User login
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
On the basis of the patient's presentation and imaging results, the likely diagnosis is non–small cell cancer (NSCLC) of an adenocarcinoma subtype. NSCLC makes up about 80% of all lung cancer cases. Adenocarcinoma in particular is the most common type of lung cancer in the United States, accounting for about 40% of cases, and it is the most common histology among nonsmokers. Women are more likely to develop this subtype of NSCLC and are generally younger when they present with symptoms. This type of cancer arises from the bronchial mucosal glands and usually develops in a peripheral location within the lung.
In the course of workup, immunohistochemical (IHC) analyses are used to identify tumor type and lineage (adenocarcinoma, squamous cell carcinoma, metastatic malignancy, or primary pleural mesothelioma). Separate IHC analyses are then used to guide treatment decisions, identifying whether ALK inhibitor therapy or programmed cell death protein ligand 1 (PD-L1) inhibitor therapy would be appropriate.
Tissue should also be conserved for molecular testing. NCCN guidelines advise that all patients with adenocarcinoma should be tested for EGFR mutations, and DNA mutational analysis is the preferred method for assessment. Patients should also undergo routine biomarker testing, with an eye toward ALK, RET, and ROS1 rearrangements, BRAF mutations, c-MET and exon 14 skipping mutations, and PD-L1 expression levels. For patients with metastatic NSCLC, PD-L1 IHC testing is recommended.
Most cases of lung cancer are diagnosed at a late stage, when symptoms have already begun to manifest. Of note, however, women with adenocarcinoma are more likely to present with localized disease. Treatment is largely influenced by the presence of targetable mutations. Among adenocarcinoma cases, the most common mutations are in the EGFR and KRAS genes.
For patients who are EGFR mutation positive (exon 10 deletion or L858R), osimertinib is the recommended first-line therapy. For patients who are positive for the EGFR exon 20 insertion mutation, initial systemic therapy options for adenocarcinoma are appropriate; the preferred regimen being pembrolizumab-carboplatin-pemetrexed if there are no contraindications to programmed cell death protein 1 (PD-1) or PD-L1 inhibitors.
KRAS mutations, unlike EGFR mutations, are associated with smoking. Because overlapping targetable alterations are uncommon, identification of KRAS mutations suggests that these patients will not benefit from additional molecular testing. Again, initial systemic therapy options for adenocarcinoma are appropriate, but the presence of KRAS mutation predicts a poor response to EGFR tyrosine kinase inhibitors. The FDA approved a KRAS inhibitor in June 2021 and immune checkpoint inhibitors appear to be beneficial in this population.
Maurie Markman, MD, President, Department of Medical Oncology, Cancer Treatment Centers of America.
Maurie Markman, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Merck
Serve(d) as a speaker or a member of a speakers bureau for: AstraZeneca; Novis; Glaxo Smith Kline
Received research grant from: AstraZeneca; Novis; GSK; Merck
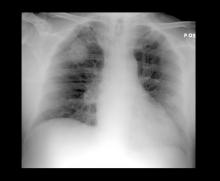
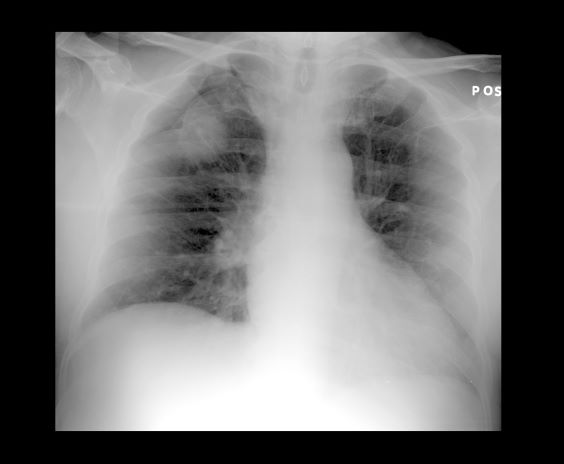
A 52-year-old woman presents with dyspnea and a persistent cough. She is 5 ft 5 in and weighs 155 lb, with no recent significant weight loss. She has been experiencing symptoms for a few months, which she originally thought might be related to her history of GERD. She reports that she was a light smoker before she had children but has not smoked regularly in about 20 years. Because of the patient's respiratory symptoms, chest radiography is ordered.
This frontal projection chest radiography clearly demonstrates a mass in the upper lobe of the right lung that represents the appearance of lung cancer (malignancy).