User login
ID Practitioner is an independent news source that provides infectious disease specialists with timely and relevant news and commentary about clinical developments and the impact of health care policy on the infectious disease specialist’s practice. Specialty focus topics include antimicrobial resistance, emerging infections, global ID, hepatitis, HIV, hospital-acquired infections, immunizations and vaccines, influenza, mycoses, pediatric infections, and STIs. Infectious Diseases News is owned by Frontline Medical Communications.
sofosbuvir
ritonavir with dasabuvir
discount
support path
program
ritonavir
greedy
ledipasvir
assistance
viekira pak
vpak
advocacy
needy
protest
abbvie
paritaprevir
ombitasvir
direct-acting antivirals
dasabuvir
gilead
fake-ovir
support
v pak
oasis
harvoni
section[contains(@class, 'footer-nav-section-wrapper')]
div[contains(@class, 'pane-pub-article-idp')]
div[contains(@class, 'pane-medstat-latest-articles-articles-section')]
div[contains(@class, 'pane-pub-home-idp')]
div[contains(@class, 'pane-pub-topic-idp')]
Babies of pregnant women who get RSV vaccine likely to be prescribed fewer antimicrobials
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
Babies born to moms who were vaccinated against respiratory syncytial virus (RSV) while pregnant appear to need fewer antimicrobial prescriptions than babies of unvaccinated moms, according to authors of a recent study.
To fight antimicrobial resistance, we need to use fewer antimicrobial drugs, the authors write in Proceedings of the National Academy of Sciences.
“In this study, an RSV vaccine was administered to pregnant women to prevent infection in their infants by the transfer of protective antibody to the infant,” Kathryn M. Edwards, MD, a professor of pediatrics and the scientific director of the Vanderbilt vaccine research program at Vanderbilt University in Nashville, Tenn., told this news organization. Dr. Edwards was not involved in the study.
“The authors investigated the impact of the vaccine on the use of antibiotics in infants during the first 90 days of life,” Dr. Edwards added in an email. “They found that the use of antibiotics was less in infants born to mothers who received the RSV vaccine than in infants born to mothers who received placebo. … They suggest that reducing RSV infection in infants will reduce respiratory infections that trigger antibiotic use.”
Senior author Ramanan Laxminarayan, PhD, MPH, director and senior fellow at the Center for Disease Dynamics, Economics & Policy in Washington and his colleagues conducted a secondary analysis of a double-blind, randomized controlled trial at 87 sites in 11 countries on several continents.
In the original study, which was conducted between December 2015 and May 2018, 3,005 maternal participants and 2,978 infant participants received the experimental RSV F vaccine, and 1,573 maternal participants and 1,546 infants received a placebo shot. Baseline characteristics of mothers and infants were well balanced, according to the authors.
In the current study, infants born to mothers who received the RSV vaccine were found to be 12.9% (95% confidence interval, 1.3%-23.1%) less likely to be prescribed antimicrobials during their first 3 months of life, compared with infants whose mothers received placebo. Vaccine efficacy against antimicrobial prescriptions for acute lower respiratory tract infections was 16.9% (95% CI, 1.4%-29.4%).
During the first 3 months of life, for every 100 infants born, maternal vaccination prevented 3.6 courses of antimicrobials in high-income countries (20.2% of all antimicrobial prescribing), and 5.1 courses in low- and middle-income countries (10.9% of all antimicrobial prescribing).
In addition to finding that lower respiratory tract infections accounted for 69%-73% of all antimicrobial prescribing prevented by maternal vaccination, the researchers found marked vaccine efficacy (71.3% [95% CI, 28.1%-88.6%]) against acute otitis media–associated antimicrobial prescription in infants in high-income countries.
RSV vaccine is ‘one of our best investments’
RSV, the authors explain, is a major cause of upper and lower respiratory tract infections that develop as a single agent or along with bacterial pathogens.
“With decreases in bacterial pneumonia following the introduction of the pneumococcal conjugate vaccine, a vaccine against RSV represents one of our best investments to lower the burden of respiratory infections in children,” Dr. Laxminarayan said in a press release.
“These findings are not unexpected because viral infections can trigger bacterial infections such as otitis, and reducing viral infections will reduce bacterial infections,” Dr. Edwards said. “Also, viral infections are often treated with antibiotics because the provider cannot rule out a bacterial infection.”
She acknowledged the value of investigating multiple outcomes but added that “the study was underpowered to assess the full impact of the antibiotics.”
“If a more effective RSV vaccine can be designed, the impact on reducing antibiotic use will likely be even greater,” Dr. Edwards advised. “Also, the vaccine was not highly effective in preventing RSV pneumonia. If it had been more effective, the antibiotic impact would likely have been greater.”
The authors acknowledged the study’s limitations. “Results of this post hoc secondary analysis should be viewed as hypothesis generating, as the trial was not powered for determination of effects against antimicrobial prescribing, and our analyses were not adjusted for multiplicity,” they write, and they joined Dr. Edwards in recommending further related research.
First author Joseph A. Lewnard, PhD, declares financial support from Pfizer unrelated to this research, three authors are employees of Novavax, and Dr. Laxminarayan has disclosed no relevant financial relationships. Dr. Edwards reports funding from the National Institutes of Health and the Centers for Disease Control and Prevention; consultancy to BioNEt and IBM; membership on data safety and monitoring boards for Pfizer, Sanofi, GSK, Merck, X-4 Pharma, Roche, and Seqirus. The Bill & Melinda Gates Foundation supported the study.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Is cancer testing going to the dogs? Nope, ants
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
The oncologist’s new best friend
We know that dogs have very sensitive noses. They can track criminals and missing persons and sniff out drugs and bombs. They can even detect cancer cells … after months of training.
And then there are ants.
Cancer cells produce volatile organic compounds (VOCs), which can be sniffed out by dogs and other animals with sufficiently sophisticated olfactory senses. A group of French investigators decided to find out if Formica fusca is such an animal.
First, they placed breast cancer cells and healthy cells in a petri dish. The sample of cancer cells, however, included a sugary treat. “Over successive trials, the ants got quicker and quicker at finding the treat, indicating that they had learned to recognize the VOCs produced by the cancerous cells, using these as a beacon to guide their way to the sugary delight,” according to IFL Science.
When the researchers removed the treat, the ants still went straight for the cancer cells. Then they removed the healthy cells and substituted another type of breast cancer cell, with just one type getting the treat. They went for the cancer cells with the treat, “indicating that they were capable of distinguishing between the different cancer types based on the unique pattern of VOCs emitted by each one,” IFL Science explained.
It’s just another chapter in the eternal struggle between dogs and ants. Dogs need months of training to learn to detect cancer cells; ants can do it in 30 minutes. Over the course of a dog’s training, Fido eats more food than 10,000 ants combined. (Okay, we’re guessing here, but it’s got to be a pretty big number, right?)
Then there’s the warm and fuzzy factor. Just look at that picture. Who wouldn’t want a cutie like that curling up in the bed next to you?
Console War II: Battle of the Twitter users
Video games can be a lot of fun, provided you’re not playing something like Rock Simulator. Or Surgeon Simulator. Or Surgeon Simulator 2. Yes, those are all real games. But calling yourself a video gamer invites a certain negative connotation, and nowhere can that be better exemplified than the increasingly ridiculous console war.
For those who don’t know their video game history, back in the early 90s Nintendo and Sega were the main video game console makers. Nintendo had Mario, Sega had Sonic, and everyone had an opinion on which was best. With Sega now but a shell of its former self and Nintendo viewed as too “casual” for the true gaming connoisseur, today’s battle pits Playstation against Xbox, and fans of both consoles spend their time trying to one-up each other in increasingly silly online arguments.
That brings us nicely to a Twitter user named “Shreeveera,” who is very vocal about his love of Playstation and hatred of the Xbox. Importantly, for LOTME purposes, Shreeveera identified himself as a doctor on his profile, and in the middle of an argument, Xbox enthusiasts called his credentials into question.
At this point, most people would recognize that there are very few noteworthy console-exclusive video games in today’s world and that any argument about consoles essentially comes down to which console design you like or which company you find less distasteful, and they would step away from the Twitter argument. Shreeveera is not most people, and he decided the next logical move was to post a video of himself and an anesthetized patient about to undergo a laparoscopic cholecystectomy.
This move did prove that he was indeed a doctor, but the ethics of posting such a video with a patient in the room is a bit dubious at best. Since Shreeveera also listed the hospital he worked at, numerous Twitter users review bombed the hospital with one-star reviews. Shreeveera’s fate is unknown, but he did take down the video and removed “doctor by profession” from his profile. He also made a second video asking Twitter to stop trying to ruin his life. We’re sure that’ll go well. Twitter is known for being completely fair and reasonable.
Use your words to gain power
We live in the age of the emoji. The use of emojis in texts and emails is basically the new shorthand. It’s a fun and easy way to chat with people close to us, but a new study shows that it doesn’t help in a business setting. In fact, it may do a little damage.
The use of images such as emojis in communication or logos can make a person seem less powerful than someone who opts for written words, according to Elinor Amit, PhD, of Tel Aviv University and associates.
Participants in their study were asked to imagine shopping with a person wearing a T-shirt. Half were then shown the logo of the Red Sox baseball team and half saw the words “Red Sox.” In another scenario, they were asked to imagine attending a retreat of a company called Lotus. Then half were shown an employee wearing a shirt with an image of lotus flower and half saw the verbal logo “Lotus.” In both scenarios, the individuals wearing shirts with images were seen as less powerful than the people who wore shirts with words on them.
Why is that? In a Eurekalert statement, Dr. Amit said that “visual messages are often interpreted as a signal for desire for social proximity.” In a world with COVID-19, that could give anyone pause.
That desire for more social proximity, in turn, equals a suggested loss of power because research shows that people who want to be around other people more are less powerful than people who don’t.
With the reduced social proximity we have these days, we may want to keep things cool and lighthearted, especially in work emails with people who we’ve never met. It may be, however, that using your words to say thank you in the multitude of emails you respond to on a regular basis is better than that thumbs-up emoji. Nobody will think less of you.
Should Daylight Savings Time still be a thing?
This past week, we just experienced the spring-forward portion of Daylight Savings Time, which took an hour of sleep away from us all. Some of us may still be struggling to find our footing with the time change, but at least it’s still sunny out at 7 pm. For those who don’t really see the point of changing the clocks twice a year, there are actually some good reasons to do so.
Sen. Marco Rubio, sponsor of a bill to make the time change permanent, put it simply: “If we can get this passed, we don’t have to do this stupidity anymore.” Message received, apparently, since the measure just passed unanimously in the Senate.
It’s not clear if President Biden will approve it, though, because there’s a lot that comes into play: economic needs, seasonal depression, and safety.
“I know this is not the most important issue confronting America, but it’s one of those issues where there’s a lot of agreement,” Sen. Rubio said.
Not total agreement, though. The National Association of Convenience Stores is opposed to the bill, and Reuters noted that one witness at a recent hearing said the time change “is like living in the wrong time zone for almost eight months out of the year.”
Many people, however, seem to be leaning toward the permanent spring-forward as it gives businesses a longer window to provide entertainment in the evenings and kids are able to play outside longer after school.
Honestly, we’re leaning toward whichever one can reduce seasonal depression.
Study: Majority of research on homeopathic remedies unpublished or unregistered
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
Homeopathy is a form of alternative medicine based on the concept that increasing dilution of a substance leads to a stronger treatment effect.
The authors of the new paper, published in BMJ Evidence-Based Medicine, also found that a quarter of the 90 randomized published trials on homeopathic remedies they analyzed changed their results before publication.
The benefits of homeopathy touted in studies may be greatly exaggerated, suggest the authors, Gerald Gartlehner, MD, of Danube University, Krems, Austria, and colleagues.
The results raise awareness that published homeopathy trials represent a limited proportion of research, skewed toward favorable results, they wrote.
“This likely affects the validity of the body of evidence of homeopathic literature and may substantially overestimate the true treatment effect of homeopathic remedies,” they concluded.
Homeopathy as practiced today was developed approximately 200 years ago in Germany, and despite ongoing debate about its effectiveness, it remains a popular alternative to conventional medicine in many developed countries, the authors noted.
According to the National Institutes of Health, homeopathy is based on the idea of “like cures like,” meaning that a disease can be cured with a substance that produces similar symptoms in healthy people, and the “law of minimum dose,” meaning that a lower dose of medication will be more effective. “Many homeopathic products are so diluted that no molecules of the original substance remain,” according to the NIH.
Homeopathy is not subject to most regulatory requirements, so assessment of effectiveness of homeopathic remedies is limited to published data, the researchers said. “When no information is publicly available about the majority of homeopathic trials, sound conclusions about the efficacy and the risks of using homeopathic medicinal products for treating health conditions are impossible,” they wrote.
Study methods and findings
The researchers examined 17 trial registries for studies involving homeopathic remedies conducted since 2002.
The registries included clinicaltrials.gov, the EU Clinical Trials Register, and the International Clinical Trials Registry Platform up to April 2019 to identify registered homeopathy trials.
To determine whether registered trials were published and to identify trials that were published but unregistered, the researchers examined PubMed, the Allied and Complementary Medicine Database, Embase, and Google Scholar up to April 2021.
They found that approximately 38% of registered trials of homeopathy were never published, and 53% of the published randomized, controlled trials (RCTs) were not registered. Notably, 25% of the trials that were registered and published showed primary outcomes that were changed compared with the registry.
The number of registered homeopathy trials increased significantly over the past 5 years, but approximately one-third (30%) of trials published during the last 5 years were not registered, they said. In a meta-analysis, unregistered RCTs showed significantly greater treatment effects than registered RCTs, with standardized mean differences of –0.53 and –0.14, respectively.
The study findings were limited by several factors including the potential for missed records of studies not covered by the registries searched. Other limitations include the analysis of pooled data from homeopathic treatments that may not generalize to personalized homeopathy, and the exclusion of trials labeled as terminated or suspended.
Proceed with caution before recommending use of homeopathic remedies, says expert
Linda Girgis, MD, noted that prior to reading this report she had known that most homeopathic remedies didn’t have any evidence of being effective, and that, therefore, the results validated her understanding of the findings of studies of homeopathy.
The study is especially important at this time in the wake of the COVID-19 pandemic, Dr. Girgis, a family physician in private practice in South River, N.J., said in an interview.
“Many people are promoting treatments that don’t have any evidence that they are effective, and more people are turning to homeopathic treatments not knowing the risks and assuming they are safe,” she continued. “Many people are taking advantage of this and trying to cash in on this with ill-proven remedies.”
Homeopathic remedies become especially harmful when patients think they can use them instead of traditional medicine, she added.
Noting that some homeopathic remedies have been studied and show some evidence that they work, Dr. Girgis said there may be a role for certain ones in primary care.
“An example would be black cohosh or primrose oil for perimenopausal hot flashes. This could be a good alternative when you want to avoid hormonal supplements,” she said.
At the same time, Dr. Girgis advised clinicians to be cautious about suggesting homeopathic remedies to patients.
“Homeopathy seems to be a good money maker if you sell these products. However, you are not protected from liability and can be found more liable for prescribing off-label treatments or those not [Food and Drug Administration] approved,” Dr. Girgis said. Her general message to clinicians: Stick with evidence-based medicine.
Her message to patients who might want to pursue homeopathic remedies is that just because something is “homeopathic” or natural doesn’t mean that it is safe.
“There are some [homeopathic] products that have caused liver damage or other problems,” she explained. “Also, these remedies can interact with other medications.”
The study received no outside funding. The researchers and Dr. Girgis had no financial conflicts to disclose.
FROM BMJ EVIDENCE BASED MEDICINE
Norovirus vaccine candidates employ different approaches
Scientists are trying different approaches to developing vaccines against norovirus, seeking to replicate the success seen in developing shots against rotavirus.
Speaking at the 12th World Congress of the World Society for Pediatric Infectious Diseases (WSPID), Miguel O’Ryan, MD, of the University of Chile, Santiago, presented an overview of candidate vaccines. Dr. O’Ryan has been involved for many years with research on rotavirus vaccines and has branched into work with the somewhat similar norovirus.
With advances in preventing rotavirus, norovirus has emerged in recent years as a leading cause of acute gastroenteritis (AGE) in most countries worldwide. It’s associated with almost 20% of all acute diarrheal cases globally and with an estimated 685 million episodes and 212,000 deaths annually, Dr. O’Ryan and coauthors reported in a review in the journal Viruses.
If successful, norovirus vaccines may be used someday to prevent outbreaks among military personnel, as this contagious virus has the potential to disrupt missions, Dr. O’Ryan and coauthors wrote. They also said people might consider getting norovirus vaccines ahead of trips to prevent traveler’s diarrhea. But most importantly, these kinds of vaccines could reduce diarrhea-associated hospitalizations and deaths of children.
Takeda Pharmaceutical Company, for whom Dr. O’Ryan has done consulting, last year announced a collaboration with Frazier Healthcare Partners to launch HilleVax. Based in Boston, the company is intended to commercialize Takeda’s norovirus vaccine candidate.
The Takeda-HilleVax candidate vaccine injection has advanced as far as phase 2 studies, including a test done over two winter seasons in U.S. Navy recruits. Takeda and U.S. Navy scientists reported in 2020 in the journal Vaccine that the primary efficacy outcome for this test could not be evaluated due to an unexpectedly low number of cases of norovirus. Still, data taken from this study indicate that the vaccine induces a broad immune response, the scientists reported.
In his WSPID presentation, Dr. O’Ryan also mentioned an oral norovirus vaccine candidate that the company Vaxart is developing, referring to this as a “very interesting approach.”
Betting on the gut
Based in South San Francisco, California, Vaxart is pursuing a theory that a vaccine designed to generate mucosal antibodies locally in the intestine, in addition to systemic antibodies in the blood, may better protect against norovirus infection than an injectable vaccine.
“A key ability to protect against norovirus needs to come from an intestinal immune response, and injected vaccines don’t give those very well,” Sean Tucker, PhD, the founder and chief scientific officer of Vaxart, told this news organization in an interview. “We think that’s one of the reasons why our oral approaches can have significant advantages.”
Challenges to developing a norovirus vaccine have included a lack of good animal models to use in research and a lack of an ability to grow the virus well in cell culture, Dr. Tucker said.
Vaxart experienced disruptions in its research during the early stages of the pandemic but has since picked up the pace of its efforts to develop its oral vaccine, Dr. Tucker said during the interview.
In a recent filing with the Securities and Exchange Commission, Vaxart said in early 2021 it resumed its norovirus vaccine program by initiating three clinical studies. These included a phase 1b placebo-controlled dose ranging study in healthy elderly adults aged 55-80. Data from these trials may be unveiled in the coming months.
Vaxart said that this year it has already initiated a phase 2 norovirus challenge study, which will evaluate safety, immunogenicity, and clinical efficacy of a vaccine candidate against placebo.
A version of this article first appeared on Medscape.com.
Scientists are trying different approaches to developing vaccines against norovirus, seeking to replicate the success seen in developing shots against rotavirus.
Speaking at the 12th World Congress of the World Society for Pediatric Infectious Diseases (WSPID), Miguel O’Ryan, MD, of the University of Chile, Santiago, presented an overview of candidate vaccines. Dr. O’Ryan has been involved for many years with research on rotavirus vaccines and has branched into work with the somewhat similar norovirus.
With advances in preventing rotavirus, norovirus has emerged in recent years as a leading cause of acute gastroenteritis (AGE) in most countries worldwide. It’s associated with almost 20% of all acute diarrheal cases globally and with an estimated 685 million episodes and 212,000 deaths annually, Dr. O’Ryan and coauthors reported in a review in the journal Viruses.
If successful, norovirus vaccines may be used someday to prevent outbreaks among military personnel, as this contagious virus has the potential to disrupt missions, Dr. O’Ryan and coauthors wrote. They also said people might consider getting norovirus vaccines ahead of trips to prevent traveler’s diarrhea. But most importantly, these kinds of vaccines could reduce diarrhea-associated hospitalizations and deaths of children.
Takeda Pharmaceutical Company, for whom Dr. O’Ryan has done consulting, last year announced a collaboration with Frazier Healthcare Partners to launch HilleVax. Based in Boston, the company is intended to commercialize Takeda’s norovirus vaccine candidate.
The Takeda-HilleVax candidate vaccine injection has advanced as far as phase 2 studies, including a test done over two winter seasons in U.S. Navy recruits. Takeda and U.S. Navy scientists reported in 2020 in the journal Vaccine that the primary efficacy outcome for this test could not be evaluated due to an unexpectedly low number of cases of norovirus. Still, data taken from this study indicate that the vaccine induces a broad immune response, the scientists reported.
In his WSPID presentation, Dr. O’Ryan also mentioned an oral norovirus vaccine candidate that the company Vaxart is developing, referring to this as a “very interesting approach.”
Betting on the gut
Based in South San Francisco, California, Vaxart is pursuing a theory that a vaccine designed to generate mucosal antibodies locally in the intestine, in addition to systemic antibodies in the blood, may better protect against norovirus infection than an injectable vaccine.
“A key ability to protect against norovirus needs to come from an intestinal immune response, and injected vaccines don’t give those very well,” Sean Tucker, PhD, the founder and chief scientific officer of Vaxart, told this news organization in an interview. “We think that’s one of the reasons why our oral approaches can have significant advantages.”
Challenges to developing a norovirus vaccine have included a lack of good animal models to use in research and a lack of an ability to grow the virus well in cell culture, Dr. Tucker said.
Vaxart experienced disruptions in its research during the early stages of the pandemic but has since picked up the pace of its efforts to develop its oral vaccine, Dr. Tucker said during the interview.
In a recent filing with the Securities and Exchange Commission, Vaxart said in early 2021 it resumed its norovirus vaccine program by initiating three clinical studies. These included a phase 1b placebo-controlled dose ranging study in healthy elderly adults aged 55-80. Data from these trials may be unveiled in the coming months.
Vaxart said that this year it has already initiated a phase 2 norovirus challenge study, which will evaluate safety, immunogenicity, and clinical efficacy of a vaccine candidate against placebo.
A version of this article first appeared on Medscape.com.
Scientists are trying different approaches to developing vaccines against norovirus, seeking to replicate the success seen in developing shots against rotavirus.
Speaking at the 12th World Congress of the World Society for Pediatric Infectious Diseases (WSPID), Miguel O’Ryan, MD, of the University of Chile, Santiago, presented an overview of candidate vaccines. Dr. O’Ryan has been involved for many years with research on rotavirus vaccines and has branched into work with the somewhat similar norovirus.
With advances in preventing rotavirus, norovirus has emerged in recent years as a leading cause of acute gastroenteritis (AGE) in most countries worldwide. It’s associated with almost 20% of all acute diarrheal cases globally and with an estimated 685 million episodes and 212,000 deaths annually, Dr. O’Ryan and coauthors reported in a review in the journal Viruses.
If successful, norovirus vaccines may be used someday to prevent outbreaks among military personnel, as this contagious virus has the potential to disrupt missions, Dr. O’Ryan and coauthors wrote. They also said people might consider getting norovirus vaccines ahead of trips to prevent traveler’s diarrhea. But most importantly, these kinds of vaccines could reduce diarrhea-associated hospitalizations and deaths of children.
Takeda Pharmaceutical Company, for whom Dr. O’Ryan has done consulting, last year announced a collaboration with Frazier Healthcare Partners to launch HilleVax. Based in Boston, the company is intended to commercialize Takeda’s norovirus vaccine candidate.
The Takeda-HilleVax candidate vaccine injection has advanced as far as phase 2 studies, including a test done over two winter seasons in U.S. Navy recruits. Takeda and U.S. Navy scientists reported in 2020 in the journal Vaccine that the primary efficacy outcome for this test could not be evaluated due to an unexpectedly low number of cases of norovirus. Still, data taken from this study indicate that the vaccine induces a broad immune response, the scientists reported.
In his WSPID presentation, Dr. O’Ryan also mentioned an oral norovirus vaccine candidate that the company Vaxart is developing, referring to this as a “very interesting approach.”
Betting on the gut
Based in South San Francisco, California, Vaxart is pursuing a theory that a vaccine designed to generate mucosal antibodies locally in the intestine, in addition to systemic antibodies in the blood, may better protect against norovirus infection than an injectable vaccine.
“A key ability to protect against norovirus needs to come from an intestinal immune response, and injected vaccines don’t give those very well,” Sean Tucker, PhD, the founder and chief scientific officer of Vaxart, told this news organization in an interview. “We think that’s one of the reasons why our oral approaches can have significant advantages.”
Challenges to developing a norovirus vaccine have included a lack of good animal models to use in research and a lack of an ability to grow the virus well in cell culture, Dr. Tucker said.
Vaxart experienced disruptions in its research during the early stages of the pandemic but has since picked up the pace of its efforts to develop its oral vaccine, Dr. Tucker said during the interview.
In a recent filing with the Securities and Exchange Commission, Vaxart said in early 2021 it resumed its norovirus vaccine program by initiating three clinical studies. These included a phase 1b placebo-controlled dose ranging study in healthy elderly adults aged 55-80. Data from these trials may be unveiled in the coming months.
Vaxart said that this year it has already initiated a phase 2 norovirus challenge study, which will evaluate safety, immunogenicity, and clinical efficacy of a vaccine candidate against placebo.
A version of this article first appeared on Medscape.com.
TB treatment can be shortened for most children: study
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
The World Health Organization is expected to recommend truncating treatment of children with mild tuberculosis by 2 months – from 6 months to 4 – after a randomized trial found similar outcomes with the shorter regimen.
An international team of investigators found the abbreviated course of antibiotics was no less effective or safe than conventional treatment and saved an average of $17.34 per child – money that could be used to mitigate the toll of TB, which is estimated to sicken 1.1 million children worldwide each year.
The findings come as deaths from TB are rising as a result of the COVID-19 pandemic, which has hindered efforts to find and treat patients. In 2020, according to the WHO, an estimated 1.5 million people died from TB, the first year-over-year increase in such deaths since 2005.
Nearly a quarter of children with TB die, primarily because they go undiagnosed, according to the researchers, who published the study in the New England Journal of Medicine. Shorter treatment “translates into very large cost savings that could be used to improve screening and diagnosis to address the current case detection gap,” first author Anna Turkova, MD, of University College London, told this news organization.
The standard TB regimen is based on trials in adults with severe respiratory disease. However, about two-thirds of children have nonsevere infections.
For the study, Dr. Turkova and colleagues assigned 1,204 children with TB in four countries – Uganda, Zambia, South Africa, and India – to either a 4- or 6-month regimen with first-line medications rifampin, isoniazid, pyrazinamide, and ethambutol. Participants were aged 2 months to 15 years and had symptomatic nonsevere lung or lymph node infections with a negative test on a sputum smear microscopy. Eleven percent also had HIV.
After 18 months, 16 participants in the group that received the shortened treatment and 18 in the standard treatment group had experienced an unfavorable outcome – defined as treatment failure, recurrence of TB, loss to follow-up, or death (adjusted difference, -0.4 percentage points; 95% confidence interval, -2.2 to 1.5).
Similar numbers – 47 in the 4-month group and 48 in the 6-month group – experienced severe or life-threatening adverse events, most commonly chest infections, such as pneumonia, and liver problems, during treatment or up to 30 days after the last dose.
New guidelines coming soon
The WHO plans to issue new guidelines and a handbook for TB management in children and adolescents on March 24, World Tuberculosis Day, a spokesman for the agency told Medscape.
Anna Mandalakas, MD, PhD, director of the Global Tuberculosis Program at Baylor College of Medicine, department of pediatrics, Houston, said the shorter regimen should enable more children to successfully complete TB treatment.
“It can be challenging to convince young children to take medications on a regular basis for 6 months,” Dr. Mandalakas, a member of a WHO guidelines development group that reviewed the study, told this news organization. “Despite best intentions, parents often become fatigued and give up the medicine battle.”
Leo Martinez, PhD, an epidemiologist at Boston University School of Public Health who studies pediatric TB, noted that study’s cost-effectiveness analysis applies only to health care costs. Families often suffer financially through lost wages, transportation to health care facilities, and lost employment, fueling a cycle of poverty and disease in low-income countries, he said.
A WHO statement noted that long treatment regimens can add toxicity and risk of drug interactions for children with HIV.
Separate efforts have been underway to hasten TB treatment in different groups of patients. A study published in NEJM showed that 4 months of the potent antibiotic rifapentine, along with another antibiotic, moxifloxacin, was non-inferior to the standard 6-month regimen in patients aged 12 and older. According to the editorial accompanying that study, the research illustrated the potential for shorter treatment courses that would be cheaper and less cumbersome, although that particular combination poses hurdles such as adherence issues and potential bacterial resistance.
Experts agreed that improved diagnostic procedures are critical to significantly reducing TB pediatric deaths – an issue that Dr. Turkova said will be addressed in WHO’s forthcoming handbook.
Because no gold-standard test exists for TB, and symptoms often overlap with other infections, widespread screening of children in households where adults have been diagnosed with TB has been found to improve detection of the disease. “Training of health care workers, easy-to-implement diagnostic algorithms, and widely accessible training materials on chest radiography in childhood TB should also improve case finding and treatment initiation,” she said.
The trial was supported by U.K. government and charitable research funders. Dr. Turkova and Dr. Martinez reported no financial disclosures. Dr. Mandalakas reported honoraria from WHO to support the preparation of diagnostics and treatment chapters in the operational handbook, for providing lectures for Medscape, and for serving on a data safety monitoring board for Janssen Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Schizophrenia and HIV: missed opportunities for care
“People don’t think about schizophrenia when they think about HIV,” Christina Mangurian, MD, professor of clinical psychiatry and vice chair for diversity and health equity at the University of California, San Francisco (UCSF), told this news organization.
The problem is complicated. According to the Centers for Disease Control and Prevention and National Institutes of Health, roughly 6% of people with serious mental illness are living with HIV, a rate that is about 10 times higher than the general U.S. population (0.4%). However, findings from a study by Dr. Mangurian and her team, published online in the journal AIDS, demonstrated that half of Medicaid patients with schizophrenia and HIV admitted to inpatient units in New York State were not coded as such upon discharge.
These data raise the question: , lack of social support, and under-recognition by practitioners that a problem even exists?
Lost in the care continuum
Dr. Mangurian and her research team examined documentation of pre-existing HIV/AIDS diagnoses and absence of ICD-9-CM HIV/AIDS coding at psychiatric discharge among 14,602 adults (aged 18-64 years) admitted to hospital inpatient units in New York State between Jan. 1, 2012, and Dec. 31, 2013. HIV diagnoses were defined as recent (within 30 days of admission) or distant (within 30-366 days of admission), and first admission was used as the index in people with multiple hospitalizations.
People living with HIV comprised 5.1% (741) of the overall dataset; 34% were diagnosed with schizophrenia and 27.9% with bipolar disorders. Overall, 54.5% were male and 50.7% were non-Hispanic Black. Furthermore, 58.3% were discharged without HIV/AIDS ICD-9 coding, reinforcing the likelihood that they were lost in the care continuum.
Dr. Mangurian explained that this break in the chain of care upon discharge can have an important impact on efforts to break the cycle of HIV transmission.
“There’s data that people with serious mental illnesses like schizophrenia are less likely to have sex, but when they do they’re more likely to engage in risky sexual behaviors, including sex for money [and] unprotected sex with partners who use injection drugs or who have HIV,” she said.
Although the majority of patients – both with and without prior HIV diagnoses – were older, adjusted models demonstrated that people aged 18-24 years had more than twice the odds of having their HIV/AIDS undocumented at discharge, compared with older adults aged 55-64 years (adjusted odds ratio, 2.37; P = .038), as were those aged 25-34 years (aOR, 2.17; P = .003). Individuals with more distant HIV diagnoses had three times the odds for an undocumented HIV/AIDS discharge, compared with more recent diagnoses (aOR, 3.25; P < .001).
Additional factors contributing to the lack of ICD-9 discharge coding included shorter lengths of stay (0-3 days vs. 15-30 days; aOR, 0.03; P = .01) and fewer HIV claims for HIV/AIDS services before hospitalization (1-2 vs. 3-9; aOR, 0.34; P < .01). Hospitals serving medium or high levels of Medicaid patients were also less likely to document HIV/AIDS before discharge (medium aOR, 1.69, P = .01; high aOR, 1.71, P = .03).
The study is not without limitations. For example, the 10-year-old dataset might not entirely reflect more recent structural or systemic changes for improving HIV detection on inpatient psychiatric units. Moreover, there was no comparator group without psychiatric inpatient admission.
Still, “[if these patients] didn’t have a discharge diagnosis, then it’s possible that they were not managed for their HIV, or their HIV was not addressed while they were in the hospital,” Sarah Andrews, MD, assistant professor of psychiatry and behavioral sciences and AIDS psychiatrist at Johns Hopkins School of Medicine, Baltimore, explained.
Dr. Andrews, who was not involved in the study, noted that this omission is significant. “A psychiatric admission or medical admission in general is a great opportunity to further manage and treat comorbidities. When we have a patient who comes in with HIV and they haven’t been on an antiviral prior to admission, we try to get infectious disease to give us recommendations of what to start, what labs to draw, to help them re-establish care,” she said.
Severe mental health an HIV disparity
Despite the burden of HIV among patient populations with serious mental health issues and data suggesting that these populations are over-represented among new HIV infections, the study findings point to an important missed opportunity for meeting several key outcomes on the HIV/AIDS care continuum, especially linkage to and retention in care.
The challenge is multifactorial.
In an earlier publication appearing in April 2021 in The Lancet HIV, Dr. Mangurian and colleagues explore a concept known as the “purview paradox,” which refers to a practitioner’s belief about who should be responsible for offering patients a particular intervention.
Structural and systemic issues also abound, as psychiatry records are often kept separate from the rest of the medical system due to insurer billing issues. “The true integration of all psychiatric and medical care has to happen to make sure that all of our patients receive the care that they deserve,” explained Dr. Mangurian.
Dr. Andrews agrees. “HIV care, as well as psychiatry, case management, pharmacy ... putting them together really helps decrease the risk of falling through the cracks and being able to refer appropriately for mental health,” she said.
Aside from changing practitioner attitudes and awareness and changing systems to include the wrap-around care model, current guidelines also need to reflect the role that patients with HIV and psychiatric comorbidities play in HIV transmission. Dr. Andrews and Dr. Mangurian agree: Routine screening in psychiatric inpatient units might be a good start.
The study was independently supported. Dr. Mangurian has reported grant funding from Genentech Charitable Foundation. Dr. Andrews has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People don’t think about schizophrenia when they think about HIV,” Christina Mangurian, MD, professor of clinical psychiatry and vice chair for diversity and health equity at the University of California, San Francisco (UCSF), told this news organization.
The problem is complicated. According to the Centers for Disease Control and Prevention and National Institutes of Health, roughly 6% of people with serious mental illness are living with HIV, a rate that is about 10 times higher than the general U.S. population (0.4%). However, findings from a study by Dr. Mangurian and her team, published online in the journal AIDS, demonstrated that half of Medicaid patients with schizophrenia and HIV admitted to inpatient units in New York State were not coded as such upon discharge.
These data raise the question: , lack of social support, and under-recognition by practitioners that a problem even exists?
Lost in the care continuum
Dr. Mangurian and her research team examined documentation of pre-existing HIV/AIDS diagnoses and absence of ICD-9-CM HIV/AIDS coding at psychiatric discharge among 14,602 adults (aged 18-64 years) admitted to hospital inpatient units in New York State between Jan. 1, 2012, and Dec. 31, 2013. HIV diagnoses were defined as recent (within 30 days of admission) or distant (within 30-366 days of admission), and first admission was used as the index in people with multiple hospitalizations.
People living with HIV comprised 5.1% (741) of the overall dataset; 34% were diagnosed with schizophrenia and 27.9% with bipolar disorders. Overall, 54.5% were male and 50.7% were non-Hispanic Black. Furthermore, 58.3% were discharged without HIV/AIDS ICD-9 coding, reinforcing the likelihood that they were lost in the care continuum.
Dr. Mangurian explained that this break in the chain of care upon discharge can have an important impact on efforts to break the cycle of HIV transmission.
“There’s data that people with serious mental illnesses like schizophrenia are less likely to have sex, but when they do they’re more likely to engage in risky sexual behaviors, including sex for money [and] unprotected sex with partners who use injection drugs or who have HIV,” she said.
Although the majority of patients – both with and without prior HIV diagnoses – were older, adjusted models demonstrated that people aged 18-24 years had more than twice the odds of having their HIV/AIDS undocumented at discharge, compared with older adults aged 55-64 years (adjusted odds ratio, 2.37; P = .038), as were those aged 25-34 years (aOR, 2.17; P = .003). Individuals with more distant HIV diagnoses had three times the odds for an undocumented HIV/AIDS discharge, compared with more recent diagnoses (aOR, 3.25; P < .001).
Additional factors contributing to the lack of ICD-9 discharge coding included shorter lengths of stay (0-3 days vs. 15-30 days; aOR, 0.03; P = .01) and fewer HIV claims for HIV/AIDS services before hospitalization (1-2 vs. 3-9; aOR, 0.34; P < .01). Hospitals serving medium or high levels of Medicaid patients were also less likely to document HIV/AIDS before discharge (medium aOR, 1.69, P = .01; high aOR, 1.71, P = .03).
The study is not without limitations. For example, the 10-year-old dataset might not entirely reflect more recent structural or systemic changes for improving HIV detection on inpatient psychiatric units. Moreover, there was no comparator group without psychiatric inpatient admission.
Still, “[if these patients] didn’t have a discharge diagnosis, then it’s possible that they were not managed for their HIV, or their HIV was not addressed while they were in the hospital,” Sarah Andrews, MD, assistant professor of psychiatry and behavioral sciences and AIDS psychiatrist at Johns Hopkins School of Medicine, Baltimore, explained.
Dr. Andrews, who was not involved in the study, noted that this omission is significant. “A psychiatric admission or medical admission in general is a great opportunity to further manage and treat comorbidities. When we have a patient who comes in with HIV and they haven’t been on an antiviral prior to admission, we try to get infectious disease to give us recommendations of what to start, what labs to draw, to help them re-establish care,” she said.
Severe mental health an HIV disparity
Despite the burden of HIV among patient populations with serious mental health issues and data suggesting that these populations are over-represented among new HIV infections, the study findings point to an important missed opportunity for meeting several key outcomes on the HIV/AIDS care continuum, especially linkage to and retention in care.
The challenge is multifactorial.
In an earlier publication appearing in April 2021 in The Lancet HIV, Dr. Mangurian and colleagues explore a concept known as the “purview paradox,” which refers to a practitioner’s belief about who should be responsible for offering patients a particular intervention.
Structural and systemic issues also abound, as psychiatry records are often kept separate from the rest of the medical system due to insurer billing issues. “The true integration of all psychiatric and medical care has to happen to make sure that all of our patients receive the care that they deserve,” explained Dr. Mangurian.
Dr. Andrews agrees. “HIV care, as well as psychiatry, case management, pharmacy ... putting them together really helps decrease the risk of falling through the cracks and being able to refer appropriately for mental health,” she said.
Aside from changing practitioner attitudes and awareness and changing systems to include the wrap-around care model, current guidelines also need to reflect the role that patients with HIV and psychiatric comorbidities play in HIV transmission. Dr. Andrews and Dr. Mangurian agree: Routine screening in psychiatric inpatient units might be a good start.
The study was independently supported. Dr. Mangurian has reported grant funding from Genentech Charitable Foundation. Dr. Andrews has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“People don’t think about schizophrenia when they think about HIV,” Christina Mangurian, MD, professor of clinical psychiatry and vice chair for diversity and health equity at the University of California, San Francisco (UCSF), told this news organization.
The problem is complicated. According to the Centers for Disease Control and Prevention and National Institutes of Health, roughly 6% of people with serious mental illness are living with HIV, a rate that is about 10 times higher than the general U.S. population (0.4%). However, findings from a study by Dr. Mangurian and her team, published online in the journal AIDS, demonstrated that half of Medicaid patients with schizophrenia and HIV admitted to inpatient units in New York State were not coded as such upon discharge.
These data raise the question: , lack of social support, and under-recognition by practitioners that a problem even exists?
Lost in the care continuum
Dr. Mangurian and her research team examined documentation of pre-existing HIV/AIDS diagnoses and absence of ICD-9-CM HIV/AIDS coding at psychiatric discharge among 14,602 adults (aged 18-64 years) admitted to hospital inpatient units in New York State between Jan. 1, 2012, and Dec. 31, 2013. HIV diagnoses were defined as recent (within 30 days of admission) or distant (within 30-366 days of admission), and first admission was used as the index in people with multiple hospitalizations.
People living with HIV comprised 5.1% (741) of the overall dataset; 34% were diagnosed with schizophrenia and 27.9% with bipolar disorders. Overall, 54.5% were male and 50.7% were non-Hispanic Black. Furthermore, 58.3% were discharged without HIV/AIDS ICD-9 coding, reinforcing the likelihood that they were lost in the care continuum.
Dr. Mangurian explained that this break in the chain of care upon discharge can have an important impact on efforts to break the cycle of HIV transmission.
“There’s data that people with serious mental illnesses like schizophrenia are less likely to have sex, but when they do they’re more likely to engage in risky sexual behaviors, including sex for money [and] unprotected sex with partners who use injection drugs or who have HIV,” she said.
Although the majority of patients – both with and without prior HIV diagnoses – were older, adjusted models demonstrated that people aged 18-24 years had more than twice the odds of having their HIV/AIDS undocumented at discharge, compared with older adults aged 55-64 years (adjusted odds ratio, 2.37; P = .038), as were those aged 25-34 years (aOR, 2.17; P = .003). Individuals with more distant HIV diagnoses had three times the odds for an undocumented HIV/AIDS discharge, compared with more recent diagnoses (aOR, 3.25; P < .001).
Additional factors contributing to the lack of ICD-9 discharge coding included shorter lengths of stay (0-3 days vs. 15-30 days; aOR, 0.03; P = .01) and fewer HIV claims for HIV/AIDS services before hospitalization (1-2 vs. 3-9; aOR, 0.34; P < .01). Hospitals serving medium or high levels of Medicaid patients were also less likely to document HIV/AIDS before discharge (medium aOR, 1.69, P = .01; high aOR, 1.71, P = .03).
The study is not without limitations. For example, the 10-year-old dataset might not entirely reflect more recent structural or systemic changes for improving HIV detection on inpatient psychiatric units. Moreover, there was no comparator group without psychiatric inpatient admission.
Still, “[if these patients] didn’t have a discharge diagnosis, then it’s possible that they were not managed for their HIV, or their HIV was not addressed while they were in the hospital,” Sarah Andrews, MD, assistant professor of psychiatry and behavioral sciences and AIDS psychiatrist at Johns Hopkins School of Medicine, Baltimore, explained.
Dr. Andrews, who was not involved in the study, noted that this omission is significant. “A psychiatric admission or medical admission in general is a great opportunity to further manage and treat comorbidities. When we have a patient who comes in with HIV and they haven’t been on an antiviral prior to admission, we try to get infectious disease to give us recommendations of what to start, what labs to draw, to help them re-establish care,” she said.
Severe mental health an HIV disparity
Despite the burden of HIV among patient populations with serious mental health issues and data suggesting that these populations are over-represented among new HIV infections, the study findings point to an important missed opportunity for meeting several key outcomes on the HIV/AIDS care continuum, especially linkage to and retention in care.
The challenge is multifactorial.
In an earlier publication appearing in April 2021 in The Lancet HIV, Dr. Mangurian and colleagues explore a concept known as the “purview paradox,” which refers to a practitioner’s belief about who should be responsible for offering patients a particular intervention.
Structural and systemic issues also abound, as psychiatry records are often kept separate from the rest of the medical system due to insurer billing issues. “The true integration of all psychiatric and medical care has to happen to make sure that all of our patients receive the care that they deserve,” explained Dr. Mangurian.
Dr. Andrews agrees. “HIV care, as well as psychiatry, case management, pharmacy ... putting them together really helps decrease the risk of falling through the cracks and being able to refer appropriately for mental health,” she said.
Aside from changing practitioner attitudes and awareness and changing systems to include the wrap-around care model, current guidelines also need to reflect the role that patients with HIV and psychiatric comorbidities play in HIV transmission. Dr. Andrews and Dr. Mangurian agree: Routine screening in psychiatric inpatient units might be a good start.
The study was independently supported. Dr. Mangurian has reported grant funding from Genentech Charitable Foundation. Dr. Andrews has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Children and COVID: Decline in new cases reaches 7th week
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.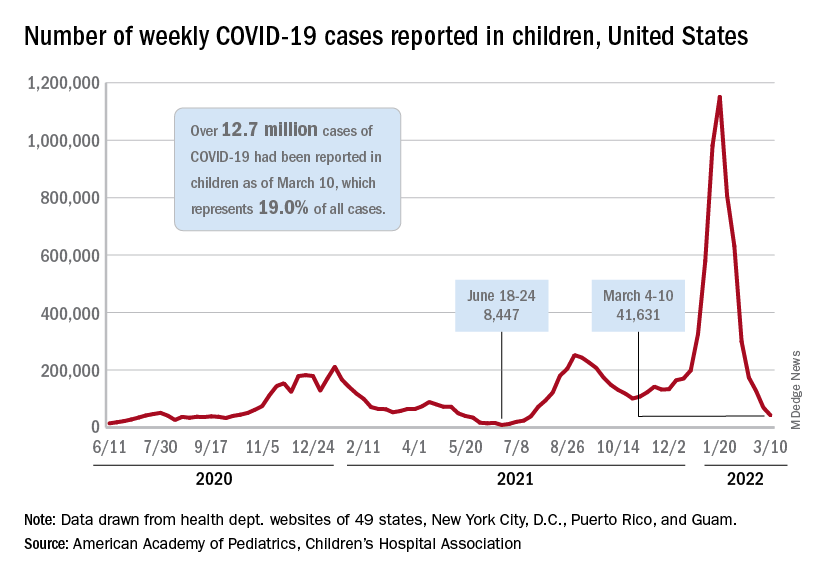
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
New cases of COVID-19 in U.S. children have fallen to their lowest level since the beginning of the Delta surge in July of 2021, according to the American Academy of Pediatrics and the Children’s Hospital Association.
. Over those 7 weeks, new cases dropped over 96% from the 1.15 million reported for Jan. 14-20, based on data collected by the AAP and CHA from state and territorial health departments.
The last time that the weekly count was below 42,000 was July 16-22, 2021, when almost 39,000 cases were reported in the midst of the Delta upsurge. That was shortly after cases had reached their lowest point, 8,447, since the early stages of the pandemic in 2020, the AAP/CHA data show.
The cumulative number of pediatric cases is now up to 12.7 million, while the overall proportion of cases occurring in children held steady at 19.0% for the 4th week in a row, the AAP and CHA said in their weekly COVID-19 report. The Centers for Disease Control and Prevention, using an age range of 0-18 versus the states’ variety of ages, puts total cases at 11.7 million and deaths at 1,656 as of March 14.
Data from the CDC’s COVID-19–Associated Hospitalization Surveillance Network show that hospitalizations with laboratory-confirmed infection were down by 50% in children aged 0-4 years, by 63% among 5- to 11-year-olds, and by 58% in those aged 12-17 years for the week of Feb. 27 to March 5, compared with the week before.
The pace of vaccination continues to follow a similar trend, as the declines seen through February have continued into March. Cumulatively, 33.7% of children aged 5-11 have received at least one dose, and 26.8% are fully vaccinated, with corresponding numbers of 68.0% and 58.0% for children aged 12-17, the CDC reported on its COVID Data Tracker.
State-level data show that children aged 5-11 in Vermont, with a rate of 65%, are the most likely to have received at least one dose of COVID vaccine, while just 15% of 5- to 11-year-olds in Alabama, Louisiana, and Mississippi have gotten their first dose. Among children aged 12-17, that rate ranges from 40% in Wyoming to 94% in Hawaii, Massachusetts, and Rhode Island, the AAP said in a separate report based on CDC data.
In a recent report involving 1,364 children aged 5-15 years, two doses of the COVID-19 vaccine reduced the risk of infection from the Omicron variant by 31% in children aged 5-11 years and by 59% among children aged 12-15 years, said Ashley L. Fowlkes, ScD, of the CDC’s COVID-19 Emergency Response Team, and associates (MMWR 2022 Mar 11;71).
Air trapping common in patients with long COVID
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to a prospective study that compared 100 COVID-19 survivors who had persistent symptoms and 106 healthy control persons.
“Something is going on in the distal airways related to either inflammation or fibrosis that is giving us a signal of air trapping,” noted senior author Alejandro P. Comellas, MD, in a press release. The study was stimulated by reports from University of Iowa clinicians noting that many patients with initial SARS-CoV-2 infection who were either hospitalized or were treated in the ambulatory setting later reported shortness of breath and other respiratory symptoms indicative of chronic lung disease.
Study results
Investigators classified patients (mean age, 48 years; 66 women) with post-acute sequelae of COVID-19 according to whether they were ambulatory (67%), hospitalized (17%), or required treatment in the intensive care unit (16%). They then compared CT findings of patients who had COVID-19 and persistent symptoms with those of a healthy control group.
COVID-19 severity did not affect the percentage of cases of lung with air trapping among these patients. Air trapping occurred at rates of 25.4% among ambulatory patients, 34.6% in hospitalized patients, and in 27.3% of those requiring intensive care (P = .10). The percentage of lungs affected by air trapping in ambulatory participants was sharply and significantly higher than in healthy controls (25.4% vs. 7.2%; P < .001). Also, air trapping persisted; it was still present in 8 of 9 participants who underwent imaging more than 200 days post diagnosis.
Qualitative analysis of chest CT images showed that the most common imaging abnormality was air trapping (58%); ground glass opacities (GGOs) were found in 51% (46/91), note Dr. Comellas and coauthors. This suggests ongoing lung inflammation, edema, or fibrosis. These symptoms are often observed during acute COVID-19, frequently in an organizing pneumonia pattern, and have been shown to persist for months after infection in survivors of severe disease. The mean percentage of total lung classified as having regional GGOs on chest CT scans was 13.2% and 28.7%, respectively, in the hospitalized and ICU groups, both very much higher than in the ambulatory group, at 3.7% (P < .001 for both). Among healthy controls, the GGO rate on chest CT was only 0.06% (P < .001).
In addition, air trapping correlated with the ratio of residual volume to total lung capacity (r = 0.6; P < .001) but not with spirometry results. In fact, the investigators did not observe airflow obstruction by spirometry in any group, suggesting that air trapping in these patients involves only small rather than large airways and that these small airways contribute little to total airway resistance. Only when a large percentage, perhaps 75% or more, of all small airways are obstructed will spirometry pick up small airways disease, the authors observe.
Continuing disease
The findings taken together suggest that functional small airways disease and air trapping are a consequence of SARS-CoV-2 infection, according to Dr. Comellas. “If a portion of patients continues to have small airways disease, then we need to think about the mechanisms behind it,” he said. “It could be something related to inflammation that’s reversible, or it may be something related to a scar that is irreversible, and then we need to look at ways to prevent further progression of the disease.” Furthermore, “studies aimed at determining the natural history of functional small airways disease in patients with post-acute sequelae of COVID-19 and the biological mechanisms that underlie these findings are urgently needed to identify therapeutic and preventative interventions,” Dr. Comellas, professor of internal medicine at Carver College of Medicine, University of Iowa, Iowa City, concluded.
The study limitations, the authors state, include the fact that theirs was a single-center study that enrolled participants infected early during the COVID-19 pandemic and did not include patients with Delta or Omicron variants, thus limiting the generalizability of the findings.
The study was published in Radiology.
The reported findings “indicate a long-term impact on bronchiolar obstruction,” states Brett M. Elicker, MD, professor of clinical radiology, University of California, San Francisco, in an accompanying editorial . Because collagen may be absorbed for months after an acute insult, it is not entirely clear whether the abnormalities seen in the current study will be permanent. He said further, “the presence of ground glass opacity and/or fibrosis on CT were most common in the patients admitted to the ICU and likely correspond to post-organizing pneumonia and/or post-diffuse alveolar damage fibrosis.”
Dr. Elicker also pointed out that organizing pneumonia is especially common among patients with COVID-19 and is usually highly steroid-responsive. The opacities improve or resolve with treatment, but sometimes residual fibrosis occurs. “Longer-term studies assessing the clinical and imaging manifestations 1-2 years after the initial infection are needed to fully ascertain the permanent manifestations of post-COVID fibrosis.”
The study was supported by grants from the National Institutes of Health. The authors and Dr. Elicker have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM RADIOLOGY
‘Overwhelming’ need to study COVID vaccine–associated tinnitus
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It’s now known that tinnitus may be an unexpected side effect of SARS-CoV-2 vaccination, and there is an urgent need to understand the precise mechanisms and best treatment for vaccine-associated tinnitus, researchers say.
As of mid-September 2021, 12,247 cases of tinnitus, or ringing in the ears, following COVID-19 vaccination had been reported to the Vaccine Adverse Event Reporting System of the U.S. Centers for Disease Control and Prevention.
“Despite several cases of tinnitus being reported following SARS-CoV-2 vaccination, the precise pathophysiology is still not clear,” write Syed Hassan Ahmed, 3rd-year MBBS student, Dow University of Health Sciences, Karachi, Pakistan, and coauthors.
The researchers review what is known and unknown about SARS-CoV-2 vaccine-associated tinnitus in an article published online Feb. 11 in Annals of Medicine and Surgery.
Molecular mimicry?
The researchers say cross-reactivity between anti-spike SARS-CoV-2 antibodies and otologic antigens is one possibility, based on the mechanisms behind other COVID-19 vaccine–induced disorders and the phenomenon of molecular mimicry.
“The heptapeptide resemblance between coronavirus spike glycoprotein and numerous human proteins further supports molecular mimicry as a potential mechanism behind such vaccine-induced disorders,” they write.
Anti-spike antibodies may react with antigens anywhere along the auditory pathway and fuel an inflammatory reaction, they point out.
“Therefore, understanding the phenomenon of cross-reactivity and molecular mimicry may be helpful in postulating potential treatment behind not only tinnitus but also the rare events of vaccination associated hearing loss and other otologic manifestations,” the authors say.
Genetic predispositions and associated conditions may also play a significant role in determining whether an individual develops vaccine-induced tinnitus.
Stress and anxiety following COVID vaccination may also play a role, inasmuch as anxiety-related adverse events following vaccination have been reported. Vaccine-related anxiety as a potential cause of tinnitus developing after vaccination needs to be explored, they write.
Jury out on best management
How best to manage COVID vaccine-associated tinnitus also remains unclear, but it starts with a well-established diagnosis, the authors say.
A well-focused and detailed history and examination are essential, with particular emphasis placed on preexisting health conditions, specifically, autoimmune diseases, such as Hashimoto thyroiditis; otologic conditions, such as sensorineural hearing loss; glaucoma; and psychological well-being. According to the review, patients often present with a history of one or more of these disorders.
“However, any such association has not yet been established and requires further investigation to be concluded as potential risk factors for vaccine-induced tinnitus,” they caution.
Routine cranial nerve examination, otoscopy, Weber test, and Rinne test, which are used for tinnitus diagnosis in general, may be helpful for confirmation of vaccine-associated tinnitus.
Owing to the significant association between tinnitus and hearing impairment, audiology should also performed, the authors say.
Although treatments for non–vaccine-induced tinnitus vary significantly, corticosteroids are the top treatment choice for SARS-CoV-2 vaccine-induced tinnitus reported in the literature.
Trials of other drug and nondrug interventions that may uniquely help with vaccine-associated tinnitus are urgently needed, the authors say.
Summing up, the reviewers say, “Although the incidence of COVID-19 vaccine-associated tinnitus is rare, there is an overwhelming need to discern the precise pathophysiology and clinical management as a better understanding of adverse events may help in encountering vaccine hesitancy and hence fostering the COVID-19 global vaccination program.
“Despite the incidence of adverse events, the benefits of the SARS-CoV-2 vaccine in reducing hospitalization and deaths continue to outweigh the rare ramifications,” they conclude.
The research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF MEDICINE AND SURGERY
Biden administration’s new test-to-treat program pits pharmacists against physicians
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.
The Biden administration’s new test-to-treat program is simple on the surface: if you feel like you may have COVID-19, go to a pharmacy, get tested, and, if positive, get treated with an antiviral medication on the spot.
One large physicians’ group is concerned that the program leaves doctors on the margins, and may put patients at risk if there are adverse effects from the medications. Pharmacists groups, on the other hand, say the program is too restrictive, according to an article by the research group Advisory Board.
Recently, the White House announced that more than 1,000 pharmacy clinics across the United States had registered to participate in the initiative, according to CNN. Ordering of the drugs is underway in many of these clinics, a White House official told the network.
Besides retail clinics in chain pharmacies, the antivirals will also be available in community health centers, long-term-care facilities, and Veterans Health Administration clinics, according to a statement from the U.S. Department of Health and Human Services.
The two antiviral pills authorized by the U.S. Food and Drug Administration include Pfizer’s Paxlovid, for people 12 and older, and Merck’s molnupiravir, for adults. Either drug has to be taken within 5 days after symptoms appear to be effective in preventing serious illness.
The need for speed is a major reason why the government chose to work with retail clinics that are more accessible than most primary care offices. However, the American Medical Association (AMA), the National Community Pharmacists Association (NCPA), and the American Pharmacists Association (APhA) have publicly criticized the administration’s approach.
The pharmacists’ groups are concerned that the program is limited only to pharmacies with clinics on site, thus restricting the number of pharmacies qualified to participate. Fourteen pharmacy groups, including the NCPA and the APhA, have also sent a letter to the Biden administration urging it to remove barriers to pharmacies ordering the medications.
The groups also want permission as “clinically trained medication experts” to prescribe the drugs and ensure their safe use.
The AMA on March 4 took issue with the prescribing component, saying that “the pharmacy-based clinic component of the test-to-treat plan flouts patient safety and risks significant negative health outcomes.”
In the AMA’s view, prescribing Paxlovid without a patient’s physician being present poses a risk for adverse drug interactions, as neither the nurse practitioners in retail clinics nor the pharmacists who dispense the drug have full knowledge of a patient›s medical history.
The next day, the AMA released another statement, saying it was reassured by comments from administration officials “that patients who have access to a regular source of care should contact their physician shortly after testing positive for COVID-19 to assess their treatment options.”
“Traditional doctor-only approach”
Having patients call their doctors after testing positive for COVID in a pharmacy “strikes me as unnecessary in the vast majority of cases, and it will delay treatment,” Robert Wachter, MD, professor and chair of the department of medicine at the University of California San Francisco, said in an interview. “In this case, it seems like the AMA is taking a very traditional doctor-only approach. And the world has changed. It’s much more of a team sport than an individual sport, the way it was years ago.”
Dr. Wachter said he has the utmost respect for pharmacists’ ability to screen prescriptions for adverse drug interactions. “We’re required to do medication reconciliation when patients see us,” he says. “And in many hospitals, we delegate that to pharmacists. They’re at least as good at it if not better than physicians are.”
While it’s essential to know what other medications a patient is taking, he noted, pharmacies have computer records of all the prescriptions they’ve filled for patients. In addition, pharmacies have access to complete medication histories through Surescripts, the company that enables electronic prescribing transactions between prescribers and pharmacies.
Drug interactions “not trivial”
Preeti Malani, MD, the chief health officer and a professor of medicine in the division of infectious diseases at the University of Michigan in Ann Arbor, told this news organization that the potential interactions between Paxlovid and some other medications are “not trivial.”
However, she said, “The really dangerous drugs are the ones for people who have had organ transplants and the like. Those aren’t individuals who are going to shop at a pharmacy.”
Besides the antirejection drugs, Dr. Wachter said, there can be serious interactions with cholesterol-lowering medications. If a person is taking Lipitor, for instance, “Someone would have to make the decision on whether it’s ok for me to stop it for a while, or to lower the dose. But I trust the pharmacist to do that as well as anybody.”
Except for these potential drug interactions with Paxlovid, the antiviral medications are “quite safe,” he said, adding that being able to treat people who test positive for COVID-19 right away is a big advantage of the test-to-treat program, considering how difficult it is for many people to get access to a doctor. That delay could mean that the antivirals are not prescribed and taken until they are no longer effective.
Both Dr. Wachter and Dr. Malani said that the widespread distribution of pharmacies and their extended hours are other big pluses, especially for people who can’t easily leave work or travel far to visit a physician.
Dr. Malani cautioned that there are still kinks to work out in the test-to-treat program. It will be a while before the retail clinics all have the antiviral drugs, and many pharmacies don’t have clinics on site.
Still, she said people can still go to their physicians to be tested, and presumably those doctors can also write antiviral prescriptions. But it’s not clear where the antivirals will be available in the near term.
“Right now, we’re playing catch-up,” Dr. Malani said. “But pharmacies are an important piece of the puzzle.”
Looking at the big picture, she said, “We know that neither vaccination nor natural infection provides long lasting immunity, and so there will be a role for antivirals in order to make this a manageable illness. And when you’re talking about millions of cases, as we were having a few months ago, the health system can’t field all those patients. So we do need a system where I can go to a pharmacy and get a test and treatment.”
A version of this article first appeared on Medscape.com.









