User login
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
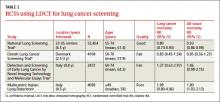
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
The US Preventive Services Task Force (USPSTF) recently released a draft recommendation on lung cancer screen- ing, advising annual screening with low-dose computed tomography (LDCT) for individuals at high risk for lung cancer based on age and smoking history. Once finalized, this recommendation will replace its “I” rating, which indicated that evidence was insufficient to recommend for or against screening for lung cancer.
While the wording of the new recommendation is nonspecific regarding who should be screened, the Task Force elaborates in its follow-on commentary: Screening should start at age 55 and continue through age 79 for those who have ≥30 pack-year history of smoking and are either current smokers or past smokers who quit <15 years earlier.1 The draft recommendation advises caution in screening those with significant comorbidities, as well as individuals in their late 70s. Examples of how these specifications would work in practice are included in TABLE 1.
Lung cancer epidemiology
Lung cancer is the second most common cancer in both men and women and the leading cause of cancer deaths in the United States, accounting for more than 158,000 deaths in 2010.2 Lung cancer is highly lethal, with >90% mortality rate and a 5-year survival rate <20%.1 However, non-small cell lung cancer (NSCLC), which can be cured with surgical resection if caught early, is responsible for 80% of cases.3 The incidence of lung cancer increases markedly after age 50, with >80% of cases occurring in those 60 years or older.3
Smoking causes >90% of lung cancers,2 which are preventable with avoidance of smoking and smoking cessation programs. Currently, 19% of Americans smoke and 37% are current or former smokers.1
Evidence report
The systematic review4 that the new draft rec- ommendation was based on found 4 clinical trials of LDCT screening that met inclusion criteria (TABLE 2). One, the National Lung Screening Trial (NLST), was a large study involving 33 centers in the United States and 53,454 current and former smokers ages 55 to 74 years. Participants had a mean age of 61.4 years and ≥30 pack-year history of smoking, with a mean of 56 pack-years.5
The study population was relatively young and healthy; only 8.8% of participants were older than 70. The researchers excluded anyone with a significant comorbidity that would make it unlikely that they would undergo surgery if cancer were detected.
Participants were randomized to either LDCT or chest x-ray, given 3 annual screens, and followed for a mean of 6.5 years. In the LDCT group, there was a 20% reduction in lung cancer mortality and a 7% decrease in overall mortality. This translates to a number needed to screen (NNS) of 320 to prevent one lung cancer death, which compares favorably with other cancer screening tests. Mammography has an NNS of about 1339 for women ages 50 to 59, for example, and colon cancer screening using flexible sigmoidoscopy has an NNS of 817 among individuals ages 55 to 74 years.4
The other 3 studies in the systematic review were conducted in other countries, and were smaller, of shorter duration, and of lower quality.6-8 None demonstrated a reduction in either lung cancer or all-cause mortality, and one showed a small increase in all-cause mortality.8 A Forest plot of all 4 studies raises questions about the significance of the decline in all-cause or lung-cancer mortality.4 However, a meta-analysis that deletes the one poor quality study did demonstrate a 19% decrease in lung cancer mortality, but no decline in all- cause mortality.9
The evidence report included an assessment of 15 studies on the accuracy of LDCT screening. Sensitivity varied from 80% to 100% and specificity ranged from 28% to 100%. The positive predictive value (PPV) for lung cancer ranged from 2% to 42%; however, most abnormal findings resolved with further imaging. As a result, the PPV for those who had a biopsy or surgery after retesting was 50% to 92%.4
Potential harms in the recommendation
Radiation exposure from an LDCT is slightly greater than that of a mammogram. The long-term effects of annual LDCT plus follow-up of abnormal findings is not fully known. There is some concern about the potential for lung cancer screening to have a negative effect on smoking cessation efforts. However, evidence suggests that the use of LDCT as a lung cancer screening tool has no influence on smoking cessation.4
Extrapolating results. The NLST was a well-controlled trial conducted at academic health centers, with strict procedures for conservative follow-up of suspicious lesions. A potential for harm exists in extrapolating results from such a study to the community at large, where work-ups may be more aggressive and include biopsy.
Overdiagnosis. Routine LDCT will likely result in some degree of overdiagnosis—eg, detection of low-grade cancers that would either regress on their own or simply not progress—and overtreatment, with the potential for complications.
Full impact is unknown
The ultimate balance of benefits and harms of the USPSTF’s lung cancer screening draft recommendation rests on some unknowns. Widespread screening is unlikely to achieve the same results as did the NLST. As already noted, those enrolled in the NLST were relatively young and had large pack-year smoking histories. The Task Force acknowledges that the 20% reduction in lung cancer mortality achieved in the NLST is unlikely to be duplicated in older patients and individuals with less significant smoking histories. Additional harms will likely accrue if suspicious findings are more aggressively pursued than they were in this study. The potential harms, as well as benefits, from incidental findings on chest LDCT scans are also unknown.
The number of screenings. The potential for benefits beyond 3 screenings is also unknown, as the USPSTF’s projections in such cases are based on modeling. The degree of overdiagnosis is not fully understood, nor is the harm that could result from the accumulated radiation of what could be an annual LDCT for 25 years. The harm/benefit ratio will become clearer with time and can then be compared with other medical interventions.
Financial burden. While it may appear to some that the draft recommendation would unfairly benefit smokers by allowing them to undergo free annual CT screening, patients are likely to incur significant financial obligations as a result of doing so. The Affordable Care Act mandates that the annual LDCT screening would have to be offered with no patient cost sharing, but follow-up CTs for questionable findings, biopsies, and treatment will all be subject to deductibles and copayments.
Recommendations of others
Other organizations have adopted recommendations on lung cancer screening similar to the USPSTF proposal. These include the American Association for Thoracic Surgery, American Cancer Society, American College of Chest Physicians, American Lung Association, American Society of Clinical Oncology, and American Thoracic Society. Most apply to those ages 55 to 74 years and use other inclusion criteria of the NLST. Some stipulate that patients should be in good enough health to benefit from early detection, and most include a reference to the quality of the centers at which screening should occur. The American Academy of Family Physicians is currently considering what its recommendation on lung cancer screening will be.
Final USPSTF recommendation expected soon
Noticeably absent from the news coverage of the proposed USPSTF recommendation was the word “draft.” The Task Force has now collected public comments about its proposed recommendation and will be considering potential changes to the wording. Publication of the final recommendation is expected in December—shortly after press time.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.
1. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement Draft. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservices- taskforce.org/uspstf13/lungcan/lungcandraftrec.htm. Accessed October 2, 2013.
2. Lung Cancer Statistics. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/lung/ statistics/. Updated October 23, 2013. Accessed November 15, 2013.
3. Lung Cancer Fact Sheet. American Lung Association Web site. Available at: http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html#Prevalence_ and_Incidence. Accessed October 2, 2013.
4. Humphrey LL, Deffeback M, Pappas M, et al. Screening for lung cancer using low-dose computed tomography. a systematic review to update the US Preventive Services Task Force Recom- mendation. Ann Intern Med. 2013;159:411-420.
5. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409.
6. Saghir Z, Dirksen A, Ashraf H, et al. CT screening for lung cancer brings forward early disease. The randomised Danish Lung Cancer Screening Trial: status after five annual screening rounds with low-dose CT. Thorax. 2012;67:296-301.
7. Infante M, Cavuto S, Lutman FR, et al; DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445-453.
8. Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev. 2012;21: 308-315.
9. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US Preventive Services Task Force Recommendation. Evidence Synthesis No. 105. AHRQ Publication No. 13-05188-EF-1. Rockville, MD: Agency for Health- care Research and Quality; 2013. Available at: http://www.uspre- ventiveservicestaskforce.org/uspstf13/lungcan/lungcanes105. pdf. Accessed October 2, 2013.