User login
American College of Chest Physicians (ACCP): Annual International Scientific Assembly (CHEST 2014)
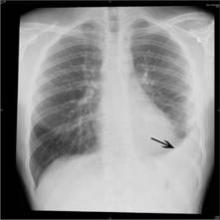
OSA confers survival advantage for ventilated pneumonia patients
AUSTIN, TEX. – Obstructive sleep apnea appeared to provide protection against in-hospital mortality and nonroutine discharge among mechanically ventilated patients with pneumonia who were included in the National Inpatient Sample from 2009 to 2011.
Patients included in the analysis were 20,652 adults with a mean age of 65 years who were hospitalized with a primary diagnosis of pneumonia requiring invasive mechanical ventilation, representing nearly 107,000 such discharges nationally. About 8% of the patients had obstructive sleep apnea (OSA), and 11% were obese. Overall mortality was 31%, and the overall rate of nonroutine discharge, defined as discharge to a skilled nursing facility or to home with home health care, was 84%, Dr. Charlisa Gibson reported at the annual meeting of the American College of Chest Physicians.
Though limited by its retrospective nature and possible underreporting of OSA, this study demonstrates that OSA in patients with pneumonia requiring invasive mechanical ventilation confers a survival benefit, she said.
Those with OSA had a significantly higher rate of tracheostomy (9.2% vs. 8.3%), a lower rate of in-hospital mortality (19% vs. 31%), and a lower rate of nonroutine discharge (77% vs. 84%), compared with non-OSA patients. Length of stay was about 14 days in both groups, said Dr. Gibson, of Mount Sinai St. Luke’s-Roosevelt Hospital, New York.
Those in the non-OSA group had higher rates of shock and septicemia.
After adjustment for age, sex, obesity, comorbidities, and disease severity, OSA was a significant predictor of decreased in-hospital mortality (odds ratio, 0.74) and nonroutine discharge (odds ratio, 0.73), Dr. Gibson said.
“In pretty much all of the conditions of interest we looked at, we consistently saw that mortality was lower in the OSA group, whether they were obese or not … and whether or not they were deemed to have a low, moderate, or severe [Charlson Comorbidity Index],” she said.
OSA is an important and likely underdiagnosed comorbidity in hospitalized patients, and pneumonia remains a significant infectious cause of morbidity and mortality in hospitalized patients, she said. OSA affects about 5%-24% of the general population, but the percentage of hospitalized patients with OSA is uncertain.
About 20% of hospitalized patients with pneumonia end up in the intensive care unit for supportive treatment with mechanical ventilation.
“Once they are vented, there are data to suggest that early tracheostomy may shorten time on mechanical ventilation and hospital length of stay, but whether or not there’s an actual impact on mortality is controversial,” she said.
While prospective randomized controlled studies are needed to better identify risk factors for mortality, Dr. Gibson said there are several possible explanations for the findings of a protective effect of OSA in hospitalized patients with acute respiratory failure due to pneumonia.
First, non-OSA patients had more septicemia and shock, which suggests they may have had multisystem organ failure and required treatments like renal replacement therapy that independently increased their risk of mortality.
Also, the increased incidence of tracheostomy in the OSA patients may indicate that clinicians were more aggressive in treating patients with OSA, and that those patients may have benefited from earlier tracheostomy, she said.
There is some evidence to suggest that OSA patients have additional coronary collateral circulation, which means that they may have less severe cardiac injury because of this adaptation, and thus may have a lower risk of experiencing a fatal heart attack, compared with non-OSA patients, she explained.
The “obesity paradox” might also work in OSA patients’ favor, she said. There is some evidence that obese patients have increased metabolic reserve that results in lower complication rates, compared with normal weight patients.
“However, we do recommend that regardless of what the reason is, when these patients do come to the unit we should be aggressive and treat them with invasive mechanical ventilation if needed,” she said.
Dr. Gibson reported having no disclosures.
AUSTIN, TEX. – Obstructive sleep apnea appeared to provide protection against in-hospital mortality and nonroutine discharge among mechanically ventilated patients with pneumonia who were included in the National Inpatient Sample from 2009 to 2011.
Patients included in the analysis were 20,652 adults with a mean age of 65 years who were hospitalized with a primary diagnosis of pneumonia requiring invasive mechanical ventilation, representing nearly 107,000 such discharges nationally. About 8% of the patients had obstructive sleep apnea (OSA), and 11% were obese. Overall mortality was 31%, and the overall rate of nonroutine discharge, defined as discharge to a skilled nursing facility or to home with home health care, was 84%, Dr. Charlisa Gibson reported at the annual meeting of the American College of Chest Physicians.
Though limited by its retrospective nature and possible underreporting of OSA, this study demonstrates that OSA in patients with pneumonia requiring invasive mechanical ventilation confers a survival benefit, she said.
Those with OSA had a significantly higher rate of tracheostomy (9.2% vs. 8.3%), a lower rate of in-hospital mortality (19% vs. 31%), and a lower rate of nonroutine discharge (77% vs. 84%), compared with non-OSA patients. Length of stay was about 14 days in both groups, said Dr. Gibson, of Mount Sinai St. Luke’s-Roosevelt Hospital, New York.
Those in the non-OSA group had higher rates of shock and septicemia.
After adjustment for age, sex, obesity, comorbidities, and disease severity, OSA was a significant predictor of decreased in-hospital mortality (odds ratio, 0.74) and nonroutine discharge (odds ratio, 0.73), Dr. Gibson said.
“In pretty much all of the conditions of interest we looked at, we consistently saw that mortality was lower in the OSA group, whether they were obese or not … and whether or not they were deemed to have a low, moderate, or severe [Charlson Comorbidity Index],” she said.
OSA is an important and likely underdiagnosed comorbidity in hospitalized patients, and pneumonia remains a significant infectious cause of morbidity and mortality in hospitalized patients, she said. OSA affects about 5%-24% of the general population, but the percentage of hospitalized patients with OSA is uncertain.
About 20% of hospitalized patients with pneumonia end up in the intensive care unit for supportive treatment with mechanical ventilation.
“Once they are vented, there are data to suggest that early tracheostomy may shorten time on mechanical ventilation and hospital length of stay, but whether or not there’s an actual impact on mortality is controversial,” she said.
While prospective randomized controlled studies are needed to better identify risk factors for mortality, Dr. Gibson said there are several possible explanations for the findings of a protective effect of OSA in hospitalized patients with acute respiratory failure due to pneumonia.
First, non-OSA patients had more septicemia and shock, which suggests they may have had multisystem organ failure and required treatments like renal replacement therapy that independently increased their risk of mortality.
Also, the increased incidence of tracheostomy in the OSA patients may indicate that clinicians were more aggressive in treating patients with OSA, and that those patients may have benefited from earlier tracheostomy, she said.
There is some evidence to suggest that OSA patients have additional coronary collateral circulation, which means that they may have less severe cardiac injury because of this adaptation, and thus may have a lower risk of experiencing a fatal heart attack, compared with non-OSA patients, she explained.
The “obesity paradox” might also work in OSA patients’ favor, she said. There is some evidence that obese patients have increased metabolic reserve that results in lower complication rates, compared with normal weight patients.
“However, we do recommend that regardless of what the reason is, when these patients do come to the unit we should be aggressive and treat them with invasive mechanical ventilation if needed,” she said.
Dr. Gibson reported having no disclosures.
AUSTIN, TEX. – Obstructive sleep apnea appeared to provide protection against in-hospital mortality and nonroutine discharge among mechanically ventilated patients with pneumonia who were included in the National Inpatient Sample from 2009 to 2011.
Patients included in the analysis were 20,652 adults with a mean age of 65 years who were hospitalized with a primary diagnosis of pneumonia requiring invasive mechanical ventilation, representing nearly 107,000 such discharges nationally. About 8% of the patients had obstructive sleep apnea (OSA), and 11% were obese. Overall mortality was 31%, and the overall rate of nonroutine discharge, defined as discharge to a skilled nursing facility or to home with home health care, was 84%, Dr. Charlisa Gibson reported at the annual meeting of the American College of Chest Physicians.
Though limited by its retrospective nature and possible underreporting of OSA, this study demonstrates that OSA in patients with pneumonia requiring invasive mechanical ventilation confers a survival benefit, she said.
Those with OSA had a significantly higher rate of tracheostomy (9.2% vs. 8.3%), a lower rate of in-hospital mortality (19% vs. 31%), and a lower rate of nonroutine discharge (77% vs. 84%), compared with non-OSA patients. Length of stay was about 14 days in both groups, said Dr. Gibson, of Mount Sinai St. Luke’s-Roosevelt Hospital, New York.
Those in the non-OSA group had higher rates of shock and septicemia.
After adjustment for age, sex, obesity, comorbidities, and disease severity, OSA was a significant predictor of decreased in-hospital mortality (odds ratio, 0.74) and nonroutine discharge (odds ratio, 0.73), Dr. Gibson said.
“In pretty much all of the conditions of interest we looked at, we consistently saw that mortality was lower in the OSA group, whether they were obese or not … and whether or not they were deemed to have a low, moderate, or severe [Charlson Comorbidity Index],” she said.
OSA is an important and likely underdiagnosed comorbidity in hospitalized patients, and pneumonia remains a significant infectious cause of morbidity and mortality in hospitalized patients, she said. OSA affects about 5%-24% of the general population, but the percentage of hospitalized patients with OSA is uncertain.
About 20% of hospitalized patients with pneumonia end up in the intensive care unit for supportive treatment with mechanical ventilation.
“Once they are vented, there are data to suggest that early tracheostomy may shorten time on mechanical ventilation and hospital length of stay, but whether or not there’s an actual impact on mortality is controversial,” she said.
While prospective randomized controlled studies are needed to better identify risk factors for mortality, Dr. Gibson said there are several possible explanations for the findings of a protective effect of OSA in hospitalized patients with acute respiratory failure due to pneumonia.
First, non-OSA patients had more septicemia and shock, which suggests they may have had multisystem organ failure and required treatments like renal replacement therapy that independently increased their risk of mortality.
Also, the increased incidence of tracheostomy in the OSA patients may indicate that clinicians were more aggressive in treating patients with OSA, and that those patients may have benefited from earlier tracheostomy, she said.
There is some evidence to suggest that OSA patients have additional coronary collateral circulation, which means that they may have less severe cardiac injury because of this adaptation, and thus may have a lower risk of experiencing a fatal heart attack, compared with non-OSA patients, she explained.
The “obesity paradox” might also work in OSA patients’ favor, she said. There is some evidence that obese patients have increased metabolic reserve that results in lower complication rates, compared with normal weight patients.
“However, we do recommend that regardless of what the reason is, when these patients do come to the unit we should be aggressive and treat them with invasive mechanical ventilation if needed,” she said.
Dr. Gibson reported having no disclosures.
Key clinical point: Pneumonia patients with OSA who require mechanical ventilation have a survival advantage, but should still be treated aggressively.
Major finding: OSA was a significant predictor of in-hospital mortality and nonroutine discharge (odds ratios, 0.74 and 0.73).
Data source: A retrospective analysis of data from 20,652 patients in the National Inpatient Sample.
Disclosures: Dr. Gibson reported having no disclosures.
LAMA/LABA provides little benefit over LAMA for early COPD
AUSTIN, TEX. – Adding a long-acting beta2-agonist to long-acting muscarinic antagonist therapy in patients with low- or moderate-complexity chronic obstructive pulmonary disease appeared to increase costs without providing significant benefit, according to findings from a retrospective cohort study.
The mean all-cause drug-related cost was $5,808 among 274 patients who received LAMA/LABA combination therapy between Jan. 1, 2009, and March 31, 2012, according to the MarketScan database, compared with $7,902 among 1,370 patients receiving LAMA monotherapy, Mona Khalid of Epstein Health, New York, reported at the annual meeting of the American College of Chest Physicians.
After adjustment for observed differences in baseline characteristics, such as disease complexity and comorbidities as assessed by Charlson comorbidity index score, the combination and monotherapy patients had similar all-cause health care resource utilization, with comparable rates of inpatient visits, office visits, and emergency department visits.
“Where we did see some difference was in COPD-related office visits. The patients on combination therapy were twice as likely to have an office visit,” Ms. Khalid said, noting that this difference between groups was statistically significant, and resulted in a mean of $92 more in costs in the combination therapy group.
The overall drug-related costs after adjustment were $1,003 higher in the combination therapy group, she said.
As for treatment adherence, 17% of patients in the combination therapy group had 80% or more days covered with therapy, compared with 25% of those in the monotherapy group. The medication possession ratio was 41% in the combination group, compared with 46% in the monotherapy group (adjusted difference about 5%).
The combination therapy patients included all patients receiving LAMA/LABA therapy identified in the database – which includes commercially insured patients and Medicare patients with supplemental coverage through an employer – during the study period. All were older than 40 years, had medical and pharmacy claims data available for at least 1 year before and after the index date, and had at least one COPD-related claim – but no claims for asthma, nonspecified bronchitis, respiratory cancer, cystic fibrosis, or LABA or LAMA use within the past year. LAMA patients were randomly selected using those same criteria and matched in a 5:1 ratio.
Most of the patients had low-complexity or moderate-complexity disease, although a few patients had severe disease, Ms. Khalid noted.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines call for the use of LAMA monotherapy early in the course of COPD among patients with low to moderate disease complexity. Some patients, however, initiate combination therapy during that phase of disease. The findings support the GOLD monotherapy recommendation in these patients, Ms. Khalid said, although they are limited by factors associated with the retrospective study design and an inability to control well for disease severity because of the use of “claims history as proxy.”
The study was conducted prior to approval of the single-inhaler LABA/LAMA combination therapy, she cautioned, so the results can’t necessarily be extended to that therapy. In addition, because of the use of the MarketScan database, the study findings can’t necessarily be extended to the uninsured population or the Medicaid population, Ms. Khalid noted.
The study was funded by Forest Laboratories. Ms. Khalid’s employer, Epstein Health, received consulting fees from Forest to conduct the study.
AUSTIN, TEX. – Adding a long-acting beta2-agonist to long-acting muscarinic antagonist therapy in patients with low- or moderate-complexity chronic obstructive pulmonary disease appeared to increase costs without providing significant benefit, according to findings from a retrospective cohort study.
The mean all-cause drug-related cost was $5,808 among 274 patients who received LAMA/LABA combination therapy between Jan. 1, 2009, and March 31, 2012, according to the MarketScan database, compared with $7,902 among 1,370 patients receiving LAMA monotherapy, Mona Khalid of Epstein Health, New York, reported at the annual meeting of the American College of Chest Physicians.
After adjustment for observed differences in baseline characteristics, such as disease complexity and comorbidities as assessed by Charlson comorbidity index score, the combination and monotherapy patients had similar all-cause health care resource utilization, with comparable rates of inpatient visits, office visits, and emergency department visits.
“Where we did see some difference was in COPD-related office visits. The patients on combination therapy were twice as likely to have an office visit,” Ms. Khalid said, noting that this difference between groups was statistically significant, and resulted in a mean of $92 more in costs in the combination therapy group.
The overall drug-related costs after adjustment were $1,003 higher in the combination therapy group, she said.
As for treatment adherence, 17% of patients in the combination therapy group had 80% or more days covered with therapy, compared with 25% of those in the monotherapy group. The medication possession ratio was 41% in the combination group, compared with 46% in the monotherapy group (adjusted difference about 5%).
The combination therapy patients included all patients receiving LAMA/LABA therapy identified in the database – which includes commercially insured patients and Medicare patients with supplemental coverage through an employer – during the study period. All were older than 40 years, had medical and pharmacy claims data available for at least 1 year before and after the index date, and had at least one COPD-related claim – but no claims for asthma, nonspecified bronchitis, respiratory cancer, cystic fibrosis, or LABA or LAMA use within the past year. LAMA patients were randomly selected using those same criteria and matched in a 5:1 ratio.
Most of the patients had low-complexity or moderate-complexity disease, although a few patients had severe disease, Ms. Khalid noted.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines call for the use of LAMA monotherapy early in the course of COPD among patients with low to moderate disease complexity. Some patients, however, initiate combination therapy during that phase of disease. The findings support the GOLD monotherapy recommendation in these patients, Ms. Khalid said, although they are limited by factors associated with the retrospective study design and an inability to control well for disease severity because of the use of “claims history as proxy.”
The study was conducted prior to approval of the single-inhaler LABA/LAMA combination therapy, she cautioned, so the results can’t necessarily be extended to that therapy. In addition, because of the use of the MarketScan database, the study findings can’t necessarily be extended to the uninsured population or the Medicaid population, Ms. Khalid noted.
The study was funded by Forest Laboratories. Ms. Khalid’s employer, Epstein Health, received consulting fees from Forest to conduct the study.
AUSTIN, TEX. – Adding a long-acting beta2-agonist to long-acting muscarinic antagonist therapy in patients with low- or moderate-complexity chronic obstructive pulmonary disease appeared to increase costs without providing significant benefit, according to findings from a retrospective cohort study.
The mean all-cause drug-related cost was $5,808 among 274 patients who received LAMA/LABA combination therapy between Jan. 1, 2009, and March 31, 2012, according to the MarketScan database, compared with $7,902 among 1,370 patients receiving LAMA monotherapy, Mona Khalid of Epstein Health, New York, reported at the annual meeting of the American College of Chest Physicians.
After adjustment for observed differences in baseline characteristics, such as disease complexity and comorbidities as assessed by Charlson comorbidity index score, the combination and monotherapy patients had similar all-cause health care resource utilization, with comparable rates of inpatient visits, office visits, and emergency department visits.
“Where we did see some difference was in COPD-related office visits. The patients on combination therapy were twice as likely to have an office visit,” Ms. Khalid said, noting that this difference between groups was statistically significant, and resulted in a mean of $92 more in costs in the combination therapy group.
The overall drug-related costs after adjustment were $1,003 higher in the combination therapy group, she said.
As for treatment adherence, 17% of patients in the combination therapy group had 80% or more days covered with therapy, compared with 25% of those in the monotherapy group. The medication possession ratio was 41% in the combination group, compared with 46% in the monotherapy group (adjusted difference about 5%).
The combination therapy patients included all patients receiving LAMA/LABA therapy identified in the database – which includes commercially insured patients and Medicare patients with supplemental coverage through an employer – during the study period. All were older than 40 years, had medical and pharmacy claims data available for at least 1 year before and after the index date, and had at least one COPD-related claim – but no claims for asthma, nonspecified bronchitis, respiratory cancer, cystic fibrosis, or LABA or LAMA use within the past year. LAMA patients were randomly selected using those same criteria and matched in a 5:1 ratio.
Most of the patients had low-complexity or moderate-complexity disease, although a few patients had severe disease, Ms. Khalid noted.
Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines call for the use of LAMA monotherapy early in the course of COPD among patients with low to moderate disease complexity. Some patients, however, initiate combination therapy during that phase of disease. The findings support the GOLD monotherapy recommendation in these patients, Ms. Khalid said, although they are limited by factors associated with the retrospective study design and an inability to control well for disease severity because of the use of “claims history as proxy.”
The study was conducted prior to approval of the single-inhaler LABA/LAMA combination therapy, she cautioned, so the results can’t necessarily be extended to that therapy. In addition, because of the use of the MarketScan database, the study findings can’t necessarily be extended to the uninsured population or the Medicaid population, Ms. Khalid noted.
The study was funded by Forest Laboratories. Ms. Khalid’s employer, Epstein Health, received consulting fees from Forest to conduct the study.
Key clinical point: LAMA monotherapy appears preferable to LABA/LAMA therapy for low- and moderate-complexity COPD.
Major finding: Overall drug-related costs after adjustment were $1,003 higher in the combination therapy group.
Data source: A retrospective cohort study of 1,644 patients.
Disclosures: The study was funded by Forest Laboratories. Ms. Khalid’s employer, Epstein Health, received consulting fees from Forest to conduct the study.
Quality improvement initiative improved catheter utilization
AUSTIN, TEX. – A quality improvement initiative reduced the number of days on a urinary catheter and improved catheter utilization among patients in a chronic ventilator-dependent unit.
During a preintervention period, 24 of 37 patients (65%) were catheterized for a total of 107 urinary catheter days (mean of 4.5 per patient); after the 2-month intervention that relied on a standardized electronic checklist and visual reminders, 18 of 35 patients (51%) were catheterized for a total of 58 urinary catheter days (mean of 3.2 per patient). Further, the device utilization ratio decreased from 0.299 before the intervention to 0.212 after, Dr. Perliveh Carrera reported at the annual meeting of the American College of Chest Physicians.
The catheter utilization rates on the unit were below the national average of 0.45, and the rates of catheter-associated urinary tract infections (CAUTI) were relatively low. The unit’s rates had increased in 2012 and 2013, however, prompting this effort to reduce infections by reducing utilization.
“CAUTI is the most common health care–associated infection, affecting up to 20% of patients,” said Dr. Carrera of the Mayo Clinic in Rochester, Minn.
About 80% of CAUTIs are precipitated by an indwelling catheter, and up to half of catheterized patients don’t have an indication for catheter placement, she said. Data suggest that 20%-50% of catheters are inappropriately placed, and catheter placement is an important modifiable risk factor for preventing UTI.
The quality initiative was implemented on a nine-bed chronic ventilator unit where adult patients had an average length of stay of about 2 weeks. The intervention involved the use of a Define, Measure, Analyze, Improve, and Control (DMAIC) framework and included a combination of multidisciplinary teamwork and tools for promoting adherence. These tools included educational presentations to staff, posters, reminder cards on patients’ doors, and promotion of an electronic checklist that required input of an appropriate indication for catheterization. A charge nurse was provided with a portable tablet to track use of the electronic checklist.
The approach was developed after an initial survey of nursing staff identified low utilization of a paper checklist and knowledge gaps about catheter utilization and infection control efforts, Dr. Carrera said.
The initiative was associated with a significant increase in electronic checklist compliance – from 33% to 79% – and with a trend toward a reduction in the number of urinary catheter days and catheter utilization, she said.
As a quality metric, catheter days and utilization are more stable than CAUTI, which has been recognized as labile and subject to wide variation, she added.
Dr. Carrera reported having no disclosures.
AUSTIN, TEX. – A quality improvement initiative reduced the number of days on a urinary catheter and improved catheter utilization among patients in a chronic ventilator-dependent unit.
During a preintervention period, 24 of 37 patients (65%) were catheterized for a total of 107 urinary catheter days (mean of 4.5 per patient); after the 2-month intervention that relied on a standardized electronic checklist and visual reminders, 18 of 35 patients (51%) were catheterized for a total of 58 urinary catheter days (mean of 3.2 per patient). Further, the device utilization ratio decreased from 0.299 before the intervention to 0.212 after, Dr. Perliveh Carrera reported at the annual meeting of the American College of Chest Physicians.
The catheter utilization rates on the unit were below the national average of 0.45, and the rates of catheter-associated urinary tract infections (CAUTI) were relatively low. The unit’s rates had increased in 2012 and 2013, however, prompting this effort to reduce infections by reducing utilization.
“CAUTI is the most common health care–associated infection, affecting up to 20% of patients,” said Dr. Carrera of the Mayo Clinic in Rochester, Minn.
About 80% of CAUTIs are precipitated by an indwelling catheter, and up to half of catheterized patients don’t have an indication for catheter placement, she said. Data suggest that 20%-50% of catheters are inappropriately placed, and catheter placement is an important modifiable risk factor for preventing UTI.
The quality initiative was implemented on a nine-bed chronic ventilator unit where adult patients had an average length of stay of about 2 weeks. The intervention involved the use of a Define, Measure, Analyze, Improve, and Control (DMAIC) framework and included a combination of multidisciplinary teamwork and tools for promoting adherence. These tools included educational presentations to staff, posters, reminder cards on patients’ doors, and promotion of an electronic checklist that required input of an appropriate indication for catheterization. A charge nurse was provided with a portable tablet to track use of the electronic checklist.
The approach was developed after an initial survey of nursing staff identified low utilization of a paper checklist and knowledge gaps about catheter utilization and infection control efforts, Dr. Carrera said.
The initiative was associated with a significant increase in electronic checklist compliance – from 33% to 79% – and with a trend toward a reduction in the number of urinary catheter days and catheter utilization, she said.
As a quality metric, catheter days and utilization are more stable than CAUTI, which has been recognized as labile and subject to wide variation, she added.
Dr. Carrera reported having no disclosures.
AUSTIN, TEX. – A quality improvement initiative reduced the number of days on a urinary catheter and improved catheter utilization among patients in a chronic ventilator-dependent unit.
During a preintervention period, 24 of 37 patients (65%) were catheterized for a total of 107 urinary catheter days (mean of 4.5 per patient); after the 2-month intervention that relied on a standardized electronic checklist and visual reminders, 18 of 35 patients (51%) were catheterized for a total of 58 urinary catheter days (mean of 3.2 per patient). Further, the device utilization ratio decreased from 0.299 before the intervention to 0.212 after, Dr. Perliveh Carrera reported at the annual meeting of the American College of Chest Physicians.
The catheter utilization rates on the unit were below the national average of 0.45, and the rates of catheter-associated urinary tract infections (CAUTI) were relatively low. The unit’s rates had increased in 2012 and 2013, however, prompting this effort to reduce infections by reducing utilization.
“CAUTI is the most common health care–associated infection, affecting up to 20% of patients,” said Dr. Carrera of the Mayo Clinic in Rochester, Minn.
About 80% of CAUTIs are precipitated by an indwelling catheter, and up to half of catheterized patients don’t have an indication for catheter placement, she said. Data suggest that 20%-50% of catheters are inappropriately placed, and catheter placement is an important modifiable risk factor for preventing UTI.
The quality initiative was implemented on a nine-bed chronic ventilator unit where adult patients had an average length of stay of about 2 weeks. The intervention involved the use of a Define, Measure, Analyze, Improve, and Control (DMAIC) framework and included a combination of multidisciplinary teamwork and tools for promoting adherence. These tools included educational presentations to staff, posters, reminder cards on patients’ doors, and promotion of an electronic checklist that required input of an appropriate indication for catheterization. A charge nurse was provided with a portable tablet to track use of the electronic checklist.
The approach was developed after an initial survey of nursing staff identified low utilization of a paper checklist and knowledge gaps about catheter utilization and infection control efforts, Dr. Carrera said.
The initiative was associated with a significant increase in electronic checklist compliance – from 33% to 79% – and with a trend toward a reduction in the number of urinary catheter days and catheter utilization, she said.
As a quality metric, catheter days and utilization are more stable than CAUTI, which has been recognized as labile and subject to wide variation, she added.
Dr. Carrera reported having no disclosures.
Key clinical point: With education and proper tools, improvements can be made in catheter utilization.
Major finding: The percentage of catheterized patients decreased from 65% to 51%, and the mean number of catheter days decreased from 4.5 to 3.2 per patient.
Data source: A comparison of pre- and postintervention outcomes among 37 and 34 patients, respectively.
Disclosures: Dr. Carrera reported having no disclosures.
Primary care screening tool reveals sleep disorders risk
AUSTIN, TEX. – An online, self-reported tool to determine risk of sleep disorders such as insomnia and restless legs syndrome outperformed assessment by a sleep specialist, new data show.
“Our results suggest that [the online survey] may be used in the primary care setting as a screening method for several sleep disorders,” Dr. Michael Morgenstern, a neurologist at the North Shore Long Island Jewish Medical Center, New Hyde Park, N.Y., said during an original investigation session at the annual meeting of the American College of Chest Physicians.
“The SNORE [Sleep Disorder and Narcolepsy Online Reference for Evaluation] online screening tool performs at least as well as the interviewer in detecting the risk of common sleep disorders, and at times it performs better,” Dr. Morgenstern said.
For obstructive sleep apnea and sleep disruption, the online screening tool and sleep physician interview were equal at assessing risk.
In a random, prospective, crossover trial of 53 adult sleep-clinic patients and 24 adult primary care–setting patients, two-thirds of whom were male, the SNORE self-report screening tool demonstrated both high sensitivity and high specificity for determining sleep disorder risks: between 94% and 85%, and 76% and 65%, respectively.
According to the American Academy of Sleep Medicine’s criteria for sleep disorder assessment, sleep specialists accurately determined the risk for insomnia 40.2% of the time, compared with SNORE’s 51.4%.
The online tool also bested a sleep specialist when it came to determining the risk of restless legs syndrome: The tool identified 44.1% of at-risk cases, while the specialist identified 23.3%.
Sleep specialists were slightly better than SNORE at determining patients at risk for sleep apnea: 78% for an interview with a specialist vs. 75.3% using the self-report tool.
Sleep disruption risk was determined equally by either method: 60% by a sleep specialist vs. 60% by SNORE.
Patients in the study were asked to complete the SNORE survey before they were interviewed by a sleep specialist. The sleep specialist was blinded to the patient’s history and survey results. Polysomnography was not included in the assessment, because risk assessment, not diagnosis, was the goal. A third of all participants were randomly assigned to take the SNORE test first or to be interviewed first.
The median overall time necessary to take the entire survey was 6 minutes. If a shortened version was used, the survey took an average of 4 minutes.
Using the survey in the primary care setting could lead to earlier detection and treatment of sleep disorders, Dr. Morgenstern noted, which would aid in overall health.
“For a lot of people who come to a primary care clinic, they don’t think to mention sleep-related symptoms, and the primary care doctor doesn’t really have time to ask about complaints the person isn’t there to talk about,” Dr. Morgenstern said. “But sleep is part of a person’s overall picture of health. Wouldn’t it be great if we had a way to identify issues so patients and providers could talk about it?”
Dr. Morgenstern said he had no relevant financial disclosures.
On Twitter @whitneymcknight
AUSTIN, TEX. – An online, self-reported tool to determine risk of sleep disorders such as insomnia and restless legs syndrome outperformed assessment by a sleep specialist, new data show.
“Our results suggest that [the online survey] may be used in the primary care setting as a screening method for several sleep disorders,” Dr. Michael Morgenstern, a neurologist at the North Shore Long Island Jewish Medical Center, New Hyde Park, N.Y., said during an original investigation session at the annual meeting of the American College of Chest Physicians.
“The SNORE [Sleep Disorder and Narcolepsy Online Reference for Evaluation] online screening tool performs at least as well as the interviewer in detecting the risk of common sleep disorders, and at times it performs better,” Dr. Morgenstern said.
For obstructive sleep apnea and sleep disruption, the online screening tool and sleep physician interview were equal at assessing risk.
In a random, prospective, crossover trial of 53 adult sleep-clinic patients and 24 adult primary care–setting patients, two-thirds of whom were male, the SNORE self-report screening tool demonstrated both high sensitivity and high specificity for determining sleep disorder risks: between 94% and 85%, and 76% and 65%, respectively.
According to the American Academy of Sleep Medicine’s criteria for sleep disorder assessment, sleep specialists accurately determined the risk for insomnia 40.2% of the time, compared with SNORE’s 51.4%.
The online tool also bested a sleep specialist when it came to determining the risk of restless legs syndrome: The tool identified 44.1% of at-risk cases, while the specialist identified 23.3%.
Sleep specialists were slightly better than SNORE at determining patients at risk for sleep apnea: 78% for an interview with a specialist vs. 75.3% using the self-report tool.
Sleep disruption risk was determined equally by either method: 60% by a sleep specialist vs. 60% by SNORE.
Patients in the study were asked to complete the SNORE survey before they were interviewed by a sleep specialist. The sleep specialist was blinded to the patient’s history and survey results. Polysomnography was not included in the assessment, because risk assessment, not diagnosis, was the goal. A third of all participants were randomly assigned to take the SNORE test first or to be interviewed first.
The median overall time necessary to take the entire survey was 6 minutes. If a shortened version was used, the survey took an average of 4 minutes.
Using the survey in the primary care setting could lead to earlier detection and treatment of sleep disorders, Dr. Morgenstern noted, which would aid in overall health.
“For a lot of people who come to a primary care clinic, they don’t think to mention sleep-related symptoms, and the primary care doctor doesn’t really have time to ask about complaints the person isn’t there to talk about,” Dr. Morgenstern said. “But sleep is part of a person’s overall picture of health. Wouldn’t it be great if we had a way to identify issues so patients and providers could talk about it?”
Dr. Morgenstern said he had no relevant financial disclosures.
On Twitter @whitneymcknight
AUSTIN, TEX. – An online, self-reported tool to determine risk of sleep disorders such as insomnia and restless legs syndrome outperformed assessment by a sleep specialist, new data show.
“Our results suggest that [the online survey] may be used in the primary care setting as a screening method for several sleep disorders,” Dr. Michael Morgenstern, a neurologist at the North Shore Long Island Jewish Medical Center, New Hyde Park, N.Y., said during an original investigation session at the annual meeting of the American College of Chest Physicians.
“The SNORE [Sleep Disorder and Narcolepsy Online Reference for Evaluation] online screening tool performs at least as well as the interviewer in detecting the risk of common sleep disorders, and at times it performs better,” Dr. Morgenstern said.
For obstructive sleep apnea and sleep disruption, the online screening tool and sleep physician interview were equal at assessing risk.
In a random, prospective, crossover trial of 53 adult sleep-clinic patients and 24 adult primary care–setting patients, two-thirds of whom were male, the SNORE self-report screening tool demonstrated both high sensitivity and high specificity for determining sleep disorder risks: between 94% and 85%, and 76% and 65%, respectively.
According to the American Academy of Sleep Medicine’s criteria for sleep disorder assessment, sleep specialists accurately determined the risk for insomnia 40.2% of the time, compared with SNORE’s 51.4%.
The online tool also bested a sleep specialist when it came to determining the risk of restless legs syndrome: The tool identified 44.1% of at-risk cases, while the specialist identified 23.3%.
Sleep specialists were slightly better than SNORE at determining patients at risk for sleep apnea: 78% for an interview with a specialist vs. 75.3% using the self-report tool.
Sleep disruption risk was determined equally by either method: 60% by a sleep specialist vs. 60% by SNORE.
Patients in the study were asked to complete the SNORE survey before they were interviewed by a sleep specialist. The sleep specialist was blinded to the patient’s history and survey results. Polysomnography was not included in the assessment, because risk assessment, not diagnosis, was the goal. A third of all participants were randomly assigned to take the SNORE test first or to be interviewed first.
The median overall time necessary to take the entire survey was 6 minutes. If a shortened version was used, the survey took an average of 4 minutes.
Using the survey in the primary care setting could lead to earlier detection and treatment of sleep disorders, Dr. Morgenstern noted, which would aid in overall health.
“For a lot of people who come to a primary care clinic, they don’t think to mention sleep-related symptoms, and the primary care doctor doesn’t really have time to ask about complaints the person isn’t there to talk about,” Dr. Morgenstern said. “But sleep is part of a person’s overall picture of health. Wouldn’t it be great if we had a way to identify issues so patients and providers could talk about it?”
Dr. Morgenstern said he had no relevant financial disclosures.
On Twitter @whitneymcknight
AT CHEST 2014
Key clinical point: A self-reported online screening tool may effectively assess a patient’s risk of sleep disorders.
Major finding: The SNORE screening survey performed significantly better than a sleep specialist at identifying risk of insomnia and restless legs syndrome.
Data source: A random, prospective, crossover trial of 53 sleep-clinic patients and 24 primary care–setting patients given the survey and a sleep specialist interview.
Disclosures: Dr. Morgenstern said he had no relevant financial disclosures.
CPAP compliance compatible with good sex
AUSTIN, TEX. – Patients who consider themselves too sexy for their continuous positive airway pressure devices should reconsider, according to a presenter at the annual meeting of the American College of Chest Physicians.
“Despite the unsexy appearance of a positive airway pressure device in the bedroom, patients who don’t comply with their CPAP [protocols] do not have a better sexual quality of life,” said Dr. Salman Alim, who presented the original investigation during a quality and clinical improvement session.
Sexual quality of life questionnaires were distributed to 52 men being treated at a single site with continuous positive airway pressure (CPAP) for obstructive sleep apnea. The 10-question survey used a scale of 1-8, with 80 being the highest score, to evaluate aspects of the participants’ emotional and physical satisfaction with their sex lives. Patients were considered CPAP-compliant if they used their device 4 or more hours nightly at least 70% of the time before going to sleep.
The compliant cohort of 27 men, whose average age was 59 years, had a sexual quality of life score of about 38. The noncompliant group of 25 men, whose average age was 56 years, had a score of about 48.
After adjusting for confounding variables such as age, body mass index, erectile dysfunction, use of phosphodiesterase inhibitors, and depression, CPAP compliance did not predict one’s sexual quality of life, reported Dr. Alim, who was with Rosalind Franklin University in North Chicago, Ill., at the time of the study and is now with Physicians Regional Healthcare System in Naples, Fla.
“Although this is not a validated survey … the study’s findings can be the basis to develop a hypothesis that can be tested more rigorously. At the least, the results provide clinicians with useful information on counseling patients on adherence with CPAP,” Dr. Mark Rosen, medical director of the American College of Chest Physicians, said in an interview.
The authors of the study said that they had no relevant disclosures.
On Twitter @whitneymcknight
Dr. Octavian C. Ioachimescu, FCCP, comments: Adherence to CPAP therapy for obstructive sleep apnea (OSA) remains a problem in day-to-day practice. Whenever a patient, often but not always a young patient, brings up the issue of CPAP not being 'sexy' in the bedroom, I ask a simple question: "How sexy do you think it is to be snoring, snorting, gasping, choking, or drooling?" That realization seems to work, at least for a while.
If validated, a sexual quality of life questionnaire may be a good instrument to assess this domain of OSA symptoms. If findings of better CPAP adherence being correlated with better sexual quality of life are reproduced in a rigorous trial, then we would welcome another tool in our armamentarium to motivate, engage and empower our patients to participate in the act of therapy. Why not 6 hours and 80% of the nights? Or 8 hours and 100% of the nights?
Dr. Ioachimescu is an associate professor of pulmonary medicine at the Emory University Atlanta VA Medical Center in Decatur, Georgia.
Dr. Octavian C. Ioachimescu, FCCP, comments: Adherence to CPAP therapy for obstructive sleep apnea (OSA) remains a problem in day-to-day practice. Whenever a patient, often but not always a young patient, brings up the issue of CPAP not being 'sexy' in the bedroom, I ask a simple question: "How sexy do you think it is to be snoring, snorting, gasping, choking, or drooling?" That realization seems to work, at least for a while.
If validated, a sexual quality of life questionnaire may be a good instrument to assess this domain of OSA symptoms. If findings of better CPAP adherence being correlated with better sexual quality of life are reproduced in a rigorous trial, then we would welcome another tool in our armamentarium to motivate, engage and empower our patients to participate in the act of therapy. Why not 6 hours and 80% of the nights? Or 8 hours and 100% of the nights?
Dr. Ioachimescu is an associate professor of pulmonary medicine at the Emory University Atlanta VA Medical Center in Decatur, Georgia.
Dr. Octavian C. Ioachimescu, FCCP, comments: Adherence to CPAP therapy for obstructive sleep apnea (OSA) remains a problem in day-to-day practice. Whenever a patient, often but not always a young patient, brings up the issue of CPAP not being 'sexy' in the bedroom, I ask a simple question: "How sexy do you think it is to be snoring, snorting, gasping, choking, or drooling?" That realization seems to work, at least for a while.
If validated, a sexual quality of life questionnaire may be a good instrument to assess this domain of OSA symptoms. If findings of better CPAP adherence being correlated with better sexual quality of life are reproduced in a rigorous trial, then we would welcome another tool in our armamentarium to motivate, engage and empower our patients to participate in the act of therapy. Why not 6 hours and 80% of the nights? Or 8 hours and 100% of the nights?
Dr. Ioachimescu is an associate professor of pulmonary medicine at the Emory University Atlanta VA Medical Center in Decatur, Georgia.
AUSTIN, TEX. – Patients who consider themselves too sexy for their continuous positive airway pressure devices should reconsider, according to a presenter at the annual meeting of the American College of Chest Physicians.
“Despite the unsexy appearance of a positive airway pressure device in the bedroom, patients who don’t comply with their CPAP [protocols] do not have a better sexual quality of life,” said Dr. Salman Alim, who presented the original investigation during a quality and clinical improvement session.
Sexual quality of life questionnaires were distributed to 52 men being treated at a single site with continuous positive airway pressure (CPAP) for obstructive sleep apnea. The 10-question survey used a scale of 1-8, with 80 being the highest score, to evaluate aspects of the participants’ emotional and physical satisfaction with their sex lives. Patients were considered CPAP-compliant if they used their device 4 or more hours nightly at least 70% of the time before going to sleep.
The compliant cohort of 27 men, whose average age was 59 years, had a sexual quality of life score of about 38. The noncompliant group of 25 men, whose average age was 56 years, had a score of about 48.
After adjusting for confounding variables such as age, body mass index, erectile dysfunction, use of phosphodiesterase inhibitors, and depression, CPAP compliance did not predict one’s sexual quality of life, reported Dr. Alim, who was with Rosalind Franklin University in North Chicago, Ill., at the time of the study and is now with Physicians Regional Healthcare System in Naples, Fla.
“Although this is not a validated survey … the study’s findings can be the basis to develop a hypothesis that can be tested more rigorously. At the least, the results provide clinicians with useful information on counseling patients on adherence with CPAP,” Dr. Mark Rosen, medical director of the American College of Chest Physicians, said in an interview.
The authors of the study said that they had no relevant disclosures.
On Twitter @whitneymcknight
AUSTIN, TEX. – Patients who consider themselves too sexy for their continuous positive airway pressure devices should reconsider, according to a presenter at the annual meeting of the American College of Chest Physicians.
“Despite the unsexy appearance of a positive airway pressure device in the bedroom, patients who don’t comply with their CPAP [protocols] do not have a better sexual quality of life,” said Dr. Salman Alim, who presented the original investigation during a quality and clinical improvement session.
Sexual quality of life questionnaires were distributed to 52 men being treated at a single site with continuous positive airway pressure (CPAP) for obstructive sleep apnea. The 10-question survey used a scale of 1-8, with 80 being the highest score, to evaluate aspects of the participants’ emotional and physical satisfaction with their sex lives. Patients were considered CPAP-compliant if they used their device 4 or more hours nightly at least 70% of the time before going to sleep.
The compliant cohort of 27 men, whose average age was 59 years, had a sexual quality of life score of about 38. The noncompliant group of 25 men, whose average age was 56 years, had a score of about 48.
After adjusting for confounding variables such as age, body mass index, erectile dysfunction, use of phosphodiesterase inhibitors, and depression, CPAP compliance did not predict one’s sexual quality of life, reported Dr. Alim, who was with Rosalind Franklin University in North Chicago, Ill., at the time of the study and is now with Physicians Regional Healthcare System in Naples, Fla.
“Although this is not a validated survey … the study’s findings can be the basis to develop a hypothesis that can be tested more rigorously. At the least, the results provide clinicians with useful information on counseling patients on adherence with CPAP,” Dr. Mark Rosen, medical director of the American College of Chest Physicians, said in an interview.
The authors of the study said that they had no relevant disclosures.
On Twitter @whitneymcknight
AT CHEST 2014
Key clinical point: Counsel CPAP patients that they can still enjoy sexual intimacy while remaining compliant with sleep apnea treatment.
Major finding: Sexual quality of life scores on a scale up to 80 were 38 in compliant and 48 in noncompliant CPAP patients.
Data source: Survey of 52 obstructive sleep apnea patients treated with CPAP.
Disclosures: The authors of this study said that they had no relevant disclosures.
Comborbidities likely explain opioid + sleep apnea mortality risk
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
AUSTIN, TEX. – Any association between opioid use and death in patients with sleep apnea cannot be explained by sleep apnea alone, according to a retrospective analysis of data from the prospective observational DREAM study.
In 1,867 patients with moderate or severe sleep apnea who were on an opioid medication, no association was seen between opioid use and severity of sleep-disordered breathing, even with increasing doses, Dr. Husham Sharifi reported at the annual meeting of the American College of Chest Physicians.
Further, when opioid use was analyzed as an unadjusted variable, it was associated with an increase in mortality (odds ratio, 1.53), but this effect was attenuated by adjustment for sleep apnea (OR, 1.52), and was further attenuated – to the point where it was no longer statistically significant – by adjustment for both sleep apnea and Charlson Comorbidity Index (OR, 1.37), said Dr. Sharifi, who was an attending physician at Brigham and Women’s Hospital, Boston, at the time he completed this research. He is now a fellow at Stanford (Calif.) University.
Sleep apnea remained an independent predictor of mortality even after adjustment for opioid use and Charlson Comorbidity Index, he said.
The DREAM study, which stands for Determining Risk of Vascular Events by Apnea Monitoring, includes a well-defined cohort of patients at three Veterans Administration sleep centers who were referred for overnight polysomnography for suspected sleep-disordered breathing between Jan. 1, 2000, and Dec. 31, 2004. All patients had an Apnea-Hypopnea Index score greater than 15, indicating moderate to severe sleep apnea, and all were on opioid medication. The patients were followed for between 3 and 10 years, with follow-up ending Dec. 31, 2010.
Opioid use has increased dramatically over the past 20 years – by more than 700%, Dr. Sharifi said.
While there does not appear to be a significant impact of opioid use on daytime respiration, there are some limited data suggesting that it may be associated with sleep-disordered breathing, he said.
The current findings, though limited by a number of factors including the observational nature of the study and possible selection bias as the DREAM cohort is a referral population, suggest that the relationship between opioid use and sleep apnea death is likely explained not by sleep apnea, but by an increased prevalence of known risk factors for morbidity and mortality in patients who take opioids and have sleep apnea, he concluded.
Dr. Sharifi reported having no disclosures.
Key clinical point: Comorbid conditions may explain the relationship between opioid use, sleep apnea, and death.
Major finding: Opioid use was not significantly associated with mortality after adjustment for sleep apnea and comorbidities (odds ratio, 1.37).
Data source: A retrospective analysis of data for 1,867 patients from the prospective observational DREAM cohort.
Disclosures: Dr. Sharifi reported having no disclosures.
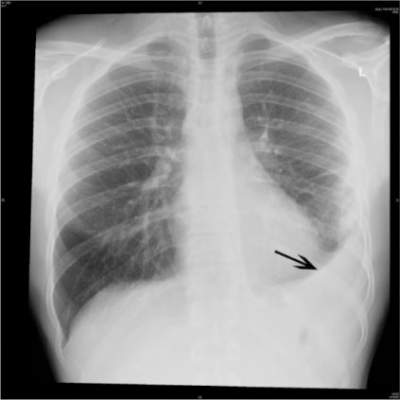
Pneumonia-related readmissions plummeted under QI initiative
AUSTIN, TEX. – A multidisciplinary intervention, including implementation of a diagnostic scoring system and daily interdepartmental meetings to review cases, significantly decreased readmission rates for patients at a tertiary care center who were discharged with a diagnosis of pneumonia.
From November 2012 to January 2013 – a 3-month period after implementation of the quality improvement (QI) initiative – the all-cause readmission rates among 227 patients discharged with a diagnosis of pneumonia declined by 7.5 percentage points, compared with the all-cause readmission rates among 236 patients discharged during the same period in the prior year, before implementation of the initiative (from 20.7% to 13.2%), Dr. Hussein Hussein, a fellow at the University of Oklahoma Health Sciences Center, Oklahoma City, reported at the annual meeting of the American College of Chest Physicians.
A similar reduction was seen for pneumonia-related readmissions, which declined from 10.5% to 3% during the same period, said Dr. Hussein, who was with Yale New Haven Hospital, New Haven, Conn., at the time the research was conducted.
Further, after implementation of the scoring system – a modified Clinical Pulmonary Infection Score (MCPIS) calculated based on patient temperature, white blood cell count, sputum cultures, oxygen requirements, and radiographic appearance, which was administered at admission and again at 32 hours – the accuracy of pneumonia diagnoses appeared to improve; the mean MCPIS scores among patients with a discharge diagnosis of pneumonia increased significantly after implementation (from 4 to 6); the proportion of patients considered unlikely to have pneumonia decreased from 42.6% to 3.6%; the proportion considered to probably have pneumonia decreased from 31.9% to 17.9%; and the number deemed likely to have pneumonia increased from 25.5% to 78.6%.
All of the changes were statistically significant, and the improved accuracy of diagnosis was likely a result of provider education that led to increased use of sputum cultures, Dr. Hussein noted.
Pneumonia is the second most common discharge diagnosis among Medicare beneficiaries, and nearly 20% of these patients are readmitted within 30 days at a cost exceeding $17 billion annually.
“This is why the Centers for Medicare & Medicaid Services is penalizing poor-performing hospitals with high rates of readmission,” he said.
The MCPIS was implemented in 2012 as a quality improvement tool. Based on the scores, patients were categorized as unlikely to have pneumonia (score of 3 or less), probably having pneumonia (score of 4-5), and likely to have pneumonia (score of 6 or greater). The daily meetings during which patients admitted with pneumonia were reviewed, involved participation of physicians from different medical divisions and representatives from nursing, social work, and continuing care.
The primary goal of these rounds was to ensure timely follow-up after discharge, Dr. Hussein explained, adding that if the diagnosis was felt to be incorrect, the case was discussed with the team that was caring for the patient.
To assess the effects of this intervention, he and his colleagues conducted a retrospective chart review of all patients discharged with a diagnosis of pneumonia during each of the two assessment periods.
“Our multidisciplinary intervention resulted in a significant decrease in readmission rates in patients discharged with a diagnosis of pneumonia, as well as improvement in the accuracy of diagnosis,” he said.
Dr. Hussein reported having no disclosures.
AUSTIN, TEX. – A multidisciplinary intervention, including implementation of a diagnostic scoring system and daily interdepartmental meetings to review cases, significantly decreased readmission rates for patients at a tertiary care center who were discharged with a diagnosis of pneumonia.
From November 2012 to January 2013 – a 3-month period after implementation of the quality improvement (QI) initiative – the all-cause readmission rates among 227 patients discharged with a diagnosis of pneumonia declined by 7.5 percentage points, compared with the all-cause readmission rates among 236 patients discharged during the same period in the prior year, before implementation of the initiative (from 20.7% to 13.2%), Dr. Hussein Hussein, a fellow at the University of Oklahoma Health Sciences Center, Oklahoma City, reported at the annual meeting of the American College of Chest Physicians.
A similar reduction was seen for pneumonia-related readmissions, which declined from 10.5% to 3% during the same period, said Dr. Hussein, who was with Yale New Haven Hospital, New Haven, Conn., at the time the research was conducted.
Further, after implementation of the scoring system – a modified Clinical Pulmonary Infection Score (MCPIS) calculated based on patient temperature, white blood cell count, sputum cultures, oxygen requirements, and radiographic appearance, which was administered at admission and again at 32 hours – the accuracy of pneumonia diagnoses appeared to improve; the mean MCPIS scores among patients with a discharge diagnosis of pneumonia increased significantly after implementation (from 4 to 6); the proportion of patients considered unlikely to have pneumonia decreased from 42.6% to 3.6%; the proportion considered to probably have pneumonia decreased from 31.9% to 17.9%; and the number deemed likely to have pneumonia increased from 25.5% to 78.6%.
All of the changes were statistically significant, and the improved accuracy of diagnosis was likely a result of provider education that led to increased use of sputum cultures, Dr. Hussein noted.
Pneumonia is the second most common discharge diagnosis among Medicare beneficiaries, and nearly 20% of these patients are readmitted within 30 days at a cost exceeding $17 billion annually.
“This is why the Centers for Medicare & Medicaid Services is penalizing poor-performing hospitals with high rates of readmission,” he said.
The MCPIS was implemented in 2012 as a quality improvement tool. Based on the scores, patients were categorized as unlikely to have pneumonia (score of 3 or less), probably having pneumonia (score of 4-5), and likely to have pneumonia (score of 6 or greater). The daily meetings during which patients admitted with pneumonia were reviewed, involved participation of physicians from different medical divisions and representatives from nursing, social work, and continuing care.
The primary goal of these rounds was to ensure timely follow-up after discharge, Dr. Hussein explained, adding that if the diagnosis was felt to be incorrect, the case was discussed with the team that was caring for the patient.
To assess the effects of this intervention, he and his colleagues conducted a retrospective chart review of all patients discharged with a diagnosis of pneumonia during each of the two assessment periods.
“Our multidisciplinary intervention resulted in a significant decrease in readmission rates in patients discharged with a diagnosis of pneumonia, as well as improvement in the accuracy of diagnosis,” he said.
Dr. Hussein reported having no disclosures.
AUSTIN, TEX. – A multidisciplinary intervention, including implementation of a diagnostic scoring system and daily interdepartmental meetings to review cases, significantly decreased readmission rates for patients at a tertiary care center who were discharged with a diagnosis of pneumonia.
From November 2012 to January 2013 – a 3-month period after implementation of the quality improvement (QI) initiative – the all-cause readmission rates among 227 patients discharged with a diagnosis of pneumonia declined by 7.5 percentage points, compared with the all-cause readmission rates among 236 patients discharged during the same period in the prior year, before implementation of the initiative (from 20.7% to 13.2%), Dr. Hussein Hussein, a fellow at the University of Oklahoma Health Sciences Center, Oklahoma City, reported at the annual meeting of the American College of Chest Physicians.
A similar reduction was seen for pneumonia-related readmissions, which declined from 10.5% to 3% during the same period, said Dr. Hussein, who was with Yale New Haven Hospital, New Haven, Conn., at the time the research was conducted.
Further, after implementation of the scoring system – a modified Clinical Pulmonary Infection Score (MCPIS) calculated based on patient temperature, white blood cell count, sputum cultures, oxygen requirements, and radiographic appearance, which was administered at admission and again at 32 hours – the accuracy of pneumonia diagnoses appeared to improve; the mean MCPIS scores among patients with a discharge diagnosis of pneumonia increased significantly after implementation (from 4 to 6); the proportion of patients considered unlikely to have pneumonia decreased from 42.6% to 3.6%; the proportion considered to probably have pneumonia decreased from 31.9% to 17.9%; and the number deemed likely to have pneumonia increased from 25.5% to 78.6%.
All of the changes were statistically significant, and the improved accuracy of diagnosis was likely a result of provider education that led to increased use of sputum cultures, Dr. Hussein noted.
Pneumonia is the second most common discharge diagnosis among Medicare beneficiaries, and nearly 20% of these patients are readmitted within 30 days at a cost exceeding $17 billion annually.
“This is why the Centers for Medicare & Medicaid Services is penalizing poor-performing hospitals with high rates of readmission,” he said.
The MCPIS was implemented in 2012 as a quality improvement tool. Based on the scores, patients were categorized as unlikely to have pneumonia (score of 3 or less), probably having pneumonia (score of 4-5), and likely to have pneumonia (score of 6 or greater). The daily meetings during which patients admitted with pneumonia were reviewed, involved participation of physicians from different medical divisions and representatives from nursing, social work, and continuing care.
The primary goal of these rounds was to ensure timely follow-up after discharge, Dr. Hussein explained, adding that if the diagnosis was felt to be incorrect, the case was discussed with the team that was caring for the patient.
To assess the effects of this intervention, he and his colleagues conducted a retrospective chart review of all patients discharged with a diagnosis of pneumonia during each of the two assessment periods.
“Our multidisciplinary intervention resulted in a significant decrease in readmission rates in patients discharged with a diagnosis of pneumonia, as well as improvement in the accuracy of diagnosis,” he said.
Dr. Hussein reported having no disclosures.
Key clinical point: A scoring system and multidisciplinary effort improved pneumonia patient readmission rates and diagnostic accuracy.
Major finding: All-cause and pneumonia-related readmission rates declined from 20.7% to 13.2%, and from 10.5% to 3%, respectively.
Data source: A retrospective review of the charts of 463 patients.
Disclosures: Dr. Hussein reported having no disclosures.
VIDEO: Should physicians reevaluate the role of clopidogrel?
AUSTIN, TEX.– Genetic testing holds promise for guiding the prescribing of antiplatelet therapies, particularly clopidogrel, but “we’re not there yet,” according to Dr. Steven Hollenberg, director of the coronary care unit at Cooper University Hospital in Camden, N.J.Genetic testing for clopidogrel responsiveness “certainly makes good sense, but I think we’re going to have to wait for good data” that better informs clinical decision making.Dr. Hollenberg discussed the implications of the negative results of the ARCTIC trial, which showed platelet function testing with antiplatelet therapy adjustment failed to improve clinical outcomes compared with standard unmonitored thienopyridine therapy in elective PCI. He also analyzed the results of other studies relevant to optimal antiplatelet and anticoagulant therapy, including the surprising outcomes of the WOEST trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AUSTIN, TEX.– Genetic testing holds promise for guiding the prescribing of antiplatelet therapies, particularly clopidogrel, but “we’re not there yet,” according to Dr. Steven Hollenberg, director of the coronary care unit at Cooper University Hospital in Camden, N.J.Genetic testing for clopidogrel responsiveness “certainly makes good sense, but I think we’re going to have to wait for good data” that better informs clinical decision making.Dr. Hollenberg discussed the implications of the negative results of the ARCTIC trial, which showed platelet function testing with antiplatelet therapy adjustment failed to improve clinical outcomes compared with standard unmonitored thienopyridine therapy in elective PCI. He also analyzed the results of other studies relevant to optimal antiplatelet and anticoagulant therapy, including the surprising outcomes of the WOEST trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AUSTIN, TEX.– Genetic testing holds promise for guiding the prescribing of antiplatelet therapies, particularly clopidogrel, but “we’re not there yet,” according to Dr. Steven Hollenberg, director of the coronary care unit at Cooper University Hospital in Camden, N.J.Genetic testing for clopidogrel responsiveness “certainly makes good sense, but I think we’re going to have to wait for good data” that better informs clinical decision making.Dr. Hollenberg discussed the implications of the negative results of the ARCTIC trial, which showed platelet function testing with antiplatelet therapy adjustment failed to improve clinical outcomes compared with standard unmonitored thienopyridine therapy in elective PCI. He also analyzed the results of other studies relevant to optimal antiplatelet and anticoagulant therapy, including the surprising outcomes of the WOEST trial.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @whitneymcknight
AT CHEST 2014
LAMA/LABA combo tops fluticasone/salmeterol in moderate/severe COPD
AUSTIN, TEX.– Once daily combination treatment with umeclidinium and vilanterol was more effective than twice-daily combination treatment with fluticasone and salmeterol in patients with moderate to severe chronic obstructive pulmonary disease in two 12-week double-blind, parallel-group double-dummy studies.
In the two multicenter studies, 706 and 697 patients, respectively, were randomized to receive either 62.5 mcg of the long-acting muscarinic antagonist (LAMA) umeclidinium and 25 mcg of the long-acting beta-2 agonist (LABA) vilanterol – a recently approved combination bronchodilator maintenance treatment for COPD – or a combination of 250 mcg of the inhaled corticosteroid (ICS) fluticasone and 50 mcg of the LABA salmeterol, which is also indicated as a maintenance therapy for COPD.
The patients in the LAMA/LABA groups, who were treated once daily for 12 weeks, had significantly greater improvements on all lung function measures, compared with those in the ICS/LABA groups, who were treated twice daily, Dr. James F. Donohue of the University of North Carolina at Chapel Hill reported at the annual meeting of the American College of Chest Physicians.
In the first study, the improvement from baseline to day 84 (the primary study endpoint) in 0- to 24-hour weighted mean forced expiratory volume in 1 second (FEV1) was 165 mL for the LAMA/LABA group, compared with 91 mL in the ICS/LABA group. In the second study, the improvement in the two groups was 213 mL and 112 mL.
The LAMA/LABA combination also improved trough FEV1 on day 85 by 82 mL and 98 mL more than did the ICS/LABA combination in the two studies, respectively.
Both combinations provided clinically meaningful improvements in dyspnea and quality of life scores, Dr. Donohue said.
Adverse events occurred during treatment in a similar proportion of patients in both treatment groups in both studies: 26% and 27% in the LAMA/LABA and ICS/LABA patients in the first study, and 30% and 31%, respectively, in the second study. The most common adverse events were headache and nasopharyngitis.
In the first study, serious adverse events occurred in 2% of the LAMA/LABA patients and 3% of ICS/LABA patients, and in 3% and 4% of patients in the second study.
One death occurred in the ICS/LABA group in the first study, but it was not considered study related. Five deaths occurred in the second study, including two in the LAMA/LABA patients and three in the ICS/LABA patients. One of the deaths in the ICS/LABA group was because of pneumonia and was reported as drug related by the investigator.
No new safety signals were detected in these studies, Dr. Donohue said.
Patients in both studies had FEV1 between 30% and 70%, and had not experienced a COPD exacerbation within the previous year. The LAMA/LABA therapy was delivered via Ellipta inhaler, and the ICS/LABA therapy was delivered via Diskus inhaler.
The Food and Drug Administration approved umeclidinium/vilanterol combination therapy (Anoro Ellipta) in December 2013, the first LAMA/LABA therapy approved in the United States. Dr. Donohue and his colleagues conducted the pivotal regulatory trial of the drug combination, which was published in July 2013 (Respir. Med. 2013;107:1538-46).
The current trials represent an effort to determine where the treatment fits into the armamentarium for treating patients with COPD, he said.
The “really robust findings as befits two bronchodilators” suggest umeclidinium/vilanterol combination therapy is an effective treatment option that provides greater lung function than fluticasone/salmeterol for moderate to severe COPD in patients with infrequent exacerbations, Dr. Donohue said.
GSK, which developed the umeclidinium/vilanterol combination product with Theravance, funded the studies. Dr. Donohue reported receiving consultant fees and/or serving on an advisory committee for Almirall, AstraZeneca, Boehringer Ingelheim, Dey, Elevation Pharmaceutical, Forest Laboratories, GlaxoSmithKline, Novartis, Pearl Pharmaceuticals, Pfizer, and Sunovion. He has also served as a member of drug safety monitoring boards for the National Institutes of Health, Novartis, Otsuda, Pearl, and Teva.
AUSTIN, TEX.– Once daily combination treatment with umeclidinium and vilanterol was more effective than twice-daily combination treatment with fluticasone and salmeterol in patients with moderate to severe chronic obstructive pulmonary disease in two 12-week double-blind, parallel-group double-dummy studies.
In the two multicenter studies, 706 and 697 patients, respectively, were randomized to receive either 62.5 mcg of the long-acting muscarinic antagonist (LAMA) umeclidinium and 25 mcg of the long-acting beta-2 agonist (LABA) vilanterol – a recently approved combination bronchodilator maintenance treatment for COPD – or a combination of 250 mcg of the inhaled corticosteroid (ICS) fluticasone and 50 mcg of the LABA salmeterol, which is also indicated as a maintenance therapy for COPD.
The patients in the LAMA/LABA groups, who were treated once daily for 12 weeks, had significantly greater improvements on all lung function measures, compared with those in the ICS/LABA groups, who were treated twice daily, Dr. James F. Donohue of the University of North Carolina at Chapel Hill reported at the annual meeting of the American College of Chest Physicians.
In the first study, the improvement from baseline to day 84 (the primary study endpoint) in 0- to 24-hour weighted mean forced expiratory volume in 1 second (FEV1) was 165 mL for the LAMA/LABA group, compared with 91 mL in the ICS/LABA group. In the second study, the improvement in the two groups was 213 mL and 112 mL.
The LAMA/LABA combination also improved trough FEV1 on day 85 by 82 mL and 98 mL more than did the ICS/LABA combination in the two studies, respectively.
Both combinations provided clinically meaningful improvements in dyspnea and quality of life scores, Dr. Donohue said.
Adverse events occurred during treatment in a similar proportion of patients in both treatment groups in both studies: 26% and 27% in the LAMA/LABA and ICS/LABA patients in the first study, and 30% and 31%, respectively, in the second study. The most common adverse events were headache and nasopharyngitis.
In the first study, serious adverse events occurred in 2% of the LAMA/LABA patients and 3% of ICS/LABA patients, and in 3% and 4% of patients in the second study.
One death occurred in the ICS/LABA group in the first study, but it was not considered study related. Five deaths occurred in the second study, including two in the LAMA/LABA patients and three in the ICS/LABA patients. One of the deaths in the ICS/LABA group was because of pneumonia and was reported as drug related by the investigator.
No new safety signals were detected in these studies, Dr. Donohue said.
Patients in both studies had FEV1 between 30% and 70%, and had not experienced a COPD exacerbation within the previous year. The LAMA/LABA therapy was delivered via Ellipta inhaler, and the ICS/LABA therapy was delivered via Diskus inhaler.
The Food and Drug Administration approved umeclidinium/vilanterol combination therapy (Anoro Ellipta) in December 2013, the first LAMA/LABA therapy approved in the United States. Dr. Donohue and his colleagues conducted the pivotal regulatory trial of the drug combination, which was published in July 2013 (Respir. Med. 2013;107:1538-46).
The current trials represent an effort to determine where the treatment fits into the armamentarium for treating patients with COPD, he said.
The “really robust findings as befits two bronchodilators” suggest umeclidinium/vilanterol combination therapy is an effective treatment option that provides greater lung function than fluticasone/salmeterol for moderate to severe COPD in patients with infrequent exacerbations, Dr. Donohue said.
GSK, which developed the umeclidinium/vilanterol combination product with Theravance, funded the studies. Dr. Donohue reported receiving consultant fees and/or serving on an advisory committee for Almirall, AstraZeneca, Boehringer Ingelheim, Dey, Elevation Pharmaceutical, Forest Laboratories, GlaxoSmithKline, Novartis, Pearl Pharmaceuticals, Pfizer, and Sunovion. He has also served as a member of drug safety monitoring boards for the National Institutes of Health, Novartis, Otsuda, Pearl, and Teva.
AUSTIN, TEX.– Once daily combination treatment with umeclidinium and vilanterol was more effective than twice-daily combination treatment with fluticasone and salmeterol in patients with moderate to severe chronic obstructive pulmonary disease in two 12-week double-blind, parallel-group double-dummy studies.
In the two multicenter studies, 706 and 697 patients, respectively, were randomized to receive either 62.5 mcg of the long-acting muscarinic antagonist (LAMA) umeclidinium and 25 mcg of the long-acting beta-2 agonist (LABA) vilanterol – a recently approved combination bronchodilator maintenance treatment for COPD – or a combination of 250 mcg of the inhaled corticosteroid (ICS) fluticasone and 50 mcg of the LABA salmeterol, which is also indicated as a maintenance therapy for COPD.
The patients in the LAMA/LABA groups, who were treated once daily for 12 weeks, had significantly greater improvements on all lung function measures, compared with those in the ICS/LABA groups, who were treated twice daily, Dr. James F. Donohue of the University of North Carolina at Chapel Hill reported at the annual meeting of the American College of Chest Physicians.
In the first study, the improvement from baseline to day 84 (the primary study endpoint) in 0- to 24-hour weighted mean forced expiratory volume in 1 second (FEV1) was 165 mL for the LAMA/LABA group, compared with 91 mL in the ICS/LABA group. In the second study, the improvement in the two groups was 213 mL and 112 mL.
The LAMA/LABA combination also improved trough FEV1 on day 85 by 82 mL and 98 mL more than did the ICS/LABA combination in the two studies, respectively.
Both combinations provided clinically meaningful improvements in dyspnea and quality of life scores, Dr. Donohue said.
Adverse events occurred during treatment in a similar proportion of patients in both treatment groups in both studies: 26% and 27% in the LAMA/LABA and ICS/LABA patients in the first study, and 30% and 31%, respectively, in the second study. The most common adverse events were headache and nasopharyngitis.
In the first study, serious adverse events occurred in 2% of the LAMA/LABA patients and 3% of ICS/LABA patients, and in 3% and 4% of patients in the second study.
One death occurred in the ICS/LABA group in the first study, but it was not considered study related. Five deaths occurred in the second study, including two in the LAMA/LABA patients and three in the ICS/LABA patients. One of the deaths in the ICS/LABA group was because of pneumonia and was reported as drug related by the investigator.
No new safety signals were detected in these studies, Dr. Donohue said.
Patients in both studies had FEV1 between 30% and 70%, and had not experienced a COPD exacerbation within the previous year. The LAMA/LABA therapy was delivered via Ellipta inhaler, and the ICS/LABA therapy was delivered via Diskus inhaler.
The Food and Drug Administration approved umeclidinium/vilanterol combination therapy (Anoro Ellipta) in December 2013, the first LAMA/LABA therapy approved in the United States. Dr. Donohue and his colleagues conducted the pivotal regulatory trial of the drug combination, which was published in July 2013 (Respir. Med. 2013;107:1538-46).
The current trials represent an effort to determine where the treatment fits into the armamentarium for treating patients with COPD, he said.
The “really robust findings as befits two bronchodilators” suggest umeclidinium/vilanterol combination therapy is an effective treatment option that provides greater lung function than fluticasone/salmeterol for moderate to severe COPD in patients with infrequent exacerbations, Dr. Donohue said.
GSK, which developed the umeclidinium/vilanterol combination product with Theravance, funded the studies. Dr. Donohue reported receiving consultant fees and/or serving on an advisory committee for Almirall, AstraZeneca, Boehringer Ingelheim, Dey, Elevation Pharmaceutical, Forest Laboratories, GlaxoSmithKline, Novartis, Pearl Pharmaceuticals, Pfizer, and Sunovion. He has also served as a member of drug safety monitoring boards for the National Institutes of Health, Novartis, Otsuda, Pearl, and Teva.
AT CHEST 2014
Key clinical point: Umeclidinium/vilanterol is an effective treatment option for moderate to severe COPD patients with infrequent exacerbations.
Major finding: The improvement in 0- to 24-hour weighted mean FEV1 on day 84 with LAMA/LABA vs. ICS/LABA was 165 mL vs. 91 mL and 213 mL vs. 112 mL in two randomized trials, respectively.
Data source: Two randomized double-blind, parallel-group double-dummy studies involving 706 and 697 patients.
Disclosures: GSK, which developed the umeclidinium/vilanterol combination product with Theravance, funded the studies. Dr. Donohue reported receiving consultant fees and/or serving on an advisory committee for Almirall, AstraZeneca, Boehringer Ingelheim, Dey, Elevation Pharmaceutical, Forest Laboratories, GlaxoSmithKline, Novartis, Pearl Pharmaceuticals, Pfizer, and Sunovion. He has also served as a member of drug safety monitoring boards for the National Institutes of Health, Novartis, Otsuda, Pearl, and Teva.
VIDEO: How U.S. health providers can contain Ebola
AUSTIN, TEX. – Why would travel restrictions make the Ebola epidemic worse in West Africa and expand its spread to U.S. shores? What can American intensive care units do to prepare, even if they aren’t designated Ebola centers? And are there lessons medical professionals and policymakers can apply from the successes and failures of the AIDS epidemic 30 years ago?
In a video interview at the annual meeting of the American College of Chest Physicians, Dr. Lewis Rubinson offered answers to those questions and perspectives on the current American response to the Ebola outbreak. Dr. Rubinson recently returned from treating more than 300 Ebola patients in Sierra Leone as a consulting physician for the World Health Organization.
“Honestly, I think if we don’t get it under control in the next few months in West Africa, there will be sporadic cases coming back [to the United States] for as long as we can think of,” cautioned Dr. Rubinson, director of the R. Adams Cowley Trauma Shock Center at the University of Maryland, Baltimore.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AUSTIN, TEX. – Why would travel restrictions make the Ebola epidemic worse in West Africa and expand its spread to U.S. shores? What can American intensive care units do to prepare, even if they aren’t designated Ebola centers? And are there lessons medical professionals and policymakers can apply from the successes and failures of the AIDS epidemic 30 years ago?
In a video interview at the annual meeting of the American College of Chest Physicians, Dr. Lewis Rubinson offered answers to those questions and perspectives on the current American response to the Ebola outbreak. Dr. Rubinson recently returned from treating more than 300 Ebola patients in Sierra Leone as a consulting physician for the World Health Organization.
“Honestly, I think if we don’t get it under control in the next few months in West Africa, there will be sporadic cases coming back [to the United States] for as long as we can think of,” cautioned Dr. Rubinson, director of the R. Adams Cowley Trauma Shock Center at the University of Maryland, Baltimore.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AUSTIN, TEX. – Why would travel restrictions make the Ebola epidemic worse in West Africa and expand its spread to U.S. shores? What can American intensive care units do to prepare, even if they aren’t designated Ebola centers? And are there lessons medical professionals and policymakers can apply from the successes and failures of the AIDS epidemic 30 years ago?
In a video interview at the annual meeting of the American College of Chest Physicians, Dr. Lewis Rubinson offered answers to those questions and perspectives on the current American response to the Ebola outbreak. Dr. Rubinson recently returned from treating more than 300 Ebola patients in Sierra Leone as a consulting physician for the World Health Organization.
“Honestly, I think if we don’t get it under control in the next few months in West Africa, there will be sporadic cases coming back [to the United States] for as long as we can think of,” cautioned Dr. Rubinson, director of the R. Adams Cowley Trauma Shock Center at the University of Maryland, Baltimore.
On Twitter @whitneymcknight
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT CHEST 2014