User login
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).
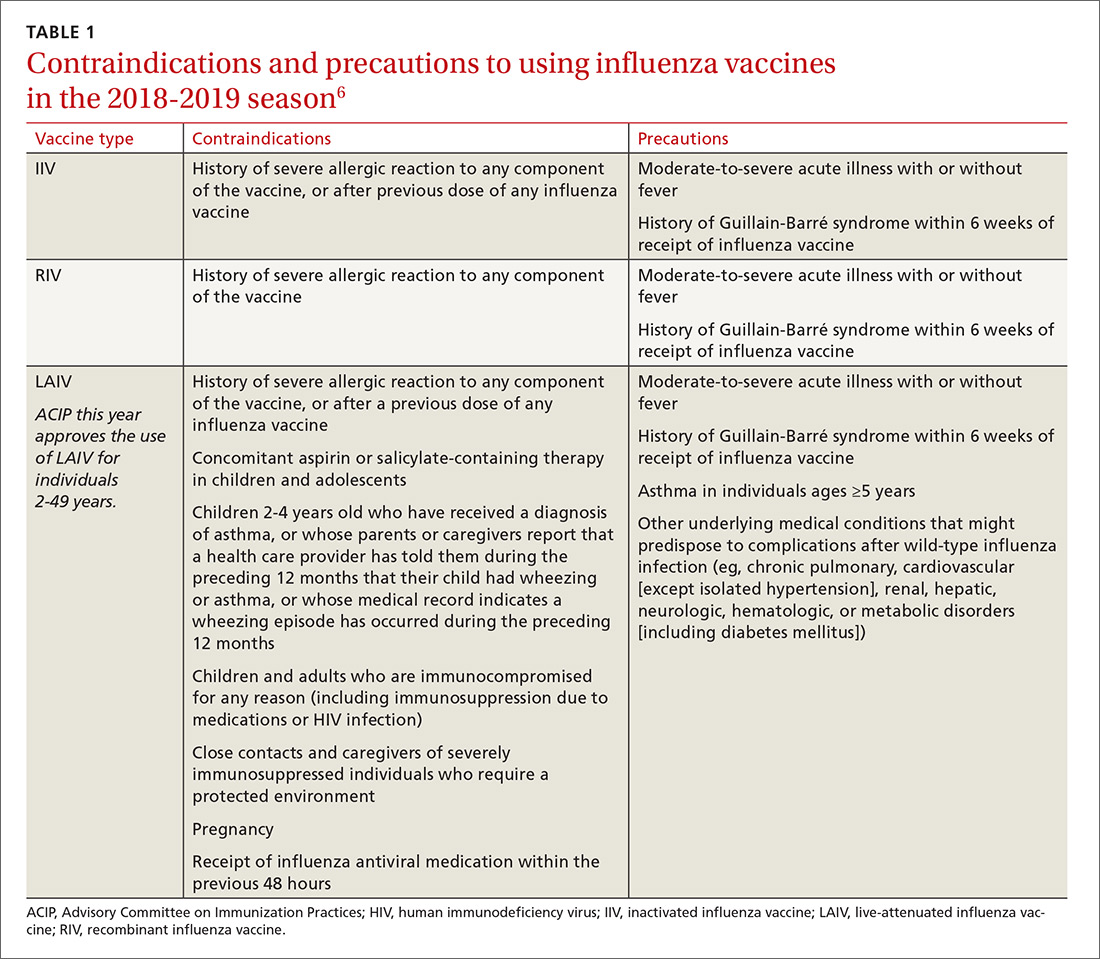
After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
The 2017-2018 influenza season was one of the most severe in this century, according to every indicator measured by the Centers for Disease Control and Prevention (CDC). The proportion of outpatient visits due to influenza-like illness (ILI) was elevated nationally above a baseline of 2.2% for 19 straight weeks, and for 3 weeks it was over 7%.1 High ILI activity was widespread and included all 50 states in January.
From October 2017 through April 2018, the CDC estimates that the influenza-related hospitalization rate was 106.6 per 100,000 population, with the highest rates among children 0 to 4 years (74.3/100,000), adults 50 to 64 years (115.7/100,000), and adults 65 years and older (460.9/100,000). More than 90% of adults hospitalized had a chronic condition, such as heart or lung disease, diabetes, or obesity, placing them at high risk for influenza complications.1
Influenza severity is also measured as the proportion of deaths due to pneumonia and influenza, which was above the epidemic threshold for 16 weeks in 2017-2018 and was above 10% for 4 weeks in January.1 Based on all of these indicators, the 2017-2018 influenza season was classified as high severity overall and for all age groups, the first time this has happened since the 2003-2004 season. There were 171 pediatric deaths attributed to influenza, and more than three-quarters of vaccine-eligible children who died from influenza this season had not received influenza vaccine.1
The type of influenza predominating last season was influenza A from early- through mid-season, and was influenza B later in the season (see https://stacks.cdc.gov/view/cdc/54974).1 For the entire season, 71.2% of specimens that tested positive for influenza in public health labs were Influenza A and 84.9% of these were H3N2.1
Effectiveness of influenza vaccine last season. As measured by preventing respiratory illness needing medical attention, vaccine effectiveness was 36% overall: 25% against influenza A (H3N2), 67% against influenza A (H1N1), and 42% against influenza B.1 Effectiveness varied by age, being the highest in those 8 years and younger.2 Effectiveness was questionable in those older than 65, with an estimated effectiveness of 23% but confidence intervals including 0.2
While the effectiveness of influenza vaccines remains suboptimal, the morbidity and mortality they prevent is still considerable. The CDC estimates that in 2016-2017, more than 5 million influenza illnesses, 2.6 million medical visits, and 84,700 hospitalizations were prevented.3 And effectiveness last season was similar to, or better than, what has been seen in each of the past 10 years (FIGURE).4

Three drugs were recommended for use to treat influenza in 2017-1018 (oseltamivir, peramivir, and zanamivir), and no resistance was found except in 1% of influenza A (H1N1) tested.1 No resistance was found in other A or any B viruses tested.1
Continue to: Safety
Safety
The safety of influenza vaccines is studied each year by both the CDC and US Food and Drug Administration (FDA). This past year, studies were conducted using the CDC-supported Safety Datalink System, looking for increased rates of acute disseminated encephalomyelitis, anaphylaxis, Bell’s palsy, encephalitis, Guillain-Barré syndrome (GBS), seizures, and transverse myelitis.5 No safety signals were detected. However, for some of the newer vaccines, the numbers of vaccinated individuals studied were small. The FDA studied the incidence of GBS using Medicare data and found no increased rates in those vaccinated.5
2018-2019 Recommendations
There are only a few changes to the recommendations for the upcoming influenza season. The Advisory Committee on Immunization Practices (ACIP) still recommends universal vaccination for anyone age 6 months and older who does not have a contraindication (TABLE 16). Two of the antigens in the vaccines for this coming season are slightly different from last season (TABLE 27).

After 2 years of recommending against the use of live attenuated influenza vaccine (LAIV) because of its low effectiveness in children against influenza A (H1N1), ACIP now includes it as an option for the upcoming season in individuals ages 2 through 49 years.8 The basis of this revised recommendation was 2-fold: 1) evidence of LAIV effectiveness comparable to that of inactivated products against A (H3N2) and B viruses; and 2) evidence that a new strain of A (H1N1) now used to produce the vaccine (A/Slovenia) produces a significantly higher antibody response than the strain (A/Bolivia) used in the years when the vaccine was not effective against A (H1N1).

However, the new formulation’s clinical effectiveness against A (H1N1) has not been demonstrated, leading the American Academy of Pediatrics to recommend that LAIV should be used in children only if other options are not available or if injectable vaccine is refused.9 Contraindications to the use of LAIV remain the same as the previous version of the vaccine (TABLE 16).
Individuals with non-severe egg allergies can receive any licensed, recommended age-appropriate influenza vaccine and no longer have to be monitored for 30 minutes after receiving the vaccine. People who have severe egg allergies should be vaccinated with an egg-free product or in a medical setting and be supervised by a health care provider who is able to recognize and manage severe allergic conditions.
Continue to: Children 6 months through 8 years...
Children 6 months through 8 years who have previously received an influenza vaccine, either trivalent or quadrivalent, need only 1 dose; those who have not received vaccination need 2 doses separated by at least 4 weeks.
Available vaccine products
A table found on the CDC influenza Web site lists the vaccine products available in the United States and the ages for which they are approved.6 The options now include 2 standard-dose trivalent inactivated influenza vaccines (IIV3), 4 standard-dose quadrivalent inactivated influenza vaccines (IIV4), one cell culture-based IIV4 (ccIIV4), one standard dose IIV4 intradermal option, a trivalent and a quadrivalent recombinant influenza vaccine (RIV3, RIV4), one LAIV, and 2 products for those 65 years and older—an adjuvanted IIV3 (aIIV3) and a high dose IIV3. Three of these products do not depend on egg-based technology: RIV3, RIV4, and ccIIV4.
Comparative effectiveness studies of these vaccine options, including those available for the elderly, are being conducted. Studies presented at the June 2018 ACIP meeting show comparable effectiveness of egg-based and non–egg-based products.6 At this time, ACIP does not make a preferential recommendation for any influenza vaccine product for any age group.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.
1. Garten R, Blanton L, Elal AIA, eta al. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-2019 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67;634-642.
2. Flannery B, Chung JR, Belongia EA, et al. Interim estimates of 2017-18 seasonal influenza vaccine effectiveness – United States, February 2018. MMWR Morb Mortal Wkly Rep. 2018;67:180-185.
3. Flannery B, Chung J, Ferdinands J. Preliminary estimates of 2017-2018 seasonal influenza vaccine effectiveness against laboratory-confirmed influenza from the US Flu VE and HAIVEN network. Meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-02-Flannery-508.pdf. Accessed August 11, 2018.
4. CDC. Seasonal influenza vaccine effectiveness, 2005-2018. Available at: https://www.cdc.gov/flu/professionals/vaccination/effectiveness-studies.htm. Accessed July 27, 2018.
5. Shimabukuro T. End-of-season update: 2017-2018 influenza vaccine safety monitoring. Presented at: meeting of the Advisory Committee on Immunization Practices; June 20, 2018; Atlanta, Ga. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2018-06/flu-04-Shimabukuro-508.pdf. Accessed August 11, 2018.
6. CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018–19 Influenza Season. Available at: https://www.cdc.gov/mmwr/volumes/67/rr/rr6703a1.htm?s_cid=rr6703a1_w. Accessed August 23, 2018.
7. CDC. Update: Influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. Available at: https://www.cdc.gov/mmwr/volumes/67/wr/mm6722a4.htm. Accessed July 27, 2018.
8. Grohskopf LA, Sokolow LZ, Fry AM, et al. Update: ACIP recommendations for the use of quadrivalent live attenuated influenza vaccine (LAIV4) — United States, 2018–19 influenza season. MMWR Morb Mortal Wkly Rep. 2018;67:643-645.
9. Jenco M. AAP: Give children IIV flu shot; use LAIV as last resort. Available at: http://www.aappublications.org/news/2018/05/21/fluvaccine051818. Accessed August 1, 2018.