User login
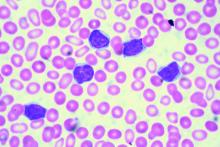
Review reveals lack of data on mild hemophilia A
A literature review has failed to provide new insights regarding the burden of mild hemophilia A.
In the 17 studies reviewed, mean annual bleeding rates (ABRs) were largely unreported. Data on joint pain and damage, quality of life (QOL), societal impacts, and costs of care were limited and inconsistent across the studies.
The review “revealed a lack of evidence” in adults with mild hemophilia A, Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues wrote in Haemophilia.
The researchers reviewed data from 10 studies conducted in Europe, 6 in North America, and 1 in Japan. Six studies were prospective cohort or registry studies, six were retrospective, and five studies were surveys or outcomes research.
The studies included 3,213 patients with mild hemophilia A aged 13 years or older. There were few details on treatment protocols, but patients received factor VIII concentrates, recombinant factor VIII, and desmopressin.
Most studies did not report mean ABRs. For the three that did, the mean ABRs were 0.44, 0.56, and 4.5. Six studies reported the percentage of patients with bleeding events, and those numbers ranged from 5.5% (1/18) to 90.7% (68/75).
Data on joint pain and damage were not standardized across studies, so the researchers were unable to draw any conclusions. One study showed no significant difference in Health Assessment Questionnaire pain score between patients with mild hemophilia A and control subjects. In another study, 5% of patients with mild hemophilia A reported having severe joint pain in the previous year, and 15% of patients reported moderate joint pain.
The researchers also found it difficult to draw conclusions about QOL. Three studies reported QOL data, and all used a different instrument.
In a study using the SF-36, general health and emotional role functioning were both significantly lower for patients with mild hemophilia A than for age-matched healthy control subjects (P less than .05). In a study using the SF-12, the physical component summary was significantly higher for patients with mild hemophilia A than for those with severe disease (P = .014).
In a study using the Haemo-QOL-A, there were no significant differences between patients with mild and severe hemophilia A. However, Dr. Peyvandi and colleagues noted that this study required long-term use of factor VIII concentrate, so the mild hemophilia A patients in this group were “probably not representative” of the overall mild hemophilia A population.
Societal impacts were difficult to assess because of a lack of standardization across studies. One study showed no significant difference in employment between patients with mild hemophilia A and healthy controls. In a U.S.-based study, patients with mild hemophilia A missed an average of 6.2 workdays per year, and 4.7 days were caused by their hemophilia. A study in Italy showed that patients with mild hemophilia A missed an average of 3.4 workdays per year.
Just two studies included data on health care costs for patients with mild hemophilia A. The mean cost of care was €793 per year in a study from Portugal published in 2015. In a U.S. study published in 1995, the annual cost of care was $22,182.
“Considering the limitations of the current body of evidence, higher-quality studies in this area are needed,” Dr. Peyvandi and colleagues wrote. “Such studies would report both bleeding and other clinical outcomes based on common definitions and for a representative population of mild [hemophilia A] adults. Areas for further research include more robust comparison to healthy controls or population norms, especially for QOL and other patient-reported outcomes.”
Seven of the eight researchers reported relationships, including employment, with BioMarin. Dr. Peyvandi reported relationships with Sanofi, Grifols, Novo Nordisk, Roche, Takeda, Sobi, Bioverativ, Spark Therapeutics, Sysmex, and CSL Behring.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Jul 11. doi: 10.1111/hae.13777.
A literature review has failed to provide new insights regarding the burden of mild hemophilia A.
In the 17 studies reviewed, mean annual bleeding rates (ABRs) were largely unreported. Data on joint pain and damage, quality of life (QOL), societal impacts, and costs of care were limited and inconsistent across the studies.
The review “revealed a lack of evidence” in adults with mild hemophilia A, Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues wrote in Haemophilia.
The researchers reviewed data from 10 studies conducted in Europe, 6 in North America, and 1 in Japan. Six studies were prospective cohort or registry studies, six were retrospective, and five studies were surveys or outcomes research.
The studies included 3,213 patients with mild hemophilia A aged 13 years or older. There were few details on treatment protocols, but patients received factor VIII concentrates, recombinant factor VIII, and desmopressin.
Most studies did not report mean ABRs. For the three that did, the mean ABRs were 0.44, 0.56, and 4.5. Six studies reported the percentage of patients with bleeding events, and those numbers ranged from 5.5% (1/18) to 90.7% (68/75).
Data on joint pain and damage were not standardized across studies, so the researchers were unable to draw any conclusions. One study showed no significant difference in Health Assessment Questionnaire pain score between patients with mild hemophilia A and control subjects. In another study, 5% of patients with mild hemophilia A reported having severe joint pain in the previous year, and 15% of patients reported moderate joint pain.
The researchers also found it difficult to draw conclusions about QOL. Three studies reported QOL data, and all used a different instrument.
In a study using the SF-36, general health and emotional role functioning were both significantly lower for patients with mild hemophilia A than for age-matched healthy control subjects (P less than .05). In a study using the SF-12, the physical component summary was significantly higher for patients with mild hemophilia A than for those with severe disease (P = .014).
In a study using the Haemo-QOL-A, there were no significant differences between patients with mild and severe hemophilia A. However, Dr. Peyvandi and colleagues noted that this study required long-term use of factor VIII concentrate, so the mild hemophilia A patients in this group were “probably not representative” of the overall mild hemophilia A population.
Societal impacts were difficult to assess because of a lack of standardization across studies. One study showed no significant difference in employment between patients with mild hemophilia A and healthy controls. In a U.S.-based study, patients with mild hemophilia A missed an average of 6.2 workdays per year, and 4.7 days were caused by their hemophilia. A study in Italy showed that patients with mild hemophilia A missed an average of 3.4 workdays per year.
Just two studies included data on health care costs for patients with mild hemophilia A. The mean cost of care was €793 per year in a study from Portugal published in 2015. In a U.S. study published in 1995, the annual cost of care was $22,182.
“Considering the limitations of the current body of evidence, higher-quality studies in this area are needed,” Dr. Peyvandi and colleagues wrote. “Such studies would report both bleeding and other clinical outcomes based on common definitions and for a representative population of mild [hemophilia A] adults. Areas for further research include more robust comparison to healthy controls or population norms, especially for QOL and other patient-reported outcomes.”
Seven of the eight researchers reported relationships, including employment, with BioMarin. Dr. Peyvandi reported relationships with Sanofi, Grifols, Novo Nordisk, Roche, Takeda, Sobi, Bioverativ, Spark Therapeutics, Sysmex, and CSL Behring.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Jul 11. doi: 10.1111/hae.13777.
A literature review has failed to provide new insights regarding the burden of mild hemophilia A.
In the 17 studies reviewed, mean annual bleeding rates (ABRs) were largely unreported. Data on joint pain and damage, quality of life (QOL), societal impacts, and costs of care were limited and inconsistent across the studies.
The review “revealed a lack of evidence” in adults with mild hemophilia A, Flora Peyvandi, MD, PhD, of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan and colleagues wrote in Haemophilia.
The researchers reviewed data from 10 studies conducted in Europe, 6 in North America, and 1 in Japan. Six studies were prospective cohort or registry studies, six were retrospective, and five studies were surveys or outcomes research.
The studies included 3,213 patients with mild hemophilia A aged 13 years or older. There were few details on treatment protocols, but patients received factor VIII concentrates, recombinant factor VIII, and desmopressin.
Most studies did not report mean ABRs. For the three that did, the mean ABRs were 0.44, 0.56, and 4.5. Six studies reported the percentage of patients with bleeding events, and those numbers ranged from 5.5% (1/18) to 90.7% (68/75).
Data on joint pain and damage were not standardized across studies, so the researchers were unable to draw any conclusions. One study showed no significant difference in Health Assessment Questionnaire pain score between patients with mild hemophilia A and control subjects. In another study, 5% of patients with mild hemophilia A reported having severe joint pain in the previous year, and 15% of patients reported moderate joint pain.
The researchers also found it difficult to draw conclusions about QOL. Three studies reported QOL data, and all used a different instrument.
In a study using the SF-36, general health and emotional role functioning were both significantly lower for patients with mild hemophilia A than for age-matched healthy control subjects (P less than .05). In a study using the SF-12, the physical component summary was significantly higher for patients with mild hemophilia A than for those with severe disease (P = .014).
In a study using the Haemo-QOL-A, there were no significant differences between patients with mild and severe hemophilia A. However, Dr. Peyvandi and colleagues noted that this study required long-term use of factor VIII concentrate, so the mild hemophilia A patients in this group were “probably not representative” of the overall mild hemophilia A population.
Societal impacts were difficult to assess because of a lack of standardization across studies. One study showed no significant difference in employment between patients with mild hemophilia A and healthy controls. In a U.S.-based study, patients with mild hemophilia A missed an average of 6.2 workdays per year, and 4.7 days were caused by their hemophilia. A study in Italy showed that patients with mild hemophilia A missed an average of 3.4 workdays per year.
Just two studies included data on health care costs for patients with mild hemophilia A. The mean cost of care was €793 per year in a study from Portugal published in 2015. In a U.S. study published in 1995, the annual cost of care was $22,182.
“Considering the limitations of the current body of evidence, higher-quality studies in this area are needed,” Dr. Peyvandi and colleagues wrote. “Such studies would report both bleeding and other clinical outcomes based on common definitions and for a representative population of mild [hemophilia A] adults. Areas for further research include more robust comparison to healthy controls or population norms, especially for QOL and other patient-reported outcomes.”
Seven of the eight researchers reported relationships, including employment, with BioMarin. Dr. Peyvandi reported relationships with Sanofi, Grifols, Novo Nordisk, Roche, Takeda, Sobi, Bioverativ, Spark Therapeutics, Sysmex, and CSL Behring.
SOURCE: Peyvandi F et al. Haemophilia. 2019 Jul 11. doi: 10.1111/hae.13777.
FROM HAEMOPHILIA
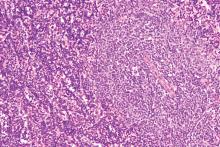
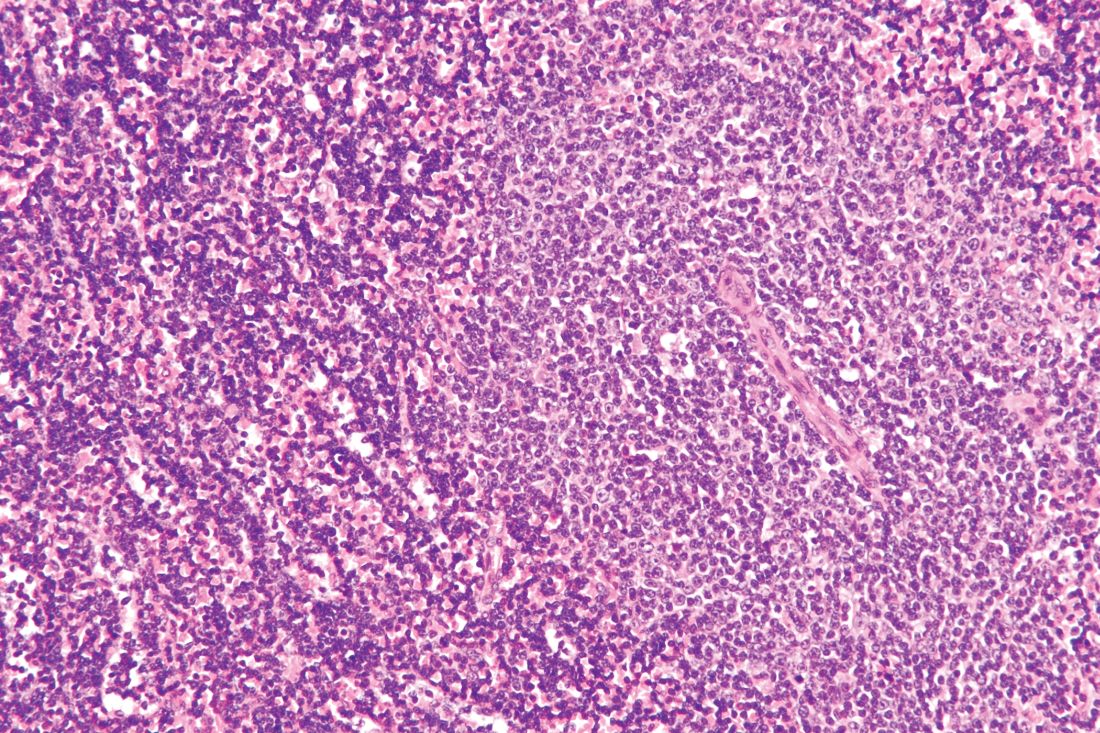
Ibrutinib/rituximab effective, safe as frontline treatment for older patients with MCL
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
LUGANO, SWITZERLAND – The chemotherapy-free combination of ibrutinib (Imbruvica) and rituximab is highly effective as frontline therapy for older, transplant-ineligible patients with nonblastoid mantle cell lymphoma, according to investigators.
In a phase 2 study of patients with a median age of 71 years, 38 of 41 patients (93%) had an objective response, and the regimen was both safe and easy to administer, reported Preetesh Jain, MD, PhD, of the University of Texas MD Anderson Cancer Center in Houston.
“The adverse event profile was generally favorable, with specific monitoring recommended for patients with cardiovascular comorbidities and a history of atrial fibrillation,” he said at the International Conference on Malignant Lymphoma.
The investigators enrolled 48 patients aged 65 years and older with previously untreated mantle cell lymphoma (MCL), of whom 41 were evaluable for the primary endpoints of overall response rate (ORR) and safety. The patients had good performance status and normal organ function, with the largest tumor size less than 10 cm. Patients with atrial fibrillation could participate, if the fibrillation was controlled. Patients with Ki-67 protein levels of 50% or greater and blastoid/pleomorphic histology were excluded.
Patients were treated with ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
Of the 41 patients evaluable for response, 26 (64%) had a complete response (CR) and 12 (29%) had a partial response. Three additional patients had stable disease, for an objective response rate of 93%.
Of 34 patients with PET scans, all had negative scans. Of 37 patients evaluable for minimal residual disease (MRD) by flow cytometry, 21 (58%) were MRD negative.
Patients with low or intermediate Mantle Cell Lymphoma International Prognostic Index (MIPI) scores had a higher ORR (100% vs. 89% for patients with high MIPI scores), and patients with low Ki-67 levels had a higher response rate than that of patients with KI-67 of 30% or greater (80% vs. 87%).
Neither median 3-year progression-free survival nor median 3-year overall survival have been reached, with respective 3-year rates of 87% and 95%.
Four patients experienced disease progression at 4, 10, 13 and 33 months of treatment. Three of these patients had disease that had transformed to blastoid/pleomorphic variant, two had Ki-67 of 30% or greater, one had mutations in TP53, and one had FAT1 and SF3B1 mutations.
Two patients died after ibrutinib therapy, one who had discontinued therapy because of bleeding, and the other who died on treatment at 13 months from transformed disease. Both of these patients had high Ki-67 levels.
Grade 3 or 4 hematological adverse events were neutropenia in four patients, and thrombocytopenia in two patients. There were no cases of grade 3 or 4 anemia.
Grade 3 or 4 nonhematological adverse events were fatigue, myalgia, and atrial fibrillation in seven patients each, diarrhea in six patients, and petechiae/bleeding in three patients.
Patients will continue to be followed for late adverse events, secondary cancers, and relapses, and further studies on clonal evolution, mutation profiling, and MRD are ongoing and will be reported at a later date, Dr. Jain said.
The National Cancer Institute supported the study. Dr. Jain reported having no financial disclosures.
SOURCE: Jain P et al. 15-ICML. Abstract 011.
REPORTING FROM 15-ICML
HDAC/HMA combo shows ‘remarkable’ activity in PTCL
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
LUGANO, SWITZERLAND – A combination of 5-azacytidine and romidepsin showed promising activity in patients with peripheral T cell lymphomas, particularly angioimmunoblastic T-cell lymphoma (AITL) and primary cutaneous follicular helper T-cell lymphoma (PTCL-TFH), results of a phase 2 study showed.
Of 16 patients with AITL or PTCL-TFH, 11 (69%) had a clinical response to the 5-azacytidine (AZA)/romidepsin combination, including 8 (50%) with complete responses (CRs), and 3 with partial responses (PRs), reported Lorenzo Falchi, MD, of Columbia University Medical Center and New York Presbyterian Hospital, New York, and colleagues.
“We show that the combination of oral AZA/romidepsin is remarkably active in patients with T-cell lymphomas. Clearly more patients with other subtypes are needed to better evaluate this combination,” Dr. Falchi said at the International Conference on Malignant Lymphoma.
The combination is intended to target epigenetic changes in PTCLs, which often bear mutations in TET2, DNMT3A, and IDH2. These mutations create global hypermethylation and cause transcriptional silencing of tumor suppressor genes, Dr. Falchi said.
Both histone deacetylase inhibitors such as romidepsin, and hypomethylating agents such as AZA have been shown to have single-agent activity against PTCL, and as previously reported at the 2018 T-cell Lymphoma Forum, the combination produced a higher overall response rate (ORR) and prolonged progression-free survival (PFS) in patients with T-cell lymphomas.
Dr. Falchi presented the phase 2 results at 15-ICML. A total of 25 patients with newly diagnosed or relapsed/refractory PTCL were treated with AZA 300 mg daily on days 1-14 and romidepsin 14 mg/m2 on days 8, 15, and 22, every 35 days.
A total of 24 patients were evaluable for response. The ORR – the primary endpoint – was achieved in 14 patients (58%), and included 10 CRs and 4 PRs. Three additional patients had stable disease, and six patients experienced disease progression (response data for one patient was not complete at the time of the presentation).
In total, 11 of 16 patients with AITL/PTCL-TFH had responses, compared with 3 of 8 patients with other histologies.
A secondary analysis of 16 patients with information on mutational status showed that 10 of 12 patients with TET2 mutations (83%) had responses, including 8 CRs and 2 PRs. Two additional patients with TET2 mutations had disease progression. In contrast, among four patients without TET2 mutations, one had a CR, one a PR, and two had disease progression.
Of the 10 patients overall with CRs, 5 patients were receiving the combination in the first line, and 5 patients were receiving it for relapsed/refractory disease.
Median PFS among all patients was 8.7 months. The median overall survival has not been reached. Among patients with the AITL or PTCL-TFH subtypes, median PFS was 8.7 months, compared with 2.3 months for patients with other histologies.
The most frequent hematologic grade 3 or 4 adverse events were thrombocytopenia and neutropenia. The most frequent nonhematologic grade 3 or 4 events included lung infection and febrile neutropenia. Common grade 1 or 2 toxicities included anemia, diarrhea, fatigue, nausea, and vomiting. No patients discontinued therapy because of adverse events.
Dr. Falchi noted that a phase 1 trial evaluating the immune checkpoint inhibitor durvalumab (Imfinzi) with AZA or romidepsin alone or in combination, or pralatrexate and romidepsin, is currently recruiting.
Dr. Falchi reported having no financial disclosures. Other investigators reported funding from Celgene, which supported the study.
SOURCE: Falchi L et al. 15-ICML, Abstract 129.
REPORTING FROM 15-ICML
Timing, volume of transfusion may not matter in children with severe anemia
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
Trial results suggest African children with uncomplicated, severe anemia may not require immediate blood transfusion, and the volume of transfusion may only matter in the context of fever.
The TRACT trial showed no significant differences in 28-day mortality or other clinical outcomes between children who received immediate transfusions and those who did not.
Similarly, there was no significant difference in 28-day mortality among children who received transfusions of 20 mL/kg and those who received transfusions of 30 mL/kg. There was evidence to suggest a higher transfusion volume may benefit children without fevers, but this was an exploratory endpoint. The findings were published in the New England Journal of Medicine.
These results suggest “there is no credible reason to transfuse immediately or to transfuse a higher volume of blood, at least in pediatric populations in regions such as these two sub-Saharan countries [Uganda and Malawi],” Julie R. Ingelfinger, MD, of Massachusetts General Hospital in Boston, wrote in an accompanying editorial, also published in the New England Journal of Medicine (2019;381:475-6).
“The possible effect of higher volume transfusion in patients with fever may trigger additional and potentially useful studies,” she added.
Immediate transfusion
One goal of the TRACT trial was to determine if blood transfusion is the best treatment for children with severe anemia. With this in mind, Kathryn Maitland, MD, PhD, of Imperial College London and colleagues evaluated 1,565 Ugandan and Malawian children with uncomplicated, severe anemia. The patients’ median age was 26 months, and 984 (62.9%) had malaria.
The children were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787). Children who did not have an immediate transfusion (control group) could receive a transfusion if they exhibited new signs of clinical severity or had their hemoglobin decrease to below 4 g/dL.
All children in the immediate-transfusion group received a transfusion, as did 386 (49.0%) in the control group. The median time to transfusion was 1.3 hours in the immediate group and 24.9 hours in the control group. The mean total blood volume transfused per child was 314 plus or minus 228 mL and 142 plus or minus 224, respectively. The follow-up period was 180 days, and 4.5% of patients (n = 71) were lost to follow-up.
The researchers found no significant difference between the treatment groups with regard to mortality, other clinical outcomes, or the cost of care.
The 28-day mortality rate was 0.9% in the immediate-transfusion group and 1.7% in the control group (hazard ratio, 0.54; 95% confidence interval, 0.22-1.36; P = .19). The 180-day mortality was 4.5% and 6.0%, respectively (HR, 0.75; 95% CI, 0.48-1.15).
Transfusion volume
To assess the effects of transfusion volume, Dr. Maitland and colleagues evaluated 3,196 Ugandan and Malawian children with severe anemia. The median age of the children was 37 months, and 2,050 (64.1%) had malaria.
The children received a transfusion of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596) at a median of 1.2 hours after randomization. Some children – 197 in the 30-mL/kg group and 300 in the 20-mL/kg group – received additional transfusions. The mean volume of total blood transfused per child was 475 plus or minus 385 mL, and 353 plus or minus 348 mL, respectively.
Overall, there was no significant between-group difference with regard to mortality. The 28-day mortality rate was 3.4% in the 30 mL/kg group and 4.5% in the 20 mL/kg group (HR = 0.76; 95% CI, 0.54 to 1.08; P = .12).
However, the 28-day mortality rate did differ according to the presence of fever at screening. The mortality rate was lower in the 30 mL/kg group for children without fevers (HR = 0.43; 95% CI, 0.27 to 0.69) but higher in the 30 mL/kg group for febrile children (HR = 1.91; 95% CI, 1.04 to 3.49).
For other outcomes, including readmissions and serious adverse events, the researchers found no significant between-group differences.
This trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator. Dr. Ingelfinger is a deputy editor at the New England Journal of Medicine. No other relevant conflicts of interest were reported.
SOURCES: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: The 28-day mortality was 0.9% in patients who had immediate transfusions and 1.7% in those who did not (hazard ratio, 0.54; P = .19). The 28-day mortality rate was 3.4% in patients who received transfusions of 30 mL/kg and 4.5% in those who received transfusions of 20 mL/kg (HR, 0.76; P = .12). However, the mortality rate was lower in the 30-mL/kg group for children without fevers (HR, 0.43) and higher in the 30-mL/kg group for febrile children (HR, 1.91).
Study details: A phase 3 trial of African children with severe anemia who were randomized to immediate transfusion (n = 778) or no immediate transfusion (n = 787) and transfusions of 30 mL/kg (n = 1,592) or 20 mL/kg (n = 1,596)
Disclosures: The trial was supported by a grant from the United Kingdom Medical Research Council through a concordat with the Department for International Development. One researcher has a Wellcome Senior Research Fellowship, and another is a National Institute for Health Research Senior Investigator.
Sources: Maitland K et al. N Engl J Med. 2019;381:407-19. Maitland K et al. N Engl J Med. 2019;381:420-31.
ICYMI: Ibrutinib/rituximab combo improves CLL survival
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
RNA interference drug fitusiran looks effective in both hemophilia A and B
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
MELBOURNE – An investigational RNA interference therapeutic that suppresses the production of antithrombin has shown significant reductions in bleeding rates with no major safety events, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Fitusiran is a once-monthly, fixed-dose subcutaneous therapy that uses RNA interference to silence the gene for the endogenous anticoagulant antithrombin.
“The therapeutic hypothesis is based on the fact that hemophilia A and B are essentially thrombin-deficiency disorders, so if we lack factor VIII or factor IX, we can’t generate enough thrombin and we can’t produce a significant and substantial blood clot,” John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust. “If we, however, administer fitusiran, which will suppress antithrombin production, we can rebalance coagulation, generate more thrombin and form a much more substantial clot.”
Dr. Pasi presented results of an interim analysis of safety and efficacy data from an open-label, phase 2 extension study in 34 individuals with hemophilia A or B, with or without inhibitors, who were treated either with 50-mg or 80-mg doses of fitusiran for a median of at least 2 years.
Researchers saw significant declines in annualized bleeding rates in patients with hemophilia A and B, with and without inhibitors. Among those without inhibitors, the median annualized bleeding rate declined from 2.00 in patients already on hemophilia prophylaxis and 12.00 in those using on-demand treatment to 1.08 overall. In patients with inhibitors, the median annualized bleeding rate dropped from 42.00 to 1.04.
The treatment was also associated with substantial reductions in antithrombin production and increases in thrombin generation.
One patient in the phase 1 study experienced a fatal cerebral venous sinus thrombosis, which subsequently led to introduction of a bleed management protocol.
“Following that last case, we revised and reviewed the bleed management guidelines in view of the fact that there might potentially be an interaction between the amount of replacement therapy and thrombin generation,” Dr. Pasi said. Since introduction of that protocol, there have been no related thrombotic events.
The majority of adverse events reported were mild and deemed not related to the study drug, Dr. Pasi said. These included headache, injection site erythema, and arthralgia. A total of 14 subjects – all of whom were positive for hepatitis C at baseline – experienced rises in ALT levels but these were asymptomatic and resolved spontaneously.
One patient with chronic active hepatitis C infection also showed significant ALT/AST elevation which led to discontinuation of treatment.
In an interview, Dr. Pasi said one of the biggest advantages of fitusiran was that it could be used in patients with hemophilia A and B. “You’ve got patients with hemophilia B who’ve got no options at the moment. That would be an obvious specific group that would gain from this.”
Another advantage was fitusiran’s stability and dosing, he said, pointing out that the treatment was fixed dosing and stable at room temperature. Fitusiran is now undergoing phase 3 trials.
The study was funded by Sanofi Genzyme and Alnylam Pharmaceuticals, and six authors were employees of Sanofi Genzyme. Dr. Pasi reported financial relationships with pharmaceutical companies, including Alnylam.
SOURCE: Pasi J et al. 2019 ISTH Congress, Abstract OC 11.3.
REPORTING FROM 2019 ISTH CONGRESS
Early phase trial shows durable responses to gene therapy for hemophilia A
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
MELBOURNE – A gene therapy treatment for hemophilia A has shown sustained reductions in bleeding rates 3 years after treatment, with no major safety issues, according to findings presented at the International Society on Thrombosis and Haemostasis congress.
Valoctocogene roxaparvovec is an investigational gene therapy that involves using an adenovirus-associated virus to deliver the gene for clotting factor VIII.
John Pasi, MBChB, PhD, of the Royal London Haemophilia Centre, Barts Health NHS Trust, presented the 3-year efficacy and safety results from the phase 1/2 trial of the therapy, involving 15 men with hemophilia A without inhibitors who received a single intravenous dose – either 4 x 1013 vector genomes (vg) per kg or 6 x 1013 vg/kg – of the therapy.
Participants’ mean annualized bleeding rate at baseline ranged from 6.5 among men who had been receiving prophylactic therapy to 25 among those who had been historically been treated on demand.
The treatment was associated with a substantial, significant reduction in mean annualized bleed rates; a 96% reduction in the 6 x 1013 vg/kg group by year 3, and 92% reduction in the 4 x 1013 vg/kg group by year 2.
By year 3, 86% of patients in the higher dose group had not experienced a bleed in the prior 12 months, all patients were off prophylaxis, and all had experienced resolution of target joints.
Mean factor VIII usage also decreased significantly, with a 96% reduction by year 3 in the higher dose cohort, and a 97% reduction by year 2 in the lower dose cohort.
The study also showed significant improvements in quality of life across all domains, Dr. Pasi reported.
There were no significant safety concerns raised during the study. Several patients experienced mild to moderate, transient rises in alanine aminotransferase levels at around 8-16 weeks after treatment, but there was no significant impact on liver function or on corticosteroid use. Two patients reported mild infusion reactions, which resolved within 48 hours with altering treatment.
The researchers also examined durability of factor VIII activity levels following the gene therapy, which was monitored using chromogenic assays. This revealed that after the initial increase following therapy, the factor VIII levels plateaued between years 2 and 3.
“We’ve got what we feel is really good clinical evidence of a persistent effect and we think this is dramatic,” Dr. Pasi said. A phase 3 trial is now underway.
A commenter from the audience, who remarked that the data were incredible and would make a huge difference for patients, asked about whether this represented a possible cure for the disease.
It’s premature to talk about a cure, Dr. Pasi said.
“It’s like watching paint dry; it’s going to take years before we know where we are,” he said in an interview.
However, this could represent massive and transformational change in the management of hemophilia A, he added.
On the question of whether this approach might also work in patients with inhibitors, Dr. Pasi said there were animal data suggesting that gene therapy could work in individuals with inhibitors, but the focus for the moment was on patients without inhibitors.
“But for patients that previously had a history of inhibitors and are now tolerant, that’s quite a significant group of patients that we were going to have to think about how we deal with that in due course,” he said.
The study was sponsored by manufacturer BioMarin Pharmaceutical. Dr. Pasi reported financial relationships with the study sponsor and other companies.
SOURCE: Pasi KJ et al. 2019 ISTH Congress, Abstract LB 01.2.
REPORTING FROM 2019 ISTH CONGRESS
BRCA2 mutations linked to childhood NHL
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
Pediatric non-Hodgkin lymphomas should be added to the list of cancers associated with BRCA2 mutations, and survivors of childhood NHL should be considered for genetic counseling, investigators suggest.
Among 1,380 survivors of childhood lymphomas, those who were retrospectively found to be carriers of BRCA2 mutations had a fivefold higher risk for non-Hodgkin lymphoma than controls without cancer, reported Zhaoming Wang, PhD, and colleagues from St. Jude Children’s Research Hospital in Memphis.
“Genetic counseling and the option of BRCA2 genetic testing should be offered to survivors of pediatric or adolescent non–Hodgkin lymphoma, particularly those with a family history of BRCA2-associated cancers,” they wrote in JAMA Oncology.
The investigators had previously reported that BRCA2 was the third-most frequently mutated gene among 3006 survivors of childhood cancers, with the highest number of mutations seen in lymphoma survivors. In that study, 7 of 586 survivors of Hodgkin and non-Hodgkin lymphoma (1.2%) were found to carry BRCA2 mutations.
In the current study, the investigators performed germline whole-genome sequencing on samples from 815 survivors of childhood Hodgkin lymphoma and 748 survivors of non-Hodgkin lymphoma from the St. Jude Lifetime Cohort and Childhood Cancer Survivor studies and compared the data with those of controls without cancer from the Genome Aggregation Database.
They identified mutations in five Hodgkin lymphoma survivors (0.6%) and eight non-Hodgkin lymphoma survivors.
A comparison of cancer risk among lymphoma survivors and controls found that non-Hodgkin lymphoma survivors and BRCA2 carriers had an odds ratio for cancer of 5.0, compared with controls who were not BRCA2 carriers (P less than .001). Among Hodgkin lymphoma survivors the OR for carriers vs. controls was 2.1, but was not statistically significant.
Available family histories for seven of the eight non-Hodgkin lymphoma BRCA2 mutation carriers showed histories of BRCA2-linked cancers, including breast, prostate, and pancreas tumors and malignant melanoma.
“Survivors whose test results are positive for mutation should be offered surveillance for BRCA2-associated cancers, such as breast and ovarian, and counseled about cancer risk–reducing strategies. Currently, it remains unclear whether surveillance for non–Hodgkin lymphoma is associated with early detection of lymphomas or with other medical advantages,” the investigators wrote.
“This study was funded by a grant to St Jude Children’s Research Hospital from the American Lebanese Syrian Associated Charities and by grants to St Jude Children’s Research Hospital from the National Institutes of Health. The authors reported having no conflicts of interest.
SOURCE: Wang Z et al. JAMA Oncology. 2019 Jul 25. doi: 10.1001/jamaoncol.2019.2203.
FROM JAMA ONCOLOGY
Lymphoma risk prompts FDA recall of Allergan’s textured breast implants
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
The Food and Drug Administration requested on July 24 that Allergan pull six brands of textured breast implants and breast expanders from the U.S. market, an action the agency took because of new data that substantially increased the number of women who developed a rare cancer – anaplastic large-cell lymphoma – in association with receiving these textured breast devices.
This is the first product recall the FDA has made to address the issue of breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL), a complication that first came to national attention with a 2011 FDA report that had tallied 60 identified BIA-ALCL cases worldwide. By the end of September 2018, the number of reported worldwide BIA-ALCL cases had jumped to 457 cases reported to the agency via medical device reporting. In July 2019, the FDA cited a total of 573 unique, global case reports for BIA-ALCL sent to the agency through July 6, including 33 episodes that led to death.
It was inclusion of these additional 116 cases since September 2018 and 24 additional deaths that led FDA researchers to conclude that “the risk of BIA-ALCL with Allergan BIOCELL textured implants is approximately six-times the risk of BIA-ALCL with textured implants from other manufacturers marketing in the U.S.,” according to a statement from the agency.
The FDA is not recommending that patients who received one of the six products covered by the recall have the material removed if symptoms have not appeared because of the potential risk from explantation.
The agency also stressed that its investigation of the risk posed by placement of other brands of textured breast implants is ongoing and that overall less than 5% of all breast implants performed in current U.S. practice involve the macrotextured implants of the type specified in the Allergan recall.
This U.S. recall follows similar actions taken in France (and the rest of the European Union), Canada, and Australia, and it contrasts with the agency’s prior decision in May 2019 not to start a recall or ban of textured implants following a advisory committee meeting that discussed BIA-ALCL.
The six products that Allergan agreed to recall from marketing at the FDA’s request are four textured breast implants (Natrelle Saline-Filled Breast Implants, Natrelle Silicone-Filled Breast Implants, Natrelle Inspira Silicone-Filled breast Implants, and Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled Breast Implants) and two tissue expanders used prior to a breast implant (Natrelle 133 Plus Tissue Expander and the Natrelle 133 Tissue Expander with Suture Tabs).
FDA officials said they are considering recommendations for changes to the labeling of breast implant products, including a possible boxed warning and beefed up patient information.
“The recall of these textured implants is a big deal in protecting women from the potential risks of developing, and dying from, this rare type of aggressive lymphoma,” Joshua Brody, MD, a medical oncologist and director of the lymphoma immunotherapy program at Mount Sinai Medical Center in New York said in a statement. “While case reports have suggested a potential link between some types of breast implants and this disease – anaplastic lymphoma – for over 20 years, it has taken time to gain sufficient evidence to suggest, and understand, the causality. Some types of implants induce inflammation, which can both increase the chance of developing cancer, and also help to ‘hide’ developing cancers from the immune system. By preventing further use of these implants, the FDA is helping women to protect themselves from the medically serious and emotionally exhausting effects of these risks.”
Dr. Brody reported having no relevant disclosures.
BTK mutations linked to CLL progression on ibrutinib
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
FROM BLOOD