User login
American Heart Association (AHA): Scientific Sessions 2014
Elevated troponin present in 40% with T2D and stable heart disease
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
CHICAGO– Abnormal levels of high-sensitivity cardiac troponin T are present in 40% of type 2 diabetic patients with stable ischemic heart disease, and they do not bode well, according to a new secondary analysis of the BARI 2D study.
In BARI 2D, an abnormal high-sensitivity cardiac troponin T (hsTnT), defined as 14 ng/L or greater, a powerful marker of ongoing myocardial injury, was independently associated with a doubled 5-year risk of the composite endpoint of cardiovascular death, MI, or stroke. Moreover, and discouragingly so, prompt coronary revascularization did nothing to mitigate that risk, Dr. Brendan M. Everett reported at the American Heart Association Scientific Sessions.
Further, early coronary revascularization did not result in a reduction in abnormal hsTnT at 1 year of follow-up, said Dr. Everett, director of the general cardiology inpatient service at Brigham and Women’s Hospital, Boston.
“To better address the risk represented by an abnormal hsTnT, we need to gain an improved understanding of the biology of troponin release in this population,” he observed. “The fact that we saw an overall decrease of about 0.5% in hemoglobin A1c and an LDL reduction of 16 mg/dL at 1 year and still there was no change in hsTnT leaves me scratching my head. The abnormal hsTnT is clearly a marker of badness, but where is it coming from? Can we address it? Or are we just left to look at it and worry about our patients who have an abnormal hsTnT?”
The BARI 2D trial was designed to learn whether patients with type 2 diabetes and stable ischemic heart disease benefit from prompt coronary revascularization plus intensive medical therapy as compared with intensive medical therapy alone. As previously reported (N. Engl. J. Med. 2009;360:2503-15), this proved not to be the case; prompt revascularization conferred no outcome advantage.
The aim of Dr. Everett’s new secondary analysis of BARI 2D was to learn if the hsTnT assay can be used to identify a subgroup of patients with type 2 diabetes and stable ischemic heart disease who might benefit from prompt coronary revascularization. The rationale was that, in patients with acute coronary syndromes, it’s well established that an abnormal hsTnT is associated with poor prognosis, and such patients would benefit from early revascularization.
The secondary analysis included 2,285 type 2 diabetics with stable ischemic heart disease whose physicians first decided whether they were better candidates for percutaneous coronary intervention or CABG surgery. Patients were then randomized to prompt revascularization by the preferred method plus intensive medical therapy or to intensive medical therapy alone.
Forty percent of participants had an abnormal hsTnT at baseline. Their 5-year rate of the composite primary endpoint of cardiovascular death, MI, or stroke was 27.1%, compared with 12.9% in patients with a baseline hsTnT below 14 ng/mL. After adjusting in a multivariate analysis for various potential confounders – including age, race, and the standard cardiovascular risk factors – the group with an abnormal baseline hsTnT had a 2.09-fold increased risk of a major cardiovascular event.
Early revascularization, regardless of whether by percutaneous coronary intervention or coronary artery bypass graft surgery, provided no benefit no matter what the patient’s baseline hsTnT level. In patients with an hsTnT of 14 ng/L or greater, the 5-year rate of the composite outcome was 26.5% with early revascularization compared with 27.6% with intensive medical therapy. In those with an hsTnT below 14 ng/L, the rate was 11.8% in the early revascularization group and 14% with medical management, a trend favoring prompt revascularization that didn’t achieve statistical significance, according to Dr. Everett.
Of patients with an abnormal hsTnT at baseline, 77% still had an abnormal value at 1 year, regardless of whether they underwent prompt revascularization or intensive medical therapy alone.
Session moderator Dr. Mikhail N. Kosiborod commented that the new BARI 2D substudy highlights a dilemma: “We know that a large population of patients with diabetes, and to some extent those with prediabetes, have elevated hsTnT levels, and we know those patients don’t do well. What we don’t know is what to do about it.”
“What [Dr. Everett’s] study clearly demonstrates is that this does not appear to be driven by epicardial coronary artery disease. If we fix the epicardial CAD, it has absolutely no impact on the outcomes nor on the actual troponin level at follow-up. As far as I can tell, it doesn’t appear to be a glycemic control issue, either. It appears that this is a humoral issue. There are ‘evil humors’ – whatever they are – and we don’t really understand what they are or what to do about it,” said Dr. Kosiborod, professor of medicine at the University of Missouri, Kansas City.
“The truth of the matter is we have no idea what’s causing this low-grade myocardial necrosis, and it’s a hugely important thing,” he continued. “There is absolutely no question that elevated hsTnT, even at very low levels, has a huge impact on subsequent risk of heart failure. We know what the public health effects of heart failure are. And patients with diabetes and heart failure tend to do particularly poorly.”
The BARI 2D trial was funded by the National Institutes of Health. Dr. Everett’s secondary analysis was funded by Roche Diagnostics. He reported receiving research grants from Roche and Novartis.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Forty percent of patients with type 2 diabetes and stable ischemic heart disease are walking around with abnormal levels of hsTnT, placing them at substantial risk of a major cardiovascular event within 5 years.
Major finding: Prompt coronary revascularization does not reduce this risk, nor does it reduce the elevated troponin T level.
Data source: A secondary analysis of the randomized, prospective BARI 2D study involving 2,285 participants with type 2 diabetes and stable ischemic heart disease.
Disclosures: The BARI 2D study was funded by the National Institutes of Health. This new secondary analysis was funded by a research grant from Roche Diagnostics.
Insulin glargine shows cardiac safety in ORIGIN-ECHO
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
CHICAGO – Insulin glargine showed no effects on left ventricular mass or function during 3 years of follow-up in dysglycemic patients at high cardiovascular risk in the ORIGIN echocardiographic substudy.
This echocardiographic study of the ORIGIN (Outcome Reduction With an Initial Glargine Intervention) trial, the largest reported study of the effects of exogenous insulin on left ventricular mass and LV systolic and diastolic function, provides reassuring new evidence that insulin glargine is safe from a cardiac standpoint, Dr. Michelle Haroun said at the American Heart Association scientific sessions.
“The key here is that we didn’t see any signal whatsoever to suggest that insulin is putting patients at increased risk. We think that this finding is important. While you need to follow patients for a very long time to detect changes in clinical heart failure outcomes, we think we’d be able to detect subtle changes in endpoints like LV mass over a 3-year period if insulin was of harm to patients,” said Dr. Haroun of the Population Health Research Institute at McMaster University in Hamilton, Ont.
ORIGIN-ECHO involved 564 dysglycemic patients at high cardiovascular risk who were randomized to insulin glargine (Lantus) or standard therapy. All had echocardiograms at baseline and after 3 years of therapy. Participants had to have impaired fasting blood glucose, impaired glucose tolerance, or early type 2 diabetes managed with no more than one oral antiglycemic drug at baseline. This was a group at high cardiovascular risk: 32% had a prior MI, 84% had a history of hypertension, obesity was common, and the average age was 64. However, none of the participants had heart failure at baseline.
The study was undertaken because some of the medications used to treat hyperglycemia are associated with increased risk of heart failure. Regulatory agencies, physicians, and patients want to see evidence of cardiovascular safety, and until ORIGIN-ECHO, the effects of exogenous insulin on LV mass and function hadn’t been well studied.
Baseline LV mass and function values were within normal range and did not change significantly over 3 years of follow-up in either treatment arm. For example, left ventricular mass/height averaged 116 g/m at baseline and 115 g/m after 3 years on insulin glargine, and was comparable at 113 and 114 g/m, respectively, with standard therapy. This was an unexpected finding, according to Dr. Haroun.
“We thought patients with diabetes on standard therapy were going to develop left ventricular hypertrophy over a 3-year follow-up period, and they didn’t. That came as a bit of a surprise to us. We expected to see a lower rate of LVH in the patients on insulin glargine. This patient population was relatively early in their course of diabetes, and we believe our findings suggest that adequate management of cardiovascular risk factors – especially hypertension– and the use of cardioprotective drugs in this population may prevent or delay abnormalities in LV structure and function,” she said.
The primary outcomes of the full ORIGIN study involving more than 12,000 patients have previously been published (N. Engl. J. Med. 2012; 367:319-28). ORIGIN was sponsored by Sanofi. Dr. Haroun reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The cardiac safety of insulin glargine in dysglycemic patients at high cardiovascular risk has received strong support from a 3-year echocardiographic study.
Major finding: Left ventricular mass over height was 116 g/m at baseline and 115 g/m after 3 years on insulin glargine.
Data source: The ORIGIN-ECHO substudy included 564 dysglycemic patients at high cardiovascular risk who were randomized to 3 years of insulin glargine or standard therapy.
Disclosures: The ORIGIN trial was sponsored by Sanofi. The presenter reported having no financial conflicts.
Takotsubo cardiomyopathy: predicting in-hospital mortality
CHICAGO – A novel risk score has been developed for predicting the risk of in-hospital mortality in patients with takotsubo cardiomyopathy.
The new risk score’s unique strength is that it was developed using hospital data on a huge patient population with this rare and incompletely understood cardiac condition: 10,582 patients hospitalized for takotsubo cardiomyopathy in seven U.S. states, Dr. David P. Kao reported at the American Heart Association scientific sessions.
He used multivariate logistic regression analysis to identify seven independent characteristics predictive of mortality in the study population. Their collective area under the curve as predictors was 0.70, which is considered good. And while the overall in-hospital mortality rate was low at 4.4%, it varied enormously depending upon how many of the seven risk factors were present, according to Dr. Kao of the University of Colorado, Denver.
In-hospital mortality ranged from 1.6% in the 2,585 patients with none of the risk factors to 19.6% in those with three or more. The 616 patients with at least three risk factors accounted for 22% of all in-hospital deaths in patients with takotsubo cardiomyopathy in California, New York, New Jersey, Colorado, West Virginia, New Hampshire, and Vermont during 2006-2012.
Intracranial hemorrhage, which was present in 2% of hospitalized takotsubo cardiomyopathy patients, was the most potent predictor both of in-hospital mortality and major adverse events. In multivariate analysis, intracranial hemorrhage was independently associated with a 6.8-fold increased risk of in-hospital mortality. The other mortality risk factors and their associated odds ratios were age 60 years or older, with a 1.8-fold risk; Asian race, 1.8-fold; male sex, 1.9-fold; acute renal failure, 4.1-fold; atrial fibrillation or flutter, 1.7-fold; and stroke, 2.9-fold.
The simple, user-friendly risk score is derived by totaling the number of risk factors present in a given hospitalized patient. The presence of each additional risk factor increased the odds of in-hospital death by 2.2-fold, according to Dr. Kao.
Takotsubo cardiomyopathy is marked by acute, typically rapidly reversible left ventricular dysfunction without evidence of epicardial coronary artery occlusion. It occurs most often in postmenopausal women in response to emotional or physical stress. Indeed, 89% of the more than 10,000 affected patients in this series were women.
Most adverse events in patients with takotsubo cardiomyopathy occur during their first hospitalization for the disorder. In this large series, 22% of patients experienced major adverse events, including ventricular arrhythmias, acute heart failure, cardiogenic shock, pulmonary edema, or ventricular rupture.
In a separate multiple logistic regression analysis, Dr. Kao and coinvestigator Dr. JoAnn Lindenfeld, also of the University of Colorado, identified eight characteristics independently predictive of in-hospital major adverse events. Five of them were also predictors of in-hospital mortality: intracranial hemorrhage, male gender, stroke, atrial fibrillation/flutter, and acute renal failure. The other three were age less than 60, substance abuse, and anemia. The major adverse event rate ranged from 10% in patients with none of the risk factors to 56% in the 242 patients having four or more. The 1,057 patients with three or more risk factors had a 47% major adverse event rate and accounted for 20% of all such events.
Dr. Kao reported having no financial conflicts related to this study.
CHICAGO – A novel risk score has been developed for predicting the risk of in-hospital mortality in patients with takotsubo cardiomyopathy.
The new risk score’s unique strength is that it was developed using hospital data on a huge patient population with this rare and incompletely understood cardiac condition: 10,582 patients hospitalized for takotsubo cardiomyopathy in seven U.S. states, Dr. David P. Kao reported at the American Heart Association scientific sessions.
He used multivariate logistic regression analysis to identify seven independent characteristics predictive of mortality in the study population. Their collective area under the curve as predictors was 0.70, which is considered good. And while the overall in-hospital mortality rate was low at 4.4%, it varied enormously depending upon how many of the seven risk factors were present, according to Dr. Kao of the University of Colorado, Denver.
In-hospital mortality ranged from 1.6% in the 2,585 patients with none of the risk factors to 19.6% in those with three or more. The 616 patients with at least three risk factors accounted for 22% of all in-hospital deaths in patients with takotsubo cardiomyopathy in California, New York, New Jersey, Colorado, West Virginia, New Hampshire, and Vermont during 2006-2012.
Intracranial hemorrhage, which was present in 2% of hospitalized takotsubo cardiomyopathy patients, was the most potent predictor both of in-hospital mortality and major adverse events. In multivariate analysis, intracranial hemorrhage was independently associated with a 6.8-fold increased risk of in-hospital mortality. The other mortality risk factors and their associated odds ratios were age 60 years or older, with a 1.8-fold risk; Asian race, 1.8-fold; male sex, 1.9-fold; acute renal failure, 4.1-fold; atrial fibrillation or flutter, 1.7-fold; and stroke, 2.9-fold.
The simple, user-friendly risk score is derived by totaling the number of risk factors present in a given hospitalized patient. The presence of each additional risk factor increased the odds of in-hospital death by 2.2-fold, according to Dr. Kao.
Takotsubo cardiomyopathy is marked by acute, typically rapidly reversible left ventricular dysfunction without evidence of epicardial coronary artery occlusion. It occurs most often in postmenopausal women in response to emotional or physical stress. Indeed, 89% of the more than 10,000 affected patients in this series were women.
Most adverse events in patients with takotsubo cardiomyopathy occur during their first hospitalization for the disorder. In this large series, 22% of patients experienced major adverse events, including ventricular arrhythmias, acute heart failure, cardiogenic shock, pulmonary edema, or ventricular rupture.
In a separate multiple logistic regression analysis, Dr. Kao and coinvestigator Dr. JoAnn Lindenfeld, also of the University of Colorado, identified eight characteristics independently predictive of in-hospital major adverse events. Five of them were also predictors of in-hospital mortality: intracranial hemorrhage, male gender, stroke, atrial fibrillation/flutter, and acute renal failure. The other three were age less than 60, substance abuse, and anemia. The major adverse event rate ranged from 10% in patients with none of the risk factors to 56% in the 242 patients having four or more. The 1,057 patients with three or more risk factors had a 47% major adverse event rate and accounted for 20% of all such events.
Dr. Kao reported having no financial conflicts related to this study.
CHICAGO – A novel risk score has been developed for predicting the risk of in-hospital mortality in patients with takotsubo cardiomyopathy.
The new risk score’s unique strength is that it was developed using hospital data on a huge patient population with this rare and incompletely understood cardiac condition: 10,582 patients hospitalized for takotsubo cardiomyopathy in seven U.S. states, Dr. David P. Kao reported at the American Heart Association scientific sessions.
He used multivariate logistic regression analysis to identify seven independent characteristics predictive of mortality in the study population. Their collective area under the curve as predictors was 0.70, which is considered good. And while the overall in-hospital mortality rate was low at 4.4%, it varied enormously depending upon how many of the seven risk factors were present, according to Dr. Kao of the University of Colorado, Denver.
In-hospital mortality ranged from 1.6% in the 2,585 patients with none of the risk factors to 19.6% in those with three or more. The 616 patients with at least three risk factors accounted for 22% of all in-hospital deaths in patients with takotsubo cardiomyopathy in California, New York, New Jersey, Colorado, West Virginia, New Hampshire, and Vermont during 2006-2012.
Intracranial hemorrhage, which was present in 2% of hospitalized takotsubo cardiomyopathy patients, was the most potent predictor both of in-hospital mortality and major adverse events. In multivariate analysis, intracranial hemorrhage was independently associated with a 6.8-fold increased risk of in-hospital mortality. The other mortality risk factors and their associated odds ratios were age 60 years or older, with a 1.8-fold risk; Asian race, 1.8-fold; male sex, 1.9-fold; acute renal failure, 4.1-fold; atrial fibrillation or flutter, 1.7-fold; and stroke, 2.9-fold.
The simple, user-friendly risk score is derived by totaling the number of risk factors present in a given hospitalized patient. The presence of each additional risk factor increased the odds of in-hospital death by 2.2-fold, according to Dr. Kao.
Takotsubo cardiomyopathy is marked by acute, typically rapidly reversible left ventricular dysfunction without evidence of epicardial coronary artery occlusion. It occurs most often in postmenopausal women in response to emotional or physical stress. Indeed, 89% of the more than 10,000 affected patients in this series were women.
Most adverse events in patients with takotsubo cardiomyopathy occur during their first hospitalization for the disorder. In this large series, 22% of patients experienced major adverse events, including ventricular arrhythmias, acute heart failure, cardiogenic shock, pulmonary edema, or ventricular rupture.
In a separate multiple logistic regression analysis, Dr. Kao and coinvestigator Dr. JoAnn Lindenfeld, also of the University of Colorado, identified eight characteristics independently predictive of in-hospital major adverse events. Five of them were also predictors of in-hospital mortality: intracranial hemorrhage, male gender, stroke, atrial fibrillation/flutter, and acute renal failure. The other three were age less than 60, substance abuse, and anemia. The major adverse event rate ranged from 10% in patients with none of the risk factors to 56% in the 242 patients having four or more. The 1,057 patients with three or more risk factors had a 47% major adverse event rate and accounted for 20% of all such events.
Dr. Kao reported having no financial conflicts related to this study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: The risk of in-hospital mortality in patients with takotsubo cardiomyopathy can be predicted using a simple risk score based upon information readily available at the bedside.
Major finding: In-hospital mortality ranged from just 1.6% in patients with no risk factors to 19.6% in those with any three or more.
Data source: The seven independent characteristics associated with in-hospital mortality were identified through multivariate logistic regression analysis applied to the hospital records of 10,582 patients with takotsubo cardiomyopathy in seven states.
Disclosures: The presenter reported having no financial conflicts in connection with this study, conducted free of commercial interests.
Introducing a better bleeding risk score in atrial fib
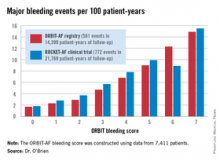
CHICAGO – The ORBIT-AF bleeding risk score is a simple, user-friendly new tool for assessing the risk of major bleeding in patients with atrial fibrillation on oral anticoagulation, Emily C. O’Brien, Ph.D., announced at the American Heart Association scientific sessions.
This novel score offers significant advantages over existing bleeding risk scores, including HAS-BLED and ATRIA. Those scores were developed on the basis of small numbers of bleeding events, they show inconsistent performance, and their calculation requires data that’s often not readily accessible in busy daily practice, according to Dr. O’Brien of the Duke Clinical Research Institute in Durham, N.C.
The new score was derived from the ORBIT-AF registry, the largest prospective U.S. registry of patients with atrial fibrillation (AF).
The score was constructed using data on 7,411 AF patients in community practice settings at 173 U.S. sites. All subjects were on oral anticoagulant therapy at baseline. During 2 years of prospective follow-up, 581 patients (7.8%) experienced a major bleeding event as defined by International Society on Thrombosis and Haemostasis criteria.
After sifting through numerous potential candidate variables, Dr. O’Brien and coinvestigators settled upon five they identified as the most potent and practical baseline predictors of major bleeding risk while on oral anticoagulation. Then they packaged them in a convenient acronym: ORBIT, for Older than 74, Renal insufficiency with an estimated glomerular filtration rate below 60 mL/minute per/1.73 m2, Bleeding history, Insufficient hemoglobin/hematocrit or anemia, and Treatment with an antiplatelet agent. The two strongest predictors – renal insufficiency and bleeding history– were awarded two points each; the others are worth one point each.
The observed major bleeding rate among patients enrolled in the ORBIT-AF registry rose with an increasing risk score. The same was true upon application of the ORBIT bleeding score to an independent study sample comprised of participants in the ROCKET-AF randomized clinical trial.
Dr. O’Brien also compared the performance of the ORBIT bleeding score to that of two existing bleeding risk scores – HAS-BLED and ATRIA – in the ORBIT-AF and ROCKET-AF cohorts. The simpler, more user friendly ORBIT bleeding score had a C-statistic of 0.67, similar to the 0.64 for HAS-BLED and 0.66 for ATRIA.
Thus, the ORBIT bleeding score is a practical new tool for use alongside the CHA2DS2-VASc stroke risk score to support clinical decision making regarding whether or not to place an individual AF patient on oral anticoagulation, Dr. O’Brien concluded.
She reported having no financial conflicts regarding this study. The ORBIT-AF registry is sponsored by Janssen.
CHICAGO – The ORBIT-AF bleeding risk score is a simple, user-friendly new tool for assessing the risk of major bleeding in patients with atrial fibrillation on oral anticoagulation, Emily C. O’Brien, Ph.D., announced at the American Heart Association scientific sessions.
This novel score offers significant advantages over existing bleeding risk scores, including HAS-BLED and ATRIA. Those scores were developed on the basis of small numbers of bleeding events, they show inconsistent performance, and their calculation requires data that’s often not readily accessible in busy daily practice, according to Dr. O’Brien of the Duke Clinical Research Institute in Durham, N.C.
The new score was derived from the ORBIT-AF registry, the largest prospective U.S. registry of patients with atrial fibrillation (AF).
The score was constructed using data on 7,411 AF patients in community practice settings at 173 U.S. sites. All subjects were on oral anticoagulant therapy at baseline. During 2 years of prospective follow-up, 581 patients (7.8%) experienced a major bleeding event as defined by International Society on Thrombosis and Haemostasis criteria.
After sifting through numerous potential candidate variables, Dr. O’Brien and coinvestigators settled upon five they identified as the most potent and practical baseline predictors of major bleeding risk while on oral anticoagulation. Then they packaged them in a convenient acronym: ORBIT, for Older than 74, Renal insufficiency with an estimated glomerular filtration rate below 60 mL/minute per/1.73 m2, Bleeding history, Insufficient hemoglobin/hematocrit or anemia, and Treatment with an antiplatelet agent. The two strongest predictors – renal insufficiency and bleeding history– were awarded two points each; the others are worth one point each.
The observed major bleeding rate among patients enrolled in the ORBIT-AF registry rose with an increasing risk score. The same was true upon application of the ORBIT bleeding score to an independent study sample comprised of participants in the ROCKET-AF randomized clinical trial.
Dr. O’Brien also compared the performance of the ORBIT bleeding score to that of two existing bleeding risk scores – HAS-BLED and ATRIA – in the ORBIT-AF and ROCKET-AF cohorts. The simpler, more user friendly ORBIT bleeding score had a C-statistic of 0.67, similar to the 0.64 for HAS-BLED and 0.66 for ATRIA.
Thus, the ORBIT bleeding score is a practical new tool for use alongside the CHA2DS2-VASc stroke risk score to support clinical decision making regarding whether or not to place an individual AF patient on oral anticoagulation, Dr. O’Brien concluded.
She reported having no financial conflicts regarding this study. The ORBIT-AF registry is sponsored by Janssen.
CHICAGO – The ORBIT-AF bleeding risk score is a simple, user-friendly new tool for assessing the risk of major bleeding in patients with atrial fibrillation on oral anticoagulation, Emily C. O’Brien, Ph.D., announced at the American Heart Association scientific sessions.
This novel score offers significant advantages over existing bleeding risk scores, including HAS-BLED and ATRIA. Those scores were developed on the basis of small numbers of bleeding events, they show inconsistent performance, and their calculation requires data that’s often not readily accessible in busy daily practice, according to Dr. O’Brien of the Duke Clinical Research Institute in Durham, N.C.
The new score was derived from the ORBIT-AF registry, the largest prospective U.S. registry of patients with atrial fibrillation (AF).
The score was constructed using data on 7,411 AF patients in community practice settings at 173 U.S. sites. All subjects were on oral anticoagulant therapy at baseline. During 2 years of prospective follow-up, 581 patients (7.8%) experienced a major bleeding event as defined by International Society on Thrombosis and Haemostasis criteria.
After sifting through numerous potential candidate variables, Dr. O’Brien and coinvestigators settled upon five they identified as the most potent and practical baseline predictors of major bleeding risk while on oral anticoagulation. Then they packaged them in a convenient acronym: ORBIT, for Older than 74, Renal insufficiency with an estimated glomerular filtration rate below 60 mL/minute per/1.73 m2, Bleeding history, Insufficient hemoglobin/hematocrit or anemia, and Treatment with an antiplatelet agent. The two strongest predictors – renal insufficiency and bleeding history– were awarded two points each; the others are worth one point each.
The observed major bleeding rate among patients enrolled in the ORBIT-AF registry rose with an increasing risk score. The same was true upon application of the ORBIT bleeding score to an independent study sample comprised of participants in the ROCKET-AF randomized clinical trial.
Dr. O’Brien also compared the performance of the ORBIT bleeding score to that of two existing bleeding risk scores – HAS-BLED and ATRIA – in the ORBIT-AF and ROCKET-AF cohorts. The simpler, more user friendly ORBIT bleeding score had a C-statistic of 0.67, similar to the 0.64 for HAS-BLED and 0.66 for ATRIA.
Thus, the ORBIT bleeding score is a practical new tool for use alongside the CHA2DS2-VASc stroke risk score to support clinical decision making regarding whether or not to place an individual AF patient on oral anticoagulation, Dr. O’Brien concluded.
She reported having no financial conflicts regarding this study. The ORBIT-AF registry is sponsored by Janssen.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: A simple new score is available to assess major bleeding risk in patients with atrial fibrillation on oral anticoagulation.
Major finding: The major bleeding risk in patients with atrial fibrillation on oral anticoagulation ranged from 1.7 per 100 patient-years in those with an ORBIT risk score of 0% to 14.9% in those with a maximum score of 7.
Data source: The risk score was derived by analyzing prospective 2-year follow-up data on 7,411 U.S. patients with atrial fibrillation in a large registry.
Disclosures: The ORBIT-AF registry is sponsored by Janssen. The presenter reported having no financial conflicts.
Hormone therapy ‘timing hypothesis’ gains ground in ELITE
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.
 |
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.
 |
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
The results from the ELITE study are consistent with what we found in the Women’s Health Initiative. Last year, my collaborators from the Women’s Health Initiative and I reported that among the subgroup of women treated with conjugated equine estrogens alone, women who entered the study at age 50-59 years showed a net reduction, compared with the placebo-treated controls, in both total mortality and by a composite tally of death plus nonfatal events that included coronary heart disease, stroke, and five other adverse outcomes (JAMA 2013;310:1358-68). Women aged 60-69 years on estrogen alone had a virtually identical number of events as the placebo group, and women aged 70-79 years had a substantial excess of deaths and composite events, compared with their placebo counterparts. This age-based difference in response was statistically significant, and a remarkable pattern that we did not see in women who received with estrogen plus systemic treatment with medroxyprogesterone.
It was clear that age, as well as time from the start of menopause, played a role in the estrogen-alone arm of the Women’s Health Initiative.
 |
| Whitney McKnight/Frontline Medical News Dr. JoAnn E. Manson |
These findings prompted me and my associates to formulate an algorithm and mobile app for managing menopausal symptoms and deciding whether or not a postmenopausal woman is a good candidate for hormonal therapy (Menopause 2014; 22:1-7). We do not endorse hormone therapy for any purpose other than suggesting it as an option to consider for postmenopausal women who experience moderate to severe hot flashes, night sweats, or both. Among these women, we suggest that hormone therapy is a reasonable option for women who are 10 or fewer years removed from menopause onset and if their 10-year risk for an atherosclerotic cardiovascular disease event is 5% or less as calculated by the 2013 risk calculator developed by the American Heart Association and American College of Cardiology. If these women 10 or fewer years out from their menopause start have a 6%-10% 10-year risk we also endorse hormone therapy as an option, but in a transdermal formulation. For women more than 10 years out from menopause, and for those with a greater than 10% 10-year risk, we suggest avoiding hormone therapy.
Dr. JoAnn E. Manson is professor of medicine and chief of preventive medicine at Harvard University and Brigham and Women’s Hospital, both in Boston. She had no disclosures. She made these comments in a lecture at the American Heart Association Scientific Sessions. Dr. Manson has served as lead investigator from the Women’s Health Initiative since the study began.
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
CHICAGO– The “timing hypothesis” of estrogen’s ability to slow progression of atherosclerotic disease in relatively young postmenopausal women received a boost in results from a randomized trial with 643 participants.
The trial, designed about a decade ago to test the timing hypothesis, produced results showing that systemic treatment with 17beta-estradiol alone (plus topical progesterone) slowed progression of carotid intima-media thickness compared with placebo in women fewer than 6 years out from menopause, while the same treatment had no effect compared with placebo in women 10 or more years removed from menopause, Dr. Howard N. Hodis said at the American Heart Association Scientific Sessions.
The new results “support the timing hypothesis, whereby women who start hormone therapy within 6 years of menopause show a significant slowing of subclinical carotid-artery atherosclerosis, whereas women who are more than 10 years postmenopausal when starting hormone therapy show no difference from placebo,” said Dr. Hodis, professor of medicine and director of the atherosclerosis research unit at the University of Southern California, Los Angeles. The findings “are consistent with the majority of the literature” that previously reported evidence supporting a net benefit from hormone therapy when started in women who are young, and not long after they entered menopause, he added.
The ELITE (Early Versus Late Intervention Trial With Estradiol) investigators enrolled 643 postmenopausal women at the University of Southern California and stratified them into two subgroups: women fewer than 6 years removed from the onset of menopause, and women 10 or more years out from menopause. The average age of women in the younger group was about 55 years, and they averaged just under 4 years since menopause onset. Women in the older group averaged about 64 years and were an average of about 14 years removed from menopause onset.
Dr. Hodis and his associates randomized women within each of the two subgroups to daily treatment with 1 mg oral, micronized 17beta-estradiol or placebo. Women treated with estradiol who had an intact uterus also applied a micronized progesterone gel on 12 days out of every cycle, while those who received a daily placebo also applied a placebo gel. The study’s primary endpoint was the rate of change in the intima-media thickness (IMT) of the common carotid artery, measured once every 6 months. Compliance with the assigned regimens reached or exceeded 98% among all women in the study.
During 6 years of follow-up, the rate of increase in carotid IMT among the women who entered the study at least 10 years after the start of menopause tracked nearly identically between those on hormone therapy and those on placebo, with a difference between the two arms that was not statistically significant. In contrast, the women who began hormone therapy fewer than 6 years after menopause onset showed a statically significant difference in the rate of change in their carotid IMT, depending on their treatment assignment. After 6 years, the average increase in carotid IMT was roughly 40% less in women who received hormone therapy, compared with those randomized to placebo. The difference in IMT change between the older women and the younger women was also statistically significant, Dr. Hodis reported.
Dr. Hodis had no disclosures.
On Twitter@mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Hormone therapy cut atherosclerotic progression, compared with placebo, in women 6 or fewer years out from menopause start, but not in those 10 or more years out.
Major finding: In women up to 6 years out from menopause, hormone therapy was linked with 40% less atherosclerotic progression than with placebo.
Data source: ELITE,a single-center, randomized study with 643 postmenopausal women.
Disclosures: Dr. Hodis had no disclosures.
Open TAA repair surpasses TEVAR survival
CHICAGO – Endovascular repair of thoracic aortic aneurysms confers an immediate but short-lived survival advantage over open repair that completely disappears and then reverses 4.5 years after intervention, a study showed.
After 4.5 years, patients who underwent open repair of a thoracic aortic aneurysm had better survival than patients who underwent endovascular thoracic aneurysm repair, based on a propensity-score adjusted analysis of more than 3,000 Medicare patients.
The findings also showed a striking interhospital variability among U.S. centers performing thoracic aortic aneurysm (TAA) repair that influenced patient survival by 50%. The tertile of hospitals with the best survival outcomes collectively had a 50% reduced rate of deaths during follow-up, compared with all other hospitals, Dr. Justin M. Schaffer said at the American Heart Association scientific sessions.
“Simply by picking the right hospital, patients could reduce their mortality by half. That is pretty amazing,” said Dr. Schaffer, a cardiothoracic surgeon at Stanford (Calif.) University.
Perhaps even more notable than this undefined hospital effect was the pattern of mortality effects associated with open TAA repair, compared with thoracic endovascular aortic repair (TEVAR). For these analyses, Dr. Schaffer and his associates focused on the 1,037 Medicare beneficiaries who underwent open repair of an isolated, nonruptured TAA during 1999-2011, and 2,010 similar patients treated with TEVAR during the same period. It was relatively uncommon for patients to need repair for a TAA that was neither ruptured nor presented with another cardiac problem. This subgroup constituted 19% of all open TAA repairs on Medicare patients during those years, and 25% of all TEVARs performed.
Comparison of the TEVAR and open repair patients showed that those who underwent TEVAR were older and had consistently higher prevalence rates of a long list of comorbidities, including diabetes, chronic kidney disease, atrial fibrillation, and heart failure. The two populations also showed substantial differences for several other potential confounders. To adjust for all these, the researchers used a form of propensity scoring to balance the variability between the TEVAR and open surgery subgroups.
After adjustment, the mortality analysis showed that immediately following TEVAR, patients had a large mortality advantage, but that this immediately began to diminish such that by about 6 months after the procedure, the instantaneous advantage from TEVAR reached zero and then began tilting toward an advantage from open surgery.
Because of TEVAR’s sizable early lead the cumulative mortality numbers took awhile to reflect this. When tallied at 12 months after the procedure, cumulative survival in the TEVAR group stood at 85%, and 75% among the open surgery patients, a statistically significant advantage for TEVAR for 1-year survival.
But at 4.5 years after the procedure, the cumulative mortality rate in the TEVAR group caught up to that in the open surgery group, and following that, the TEVAR patients had a higher cumulative mortality. At 5-year follow-up, cumulative survival was 56% in the open surgery group and 55% in the TEVAR group, a statistically significant difference, Dr. Schaffer reported.
“There is a clear trade-off” between the two repair options, he said. “If a patient’s expected survival [following surgery] is limited, then TEVAR is reasonable, and if the anatomy also makes it feasible,” he said. “But for healthier patients with better expected survival, open repair is more complete, more durable, and superior.”
Although the analysis has not yet identified the clinical factors that contribute to worse survival after TEVAR, “it appears to be a higher reintervention rate,” based on preliminary assessments of the data, Dr. Schaffer said.
The analysis also showed other factors that significantly linked with mortality among all patients, regardless of whether they underwent TEVAR or open surgery. In addition to showing significant incremental risk effects from each of a range of comorbidities, the results showed that over the period 1999-2011, patients had a progressive, relative reduction of 6% fewer deaths each year, and that centers with the highest repair volumes produced 11% fewer deaths, compared with medium-volume hospitals.
Dr. Schaffer said he had no relevant financial disclosures.
On Twitter @mitchelzoler
CHICAGO – Endovascular repair of thoracic aortic aneurysms confers an immediate but short-lived survival advantage over open repair that completely disappears and then reverses 4.5 years after intervention, a study showed.
After 4.5 years, patients who underwent open repair of a thoracic aortic aneurysm had better survival than patients who underwent endovascular thoracic aneurysm repair, based on a propensity-score adjusted analysis of more than 3,000 Medicare patients.
The findings also showed a striking interhospital variability among U.S. centers performing thoracic aortic aneurysm (TAA) repair that influenced patient survival by 50%. The tertile of hospitals with the best survival outcomes collectively had a 50% reduced rate of deaths during follow-up, compared with all other hospitals, Dr. Justin M. Schaffer said at the American Heart Association scientific sessions.
“Simply by picking the right hospital, patients could reduce their mortality by half. That is pretty amazing,” said Dr. Schaffer, a cardiothoracic surgeon at Stanford (Calif.) University.
Perhaps even more notable than this undefined hospital effect was the pattern of mortality effects associated with open TAA repair, compared with thoracic endovascular aortic repair (TEVAR). For these analyses, Dr. Schaffer and his associates focused on the 1,037 Medicare beneficiaries who underwent open repair of an isolated, nonruptured TAA during 1999-2011, and 2,010 similar patients treated with TEVAR during the same period. It was relatively uncommon for patients to need repair for a TAA that was neither ruptured nor presented with another cardiac problem. This subgroup constituted 19% of all open TAA repairs on Medicare patients during those years, and 25% of all TEVARs performed.
Comparison of the TEVAR and open repair patients showed that those who underwent TEVAR were older and had consistently higher prevalence rates of a long list of comorbidities, including diabetes, chronic kidney disease, atrial fibrillation, and heart failure. The two populations also showed substantial differences for several other potential confounders. To adjust for all these, the researchers used a form of propensity scoring to balance the variability between the TEVAR and open surgery subgroups.
After adjustment, the mortality analysis showed that immediately following TEVAR, patients had a large mortality advantage, but that this immediately began to diminish such that by about 6 months after the procedure, the instantaneous advantage from TEVAR reached zero and then began tilting toward an advantage from open surgery.
Because of TEVAR’s sizable early lead the cumulative mortality numbers took awhile to reflect this. When tallied at 12 months after the procedure, cumulative survival in the TEVAR group stood at 85%, and 75% among the open surgery patients, a statistically significant advantage for TEVAR for 1-year survival.
But at 4.5 years after the procedure, the cumulative mortality rate in the TEVAR group caught up to that in the open surgery group, and following that, the TEVAR patients had a higher cumulative mortality. At 5-year follow-up, cumulative survival was 56% in the open surgery group and 55% in the TEVAR group, a statistically significant difference, Dr. Schaffer reported.
“There is a clear trade-off” between the two repair options, he said. “If a patient’s expected survival [following surgery] is limited, then TEVAR is reasonable, and if the anatomy also makes it feasible,” he said. “But for healthier patients with better expected survival, open repair is more complete, more durable, and superior.”
Although the analysis has not yet identified the clinical factors that contribute to worse survival after TEVAR, “it appears to be a higher reintervention rate,” based on preliminary assessments of the data, Dr. Schaffer said.
The analysis also showed other factors that significantly linked with mortality among all patients, regardless of whether they underwent TEVAR or open surgery. In addition to showing significant incremental risk effects from each of a range of comorbidities, the results showed that over the period 1999-2011, patients had a progressive, relative reduction of 6% fewer deaths each year, and that centers with the highest repair volumes produced 11% fewer deaths, compared with medium-volume hospitals.
Dr. Schaffer said he had no relevant financial disclosures.
On Twitter @mitchelzoler
CHICAGO – Endovascular repair of thoracic aortic aneurysms confers an immediate but short-lived survival advantage over open repair that completely disappears and then reverses 4.5 years after intervention, a study showed.
After 4.5 years, patients who underwent open repair of a thoracic aortic aneurysm had better survival than patients who underwent endovascular thoracic aneurysm repair, based on a propensity-score adjusted analysis of more than 3,000 Medicare patients.
The findings also showed a striking interhospital variability among U.S. centers performing thoracic aortic aneurysm (TAA) repair that influenced patient survival by 50%. The tertile of hospitals with the best survival outcomes collectively had a 50% reduced rate of deaths during follow-up, compared with all other hospitals, Dr. Justin M. Schaffer said at the American Heart Association scientific sessions.
“Simply by picking the right hospital, patients could reduce their mortality by half. That is pretty amazing,” said Dr. Schaffer, a cardiothoracic surgeon at Stanford (Calif.) University.
Perhaps even more notable than this undefined hospital effect was the pattern of mortality effects associated with open TAA repair, compared with thoracic endovascular aortic repair (TEVAR). For these analyses, Dr. Schaffer and his associates focused on the 1,037 Medicare beneficiaries who underwent open repair of an isolated, nonruptured TAA during 1999-2011, and 2,010 similar patients treated with TEVAR during the same period. It was relatively uncommon for patients to need repair for a TAA that was neither ruptured nor presented with another cardiac problem. This subgroup constituted 19% of all open TAA repairs on Medicare patients during those years, and 25% of all TEVARs performed.
Comparison of the TEVAR and open repair patients showed that those who underwent TEVAR were older and had consistently higher prevalence rates of a long list of comorbidities, including diabetes, chronic kidney disease, atrial fibrillation, and heart failure. The two populations also showed substantial differences for several other potential confounders. To adjust for all these, the researchers used a form of propensity scoring to balance the variability between the TEVAR and open surgery subgroups.
After adjustment, the mortality analysis showed that immediately following TEVAR, patients had a large mortality advantage, but that this immediately began to diminish such that by about 6 months after the procedure, the instantaneous advantage from TEVAR reached zero and then began tilting toward an advantage from open surgery.
Because of TEVAR’s sizable early lead the cumulative mortality numbers took awhile to reflect this. When tallied at 12 months after the procedure, cumulative survival in the TEVAR group stood at 85%, and 75% among the open surgery patients, a statistically significant advantage for TEVAR for 1-year survival.
But at 4.5 years after the procedure, the cumulative mortality rate in the TEVAR group caught up to that in the open surgery group, and following that, the TEVAR patients had a higher cumulative mortality. At 5-year follow-up, cumulative survival was 56% in the open surgery group and 55% in the TEVAR group, a statistically significant difference, Dr. Schaffer reported.
“There is a clear trade-off” between the two repair options, he said. “If a patient’s expected survival [following surgery] is limited, then TEVAR is reasonable, and if the anatomy also makes it feasible,” he said. “But for healthier patients with better expected survival, open repair is more complete, more durable, and superior.”
Although the analysis has not yet identified the clinical factors that contribute to worse survival after TEVAR, “it appears to be a higher reintervention rate,” based on preliminary assessments of the data, Dr. Schaffer said.
The analysis also showed other factors that significantly linked with mortality among all patients, regardless of whether they underwent TEVAR or open surgery. In addition to showing significant incremental risk effects from each of a range of comorbidities, the results showed that over the period 1999-2011, patients had a progressive, relative reduction of 6% fewer deaths each year, and that centers with the highest repair volumes produced 11% fewer deaths, compared with medium-volume hospitals.
Dr. Schaffer said he had no relevant financial disclosures.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Medicare patients who underwent open repair for a thoracic aortic aneurysm had better long-term survival than matched Medicare patients who had endovascular repair.
Major finding: After 5 years, survival was 56% after open thoracic aortic aneurysm repa
ir and 55% after endovascular repair.
Data source: A retrospective study of 3,047 Medicare patients who underwent repair of a nonruptured, isolated thoracic aortic aneurysm during 1999-2011.
Disclosures: Dr. Schaffer had no relevant financial disclosures.
ICD lead extraction complication rates warrant surgical backup
CHICAGO– Transvenous lead extraction was associated with a significant risk of urgent cardiac surgery and mortality in a real-world cohort of patients undergoing procedures across a wide spectrum of centers and operators.
Among the 11,304 extractions, the major complication rate was 2.3% and mortality rate 0.9%.
While the complication rate was in line with previously published single-center registry data, the mortality rate was more than twice that reported in recent single-center studies from high-volume centers (0.9% vs. 0.4%), Dr. Nitesh Sood reported at the American Heart Association annual scientific sessions.
Of the 98 perioperative deaths, 18 occurred during the lead extraction procedure.
Another 41 patients (16%) required urgent cardiac surgery, of whom 14 (34%) died during or in the immediate postoperative period after surgery.
“Thus, while overall rate of major complications remains low, there exists a significant risk of urgent cardiac surgery and mortality during transvenous lead extractions [TLE] performed in the ‘real world.’ Appropriate training of all personnel involved and optimal cardiothoracic surgical back-up at centers performing TLE is imperative,” Dr. Sood of the Southcoast Health System, Fall River, Mass., concluded.
The analysis is the largest real-world cohort of TLE involving 11,304 patients with an implantable cardioverter defibrillator (ICD) in the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) ICD Registry with lead extraction data submitted between April 2010 and July 2012. Major complication was a combined endpoint of major operative complications, postoperative or in-hospital mortality, as defined by the NCDR ICD Registry.
The 258 complications included 62 cardiac arrests, 55 pericardial tamponades, 47 pneumothoraces, and 40 cardiac perforations.
In multivariate analysis, significant predictors of any complication were female sex (adjusted odds ratio, 1.46), heart failure admission vs. lead extraction admission (OR, 2.6), noncardiac admission vs. lead extraction admission (OR, 2.4), lead-only procedure vs. extraction during generator change/upgrade (OR, 1.76), age of lead (OR, 1.08), and clinical status requiring lead replacement (OR, 2.2). Dr. Sood reported.
Among lead characteristics, multivariate predictors of major perioperative complications included at least three concurrent leads extracted (OR, 2.13), longer implant duration (OR, 1.13), flat coil design vs. round (OR, 2.68), greater proximal coil surface area (OR, 1.04), and dislodgement of other leads during extraction (OR, 3.97), he noted.
CHICAGO– Transvenous lead extraction was associated with a significant risk of urgent cardiac surgery and mortality in a real-world cohort of patients undergoing procedures across a wide spectrum of centers and operators.
Among the 11,304 extractions, the major complication rate was 2.3% and mortality rate 0.9%.
While the complication rate was in line with previously published single-center registry data, the mortality rate was more than twice that reported in recent single-center studies from high-volume centers (0.9% vs. 0.4%), Dr. Nitesh Sood reported at the American Heart Association annual scientific sessions.
Of the 98 perioperative deaths, 18 occurred during the lead extraction procedure.
Another 41 patients (16%) required urgent cardiac surgery, of whom 14 (34%) died during or in the immediate postoperative period after surgery.
“Thus, while overall rate of major complications remains low, there exists a significant risk of urgent cardiac surgery and mortality during transvenous lead extractions [TLE] performed in the ‘real world.’ Appropriate training of all personnel involved and optimal cardiothoracic surgical back-up at centers performing TLE is imperative,” Dr. Sood of the Southcoast Health System, Fall River, Mass., concluded.
The analysis is the largest real-world cohort of TLE involving 11,304 patients with an implantable cardioverter defibrillator (ICD) in the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) ICD Registry with lead extraction data submitted between April 2010 and July 2012. Major complication was a combined endpoint of major operative complications, postoperative or in-hospital mortality, as defined by the NCDR ICD Registry.
The 258 complications included 62 cardiac arrests, 55 pericardial tamponades, 47 pneumothoraces, and 40 cardiac perforations.
In multivariate analysis, significant predictors of any complication were female sex (adjusted odds ratio, 1.46), heart failure admission vs. lead extraction admission (OR, 2.6), noncardiac admission vs. lead extraction admission (OR, 2.4), lead-only procedure vs. extraction during generator change/upgrade (OR, 1.76), age of lead (OR, 1.08), and clinical status requiring lead replacement (OR, 2.2). Dr. Sood reported.
Among lead characteristics, multivariate predictors of major perioperative complications included at least three concurrent leads extracted (OR, 2.13), longer implant duration (OR, 1.13), flat coil design vs. round (OR, 2.68), greater proximal coil surface area (OR, 1.04), and dislodgement of other leads during extraction (OR, 3.97), he noted.
CHICAGO– Transvenous lead extraction was associated with a significant risk of urgent cardiac surgery and mortality in a real-world cohort of patients undergoing procedures across a wide spectrum of centers and operators.
Among the 11,304 extractions, the major complication rate was 2.3% and mortality rate 0.9%.
While the complication rate was in line with previously published single-center registry data, the mortality rate was more than twice that reported in recent single-center studies from high-volume centers (0.9% vs. 0.4%), Dr. Nitesh Sood reported at the American Heart Association annual scientific sessions.
Of the 98 perioperative deaths, 18 occurred during the lead extraction procedure.
Another 41 patients (16%) required urgent cardiac surgery, of whom 14 (34%) died during or in the immediate postoperative period after surgery.
“Thus, while overall rate of major complications remains low, there exists a significant risk of urgent cardiac surgery and mortality during transvenous lead extractions [TLE] performed in the ‘real world.’ Appropriate training of all personnel involved and optimal cardiothoracic surgical back-up at centers performing TLE is imperative,” Dr. Sood of the Southcoast Health System, Fall River, Mass., concluded.
The analysis is the largest real-world cohort of TLE involving 11,304 patients with an implantable cardioverter defibrillator (ICD) in the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR) ICD Registry with lead extraction data submitted between April 2010 and July 2012. Major complication was a combined endpoint of major operative complications, postoperative or in-hospital mortality, as defined by the NCDR ICD Registry.
The 258 complications included 62 cardiac arrests, 55 pericardial tamponades, 47 pneumothoraces, and 40 cardiac perforations.
In multivariate analysis, significant predictors of any complication were female sex (adjusted odds ratio, 1.46), heart failure admission vs. lead extraction admission (OR, 2.6), noncardiac admission vs. lead extraction admission (OR, 2.4), lead-only procedure vs. extraction during generator change/upgrade (OR, 1.76), age of lead (OR, 1.08), and clinical status requiring lead replacement (OR, 2.2). Dr. Sood reported.
Among lead characteristics, multivariate predictors of major perioperative complications included at least three concurrent leads extracted (OR, 2.13), longer implant duration (OR, 1.13), flat coil design vs. round (OR, 2.68), greater proximal coil surface area (OR, 1.04), and dislodgement of other leads during extraction (OR, 3.97), he noted.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: Appropriate training and optimal cardiothoracic surgical backup is necessary at all centers performing lead extractions, because of a significant risk of urgent cardiac surgery and death.
Major finding: The major complication rate was 2.3% and mortality rate 0.9%.
Data source: Retrospective analysis of 11,304 patients with transvenous lead extraction in the NCDR ICD Registry.
Disclosures: The study was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry. Dr. Sood reported having no financial disclosures. Three coauthors reported relationships with device makers.
CABG plus mitral repair put under spotlight
CHICAGO – Early results from a randomized trial cast doubt on the benefits of routinely repairing a leaky mitral valve during coronary artery bypass grafting in patients with moderate ischemic mitral regurgitation.
At 1 year, there was no significant difference between patients undergoing CABG alone or CABG plus mitral repair in the primary endpoint of left ventricular reverse modeling, as defined by changes in LV end-systolic volume index (LVESVI) at 1 year (z score 0.50).
Both groups achieved significant reductions in LVESVI, with a median reduction of about 6 mL/m2 from baseline, Dr. Robert Michler, chair of cardiovascular and thoracic surgery at Montefiore Medical Center, New York, reported at the American Heart Association scientific sessions.
At 1 year, patients who underwent CABG and mitral valve repair had significantly less residual moderate or severe mitral regurgitation (11% vs. 31%; P < .001).
On the other hand, the combination procedure was associated with significantly higher rates of any neurologic event (9.6% vs. 3.1%; P = .03), longer cross-clamp (117 vs. 74 minutes) and cardiopulmonary bypass times (163 vs. 106 minutes; P values both < .001), and longer ICU (4.8 vs. 4.0 days; P = .006) and hospital length of stay (11.3 vs. 9.4 days; P = .002), according to the results, also published online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1410490]).
“The trial did not demonstrate a clinically meaningful advantage to the routine addition of mitral valve repair to CABG,” Dr. Michler said, on behalf of the Cardiothoracic Surgical Trials Network investigators.
The 2014 AHA/American College of Cardiology mitral valve guidelines give a weak class IIb recommendation for mitral valve repair for patients with chronic mitral regurgitation (MR) who are undergoing other cardiac surgery, he noted.
The study evenly randomized 301 patients with moderate ischemic mitral regurgitation to CABG alone or CABG plus valve repair with an undersized ring (average, 28.3 mm for men and 27.1 mm for women).
The mean LVESVI at baseline was 54.8 mL/m2 in the CABG-alone group and 59.6 mL/m2 in the combined procedure group.
At 12 months, mean LVESVIs were 46.1 mL/m2 and 49.6 mL/m2, respectively.
The trial lacked a clinical primary endpoint, and longer follow-up is ongoing to determine whether the lower incidence of moderate or severe MR at 1 year will translate into a net clinical benefit for patients undergoing CABG plus mitral repair, Dr. Michler said.
Designated discussant Dr. David Adams, director of Mount Sinai Hospital’s mitral valve repair reference center in New York, cautioned the audience on the length of the study and called for a full 5 years of follow-up rather than the 2 years as planned.
“Ischemic mitral regurgitation is a disease that hurts you over time. That’s in patients that have had MI, had previous CABG, had attempted mitral valve repair, and had PCI [percutaneous coronary intervention]. So we need much longer term follow-up of this very important data set to really understand its implications,” he said.
In light of roughly half of the patients being in heart failure at baseline, session cochair Dr. Robert Bonow, vice chair of medicine, Northwestern University, Chicago, questioned whether there were differences in outcomes related to changes in baseline ejection fraction (EF) or whether improvement in EF in patients with low EF correlated with reduction in mitral regurgitation with CABG alone.
What is known right now is that mean LVEF increased to the same degree after CABG in both groups, Dr. Michler responded. This was true despite this being a “sick population of patients,” with more than half having diabetes, 50% with heart failure, 20% with renal insufficiency, 10% with prior stroke, and a mean ejection fraction of 40% in both groups.
“What we have yet to identify and plan to explore is the correlation between reverse ventricular remodeling, ejection fraction, and outcome, meaning both the degree of mitral regurgitation and whether there is any signal with respect to repeat hospitalizations, heart failure, or possibly even mortality,” Dr. Michler said.
Both Dr. Michler and Dr. Adams remarked that surgery was extremely safe for the CABG alone and CABG plus MR groups, as reflected by the low mortality at 30 days (2.7% vs. 1.3%) and 1 year (7.3% vs. 6.7%).
The composite endpoint of major adverse cardiac or cerebrovascular events was also similar between groups.
The trial was funded by the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Michler reported grant support from NIH during the conduct of the study. Dr. Adams reported coinventing a mitral valve repair ring.
CHICAGO – Early results from a randomized trial cast doubt on the benefits of routinely repairing a leaky mitral valve during coronary artery bypass grafting in patients with moderate ischemic mitral regurgitation.
At 1 year, there was no significant difference between patients undergoing CABG alone or CABG plus mitral repair in the primary endpoint of left ventricular reverse modeling, as defined by changes in LV end-systolic volume index (LVESVI) at 1 year (z score 0.50).
Both groups achieved significant reductions in LVESVI, with a median reduction of about 6 mL/m2 from baseline, Dr. Robert Michler, chair of cardiovascular and thoracic surgery at Montefiore Medical Center, New York, reported at the American Heart Association scientific sessions.
At 1 year, patients who underwent CABG and mitral valve repair had significantly less residual moderate or severe mitral regurgitation (11% vs. 31%; P < .001).
On the other hand, the combination procedure was associated with significantly higher rates of any neurologic event (9.6% vs. 3.1%; P = .03), longer cross-clamp (117 vs. 74 minutes) and cardiopulmonary bypass times (163 vs. 106 minutes; P values both < .001), and longer ICU (4.8 vs. 4.0 days; P = .006) and hospital length of stay (11.3 vs. 9.4 days; P = .002), according to the results, also published online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1410490]).
“The trial did not demonstrate a clinically meaningful advantage to the routine addition of mitral valve repair to CABG,” Dr. Michler said, on behalf of the Cardiothoracic Surgical Trials Network investigators.
The 2014 AHA/American College of Cardiology mitral valve guidelines give a weak class IIb recommendation for mitral valve repair for patients with chronic mitral regurgitation (MR) who are undergoing other cardiac surgery, he noted.
The study evenly randomized 301 patients with moderate ischemic mitral regurgitation to CABG alone or CABG plus valve repair with an undersized ring (average, 28.3 mm for men and 27.1 mm for women).
The mean LVESVI at baseline was 54.8 mL/m2 in the CABG-alone group and 59.6 mL/m2 in the combined procedure group.
At 12 months, mean LVESVIs were 46.1 mL/m2 and 49.6 mL/m2, respectively.
The trial lacked a clinical primary endpoint, and longer follow-up is ongoing to determine whether the lower incidence of moderate or severe MR at 1 year will translate into a net clinical benefit for patients undergoing CABG plus mitral repair, Dr. Michler said.
Designated discussant Dr. David Adams, director of Mount Sinai Hospital’s mitral valve repair reference center in New York, cautioned the audience on the length of the study and called for a full 5 years of follow-up rather than the 2 years as planned.
“Ischemic mitral regurgitation is a disease that hurts you over time. That’s in patients that have had MI, had previous CABG, had attempted mitral valve repair, and had PCI [percutaneous coronary intervention]. So we need much longer term follow-up of this very important data set to really understand its implications,” he said.
In light of roughly half of the patients being in heart failure at baseline, session cochair Dr. Robert Bonow, vice chair of medicine, Northwestern University, Chicago, questioned whether there were differences in outcomes related to changes in baseline ejection fraction (EF) or whether improvement in EF in patients with low EF correlated with reduction in mitral regurgitation with CABG alone.
What is known right now is that mean LVEF increased to the same degree after CABG in both groups, Dr. Michler responded. This was true despite this being a “sick population of patients,” with more than half having diabetes, 50% with heart failure, 20% with renal insufficiency, 10% with prior stroke, and a mean ejection fraction of 40% in both groups.
“What we have yet to identify and plan to explore is the correlation between reverse ventricular remodeling, ejection fraction, and outcome, meaning both the degree of mitral regurgitation and whether there is any signal with respect to repeat hospitalizations, heart failure, or possibly even mortality,” Dr. Michler said.
Both Dr. Michler and Dr. Adams remarked that surgery was extremely safe for the CABG alone and CABG plus MR groups, as reflected by the low mortality at 30 days (2.7% vs. 1.3%) and 1 year (7.3% vs. 6.7%).
The composite endpoint of major adverse cardiac or cerebrovascular events was also similar between groups.
The trial was funded by the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Michler reported grant support from NIH during the conduct of the study. Dr. Adams reported coinventing a mitral valve repair ring.
CHICAGO – Early results from a randomized trial cast doubt on the benefits of routinely repairing a leaky mitral valve during coronary artery bypass grafting in patients with moderate ischemic mitral regurgitation.
At 1 year, there was no significant difference between patients undergoing CABG alone or CABG plus mitral repair in the primary endpoint of left ventricular reverse modeling, as defined by changes in LV end-systolic volume index (LVESVI) at 1 year (z score 0.50).
Both groups achieved significant reductions in LVESVI, with a median reduction of about 6 mL/m2 from baseline, Dr. Robert Michler, chair of cardiovascular and thoracic surgery at Montefiore Medical Center, New York, reported at the American Heart Association scientific sessions.
At 1 year, patients who underwent CABG and mitral valve repair had significantly less residual moderate or severe mitral regurgitation (11% vs. 31%; P < .001).
On the other hand, the combination procedure was associated with significantly higher rates of any neurologic event (9.6% vs. 3.1%; P = .03), longer cross-clamp (117 vs. 74 minutes) and cardiopulmonary bypass times (163 vs. 106 minutes; P values both < .001), and longer ICU (4.8 vs. 4.0 days; P = .006) and hospital length of stay (11.3 vs. 9.4 days; P = .002), according to the results, also published online (N. Engl. J. Med. 2014 [doi: 10.1056/NEJMoa1410490]).
“The trial did not demonstrate a clinically meaningful advantage to the routine addition of mitral valve repair to CABG,” Dr. Michler said, on behalf of the Cardiothoracic Surgical Trials Network investigators.
The 2014 AHA/American College of Cardiology mitral valve guidelines give a weak class IIb recommendation for mitral valve repair for patients with chronic mitral regurgitation (MR) who are undergoing other cardiac surgery, he noted.
The study evenly randomized 301 patients with moderate ischemic mitral regurgitation to CABG alone or CABG plus valve repair with an undersized ring (average, 28.3 mm for men and 27.1 mm for women).
The mean LVESVI at baseline was 54.8 mL/m2 in the CABG-alone group and 59.6 mL/m2 in the combined procedure group.
At 12 months, mean LVESVIs were 46.1 mL/m2 and 49.6 mL/m2, respectively.
The trial lacked a clinical primary endpoint, and longer follow-up is ongoing to determine whether the lower incidence of moderate or severe MR at 1 year will translate into a net clinical benefit for patients undergoing CABG plus mitral repair, Dr. Michler said.
Designated discussant Dr. David Adams, director of Mount Sinai Hospital’s mitral valve repair reference center in New York, cautioned the audience on the length of the study and called for a full 5 years of follow-up rather than the 2 years as planned.
“Ischemic mitral regurgitation is a disease that hurts you over time. That’s in patients that have had MI, had previous CABG, had attempted mitral valve repair, and had PCI [percutaneous coronary intervention]. So we need much longer term follow-up of this very important data set to really understand its implications,” he said.
In light of roughly half of the patients being in heart failure at baseline, session cochair Dr. Robert Bonow, vice chair of medicine, Northwestern University, Chicago, questioned whether there were differences in outcomes related to changes in baseline ejection fraction (EF) or whether improvement in EF in patients with low EF correlated with reduction in mitral regurgitation with CABG alone.
What is known right now is that mean LVEF increased to the same degree after CABG in both groups, Dr. Michler responded. This was true despite this being a “sick population of patients,” with more than half having diabetes, 50% with heart failure, 20% with renal insufficiency, 10% with prior stroke, and a mean ejection fraction of 40% in both groups.
“What we have yet to identify and plan to explore is the correlation between reverse ventricular remodeling, ejection fraction, and outcome, meaning both the degree of mitral regurgitation and whether there is any signal with respect to repeat hospitalizations, heart failure, or possibly even mortality,” Dr. Michler said.
Both Dr. Michler and Dr. Adams remarked that surgery was extremely safe for the CABG alone and CABG plus MR groups, as reflected by the low mortality at 30 days (2.7% vs. 1.3%) and 1 year (7.3% vs. 6.7%).
The composite endpoint of major adverse cardiac or cerebrovascular events was also similar between groups.
The trial was funded by the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Michler reported grant support from NIH during the conduct of the study. Dr. Adams reported coinventing a mitral valve repair ring.
AT THE AHA SCIENTIFIC SESSIONS 2014
Key clinical point: CABG plus mitral repair did not significantly improve left ventricular remodeling at 1 year, and was associated with some untoward events.
Major finding: At 12 months, mean LVESVI was 46.1 mL/m2 with CABG alone and 49.6 mL/m2 with CABG plus mitral repair.
Data source: A randomized trial in 301 patients with moderate mitral regurgitation.
Disclosures: The trial was funded by the National Institutes of Health and the Canadian Institutes for Health Research. Dr. Michler reported grant support from NIH during the conduct of the study. Dr. Adams reported coinventing a mitral valve repair ring.
TAVR survival improved during 2009-2012
CHICAGO – During 2009-2012, U.S. interventionalists and surgeons performing transcatheter aortic valve replacements made substantial strides in improving the safety and efficacy of the procedure, cutting first-year mortality rates by about a third, according to a review of more than 1,000 patients treated during that time.
Factors contributing to these gains included patient selection and an increased percentage of patients who emerged from their transcatheter aortic valve replacement (TAVR) procedure with mild, trace, or no paravalvular leak. Other developments that may have boosted patient survival included increased operator experience and changes in the TAVR delivery system, Dr. Nirat Beohar said at the American Heart Association scientific sessions.
“I think most important has been sizing the valve, which seemed to evolve” during the course of the 3-year period studied, said Dr. Beohar, director of the cardiac catheterization laboratory at Mount Sinai Medical Center in Miami Beach.
His analysis used data collected during the PARTNER continued-access program that allowed centers that had participated in the PARTNER trial, the first U.S. pivotal trial of the SAPIEN TAVR system marketed by Edwards, to continue to offer TAVR to patients during March 2009-January 2012, a period that mostly preceded Food and Drug Administration approval of the SAPIEN system for U.S. use in November 2011.
To assess changes in outcomes over time, Dr. Beohar and his associates divided the 1,063 patients who received TAVR through the continued-access program during this time into three tertiles: The 353 patients who underwent TAVR during March 2009-July 2010, 355 patients treated during July 2010-March 2011, and 355 patients treated during March 2011-January 2012.
Compared with patients treated during the earliest period, those treated during the most recent period were significantly older, with an average age of 86 years versus 84 years at the start of the program. The most recent patients also had a significantly reduced prevalence of comorbidities. For example, chronic obstructive pulmonary disease dropped from a 52% prevalence early to 37% during the most recent period. Patients with a porcelain aorta fell from 7% to 1%, and patients considered inoperable fell from 40% of the first tertile cohort to 8% of the patients in the most recent tertile.
All 1,063 patients in the analysis underwent TAVR using transfemoral access, but the use of fully percutaneous access (as opposed to surgical cutdown access) doubled from 31% during the first period to 62% during the most recent period.
All-cause mortality improved significantly at both 1-year and 2-year follow-up. Patients treated during the first time period, March 2009-July 2010, had a 23% mortality rate after 1 year and 35% after 2 years. During the most recent period studied, March 2011-January 2012, first-year deaths fell to 14%, and mortality was 24% after 2-year follow-up, said Dr. Beohar.
Despite this improvement in survival, the rate of nonfatal major complications during the first 30 days after treatment remained stable, occurring in 17% of patients in the first tertile, 13% in the second tertile, and 15% in the third tertile.
In contrast, the prevalence of moderate or severe paravalvular leak (PVL) following TAVR fell during the entire period studied and appeared to strongly correlate with long-term survival. The prevalence of moderate or severe PVL by 30 days after TAVR was 19% among patients in the first tertile and 10% among those in the third tertile, a statistically significant difference.
In a multivariate analysis that adjusted for several demographic and clinical factors, the appearance of moderate or severe PVL during the 30 days following TAVR was linked with a statistically significant, roughly fourfold increase in the rate of 1-year mortality, Dr. Beohar reported. Other factors that had statistically significant association with 1-year mortality were the occurrence of a nonfatal major complication, which linked with a threefold increased mortality rate; reduced renal function, which was associated with a roughly doubled mortality rate; male sex, which was tied to a nearly doubled mortality rate; and treatment with TAVR during the most recent period, which was linked with an approximately 20% reduction in mortality.
Another factor that distinguished the tertile 1 patients from those treated in tertile 3 was that the prevailing device used during the most recent period was the SAPIEN XT device, while patients treated during the first two tertile periods primarily received treatment using the original SAPIEN TAVR system.
On Twitter @mitchelzoler
CHICAGO – During 2009-2012, U.S. interventionalists and surgeons performing transcatheter aortic valve replacements made substantial strides in improving the safety and efficacy of the procedure, cutting first-year mortality rates by about a third, according to a review of more than 1,000 patients treated during that time.
Factors contributing to these gains included patient selection and an increased percentage of patients who emerged from their transcatheter aortic valve replacement (TAVR) procedure with mild, trace, or no paravalvular leak. Other developments that may have boosted patient survival included increased operator experience and changes in the TAVR delivery system, Dr. Nirat Beohar said at the American Heart Association scientific sessions.
“I think most important has been sizing the valve, which seemed to evolve” during the course of the 3-year period studied, said Dr. Beohar, director of the cardiac catheterization laboratory at Mount Sinai Medical Center in Miami Beach.
His analysis used data collected during the PARTNER continued-access program that allowed centers that had participated in the PARTNER trial, the first U.S. pivotal trial of the SAPIEN TAVR system marketed by Edwards, to continue to offer TAVR to patients during March 2009-January 2012, a period that mostly preceded Food and Drug Administration approval of the SAPIEN system for U.S. use in November 2011.
To assess changes in outcomes over time, Dr. Beohar and his associates divided the 1,063 patients who received TAVR through the continued-access program during this time into three tertiles: The 353 patients who underwent TAVR during March 2009-July 2010, 355 patients treated during July 2010-March 2011, and 355 patients treated during March 2011-January 2012.
Compared with patients treated during the earliest period, those treated during the most recent period were significantly older, with an average age of 86 years versus 84 years at the start of the program. The most recent patients also had a significantly reduced prevalence of comorbidities. For example, chronic obstructive pulmonary disease dropped from a 52% prevalence early to 37% during the most recent period. Patients with a porcelain aorta fell from 7% to 1%, and patients considered inoperable fell from 40% of the first tertile cohort to 8% of the patients in the most recent tertile.
All 1,063 patients in the analysis underwent TAVR using transfemoral access, but the use of fully percutaneous access (as opposed to surgical cutdown access) doubled from 31% during the first period to 62% during the most recent period.
All-cause mortality improved significantly at both 1-year and 2-year follow-up. Patients treated during the first time period, March 2009-July 2010, had a 23% mortality rate after 1 year and 35% after 2 years. During the most recent period studied, March 2011-January 2012, first-year deaths fell to 14%, and mortality was 24% after 2-year follow-up, said Dr. Beohar.
Despite this improvement in survival, the rate of nonfatal major complications during the first 30 days after treatment remained stable, occurring in 17% of patients in the first tertile, 13% in the second tertile, and 15% in the third tertile.
In contrast, the prevalence of moderate or severe paravalvular leak (PVL) following TAVR fell during the entire period studied and appeared to strongly correlate with long-term survival. The prevalence of moderate or severe PVL by 30 days after TAVR was 19% among patients in the first tertile and 10% among those in the third tertile, a statistically significant difference.
In a multivariate analysis that adjusted for several demographic and clinical factors, the appearance of moderate or severe PVL during the 30 days following TAVR was linked with a statistically significant, roughly fourfold increase in the rate of 1-year mortality, Dr. Beohar reported. Other factors that had statistically significant association with 1-year mortality were the occurrence of a nonfatal major complication, which linked with a threefold increased mortality rate; reduced renal function, which was associated with a roughly doubled mortality rate; male sex, which was tied to a nearly doubled mortality rate; and treatment with TAVR during the most recent period, which was linked with an approximately 20% reduction in mortality.
Another factor that distinguished the tertile 1 patients from those treated in tertile 3 was that the prevailing device used during the most recent period was the SAPIEN XT device, while patients treated during the first two tertile periods primarily received treatment using the original SAPIEN TAVR system.
On Twitter @mitchelzoler
CHICAGO – During 2009-2012, U.S. interventionalists and surgeons performing transcatheter aortic valve replacements made substantial strides in improving the safety and efficacy of the procedure, cutting first-year mortality rates by about a third, according to a review of more than 1,000 patients treated during that time.
Factors contributing to these gains included patient selection and an increased percentage of patients who emerged from their transcatheter aortic valve replacement (TAVR) procedure with mild, trace, or no paravalvular leak. Other developments that may have boosted patient survival included increased operator experience and changes in the TAVR delivery system, Dr. Nirat Beohar said at the American Heart Association scientific sessions.
“I think most important has been sizing the valve, which seemed to evolve” during the course of the 3-year period studied, said Dr. Beohar, director of the cardiac catheterization laboratory at Mount Sinai Medical Center in Miami Beach.
His analysis used data collected during the PARTNER continued-access program that allowed centers that had participated in the PARTNER trial, the first U.S. pivotal trial of the SAPIEN TAVR system marketed by Edwards, to continue to offer TAVR to patients during March 2009-January 2012, a period that mostly preceded Food and Drug Administration approval of the SAPIEN system for U.S. use in November 2011.
To assess changes in outcomes over time, Dr. Beohar and his associates divided the 1,063 patients who received TAVR through the continued-access program during this time into three tertiles: The 353 patients who underwent TAVR during March 2009-July 2010, 355 patients treated during July 2010-March 2011, and 355 patients treated during March 2011-January 2012.
Compared with patients treated during the earliest period, those treated during the most recent period were significantly older, with an average age of 86 years versus 84 years at the start of the program. The most recent patients also had a significantly reduced prevalence of comorbidities. For example, chronic obstructive pulmonary disease dropped from a 52% prevalence early to 37% during the most recent period. Patients with a porcelain aorta fell from 7% to 1%, and patients considered inoperable fell from 40% of the first tertile cohort to 8% of the patients in the most recent tertile.
All 1,063 patients in the analysis underwent TAVR using transfemoral access, but the use of fully percutaneous access (as opposed to surgical cutdown access) doubled from 31% during the first period to 62% during the most recent period.
All-cause mortality improved significantly at both 1-year and 2-year follow-up. Patients treated during the first time period, March 2009-July 2010, had a 23% mortality rate after 1 year and 35% after 2 years. During the most recent period studied, March 2011-January 2012, first-year deaths fell to 14%, and mortality was 24% after 2-year follow-up, said Dr. Beohar.
Despite this improvement in survival, the rate of nonfatal major complications during the first 30 days after treatment remained stable, occurring in 17% of patients in the first tertile, 13% in the second tertile, and 15% in the third tertile.
In contrast, the prevalence of moderate or severe paravalvular leak (PVL) following TAVR fell during the entire period studied and appeared to strongly correlate with long-term survival. The prevalence of moderate or severe PVL by 30 days after TAVR was 19% among patients in the first tertile and 10% among those in the third tertile, a statistically significant difference.
In a multivariate analysis that adjusted for several demographic and clinical factors, the appearance of moderate or severe PVL during the 30 days following TAVR was linked with a statistically significant, roughly fourfold increase in the rate of 1-year mortality, Dr. Beohar reported. Other factors that had statistically significant association with 1-year mortality were the occurrence of a nonfatal major complication, which linked with a threefold increased mortality rate; reduced renal function, which was associated with a roughly doubled mortality rate; male sex, which was tied to a nearly doubled mortality rate; and treatment with TAVR during the most recent period, which was linked with an approximately 20% reduction in mortality.
Another factor that distinguished the tertile 1 patients from those treated in tertile 3 was that the prevailing device used during the most recent period was the SAPIEN XT device, while patients treated during the first two tertile periods primarily received treatment using the original SAPIEN TAVR system.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: During May 2009-January 2012, survival of patients undergoing transcatheter aortic valve replacement using Sapien devices showed steady improvement.
Major finding: Patients treated during March 2009-July 2010 had a 23% 1-year mortality rate, compared with a 14% rate during March 2011-January 2012.
Data source: Review of 1,063 patients who underwent transcatheter aortic valve replacement as part of the PARTNER continued access program during March 2009-January 2012.
Disclosures: The PARTNER studies and the continued access program was sponsored by Edwards, the company that markets SAPIEN TAVR devices. Dr. Beohar has been a PARTNER investigator but said he had no disclosures.
Implications of cholesterol guidelines for cardiology practices
CHICAGO – Cardiologists certainly have their work cut out in order to bring their patients into concordance with the 2013 American College of Cardiology/American Heart Association cholesterol guidelines, according to Dr. Thomas M. Maddox.
An analysis of nearly 1.2 million patients in U.S. outpatient cardiology practices showed that one in three who appeared to have an indication for statin therapy under the latest guidelines weren’t on a statin as of 2012. That constitutes a sizable “statin gap” that cardiologists need to address, he said at the American Heart Association scientific sessions.
Dr. Maddox presented an analysis of 1,174,535 adult patients under cardiologists’ care during 2008-2012 in more than 100 U.S. outpatient cardiology practices participating in the voluntary National Cardiovascular Data Registry’s Practice Innovation and Clinical Excellence Registry (NCDR PINNACLE). Under this national office-based quality improvement program sponsored by the ACC, patient electronic medical record (EMR) data gets uploaded to the registry nightly.
The 2013 ACC/AHA cholesterol guidelines in some ways greatly simplified patient management. The guidelines redefined the risk groups warranting treatment: basically, patients with known atherosclerotic cardiovascular disease (ASCVD), diabetes, an off-treatment LDL of 190 mg/dL or more, or a 10-year ASCVD risk of 7.5% or greater using the risk calculator incorporated in the guidelines (Circulation 2014; 129:S1-45). Also, physicians were advised to use fixed-dose statins and no longer to treat to an LDL target, thereby making repeated LDL testing unnecessary.
The purposes of this new NCDR PINNACLE study were to evaluate the potential impact of the new guidelines on current cardiology practice through an assessment of current treatment and testing patterns, and to make a determination of the scope of changes necessary under the 2013 guidelines, explained Dr. Maddox, a cardiologist at the Veterans Affairs Eastern Colorado Health Care System and the University of Colorado at Denver.
Under the new guidelines, 1,129,205 adult cardiology patients, or 96% of the study population, appeared to be candidates for statin therapy, most often because they had known ASCVD, as was the case in 88%, or diabetes without known ASCVD, accounting for another 6%.
Among the statin-eligible patients, 29% were not on any lipid-lowering therapy, and another 3% were on nonstatin lipid-lowering agents only, which is not recommended in the guidelines. Thus, 32% of the cardiologists’ patients for whom statin therapy appeared to be indicated under the 2013 guidelines weren’t on it.
In addition, 29% of statin-eligible patients were on combined lipid-lowering therapy with a statin plus a nonstatin, such as niacin, a fibrate, or ezetimibe. The guidelines don’t recommend the use of nonstatins because of the lack of evidence of clinical benefit, so cardiologists will want to reconsider their use of combination therapy in this sizable group. The major caveat here is that the guidelines are likely to be revised to embrace the selective use of a moderate-intensity statin plus ezetimibe on the basis of the positive findings of the IMPROVE-IT trial, also presented at the AHA meeting, Dr. Maddox noted.
The registry analysis also pointed to a need to reduce repeated LDL testing, which the guidelines characterize as costly, inconvenient, and unnecessary. Nearly 21% of subjects had at least two LDL assessments during the 4-year period, and 7% had more than four. And those figures probably underestimate the true rate of LDL testing, since many patients may have also had LDL measurements taken in primary care settings.
Several audience members rose to decry the one-in-three-patient statin gap as evidence of widespread substandard care by cardiologists, especially given that 28% of the patients with known ASCVD and 36% with diabetes were not receiving any lipid-lowering therapy, contrary to recommendations both in the current ACC/AHA guidelines and the guidelines in place in 2012. There is good evidence to show that putting such patients on statin therapy would result in roughly a 25% reduction in cardiovascular events.
But Dr. Maddox took a more sanguine view of the statin gap. Although it’s likely there is some heterogeneity in clinical practice that needs to be corrected, he cautioned that the limitations of an analysis based upon EMR data must be borne in mind. Some cardiologists probably didn’t record the use of statins at every visit, and they may not have always reliably documented patients’ intolerance of statins in the EMR.
The NCDR PINNACLE Registry is supported by the American College of Cardiology Foundation. Dr. Maddox reported having no relevant financial conflicts of interest.
CHICAGO – Cardiologists certainly have their work cut out in order to bring their patients into concordance with the 2013 American College of Cardiology/American Heart Association cholesterol guidelines, according to Dr. Thomas M. Maddox.
An analysis of nearly 1.2 million patients in U.S. outpatient cardiology practices showed that one in three who appeared to have an indication for statin therapy under the latest guidelines weren’t on a statin as of 2012. That constitutes a sizable “statin gap” that cardiologists need to address, he said at the American Heart Association scientific sessions.
Dr. Maddox presented an analysis of 1,174,535 adult patients under cardiologists’ care during 2008-2012 in more than 100 U.S. outpatient cardiology practices participating in the voluntary National Cardiovascular Data Registry’s Practice Innovation and Clinical Excellence Registry (NCDR PINNACLE). Under this national office-based quality improvement program sponsored by the ACC, patient electronic medical record (EMR) data gets uploaded to the registry nightly.
The 2013 ACC/AHA cholesterol guidelines in some ways greatly simplified patient management. The guidelines redefined the risk groups warranting treatment: basically, patients with known atherosclerotic cardiovascular disease (ASCVD), diabetes, an off-treatment LDL of 190 mg/dL or more, or a 10-year ASCVD risk of 7.5% or greater using the risk calculator incorporated in the guidelines (Circulation 2014; 129:S1-45). Also, physicians were advised to use fixed-dose statins and no longer to treat to an LDL target, thereby making repeated LDL testing unnecessary.
The purposes of this new NCDR PINNACLE study were to evaluate the potential impact of the new guidelines on current cardiology practice through an assessment of current treatment and testing patterns, and to make a determination of the scope of changes necessary under the 2013 guidelines, explained Dr. Maddox, a cardiologist at the Veterans Affairs Eastern Colorado Health Care System and the University of Colorado at Denver.
Under the new guidelines, 1,129,205 adult cardiology patients, or 96% of the study population, appeared to be candidates for statin therapy, most often because they had known ASCVD, as was the case in 88%, or diabetes without known ASCVD, accounting for another 6%.
Among the statin-eligible patients, 29% were not on any lipid-lowering therapy, and another 3% were on nonstatin lipid-lowering agents only, which is not recommended in the guidelines. Thus, 32% of the cardiologists’ patients for whom statin therapy appeared to be indicated under the 2013 guidelines weren’t on it.
In addition, 29% of statin-eligible patients were on combined lipid-lowering therapy with a statin plus a nonstatin, such as niacin, a fibrate, or ezetimibe. The guidelines don’t recommend the use of nonstatins because of the lack of evidence of clinical benefit, so cardiologists will want to reconsider their use of combination therapy in this sizable group. The major caveat here is that the guidelines are likely to be revised to embrace the selective use of a moderate-intensity statin plus ezetimibe on the basis of the positive findings of the IMPROVE-IT trial, also presented at the AHA meeting, Dr. Maddox noted.
The registry analysis also pointed to a need to reduce repeated LDL testing, which the guidelines characterize as costly, inconvenient, and unnecessary. Nearly 21% of subjects had at least two LDL assessments during the 4-year period, and 7% had more than four. And those figures probably underestimate the true rate of LDL testing, since many patients may have also had LDL measurements taken in primary care settings.
Several audience members rose to decry the one-in-three-patient statin gap as evidence of widespread substandard care by cardiologists, especially given that 28% of the patients with known ASCVD and 36% with diabetes were not receiving any lipid-lowering therapy, contrary to recommendations both in the current ACC/AHA guidelines and the guidelines in place in 2012. There is good evidence to show that putting such patients on statin therapy would result in roughly a 25% reduction in cardiovascular events.
But Dr. Maddox took a more sanguine view of the statin gap. Although it’s likely there is some heterogeneity in clinical practice that needs to be corrected, he cautioned that the limitations of an analysis based upon EMR data must be borne in mind. Some cardiologists probably didn’t record the use of statins at every visit, and they may not have always reliably documented patients’ intolerance of statins in the EMR.
The NCDR PINNACLE Registry is supported by the American College of Cardiology Foundation. Dr. Maddox reported having no relevant financial conflicts of interest.
CHICAGO – Cardiologists certainly have their work cut out in order to bring their patients into concordance with the 2013 American College of Cardiology/American Heart Association cholesterol guidelines, according to Dr. Thomas M. Maddox.
An analysis of nearly 1.2 million patients in U.S. outpatient cardiology practices showed that one in three who appeared to have an indication for statin therapy under the latest guidelines weren’t on a statin as of 2012. That constitutes a sizable “statin gap” that cardiologists need to address, he said at the American Heart Association scientific sessions.
Dr. Maddox presented an analysis of 1,174,535 adult patients under cardiologists’ care during 2008-2012 in more than 100 U.S. outpatient cardiology practices participating in the voluntary National Cardiovascular Data Registry’s Practice Innovation and Clinical Excellence Registry (NCDR PINNACLE). Under this national office-based quality improvement program sponsored by the ACC, patient electronic medical record (EMR) data gets uploaded to the registry nightly.
The 2013 ACC/AHA cholesterol guidelines in some ways greatly simplified patient management. The guidelines redefined the risk groups warranting treatment: basically, patients with known atherosclerotic cardiovascular disease (ASCVD), diabetes, an off-treatment LDL of 190 mg/dL or more, or a 10-year ASCVD risk of 7.5% or greater using the risk calculator incorporated in the guidelines (Circulation 2014; 129:S1-45). Also, physicians were advised to use fixed-dose statins and no longer to treat to an LDL target, thereby making repeated LDL testing unnecessary.
The purposes of this new NCDR PINNACLE study were to evaluate the potential impact of the new guidelines on current cardiology practice through an assessment of current treatment and testing patterns, and to make a determination of the scope of changes necessary under the 2013 guidelines, explained Dr. Maddox, a cardiologist at the Veterans Affairs Eastern Colorado Health Care System and the University of Colorado at Denver.
Under the new guidelines, 1,129,205 adult cardiology patients, or 96% of the study population, appeared to be candidates for statin therapy, most often because they had known ASCVD, as was the case in 88%, or diabetes without known ASCVD, accounting for another 6%.
Among the statin-eligible patients, 29% were not on any lipid-lowering therapy, and another 3% were on nonstatin lipid-lowering agents only, which is not recommended in the guidelines. Thus, 32% of the cardiologists’ patients for whom statin therapy appeared to be indicated under the 2013 guidelines weren’t on it.
In addition, 29% of statin-eligible patients were on combined lipid-lowering therapy with a statin plus a nonstatin, such as niacin, a fibrate, or ezetimibe. The guidelines don’t recommend the use of nonstatins because of the lack of evidence of clinical benefit, so cardiologists will want to reconsider their use of combination therapy in this sizable group. The major caveat here is that the guidelines are likely to be revised to embrace the selective use of a moderate-intensity statin plus ezetimibe on the basis of the positive findings of the IMPROVE-IT trial, also presented at the AHA meeting, Dr. Maddox noted.
The registry analysis also pointed to a need to reduce repeated LDL testing, which the guidelines characterize as costly, inconvenient, and unnecessary. Nearly 21% of subjects had at least two LDL assessments during the 4-year period, and 7% had more than four. And those figures probably underestimate the true rate of LDL testing, since many patients may have also had LDL measurements taken in primary care settings.
Several audience members rose to decry the one-in-three-patient statin gap as evidence of widespread substandard care by cardiologists, especially given that 28% of the patients with known ASCVD and 36% with diabetes were not receiving any lipid-lowering therapy, contrary to recommendations both in the current ACC/AHA guidelines and the guidelines in place in 2012. There is good evidence to show that putting such patients on statin therapy would result in roughly a 25% reduction in cardiovascular events.
But Dr. Maddox took a more sanguine view of the statin gap. Although it’s likely there is some heterogeneity in clinical practice that needs to be corrected, he cautioned that the limitations of an analysis based upon EMR data must be borne in mind. Some cardiologists probably didn’t record the use of statins at every visit, and they may not have always reliably documented patients’ intolerance of statins in the EMR.
The NCDR PINNACLE Registry is supported by the American College of Cardiology Foundation. Dr. Maddox reported having no relevant financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point: As U.S. cardiologists increasingly “get with the guidelines” regarding cholesterol lowering, expect to see large increases in statin use, much less prescribing of nonstatin therapies, and a lot less repeat LDL testing.
Major finding: Nearly one in three U.S. patients under a cardiologist’s care who appear to have an indication for statin therapy under the 2013 ACC/AHA cholesterol guidelines weren’t on a statin as of 2012.
Data source: An analysis of nearly 1.2 million patients in an ongoing nationwide voluntary prospective registry aimed at improving the quality of cardiovascular care.
Disclosures: The NCDR PINNACLE Registry is supported by the American College of Cardiology Foundation. The presenter reported having no relevant financial conflicts.