User login
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.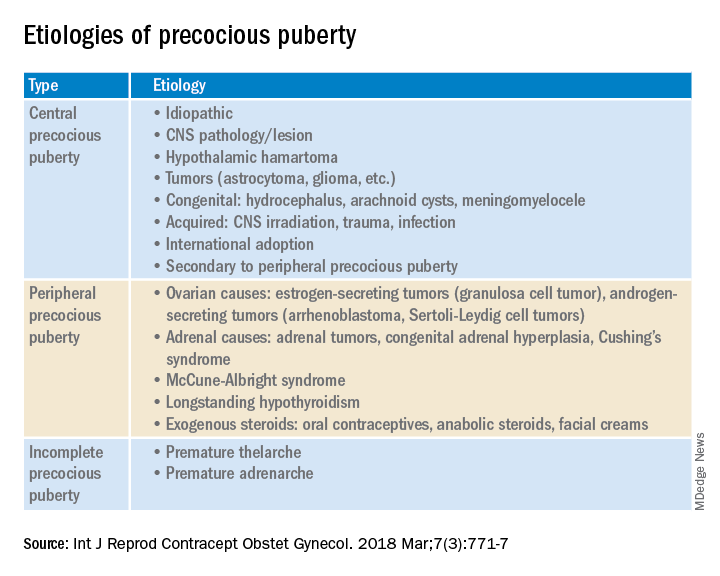
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A 6-year-old girl presents with breast development. Her medical history is unremarkable. The parents are of average height, and the mother reports her thelarche was age 11 years. The girl is at the 97th percentile for her height and 90th percentile for her weight. She has Tanner stage 3 breast development and Tanner stage 2 pubic hair development. She has grown slightly more than 3 inches over the past year. How should she be evaluated and managed (N Engl J Med. 2008;358:2366-77)?
The premature onset of puberty, i.e., precocious puberty (PP), can be an emotionally traumatic event for the child and parents. Over the past century, improvements in public health and nutrition, and, more recently, increased obesity, have been associated with earlier puberty and the dominant factor has been attributed to genetics (Curr Opin Endocrinol Diabetes Obes. 2018;25[1]:49-54). This month’s article will focus on understanding what is considered “early” puberty, evaluating for causes, and managing precocious puberty.
More commonly seen in girls than boys, PP is defined as the onset of secondary sexual characteristics before age 7.5 years in Black and Hispanic girls, and prior to 8 years in White girls, which is 2-2.5 standard deviations below the average age of pubertal onset in healthy children (J Pediatr Adolesc Gynecol. 2019;32:455-9). As a comparison, PP is diagnosed with onset before age 9 years in boys. For White compared with Black girls, the average timing of thelarche is age 10 vs. 9.5 years, peak growth velocity is age 11.5, menarche is age 12.5 vs. 12, while completion of puberty is near age 14.5 vs. 13.5, respectively (J Pediatr. 1985;107:317). Fortunately, most girls with PP have common variants rather than serious pathology.
Classification: Central (CPP) vs. peripheral (PPP)
CPP is gonadotropin dependent, meaning the hypothalamic-pituitary-ovarian axis (HPO) is prematurely activated resulting in the normal progression of puberty.
PPP is gonadotropin independent, caused by sex steroid secretion from any source – ovaries, adrenal gland, exogenous or ectopic production, e.g., germ-cell tumor. This results in a disordered progression of pubertal milestones.
Whereas CPP is typically isosexual development, i.e., consistent with the child’s gender, PPP can be isosexual or contrasexual, e.g., virilization of girls. A third classification is “benign or nonprogressive pubertal variants” manifesting as isolated premature thelarche or adrenarche.
Causes (see table)
CPP. Idiopathic causes account for 80%-90% of presentations in girls and 25%-80% in boys. Remarkably, international and domestic adoption, as well as a family history of PP increases the likelihood of CPP in girls. Other etiologies include CNS lesions, e.g., hamartomas, which are the most common cause of PP in young children. MRI with contrast has been the traditional mode of diagnosis for CNS tumors, yet the yield is dubious in girls above age 6. Genetic causes are found in only a small percentage of PP cases. Rarely, CPP can result from gonadotropin-secreting tumors because of elevated luteinizing hormone levels.
PPP. As a result of sex steroid secretion, peripheral causes of PPP include ovarian cysts and ovarian tumors that increase circulating estradiol, such as granulosa cell tumors, which would cause isosexual PPP and Sertoli-Leydig cell tumors that secrete testosterone, which can result in contrasexual PPP. Mild congenital adrenal hyperplasia can result in PPP with virilization (contrasexual) and markedly advanced bone age.
McCune-Albright syndrome is rare and presents with the classic triad of PPP, skin pigmentation called café-au-lait, and fibrous dysplasia of bone. The pathophysiology of McCune-Albright syndrome is autoactivation of the G-protein leading to activation of ovarian tissue that results in formation of large ovarian cysts and extreme elevations in serum estradiol as well as the potential production of other hormones, e.g., thyrotoxicosis, excess growth hormone (acromegaly), and Cushing syndrome.
Premature thelarche. Premature thelarche typically occurs in girls between the ages of 1 and 3 years and is limited to breast enlargement. While no cause has been determined, the plausible explanations include partial activation of the HPO axis, endocrine-disrupting chemicals (EDCs), or a genetic origin. A small percentage of these girls progress to CPP.
EDCs have been considered as potential influencers of early puberty, but no consensus has been established. (Examples of EDCs in the environment include air, soil, or water supply along with food sources, personal care products, and manufactured products that can affect the endocrine system.)
Premature adenarche. Premature adrenarche presents with adult body odor and/or body hair (pubic and/or axillary) in girls who have an elevated body mass index, most commonly at the ages of 6-7 years. The presumed mechanism is normal maturation of the adrenal gland with resultant elevation of circulating androgens. Bone age may be mildly accelerated and DHEAS is prematurely elevated for age. These girls appear to be at increased risk for polycystic ovary syndrome.
Evaluation
The initial step in the evaluation of PP is to determine whether the cause is CPP or PPP; the latter includes distinguishing isosexual from contrasexual development. A thorough history (growth, headaches, behavior or visual change, seizures, abdominal pain), physical exam, including Tanner staging, and bone age is required. However, with isolated premature thelarche or adrenarche, a bone age may not be necessary, as initial close clinical observation for pubertal progression is likely sufficient.
For CPP, the diagnosis is based on serum LH, whether random levels or elevations follow GnRH stimulation. Puberty milestones progress normally although adrenarche is not consistently apparent. For girls younger than age 6, a brain MRI is recommended but not in asymptomatic older girls with CPP. LH and FSH along with estradiol or testosterone, the latter especially in boys, are the first line of serum testing. Serum TSH is recommended for suspicion of primary hypothyroidism. In girls with premature adrenarche, a bone age, testosterone, DHEAS, and 17-OHP to rule out adrenal hyperplasia should be obtained. Pelvic ultrasound may be a useful adjunct to assess uterine volume and/or ovarian cysts/tumors.
Rapidity of onset can also lead the evaluation since a normal growth chart and skeletal maturation suggests a benign pubertal variant whereas a more rapid rate can signal CPP or PPP. Of note, health care providers should ensure prescription, over-the-counter oral or topical sources of hormones, and EDCs are ruled out.
Consequences
An association between childhood sexual abuse and earlier pubertal onset has been cited. These girls may be at increased risk for psychosocial difficulties, menstrual and fertility problems, and even reproductive cancers because of prolonged exposure to sex hormones (J Adolesc Health. 2016;60[1]:65-71).
Treatment
The mainstay of CPP treatment is maximizing adult height, typically through the use of a GnRH agonist for HPO suppression from pituitary downregulation. For girls above age 8 years, attempts at improving adult height have not shown a benefit.
In girls with PPP, treatment is directed at the prevailing pathology. Interestingly, early PPP can activate the HPO axis thereby converting to “secondary” CPP. In PPP, McCune-Albright syndrome treatment targets reducing circulating estrogens through letrozole or tamoxifen as well as addressing other autoactivated hormone production. Ovarian and adrenal tumors, albeit rare, can cause PP; therefore, surgical excision is the goal of treatment.
PP should be approached with equal concerns about the physical and emotional effects while including the family to help them understand the pathophysiology and psychosocial risks.
Dr. Mark P. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.

