User login
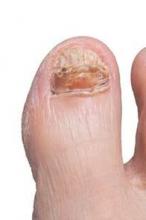
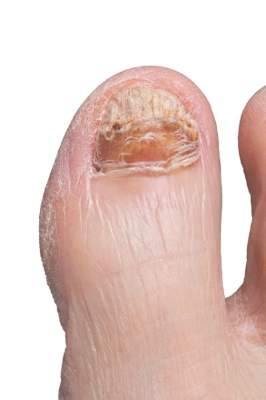
Topical efinaconazole’s ability to permeate the nail to the infection in onychomycosis is not hampered by the presence of infection or nail thickness, according to data from a multicenter, open-label study.
Adult patients with onychomycosis treated with efinaconazole 10% solution for 4 weeks had drug concentrations in both big and second toenails much higher than minimum inhibitory concentration (MIC) values for common onychomycosis pathogens, the researchers reported in the Journal of Drugs in Dermatology (J. Drugs Dermatol. 2014;13:1388-92).
The topical triazole efinaconazole has a broad spectrum of activity that is particularly potent against the common onychomycosis pathogens Trichophyton rubrum,T. mentagrophytes, and Candida albicans, the investigators, led by Misao Sakamoto of Kaken Pharmaceutical in Tokyo, noted. The treatment is an alternative to oral antifungal therapy, which can have systemic side effects or drug interactions. However, transungual delivery of effective topical treatments has been hampered by low permeation rates. The goal of this study was to assess the transungual delivery of efinaconazole in onychomycosis and its fungicidal activity in the toenail.
A total of 40 patients treated their toenails with efinaconazole 5% or 10% topical solution once daily before bedtime for 28 days. Patients applied two drops of solution to both great toes and one drop to all other toenails. Nail samples were taken from the big toenails at weeks 2, 4, and 6. Fungicidal activity against T. rubrum in the ventral layer of the nails was assessed by using an in vitro human nail infection model. Concentrations of the antifungal in the toenail were similar at weeks 2 and 4 with 10% solution, whereas they were lower at week 2 than at week 4 with 5% treatment. For both doses, efinaconazole concentrations peaked at the end of week 4 and declined at week 6. Great-toenail concentrations at week 4 for the 5% and 10% solutions were 5.6 and 6.0 mg/g, respectively.
This finding might be explained by the drug diffusing into the nail bed, the study authors said. No differences in the concentrations were seen in normal or affected nails, suggesting that transungual delivery of efinaconazole was not influenced by the presence of disease.
Concentrations of the antifungal were similar in the great and second toenails, suggesting nail thickness did not affect drug accumulation. In the in vitro nail model, efinaconazole was effective in reducing fungal viability, which suggested that sufficient amounts of the antifungal were being delivered to the ventral layer of the nail plate, the researchers noted.
“The high efinaconazole concentrations in patients’ toenails and fungicidal activity in vitro potentially contribute to the clinical efficacy reported in phase III studies,” they concluded.
All authors work for Kaken Pharmaceutical or Dow Pharmaceutical Sciences (a division of Valeant Pharmaceuticals, manufacturer of efinaconazole). The study was funded by Kaken and Valeant.
Topical efinaconazole’s ability to permeate the nail to the infection in onychomycosis is not hampered by the presence of infection or nail thickness, according to data from a multicenter, open-label study.
Adult patients with onychomycosis treated with efinaconazole 10% solution for 4 weeks had drug concentrations in both big and second toenails much higher than minimum inhibitory concentration (MIC) values for common onychomycosis pathogens, the researchers reported in the Journal of Drugs in Dermatology (J. Drugs Dermatol. 2014;13:1388-92).
The topical triazole efinaconazole has a broad spectrum of activity that is particularly potent against the common onychomycosis pathogens Trichophyton rubrum,T. mentagrophytes, and Candida albicans, the investigators, led by Misao Sakamoto of Kaken Pharmaceutical in Tokyo, noted. The treatment is an alternative to oral antifungal therapy, which can have systemic side effects or drug interactions. However, transungual delivery of effective topical treatments has been hampered by low permeation rates. The goal of this study was to assess the transungual delivery of efinaconazole in onychomycosis and its fungicidal activity in the toenail.
A total of 40 patients treated their toenails with efinaconazole 5% or 10% topical solution once daily before bedtime for 28 days. Patients applied two drops of solution to both great toes and one drop to all other toenails. Nail samples were taken from the big toenails at weeks 2, 4, and 6. Fungicidal activity against T. rubrum in the ventral layer of the nails was assessed by using an in vitro human nail infection model. Concentrations of the antifungal in the toenail were similar at weeks 2 and 4 with 10% solution, whereas they were lower at week 2 than at week 4 with 5% treatment. For both doses, efinaconazole concentrations peaked at the end of week 4 and declined at week 6. Great-toenail concentrations at week 4 for the 5% and 10% solutions were 5.6 and 6.0 mg/g, respectively.
This finding might be explained by the drug diffusing into the nail bed, the study authors said. No differences in the concentrations were seen in normal or affected nails, suggesting that transungual delivery of efinaconazole was not influenced by the presence of disease.
Concentrations of the antifungal were similar in the great and second toenails, suggesting nail thickness did not affect drug accumulation. In the in vitro nail model, efinaconazole was effective in reducing fungal viability, which suggested that sufficient amounts of the antifungal were being delivered to the ventral layer of the nail plate, the researchers noted.
“The high efinaconazole concentrations in patients’ toenails and fungicidal activity in vitro potentially contribute to the clinical efficacy reported in phase III studies,” they concluded.
All authors work for Kaken Pharmaceutical or Dow Pharmaceutical Sciences (a division of Valeant Pharmaceuticals, manufacturer of efinaconazole). The study was funded by Kaken and Valeant.
Topical efinaconazole’s ability to permeate the nail to the infection in onychomycosis is not hampered by the presence of infection or nail thickness, according to data from a multicenter, open-label study.
Adult patients with onychomycosis treated with efinaconazole 10% solution for 4 weeks had drug concentrations in both big and second toenails much higher than minimum inhibitory concentration (MIC) values for common onychomycosis pathogens, the researchers reported in the Journal of Drugs in Dermatology (J. Drugs Dermatol. 2014;13:1388-92).
The topical triazole efinaconazole has a broad spectrum of activity that is particularly potent against the common onychomycosis pathogens Trichophyton rubrum,T. mentagrophytes, and Candida albicans, the investigators, led by Misao Sakamoto of Kaken Pharmaceutical in Tokyo, noted. The treatment is an alternative to oral antifungal therapy, which can have systemic side effects or drug interactions. However, transungual delivery of effective topical treatments has been hampered by low permeation rates. The goal of this study was to assess the transungual delivery of efinaconazole in onychomycosis and its fungicidal activity in the toenail.
A total of 40 patients treated their toenails with efinaconazole 5% or 10% topical solution once daily before bedtime for 28 days. Patients applied two drops of solution to both great toes and one drop to all other toenails. Nail samples were taken from the big toenails at weeks 2, 4, and 6. Fungicidal activity against T. rubrum in the ventral layer of the nails was assessed by using an in vitro human nail infection model. Concentrations of the antifungal in the toenail were similar at weeks 2 and 4 with 10% solution, whereas they were lower at week 2 than at week 4 with 5% treatment. For both doses, efinaconazole concentrations peaked at the end of week 4 and declined at week 6. Great-toenail concentrations at week 4 for the 5% and 10% solutions were 5.6 and 6.0 mg/g, respectively.
This finding might be explained by the drug diffusing into the nail bed, the study authors said. No differences in the concentrations were seen in normal or affected nails, suggesting that transungual delivery of efinaconazole was not influenced by the presence of disease.
Concentrations of the antifungal were similar in the great and second toenails, suggesting nail thickness did not affect drug accumulation. In the in vitro nail model, efinaconazole was effective in reducing fungal viability, which suggested that sufficient amounts of the antifungal were being delivered to the ventral layer of the nail plate, the researchers noted.
“The high efinaconazole concentrations in patients’ toenails and fungicidal activity in vitro potentially contribute to the clinical efficacy reported in phase III studies,” they concluded.
All authors work for Kaken Pharmaceutical or Dow Pharmaceutical Sciences (a division of Valeant Pharmaceuticals, manufacturer of efinaconazole). The study was funded by Kaken and Valeant.
FROM THE JOURNAL OF DRUGS IN DERMATOLOGY
Key clinical point: Topical 10% efinaconazole was effective in permeating the nail to reach the infection site in patients with onychomycosis.
Major finding: Treatment with efinaconazole 10% solution resulted in drug concentrations much higher than MIC values of common onychomycosis pathogens in both the great toenails and second toenails of onychomycosis patients.
Data source: Multicenter, open-label study investigating the transungual delivery of efinaconazole in 40 patients with onychomycosis.
Disclosures: All authors work for Kaken Pharmaceutical or Dow Pharmaceutical Sciences (a division of Valeant Pharmaceuticals, manufacturer of efinaconazole). The study was funded by Kaken and Valeant.