User login
Dr. Matt Kalaycio’s top 10 hematologic oncology abstracts for ASCO 2016
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
Hematology News’ Editor-in-Chief Matt Kalaycio selected the following as his “top 10” picks for hematologic oncology abstracts at ASCO 2016:
Abstract 7000: Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML
Comment: When any treatment appears to improve survival, compared with 7+3 for AML, all must take notice.
Abstract 7001: Treatment-free remission (TFR) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP) treated with frontline nilotinib: Results from the ENESTFreedom study
Comment: About 50% of the CML patients treated with frontline nilotinib are eventually able to stop the drug and successfully stay off of it. That means more patients in treatment-free remission, compared with those initially treated with imatinib.
Link to abstract 7001
Abstract 7007: Phase Ib/2 study of venetoclax with low-dose cytarabine in treatment-naive patients age ≥ 65 with acute myelogenous leukemia
Abstract 7009: Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥ 65 years ineligible for standard induction therapy
Comment: The response rates in these older AML patients are remarkable and challenge results typically seen with 7+3 in a younger population.
Link to abstract 7007 and 7009
Abstract 7501: A prospective, multicenter, randomized study of anti-CCR4 monoclonal antibody mogamulizumab (moga) vs investigator’s choice (IC) in the treatment of patients (pts) with relapsed/refractory (R/R) adult T-cell leukemia-lymphoma (ATL)
Comment: The response rate to mogamulizumab was outstanding in the largest randomized clinical trial thus far conducted for this cancer. Although rare in the USA, ATL is more common in Asia.
Link to abstract 7501
Abstract 7507: Effect of bortezomib on complete remission (CR) rate when added to bendamustine-rituximab (BR) in previously untreated high-risk (HR) follicular lymphoma (FL): A randomized phase II trial of the ECOG-ACRIN Cancer Research Group (E2408)
Comment: This interesting observation of improved complete remission needs longer follow-up.
Link to abstract 7507
Abstract 7519: Venetoclax activity in CLL patients who have relapsed after or are refractory to ibrutinib or idelalisib
Comment: This study has implications for practice. Venetoclax elicits a 50%-60% response rate after patients with CLL progress during treatment with B-cell receptor pathway inhibitors.
Link to abstract 7519
Abstract 7521: Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL)
Comment: This next-generation variation on ibrutinib was associated with a 96% overall response rate with fewer adverse effects such as atrial fibrillation.
Link to abstract 7521
Abstract 8000: Upfront autologous stem cell transplantation (ASCT) versus novel agent-based therapy for multiple myeloma (MM): A randomized phase 3 study of the European Myeloma Network (EMN02/HO95 MM trial)
Comment: Other trials are underway to address the role of upfront ASCT for newly diagnosed multiple myeloma. While the last word on this issue has yet to be written, ASCT remains the standard of care for MM patients after induction.
Link to abstract 8000
LBA4: Phase III randomized controlled study of daratumumab, bortezomib, and dexamethasone (DVd) versus bortezomib and dexamethasone (Vd) in patients (pts) with relapsed or refractory multiple myeloma (RRMM): CASTOR study
Comment: As predicted by most, the addition of daratumumab to bortezomib-based therapy increases response rates, compared with bortezomib-based alone. Efficacy is becoming less of a concern with myeloma treatment than is economics..
Look for the full, final text of this abstract to be posted online at 7:30 AM (EDT) on Sunday, June 5.
ASCT still a player for multiple myeloma
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
Even in this era of novel therapies for multiple myeloma, for patients with newly diagnosed disease, autologous stem cell transplant (ASCT) after chemotherapy provides benefits in terms of disease progression and extent of response, compared with chemotherapy alone. The benefit of ASCT was especially pronounced among certain groups of high-risk patients.
Novel proteasome inhibitors and immunomodulators “have dramatically increased the complete response rate and significantly extended progression-free survival and overall survival in previously untreated multiple myeloma patients,” Dr. Michele Cavo, head of the Seragnoli Institute of Hematology at the University of Bologna School of Medicine in Italy, said at a presscast in advance of the annual meeting of the American Society of Clinical Oncology.
But questions remain about how these newer agents perform, compared with high-dose melphalan (HDM) followed by ASCT, traditionally seen as the standard of care for younger and fit patients with newly diagnosed disease.
EMN02/HO95 is a large, prospective, multicenter, intergroup, randomized phase III study that addresses this question, as well as single vs. double ASCT and the use of consolidation therapy or not. The study includes patients 65 years old or younger, and the trial protocol involves induction therapy with bortezomib (Velcade)–cyclophosphamide-dexamethasone (VCD) and subsequent collection of peripheral blood stem cells.
Patients were then randomly assigned to receive bortezomib-melphalan-prednisone (VMP) or HDM as intensification therapy in centers that had a single ASCT policy. For those centers doing double (tandem) ASCT procedures, the randomization was to VMP vs. HDM + single ASCT vs. HDM + double ASCT.
Patients in each treatment arm then underwent another randomization to consolidation therapy with bortezomib-lenalidomide (Revlimid)–dexamethasone or no consolidation. All patients received lenalidomide maintenance until disease progression or toxicity. At the time of a preliminary analysis of trial data in January 2016, results from the second randomization to consolidation or no consolidation therapy were not yet complete. This first prespecified interim analysis was performed after at least 33% of the required events had occurred.
Early results show ASCT benefit
Early results on 1,266 patients (VMP, n = 512; HDM, n = 754) show that a median progression-free survival (PFS) was not yet reached after a median follow-up of 23.9 months from the first randomization (to VMP vs. HDM+ASCT), the primary endpoint of the trial.
In the overall patient population, patients achieved a significant 24% benefit in PFS when given HDM+ASCT up front (hazard ratio, 0.76 vs. VMP), and this benefit extended to certain patient subgroups, as well.
“PFS benefit with bortezomib-based ASCT was of relevance for patients at high risk of early relapse, in particular for those with revised ISS [International Staging System] stage III and high-risk cytogenetic profiles, who had a relative reduction in the risk of progression or death of 48% and 28%, respectively,” Dr. Cavo said.
Other predictors of longer PFS were ISS stage I (HR, 0.44; 95% confidence interval, 0.28-067; P less than .0001), standard risk cytogenetics (HR, 0.57; 95% CI, 0.41-0.78; P less than .0001), randomization to the HDM+ASCT arm (HR, 0.61; 95% CI, 0.45-0.82; P = .001), and less than 60% bone marrow plasma cells (HR, 0.67; 95% CI, 0.48-0.99; P = .014).
More patients receiving ASCT up front had a significantly greater reduction in tumor volume of at least 90%, as indicated by the composite of very good partial remission, complete response, and stringent complete response, which was achieved in 74.0% in the VMP arm and in 84.4% of the HDM+ASCT arm (P less than .0001).
For patients at low risk of relapse, Dr. Cavo said longer follow up will be needed to compare the different arms of the study, and future analyses will delineate the effects of consolidation or no consolidation therapy and the use of the VMP regimen, compared with single or double ASCT.
ASCO president Dr. Julie Vose said that even with effective novel agents available, older, proven approaches still retain their value. “This study demonstrated that combining the best of both worlds – initial therapy with a novel agent followed by stem cell transplant – resulted in the best patient outcomes,” she said.
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: ASCT bested bortezomib for newly diagnosed younger multiple myeloma patients.
Major finding: Multiple myeloma patients showed 24% PFS prolongation with up front HDM+ASCT.
Data source: EMN02/HO95, a prospective, multicenter, intergroup, randomized phase III study of 1,266 patients.
Disclosures: The study was funded by HOVON, the Hemato Oncology Foundation for Adults in the Netherlands. Dr. Cavo disclosed relationships with Janssen, Takeda, Amgen, Bristol-Myers Squibb, and Celgene. Dr. Vose disclosed relationships with Sanofi Aventis, Seattle Genetics, Acerta, Bristol-Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Incyte, Janssen Biotech, Kite Pharma, Pharmacyclics, and Spectrum Pharmaceuticals.
Precision targeting yields better phase I efficacy outcomes
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Patients had significantly better outcomes when treatments were selected based on biomarker analysis of their tumors, in a meta-analysis of 346 phase I trials involving 351 study arms and 13,203 cancer patients.
Up to now, the purpose of phase I trials has been to determine safety based on adverse effects and tolerability. “Our analysis really shows that these days it is completely outdated and that with biomarker selection and especially genomic biomarkers, we can reach high response rates even in phase I trials,” Maria Schwaederle, Pharm.D., of the center for personalized cancer therapy at the University of California, San Diego, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
For the meta-analysis, Dr. Schwaederle and her colleagues performed a PubMed search on phase I clinical trials in cancer published in the years 2011-2013. They included studies using a single agent that reported adequate efficacy endpoints for response rate and progression-free survival (PSF). Overall survival was rarely reported and so was excluded.
Personalized therapy was defined as molecular biomarker–based selection of a treatment or at least half the patients in a trial having a specific tumor known to harbor the biomarker. The study compared outcomes based on a personalized strategy with those that were not.
“We think that the results were striking here,” Dr. Schwaederle said. “We can see that personalized therapy that is [based on] biomarker-selected treatments was really associated with significantly higher response rates and progression-free survival.” Multivariable analysis showed that response rates were sixfold higher with the personalized approach (30.6%, n = 58 in the personalized trials, vs. 4.9%, n = 293 in those not personalized; P less than .0001), and median PFS practically doubled (5.7 months, n = 7 in personalized trials vs. 2.95 months, n = 38 in those not personalized; P = .0002).
Most (98.3%) of the personalized arms used targeted agents. However, 76% of the arms using targeted agents did not select patients using a related biomarker and were thus nonpersonalized in their approach to treatment. A subanalysis showed that targeted drugs that were applied in a nontargeted manner had much worse response rates than targeted therapies applied using biomarker-based selection and were equivalent to cytotoxic therapies (personalized approach, 31.1%; nontargeted approach, 5.1%; P less than 0.0001; cytotoxic drugs, 4.7%, P = .63 vs. nonpersonalized approach). The median PFS was also similar for the nonpersonalized and cytotoxic strategies (3.3 vs. 2.5 months, P = .22).
Better targeting with genomic biomarkers
The investigators found that patients selected based on genomic (DNA) biomarkers had almost double the response rates (42%) of patients selected based on protein biomarkers (22.4%, P = .001). The reasons for this difference were not clear. “We might think that the target in the end is a protein, so we would expect maybe to see better results with protein expression,” Dr. Schwaederle said. But she pointed to the example of patients with lung cancer and epidermal growth factor receptor genetic alterations. If looking only for epidermal growth factor receptor protein overexpression, she said, “we wouldn’t see any response, but only the patients that have specific alterations in this gene respond very well.” So in that case, genetic detection appears to be more sensitive than a protein biomarker–based test.
Dr. Schwaederle addressed the question that has often arisen of whether targeted agents are just better therapies. “Our analysis showed that it is not just that the therapies are better but that targeted therapies must be given to the right patients,” she said. “Indeed, when targeted therapies were given to patients without a biomarker selection, the response rates were only about 5%.”
The response rate in the range of 40% using genetic biomarkers in these phase I trials suggests that incorporating such targeted approaches even at this early stage of drug testing may potentially yield useful information on efficacy.
Dr. Don Dizon, presscast moderator and chair of ASCO’s Cancer Communications Committee, said that precision medicine “is here and that we can use patient selection using either changes in a tumor’s DNA, called genomic changes, or even protein biomarkers and do much better than we have done in the past.”
Dr. Schwaederle noted that one limitation of her study is that more recent trials, unpublished at the time of her analysis, could influence the results today.
The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Precision drug targeting in phase I trials may yield efficacy information.
Major finding: Genomic targeting yielded a 42% response rate vs. 5% without targeting.
Data source: A meta-analysis of 346 phase I trials with 351 treatment arms and 13,203 cancer patients.
Disclosures: The study received funding from the Joan and Irwin Jacobs Philanthropic Fund. Two coauthors reported extensive financial relationships with pharmaceutical or other commercial and noncommercial entities.
Colon tumor side predicts outcomes from therapies
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
In this election year, the focus on left vs. right has even reached the discussion of colon cancer. In a retrospective analysis of data from the CALGB/SWOG 80405 trial of FOLFIRI or FOLFOX chemotherapy in combination with cetuximab or with bevacizumab as first-line treatment for metastatic colorectal cancer, patients with primary tumors that arose on the left side of the colon (descending and sigmoid colon and rectum; n = 732) survived significantly longer than did patients whose tumors originated on the right side (n = 293), comprising the cecum and ascending colon. And the metastatic disease responded differently to the two biologic agents.
The main cohort of the trial involved only patients with tumors with KRAS wild type gene. Patients were randomly assigned to receive cetuximab or bevacizumab in an open-label fashion. The choice of the FOLFIRI or FOLFOX regimen was at the physician’s discretion.
Although there was no significant difference in overall survival between the two treatment arms, “when we looked at patients whose tumors started on the left side, they had a median overall survival of 33 months compared to 19 months if the cancer began on the right side,” Dr. Alan Venook, professor of medicine at the University of California, San Francisco, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This is statistically significant with a hazard ratio [HR] of 1.60 describing the magnitude of difference in these outcomes,” he said.
For patients who received cetuximab, the difference in median overall survival approached 20 months. Patients with left-sided tumors had a median survival of 36.0 months vs. 16.7 months for right-sided tumors (HR = 1.987; 95% CI 1.60-2.46; P less than .001).
“This is really a dramatic finding that I think really was surprising to most of us or all of us given our understanding or belief beforehand that this really was not likely to make a big difference,” Dr. Venook said. The results for cetuximab are in line with a smaller European study, FIRE-3, that found a 22.6 month longer median overall survival for tumors on the left vs. the right.
Although not as large a benefit as seen with cetuximab, patients who received bevacizumab also did better if their tumors arose on the left side (n = 356) than if they arose on the right (n = 150): 31.4 months vs. 24.2 months, respectively (HR = 1.297; 95% CI 1.05-1.60; P = .017).
Including the few tumors found in the transverse colon did not change any of the results, so they were eliminated from analysis.
Agent, side matter for PFS
Data for progression-free survival (PFS) showed differential effects for the two biologic agents, depending on the side of the primary tumor. “We see that bevacizumab seemed to be more helpful on the right side … and cetuximab more helpful on the left side,” Dr. Venook said.
Considering both progression-free and overall survival, bevacizumab was superior to cetuximab (P = .03) for right-sided primaries, and cetuximab was superior for left-sided primaries (P = .04). These results showed a clear interaction of sidedness and the biologic agent used, with a P(interaction) = .003.
Practice implications and biologic explanations
“The 14 months’ improved survival in left vs. right side primary tumors is really striking for patients who present with metastatic disease,” Dr. Venook said. “Cetuximab added to first-line chemotherapy is associated with more favorable outcomes in patients with left-sided primary and appears to add more than bevacizumab to chemotherapy in these patients with left-sided primaries. Patients with right-sided primaries appear to benefit more from bevacizumab.”
He said he believes that the side is a surrogate marker for some biological explanation, and molecular analyses of tumor tissues are now in progress. “Until we have sorted that out, colon cancer originating on the right side should be treated differently than colon cancer on the left side,” he recommended. “And although this is retrospective, these data and other findings [presented at ASCO] and in press suggest that patients with right-sided primary metastatic colon cancer should get little to no benefit from cetuximab.”
He noted that the left and right colon segments arise from different embryonic tissues, the left coming from the hindgut and the right from the midgut. Therefore, based on the behavior of the tumors from this and previous studies as well as the tissues of origin of the gut segments, the left and right colon may have different biologies.
Session moderator Dr. Julie Vose, ASCO president and chief of the oncology/hematology division at the University of Nebraska Medical Center in Omaha, emphasized the role of the federal government in studies of this kind. “I think this study really points out how very large federally funded studies such as this one can really help us to differentiate some of the issues that we need to understand to treat our patients very specifically in a personalized way,” she said. And besides yielding information from the trial at hand, “it definitely is going to help us to generate hypotheses for future studies to hopefully take advantage of this information.”
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Cetuximab and bevacizumab have differential effects on overall survival and progression-free survival in metastatic colon cancer depending on which side of the colon the primary tumor originated.
Major finding: Survival with cetuximab was longer for left vs. right primaries.
Data source: Retrospective analysis involving a cohort of 1,085 patients with metastatic colon cancer from the CALGB/SWOG 80405 trial (patients randomized to cetuximab or bevacizumab in combination with FOLFOX or FOLFIRI chemotherapy).
Disclosures: The study received funding and support from Bristol-Myers Squibb, Genentech, and ImClone in collaboration with the National Cancer Institute. Dr. Venook has received compensation and research funding from several companies, including Bristol-Myers Squibb.
Upfront ASCT still preferred for young MM patients

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
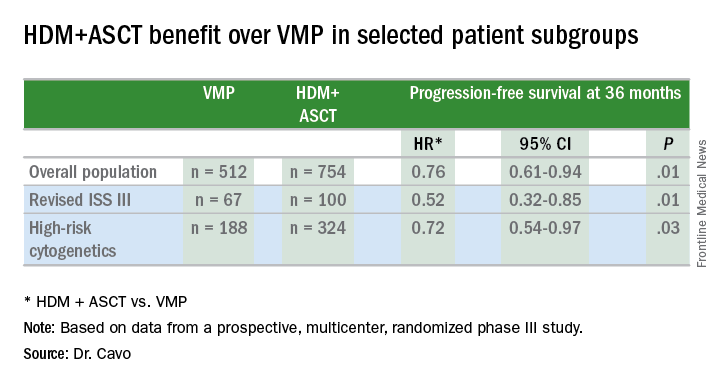
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()

Photo by Chad McNeeley
CHICAGO—An interim analysis of a large, phase 3 study has confirmed that upfront autologous stem cell transplantation (ASCT) is still the preferred
treatment for newly diagnosed, young multiple myeloma (MM) patients, even in the age of novel agents such as bortezomib.
Investigators compared 4 cycles of bortezomib-melphalan-prednisone (VMP) with high-dose melphalan (HDM) and single or double ASCT, depending upon the policy of the treating institution.
At a median follow-up of 24 months, the 3-year progression-free survival (PFS) was significantly better for patients who had received ASCT.
Michele Cavo, MD, of Seràgnoli Institute of Hematology in Bologna, Italy, reported the results of this first interim analysis of the European Myeloma Network trial (EMN/HO95 MM) at a press briefing preceding the 2016 ASCO Annual Meeting. More details will be presented at the meeting itself (abstract 8000).
Study investigators enrolled 1503 patients from February 2011 through April 2014. They performed the specified interim analysis in January 2016.
Patients were 65 years or younger, and all received bortezomib-based induction therapy followed by stem cell collection. Investigators then randomized 1266 patients to receive either VMP (n=754) or HDM plus single or double ASCT (n=512).
Patients underwent a second randomization to either 2 cycles of bortezomib-based consolidation or no consolidation therapy.
All patients received lenalidomide maintenance until disease progression. The primary endpoint was PFS after the first randomization.
Results
PFS was significantly longer in patients who had received a transplant, with a hazard ratio (HR) of 0.76, 95% confidence interval (CI) of 0.61-0.94, and P value of 0.01.
This benefit held true for patients with revised ISS stage III (HR=0.52, 95% CI 0.32-0.84, P=0.01).
And patients with high-risk cytogenetics also retained the benefit (HR=0.72, 95% CI 0.54-0.97, P=0.03). High-risk was defined as t(4;14), del(17p), del(1p), or gain of 1q.
Investigators also performed a multivariate analysis and found randomization to the HDM arm to be an independent predictor of prolonged PFS (HR=0.61, 95% CI 0.45-0.82, P=0.001).
There was no significant difference between the 2 arms in terms of stringent complete response and complete response.
However, when very good partial response was included in the best-response analysis, patients in the transplant arm fared significantly better (P<0.0001) than patients in the VMP arm—84% and 74%, respectively.
Investigators have not yet completed the interim data analysis related to the second randomization. The study is ongoing, and future analyses will include overall survival, toxicity, quality of life, and other measures.
This study was funded by the Haemato Oncology Foundation for Adults in the Netherlands (HOVON). ![]()
Pembrolizumab benefit holds long-term for some melanoma patients
The anti–PD-1 immunotherapy pembrolizumab increases long-term survival in some patients with advanced melanoma, according to updated results of KEYNOTE-001.
Among the 655 patients studied in the phase 1b trial, the 3-year overall survival rate for advanced melanoma patients treated with pembrolizumab was 40%, Dr. Caroline Robert reported in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
Median overall survival was 24.4 months. Before 2011, patients with advanced melanoma had a median overall survival of less than one year, said Dr. Robert, head of the dermatology unit at the Institut Gustave-Roussy in Paris.
Study participants received pembrolizumab at either 2 or 10 mg/kg every 3 weeks. During the trial, 2 mg/kg every 3 weeks was determined to be the optimal dosing regimen. Patients remained on the treatment until disease progression, intolerable toxicity, or investigator decision. Eight percent of patients stopped treatment due to drug-related symptoms, and there were no drug-related deaths.
Survival rates differed slightly based on prior melanoma treatment. Of the 655 patients in the study, 75% had received previous treatments; patients who had not received prior treatment had slightly higher survival at 45%.
Overall response rate was 33%. Responses were durable as 73% of patients had a response rate of two or more years. Ninety-five patients had a complete response, and 61 of those patients stopped treatment following the complete response.
“I really hope for a cure for these people,” Dr. Robert said.
Pembrolizumab was generally well tolerated with the most common adverse events being fatigue (40%), itchiness (28%), and rash (23%).
ASCO spokesperson and moderator, Dr. Don Dizon, said the results of this study are “incredibly exciting given they came from a phase I trial. This is a new treatment and the longest follow-up of people [with melanoma] who have received pembrolizumab.” Dr. Dizon hopes to “potentially see a cure [for] melanoma” given the reported durability and response rate to the drug.
On Twitter @JessCraig_OP
The anti–PD-1 immunotherapy pembrolizumab increases long-term survival in some patients with advanced melanoma, according to updated results of KEYNOTE-001.
Among the 655 patients studied in the phase 1b trial, the 3-year overall survival rate for advanced melanoma patients treated with pembrolizumab was 40%, Dr. Caroline Robert reported in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
Median overall survival was 24.4 months. Before 2011, patients with advanced melanoma had a median overall survival of less than one year, said Dr. Robert, head of the dermatology unit at the Institut Gustave-Roussy in Paris.
Study participants received pembrolizumab at either 2 or 10 mg/kg every 3 weeks. During the trial, 2 mg/kg every 3 weeks was determined to be the optimal dosing regimen. Patients remained on the treatment until disease progression, intolerable toxicity, or investigator decision. Eight percent of patients stopped treatment due to drug-related symptoms, and there were no drug-related deaths.
Survival rates differed slightly based on prior melanoma treatment. Of the 655 patients in the study, 75% had received previous treatments; patients who had not received prior treatment had slightly higher survival at 45%.
Overall response rate was 33%. Responses were durable as 73% of patients had a response rate of two or more years. Ninety-five patients had a complete response, and 61 of those patients stopped treatment following the complete response.
“I really hope for a cure for these people,” Dr. Robert said.
Pembrolizumab was generally well tolerated with the most common adverse events being fatigue (40%), itchiness (28%), and rash (23%).
ASCO spokesperson and moderator, Dr. Don Dizon, said the results of this study are “incredibly exciting given they came from a phase I trial. This is a new treatment and the longest follow-up of people [with melanoma] who have received pembrolizumab.” Dr. Dizon hopes to “potentially see a cure [for] melanoma” given the reported durability and response rate to the drug.
On Twitter @JessCraig_OP
The anti–PD-1 immunotherapy pembrolizumab increases long-term survival in some patients with advanced melanoma, according to updated results of KEYNOTE-001.
Among the 655 patients studied in the phase 1b trial, the 3-year overall survival rate for advanced melanoma patients treated with pembrolizumab was 40%, Dr. Caroline Robert reported in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
Median overall survival was 24.4 months. Before 2011, patients with advanced melanoma had a median overall survival of less than one year, said Dr. Robert, head of the dermatology unit at the Institut Gustave-Roussy in Paris.
Study participants received pembrolizumab at either 2 or 10 mg/kg every 3 weeks. During the trial, 2 mg/kg every 3 weeks was determined to be the optimal dosing regimen. Patients remained on the treatment until disease progression, intolerable toxicity, or investigator decision. Eight percent of patients stopped treatment due to drug-related symptoms, and there were no drug-related deaths.
Survival rates differed slightly based on prior melanoma treatment. Of the 655 patients in the study, 75% had received previous treatments; patients who had not received prior treatment had slightly higher survival at 45%.
Overall response rate was 33%. Responses were durable as 73% of patients had a response rate of two or more years. Ninety-five patients had a complete response, and 61 of those patients stopped treatment following the complete response.
“I really hope for a cure for these people,” Dr. Robert said.
Pembrolizumab was generally well tolerated with the most common adverse events being fatigue (40%), itchiness (28%), and rash (23%).
ASCO spokesperson and moderator, Dr. Don Dizon, said the results of this study are “incredibly exciting given they came from a phase I trial. This is a new treatment and the longest follow-up of people [with melanoma] who have received pembrolizumab.” Dr. Dizon hopes to “potentially see a cure [for] melanoma” given the reported durability and response rate to the drug.
On Twitter @JessCraig_OP
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Pembrolizumab increases long-term survival in some patients with advanced melanoma.
Major finding: The 3-year overall survival rate for advanced melanoma patients treated with pembrolizumab was 40%. The median overall survival was 24.4 months.
Data source: A phase Ib clinical trial of 655 patients with advanced melanoma.
Disclosures: This study was funded by Merck. All of the investigators reported serving in advisory/consultatory roles for, having stock in, or receiving financial compensation or honoraria from several companies.
Early palliative care for cancer patients benefits caregivers
Introducing palliative care in combination with standard oncology care immediately following a cancer diagnosis results in improved quality of life and lower incidence of depression for caregivers of cancer patients.
“The integration of palliative care can improve patient care but the evidence is lacking about whether or not there are benefits [for] caregivers,” Dr. Areej El-Jawahri of Massachusetts General Hospital, Boston, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This study suggests that early palliative care creates a powerful positive feedback loop in families facing cancer. While patients receive a direct benefit from early palliative care, their caregivers experience a positive downstream effect, which may make it easier for them to care for their loved ones,” she said.
Investigators enrolled 275 family caregivers of patients newly diagnosed with incurable lung or gastrointestinal cancers. Patients were randomly assigned to receive early palliative care in addition to standard oncology care or to receive standard oncology care alone.
Palliative care involved a multifaceted team including nurses, social workers, and psychologists. The palliative care intervention was patient focused, and caregivers, who were defined as a relative or friend identified by the patient as the primary caregiver, were not required to attend palliative care appointments. However, about 50% of caregivers did attend, according to Dr. El-Jawahri.
At time of enrollment and then at time points 12 and 14 weeks post enrollment, caregivers completed standard questionnaires, the 36-Item Short Form Health Survey and the Hospital Anxiety and Depression Scale, that assessed quality of life and mood.
Twelve weeks after the cancer diagnosis, caregivers who received early palliative care reported significantly lower depression symptoms while vitality and social functioning improved. For patients who did not receive early palliative care, their caregivers’ vitality and social functioning decreased.
“This is the first study showing a positive impact of a patient-focused palliative care intervention on family caregivers,” said Dr. El-Jawahri.
“This study really points out that we have so many ways to help our patients and their families,” Dr. Julie Vose, president of ASCO, said during the presscast.
On Twitter @JessCraig_OP
Introducing palliative care in combination with standard oncology care immediately following a cancer diagnosis results in improved quality of life and lower incidence of depression for caregivers of cancer patients.
“The integration of palliative care can improve patient care but the evidence is lacking about whether or not there are benefits [for] caregivers,” Dr. Areej El-Jawahri of Massachusetts General Hospital, Boston, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This study suggests that early palliative care creates a powerful positive feedback loop in families facing cancer. While patients receive a direct benefit from early palliative care, their caregivers experience a positive downstream effect, which may make it easier for them to care for their loved ones,” she said.
Investigators enrolled 275 family caregivers of patients newly diagnosed with incurable lung or gastrointestinal cancers. Patients were randomly assigned to receive early palliative care in addition to standard oncology care or to receive standard oncology care alone.
Palliative care involved a multifaceted team including nurses, social workers, and psychologists. The palliative care intervention was patient focused, and caregivers, who were defined as a relative or friend identified by the patient as the primary caregiver, were not required to attend palliative care appointments. However, about 50% of caregivers did attend, according to Dr. El-Jawahri.
At time of enrollment and then at time points 12 and 14 weeks post enrollment, caregivers completed standard questionnaires, the 36-Item Short Form Health Survey and the Hospital Anxiety and Depression Scale, that assessed quality of life and mood.
Twelve weeks after the cancer diagnosis, caregivers who received early palliative care reported significantly lower depression symptoms while vitality and social functioning improved. For patients who did not receive early palliative care, their caregivers’ vitality and social functioning decreased.
“This is the first study showing a positive impact of a patient-focused palliative care intervention on family caregivers,” said Dr. El-Jawahri.
“This study really points out that we have so many ways to help our patients and their families,” Dr. Julie Vose, president of ASCO, said during the presscast.
On Twitter @JessCraig_OP
Introducing palliative care in combination with standard oncology care immediately following a cancer diagnosis results in improved quality of life and lower incidence of depression for caregivers of cancer patients.
“The integration of palliative care can improve patient care but the evidence is lacking about whether or not there are benefits [for] caregivers,” Dr. Areej El-Jawahri of Massachusetts General Hospital, Boston, said in a presscast leading up to the annual meeting of the American Society of Clinical Oncology.
“This study suggests that early palliative care creates a powerful positive feedback loop in families facing cancer. While patients receive a direct benefit from early palliative care, their caregivers experience a positive downstream effect, which may make it easier for them to care for their loved ones,” she said.
Investigators enrolled 275 family caregivers of patients newly diagnosed with incurable lung or gastrointestinal cancers. Patients were randomly assigned to receive early palliative care in addition to standard oncology care or to receive standard oncology care alone.
Palliative care involved a multifaceted team including nurses, social workers, and psychologists. The palliative care intervention was patient focused, and caregivers, who were defined as a relative or friend identified by the patient as the primary caregiver, were not required to attend palliative care appointments. However, about 50% of caregivers did attend, according to Dr. El-Jawahri.
At time of enrollment and then at time points 12 and 14 weeks post enrollment, caregivers completed standard questionnaires, the 36-Item Short Form Health Survey and the Hospital Anxiety and Depression Scale, that assessed quality of life and mood.
Twelve weeks after the cancer diagnosis, caregivers who received early palliative care reported significantly lower depression symptoms while vitality and social functioning improved. For patients who did not receive early palliative care, their caregivers’ vitality and social functioning decreased.
“This is the first study showing a positive impact of a patient-focused palliative care intervention on family caregivers,” said Dr. El-Jawahri.
“This study really points out that we have so many ways to help our patients and their families,” Dr. Julie Vose, president of ASCO, said during the presscast.
On Twitter @JessCraig_OP
FROM THE 2016 ASCO ANNUAL MEETING
Key clinical point: Early palliative care for cancer patients improved quality of life and lowered the incidence of depression for caregivers.
Major finding: After 12 weeks, caregivers of patients who received early palliative care reported significantly lower depression symptoms. Vitality and social functioning also improved.
Data source: A randomized clinical trial of 275 family caregivers of patients newly diagnosed with incurable lung or gastrointestinal cancers.
Disclosures: The National Institutes of Health funded the study. Three of the investigators reported serving in advisory roles or receiving financial compensation or honoraria from several companies.