User login
Disseminated Cryptococcosis in a Diabetic Patient
Cryptococcosis is a granulomatous infection caused by Cryptococcus neoformans1,2 that usually develops in immunocompromised hosts, especially those with human immunodeficiency virus (HIV) infection. Cryptococcosis rarely has been described in patients with diabetes mellitus (DM).3 Patients with uncontrolled DM are prone to numerous infections, as glucose-rich blood serves as an excellent medium for growth of organisms and hyperglycemia has been shown to cause numerous defects in host defense mechanisms. We present a rare case of disseminated cryptococcosis in an HIV-negative patient with DM.
Case Report
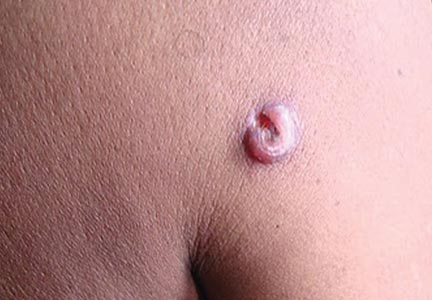
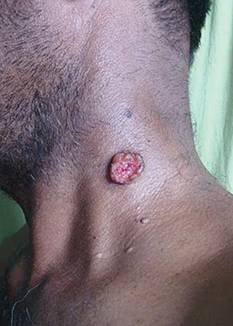
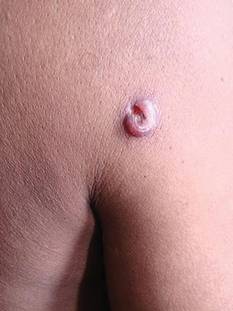
A 48-year-old man presented with raised lesions on the left side of the neck and the back of the right shoulder that had gradually increased in size over the last 4 months. The patient denied any history of antecedent trauma. Physical examination revealed a well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck (Figure 1) and a single 1×1-cm erythematous nodule with a central ulcerated core on the right shoulder (Figure 2). Both lesions showed minimal seropurulent discharge. Genital examination showed evidence of candidal balanitis. Systemic examination was normal.
|
| Figure 1. A well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck. |
|
| Figure 2. An erythematous 1×1-cm nodule with a central ulcerated core on the right shoulder. |
|
|
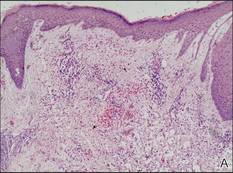
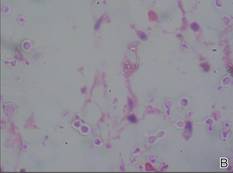
Figure 3. Skin biopsy revealed granulomatous and gelatinous tissue reaction with ovoid encapsulated spores (A)(H&E, original magnification ×40). Ovoid, budding, encapsulated spores of Cryptococcus were seen on higher power (B)(H&E, original magnification ×400). |
|
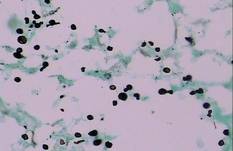
| Figure 4. Black-colored, ovoid, budding spores of Cryptococcus were seen (Gomori methenamine-silver, original magnification ×100). |
|
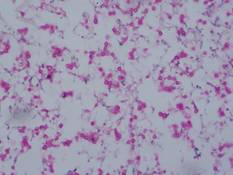
| Figure 5. Mucicarmine stain showed multiple red-colored, budding, capsulated spores (original magnification ×100). |
Keratoacanthoma, secondary syphilis, cutaneous tuberculosis, leishmaniasis, cryptococcosis, histoplasmosis, and leprosy were considered in the differential diagnosis. The complete blood cell count was normal. A VDRL test and enzyme-linked immunosorbent assay for HIV-1 and HIV-2 were nonreactive. The absolute CD4 count was 885 cells/µL (reference range, 404–1612 cells/µL), CD4 was 41% (reference range, 33%–58%), absolute CD8 count was 443 cells/µL (reference range, 220–1129 cells/µL), CD8 was 20% (reference range, 13%–39%), and CD4:CD8 ratio was 2.04 (reference range, 0.8–3.0). The fasting blood glucose level was elevated at 230 mg/dL (reference range, 80–120 mg/dL) and the postprandial glucose level was 348 mg/dL (reference range, 130–150 mg/dL). A chest radiograph revealed a nodular opacity in the right upper lobe of the lung with no evidence of cavitation. Ultrasonography of the abdomen and pelvis showed mild splenomegaly.
Gram stain and a potassium hydroxide (KOH) mount of a smear from the ulcerated lesion on the neck showed encapsulated budding spores. Histopathologic study of hematoxylin and eosin–stained sections from the noduloulcerative lesion on the shoulder demonstrated irregular acanthosis in the epidermis; a granulomatous reaction comprised of lymphocytes, histiocytes, neutrophils, plasma cells, and few giant cells; and a gelatinous tissue reaction with numerous ovoid encapsulated spores (Figure 3). Gomori methenamine-silver stain revealed black-colored, ovoid, budding spores (Figure 4), and periodic acid–Schiff stain also revealed narrow-based budding spores. Mucicarmine staining showed multiple red-colored, budding, capsulated spores (Figure 5). A culture on Sabouraud dextrose agar confirmed growth of C neoformans. Examination of sputum by KOH mount and india ink preparation demonstrated encapsulated spores of Cryptococcus. India ink staining of cerebrospinal fluid and urine was negative for Cryptococcus. Cerebrospinal fluid cytochemistry was within reference range. The final diagnosis was disseminated cryptococcosis in the setting of uncontrolled DM.
The patient was treated with oral fluconazole 200 mg twice daily. He also was started on metformin 500 mg daily, glipizide 5 mg twice daily, and pioglitazone 15 mg daily for treatment of DM. The lesions resolved with scarring after 8 months of treatment.
Comment
Cryptococcosis is a disease caused by C neoformans, an encapsulated yeast. There are 2 variants of cryptococcosis and 5 serotypes: C neoformans var neoformans (serotypes A, D, and AD) and C neoformans var gattii (serotypes B and C).1,2 The former is more common in Europe and much of the United States, while the latter is more common in the tropics.3Cryptococcus neoformans var neoformans is a saprophyte that commonly is found in soil rich in pigeon droppings, while C neoformans var gattii is found in tree bark (eg, eucalyptus).
Cryptococcal infection usually is acquired by respiratory transmission, though primary cutaneous lesions rarely may occur.4 The organisms are less than 2 µm in size and are unencapsulated; therefore, they can easily be inhaled into the alveolar space.2,5 The most important host response to cryptococcal infection is cell-mediated immunity, which explains the increased incidence of infection in immunosuppressed patients, especially in patients with HIV infection. It has been sporadically reported in HIV-negative patients in the setting of other causes of immunosuppression (eg, chronic corticosteroid use, organ transplantation, chemotherapy, hematologic malignancies, systemic lupus erythematosus, sarcoidosis, liver failure, renal failure, idiopathic CD4+ lymphocytopenia, monoclonal antibody therapy [eg, alemtuzumab, infliximab]).1,6,7
It occasionally has been documented in patients with DM.3 Hyperglycemia is known to cause impaired polymorphonuclear chemotaxis, abnormal phagocytosis, poor blast transformation of lymphocytes, and defective opsonization. The level of hyperglycemia has a direct influence on microbicidal function of macrophages. Temporary high glucose levels (eg, 200 mg/dL) can substantially impair the respiratory burst of macrophages. In a series of 40 HIV-negative patients with cryptococcosis, 14% (5/37) had DM as a predisposing factor.8 In another series of 94 HIV-negative patients with cryptococcal meningitis, 8.5% (8/94) had DM.9
After entry into the respiratory system, Cryptococcus can lead to latent infection or acute disease depending on the immune status of the host. Disseminated cryptococcosis is defined as the recovery of C neoformans from blood, sterile body fluids, or tissues other than pulmonary tissue.6 Hematogenous dissemination occurs most commonly to the central nervous system (CNS). The skin is the most common site for extraneural dissemination and has been reported in 10% to 20% of patients.5 Cuta-neous cryptococcosis may be present for 2 to 8 months before development of systemic signs of infection, providing a window of opportunity for treatment of patients before fatal effects of dissemination occur.
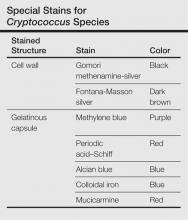
Cutaneous manifestations of cryptococcosis are variable and can include papules, pustules, ulcerated nodules, molluscoid lesions, and cellulitis.5 Acneform lesions as well as lesions resembling erythema nodosum, Kaposi sarcoma, keratoacanthoma, and herpes virus infection also have been described in the literature. Lesions most commonly are located on the head and neck.5 The 2 types of histologic reactions seen in cryptococcal infections are gelatinous and granulomatous, which can occur individually or simultaneously. Gelatinous reactions show numerous spores with large polysaccharide capsules and little tissue reaction. In contrast, granulomatous reactions show pronounced tissue reactions consisting of histiocytes, giant cells, lymphoid cells, and fibroblasts with few spores, which are mainly found within giant cells and histiocytes and occasionally are free in the tissue.5Cryptococcus neoformans is a round to ovoid spore measuring 4 to 12 µm in gelatinous reactions and 2 to 4 µm in granulomatous reactions. Special stains are helpful for demonstration of Cryptococcus species (Table). Diagnosis of cryptococcosis also may be made by india ink staining, KOH preparation, or gram staining of a smear from the lesion. Culture on Sabouraud dextrose agar also can be performed to confirm the species. Detection of cryptococcal capsular polysaccharide antigen in serum and body fluids by latex agglutination test or enzyme-linked immunosorbent assay is useful for diagnostic as well as a prognostic evaluation.2 Because cutaneous cryptococcosis usually represents hematogenous dissemination from an internal focus, investigation to detect other organ involvement, especially the CNS, is mandatory.
The gold standard for treatment of severe cryptococcosis and CNS involvement is intravenous amphotericin B (0.7–1 mg/kg daily [total, 1–2 g]) with or without intravenous flucytosine (100 mg/kg daily) for 2 weeks,2 which is followed by treatment with oral fluconazole (400 mg daily) for 10 weeks. In cases with renal dysfunction, liposomal amphotericin B (4–6 mg/kg daily) is a better alternative. In other mild to moderate cases, only fluconazole or itraconazole (400 mg daily) for 6 to 12 months is sufficient for resolution of lesions, as in the case of our patient. Long-term suppressive treatment with fluconazole is essential in HIV-positive patients with low CD4 counts.2
Cryptococcosis in HIV-negative patients differs from HIV-positive patients. Central nervous system and extrapulmonary involvement is less common in HIV-negative patients. Response to treatment often is brisk and mortality is much lower in HIV-negative patients.10 Patients who are HIV negative exhibit a greater inflammatory response with a lesser organism load; however, an exaggerated immune response resulting in higher cerebrospinal fluid pleocytosis and protein rarely may result in fatal outcomes.11 Our case emphasizes the need for physicians to consider cryptococcosis in the differential diagnosis of neurologic and cutaneous manifestations in DM.
1. Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:2:36.89-36.91.
2. Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507-544, v-vi.
3. Thangakunam B, Christopher DJ, Kurian S, et al. Endogenous excess cortisol production and diabetes mellitus as predisposing factors for pulmonary cryptococcosis: a case report and literature review. Lung India. 2008;25:155-157.
4. Patel P, Ramanathan J, Kayser M, et al. Primary cutaneous cryptococcosis of the nose in an immunocompetent woman. J Am Acad Dermatol. 2000;43(2, pt 2):344-345.
5. Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32(5, pt 2):844-850.
6. Taneja J, Bhargava A, Loomba P, et al. Cryptococcal granulomas in an immunocompromised HIV-negative patient. Indian J Pathol Microbiol. 2008;51:553-555.
7. Hage CA, Wood KL, Winer-Muram HT, et al. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003;124:2395-2397.
8. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, et al. Cryptococcosis in human immunodeficiency virus-negative patients [published online ahead of print November 9, 2005]. Int J Infect Dis. 2006;10:72-78.
9. Shih CC, Chen YC, Chang SC, et al. Cryptococcal meningitis in non-HIV-infected patients. QJM. 2000;93:245-251.
10. Tunlayadechanont S, Viranuvatti K, Phuapradit P, et al. Cryptococcal meningitis in patients with non-HIV and HIV infection: a clinical study. Neurol J Southeast Asia. 1997;2:45-50.
11. Kushawaha A, Mobarakai N, Parikh N, et al. Cryptococcus neoformans meningitis in a diabetic patient–the perils of an overzealous immune response: a case report. Cases Journal. 2009;2:209.
Cryptococcosis is a granulomatous infection caused by Cryptococcus neoformans1,2 that usually develops in immunocompromised hosts, especially those with human immunodeficiency virus (HIV) infection. Cryptococcosis rarely has been described in patients with diabetes mellitus (DM).3 Patients with uncontrolled DM are prone to numerous infections, as glucose-rich blood serves as an excellent medium for growth of organisms and hyperglycemia has been shown to cause numerous defects in host defense mechanisms. We present a rare case of disseminated cryptococcosis in an HIV-negative patient with DM.
Case Report
A 48-year-old man presented with raised lesions on the left side of the neck and the back of the right shoulder that had gradually increased in size over the last 4 months. The patient denied any history of antecedent trauma. Physical examination revealed a well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck (Figure 1) and a single 1×1-cm erythematous nodule with a central ulcerated core on the right shoulder (Figure 2). Both lesions showed minimal seropurulent discharge. Genital examination showed evidence of candidal balanitis. Systemic examination was normal.

|
| Figure 1. A well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck. |

|
| Figure 2. An erythematous 1×1-cm nodule with a central ulcerated core on the right shoulder. |

|

|
Figure 3. Skin biopsy revealed granulomatous and gelatinous tissue reaction with ovoid encapsulated spores (A)(H&E, original magnification ×40). Ovoid, budding, encapsulated spores of Cryptococcus were seen on higher power (B)(H&E, original magnification ×400). |

|
| Figure 4. Black-colored, ovoid, budding spores of Cryptococcus were seen (Gomori methenamine-silver, original magnification ×100). |

|
| Figure 5. Mucicarmine stain showed multiple red-colored, budding, capsulated spores (original magnification ×100). |
Keratoacanthoma, secondary syphilis, cutaneous tuberculosis, leishmaniasis, cryptococcosis, histoplasmosis, and leprosy were considered in the differential diagnosis. The complete blood cell count was normal. A VDRL test and enzyme-linked immunosorbent assay for HIV-1 and HIV-2 were nonreactive. The absolute CD4 count was 885 cells/µL (reference range, 404–1612 cells/µL), CD4 was 41% (reference range, 33%–58%), absolute CD8 count was 443 cells/µL (reference range, 220–1129 cells/µL), CD8 was 20% (reference range, 13%–39%), and CD4:CD8 ratio was 2.04 (reference range, 0.8–3.0). The fasting blood glucose level was elevated at 230 mg/dL (reference range, 80–120 mg/dL) and the postprandial glucose level was 348 mg/dL (reference range, 130–150 mg/dL). A chest radiograph revealed a nodular opacity in the right upper lobe of the lung with no evidence of cavitation. Ultrasonography of the abdomen and pelvis showed mild splenomegaly.
Gram stain and a potassium hydroxide (KOH) mount of a smear from the ulcerated lesion on the neck showed encapsulated budding spores. Histopathologic study of hematoxylin and eosin–stained sections from the noduloulcerative lesion on the shoulder demonstrated irregular acanthosis in the epidermis; a granulomatous reaction comprised of lymphocytes, histiocytes, neutrophils, plasma cells, and few giant cells; and a gelatinous tissue reaction with numerous ovoid encapsulated spores (Figure 3). Gomori methenamine-silver stain revealed black-colored, ovoid, budding spores (Figure 4), and periodic acid–Schiff stain also revealed narrow-based budding spores. Mucicarmine staining showed multiple red-colored, budding, capsulated spores (Figure 5). A culture on Sabouraud dextrose agar confirmed growth of C neoformans. Examination of sputum by KOH mount and india ink preparation demonstrated encapsulated spores of Cryptococcus. India ink staining of cerebrospinal fluid and urine was negative for Cryptococcus. Cerebrospinal fluid cytochemistry was within reference range. The final diagnosis was disseminated cryptococcosis in the setting of uncontrolled DM.
The patient was treated with oral fluconazole 200 mg twice daily. He also was started on metformin 500 mg daily, glipizide 5 mg twice daily, and pioglitazone 15 mg daily for treatment of DM. The lesions resolved with scarring after 8 months of treatment.
Comment
Cryptococcosis is a disease caused by C neoformans, an encapsulated yeast. There are 2 variants of cryptococcosis and 5 serotypes: C neoformans var neoformans (serotypes A, D, and AD) and C neoformans var gattii (serotypes B and C).1,2 The former is more common in Europe and much of the United States, while the latter is more common in the tropics.3Cryptococcus neoformans var neoformans is a saprophyte that commonly is found in soil rich in pigeon droppings, while C neoformans var gattii is found in tree bark (eg, eucalyptus).
Cryptococcal infection usually is acquired by respiratory transmission, though primary cutaneous lesions rarely may occur.4 The organisms are less than 2 µm in size and are unencapsulated; therefore, they can easily be inhaled into the alveolar space.2,5 The most important host response to cryptococcal infection is cell-mediated immunity, which explains the increased incidence of infection in immunosuppressed patients, especially in patients with HIV infection. It has been sporadically reported in HIV-negative patients in the setting of other causes of immunosuppression (eg, chronic corticosteroid use, organ transplantation, chemotherapy, hematologic malignancies, systemic lupus erythematosus, sarcoidosis, liver failure, renal failure, idiopathic CD4+ lymphocytopenia, monoclonal antibody therapy [eg, alemtuzumab, infliximab]).1,6,7
It occasionally has been documented in patients with DM.3 Hyperglycemia is known to cause impaired polymorphonuclear chemotaxis, abnormal phagocytosis, poor blast transformation of lymphocytes, and defective opsonization. The level of hyperglycemia has a direct influence on microbicidal function of macrophages. Temporary high glucose levels (eg, 200 mg/dL) can substantially impair the respiratory burst of macrophages. In a series of 40 HIV-negative patients with cryptococcosis, 14% (5/37) had DM as a predisposing factor.8 In another series of 94 HIV-negative patients with cryptococcal meningitis, 8.5% (8/94) had DM.9
After entry into the respiratory system, Cryptococcus can lead to latent infection or acute disease depending on the immune status of the host. Disseminated cryptococcosis is defined as the recovery of C neoformans from blood, sterile body fluids, or tissues other than pulmonary tissue.6 Hematogenous dissemination occurs most commonly to the central nervous system (CNS). The skin is the most common site for extraneural dissemination and has been reported in 10% to 20% of patients.5 Cuta-neous cryptococcosis may be present for 2 to 8 months before development of systemic signs of infection, providing a window of opportunity for treatment of patients before fatal effects of dissemination occur.
Cutaneous manifestations of cryptococcosis are variable and can include papules, pustules, ulcerated nodules, molluscoid lesions, and cellulitis.5 Acneform lesions as well as lesions resembling erythema nodosum, Kaposi sarcoma, keratoacanthoma, and herpes virus infection also have been described in the literature. Lesions most commonly are located on the head and neck.5 The 2 types of histologic reactions seen in cryptococcal infections are gelatinous and granulomatous, which can occur individually or simultaneously. Gelatinous reactions show numerous spores with large polysaccharide capsules and little tissue reaction. In contrast, granulomatous reactions show pronounced tissue reactions consisting of histiocytes, giant cells, lymphoid cells, and fibroblasts with few spores, which are mainly found within giant cells and histiocytes and occasionally are free in the tissue.5Cryptococcus neoformans is a round to ovoid spore measuring 4 to 12 µm in gelatinous reactions and 2 to 4 µm in granulomatous reactions. Special stains are helpful for demonstration of Cryptococcus species (Table). Diagnosis of cryptococcosis also may be made by india ink staining, KOH preparation, or gram staining of a smear from the lesion. Culture on Sabouraud dextrose agar also can be performed to confirm the species. Detection of cryptococcal capsular polysaccharide antigen in serum and body fluids by latex agglutination test or enzyme-linked immunosorbent assay is useful for diagnostic as well as a prognostic evaluation.2 Because cutaneous cryptococcosis usually represents hematogenous dissemination from an internal focus, investigation to detect other organ involvement, especially the CNS, is mandatory.
The gold standard for treatment of severe cryptococcosis and CNS involvement is intravenous amphotericin B (0.7–1 mg/kg daily [total, 1–2 g]) with or without intravenous flucytosine (100 mg/kg daily) for 2 weeks,2 which is followed by treatment with oral fluconazole (400 mg daily) for 10 weeks. In cases with renal dysfunction, liposomal amphotericin B (4–6 mg/kg daily) is a better alternative. In other mild to moderate cases, only fluconazole or itraconazole (400 mg daily) for 6 to 12 months is sufficient for resolution of lesions, as in the case of our patient. Long-term suppressive treatment with fluconazole is essential in HIV-positive patients with low CD4 counts.2
Cryptococcosis in HIV-negative patients differs from HIV-positive patients. Central nervous system and extrapulmonary involvement is less common in HIV-negative patients. Response to treatment often is brisk and mortality is much lower in HIV-negative patients.10 Patients who are HIV negative exhibit a greater inflammatory response with a lesser organism load; however, an exaggerated immune response resulting in higher cerebrospinal fluid pleocytosis and protein rarely may result in fatal outcomes.11 Our case emphasizes the need for physicians to consider cryptococcosis in the differential diagnosis of neurologic and cutaneous manifestations in DM.
Cryptococcosis is a granulomatous infection caused by Cryptococcus neoformans1,2 that usually develops in immunocompromised hosts, especially those with human immunodeficiency virus (HIV) infection. Cryptococcosis rarely has been described in patients with diabetes mellitus (DM).3 Patients with uncontrolled DM are prone to numerous infections, as glucose-rich blood serves as an excellent medium for growth of organisms and hyperglycemia has been shown to cause numerous defects in host defense mechanisms. We present a rare case of disseminated cryptococcosis in an HIV-negative patient with DM.
Case Report
A 48-year-old man presented with raised lesions on the left side of the neck and the back of the right shoulder that had gradually increased in size over the last 4 months. The patient denied any history of antecedent trauma. Physical examination revealed a well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck (Figure 1) and a single 1×1-cm erythematous nodule with a central ulcerated core on the right shoulder (Figure 2). Both lesions showed minimal seropurulent discharge. Genital examination showed evidence of candidal balanitis. Systemic examination was normal.

|
| Figure 1. A well-defined, tender, indurated, ulcerated plaque measuring 3×4 cm on the left side of the neck. |

|
| Figure 2. An erythematous 1×1-cm nodule with a central ulcerated core on the right shoulder. |

|

|
Figure 3. Skin biopsy revealed granulomatous and gelatinous tissue reaction with ovoid encapsulated spores (A)(H&E, original magnification ×40). Ovoid, budding, encapsulated spores of Cryptococcus were seen on higher power (B)(H&E, original magnification ×400). |

|
| Figure 4. Black-colored, ovoid, budding spores of Cryptococcus were seen (Gomori methenamine-silver, original magnification ×100). |

|
| Figure 5. Mucicarmine stain showed multiple red-colored, budding, capsulated spores (original magnification ×100). |
Keratoacanthoma, secondary syphilis, cutaneous tuberculosis, leishmaniasis, cryptococcosis, histoplasmosis, and leprosy were considered in the differential diagnosis. The complete blood cell count was normal. A VDRL test and enzyme-linked immunosorbent assay for HIV-1 and HIV-2 were nonreactive. The absolute CD4 count was 885 cells/µL (reference range, 404–1612 cells/µL), CD4 was 41% (reference range, 33%–58%), absolute CD8 count was 443 cells/µL (reference range, 220–1129 cells/µL), CD8 was 20% (reference range, 13%–39%), and CD4:CD8 ratio was 2.04 (reference range, 0.8–3.0). The fasting blood glucose level was elevated at 230 mg/dL (reference range, 80–120 mg/dL) and the postprandial glucose level was 348 mg/dL (reference range, 130–150 mg/dL). A chest radiograph revealed a nodular opacity in the right upper lobe of the lung with no evidence of cavitation. Ultrasonography of the abdomen and pelvis showed mild splenomegaly.
Gram stain and a potassium hydroxide (KOH) mount of a smear from the ulcerated lesion on the neck showed encapsulated budding spores. Histopathologic study of hematoxylin and eosin–stained sections from the noduloulcerative lesion on the shoulder demonstrated irregular acanthosis in the epidermis; a granulomatous reaction comprised of lymphocytes, histiocytes, neutrophils, plasma cells, and few giant cells; and a gelatinous tissue reaction with numerous ovoid encapsulated spores (Figure 3). Gomori methenamine-silver stain revealed black-colored, ovoid, budding spores (Figure 4), and periodic acid–Schiff stain also revealed narrow-based budding spores. Mucicarmine staining showed multiple red-colored, budding, capsulated spores (Figure 5). A culture on Sabouraud dextrose agar confirmed growth of C neoformans. Examination of sputum by KOH mount and india ink preparation demonstrated encapsulated spores of Cryptococcus. India ink staining of cerebrospinal fluid and urine was negative for Cryptococcus. Cerebrospinal fluid cytochemistry was within reference range. The final diagnosis was disseminated cryptococcosis in the setting of uncontrolled DM.
The patient was treated with oral fluconazole 200 mg twice daily. He also was started on metformin 500 mg daily, glipizide 5 mg twice daily, and pioglitazone 15 mg daily for treatment of DM. The lesions resolved with scarring after 8 months of treatment.
Comment
Cryptococcosis is a disease caused by C neoformans, an encapsulated yeast. There are 2 variants of cryptococcosis and 5 serotypes: C neoformans var neoformans (serotypes A, D, and AD) and C neoformans var gattii (serotypes B and C).1,2 The former is more common in Europe and much of the United States, while the latter is more common in the tropics.3Cryptococcus neoformans var neoformans is a saprophyte that commonly is found in soil rich in pigeon droppings, while C neoformans var gattii is found in tree bark (eg, eucalyptus).
Cryptococcal infection usually is acquired by respiratory transmission, though primary cutaneous lesions rarely may occur.4 The organisms are less than 2 µm in size and are unencapsulated; therefore, they can easily be inhaled into the alveolar space.2,5 The most important host response to cryptococcal infection is cell-mediated immunity, which explains the increased incidence of infection in immunosuppressed patients, especially in patients with HIV infection. It has been sporadically reported in HIV-negative patients in the setting of other causes of immunosuppression (eg, chronic corticosteroid use, organ transplantation, chemotherapy, hematologic malignancies, systemic lupus erythematosus, sarcoidosis, liver failure, renal failure, idiopathic CD4+ lymphocytopenia, monoclonal antibody therapy [eg, alemtuzumab, infliximab]).1,6,7
It occasionally has been documented in patients with DM.3 Hyperglycemia is known to cause impaired polymorphonuclear chemotaxis, abnormal phagocytosis, poor blast transformation of lymphocytes, and defective opsonization. The level of hyperglycemia has a direct influence on microbicidal function of macrophages. Temporary high glucose levels (eg, 200 mg/dL) can substantially impair the respiratory burst of macrophages. In a series of 40 HIV-negative patients with cryptococcosis, 14% (5/37) had DM as a predisposing factor.8 In another series of 94 HIV-negative patients with cryptococcal meningitis, 8.5% (8/94) had DM.9
After entry into the respiratory system, Cryptococcus can lead to latent infection or acute disease depending on the immune status of the host. Disseminated cryptococcosis is defined as the recovery of C neoformans from blood, sterile body fluids, or tissues other than pulmonary tissue.6 Hematogenous dissemination occurs most commonly to the central nervous system (CNS). The skin is the most common site for extraneural dissemination and has been reported in 10% to 20% of patients.5 Cuta-neous cryptococcosis may be present for 2 to 8 months before development of systemic signs of infection, providing a window of opportunity for treatment of patients before fatal effects of dissemination occur.
Cutaneous manifestations of cryptococcosis are variable and can include papules, pustules, ulcerated nodules, molluscoid lesions, and cellulitis.5 Acneform lesions as well as lesions resembling erythema nodosum, Kaposi sarcoma, keratoacanthoma, and herpes virus infection also have been described in the literature. Lesions most commonly are located on the head and neck.5 The 2 types of histologic reactions seen in cryptococcal infections are gelatinous and granulomatous, which can occur individually or simultaneously. Gelatinous reactions show numerous spores with large polysaccharide capsules and little tissue reaction. In contrast, granulomatous reactions show pronounced tissue reactions consisting of histiocytes, giant cells, lymphoid cells, and fibroblasts with few spores, which are mainly found within giant cells and histiocytes and occasionally are free in the tissue.5Cryptococcus neoformans is a round to ovoid spore measuring 4 to 12 µm in gelatinous reactions and 2 to 4 µm in granulomatous reactions. Special stains are helpful for demonstration of Cryptococcus species (Table). Diagnosis of cryptococcosis also may be made by india ink staining, KOH preparation, or gram staining of a smear from the lesion. Culture on Sabouraud dextrose agar also can be performed to confirm the species. Detection of cryptococcal capsular polysaccharide antigen in serum and body fluids by latex agglutination test or enzyme-linked immunosorbent assay is useful for diagnostic as well as a prognostic evaluation.2 Because cutaneous cryptococcosis usually represents hematogenous dissemination from an internal focus, investigation to detect other organ involvement, especially the CNS, is mandatory.
The gold standard for treatment of severe cryptococcosis and CNS involvement is intravenous amphotericin B (0.7–1 mg/kg daily [total, 1–2 g]) with or without intravenous flucytosine (100 mg/kg daily) for 2 weeks,2 which is followed by treatment with oral fluconazole (400 mg daily) for 10 weeks. In cases with renal dysfunction, liposomal amphotericin B (4–6 mg/kg daily) is a better alternative. In other mild to moderate cases, only fluconazole or itraconazole (400 mg daily) for 6 to 12 months is sufficient for resolution of lesions, as in the case of our patient. Long-term suppressive treatment with fluconazole is essential in HIV-positive patients with low CD4 counts.2
Cryptococcosis in HIV-negative patients differs from HIV-positive patients. Central nervous system and extrapulmonary involvement is less common in HIV-negative patients. Response to treatment often is brisk and mortality is much lower in HIV-negative patients.10 Patients who are HIV negative exhibit a greater inflammatory response with a lesser organism load; however, an exaggerated immune response resulting in higher cerebrospinal fluid pleocytosis and protein rarely may result in fatal outcomes.11 Our case emphasizes the need for physicians to consider cryptococcosis in the differential diagnosis of neurologic and cutaneous manifestations in DM.
1. Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:2:36.89-36.91.
2. Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507-544, v-vi.
3. Thangakunam B, Christopher DJ, Kurian S, et al. Endogenous excess cortisol production and diabetes mellitus as predisposing factors for pulmonary cryptococcosis: a case report and literature review. Lung India. 2008;25:155-157.
4. Patel P, Ramanathan J, Kayser M, et al. Primary cutaneous cryptococcosis of the nose in an immunocompetent woman. J Am Acad Dermatol. 2000;43(2, pt 2):344-345.
5. Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32(5, pt 2):844-850.
6. Taneja J, Bhargava A, Loomba P, et al. Cryptococcal granulomas in an immunocompromised HIV-negative patient. Indian J Pathol Microbiol. 2008;51:553-555.
7. Hage CA, Wood KL, Winer-Muram HT, et al. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003;124:2395-2397.
8. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, et al. Cryptococcosis in human immunodeficiency virus-negative patients [published online ahead of print November 9, 2005]. Int J Infect Dis. 2006;10:72-78.
9. Shih CC, Chen YC, Chang SC, et al. Cryptococcal meningitis in non-HIV-infected patients. QJM. 2000;93:245-251.
10. Tunlayadechanont S, Viranuvatti K, Phuapradit P, et al. Cryptococcal meningitis in patients with non-HIV and HIV infection: a clinical study. Neurol J Southeast Asia. 1997;2:45-50.
11. Kushawaha A, Mobarakai N, Parikh N, et al. Cryptococcus neoformans meningitis in a diabetic patient–the perils of an overzealous immune response: a case report. Cases Journal. 2009;2:209.
1. Hay RJ, Ashbee HR. Mycology. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. 8th ed. Oxford, England: Wiley-Blackwell; 2010:2:36.89-36.91.
2. Chayakulkeeree M, Perfect JR. Cryptococcosis. Infect Dis Clin North Am. 2006;20:507-544, v-vi.
3. Thangakunam B, Christopher DJ, Kurian S, et al. Endogenous excess cortisol production and diabetes mellitus as predisposing factors for pulmonary cryptococcosis: a case report and literature review. Lung India. 2008;25:155-157.
4. Patel P, Ramanathan J, Kayser M, et al. Primary cutaneous cryptococcosis of the nose in an immunocompetent woman. J Am Acad Dermatol. 2000;43(2, pt 2):344-345.
5. Dimino-Emme L, Gurevitch AW. Cutaneous manifestations of disseminated cryptococcosis. J Am Acad Dermatol. 1995;32(5, pt 2):844-850.
6. Taneja J, Bhargava A, Loomba P, et al. Cryptococcal granulomas in an immunocompromised HIV-negative patient. Indian J Pathol Microbiol. 2008;51:553-555.
7. Hage CA, Wood KL, Winer-Muram HT, et al. Pulmonary cryptococcosis after initiation of anti-tumor necrosis factor-alpha therapy. Chest. 2003;124:2395-2397.
8. Kiertiburanakul S, Wirojtananugoon S, Pracharktam R, et al. Cryptococcosis in human immunodeficiency virus-negative patients [published online ahead of print November 9, 2005]. Int J Infect Dis. 2006;10:72-78.
9. Shih CC, Chen YC, Chang SC, et al. Cryptococcal meningitis in non-HIV-infected patients. QJM. 2000;93:245-251.
10. Tunlayadechanont S, Viranuvatti K, Phuapradit P, et al. Cryptococcal meningitis in patients with non-HIV and HIV infection: a clinical study. Neurol J Southeast Asia. 1997;2:45-50.
11. Kushawaha A, Mobarakai N, Parikh N, et al. Cryptococcus neoformans meningitis in a diabetic patient–the perils of an overzealous immune response: a case report. Cases Journal. 2009;2:209.
Practice Points
- Uncontrolled diabetes mellitus can be an important etiological factor for disseminated cryptococcosis.
- Cryptococcosis in human immunodeficiency virus–negative patients tends to have less severe clinical manifestations and better prognoses versus in patients who are human immunodeficiency virus positive.