User login
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
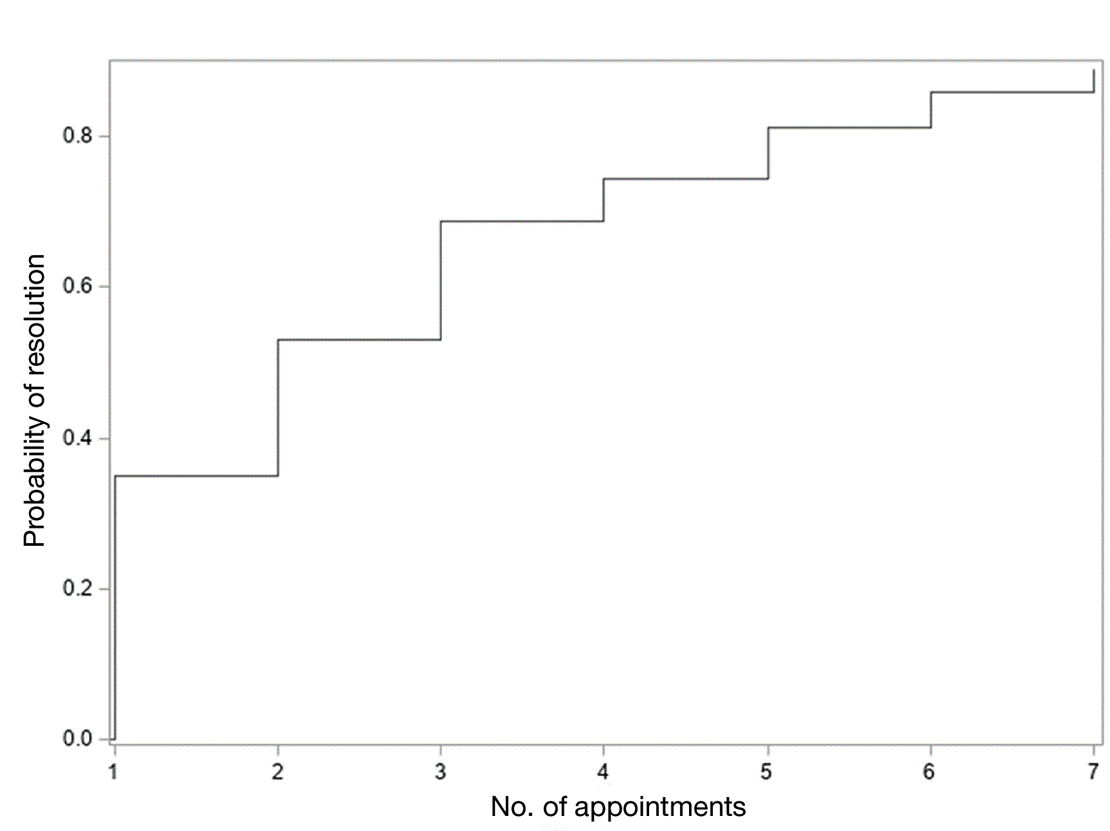
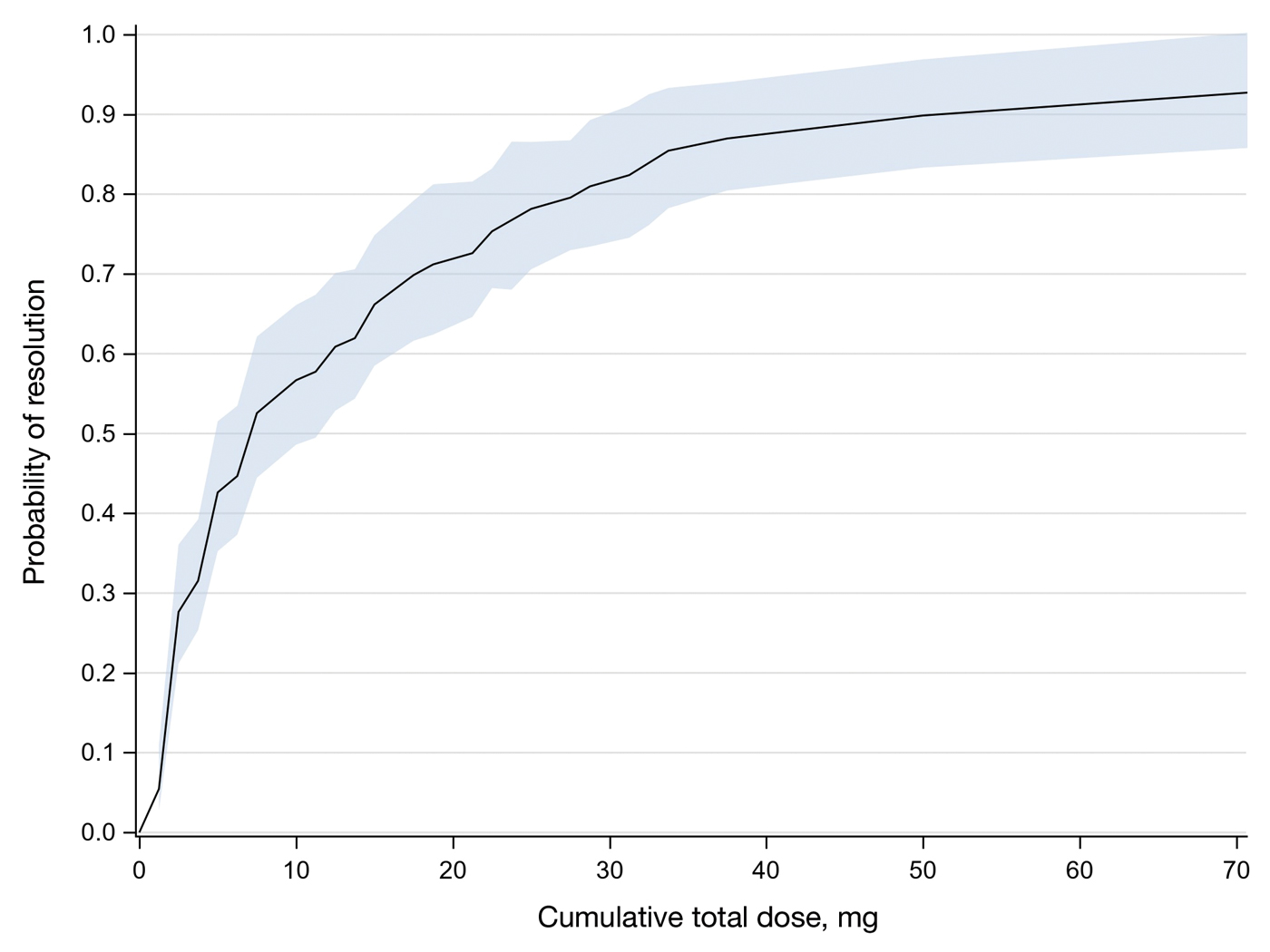
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).
Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
Squamous cell carcinoma (SCC) is the malignant proliferation of keratinocytes in the epidermis of the skin. Most SCCs are caused by UV light exposure, with sex and increased age acting as the primary known risk factors: SCCs are nearly twice as prevalent in men vs women, and the average age of presentation is the middle of the seventh decade of life.1 In the United States, there are an estimated 1.8 million new SCC cases annually.2 Although not usually life threatening, if left untreated, SCC can metastasize, thereby reducing the 10-year survival rate from above 90% with treatment to 16%.3-6
Most invasive SCC lesions are treated surgically, but intralesional methotrexate (IL-MTX) has emerged as an alternative treatment for cutaneous SCC. It offers the potential for lower-cost, efficacious outpatient treatment.7-12 Methotrexate competitively inhibits the enzyme dihydrofolate reductase, which converts dihydrofolate into tetrahydrofolate.13 In doing so, MTX indirectly prevents the synthesis of thymine, a nucleotide required for DNA synthesis. Thus, MTX can halt DNA synthesis and consequently, cell division. Intralesional MTX has been shown to successfully treat keratoacanthomas, lymphomas, and various inflammatory dermatologic conditions.8-12
Surgical options include standard excision, Mohs micrographic surgery, or electrodesiccation and curettage. Surgical treatment has high (92% to 99%) cure rates and typically requires only 1 or 2 appointments.14,15 Although costs can vary, one 2012 study using Medicare fee schedules found that total costs (including primary procedure, biopsy, follow-up appointments through 2 months, and other associated costs) for cutaneous SCC were $475 for electrodesiccation and curettage, $1302.92 for excision, and $2093.14 for Mohs micrographic surgery.16 For some patients, surgery is not an ideal option due to the tumor location, poor wound healing, anticoagulation, and cost. In these patients, photodynamic therapy, topical therapy with 5-fluorouracil or imiquimod, radiation, and cryotherapy are options listed in the American Academy of Dermatology guidelines.15 Compared with surgery, radiation is more demanding on the patient, often requiring multiple visits a week and including common undesirable adverse effects such as radiation dermatitis and prolonged wounds on the lower legs.17 Radiation also can be costly, with one study reporting costs between $2559 and $3431 for SCC of the forearm.18 Furthermore, in young patients, radiotherapy can increase the risk for developing nonmelanoma skin cancer later in life.16
Intralesional MTX is a localized treatment option that avoids the high costs of surgery, the side effects of radiotherapy, prolonged healing, and the systemic effects of chemotherapy. Treatment with IL-MTX can vary depending on the number of treatments necessary but usually only costs a few hundred dollars, rarely costing more than $1000.7 Although IL-MTX is less expensive, it typically requires several follow-up visits, whereas surgical removal may only require 1 visit.
Prior research has noted the efficacy of IL-MTX as a neoadjuvant therapy, with one study finding that IL-MTX can reduce the size of SCC lesions by an average of 0.52 cm2 prior to surgery.19 Several case studies also have documented the effectiveness of IL-MTX as a treatment for SCC.20-22 However, larger studies involving multiple patients to evaluate the efficacy of IL-MTX as a sole treatment for SCC are lacking. Gualdi et al23 looked at the outcomes (complete resolution, partial response, or no response) for SCC treated with IL-MTX and found that 62% (13/21) of patients experienced improvement, with 48% (10/21) experiencing at least 50% improvement. Although these results are promising, further research is needed.
Our study sought to examine IL-MTX efficacy as well as evaluate the dosage and number of appointments/sessions needed to achieve resolution of the lesions.
Methods
We conducted a retrospective chart review of patients who received only IL-MTX for clinically evident or biopsy-proven SCC at US Dermatology Partners clinics in Phoenix, Arizona, from January 1, 2022, to June 30, 2023. Patients aged 18 to 89 years were included, and they had not received other treatment for their SCC lesions such as radiation or systemic chemotherapy. Each patient received at least 1 dose of IL-MTX, beginning with a concentration of 12.5 mg/mL and with all subsequent doses at a concentration of 25 mg/mL (low dose vs high dose). Lesion resolution was categorized as no gross clinical tumor on follow-up. Patients received additional doses of IL-MTX based on the clinical appearance of their lesion(s).
Patient-level descriptive statistics are reported as mean (SD) or median (interquartile range [IQR]) for continuous variables as well as frequency and percentage for categorical variables. To account for the correlation of multiple lesions within individual patients, marginal Cox proportional hazard models were used. Time as well as cumulative dose to lesion resolution were evaluated and presented via the cumulative hazard function, while differences in resolution were estimated using separate Cox models for age, sex, and initial dose.
Results
In total, 107 different lesions from 21 patients were included in the analysis. The median number of lesions was 4 per patient (range, 1-15; IQR, 2-7), with a mean (SD) age of 80 (6) years. Patients were primarily female (81% [17/21]). From the data provided, the majority of lesions (83% [89/107]) resolved with IL-MTX. Of the 18 unresolved lesions, 5 (5%) were referred for a different procedure, and the remaining 13 (12%) were censored (lost to follow-up). Figure 1 provides the cumulative incidence function for lesion resolution. Approximately 50% of patient lesions resolved by the second appointment. Similarly, Figure 2 provides the cumulative dose function for lesion resolution; the median cumulative total dose for resolution was 5 mg (IQR, 2.5–12.5). Finally, concerning the ratio for case resolution, no difference in hazard ratio (HR) was observed for age (female vs male, HR: 1.01; 95% CI: 0.96-1.06), biological sex (HR, 1.01; 95% CI, 0.63-1.63), or initial dose (high vs low, HR: 1.13; 95% CI: 0.77-1.65).


Comment
Results of this study demonstrate the efficacy of IL-MTX for the treatment of cutaneous SCC. More than 80% of the lesions resolved by IL-MTX alone. This treatment approach is more cost-effective with fewer adverse effects when compared to other options. In our study, treatment with IL-MTX also proved to be reasonable in terms of the number of appointments and total dose required, with more than 50% of lesions resolving within 2 appointments and a median cumulative total dose of 5 mg. Intralesional MTX appears to be similarly efficacious in men and women, and the concentration of the initial dose (12.5 mg/mL vs 25 mg/mL) does not change the treatment outcome.
Although these data are encouraging for the use of IL-MTX in the treatment of SCC, future work should consider the relationships between lesion characteristics (such as size and location) and case resolution with IL-MTX as well as recurrence rates with lesions treated by IL-MTX compared to other treatment options.
Conclusion
This study demonstrated the efficacy of IL-MTX as a treatment for SCC that is cost-effective, avoids bothersome side effects, and can be accomplished in relatively few appointments. However, more data are needed to characterize the lesion type best suited to this treatment.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- The Skin Cancer Foundation. Skin cancer facts & statistics: what you need to know. Updated January 2026. Accessed January 20, 2026. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- Rees JR, Zens MS, Celaya MO, et al. Survival after squamous cell and basal cell carcinoma of the skin: a retrospective cohort analysis. Int J Cancer. 2015;137:878-884.
- Weinberg A, Ogle C, Shin E. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885-899.
- Varra V, Woody NM, Reddy C, et al. Suboptimal outcomes in cutaneous squamous cell cancer of the head and neck with nodal metastases. Anticancer Res. 2018;38:5825-5830. doi:10.21873/anticanres.12923
- Epstein E, Epstein NN, Bragg K, et al. Metastases from squamous cell carcinomas of the skin. Arch Dermatol. 1968;97:245-251.
- Chitwood K, Etzkorn J, Cohen G. Topical and intralesional treatment of nonmelanoma skin cancer: efficacy and cost comparisons. Dermatol Surg. 2013;39:1306-1316
- Scalvenzi M, Patrì A, Costa C, et al. Intralesional methotrexate for the treatment of keratoacanthoma: the Neapolitan experience. Dermatol Ther. 2019;9:369-372.
- Patel NP, Cervino AL. Treatment of keratoacanthoma: is intralesional methotrexate an option? Can J Plast Surg. 2011;19:E15-E18.
- Smith C, Srivastava D, Nijhawan RI. Intralesional methotrexate for keratoacanthomas: a retrospective cohort study. JAAD Int. 2020;83:904-905.
- Blume JE, Stoll HL, Cheney RT. Treatment of primary cutaneous CD30+ anaplastic large cell lymphoma with intralesional methotrexate. J Am Acad Dermatol. 2006;54(5 Suppl):S229-S230.
- Nedelcu RI, Balaban M, Turcu G, et al. Efficacy of methotrexate as anti‑inflammatory and anti‑proliferative drug in dermatology: three case reports. Exp Ther Med. 2019;18:905-910.
- Lester RS. Methotrexate. Clin Dermatol. 1989;7:128-135.
- Roenigk RK, Roenigk HH. Current surgical management of skin cancer in dermatology. J Dermatol Surg Oncol. 1990;16:136-151.
- Alam M, Armstrong A, Baum C, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560-578.
- Wilson LS, Pregenzer M, Basu R, et al. Fee comparisons of treatments for nonmelanoma skin cancer in a private practice academic setting. Dermatol Surg. 2012;38:570-584.
- DeConti RC. Chemotherapy of squamous cell carcinoma of the skin. Semin Oncol. 2012;39:145-149.
- Rogers HW, Coldiron BM. A relative value unit–based cost comparison of treatment modalities for nonmelanoma skin cancer: effect of the loss of the Mohs multiple surgery reduction exemption. J Am Acad Dermatol. 2009;61:96-103.
- Salido-Vallejo R, Cuevas-Asencio I, Garnacho-Sucedo G, et al. Neoadjuvant intralesional methotrexate in cutaneous squamous cell carcinoma: a comparative cohort study. J Eur Acad Dermatol Venereol. 2016;30:1120-1124.
- Salido-Vallejo R, Garnacho-Saucedo G, Sánchez-Arca M, et al. Neoadjuvant intralesional methotrexate before surgical treatment of invasive squamous cell carcinoma of the lower lip. Dermatol Surg. 2012;38:1849-1850.
- Vega-González LG, Morales-Pérez MI, Molina-Pérez T, et al. Successful treatment of squamous cell carcinoma with intralesional methotrexate. JAAD Case Rep. 2022;24:68-70.
- Moye MS, Clark AH, Legler AA, et al. Intralesional methotrexate for treatment of invasive squamous cell carcinomas in a patient taking vemurafenib for treatment of metastatic melanoma. J Clin Oncol. 2016;34:E134-E136.
- Gualdi G, Caravello S, Frasci F, et al. Intralesional methotrexate for the treatment of advanced keratinocytic tumors: a multi-center retrospective study. Dermatol Ther (Heidelb). 2020;10:769-777.
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
Intralesional Methotrexate: A Cost-Effective, High-Efficacy Alternative to Surgery for Cutaneous Squamous Cell Carcinoma
PRACTICE POINTS
- Intralesional methotrexate (IL-MTX) is an efficacious treatment option for cutaneous squamous cell carcinoma lesions in patients who are not good candidates for surgical excision.
- The starting concentration of the initial IL-MTX dose did not substantially impact outcomes; however, a 25 mg/mL concentration is standard for subsequent treatments to maintain efficacy.