User login
Vitamin D is viewed as a promising supplement by the medical, public health, and lay communities, potentially offering many health benefits. But enthusiasm for a new intervention too often gets far ahead of the evidence, as was the case with beta-carotene, selenium, folic acid, and vitamins C and E.
Despite the enthusiasm for vitamin D, there have been no large-scale primary prevention trials that have had either cardiovascular disease or cancer as a prespecified primary outcome. Previous randomized trials of vitamin D have focused primarily on osteoporosis, fracture, falls, and physical function. Although the investigators often reported their findings on vitamin D and cardiovascular disease or cancer, these outcomes were generally secondary or tertiary end points that were not prespecified. These studies should be viewed as hypothesis-generating rather than hypothesis-testing. The increasing prevalence of use of vitamin D supplements underscores the need for rigorous and conclusive evidence from randomized clinical trials that have cardiovascular disease and cancer as primary outcomes.
This article will explain the rationale for a large-scale, randomized clinical trial to evaluate the role of vitamin D in the prevention of cardiovascular disease and cancer. It will also describe the biological mechanisms and currently available evidence relating vitamin D to potential health benefits. Finally, the design, dosage considerations, and logistics of the Vitamin D and Omega-3 Trial (VITAL) will be presented.
EVIDENCE IS MOUNTING FOR VITAMIN D’S BIOLOGICAL IMPORTANCE
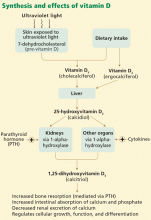
Vitamin D is undoubtedly important to health: not only is it produced endogenously, but at least 500 genes have been identified with vitamin D response elements. The vitamin D receptor is found in nearly all cells in the body, and the 1-alpha-hydroxylase enzyme is present in many tissues. Some studies suggest that almost 10% of the human genome may be at least partially regulated by vitamin D.
Although we know vitamin D is important, what our optimal intake and our blood level of 25-hydroxyvitamin D3 should be are key unknowns.
RECOMMENDATIONS FOR VITAMIN D INTAKE
During winter, late fall, and early spring, people who live above the 37th parallel (geographically, about one-half of the contiguous United States) do not get enough ultraviolet B energy from the sun to make all the vitamin D they need, even if they spend several hours outside every day. In addition, dark skin pigmentation serves as a sun block, as do sunscreens.
The Institute of Medicine (IOM) provided guidelines for vitamin D intake in 1997 and, most recently, in 2010. However, these guidelines are based on the amount of vitamin D required for bone health and do not address the amount that may be of benefit for prevention of cancer and cardiovascular disease. The latter outcomes are not addressed because the IOM committee believed that evidence was insufficient to determine the role of vitamin D in the prevention of cardiovascular disease, cancer, and other chronic diseases. Thus, current IOM guidelines, which generally recommend less than 1,000 IU of vitamin D daily, are relevant to bone health but not necessarily to other health outcomes. More research is needed to understand whether the guidelines should be modified for the prevention of other chronic diseases.
Moreover, whether or not everyone should be screened for 25-hydroxyvitamin D3 blood levels is controversial. Most experts agree that a level less than 20 ng/mL is deficient or insufficient. Conversely, potentially harmful are levels 150 ng/mL or more (> 375 nmol/L), which entail the risk of hypercalcemia, vascular soft tissue calcification, and hyperphosphatemia.
People do not reach toxic levels with ultraviolet light exposure because the amount of 25-hydroxyvitamin D3 synthesis is well regulated. Dietary supplements, however, can bring about toxic levels, and patients taking high doses need to be monitored carefully. The level that should be considered optimal is controversial and requires further study.
RISK FACTORS FOR LOW VITAMIN D LEVELS
Risk factors for low vitamin D levels include older age, living in northern latitudes, sun avoidance, dark skin pigmentation, obesity, low dietary intake, and various medical conditions, especially malabsorption syndromes. Some of these are also risk factors for cardiovascular disease, cancer, and other chronic diseases, and potentially confound outcomes in many studies. Older age, which is usually adjusted for in multivariate models, is important to recognize as a major risk factor for vitamin D deficiency, owing to reduced absorption and synthesis, less time outdoors, and low dietary intake.
Wearing sunscreen decreases the synthesis of vitamin D in the skin, but because ultra-violet light has been clearly classified as a carcinogen, it is a not advisable to increase sun exposure for the sake of increasing vitamin D levels. That is a poor trade-off, given the high incidence rate of skin cancer and the adverse effects of solar radiation on skin aging.
Obesity is a risk factor for vitamin D deficiency because vitamin D is fat-soluble and becomes sequestered in fat tissue. Vitamin D may also play a role in the differentiation of adipocytes and may affect their function. In observational studies, it is very important for researchers to adjust for body mass index, physical activity (which may be correlated with more time outdoors), and other potential confounders in their analyses.
HOW VITAMIN D MAY LOWER CANCER RISK
Because of the important effect of vitamin D in regulating cell differentiation and cell growth, there are multiple ways that it may affect cancer risk. Laboratory, cell culture, and animal studies suggest that vitamin D may lower cancer risk by inhibiting cell proliferation, angiogenesis, metastasis, and inflammation and inducing apoptosis and cellular differentiation. Several of these mechanisms are also relevant to atherosclerosis and cardiovascular disease. Although VITAL is addressing the role of vitamin D in preventing both cancer and cardiovascular disease, the remainder of this article will focus on cardiovascular outcomes.
HOW VITAMIN D MAY REDUCE CARDIOVASCULAR RISK
Vitamin D may lower cardiovascular risk via several mechanisms:
Inhibiting inflammation. Vitamin D has a powerful immunomodulatory effect: laboratory studies show that it inhibits prostaglandin and cyclooxygenase 2 pathways, reduces matrix metalloproteinase 9 and several proinflammatory cytokines, and increases interleukin 10, all of which result in suppressed inflammation.1
Inhibiting vascular muscle proliferation and vascular calcification. Animal studies indicate that in moderate doses vitamin D decreases calcium cellular influx and increases matrix Gla protein, which inhibits vascular smooth muscle proliferation and vascular calcification. These protective effects contrast with the hypercalcemia associated with a high intake of vitamin D, especially in the context of renal failure or other risk factors, which may lead to increased vascular calcification.1
Regulates blood pressure. Vitamin D decreases renin gene expression and the synthesis of renin, which reduces activity of the renin-angiotensin-aldosterone system, leading to a reduction of blood pressure and a favorable effect on volume homeostasis.1
Regulates glucose metabolism. Limited evidence shows that vitamin D may increase insulin sensitivity and regulate glucose metabolism.1
Vitamin D and cardiac hypertrophy
The vitamin D receptor is present in virtually all tissues, including cardiac myocytes and endothelial cells. Animals with vitamin D deficiency have higher blood pressures, and animals genetically altered to have no vitamin D receptors (knock-out models) develop left ventricular hypertrophy and heart failure.
Animals genetically altered to have no 1-alpha-hydroxylase (so that the most active form of vitamin D is not made) also develop left ventricular hypertrophy. They can be rescued by the administration of 1,25-dihydroxy vitamin D3.1
These findings are consistent with what is observed in patients with end-stage renal disease, who produce very little 1,25-dihydroxyvitamin D3: they often develop left ventricular hypertrophy, diastolic heart failure, atherosclerosis, and vascular calcification.
EVIDENCE FOR CARDIOVASCULAR DISEASE REDUCTION
Wang et al1 recently reviewed available prospective cohort and randomized clinical trials from 1966 to 2009 that examined vitamin D or calcium supplementation and cardiovascular disease. Comparing people with the lowest to the highest levels of serum 25-hydroxyvitamin D3 indicated that a low level is a risk factor for coronary artery disease and cardiovascular death. Unfortunately, most studies were not designed to assess primary effects on cardiovascular outcomes, and so have many potential confounders.
Prospective observational studies
Observational studies suggest that vitamin D deficiency is associated with an increased risk of cardiovascular disease. Some examples:
The Framingham Offspring Study2 followed 1,739 men and women with a mean age of 59 for 5.4 years. The study compared the incidence of cardiovascular events in those with a serum 25-dihydroxyvitamin D level of at least 37.5 nmol/L vs those with lower levels. The risk of cardiovascular disease was 1.62 times higher in those with the lowest levels of vitamin D, a statistically significant difference. However, a threshold effect was apparent (discussed below).
The Health Professionals Follow-up Study3 prospectively evaluated more than 18,000 men ages 40 to 75 for 10 years. The study compared men with a low serum level of vitamin D (< 37.5 nmol/L) to those with a more optimal level (> 75 nmol/L). The incidence of cardiovascular events was 2.09 times higher in men with low levels of vitamin D, a difference that was statistically significant.
The Third National Health and Nutrition Examination Survey (NHANES III) included data for more than 13,300 men and women age 20 years and older. Using a cohort that was followed for 8.7 years, Melamed et al4 compared the quartile with the lowest serum vitamin D level (< 44.4 nmol/L) against the quartile with the highest level (> 80.1 nmol/L). The associations were modest: those with low levels had a 1.20-times higher rate of death from cardiovascular disease and a statistically significant 1.26-times higher rate of death from all causes.
Randomized clinical trials
A meta-analysis of 18 randomized trials5 of vitamin D supplementation (300–2,000 IU/day, mean 528 IU/day vs placebo), including 57,311 participants, evaluated the rate of death from all causes and found a modest but significant reduction in risk (relative risk 0.93, 95% confidence interval [CI] 0.87–0.99). These were generally trials looking at fracture rates or physical performance, and a dose-response relationship was not evident. A recent systematic review of randomized controlled trials of vitamin D1 that included cardiovascular disease as a secondary outcome found a pooled relative risk for cardiovascular disease of 0.90 (95% CI 0.77–1.05) for vitamin D supplementation compared with placebo and 1.04 (95% CI 0.92–1.18) for combination vitamin D plus calcium supplementation vs placebo.1 Two individual trials are discussed below.
Trivedi et al6 randomized 2,686 British men and women to vitamin D3 100,000 IU given every 4 months over 5 years (equivalent to 800 IU/day) or placebo. The relative risk of cardiovascular events was 0.90 (95% CI 0.77–1.06) and of cardiovascular deaths 0.84 (95% CI 0.65–1.10). Although the results were promising, the trial was designed to assess fracture risk and was not large enough for the differences in cardiovascular outcomes to reach statistical significance.
The Women’s Health Initiative,7,8 which included 36,282 postmenopausal women aged 50 to 79, tested combined vitamin D3 (400 IU/day) with calcium (1,000 mg/day) vs placebo. No benefit was seen for preventing coronary events or stroke, which may be due to the low dosage of vitamin D. The hazard ratio for coronary disease was 1.04 (0.92–1.18). Regarding mortality, the hazard ratio for cardiovascular death was 0.92, for cerebrovascular death 0.89, for cancer death 0.89, and for other deaths 0.95. None of these hazard ratios reached statistical significance.
MORE MAY NOT BE BETTER
As is probably true for everything in biological systems, there apparently is an optimal level of intake to meet vitamin D needs.
The Framingham Offspring Study,2 which found a higher risk with vitamin D deficiency, also found a suggestion of a threshold. Participants who had levels of 50 to 65 nmol/L had the lowest risk. Higher levels did not confer lower risk and even suggested a slight upturn.
Evidence from the Women’s Health Initiative8 also indicates that high dosages may not be better than moderate dosages. The meta-analysis of vitamin D and all-cause mortality5 found a relative risk of 0.93, but one of the largest studies in that meta-analysis tested only 400 IU a day and found a similar relative risk of 0.91 (95% confidence interval, 0.83–1.01).
Moreover, the NHANES study found that with increasing serum 25-hydroxyvitamin D3 levels, the risk of all-cause mortality fell until about 100 nmol/L, but then plateaued and even increased with higher serum levels.4
VITAL: STUDY DESIGN AND LOGISTICS
In VITAL, the investigators aim to recruit 20,000 healthy men (age 60 and older) and women (65 and older) who are representative of the US population (www.vitalstudy.org). Because it is a primary prevention trial, people with a known history of cardiovascular disease or cancer will be excluded. Participants will be randomized to receive either 2,000 IU of vitamin D3 per day or placebo. Each group will be further randomized to receive either 1 g per day of fish oil (combined eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo. The mean treatment period will be 5 years. Recruitment began in early 2010.
Blood will be collected in about 80% (ideally 100%) of participants, with follow-up blood collection in at least 2,000.
Primary aims of the trial are to test whether vitamin D3 and the omega-3 fatty acids reduce the risk of total cancer and major cardiovascular events (a composite of myocardial infarction, stroke, and death due to cardiovascular events).
Secondary aims are to test whether these agents lower the risk of:
- Site-specific cancer, including colorectal, breast, and prostate cancer, and the total cancer mortality rate
- An expanded composite outcome including myocardial infarction, stroke, cardiovascular death, coronary artery bypass grafting, percutaneous coronary intervention, and its individual components.
Tertiary aims are to explore whether vitamin D3 and omega-3 fatty acids have additive effects on the primary and secondary end points. The trial will also explore whether the effects of vitamin D3 and omega-3 fatty acids on cancer and cardiovascular disease vary by baseline blood levels of these nutrients, and whether race, skin pigmentation, or body mass index modify the effects of vitamin D3.
Ancillary studies will assess the effect of the interventions on risk of diabetes, hypertension, cognitive decline, depression, fracture, infections, respiratory disorders, and autoimmune diseases. The primary sponsor of this trial is the National Cancer Institute, and the secondary sponsor is the National Heart, Lung and Blood Institute. Other institutes and agencies also are cosponsors of the study.
The timing of VITAL is optimal
There is a limited window of opportunity for conducting a randomized clinical trial: the evidence must be strong enough to justify mounting a very large trial with enough power to look at cardiovascular events and cancer, but the evidence must not be so strong that it would be unethical to have a placebo group. Thus, there must be a state of equipoise. Our trial allows the study population to have a background intake of vitamin D that is currently recommended by national guidelines. Therefore, even the placebo group should have adequate intake of vitamin D.
The growing use of vitamin D supplementation by the public underscores the need for conclusive evidence of its benefits and risks. No previous large-scale randomized clinical trial has tested moderate to high doses of vitamin D for the primary prevention of cancer and cardiovascular disease.
Setting the dosage
VITAL set the vitamin D3 dosage at 2,000 IU per day (50 μg/day), which is designed to provide the best balance of efficacy and safety. As a general rule, each microgram of vitamin D3 is expected to raise the serum 25-hydroxyvitamin D3 level about 1 nmol/L, although the response is not linear: if baseline levels are lower, the increase is greater. In the United States, people commonly have a baseline level of about 40 nmol/L, so we expect that levels of people treated in the study will reach about 90 nmol/L (range 75–100 nmol/L), about 35 to 50 nmol/L higher than in the placebo group.
The target range of 75 to 100 nmol/L is the level at which greatest efficacy has been suggested in observational studies. Previous randomized trials of vitamin D have not tested high enough doses to achieve this level of 25-hydroxyvitamin D3. VITAL will test whether reaching this serum level lowers the risk of cardiovascular disease, cancer, and other chronic diseases. This level may be associated with benefit and has minimal risk of hypercalcemia. Risk of hypercalcemia may be present in participants with an occult chronic granulomatous condition such as sarcoidosis or Wegener granulomatosis, in which activated macrophages synthesize 1,25-dihydroxyvitamin D3. These conditions are very rare, however, and the risk of hypercalcemia in the trial is exceedingly low.
VITAL participants will also be randomized to take placebo or 1 g per day of combined EPA and DHA, about 5 to 10 times more than most Americans consume.
Nationwide recruitment among senior citizens
We aim to recruit 20,000 people (10,000 men and 10,000 women) nationwide who are willing, eligible, and compliant (ie, who take more than two-thirds of study pills during a 3-month placebo “run-in” phase of the trial). The trial aims to enroll 40,000 in the run-in period, and 20,000 will be randomized. To get this many participants, we will send invitational mailings and screening questionnaires to at least 2.5 million people around the United States, with mailing lists selected by age—ie, members of the American Association of Retired Persons, health professionals, teachers, and subscription lists for selected magazines. A pilot study in 5,000 people has indicated that recruiting and randomizing 20,000 participants via large mailings should be possible.
The trial is expected to be extremely cost-effective because it will be conducted largely by mail. Medication will be mailed in calendar blister packs. Participants report outcomes, which are then confirmed by medical record review. The Centers for Medicare and Medicaid Services and the National Death Index will also be used to ascertain outcomes.
We hope to recruit a more racially diverse study population than is typically seen in US trials: 63% (12,620) whites, 25% (5,000) African Americans, 7% (1,400) Hispanics, 2.5% (500) Asians, 2% (400) American Indians and Alaska natives, and 0.4% (80) native Hawaiian and Pacific Islanders.
Eligibility criteria ensure primary prevention is tested
To enter the study, men must be at least 60 years old and women at least 65. At a minimum, a high school education is required due to the detailed forms and questionnaires to be completed. Because this is a primary prevention trial, anyone with a history of cancer (except nonmelanoma skin cancer) or cardiovascular disease (including myocardial infarction, stroke, or coronary revascularization) will be excluded, as will anyone with a history of kidney stones, renal failure or dialysis, hypercalcemia, hypoparathyroidism or hyperparathyroidism, severe liver disease (eg, cirrhosis), sarcoidosis, tuberculosis, or other granulomatous disease. People with an allergy to fish will also be excluded.
We do not expect that those in the placebo group will develop vitamin D deficiency due to their participation in the study. The trial will allow a background intake in the study population of up to 800 IU of vitamin D and 1,200 mg of calcium per day in supplements. Assuming they also get about 200 IU of vitamin D in the diet, the background intake in the placebo group may be close to 1,000 IU of vitamin D. Assuming that the active treatment group has a similar background intake, their total intake will be about 3,000 IU per day (about 1,000 IU/day from background intake plus 2,000 IU/day from the intervention).
Cohort power sufficient to see effect in 5 years
The trial is expected to have sufficient power to evaluate cardiovascular disease and cancer end points as primary outcomes during 5 years of follow-up. The trial is designed to have a power of 91% to 92% to detect a relative risk of 0.85 for the primary cancer end point of total cancer incidence and 0.80 for the cardiovascular disease end point of myocardial infarction, stroke, and cardiovascular mortality. Power will be even greater for the expanded composite outcome for cardiovascular disease.
Ancillary studies
Ancillary studies include evaluating the interventions’ role in preventing diabetes and glucose intolerance, hypertension, heart failure, atrial fibrillation, cognitive decline, mood disorders, osteoporosis and fractures, asthma and respiratory diseases, infections, macular degeneration, rheumatoid arthritis, systemic lupus erythematosus, and a composite of autoimmune diseases. Imaging studies also are planned, including dual energy x-ray absorptiometry, mammographic density, and non-invasive vascular imaging (carotid intima medial thickness, coronary calcium measurements, and two-dimensional echocardiography to assess cardiac function).
Several biomarker and genetic studies will also be carried out. We intend to perform genetic studies on most of the study population to evaluate gene variants in the vitamin D receptor, vitamin D binding protein, and other vitamin-D-related genes that may contribute to lower baseline levels of 25-hydroxyvitamin D3 or different responses to the interventions.
Clinical and Translational Science Center visits are planned to provide more detailed assessments of 1,000 participants, including blood pressure measurements, height, weight, waist circumference, other anthropometric measurements, a 2-hour glucose tolerance test, a fasting blood collection, hemoglobin A1c measurements, spirometry, and assessment of physical performance, strength, frailty, cognitive function, mood, and depression. Dual-energy x-ray absorptiometry and noninvasive vascular imaging studies are also planned for those visits.
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010; 152:315–323.
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 117:503–511.
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008; 168:1174–1180.
- Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008; 168:1629–1637.
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1730–1737.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003; 326:469.
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115:846–854.
- LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009; 64:559–567.
Vitamin D is viewed as a promising supplement by the medical, public health, and lay communities, potentially offering many health benefits. But enthusiasm for a new intervention too often gets far ahead of the evidence, as was the case with beta-carotene, selenium, folic acid, and vitamins C and E.
Despite the enthusiasm for vitamin D, there have been no large-scale primary prevention trials that have had either cardiovascular disease or cancer as a prespecified primary outcome. Previous randomized trials of vitamin D have focused primarily on osteoporosis, fracture, falls, and physical function. Although the investigators often reported their findings on vitamin D and cardiovascular disease or cancer, these outcomes were generally secondary or tertiary end points that were not prespecified. These studies should be viewed as hypothesis-generating rather than hypothesis-testing. The increasing prevalence of use of vitamin D supplements underscores the need for rigorous and conclusive evidence from randomized clinical trials that have cardiovascular disease and cancer as primary outcomes.
This article will explain the rationale for a large-scale, randomized clinical trial to evaluate the role of vitamin D in the prevention of cardiovascular disease and cancer. It will also describe the biological mechanisms and currently available evidence relating vitamin D to potential health benefits. Finally, the design, dosage considerations, and logistics of the Vitamin D and Omega-3 Trial (VITAL) will be presented.

EVIDENCE IS MOUNTING FOR VITAMIN D’S BIOLOGICAL IMPORTANCE
Vitamin D is undoubtedly important to health: not only is it produced endogenously, but at least 500 genes have been identified with vitamin D response elements. The vitamin D receptor is found in nearly all cells in the body, and the 1-alpha-hydroxylase enzyme is present in many tissues. Some studies suggest that almost 10% of the human genome may be at least partially regulated by vitamin D.
Although we know vitamin D is important, what our optimal intake and our blood level of 25-hydroxyvitamin D3 should be are key unknowns.
RECOMMENDATIONS FOR VITAMIN D INTAKE
During winter, late fall, and early spring, people who live above the 37th parallel (geographically, about one-half of the contiguous United States) do not get enough ultraviolet B energy from the sun to make all the vitamin D they need, even if they spend several hours outside every day. In addition, dark skin pigmentation serves as a sun block, as do sunscreens.
The Institute of Medicine (IOM) provided guidelines for vitamin D intake in 1997 and, most recently, in 2010. However, these guidelines are based on the amount of vitamin D required for bone health and do not address the amount that may be of benefit for prevention of cancer and cardiovascular disease. The latter outcomes are not addressed because the IOM committee believed that evidence was insufficient to determine the role of vitamin D in the prevention of cardiovascular disease, cancer, and other chronic diseases. Thus, current IOM guidelines, which generally recommend less than 1,000 IU of vitamin D daily, are relevant to bone health but not necessarily to other health outcomes. More research is needed to understand whether the guidelines should be modified for the prevention of other chronic diseases.
Moreover, whether or not everyone should be screened for 25-hydroxyvitamin D3 blood levels is controversial. Most experts agree that a level less than 20 ng/mL is deficient or insufficient. Conversely, potentially harmful are levels 150 ng/mL or more (> 375 nmol/L), which entail the risk of hypercalcemia, vascular soft tissue calcification, and hyperphosphatemia.
People do not reach toxic levels with ultraviolet light exposure because the amount of 25-hydroxyvitamin D3 synthesis is well regulated. Dietary supplements, however, can bring about toxic levels, and patients taking high doses need to be monitored carefully. The level that should be considered optimal is controversial and requires further study.
RISK FACTORS FOR LOW VITAMIN D LEVELS
Risk factors for low vitamin D levels include older age, living in northern latitudes, sun avoidance, dark skin pigmentation, obesity, low dietary intake, and various medical conditions, especially malabsorption syndromes. Some of these are also risk factors for cardiovascular disease, cancer, and other chronic diseases, and potentially confound outcomes in many studies. Older age, which is usually adjusted for in multivariate models, is important to recognize as a major risk factor for vitamin D deficiency, owing to reduced absorption and synthesis, less time outdoors, and low dietary intake.
Wearing sunscreen decreases the synthesis of vitamin D in the skin, but because ultra-violet light has been clearly classified as a carcinogen, it is a not advisable to increase sun exposure for the sake of increasing vitamin D levels. That is a poor trade-off, given the high incidence rate of skin cancer and the adverse effects of solar radiation on skin aging.
Obesity is a risk factor for vitamin D deficiency because vitamin D is fat-soluble and becomes sequestered in fat tissue. Vitamin D may also play a role in the differentiation of adipocytes and may affect their function. In observational studies, it is very important for researchers to adjust for body mass index, physical activity (which may be correlated with more time outdoors), and other potential confounders in their analyses.
HOW VITAMIN D MAY LOWER CANCER RISK
Because of the important effect of vitamin D in regulating cell differentiation and cell growth, there are multiple ways that it may affect cancer risk. Laboratory, cell culture, and animal studies suggest that vitamin D may lower cancer risk by inhibiting cell proliferation, angiogenesis, metastasis, and inflammation and inducing apoptosis and cellular differentiation. Several of these mechanisms are also relevant to atherosclerosis and cardiovascular disease. Although VITAL is addressing the role of vitamin D in preventing both cancer and cardiovascular disease, the remainder of this article will focus on cardiovascular outcomes.
HOW VITAMIN D MAY REDUCE CARDIOVASCULAR RISK
Vitamin D may lower cardiovascular risk via several mechanisms:
Inhibiting inflammation. Vitamin D has a powerful immunomodulatory effect: laboratory studies show that it inhibits prostaglandin and cyclooxygenase 2 pathways, reduces matrix metalloproteinase 9 and several proinflammatory cytokines, and increases interleukin 10, all of which result in suppressed inflammation.1
Inhibiting vascular muscle proliferation and vascular calcification. Animal studies indicate that in moderate doses vitamin D decreases calcium cellular influx and increases matrix Gla protein, which inhibits vascular smooth muscle proliferation and vascular calcification. These protective effects contrast with the hypercalcemia associated with a high intake of vitamin D, especially in the context of renal failure or other risk factors, which may lead to increased vascular calcification.1
Regulates blood pressure. Vitamin D decreases renin gene expression and the synthesis of renin, which reduces activity of the renin-angiotensin-aldosterone system, leading to a reduction of blood pressure and a favorable effect on volume homeostasis.1
Regulates glucose metabolism. Limited evidence shows that vitamin D may increase insulin sensitivity and regulate glucose metabolism.1
Vitamin D and cardiac hypertrophy
The vitamin D receptor is present in virtually all tissues, including cardiac myocytes and endothelial cells. Animals with vitamin D deficiency have higher blood pressures, and animals genetically altered to have no vitamin D receptors (knock-out models) develop left ventricular hypertrophy and heart failure.
Animals genetically altered to have no 1-alpha-hydroxylase (so that the most active form of vitamin D is not made) also develop left ventricular hypertrophy. They can be rescued by the administration of 1,25-dihydroxy vitamin D3.1
These findings are consistent with what is observed in patients with end-stage renal disease, who produce very little 1,25-dihydroxyvitamin D3: they often develop left ventricular hypertrophy, diastolic heart failure, atherosclerosis, and vascular calcification.
EVIDENCE FOR CARDIOVASCULAR DISEASE REDUCTION
Wang et al1 recently reviewed available prospective cohort and randomized clinical trials from 1966 to 2009 that examined vitamin D or calcium supplementation and cardiovascular disease. Comparing people with the lowest to the highest levels of serum 25-hydroxyvitamin D3 indicated that a low level is a risk factor for coronary artery disease and cardiovascular death. Unfortunately, most studies were not designed to assess primary effects on cardiovascular outcomes, and so have many potential confounders.
Prospective observational studies
Observational studies suggest that vitamin D deficiency is associated with an increased risk of cardiovascular disease. Some examples:
The Framingham Offspring Study2 followed 1,739 men and women with a mean age of 59 for 5.4 years. The study compared the incidence of cardiovascular events in those with a serum 25-dihydroxyvitamin D level of at least 37.5 nmol/L vs those with lower levels. The risk of cardiovascular disease was 1.62 times higher in those with the lowest levels of vitamin D, a statistically significant difference. However, a threshold effect was apparent (discussed below).
The Health Professionals Follow-up Study3 prospectively evaluated more than 18,000 men ages 40 to 75 for 10 years. The study compared men with a low serum level of vitamin D (< 37.5 nmol/L) to those with a more optimal level (> 75 nmol/L). The incidence of cardiovascular events was 2.09 times higher in men with low levels of vitamin D, a difference that was statistically significant.
The Third National Health and Nutrition Examination Survey (NHANES III) included data for more than 13,300 men and women age 20 years and older. Using a cohort that was followed for 8.7 years, Melamed et al4 compared the quartile with the lowest serum vitamin D level (< 44.4 nmol/L) against the quartile with the highest level (> 80.1 nmol/L). The associations were modest: those with low levels had a 1.20-times higher rate of death from cardiovascular disease and a statistically significant 1.26-times higher rate of death from all causes.
Randomized clinical trials
A meta-analysis of 18 randomized trials5 of vitamin D supplementation (300–2,000 IU/day, mean 528 IU/day vs placebo), including 57,311 participants, evaluated the rate of death from all causes and found a modest but significant reduction in risk (relative risk 0.93, 95% confidence interval [CI] 0.87–0.99). These were generally trials looking at fracture rates or physical performance, and a dose-response relationship was not evident. A recent systematic review of randomized controlled trials of vitamin D1 that included cardiovascular disease as a secondary outcome found a pooled relative risk for cardiovascular disease of 0.90 (95% CI 0.77–1.05) for vitamin D supplementation compared with placebo and 1.04 (95% CI 0.92–1.18) for combination vitamin D plus calcium supplementation vs placebo.1 Two individual trials are discussed below.
Trivedi et al6 randomized 2,686 British men and women to vitamin D3 100,000 IU given every 4 months over 5 years (equivalent to 800 IU/day) or placebo. The relative risk of cardiovascular events was 0.90 (95% CI 0.77–1.06) and of cardiovascular deaths 0.84 (95% CI 0.65–1.10). Although the results were promising, the trial was designed to assess fracture risk and was not large enough for the differences in cardiovascular outcomes to reach statistical significance.
The Women’s Health Initiative,7,8 which included 36,282 postmenopausal women aged 50 to 79, tested combined vitamin D3 (400 IU/day) with calcium (1,000 mg/day) vs placebo. No benefit was seen for preventing coronary events or stroke, which may be due to the low dosage of vitamin D. The hazard ratio for coronary disease was 1.04 (0.92–1.18). Regarding mortality, the hazard ratio for cardiovascular death was 0.92, for cerebrovascular death 0.89, for cancer death 0.89, and for other deaths 0.95. None of these hazard ratios reached statistical significance.
MORE MAY NOT BE BETTER
As is probably true for everything in biological systems, there apparently is an optimal level of intake to meet vitamin D needs.
The Framingham Offspring Study,2 which found a higher risk with vitamin D deficiency, also found a suggestion of a threshold. Participants who had levels of 50 to 65 nmol/L had the lowest risk. Higher levels did not confer lower risk and even suggested a slight upturn.
Evidence from the Women’s Health Initiative8 also indicates that high dosages may not be better than moderate dosages. The meta-analysis of vitamin D and all-cause mortality5 found a relative risk of 0.93, but one of the largest studies in that meta-analysis tested only 400 IU a day and found a similar relative risk of 0.91 (95% confidence interval, 0.83–1.01).
Moreover, the NHANES study found that with increasing serum 25-hydroxyvitamin D3 levels, the risk of all-cause mortality fell until about 100 nmol/L, but then plateaued and even increased with higher serum levels.4
VITAL: STUDY DESIGN AND LOGISTICS
In VITAL, the investigators aim to recruit 20,000 healthy men (age 60 and older) and women (65 and older) who are representative of the US population (www.vitalstudy.org). Because it is a primary prevention trial, people with a known history of cardiovascular disease or cancer will be excluded. Participants will be randomized to receive either 2,000 IU of vitamin D3 per day or placebo. Each group will be further randomized to receive either 1 g per day of fish oil (combined eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo. The mean treatment period will be 5 years. Recruitment began in early 2010.
Blood will be collected in about 80% (ideally 100%) of participants, with follow-up blood collection in at least 2,000.
Primary aims of the trial are to test whether vitamin D3 and the omega-3 fatty acids reduce the risk of total cancer and major cardiovascular events (a composite of myocardial infarction, stroke, and death due to cardiovascular events).
Secondary aims are to test whether these agents lower the risk of:
- Site-specific cancer, including colorectal, breast, and prostate cancer, and the total cancer mortality rate
- An expanded composite outcome including myocardial infarction, stroke, cardiovascular death, coronary artery bypass grafting, percutaneous coronary intervention, and its individual components.
Tertiary aims are to explore whether vitamin D3 and omega-3 fatty acids have additive effects on the primary and secondary end points. The trial will also explore whether the effects of vitamin D3 and omega-3 fatty acids on cancer and cardiovascular disease vary by baseline blood levels of these nutrients, and whether race, skin pigmentation, or body mass index modify the effects of vitamin D3.
Ancillary studies will assess the effect of the interventions on risk of diabetes, hypertension, cognitive decline, depression, fracture, infections, respiratory disorders, and autoimmune diseases. The primary sponsor of this trial is the National Cancer Institute, and the secondary sponsor is the National Heart, Lung and Blood Institute. Other institutes and agencies also are cosponsors of the study.
The timing of VITAL is optimal
There is a limited window of opportunity for conducting a randomized clinical trial: the evidence must be strong enough to justify mounting a very large trial with enough power to look at cardiovascular events and cancer, but the evidence must not be so strong that it would be unethical to have a placebo group. Thus, there must be a state of equipoise. Our trial allows the study population to have a background intake of vitamin D that is currently recommended by national guidelines. Therefore, even the placebo group should have adequate intake of vitamin D.
The growing use of vitamin D supplementation by the public underscores the need for conclusive evidence of its benefits and risks. No previous large-scale randomized clinical trial has tested moderate to high doses of vitamin D for the primary prevention of cancer and cardiovascular disease.
Setting the dosage
VITAL set the vitamin D3 dosage at 2,000 IU per day (50 μg/day), which is designed to provide the best balance of efficacy and safety. As a general rule, each microgram of vitamin D3 is expected to raise the serum 25-hydroxyvitamin D3 level about 1 nmol/L, although the response is not linear: if baseline levels are lower, the increase is greater. In the United States, people commonly have a baseline level of about 40 nmol/L, so we expect that levels of people treated in the study will reach about 90 nmol/L (range 75–100 nmol/L), about 35 to 50 nmol/L higher than in the placebo group.
The target range of 75 to 100 nmol/L is the level at which greatest efficacy has been suggested in observational studies. Previous randomized trials of vitamin D have not tested high enough doses to achieve this level of 25-hydroxyvitamin D3. VITAL will test whether reaching this serum level lowers the risk of cardiovascular disease, cancer, and other chronic diseases. This level may be associated with benefit and has minimal risk of hypercalcemia. Risk of hypercalcemia may be present in participants with an occult chronic granulomatous condition such as sarcoidosis or Wegener granulomatosis, in which activated macrophages synthesize 1,25-dihydroxyvitamin D3. These conditions are very rare, however, and the risk of hypercalcemia in the trial is exceedingly low.
VITAL participants will also be randomized to take placebo or 1 g per day of combined EPA and DHA, about 5 to 10 times more than most Americans consume.
Nationwide recruitment among senior citizens
We aim to recruit 20,000 people (10,000 men and 10,000 women) nationwide who are willing, eligible, and compliant (ie, who take more than two-thirds of study pills during a 3-month placebo “run-in” phase of the trial). The trial aims to enroll 40,000 in the run-in period, and 20,000 will be randomized. To get this many participants, we will send invitational mailings and screening questionnaires to at least 2.5 million people around the United States, with mailing lists selected by age—ie, members of the American Association of Retired Persons, health professionals, teachers, and subscription lists for selected magazines. A pilot study in 5,000 people has indicated that recruiting and randomizing 20,000 participants via large mailings should be possible.
The trial is expected to be extremely cost-effective because it will be conducted largely by mail. Medication will be mailed in calendar blister packs. Participants report outcomes, which are then confirmed by medical record review. The Centers for Medicare and Medicaid Services and the National Death Index will also be used to ascertain outcomes.
We hope to recruit a more racially diverse study population than is typically seen in US trials: 63% (12,620) whites, 25% (5,000) African Americans, 7% (1,400) Hispanics, 2.5% (500) Asians, 2% (400) American Indians and Alaska natives, and 0.4% (80) native Hawaiian and Pacific Islanders.
Eligibility criteria ensure primary prevention is tested
To enter the study, men must be at least 60 years old and women at least 65. At a minimum, a high school education is required due to the detailed forms and questionnaires to be completed. Because this is a primary prevention trial, anyone with a history of cancer (except nonmelanoma skin cancer) or cardiovascular disease (including myocardial infarction, stroke, or coronary revascularization) will be excluded, as will anyone with a history of kidney stones, renal failure or dialysis, hypercalcemia, hypoparathyroidism or hyperparathyroidism, severe liver disease (eg, cirrhosis), sarcoidosis, tuberculosis, or other granulomatous disease. People with an allergy to fish will also be excluded.
We do not expect that those in the placebo group will develop vitamin D deficiency due to their participation in the study. The trial will allow a background intake in the study population of up to 800 IU of vitamin D and 1,200 mg of calcium per day in supplements. Assuming they also get about 200 IU of vitamin D in the diet, the background intake in the placebo group may be close to 1,000 IU of vitamin D. Assuming that the active treatment group has a similar background intake, their total intake will be about 3,000 IU per day (about 1,000 IU/day from background intake plus 2,000 IU/day from the intervention).
Cohort power sufficient to see effect in 5 years
The trial is expected to have sufficient power to evaluate cardiovascular disease and cancer end points as primary outcomes during 5 years of follow-up. The trial is designed to have a power of 91% to 92% to detect a relative risk of 0.85 for the primary cancer end point of total cancer incidence and 0.80 for the cardiovascular disease end point of myocardial infarction, stroke, and cardiovascular mortality. Power will be even greater for the expanded composite outcome for cardiovascular disease.
Ancillary studies
Ancillary studies include evaluating the interventions’ role in preventing diabetes and glucose intolerance, hypertension, heart failure, atrial fibrillation, cognitive decline, mood disorders, osteoporosis and fractures, asthma and respiratory diseases, infections, macular degeneration, rheumatoid arthritis, systemic lupus erythematosus, and a composite of autoimmune diseases. Imaging studies also are planned, including dual energy x-ray absorptiometry, mammographic density, and non-invasive vascular imaging (carotid intima medial thickness, coronary calcium measurements, and two-dimensional echocardiography to assess cardiac function).
Several biomarker and genetic studies will also be carried out. We intend to perform genetic studies on most of the study population to evaluate gene variants in the vitamin D receptor, vitamin D binding protein, and other vitamin-D-related genes that may contribute to lower baseline levels of 25-hydroxyvitamin D3 or different responses to the interventions.
Clinical and Translational Science Center visits are planned to provide more detailed assessments of 1,000 participants, including blood pressure measurements, height, weight, waist circumference, other anthropometric measurements, a 2-hour glucose tolerance test, a fasting blood collection, hemoglobin A1c measurements, spirometry, and assessment of physical performance, strength, frailty, cognitive function, mood, and depression. Dual-energy x-ray absorptiometry and noninvasive vascular imaging studies are also planned for those visits.
Vitamin D is viewed as a promising supplement by the medical, public health, and lay communities, potentially offering many health benefits. But enthusiasm for a new intervention too often gets far ahead of the evidence, as was the case with beta-carotene, selenium, folic acid, and vitamins C and E.
Despite the enthusiasm for vitamin D, there have been no large-scale primary prevention trials that have had either cardiovascular disease or cancer as a prespecified primary outcome. Previous randomized trials of vitamin D have focused primarily on osteoporosis, fracture, falls, and physical function. Although the investigators often reported their findings on vitamin D and cardiovascular disease or cancer, these outcomes were generally secondary or tertiary end points that were not prespecified. These studies should be viewed as hypothesis-generating rather than hypothesis-testing. The increasing prevalence of use of vitamin D supplements underscores the need for rigorous and conclusive evidence from randomized clinical trials that have cardiovascular disease and cancer as primary outcomes.
This article will explain the rationale for a large-scale, randomized clinical trial to evaluate the role of vitamin D in the prevention of cardiovascular disease and cancer. It will also describe the biological mechanisms and currently available evidence relating vitamin D to potential health benefits. Finally, the design, dosage considerations, and logistics of the Vitamin D and Omega-3 Trial (VITAL) will be presented.

EVIDENCE IS MOUNTING FOR VITAMIN D’S BIOLOGICAL IMPORTANCE
Vitamin D is undoubtedly important to health: not only is it produced endogenously, but at least 500 genes have been identified with vitamin D response elements. The vitamin D receptor is found in nearly all cells in the body, and the 1-alpha-hydroxylase enzyme is present in many tissues. Some studies suggest that almost 10% of the human genome may be at least partially regulated by vitamin D.
Although we know vitamin D is important, what our optimal intake and our blood level of 25-hydroxyvitamin D3 should be are key unknowns.
RECOMMENDATIONS FOR VITAMIN D INTAKE
During winter, late fall, and early spring, people who live above the 37th parallel (geographically, about one-half of the contiguous United States) do not get enough ultraviolet B energy from the sun to make all the vitamin D they need, even if they spend several hours outside every day. In addition, dark skin pigmentation serves as a sun block, as do sunscreens.
The Institute of Medicine (IOM) provided guidelines for vitamin D intake in 1997 and, most recently, in 2010. However, these guidelines are based on the amount of vitamin D required for bone health and do not address the amount that may be of benefit for prevention of cancer and cardiovascular disease. The latter outcomes are not addressed because the IOM committee believed that evidence was insufficient to determine the role of vitamin D in the prevention of cardiovascular disease, cancer, and other chronic diseases. Thus, current IOM guidelines, which generally recommend less than 1,000 IU of vitamin D daily, are relevant to bone health but not necessarily to other health outcomes. More research is needed to understand whether the guidelines should be modified for the prevention of other chronic diseases.
Moreover, whether or not everyone should be screened for 25-hydroxyvitamin D3 blood levels is controversial. Most experts agree that a level less than 20 ng/mL is deficient or insufficient. Conversely, potentially harmful are levels 150 ng/mL or more (> 375 nmol/L), which entail the risk of hypercalcemia, vascular soft tissue calcification, and hyperphosphatemia.
People do not reach toxic levels with ultraviolet light exposure because the amount of 25-hydroxyvitamin D3 synthesis is well regulated. Dietary supplements, however, can bring about toxic levels, and patients taking high doses need to be monitored carefully. The level that should be considered optimal is controversial and requires further study.
RISK FACTORS FOR LOW VITAMIN D LEVELS
Risk factors for low vitamin D levels include older age, living in northern latitudes, sun avoidance, dark skin pigmentation, obesity, low dietary intake, and various medical conditions, especially malabsorption syndromes. Some of these are also risk factors for cardiovascular disease, cancer, and other chronic diseases, and potentially confound outcomes in many studies. Older age, which is usually adjusted for in multivariate models, is important to recognize as a major risk factor for vitamin D deficiency, owing to reduced absorption and synthesis, less time outdoors, and low dietary intake.
Wearing sunscreen decreases the synthesis of vitamin D in the skin, but because ultra-violet light has been clearly classified as a carcinogen, it is a not advisable to increase sun exposure for the sake of increasing vitamin D levels. That is a poor trade-off, given the high incidence rate of skin cancer and the adverse effects of solar radiation on skin aging.
Obesity is a risk factor for vitamin D deficiency because vitamin D is fat-soluble and becomes sequestered in fat tissue. Vitamin D may also play a role in the differentiation of adipocytes and may affect their function. In observational studies, it is very important for researchers to adjust for body mass index, physical activity (which may be correlated with more time outdoors), and other potential confounders in their analyses.
HOW VITAMIN D MAY LOWER CANCER RISK
Because of the important effect of vitamin D in regulating cell differentiation and cell growth, there are multiple ways that it may affect cancer risk. Laboratory, cell culture, and animal studies suggest that vitamin D may lower cancer risk by inhibiting cell proliferation, angiogenesis, metastasis, and inflammation and inducing apoptosis and cellular differentiation. Several of these mechanisms are also relevant to atherosclerosis and cardiovascular disease. Although VITAL is addressing the role of vitamin D in preventing both cancer and cardiovascular disease, the remainder of this article will focus on cardiovascular outcomes.
HOW VITAMIN D MAY REDUCE CARDIOVASCULAR RISK
Vitamin D may lower cardiovascular risk via several mechanisms:
Inhibiting inflammation. Vitamin D has a powerful immunomodulatory effect: laboratory studies show that it inhibits prostaglandin and cyclooxygenase 2 pathways, reduces matrix metalloproteinase 9 and several proinflammatory cytokines, and increases interleukin 10, all of which result in suppressed inflammation.1
Inhibiting vascular muscle proliferation and vascular calcification. Animal studies indicate that in moderate doses vitamin D decreases calcium cellular influx and increases matrix Gla protein, which inhibits vascular smooth muscle proliferation and vascular calcification. These protective effects contrast with the hypercalcemia associated with a high intake of vitamin D, especially in the context of renal failure or other risk factors, which may lead to increased vascular calcification.1
Regulates blood pressure. Vitamin D decreases renin gene expression and the synthesis of renin, which reduces activity of the renin-angiotensin-aldosterone system, leading to a reduction of blood pressure and a favorable effect on volume homeostasis.1
Regulates glucose metabolism. Limited evidence shows that vitamin D may increase insulin sensitivity and regulate glucose metabolism.1
Vitamin D and cardiac hypertrophy
The vitamin D receptor is present in virtually all tissues, including cardiac myocytes and endothelial cells. Animals with vitamin D deficiency have higher blood pressures, and animals genetically altered to have no vitamin D receptors (knock-out models) develop left ventricular hypertrophy and heart failure.
Animals genetically altered to have no 1-alpha-hydroxylase (so that the most active form of vitamin D is not made) also develop left ventricular hypertrophy. They can be rescued by the administration of 1,25-dihydroxy vitamin D3.1
These findings are consistent with what is observed in patients with end-stage renal disease, who produce very little 1,25-dihydroxyvitamin D3: they often develop left ventricular hypertrophy, diastolic heart failure, atherosclerosis, and vascular calcification.
EVIDENCE FOR CARDIOVASCULAR DISEASE REDUCTION
Wang et al1 recently reviewed available prospective cohort and randomized clinical trials from 1966 to 2009 that examined vitamin D or calcium supplementation and cardiovascular disease. Comparing people with the lowest to the highest levels of serum 25-hydroxyvitamin D3 indicated that a low level is a risk factor for coronary artery disease and cardiovascular death. Unfortunately, most studies were not designed to assess primary effects on cardiovascular outcomes, and so have many potential confounders.
Prospective observational studies
Observational studies suggest that vitamin D deficiency is associated with an increased risk of cardiovascular disease. Some examples:
The Framingham Offspring Study2 followed 1,739 men and women with a mean age of 59 for 5.4 years. The study compared the incidence of cardiovascular events in those with a serum 25-dihydroxyvitamin D level of at least 37.5 nmol/L vs those with lower levels. The risk of cardiovascular disease was 1.62 times higher in those with the lowest levels of vitamin D, a statistically significant difference. However, a threshold effect was apparent (discussed below).
The Health Professionals Follow-up Study3 prospectively evaluated more than 18,000 men ages 40 to 75 for 10 years. The study compared men with a low serum level of vitamin D (< 37.5 nmol/L) to those with a more optimal level (> 75 nmol/L). The incidence of cardiovascular events was 2.09 times higher in men with low levels of vitamin D, a difference that was statistically significant.
The Third National Health and Nutrition Examination Survey (NHANES III) included data for more than 13,300 men and women age 20 years and older. Using a cohort that was followed for 8.7 years, Melamed et al4 compared the quartile with the lowest serum vitamin D level (< 44.4 nmol/L) against the quartile with the highest level (> 80.1 nmol/L). The associations were modest: those with low levels had a 1.20-times higher rate of death from cardiovascular disease and a statistically significant 1.26-times higher rate of death from all causes.
Randomized clinical trials
A meta-analysis of 18 randomized trials5 of vitamin D supplementation (300–2,000 IU/day, mean 528 IU/day vs placebo), including 57,311 participants, evaluated the rate of death from all causes and found a modest but significant reduction in risk (relative risk 0.93, 95% confidence interval [CI] 0.87–0.99). These were generally trials looking at fracture rates or physical performance, and a dose-response relationship was not evident. A recent systematic review of randomized controlled trials of vitamin D1 that included cardiovascular disease as a secondary outcome found a pooled relative risk for cardiovascular disease of 0.90 (95% CI 0.77–1.05) for vitamin D supplementation compared with placebo and 1.04 (95% CI 0.92–1.18) for combination vitamin D plus calcium supplementation vs placebo.1 Two individual trials are discussed below.
Trivedi et al6 randomized 2,686 British men and women to vitamin D3 100,000 IU given every 4 months over 5 years (equivalent to 800 IU/day) or placebo. The relative risk of cardiovascular events was 0.90 (95% CI 0.77–1.06) and of cardiovascular deaths 0.84 (95% CI 0.65–1.10). Although the results were promising, the trial was designed to assess fracture risk and was not large enough for the differences in cardiovascular outcomes to reach statistical significance.
The Women’s Health Initiative,7,8 which included 36,282 postmenopausal women aged 50 to 79, tested combined vitamin D3 (400 IU/day) with calcium (1,000 mg/day) vs placebo. No benefit was seen for preventing coronary events or stroke, which may be due to the low dosage of vitamin D. The hazard ratio for coronary disease was 1.04 (0.92–1.18). Regarding mortality, the hazard ratio for cardiovascular death was 0.92, for cerebrovascular death 0.89, for cancer death 0.89, and for other deaths 0.95. None of these hazard ratios reached statistical significance.
MORE MAY NOT BE BETTER
As is probably true for everything in biological systems, there apparently is an optimal level of intake to meet vitamin D needs.
The Framingham Offspring Study,2 which found a higher risk with vitamin D deficiency, also found a suggestion of a threshold. Participants who had levels of 50 to 65 nmol/L had the lowest risk. Higher levels did not confer lower risk and even suggested a slight upturn.
Evidence from the Women’s Health Initiative8 also indicates that high dosages may not be better than moderate dosages. The meta-analysis of vitamin D and all-cause mortality5 found a relative risk of 0.93, but one of the largest studies in that meta-analysis tested only 400 IU a day and found a similar relative risk of 0.91 (95% confidence interval, 0.83–1.01).
Moreover, the NHANES study found that with increasing serum 25-hydroxyvitamin D3 levels, the risk of all-cause mortality fell until about 100 nmol/L, but then plateaued and even increased with higher serum levels.4
VITAL: STUDY DESIGN AND LOGISTICS
In VITAL, the investigators aim to recruit 20,000 healthy men (age 60 and older) and women (65 and older) who are representative of the US population (www.vitalstudy.org). Because it is a primary prevention trial, people with a known history of cardiovascular disease or cancer will be excluded. Participants will be randomized to receive either 2,000 IU of vitamin D3 per day or placebo. Each group will be further randomized to receive either 1 g per day of fish oil (combined eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo. The mean treatment period will be 5 years. Recruitment began in early 2010.
Blood will be collected in about 80% (ideally 100%) of participants, with follow-up blood collection in at least 2,000.
Primary aims of the trial are to test whether vitamin D3 and the omega-3 fatty acids reduce the risk of total cancer and major cardiovascular events (a composite of myocardial infarction, stroke, and death due to cardiovascular events).
Secondary aims are to test whether these agents lower the risk of:
- Site-specific cancer, including colorectal, breast, and prostate cancer, and the total cancer mortality rate
- An expanded composite outcome including myocardial infarction, stroke, cardiovascular death, coronary artery bypass grafting, percutaneous coronary intervention, and its individual components.
Tertiary aims are to explore whether vitamin D3 and omega-3 fatty acids have additive effects on the primary and secondary end points. The trial will also explore whether the effects of vitamin D3 and omega-3 fatty acids on cancer and cardiovascular disease vary by baseline blood levels of these nutrients, and whether race, skin pigmentation, or body mass index modify the effects of vitamin D3.
Ancillary studies will assess the effect of the interventions on risk of diabetes, hypertension, cognitive decline, depression, fracture, infections, respiratory disorders, and autoimmune diseases. The primary sponsor of this trial is the National Cancer Institute, and the secondary sponsor is the National Heart, Lung and Blood Institute. Other institutes and agencies also are cosponsors of the study.
The timing of VITAL is optimal
There is a limited window of opportunity for conducting a randomized clinical trial: the evidence must be strong enough to justify mounting a very large trial with enough power to look at cardiovascular events and cancer, but the evidence must not be so strong that it would be unethical to have a placebo group. Thus, there must be a state of equipoise. Our trial allows the study population to have a background intake of vitamin D that is currently recommended by national guidelines. Therefore, even the placebo group should have adequate intake of vitamin D.
The growing use of vitamin D supplementation by the public underscores the need for conclusive evidence of its benefits and risks. No previous large-scale randomized clinical trial has tested moderate to high doses of vitamin D for the primary prevention of cancer and cardiovascular disease.
Setting the dosage
VITAL set the vitamin D3 dosage at 2,000 IU per day (50 μg/day), which is designed to provide the best balance of efficacy and safety. As a general rule, each microgram of vitamin D3 is expected to raise the serum 25-hydroxyvitamin D3 level about 1 nmol/L, although the response is not linear: if baseline levels are lower, the increase is greater. In the United States, people commonly have a baseline level of about 40 nmol/L, so we expect that levels of people treated in the study will reach about 90 nmol/L (range 75–100 nmol/L), about 35 to 50 nmol/L higher than in the placebo group.
The target range of 75 to 100 nmol/L is the level at which greatest efficacy has been suggested in observational studies. Previous randomized trials of vitamin D have not tested high enough doses to achieve this level of 25-hydroxyvitamin D3. VITAL will test whether reaching this serum level lowers the risk of cardiovascular disease, cancer, and other chronic diseases. This level may be associated with benefit and has minimal risk of hypercalcemia. Risk of hypercalcemia may be present in participants with an occult chronic granulomatous condition such as sarcoidosis or Wegener granulomatosis, in which activated macrophages synthesize 1,25-dihydroxyvitamin D3. These conditions are very rare, however, and the risk of hypercalcemia in the trial is exceedingly low.
VITAL participants will also be randomized to take placebo or 1 g per day of combined EPA and DHA, about 5 to 10 times more than most Americans consume.
Nationwide recruitment among senior citizens
We aim to recruit 20,000 people (10,000 men and 10,000 women) nationwide who are willing, eligible, and compliant (ie, who take more than two-thirds of study pills during a 3-month placebo “run-in” phase of the trial). The trial aims to enroll 40,000 in the run-in period, and 20,000 will be randomized. To get this many participants, we will send invitational mailings and screening questionnaires to at least 2.5 million people around the United States, with mailing lists selected by age—ie, members of the American Association of Retired Persons, health professionals, teachers, and subscription lists for selected magazines. A pilot study in 5,000 people has indicated that recruiting and randomizing 20,000 participants via large mailings should be possible.
The trial is expected to be extremely cost-effective because it will be conducted largely by mail. Medication will be mailed in calendar blister packs. Participants report outcomes, which are then confirmed by medical record review. The Centers for Medicare and Medicaid Services and the National Death Index will also be used to ascertain outcomes.
We hope to recruit a more racially diverse study population than is typically seen in US trials: 63% (12,620) whites, 25% (5,000) African Americans, 7% (1,400) Hispanics, 2.5% (500) Asians, 2% (400) American Indians and Alaska natives, and 0.4% (80) native Hawaiian and Pacific Islanders.
Eligibility criteria ensure primary prevention is tested
To enter the study, men must be at least 60 years old and women at least 65. At a minimum, a high school education is required due to the detailed forms and questionnaires to be completed. Because this is a primary prevention trial, anyone with a history of cancer (except nonmelanoma skin cancer) or cardiovascular disease (including myocardial infarction, stroke, or coronary revascularization) will be excluded, as will anyone with a history of kidney stones, renal failure or dialysis, hypercalcemia, hypoparathyroidism or hyperparathyroidism, severe liver disease (eg, cirrhosis), sarcoidosis, tuberculosis, or other granulomatous disease. People with an allergy to fish will also be excluded.
We do not expect that those in the placebo group will develop vitamin D deficiency due to their participation in the study. The trial will allow a background intake in the study population of up to 800 IU of vitamin D and 1,200 mg of calcium per day in supplements. Assuming they also get about 200 IU of vitamin D in the diet, the background intake in the placebo group may be close to 1,000 IU of vitamin D. Assuming that the active treatment group has a similar background intake, their total intake will be about 3,000 IU per day (about 1,000 IU/day from background intake plus 2,000 IU/day from the intervention).
Cohort power sufficient to see effect in 5 years
The trial is expected to have sufficient power to evaluate cardiovascular disease and cancer end points as primary outcomes during 5 years of follow-up. The trial is designed to have a power of 91% to 92% to detect a relative risk of 0.85 for the primary cancer end point of total cancer incidence and 0.80 for the cardiovascular disease end point of myocardial infarction, stroke, and cardiovascular mortality. Power will be even greater for the expanded composite outcome for cardiovascular disease.
Ancillary studies
Ancillary studies include evaluating the interventions’ role in preventing diabetes and glucose intolerance, hypertension, heart failure, atrial fibrillation, cognitive decline, mood disorders, osteoporosis and fractures, asthma and respiratory diseases, infections, macular degeneration, rheumatoid arthritis, systemic lupus erythematosus, and a composite of autoimmune diseases. Imaging studies also are planned, including dual energy x-ray absorptiometry, mammographic density, and non-invasive vascular imaging (carotid intima medial thickness, coronary calcium measurements, and two-dimensional echocardiography to assess cardiac function).
Several biomarker and genetic studies will also be carried out. We intend to perform genetic studies on most of the study population to evaluate gene variants in the vitamin D receptor, vitamin D binding protein, and other vitamin-D-related genes that may contribute to lower baseline levels of 25-hydroxyvitamin D3 or different responses to the interventions.
Clinical and Translational Science Center visits are planned to provide more detailed assessments of 1,000 participants, including blood pressure measurements, height, weight, waist circumference, other anthropometric measurements, a 2-hour glucose tolerance test, a fasting blood collection, hemoglobin A1c measurements, spirometry, and assessment of physical performance, strength, frailty, cognitive function, mood, and depression. Dual-energy x-ray absorptiometry and noninvasive vascular imaging studies are also planned for those visits.
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010; 152:315–323.
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 117:503–511.
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008; 168:1174–1180.
- Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008; 168:1629–1637.
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1730–1737.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003; 326:469.
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115:846–854.
- LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009; 64:559–567.
- Wang L, Manson JE, Song Y, Sesso HD. Systematic review: vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med 2010; 152:315–323.
- Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008; 117:503–511.
- Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med 2008; 168:1174–1180.
- Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008; 168:1629–1637.
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 2007; 167:1730–1737.
- Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 2003; 326:469.
- Hsia J, Heiss G, Ren H, et al; Women’s Health Initiative Investigators. Calcium/vitamin D supplementation and cardiovascular events. Circulation 2007; 115:846–854.
- LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 2009; 64:559–567.
KEY POINTS
- Laboratory evidence suggests that vitamin D may lower cancer risk by inhibiting cell proliferation, angiogenesis, metastasis, and inflammation.
- Vitamin D may also reduce cardiovascular risk by inhibiting vascular smooth muscle proliferation, regulating blood pressure and glucose metabolism, and reducing inflammation.
- Some observational studies indicate there may be a threshold for vitamin D intake above which there is no increase in benefit and which may increase risk.
- The VITAL trial is currently randomizing 20,000 healthy older men and women throughout the United States to receive either 2,000 IU of vitamin D3 (cholecalciferol) per day or placebo, as well as 1 g of marine omega-3 fatty acids per day or placebo, for 5 years.