User login
Artificial intelligence colonoscopy system shows promise
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
A new artificial intelligence (AI) system can help expert endoscopists improve their colonoscopies, a new study indicates.
Endoscopists using the computer program SKOUT (Iterative Scopes) achieved a 27% better detection rate of adenomas per colonoscopy, compared with endoscopists working without computer assistance, said lead author Aasma Shaukat, MD, MPH, director of outcomes research in the division of gastroenterology and hepatology at New York University.
The study showed that AI colonoscopy systems can work in a routine population of U.S. patients, Dr. Shaukat said in an interview.
“As gastroenterologists, we are very excited,” she said.
The study was published online in Gastroenterology and was presented at the annual Digestive Disease® Week.
Previous research has shown that experienced endoscopists miss many polyps. To improve their detection rate, multiple companies have used machine learning to develop algorithms to identify suspicious areas.
“Once the computer sees the polyp, it puts a bounding box around it,” said Dr. Shaukat. “It draws the attention of the endoscopist to it. It assists the endoscopist but doesn’t replace the endoscopist.”
The Food and Drug Administration has approved two such systems: EndoScreener (Wision AI) and GI Genius (Cosmo Pharmaceuticals).
The SKOUT algorithm was trained on 3,616 full-length colonoscopy procedure videos from multiple centers. In bench testing, it achieved a 93.5% polyp-level true positive rate and a 2.3% false positive rate.
Randomized trial pits AI against standard procedure
To see how well the system works in the clinic, Dr. Shaukat and colleagues recruited 22 U.S. board-certified gastroenterologists from five academic and community centers. The gastroenterologists all had a minimum adenoma detection rate of 25%, defined as the number of colonoscopies in which at least one adenoma is found, divided by the number of colonoscopies performed. All the gastroenterologists had performed a minimum of 1,000 colonoscopy procedures.
The researchers randomly assigned 682 patients to undergo colonoscopy with the SKOUT and 677 to undergo colonoscopy using the standard procedure. The patients were aged 40 years or older and were scheduled for either screening or surveillance.
The endoscopists who received computer assistance detected 1.05 adenomas per colonoscopy versus 0.83 for those who did not have computer assistance, a statistically significant difference.
The proportion of resections with clinically significant histology was 71.7% with standard colonoscopies versus 67.4% with computer-assisted colonoscopies. This fell within the 14% margin that the researchers had set to show noninferiority for the computer system.
“The important thing is not just detecting all polyps but the polyps we care about, which are adenomas, and doing so without increasing the false positive rate,” said Dr. Shaukat.
The adenoma detection rate was 43.9% for the standard procedure and 47.8% for the computer-assisted procedure. This difference was not statistically significant, but Dr. Shaukat argued that the adenoma detection rate is not the best measure of success, because endoscopists sometimes stop looking for polyps once they find one.
The overall sessile serrated lesion detection rate for the standard colonoscopies was 16.0% versus 12.6% for the computer-assisted colonoscopies, which also was not statistically significant.
Next steps
This study is important because it was a large, multicenter trial in the United States, said Omer Ahmad, BSc, MBBS, MRCP, a gastroenterologist and clinical researcher at University College London, who was not involved in the study. Most of the trials of AI have been in China or Europe. “It was very important just to see this replicated in the U.S. population.”
The average procedure time was 15.41 minutes for the standard colonoscopies versus 15.82 minutes for the computer-assisted colonoscopies, which was not statistically different.
“It is important to note that the studies so far suggest that false positives do not have a significant impact on workflow,” said Dr. Ahmad.
The next crucial step in evaluating AI colonoscopy will be to track the effects over the long term, said Dr. Shaukat.
“As these technologies get approved and we see them in practice, we need to see that it’s leading to some outcome, like reduced colon cancer,” she said.
That also may be necessary before payers in the United States are willing to pay the additional cost for this technology, she added.
In the meantime, Dr. Ahmad said computer assistance is improving his own colonoscopies.
“I have found the systems have spotted some polyps that I may have otherwise missed,” he said. “There is a false positive rate, but for me, it doesn’t distract from my workflow.”
He believes the systems will be particularly helpful in improving the performance of less-skilled endoscopists.
He is also looking forward to systems that can help complete the reports needed at the end of each colonoscopy. “Most of us dislike having to write a laborious report and having to code everything at the end of the procedure,” he said.
The study was funded by Iterative Scopes. Dr. Shaukat reported having received research funding to her institution for the current study from Iterative Scopes and consulting fees from Freenome and Medtronic. Dr. Ahmad reports receiving speaker fees from the Canadian Association of Gastroenterology/Medtronic.
A version of this article first appeared on Medscape.com.
At-home colorectal cancer testing and follow-up vary by ethnicity
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Dogs can be protective, even against Crohn’s disease
Sorry, cat people and only children: Having a dog as a toddler and growing up in a large family are two things linked to a significantly lower chance of getting Crohn’s disease later in life, according to a new study.
Children who lived with a dog between the ages of 2 years and 4 years were 37% less likely to have Crohn’s disease, the study says. And those who lived with at least three other family members during the first year of life were 64% less likely to have this form of inflammatory bowel disease (IBD).
“In this study, we’re interested in environmental exposures and which ones are associated with Crohn’s disease onset,” Williams Turpin, PhD, said in a media interview May 23 at the annual Digestive Disease Week® (DDW).
Dr. Turpin and colleagues looked at other things in the environment – including living on a farm, drinking unpasteurized milk or well water, and growing up with a cat – but they did not have a significant link to a higher risk.
Two other things were associated with a slight increase in risk: having a sibling with Crohn’s disease and living with a bird at time of the study. But the number of bird owners was small; only a few people in the study had a pet bird when they enrolled.
The link to living with a dog as a toddler “was more robust,” said Dr. Turpin, a project manager at Mount Sinai Hospital in Toronto.
The study included 4,289 healthy first-degree relatives of people diagnosed with Crohn’s disease. They provided urine, blood, and stool samples and did surveys about environmental exposures at different stages of life.
Investigators followed them an average of 5.6 years, during which time 86 people got Crohn’s disease.
Gut instinct
Living with a dog early in life likely means more exposure to different microbes, boosting the strength of a person’s immune system against later challenges. This theory was supported in the study comparing the gut microbiome in people who did and not have a dog in the home early in life.
Dr. Turpin and colleagues genetically sequenced the gut microbiome of the people in the study and found differences in bacteria between groups.
“Our study also shows that just by living with a dog, it impacts your gut microbiome composition, which may have an impact on the immune response later in life,” Dr. Turpin said.
The researchers also looked at the health of the gut by measuring certain factors in the urine. One factor was higher in people who did not live with a dog at any point.
Mediated by the microbiome?
Living with a dog between the ages of 2 and 4 years and a large family size (more than three people) in the first year were significantly associated with a lower risk of Crohn’s disease onset.
It is unknown if the results apply to other populations; the researchers studied first-degree relatives of people with Crohn’s disease.
“The study needs to be replicated and validated,” Dr. Turpin said.
Future research could evaluate people who never had a dog and look for changes in their microbiome after they get one.
‘Well-crafted’ study
“It’s a really interesting study from a good group. It’s novel in terms of getting at what really drives environmental risk factors,” said Brigid Boland, MD, a gastroenterologist at UC San Diego Health, who was not affiliated with the study.
Autoimmune diseases are really complicated, in part because the risk of getting an autoimmune disease is low, and you’re going back in time to look at what put people at risk.
“The study was well crafted in choosing siblings and family members of people with IBD,” Dr. Boland said, agreeing with Dr. Turpin that more research is needed to understand this.
A version of this article first appeared on WebMD.com.
Sorry, cat people and only children: Having a dog as a toddler and growing up in a large family are two things linked to a significantly lower chance of getting Crohn’s disease later in life, according to a new study.
Children who lived with a dog between the ages of 2 years and 4 years were 37% less likely to have Crohn’s disease, the study says. And those who lived with at least three other family members during the first year of life were 64% less likely to have this form of inflammatory bowel disease (IBD).
“In this study, we’re interested in environmental exposures and which ones are associated with Crohn’s disease onset,” Williams Turpin, PhD, said in a media interview May 23 at the annual Digestive Disease Week® (DDW).
Dr. Turpin and colleagues looked at other things in the environment – including living on a farm, drinking unpasteurized milk or well water, and growing up with a cat – but they did not have a significant link to a higher risk.
Two other things were associated with a slight increase in risk: having a sibling with Crohn’s disease and living with a bird at time of the study. But the number of bird owners was small; only a few people in the study had a pet bird when they enrolled.
The link to living with a dog as a toddler “was more robust,” said Dr. Turpin, a project manager at Mount Sinai Hospital in Toronto.
The study included 4,289 healthy first-degree relatives of people diagnosed with Crohn’s disease. They provided urine, blood, and stool samples and did surveys about environmental exposures at different stages of life.
Investigators followed them an average of 5.6 years, during which time 86 people got Crohn’s disease.
Gut instinct
Living with a dog early in life likely means more exposure to different microbes, boosting the strength of a person’s immune system against later challenges. This theory was supported in the study comparing the gut microbiome in people who did and not have a dog in the home early in life.
Dr. Turpin and colleagues genetically sequenced the gut microbiome of the people in the study and found differences in bacteria between groups.
“Our study also shows that just by living with a dog, it impacts your gut microbiome composition, which may have an impact on the immune response later in life,” Dr. Turpin said.
The researchers also looked at the health of the gut by measuring certain factors in the urine. One factor was higher in people who did not live with a dog at any point.
Mediated by the microbiome?
Living with a dog between the ages of 2 and 4 years and a large family size (more than three people) in the first year were significantly associated with a lower risk of Crohn’s disease onset.
It is unknown if the results apply to other populations; the researchers studied first-degree relatives of people with Crohn’s disease.
“The study needs to be replicated and validated,” Dr. Turpin said.
Future research could evaluate people who never had a dog and look for changes in their microbiome after they get one.
‘Well-crafted’ study
“It’s a really interesting study from a good group. It’s novel in terms of getting at what really drives environmental risk factors,” said Brigid Boland, MD, a gastroenterologist at UC San Diego Health, who was not affiliated with the study.
Autoimmune diseases are really complicated, in part because the risk of getting an autoimmune disease is low, and you’re going back in time to look at what put people at risk.
“The study was well crafted in choosing siblings and family members of people with IBD,” Dr. Boland said, agreeing with Dr. Turpin that more research is needed to understand this.
A version of this article first appeared on WebMD.com.
Sorry, cat people and only children: Having a dog as a toddler and growing up in a large family are two things linked to a significantly lower chance of getting Crohn’s disease later in life, according to a new study.
Children who lived with a dog between the ages of 2 years and 4 years were 37% less likely to have Crohn’s disease, the study says. And those who lived with at least three other family members during the first year of life were 64% less likely to have this form of inflammatory bowel disease (IBD).
“In this study, we’re interested in environmental exposures and which ones are associated with Crohn’s disease onset,” Williams Turpin, PhD, said in a media interview May 23 at the annual Digestive Disease Week® (DDW).
Dr. Turpin and colleagues looked at other things in the environment – including living on a farm, drinking unpasteurized milk or well water, and growing up with a cat – but they did not have a significant link to a higher risk.
Two other things were associated with a slight increase in risk: having a sibling with Crohn’s disease and living with a bird at time of the study. But the number of bird owners was small; only a few people in the study had a pet bird when they enrolled.
The link to living with a dog as a toddler “was more robust,” said Dr. Turpin, a project manager at Mount Sinai Hospital in Toronto.
The study included 4,289 healthy first-degree relatives of people diagnosed with Crohn’s disease. They provided urine, blood, and stool samples and did surveys about environmental exposures at different stages of life.
Investigators followed them an average of 5.6 years, during which time 86 people got Crohn’s disease.
Gut instinct
Living with a dog early in life likely means more exposure to different microbes, boosting the strength of a person’s immune system against later challenges. This theory was supported in the study comparing the gut microbiome in people who did and not have a dog in the home early in life.
Dr. Turpin and colleagues genetically sequenced the gut microbiome of the people in the study and found differences in bacteria between groups.
“Our study also shows that just by living with a dog, it impacts your gut microbiome composition, which may have an impact on the immune response later in life,” Dr. Turpin said.
The researchers also looked at the health of the gut by measuring certain factors in the urine. One factor was higher in people who did not live with a dog at any point.
Mediated by the microbiome?
Living with a dog between the ages of 2 and 4 years and a large family size (more than three people) in the first year were significantly associated with a lower risk of Crohn’s disease onset.
It is unknown if the results apply to other populations; the researchers studied first-degree relatives of people with Crohn’s disease.
“The study needs to be replicated and validated,” Dr. Turpin said.
Future research could evaluate people who never had a dog and look for changes in their microbiome after they get one.
‘Well-crafted’ study
“It’s a really interesting study from a good group. It’s novel in terms of getting at what really drives environmental risk factors,” said Brigid Boland, MD, a gastroenterologist at UC San Diego Health, who was not affiliated with the study.
Autoimmune diseases are really complicated, in part because the risk of getting an autoimmune disease is low, and you’re going back in time to look at what put people at risk.
“The study was well crafted in choosing siblings and family members of people with IBD,” Dr. Boland said, agreeing with Dr. Turpin that more research is needed to understand this.
A version of this article first appeared on WebMD.com.
FROM DDW 2022
Psychological intervention looks promising in Crohn’s disease
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.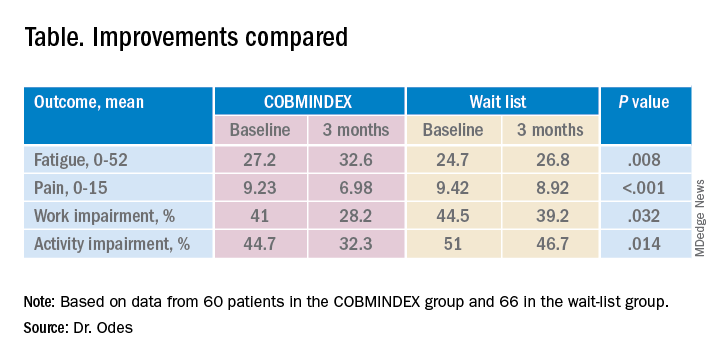
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
SAN DIEGO – A combination of cognitive-behavioral therapy and mindfulness meditation could reduce pain and fatigue from Crohn’s disease, researchers say.
Patients who followed the program not only felt better but were also more often able to show up for work and leisure activities, compared with a control group assigned to a wait list, said Shmuel Odes, MD, a professor of internal medicine at Ben-Gurion University of the Negev in Beersheba, Israel. He presented the finding at Digestive Diseases Week® (DDW) 2022.
Psychological and social factors affect the gut and vice versa, Dr. Odes said. Yet many inflammatory bowel disease clinics overlook psychological interventions.
To address these issues, Dr. Odes and colleagues developed cognitive-behavioral– and mindfulness-based stress reduction (COBMINDEX) training, which can be taught by clinical social workers over the Internet. “The patient learns to relax,” Dr. Odes told MDedge News. “He learns not to fight his condition.”
In a previous paper, published in the journal Inflammatory Bowel Diseases, Dr. Odes and colleagues reported that patients who learned the technique showed improvement on a variety of psychological and quality-of-life measures, accompanied by changes in inflammatory cytokines and cortisol.
In a follow-up analysis presented here, the researchers looked at measures of pain and fatigue and then examined whether these were associated with productivity at work and other daily activities.
The study investigators randomly assigned 72 patients to an intervention group who got COBMINDEX training right away, and another 70 to a control group assigned to a wait list of 12 weeks before they could get the training. At baseline, the two groups were not significantly different in any demographic or clinical variable the researchers could find.
Social workers provided COBMINDEX training for the patients in seven 60-minute session over 12 weeks. Five of the sessions were devoted to cognitive-behavioral therapy and two to mindfulness-based stress reduction. The social workers asked the patients to do exercises at least once a day and report outcomes through an app.
Twelve patients dropped out of the COBMINDEX group and four dropped from the wait-list group because of lack of interest, time constraints, pregnancy, or illness.
The researchers created a composite score with a 0-15 scale (with higher scores indicating greater pain) from three pain items from the Harvey-Bradshaw Index for Crohn’s Disease, the Short Inflammatory Bowel Disease Questionnaire, and the 12-Item Short Form Survey.
To measure fatigue, they used the Functional Assessment of Chronic Illness Therapy-Fatigue, which has a 0-52 scale, with lower scores indicating greater fatigue.
To measure impairment while working and other daily activities, they used the Work Productivity and Activity Impairment Questionnaire: Crohn’s Disease. Scores on this measure are expressed as a percentage, with higher values indicating greater impairment.
Both the COBMINDEX and the wait-list groups improved on all these scales, but the improvements were significantly greater for the COBMINDEX group.
Through statistical analysis, the researchers found that the improvements in pain and fatigue indirectly caused the improvements in work and activity impairment, and that pain and fatigue improvements made independent contributions of similar magnitudes. COBMINDEX did not directly improve work or activity.
Psychological interventions are too often overlooked in Crohn’s disease, said the session comoderator Paul Moayyedi, MD, a professor of gastroenterology at McMaster University in Hamilton, Ont. “We need to realize how important this is to patients and urgently make this available,” he told MDedge.
A variety of interventions are being researched, and this study makes an important contribution, he said. However, he questioned whether people on a wait list can serve as an adequate control. “If you have to wait for something, you tend to have more pain, and you could have less productivity just because of waiting,” he said. “Ideally they should do a randomized trial with a sham intervention, not a wait list.”
Dr. Odes responded that it is very difficult to recruit people to a trial if they only have a 50% chance of getting a real treatment. And he noted that the people on the wait list in this trial did not show any signs of increased symptoms.
Physicians wanting to provide psychological help to their Crohn’s disease patients can refer them to social workers or psychotherapists, Dr. Odes said, but these professionals may lack training for applying cognitive-behavioral therapy and mindfulness-based stress reduction to patients with Crohn’s disease. His team hopes to make an app publicly available soon.
Neither Dr. Odes nor Dr. Moayyedi reported any relevant financial interests. The study was supported by a grant from the Leona M. and Harry B. Helmsley Charitable Trust.
AT DDW 2022
Race-, ethnicity-based clinical guidelines miss the mark: Study
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
SAN DIEGO – Race-based recommendations and clinical algorithms may be doing more harm than good, according to a systematic review of databases and guidelines.
The study found examples of screening recommendations based on race or ethnicity that were likely misleading since these are social constructs that don’t reflect a patient’s individual risk, said Shazia Siddique, MD, who presented the study at the annual Digestive Disease Week® (DDW). “Historically, we’ve made so many clinical decisions based on somebody’s race and ethnicity. We walk into a room, we don’t even ask people which racial or ethnic category they identify with. We just look at them and we say, ‘Their skin color looks black, and therefore we’re going to apply a different equation to them.’ ”
However, a patient’s risks and unique health circumstances are much more complicated than that. They may be related to genetics, or environmental exposures, or level of access to quality health care. Race can often be inappropriately used as a stand-in for these and other factors, she explained.
“These [racial] categories are truly a social construct. It’s becoming very problematic that people are literally making decisions based on somebody’s skin color. That’s just not what the science supports. If there are specific genes or environmental factors, or differences in access to health care that then impact outcomes for certain racial or ethnic groups, we need to figure out what those are,” said Dr. Siddique, who is an assistant professor of medicine at the University of Pennsylvania, Philadelphia.
Those messages are still entrenched in medical education. “I graduated medical school in 2012, and it was taught to me to use race and ethnicity in clinical decision-making. We need to start in medical education to shift the way that we’re thinking. On the research side, we really need to think about how we can replace or remove race and ethnicity and understand the consequences of that, so that over time we can make a shift,” said Dr. Siddique.
For example, Dr. Siddique discussed recommendations that suggest Asian heritage as a risk factor for hepatitis B screening, but that’s not a good factor to consider: “People were saying that Asians should be screened at an earlier age, but it’s really people that were born and raised in Asian countries where it’s endemic or they may have gotten it from their mothers at birth. It’s a marker for how long you have had the disease and how much virus is in your bloodstream. It’s not because you’re Asian. If you’re born and raised in the United States, and you don’t have any of those risk factors, you shouldn’t be treated differently based on your identified racial and ethnic group,” said Dr. Siddique.
These questions have become even more important in recent years because of patients with multiracial identifies and other considerations. “Now the proxy for which race was being used is even messier,” said Dr. Siddique.
So, how should physicians think about assessing a patient’s personalized risks? The key, said Dr. Siddique, is to look at each patient’s individual factors, such as health care access, environmental exposures from jobs or living conditions, or the country they emigrated from if they weren’t born in the United States. “Disease prevalences are different in different areas, and that changes your index of suspicion,” she said.
And when considering current guidelines that incorporate race or ethnicity, she recommends viewing them skeptically: “If there is a current algorithm in your health system or in a guideline that you’re reading that says you should be making a change based on race and ethnicity, you should look at that with a close eye and say, “What do I think it’s being used as a proxy for, and how can I elicit that from my patient?’ ”
The issues raised by Dr. Siddique’s study are important, but there also could be concerns in taking them too far, according to Gary Falk, MD, a professor of medicine at the University of Pennsylvania who comoderated the session where Dr. Siddique presented. He was not involved in the study, but was listed on Dr. Siddique’s acknowledgement slide.
Dr. Falk coauthored Barrett’s esophagus guidelines in 2016 that incorporated White race as a risk factor.
“There are certain clear ethnic factors or country of origin factors that impact one’s risk for cancer, and there are certain diseases that are more common in certain ethnic groups. I think that if we homogenize everybody, we may potentially hurt some people in the effort to be inclusive. That’s my only concern. I think it’s totally correct that we have to get out of our comfort zone, but I hate to see us reach too far on the other end, and homogenize things to the point that people who have increased risk are not being recognized for that reason,” said Dr. Falk.
He acknowledged that White race as a risk for Barrett’s is not easy to define given the uncertainty of the genetic risk, for example, in patients with mixed heritage. “This is all very provocative. We have to think about it carefully,” said Dr. Falk.
Dr. Siddique and Dr. Falk have no relevant financial disclosures.
AT DDW 2022
Index cholecystectomy reduces readmissions after acute cholangitis
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
SAN DIEGO – Patients with acute cholangitis are twice as likely to be readmitted within 30 days if they don’t get a cholecystectomy in the same hospital admission for which they get biliary decompression, researchers say.
The readmissions result mostly from sepsis and recurrence of the acute cholangitis, said Ahmad Khan, MD, MS, a gastroenterology fellow at Case Western Reserve University in Cleveland, at Digestive Diseases Week® (DDW) 2022. “These added readmissions can cause a significant burden in terms of costs and extra days of hospitalization in these patients.”
Acute cholangitis in patients without bile duct stents is most often caused by biliary calculi, benign biliary stricture, or malignancy. A gastrointestinal emergency, it requires treatment with biliary decompression followed by cholecystectomy, but the cholecystectomy is considered an elective procedure.
Surgeons may delay it if the patient is very sick, or simply for scheduling reasons, Dr. Khan said. “There are some areas where the surgeons may be too busy,” he said. Or if the patient first presents at the end of the week, some surgeons will send the patient home so they don’t have to operate on the weekend, he said.
To understand the consequences of these decisions, Dr. Khan and his colleagues analyzed data from 2016 to 2018 from the National Readmission Database of the U.S. Agency for Healthcare Research and Quality.
They found that 11% of patients who went home before returning for a cholecystectomy had to be readmitted versus only 5.5% of those who got a cholecystectomy during the same (index) admission as their biliary decompression.
Patients who got cholecystectomies during their index admissions were slightly younger and healthier: Their mean age was 67.29 years and 20.59% had three or more comorbidities at index admission versus 70.77 years of age and 39.80% with three or more comorbidities at index admission for those who got their cholecystectomies later.
The researchers did not find any significant differences in the hospitals’ characteristics, such as being urban or academic, between the two groups.
Mortality was higher for those who received their cholecystectomy after returning home, but they spent less time in the hospital at lower total cost. The differences in outcomes between the index admission and readmission were all statistically significant (P < .01).
This observational study could not determine cause and effect, but it justifies a prospective trial that could more definitely determine which approach results in better outcomes, Dr. Khan said.
That patients are less likely to need readmission if they return home without a gall bladder after treatment for acute cholangitis “makes sense,” said session comoderator Richard Sterling, MD, MSc, chief of hepatology at Virginia Commonwealth University in Richmond.
“Should you do it immediately or can you wait a day or 2? They didn’t really address when during that admission, so we still don’t know the optimal sequence of events.”
If a patient has so many comorbidities that the surgeon and anesthesiologist don’t think the patient could survive a cholecystectomy, then the surgeon might do a cholecystostomy instead, he said.
Dr. Khan said he hopes to delve deeper into the data to determine what factors might have influenced the surgeons’ decisions to delay the cholecystectomy. “I want to see, of the patients who did not get same-admission cholecystectomies, how many had diabetes, how many had coronary artery disease, how many were on blood thinners, and things like that.”
Neither Dr. Khan nor Dr. Sterling reported any relevant financial interests.
AT DDW 2022
Eosinophilic diseases often overlap, raising costs
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
Eosinophilic GI diseases (EGIDs) often overlap with other eosinophil-associated diseases (EADs), which leads to greater health care costs, according to an analysis of the U.S. Optum Clinformatics claims database.
EADs have gained increased attention in recent years. They include eosinophilic esophagitis (EoE), eosinophilic asthma, bullous pemphigoid, eosinophilic granulomatosis with polyangiitis, eosinophilic gastritis/gastroenteritis (EG/EGE), and a subset of non–cystic fibrosis bronchiectasis. All involve infiltration of eosinophils, but the exact immune mechanisms behind them seem to vary and are poorly understood, according to Justin Kwiatek, PharmD, who presented the results at the annual Digestive Disease Week® (DDW).
“We do know that the suitable course of treatment is dependent on the organs impacted. From this study, we also know that EoE mostly exists on its own, with only a small portion also being diagnosed with asthma, while overlap with other EGIDs tends to be higher. This could be because EoE appears to be pathologically different from other EGIDs in the gastrointestinal tract such as eosinophilic gastritis in the stomach or eosinophilic gastroenteritis in the stomach and small bowel. Eosinophils are not normally present in the esophagus but are often found in the stomach or small bowel without inflammation,” said Dr. Kwiatek, who is senior global medical affairs leader, respiratory & immunology, at AstraZeneca.
The study is important, said Dhyanesh Patel, MD, who was asked to comment on the study. “There’s been a lot of interest in eosinophilic gastrointestinal diseases recently because there is lack of a clear definition. We need to define it better because we need to figure out treatment options for the patients,” said Dr. Patel, who is an assistant professor of medicine at Vanderbilt University, Nashville, Tenn.
“It highlights that a lot of the patients that have one eosinophilic disease might have other concomitant atopic diseases. [It may be that] you can use one drug to treat all of them together, so I think it’s important to have a multidisciplinary approach where you work with an allergist and you work with an immunologist and treat their eosinophilic gastritis and their asthma together with one drug. That may help reduce medication burden,” said Dr. Patel.
The researchers analyzed records from 1,326,645 diagnosed patients with at least one EAD and at least 2 years following treatment. There were 13,872 patients with EoE, 38.4% of whom had at least one overlapping EAD. Of 1,365 patients with EG/EGE, 57.9% had at least one overlapping EAD.
EADs were associated with higher Charlson Comorbidity Index scores and high blood eosinophil levels (≥ 300 cells/mcL) among EoE patients, but not among EG/EGE patients. Within the EoE group, female gender was linked to more EAD comorbidities: 35% of patients with only EoE were female; 45% of patients with one comorbidity were female, as were 55% of those with two comorbidities and 57% of those with three or more comorbidities. There was no such trend among patients with EG/EGE.
Total health care costs were lower in the absence of one overlapping EAD among both EoE ($2,061 vs. $3,766 per patient per month) and EG/EGE patients ($2,860 vs. $4,053). Costs went up with more overlap: $8,572 for EoE and three or more other EADs, and $10,397 for EG/EGE and three or more other EADs. These costs were largely driven by outpatient care.
“The data shows that patients with eosinophilic gastritis and eosinophilic gastroenteritis are more likely to have overlapping eosinophilic conditions, such as asthma. When diagnosing a patient with EG or EGE, it’s important to monitor any new symptoms closely and to educate them about the risk factors. This is particularly true for patients with elevated blood eosinophil counts. Accounting for comorbidities and establishing a treatment plan early can help to manage the higher health care spend for patients with overlapping conditions,” said Dr. Kwiatek.
Dr. Kwiatek is an employee and stockholder of AstraZeneca, which funded the study and developed benralizumab, a drug that has been granted orphan drug status for EG/EGE and EoE. Optum Clinformatics is a longitudinal database of deidentified data formed by UnitedHealth Group. Dr. Patel has no relevant financial disclosures.
FROM DDW 2022
ESG’s cardiometabolic benefits last 5 years
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
SAN DIEGO – Endoscopic sleeve gastroplasty (ESG) led to sustained weight loss and a reduction of cardiometabolic syndrome comorbidities at 5 years, according to a new retrospective analysis of prospectively collected data.
Improved cardiometabolic outcomes following bariatric surgery have been well documented, but ESG is relatively new, so its outcomes haven’t been as well described. The outcomes are encouraging, though not as good as those of bariatric surgery. “It’s still better, but only one percent of the patients undergo the surgery, even though they’re candidates,” said Donevan Westerveld, MD, who presented the study at the annual Digestive Disease Week® (DDW).
Improvements included weight, HbA1c percentage, hypertension, and low-density lipoprotein. “I was surprised that the LDL decreased numerically, not so much HbA1c and hypertension. I knew [those] would come down with weight loss,” said Dr. Westerveld, a second-year fellow at Weill Cornell Medicine, New York.
He also called for guidelines for ESG. “Given the fact there’s an improvement of comorbid conditions, it’s something we should look at,” said Dr. Westerveld.
“It’s fascinating because it tells us two important things about endoscopic sleeve gastroplasty. One, [the benefit] in the majority of cases lasts at least 5 years. The weight loss is durable. And then it tells us that there’s improvement in all the cardiometabolic factors that matter, and those effects are seen all the way up to 5 years. So very important findings that support the benefits of the endoscopic gastroplasty in obesity and cardiometabolic risks and metabolic syndrome,” said Andres Acosta, MD, PhD, a comoderator of the session where the study was presented. He is assistant professor of medicine and a consultant in gastroenterology and hepatology at Mayo Clinic in Rochester, Minn.
The findings should also encourage more innovation. “Doing these endoscopic procedures, having successful results that hold for 5 years, opens the path for new and better procedures, so we have better weight loss,” said Dr. Acosta.
Previous work by Dr. Westerveld’s group found benefits of ESG at 12 months, including improvements in mean HbA1c levels in all patients (6.1%-5.5%; P = .05) and those with diabetes or prediabetes (6.6%-5.6%; P = .02), reduction in mean waist circumference (119.66-92.75 cm; P < .001), reduction in systolic blood pressure (129.02-122.23 mg/dL; P = .023), triglycerides (131.84-92.36 mg/dL; P = .017), and alanine aminotransferase (ALT, 32.26-20.68 mg/dL; P < .001).
In the new study, the group followed 255 patients at 1, 3, and 5 years post procedure who were treated consecutively at Weill Cornell Medicine from 2013 to 2021. Among the patients were those who had failed weight loss measures and were either not candidates for surgery or had refused surgery.
The mean age was 45.5 years, 69% were female, and the mean body mass index was 38.6. Overall, 40.3% had prediabetes or diabetes, 26.7% had hypertension, 60.8% had low-density lipoprotein (LDL) above 100 mg/dL, and 29.3% had elevated ALT. Sixty-six percent had been followed up at 1 year, 78% at 3 years, and 87% at 5 years.
Weight loss averaged 15.7% at 1 year and 15.3% at year 5, and the values were statistically significant. Among patients with diabetes and prediabetes, HbA1c percentage dropped from a baseline value of 6.4% to 5.7% at year 1, 6.1% at year 3, and 5.8% at year 5 (P < .05 for all). For all patients, the value dropped from 5.8% at baseline to 5.6% at year 1, 5.7% at year 3, and 5.4% at year 5. These changes were not statistically significant.
Systolic blood pressure went down among patients with stage 1 hypertension, from 135 mm Hg at baseline to 122 at year 1 and 121 at year 3 (P < .05 or both), but the mean value increased to 129 at year 5 and was not statistically significant. LDL among all patients declined from 136 mg/dL at baseline to 125 at year 1 (nonsignificant), 115 at year 3 (P < .05), and 109 at year 5 (P < .05). Alanine transaminase values declined from about 29 at baseline to 25 at year 1, 26 at year 3, and 24 at year 5 (P < .05 for all).
Serious adverse events were rare, occurring in just two cases (< 1%).
The study was limited by lack of a sham control, and its retrospective data may have included bias because many of the procedures were not paid for by insurance, leading to high rates of self-pay.
Dr. Westerveld has no relevant financial disclosures. Dr. Acosta is a founder of Gila Therapeutics and Phenomix Sciences. Dr. Acosta consults for Amgen, Gila Therapeutics, Rhythm Pharmaceuticals, and General Mills. He has received funding from Rhythm, Novo Nordisk, Apollo Endosurgery, and USGI Medical.
At DDW 2022
H. pylori antibiotics briefly disrupt gut microbiome
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
SAN DIEGO – Treatments to eradicate Helicobacter pylori (H. pylori) infections do increase the antibiotic resistance of the gut microbiota, but for only a few months, researchers reported at Digestive Disease Week® (DDW).
The finding applies similarly to levofloxacin quadruple therapy and bismuth quadruple therapy, both of which are equally efficacious as second-line treatments, said Jyh-Ming Liou, MD, PhD, clinical professor of internal medicine at National Taiwan University in Taipei.
This provides some reassurance that increased use of antibiotics to treat these infections won’t cause long-term disruptions to the patients’ microbiomes, said Dr. Liou.
“Maybe if we have indications for antibiotic treatment, then we don’t worry about the emergence of resistance in our bodies,” he said. “But the accumulation of antibodies in the environment may induce bacteria to mutate, so maybe we still need cautious use of antibiotics.”
H. pylori infections are becoming harder to treat as more strains develop resistance to antibiotics, leading physicians to use regimens with multiple agents. This in turn has raised concerns that gut microbiota could be disrupted, with pathogens potentially developing their own resistance.
To explore these risks, Dr. Liou and colleagues recruited adults whose H. pylori infections were not successfully eradicated.
They randomly assigned 280 patients each to one of two second-line therapies, levofloxacin quadruple or bismuth quadruple. At baseline, the researchers could not find any statistically significant differences in the two groups’ demographics, cigarette and alcohol use, or ulcers, as well as antibiotic resistance in patients’ microbiome between the groups.
Levofloxacin quadruple therapy consisted of esomeprazole 40 mg and amoxicillin 1 g for the first 7 days, followed by esomeprazole 40 mg, metronidazole 500 mg, and levofloxacin 250 mg for another 7 days (all twice daily).
Bismuth quadruple therapy consisted of esomeprazole 40 mg twice daily, bismuth tripotassium dicitrate 300 mg four times a day, tetracycline 500 mg four times a day, and metronidazole 500 mg three times a day, for 10 days.
The researchers collected stool samples at baseline, week 2, week 8, and 1 year after eradication therapy and analyzed them for microbiota diversity and antibiotic susceptibility.
The H. pylori eradication rates were almost the same in the two second-line therapies: 87.9% for levofloxacin quadruple and 87.5% for bismuth quadruple. When they were used as third-line (rescue) therapies, the success rates were also statistically the same, and the cumulative second-line and third-line eradication rate was 95.6% for levofloxacin quadruple and 96.6% for bismuth quadruple.
The two treatments did differ in adverse events with 48.4% for levofloxacin quadruple and 77.3% for bismuth quadruple, which was statistically significant (P < .0001).
After a year, H. pylori reinfected 2.5% of the levofloxacin group and 3% of the bismuth quadruple group.
The researchers used metagenomic sequencing to examine the bacteria in the patients’ microbiome for antibiotic resistance. Using 16S rRNA sequencing, they found that the proportion of genera and species with significant changes in abundance at 2 weeks after treatment compared with baseline was 52.4% for levofloxacin quadruple therapy versus 45.1% for bismuth quadruple therapy.
However, 8 weeks after treatment, the proportion with significant changes had dropped to 5.8% for the levofloxacin group and 21.5% for the bismuth group. And at the end of a year, they had further dropped to 0.9% for the levofloxacin group and 8.4% for the bismuth group.
“It was generally reassuring that, even after giving these combinations of different antibiotics, eventually it doesn’t seem to affect the resistance pattern in bacteria lower down in the gut,” said session moderator Steven Moss, MD, professor of medicine at Brown University in Providence, R.I.
Still, continuing to pile on more and more antibiotics to treat H. pylori infections won’t work forever because H. pylori strains are themselves developing resistance so rapidly, he said. “We’re certainly going to have worse eradications in the future unless we can come up with new tricks.”
A hopeful development are new techniques to test H. pylori for resistance to specific antibiotics before initiating treatment, said Dr. Moss.
Dr. Moss consults with companies developing H. pylori therapies and diagnostics. Dr. Liou reported no relevant financial interests.
AT DDW 2022
Fidaxomicin favored over vancomycin in real-world C. diff study
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
Fidaxomicin (Fificid) emerged favorable to vancomycin for the treatment of both initial and recurrent Clostridioides difficile infections in a Medicare population, according to a new retrospective study.
Although fidaxomicin was about 14% more effective than vancomycin in treating the initial infection, a larger difference of 30% was found among people with recurrent C. diff. infections.
Lead investigator Erik Dubberke, MD, professor of infectious diseases at the University of Washington, St. Louis, and colleagues noted that this real-world evidence of the two agents used to treat C. diff. was “strikingly similar” to clinical trial data.
They said that their findings support the 2021 change in clinical guidelines from the Infectious Diseases Society of America recommending fidaxomicin over vancomycin.
The study was presented at Digestive Disease Week® (DDW) 2022, which was held virtually and in San Diego.
Evaluating a high-risk population
Because few real-world data exist that compare these two agents for C. diff., “particularly in a high-risk, high-prevalence population like Medicare,” the researchers evaluated Medicare Parts A, B, and D claims from 2016 to 2018 and included patients who had received fidaxomicin or vancomycin for an initial episode of C. diff. and for any recurrent episodes.
The researchers compared sustained response and recurrence of C. diff. within 4 weeks and 8 weeks after initial treatment with fidaxomicin or vancomycin. Treatment was considered successful if clinical resolution occurred 1 day after finishing therapy and there was no evidence of C. diff. recurrence.
Recurrence of C. diff. was defined as any evidence of new treatment or hospitalization for the infection within 4 or 8 weeks of when a patient filled the prescription for fidaxomicin or vancomycin.
The treatment groups were similar in age and race. However, the fidaxomicin group was at higher risk for recurrence, owing to risk factors such as history of C. diff. infection and compromised immunity. To reduce bias in comparing the groups, Dr. Dubberke and colleagues used propensity score matching. This approach yielded 190 matched pairs in the initial C. diff. episode sample and 67 matched pairs in the recurrent episode sample.
Among patients with their first C. diff. infection, fidaxomicin had a 13.5% higher rate of 4-week sustained response, compared with vancomycin (71.7% vs. 58.2%; P = .0058). There was also a 13.2% higher rate for 8-week sustained response with fidaxomicin (63.2% vs. 50.0%; P = .0114).
Sustained response at 4 weeks and 8 weeks among the patients who experienced a recurrent episode of C. diff. favored fidaxomicin over vancomycin by 30.1% (P = .0002) and 27.6% (P = .0012), respectively.
The rates of C. diff. recurrence in patients who experienced their first C. diff. infection or who experienced a recurrent bout were lower with fidaxomicin than vancomycin, but the differences were not statistically significant.
A costly edge
When asked to comment, Colleen Kelly, MD, a gastroenterologist and associate professor of medicine at Brown University, Providence, R.I., said that the study was “worthwhile” and added that “Eric Dubberke has done a lot of work in this area.”
The study “gives more evidence that fidaxomicin does have a bit of an edge in people who have already had a bout of C. diff.,” she said.
Dr. Kelly added that the cost needs to be considered. Fidaxomicin “is about 30 times more expensive than vancomycin,” she said.
In part because of the cost difference, the American College of Gastroenterology (ACG) 2021 guidelines, which Dr. Kelly helped create, recommend that fidaxomicin be held as a second-line agent. The ACG guidance reserved fidaxomicin for people with C. diff. for whom initial treatment with vancomycin failed.
“The fidaxomicin question is going to get a lot easier once the cost of the drug comes down,” Dr. Kelly said.
The study was funded by Merck. Dr. Dubberke is a consultant for Merck. Dr. Kelly reports no relevant financial relationships.
Help your patients understand their C. difficile diagnosis by sharing patient education from the AGA GI Patient Center: www.gastro.org/Cdiff.
A version of this article first appeared on Medscape.com.
AT DDW 2022



