention strategy in patients undergoing transcatheter aortic valve replacement is routine use of a cerebroembolic protection device for all, because no identifiable high-risk anatomic subsets exist, Hasan Jilaihawi, MD, said at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We looked at the anatomy in great detail. I’d hoped to find a strata that was truly high risk, but there is no clear strata that is truly higher risk. So stroke remains an unpredictable event in TAVR, and in the ideal world we would use cerebroembolic protection in everyone,” said Dr. Jilaihawi, codirector of transcatheter valve therapy at New York University.
“I put it to you that, as in carotid stenting, where we routinely use cerebroembolic protection, perhaps we need to consider the same in TAVR,” the cardiologist added.
The SENTINEL trial randomized 363 patients undergoing TAVR 2:1 to the use of the Sentinel intraluminal filter device or no neuroprotection during the procedure. The use of the cerebroembolic protection device was associated with a statistically significant 63% reduction in the incidence of neurologist-adjudicated stroke within 72 hours, from 8.2% to 3.0% (J Am Coll Cardiol. 2017 Jan 31;69[4]:367-77). The device was cleared for marketing by the Food and Drug Administration in 2017 and approved by European authorities several years earlier.
A wealth of evidence shows that the average stroke rate associated with contemporary TAVR is 4.4%, although this figure is probably on the low side because most of the data come from nonrandomized registries, which typically underreport neurologic outcomes. The stroke rate is independent of operator experience and volume, surgical risk score, and institutional TAVR volume. Moreover, in the SENTINEL trial, embolic debris was captured in 99% of patients fitted with the cerebroembolic protection device.
“A huge variety of material was captured, including thrombus, valve tissue, calcified material, and – alarmingly – foreign material in 35% of cases,” Dr. Jilaihawi noted.
Nonetheless, the issue of routine versus selective use of cardioembolic protection remains controversial at a time when interventionalists are trying to make TAVR a simpler, briefer procedure, even though the approved Sentinel device is successfully deployed in a median of only 4 minutes. This was the impetus for Dr. Jilaihawi to examine baseline anatomy as a potential predictor of stroke.
He looked at four key anatomic features: aortic arch type, aortic root angulation, aortic arch calcium, and aortic valve calcification. The bottom line: The benefit of cerebroembolic protection with the Sentinel device was consistent across all anatomic subgroups. For example, in patients with an aortic root angulation angle of less than 50 degrees, the incidence of stroke within 3 days post TAVR was 3.2% with and 5.9% without cerebroembolic protection, while in those with an angle of 50 degrees or more the stroke rate was 2.6% with and 9.1% without the Sentinel device. With a total of only 16 strokes by day 3 in the study, those stroke rates in the absence of cerebroembolic protection aren’t significantly different.
There was, however, one unexpected and counterintuitive finding: The greatest stroke reduction with cerebroembolic protection was seen in patients with the least aortic valve calcium. This prompted session cochair Alain Cribier, MD, professor of medicine at the University of Rouen, France, to observe that perhaps valve repositioning is an important factor in TAVR-related strokes. After all, he noted, valve repositioning occurs more often when a patient’s valves are softer and less calcified.
“This is a very important point,” Dr. Jilaihawi responded. “I think there is an interplay between procedural aspects and the anatomy which is not completely captured in this study because we don’t know whose valve was repositioned multiple times.”
He added that the finding that TAVR-related stroke is more common in patients with less calcified aortic valves is consistent with the earlier experience in carotid stenting.
“If you look 10 years ago in the field of carotid stenting, there were a lot of analyses done which concluded that the highest-risk lesions are the least calcified lesions, even though it’s counterintuitive,” he said.
Discussant Saibal Kar, MD, director of interventional cardiac research at Cedars-Sinai Medical Center in Los Angeles, said the take-home point from the SENTINEL analysis is clear: “Cerebroembolic protection is like a seat belt: You should wear it. All patients should wear it.”
The SENTINEL trial was sponsored by Claret Medical. Dr. Jilaihawi reported receiving research grants from Abbott and Medtronic and serving as a consultant to Edwards Lifesciences and Venus Medtech.
SOURCE: Jilaihawi H. EuroPCR 2018.
REPORTING FROM EUROPCR 2018
Key clinical point: .
Major finding: Neither aortic arch type, root angulation, nor calcium burden identifies a subgroup of TAVR patients at higher stroke risk.
Study details: The SENTINEL trial randomized 363 TAVR patients 2:1 to the use of a cerebroembolic protection device or no neuroprotection during the procedure.
Disclosures: The SENTINEL trial was sponsored by Claret Medical. The presenter reported having no financial conflicts of interest.
Source: Jilaihawi H. EuroPCR 2018.
Amplatzer Amulet slashes stroke risk in A-fib
PARIS – The Amplatzer Amulet left atrial appendage occlusion device reduced stroke risk by nearly 60% at 1 year in a large, real-world registry of patients with atrial fibrillation at dual high risk for stroke and bleeding, Ulf Landmesser, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
One of the most impressive findings was that this feat was accomplished by and large without background oral anticoagulation. Indeed, 83% of the 1,088 patients in this 61-center, 17-country study had contraindications to oral anticoagulation. Only 11% of subjects were discharged on oral anticoagulation after device implantation, while 22.5% were discharged on aspirin or clopidogrel monotherapy. By 1-3 months post implantation, 60% of patients were either on a single antiplatelet agent or no antithrombotic medication at all.
“Antithrombotic therapy was individualized by the patient’s physician. There didn’t seem to be an increased risk of device-related thrombus in these patients on single antiplatelet therapy. Our data suggest that, given the high bleeding risk, single antiplatelet therapy seems to be a good option for these patients,” said Dr. Landmesser, a professor in and the chair of the department of cardiology at Charité Medical School in Berlin.
Participants in the Global Prospective Amulet Study averaged 75 years of age, and 72% had a history of major bleeding. The average CHA2DS2-VASc score was 4.2, with a HAS-BLED score of 3.3, which emphasizes the high-risk nature of study participants.
On the basis of the CHA2DS2-VASc score, the predicted 1-year ischemic stroke rate without oral anticoagulation was 6.7%, so the actual 2.9% rate represented a 57% reduction in risk. Similarly, for the composite endpoint of ischemic stroke, transient ischemic attack, or systemic embolism, the predicted rate was 9.4%, but the achieved rate was 3.8%, which represented a 60% reduction in risk.
The annualized major bleeding rate was 10.3% despite the low usage of oral anticoagulation or dual-antiplatelet therapy. However, the rate of procedure- or device-related major bleeding was only 3.2%; the other 7.1% was unrelated to Amulet and reflected the underlying high risk of the study population.
The 1-year mortality rate was 8.4%. Thirty-five deaths had cardiovascular causes, 35 were noncardiovascular, and in 18 patients, cause of death couldn’t be determined.
The device-related thrombus rate through 1 year was 1.7%; 10 of 18 cases occurred within the first 90 days.
Dr. Landmesser emphasized that this was a particularly rigorously conducted registry. A unique feature was its use of an independent echocardiography core lab to assess procedural success, as well as an independent clinical events committee to adjudicate serious adverse events. Prior studies of other left atrial appendage (LAA) occlusion devices didn’t use these measures.
The Amplatzer Amulet is a second-generation occlusion device designed for easier placement and more complete sealing than its predecessor and comes in eight sizes to address anatomic variations. At implantation, adequate LAA occlusion as defined by the echocardiography core laboratory was achieved in 99.3% of patients; at that time, 89.4% of patients had no residual flow, and another 9.9% had a residual flow of less than 3 mm. At 1-3 months of follow-up, echocardiography showed 98.4% of patients had adequate occlusion.
Session cochair Alberto Cremonesi, MD, pronounced this to be “really important data.”
“I want to stress that these device implantations were transesophageal echocardiography–guided. In my mind this is absolutely essential to your excellent long-term results,” observed Dr. Cremonesi of Maria Cecilia Hospital in Cotignola, Italy.
Asked to speculate on what outcomes might have looked like had patients been treated with an oral anticoagulant rather than the Amulet occlusion device, Dr. Landmesser predicted the major bleeding rate would have been substantially higher than 10.3%. Most of the bleeding events in the study were gastrointestinal, and the novel oral anticoagulants are known to boost the risk of GI bleeding.
But that’s speculation. He noted that two ongoing randomized trials – one in Germany, the other in Scandinavia – are randomizing high-risk patients to a LAA occlusion device or best medical care, including a novel oral anticoagulant when not contraindicated. The Scandinavian study uses the Amulet, while the German trial uses both the Amulet and the Watchman device. The primary endpoint is the ischemic stroke rate.
The Amulet registry, which will continue for a second year of follow-up, was sponsored by Abbott Laboratories, which developed the Amulet device. Dr. Landmesser reported serving as a consultant to Abbott, as well as Biotronik, Rewa, and Bayer.
PARIS – The Amplatzer Amulet left atrial appendage occlusion device reduced stroke risk by nearly 60% at 1 year in a large, real-world registry of patients with atrial fibrillation at dual high risk for stroke and bleeding, Ulf Landmesser, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
One of the most impressive findings was that this feat was accomplished by and large without background oral anticoagulation. Indeed, 83% of the 1,088 patients in this 61-center, 17-country study had contraindications to oral anticoagulation. Only 11% of subjects were discharged on oral anticoagulation after device implantation, while 22.5% were discharged on aspirin or clopidogrel monotherapy. By 1-3 months post implantation, 60% of patients were either on a single antiplatelet agent or no antithrombotic medication at all.
“Antithrombotic therapy was individualized by the patient’s physician. There didn’t seem to be an increased risk of device-related thrombus in these patients on single antiplatelet therapy. Our data suggest that, given the high bleeding risk, single antiplatelet therapy seems to be a good option for these patients,” said Dr. Landmesser, a professor in and the chair of the department of cardiology at Charité Medical School in Berlin.
Participants in the Global Prospective Amulet Study averaged 75 years of age, and 72% had a history of major bleeding. The average CHA2DS2-VASc score was 4.2, with a HAS-BLED score of 3.3, which emphasizes the high-risk nature of study participants.
On the basis of the CHA2DS2-VASc score, the predicted 1-year ischemic stroke rate without oral anticoagulation was 6.7%, so the actual 2.9% rate represented a 57% reduction in risk. Similarly, for the composite endpoint of ischemic stroke, transient ischemic attack, or systemic embolism, the predicted rate was 9.4%, but the achieved rate was 3.8%, which represented a 60% reduction in risk.
The annualized major bleeding rate was 10.3% despite the low usage of oral anticoagulation or dual-antiplatelet therapy. However, the rate of procedure- or device-related major bleeding was only 3.2%; the other 7.1% was unrelated to Amulet and reflected the underlying high risk of the study population.
The 1-year mortality rate was 8.4%. Thirty-five deaths had cardiovascular causes, 35 were noncardiovascular, and in 18 patients, cause of death couldn’t be determined.
The device-related thrombus rate through 1 year was 1.7%; 10 of 18 cases occurred within the first 90 days.
Dr. Landmesser emphasized that this was a particularly rigorously conducted registry. A unique feature was its use of an independent echocardiography core lab to assess procedural success, as well as an independent clinical events committee to adjudicate serious adverse events. Prior studies of other left atrial appendage (LAA) occlusion devices didn’t use these measures.
The Amplatzer Amulet is a second-generation occlusion device designed for easier placement and more complete sealing than its predecessor and comes in eight sizes to address anatomic variations. At implantation, adequate LAA occlusion as defined by the echocardiography core laboratory was achieved in 99.3% of patients; at that time, 89.4% of patients had no residual flow, and another 9.9% had a residual flow of less than 3 mm. At 1-3 months of follow-up, echocardiography showed 98.4% of patients had adequate occlusion.
Session cochair Alberto Cremonesi, MD, pronounced this to be “really important data.”
“I want to stress that these device implantations were transesophageal echocardiography–guided. In my mind this is absolutely essential to your excellent long-term results,” observed Dr. Cremonesi of Maria Cecilia Hospital in Cotignola, Italy.
Asked to speculate on what outcomes might have looked like had patients been treated with an oral anticoagulant rather than the Amulet occlusion device, Dr. Landmesser predicted the major bleeding rate would have been substantially higher than 10.3%. Most of the bleeding events in the study were gastrointestinal, and the novel oral anticoagulants are known to boost the risk of GI bleeding.
But that’s speculation. He noted that two ongoing randomized trials – one in Germany, the other in Scandinavia – are randomizing high-risk patients to a LAA occlusion device or best medical care, including a novel oral anticoagulant when not contraindicated. The Scandinavian study uses the Amulet, while the German trial uses both the Amulet and the Watchman device. The primary endpoint is the ischemic stroke rate.
The Amulet registry, which will continue for a second year of follow-up, was sponsored by Abbott Laboratories, which developed the Amulet device. Dr. Landmesser reported serving as a consultant to Abbott, as well as Biotronik, Rewa, and Bayer.
PARIS – The Amplatzer Amulet left atrial appendage occlusion device reduced stroke risk by nearly 60% at 1 year in a large, real-world registry of patients with atrial fibrillation at dual high risk for stroke and bleeding, Ulf Landmesser, MD, reported at the annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
One of the most impressive findings was that this feat was accomplished by and large without background oral anticoagulation. Indeed, 83% of the 1,088 patients in this 61-center, 17-country study had contraindications to oral anticoagulation. Only 11% of subjects were discharged on oral anticoagulation after device implantation, while 22.5% were discharged on aspirin or clopidogrel monotherapy. By 1-3 months post implantation, 60% of patients were either on a single antiplatelet agent or no antithrombotic medication at all.
“Antithrombotic therapy was individualized by the patient’s physician. There didn’t seem to be an increased risk of device-related thrombus in these patients on single antiplatelet therapy. Our data suggest that, given the high bleeding risk, single antiplatelet therapy seems to be a good option for these patients,” said Dr. Landmesser, a professor in and the chair of the department of cardiology at Charité Medical School in Berlin.
Participants in the Global Prospective Amulet Study averaged 75 years of age, and 72% had a history of major bleeding. The average CHA2DS2-VASc score was 4.2, with a HAS-BLED score of 3.3, which emphasizes the high-risk nature of study participants.
On the basis of the CHA2DS2-VASc score, the predicted 1-year ischemic stroke rate without oral anticoagulation was 6.7%, so the actual 2.9% rate represented a 57% reduction in risk. Similarly, for the composite endpoint of ischemic stroke, transient ischemic attack, or systemic embolism, the predicted rate was 9.4%, but the achieved rate was 3.8%, which represented a 60% reduction in risk.
The annualized major bleeding rate was 10.3% despite the low usage of oral anticoagulation or dual-antiplatelet therapy. However, the rate of procedure- or device-related major bleeding was only 3.2%; the other 7.1% was unrelated to Amulet and reflected the underlying high risk of the study population.
The 1-year mortality rate was 8.4%. Thirty-five deaths had cardiovascular causes, 35 were noncardiovascular, and in 18 patients, cause of death couldn’t be determined.
The device-related thrombus rate through 1 year was 1.7%; 10 of 18 cases occurred within the first 90 days.
Dr. Landmesser emphasized that this was a particularly rigorously conducted registry. A unique feature was its use of an independent echocardiography core lab to assess procedural success, as well as an independent clinical events committee to adjudicate serious adverse events. Prior studies of other left atrial appendage (LAA) occlusion devices didn’t use these measures.
The Amplatzer Amulet is a second-generation occlusion device designed for easier placement and more complete sealing than its predecessor and comes in eight sizes to address anatomic variations. At implantation, adequate LAA occlusion as defined by the echocardiography core laboratory was achieved in 99.3% of patients; at that time, 89.4% of patients had no residual flow, and another 9.9% had a residual flow of less than 3 mm. At 1-3 months of follow-up, echocardiography showed 98.4% of patients had adequate occlusion.
Session cochair Alberto Cremonesi, MD, pronounced this to be “really important data.”
“I want to stress that these device implantations were transesophageal echocardiography–guided. In my mind this is absolutely essential to your excellent long-term results,” observed Dr. Cremonesi of Maria Cecilia Hospital in Cotignola, Italy.
Asked to speculate on what outcomes might have looked like had patients been treated with an oral anticoagulant rather than the Amulet occlusion device, Dr. Landmesser predicted the major bleeding rate would have been substantially higher than 10.3%. Most of the bleeding events in the study were gastrointestinal, and the novel oral anticoagulants are known to boost the risk of GI bleeding.
But that’s speculation. He noted that two ongoing randomized trials – one in Germany, the other in Scandinavia – are randomizing high-risk patients to a LAA occlusion device or best medical care, including a novel oral anticoagulant when not contraindicated. The Scandinavian study uses the Amulet, while the German trial uses both the Amulet and the Watchman device. The primary endpoint is the ischemic stroke rate.
The Amulet registry, which will continue for a second year of follow-up, was sponsored by Abbott Laboratories, which developed the Amulet device. Dr. Landmesser reported serving as a consultant to Abbott, as well as Biotronik, Rewa, and Bayer.
REPORTING FROM EUROPCR 2018
Key clinical point:
Major finding: The 1-year ischemic stroke rate in Amulet recipients was 2.9%, compared with a predicted rate of 6.7% based on CHA2DS2-VASc score.
Study details: This prospective all-comers registry included 1,088 atrial fibrillation patients who received the Amulet device at 61 centers in 17 countries.
Disclosures: The study was sponsored by Abbott Laboratories, which developed the device. The presenter reported serving as a consultant to the company, as well as Biotronik, Rewa, and Bayer.



















