User login
Think it’s ILD? Tell it to the machines
SAN FRANCISCO – Interstitial lung disease is a difficult diagnosis to make, but a combination of artificial intelligence (AI) techniques and automated language processing could help clinicians identify the early signs of ILD and start patients on therapy, investigators say.
For example, applying an AI algorithm to spirometry readings taken from patients whose data were registered in the UK Biobank identified 27% as having ILD, and of this group, 66% had ostensibly normal lung function on spirometry but were later diagnosed with ILD, reported Marko Topalovic, PhD, from the AI company ArtiQ in Leuven, Belgium, at the American Thoracic Society’s international conference.
“A diagnosis of ILD is very challenging, so you have patients who are going to be misdiagnosed or have a very late diagnosis, so we aimed to apply our AI algorithm on spirometry to see whether we could detect ILD much earlier,” he said in an interview conducted during a poster discussion session.
AI detected ILD up to 6.8 years before a clinician’s diagnosis, Dr. Topalovic said.
Reading between the lines
In a separate study, investigators at the University of California, Davis, used language analysis software to scour electronic health records for words indicative of early ILD, and found that the technique dramatically shortened the median time to a pulmonary referral, compared with historical controls.
“This is a language processing program that can essentially look through the radiology reports and look for the key words that often describe interstitial lung disease, like traction, honeycomb, fibrotic, etc. With those studies being flagged, an actual pulmonologist will then further review the scan, and see whether it meets criteria for one of the interstitial lung diseases,” lead author William Leon, MD, a resident in the department of internal medicine at the University of California, Davis, said in an interview.
“We then sent the primary care doctor a message to say: ‘Hey, this patient has ILD. You need to send them to a pulmonologist,’ ” he added.
Putting it together
Philip L. Molyneaux, MRCP (UK), MBBS, BS (Hons), from Imperial College London, who comoderated the session but was not involved in the studies, speculated that combining these and other, nontechnical interventions also discussed could help to improve diagnosis of ILD and allow clinicians to prescribe therapy earlier in the disease course.
“What’s going to give you the biggest impact for patients? Everyone working individually is coming up with great advances, and if you put them all together it’s going to provide much greater benefit for our patients,” he said in an interview.
AI Spirometry details
In collaboration with colleagues at the Laboratory of Respiratory Disease at University Hospital in Leuven, Dr. Topalovic applied AI to results of spirometry performed prior to diagnosis of ILD among 109 patients registered in the UK Biobank, a repository of information on more than 500,000 volunteers.
The patients selected had ILD listed as their cause of death, had spirometry performed up to 7 years before their deaths, and did not receive a diagnosis of ILD on the day of the index spirometry.
In all 73% of patients were men, 27% women, with an average age of 64.6 years. A large majority of the sample (77.15%) had a history of smoking, and 60 of the patients (55%) died within one year of an ILD diagnosis.
The investigators plugged the spirometry data and each patients demographic information – including gender, age, height, weight, race, and smoking status – into the AI clinical decision support program, which yielded a statistical probability for each subject of having normal lung function, asthma, COPD, ILD, another obstructive disease, or another unidentifiable respiratory disease.
In 29 patients (27%) the software listed ILD as the highest probability, and of this group 19 patients (66%) had normal lung function according to standard interpretation guidelines.
Spirometry parameters among patients identified as having probable ILD were different from those where ILD was not detected. For example, forced vital capacity (FVC) was 76% of predicted among patients with likely ILD versus 87% of predicted in those who had a diagnosis later (P = .003). Similar differences were seen in the forced expiratory volume in 1 second to FVC ratio, at 0.82 vs. 0.75, respectively (P = .007).
There were no differences in mortality or in median time between spirometry and clinician diagnosis between the groups.
Language processing details
Dr. Leon and colleagues used a language analysis software package to review CT chest reports. Reports were flagged if they contained the words traction, honeycomb, fibrotic, fibrosis, reticular, or reticulation.
The CT scan accompanying each flagged reported was reviewed by a pulmonologist for the presence of ILD, and scans with ILD identified were referred to pulmonary specialists. The results of 2,198 prospective scans followed by prospective screening were compared with those of 1,690 historical controls seen in 2015 and 2016.
The investigators found that 85 incident cases of ILD were identified in the historical controls, compared with 143 in the prospective cohort, leading to 38 and 120 pulmonary referrals, respectively.
For the primary outcome of median time from CT to pulmonary referral, the authors found that it was 1.27 months for the prospective cohort, compared with not reached (censored after 18 months) in historical controls.
The hazard ratio for a pulmonary referral in the prospective versus historical cohort was 2.79, an association that was strengthened after adjusting for sex, age, race, smoking pack-years, cough, crackles, and dyspnea (HR, 4.54; both comparisons significant according to confidence intervals).
The studies were internally funded. Dr. Topalovic is CEO and cofounder of ArtiQ. Dr. Leon and Dr. Molyneaux reported no relevant conflicts of interest.
SAN FRANCISCO – Interstitial lung disease is a difficult diagnosis to make, but a combination of artificial intelligence (AI) techniques and automated language processing could help clinicians identify the early signs of ILD and start patients on therapy, investigators say.
For example, applying an AI algorithm to spirometry readings taken from patients whose data were registered in the UK Biobank identified 27% as having ILD, and of this group, 66% had ostensibly normal lung function on spirometry but were later diagnosed with ILD, reported Marko Topalovic, PhD, from the AI company ArtiQ in Leuven, Belgium, at the American Thoracic Society’s international conference.
“A diagnosis of ILD is very challenging, so you have patients who are going to be misdiagnosed or have a very late diagnosis, so we aimed to apply our AI algorithm on spirometry to see whether we could detect ILD much earlier,” he said in an interview conducted during a poster discussion session.
AI detected ILD up to 6.8 years before a clinician’s diagnosis, Dr. Topalovic said.
Reading between the lines
In a separate study, investigators at the University of California, Davis, used language analysis software to scour electronic health records for words indicative of early ILD, and found that the technique dramatically shortened the median time to a pulmonary referral, compared with historical controls.
“This is a language processing program that can essentially look through the radiology reports and look for the key words that often describe interstitial lung disease, like traction, honeycomb, fibrotic, etc. With those studies being flagged, an actual pulmonologist will then further review the scan, and see whether it meets criteria for one of the interstitial lung diseases,” lead author William Leon, MD, a resident in the department of internal medicine at the University of California, Davis, said in an interview.
“We then sent the primary care doctor a message to say: ‘Hey, this patient has ILD. You need to send them to a pulmonologist,’ ” he added.
Putting it together
Philip L. Molyneaux, MRCP (UK), MBBS, BS (Hons), from Imperial College London, who comoderated the session but was not involved in the studies, speculated that combining these and other, nontechnical interventions also discussed could help to improve diagnosis of ILD and allow clinicians to prescribe therapy earlier in the disease course.
“What’s going to give you the biggest impact for patients? Everyone working individually is coming up with great advances, and if you put them all together it’s going to provide much greater benefit for our patients,” he said in an interview.
AI Spirometry details
In collaboration with colleagues at the Laboratory of Respiratory Disease at University Hospital in Leuven, Dr. Topalovic applied AI to results of spirometry performed prior to diagnosis of ILD among 109 patients registered in the UK Biobank, a repository of information on more than 500,000 volunteers.
The patients selected had ILD listed as their cause of death, had spirometry performed up to 7 years before their deaths, and did not receive a diagnosis of ILD on the day of the index spirometry.
In all 73% of patients were men, 27% women, with an average age of 64.6 years. A large majority of the sample (77.15%) had a history of smoking, and 60 of the patients (55%) died within one year of an ILD diagnosis.
The investigators plugged the spirometry data and each patients demographic information – including gender, age, height, weight, race, and smoking status – into the AI clinical decision support program, which yielded a statistical probability for each subject of having normal lung function, asthma, COPD, ILD, another obstructive disease, or another unidentifiable respiratory disease.
In 29 patients (27%) the software listed ILD as the highest probability, and of this group 19 patients (66%) had normal lung function according to standard interpretation guidelines.
Spirometry parameters among patients identified as having probable ILD were different from those where ILD was not detected. For example, forced vital capacity (FVC) was 76% of predicted among patients with likely ILD versus 87% of predicted in those who had a diagnosis later (P = .003). Similar differences were seen in the forced expiratory volume in 1 second to FVC ratio, at 0.82 vs. 0.75, respectively (P = .007).
There were no differences in mortality or in median time between spirometry and clinician diagnosis between the groups.
Language processing details
Dr. Leon and colleagues used a language analysis software package to review CT chest reports. Reports were flagged if they contained the words traction, honeycomb, fibrotic, fibrosis, reticular, or reticulation.
The CT scan accompanying each flagged reported was reviewed by a pulmonologist for the presence of ILD, and scans with ILD identified were referred to pulmonary specialists. The results of 2,198 prospective scans followed by prospective screening were compared with those of 1,690 historical controls seen in 2015 and 2016.
The investigators found that 85 incident cases of ILD were identified in the historical controls, compared with 143 in the prospective cohort, leading to 38 and 120 pulmonary referrals, respectively.
For the primary outcome of median time from CT to pulmonary referral, the authors found that it was 1.27 months for the prospective cohort, compared with not reached (censored after 18 months) in historical controls.
The hazard ratio for a pulmonary referral in the prospective versus historical cohort was 2.79, an association that was strengthened after adjusting for sex, age, race, smoking pack-years, cough, crackles, and dyspnea (HR, 4.54; both comparisons significant according to confidence intervals).
The studies were internally funded. Dr. Topalovic is CEO and cofounder of ArtiQ. Dr. Leon and Dr. Molyneaux reported no relevant conflicts of interest.
SAN FRANCISCO – Interstitial lung disease is a difficult diagnosis to make, but a combination of artificial intelligence (AI) techniques and automated language processing could help clinicians identify the early signs of ILD and start patients on therapy, investigators say.
For example, applying an AI algorithm to spirometry readings taken from patients whose data were registered in the UK Biobank identified 27% as having ILD, and of this group, 66% had ostensibly normal lung function on spirometry but were later diagnosed with ILD, reported Marko Topalovic, PhD, from the AI company ArtiQ in Leuven, Belgium, at the American Thoracic Society’s international conference.
“A diagnosis of ILD is very challenging, so you have patients who are going to be misdiagnosed or have a very late diagnosis, so we aimed to apply our AI algorithm on spirometry to see whether we could detect ILD much earlier,” he said in an interview conducted during a poster discussion session.
AI detected ILD up to 6.8 years before a clinician’s diagnosis, Dr. Topalovic said.
Reading between the lines
In a separate study, investigators at the University of California, Davis, used language analysis software to scour electronic health records for words indicative of early ILD, and found that the technique dramatically shortened the median time to a pulmonary referral, compared with historical controls.
“This is a language processing program that can essentially look through the radiology reports and look for the key words that often describe interstitial lung disease, like traction, honeycomb, fibrotic, etc. With those studies being flagged, an actual pulmonologist will then further review the scan, and see whether it meets criteria for one of the interstitial lung diseases,” lead author William Leon, MD, a resident in the department of internal medicine at the University of California, Davis, said in an interview.
“We then sent the primary care doctor a message to say: ‘Hey, this patient has ILD. You need to send them to a pulmonologist,’ ” he added.
Putting it together
Philip L. Molyneaux, MRCP (UK), MBBS, BS (Hons), from Imperial College London, who comoderated the session but was not involved in the studies, speculated that combining these and other, nontechnical interventions also discussed could help to improve diagnosis of ILD and allow clinicians to prescribe therapy earlier in the disease course.
“What’s going to give you the biggest impact for patients? Everyone working individually is coming up with great advances, and if you put them all together it’s going to provide much greater benefit for our patients,” he said in an interview.
AI Spirometry details
In collaboration with colleagues at the Laboratory of Respiratory Disease at University Hospital in Leuven, Dr. Topalovic applied AI to results of spirometry performed prior to diagnosis of ILD among 109 patients registered in the UK Biobank, a repository of information on more than 500,000 volunteers.
The patients selected had ILD listed as their cause of death, had spirometry performed up to 7 years before their deaths, and did not receive a diagnosis of ILD on the day of the index spirometry.
In all 73% of patients were men, 27% women, with an average age of 64.6 years. A large majority of the sample (77.15%) had a history of smoking, and 60 of the patients (55%) died within one year of an ILD diagnosis.
The investigators plugged the spirometry data and each patients demographic information – including gender, age, height, weight, race, and smoking status – into the AI clinical decision support program, which yielded a statistical probability for each subject of having normal lung function, asthma, COPD, ILD, another obstructive disease, or another unidentifiable respiratory disease.
In 29 patients (27%) the software listed ILD as the highest probability, and of this group 19 patients (66%) had normal lung function according to standard interpretation guidelines.
Spirometry parameters among patients identified as having probable ILD were different from those where ILD was not detected. For example, forced vital capacity (FVC) was 76% of predicted among patients with likely ILD versus 87% of predicted in those who had a diagnosis later (P = .003). Similar differences were seen in the forced expiratory volume in 1 second to FVC ratio, at 0.82 vs. 0.75, respectively (P = .007).
There were no differences in mortality or in median time between spirometry and clinician diagnosis between the groups.
Language processing details
Dr. Leon and colleagues used a language analysis software package to review CT chest reports. Reports were flagged if they contained the words traction, honeycomb, fibrotic, fibrosis, reticular, or reticulation.
The CT scan accompanying each flagged reported was reviewed by a pulmonologist for the presence of ILD, and scans with ILD identified were referred to pulmonary specialists. The results of 2,198 prospective scans followed by prospective screening were compared with those of 1,690 historical controls seen in 2015 and 2016.
The investigators found that 85 incident cases of ILD were identified in the historical controls, compared with 143 in the prospective cohort, leading to 38 and 120 pulmonary referrals, respectively.
For the primary outcome of median time from CT to pulmonary referral, the authors found that it was 1.27 months for the prospective cohort, compared with not reached (censored after 18 months) in historical controls.
The hazard ratio for a pulmonary referral in the prospective versus historical cohort was 2.79, an association that was strengthened after adjusting for sex, age, race, smoking pack-years, cough, crackles, and dyspnea (HR, 4.54; both comparisons significant according to confidence intervals).
The studies were internally funded. Dr. Topalovic is CEO and cofounder of ArtiQ. Dr. Leon and Dr. Molyneaux reported no relevant conflicts of interest.
AT ATS 2022
COVID-19 burnout? Turn off your mind, relax, and float downstream
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Along with first responders, health care workers in pulmonary and critical care have borne the brunt of the COVID-19 pandemic, and it’s not surprising that a large proportion have suffered from burnout, a syndrome characterized by chronic workplace stress, emotional exhaustion, cynicism about the job, and a reduced sense of personal accomplishment.
“Prior to the pandemic, 50% of providers reported burnout, and that, of course, has been exacerbated, with recent surveys showing up to 80% of health care workers reporting burnout,” said Sangeeta Joshi, MD, of the division of pulmonary, allergy, and critical care medicine at Duke University in Durham, N.C.
In a randomized clinical trial, Dr. Joshi and colleagues showed that transcendental meditation (TM) can significantly improve burnout symptoms of emotional exhaustion, anxiety, and insomnia compared with other interventions, albeit without significant improvement in acute psychological distress.
Dr. Joshi reported the results of the trial at the American Thoracic Society’s international conference.
Mind-body intervention
TM, popularized in the 1960s by the Beatles and their guru, Maharishi Mahesh Yogi, is a nonpharmacologic mind-body intervention that has been shown to reduce sympathetic arousal and to promote a state of relaxation, Dr. Joshi said.
Although the mechanism of action is not fully understood, proposed explanations for its efficacy include increased alpha coherence, as seen on electroencephalography, and increases in blood flow to the prefrontal cortex, as visualized on functional MRI.
TM has been shown to be effective for reducing symptoms of posttraumatic stress disorder in veterans and for reducing stress and burnout symptoms in teachers, Dr. Joshi noted.
Randomized trial
To see whether TM could make a difference for health care providers, Dr. Joshi and colleagues screened candidates for burnout with the single-item Columbia–Suicide Severity Rating Scale and digital autonomic reactivity, a measure of the depth of physiologic stimulus.
Their study included 80 eligible participants, who were randomly assigned to receive either TM or treatment as usual.
The participants who received the intervention were assigned to attend four TM instruction sessions over 4 consecutive days, followed by four virtual follow-up sessions over the 3-month period. The investigators hypothesized that these participants would have significant improvements in symptoms of burnout over baseline compared with those assigned to standard treatments. Participants who underwent the intervention were encouraged to perform TM at home for 20 minutes twice each day.
Participants were evaluated at baseline and at 3-month follow-up with the Brief Symptom Inventory–18 (BSI), the Maslach Burnout Inventory (MBI), the Patient Health Questionnaire–9 (PHQ-9), the Generalized Anxiety Disorder–7, the Insomnia Severity Index (ISI), and the Connor Davidson Resilience Scale (CD-RISC)–25.
At baseline, 70% of all participants reported a history of visiting a psychiatrist or other mental health worker, and 91% reported onset of a mental health condition. Only 30% reported that they had had a mental health condition that resolved with treatment.
At 3 months, there were significant improvements over baseline in the TM group compared with the treatment-as-usual group for the MBI emotional exhaustion item (P = .005), insomnia (P = .029), and anxiety (P = .010). There was trend toward significance on the PHQ-9 (P = .057), but no significant difference in the Global Severity Index (the total score of BSI items).
There were improvements in both study arms in both the MBI professional accomplishment item and in the CD-RISC scale, but the between-group differences were not significant.
The results show that “TM is a feasible, efficacious intervention in health care workers, especially during a pandemic,” Dr. Joshi said.
Future studies of TM in this setting should expand the number of participants and recruitment sites so as to have the necessary power to detect statistically significant changes in the numerical scales, she said.
Integrating TM into employee wellness
“These results are really encouraging,” said Seppo Rinne, MD, PhD, assistant professor of medicine at Boston University, who comoderated the oral abstract session in which the data were presented but was not involved in the study.
Commenting on the fact that TM is not more widely offered as part of a package of services for treating employees with symptoms of burnout, he noted that “in the burnout literature, we have a tendency to dichotomize these individual vs. organizational interventions, and the reality is that they are probably more integrated, and it’s not really helpful for us to think about these as totally separate.
“We need organizational interventions that support individual wellness,” he said.
The trial was sponsored by Duke University. Dr. Joshi and Dr. Rinne reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ATS 2022
NAVIGATOR steers uncontrolled asthma toward calmer seas
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.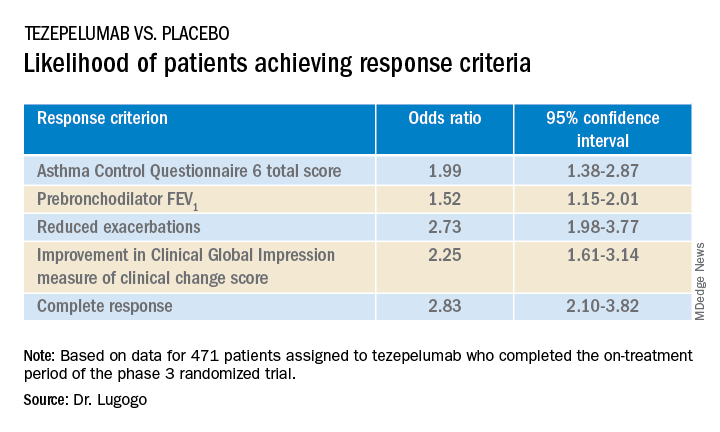
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
AT ATS 2022
Race-based spirometry may lead to missed diagnoses
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – It may be time to move beyond relying largely on spirometry to distinguish between healthy and abnormal lung function in diverse populations.
That conclusion comes from investigators who looked at patients with ostensibly normal spirometry values in a large population-based study and found that using standard equations to adjust for racial differences in lung-function measures appeared to miss emphysema in a significant proportion of Black patients.
“Our traditional measures of lung health based on spirometry may be under-recognizing impaired respiratory health in Black adults and particularly Black men,” said lead author Gabrielle Liu, MD, a fellow in the division of pulmonary and critical care medicine at the Northwestern University Feinberg School of Medicine, Chicago.
“CT imaging may be useful in the evaluation of those with suspected impaired respiratory health and normal spirometry,” she said in an oral abstract session at the American Thoracic Society International Conference 2022.
Dr. Liu and colleagues studied the association between self-identified race and visually identified emphysema among 2,674 participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study. The patients had CT scans at a mean age of 50 and spirometry at a mean age of 55.
Racial differences
The investigators found that among men with forced expiratory volume in 1 second (FEV1) ranging from 100% to 120% of predicted according to race-adjusted formulas, 14.6% of Black men had emphysema, compared with only 1.7% of White men (P < .001). Respective emphysema rates in Black women and White women were 3.8% and 1.9%; this difference was not statistically significant.
Among patients with FEV1 80% to 99% of predicted according to race-specific measures, 15.5% of Black men had emphysema, compared with 4% of White men (P < .001). Respective rates of emphysema were 6.9% for Black women versus 3.2% for White women (P = .025).
When the investigators applied race-neutral spirometry reference equations to the same population, they found that it attenuated but did not completely eliminate the racial disparity in emphysema prevalence among patients with FEV1, ranging from 80% to 120% of predicted.
Relic of the past
The results suggest that race-based adjustments of spirometry measures are a relic of less enlightened times, said Adam Gaffney, MD, MPH, assistant professor of medicine at Harvard Medical School, Boston, and a pulmonologist and critical care physician at Cambridge Health Alliance, Massachusetts.
“If the average lower lung function of Black people is being driven by adversity, structural racism, and deprivation, that means that race-specific equations are normalizing that adversity,” he said in an interview.
“In my opinion, it is time to move beyond race-based equations in clinical pulmonary medicine, particularly in the context of patients with established lung disease in whom use of race-based equations might actually lead to undertreatment,” said Dr. Gaffney, who was not involved in the study.
Dr. Liu agreed that it’s time to move to race-neutral measures and that the whole concept of race-based differences is flawed.
“The long-standing structural inequities in health likely made the reference populations have lower lung function than among Whites,” she told this news organization.
Dr. Liu said that evaluation of lung function should not rely on spirometry alone, but should also include – when appropriate – CT scans, as well as improved understanding of how symptoms may be predictive for poor outcomes.
The study was supported by grants from the National Institutes of Health. Dr. Liu and Dr. Gaffney have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ATS 2022


