User login
In reply: Postsurgical hypoparathyroidism is not primary hypoparathyroidism
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
In Reply: We thank Dr. Parmar and appreciate his important comments.
Regarding the difference between primary and secondary hypoparathyroidism, the definition varies among investigators. Some define primary hypoparathyroidism as a condition characterized by primary absence or deficiency of parathyroid hormone (PTH), which results in hypocalcemia and which can be congenital or acquired, including postsurgical hypoparathyroidism.1–4 In principle, this is similar to the classification of disorders affecting other endocrine glands as primary and secondary. For example, primary hypothyroidism refers to a state of low thyroid hormones resulting from impairment or loss of function of the thyroid gland itself, such as in Hashimoto thyroiditis, radioactive iodine therapy, or thyroidectomy, among others.5 We adopted this definition in our article. In contrast, secondary hypoparathyroidism is characterized by low PTH secretion in response to certain conditions that cause hypercalcemia. Non-PTH-mediated hypercalcemia is a more common term used to describe this state of secondary hypoparathyroidism.
Other investigators restrict the term “primary hypoparathyroidism” to nonacquired (congenital or hereditary) etiologies, while applying the term “secondary hypoparathyroidism” to acquired etiologies.6
Concerning the association between diabetes mellitus and hypomagnesemia, we agree that diabetes does not need to be uncontrolled to cause hypomagnesemia. However, the patient described in our article presented with severe hypomagnesemia (serum level 0.6 mg/dL), which is not commonly associated with diabetes. Most cases of hypomagnesemia in patients with type 2 diabetes mellitus are mild and asymptomatic, whereas severe manifestations including seizures, cardiac arrhythmias, and acute tetany are rarely encountered in clinical practice.7 Furthermore, numerous studies have shown a negative correlation between serum magnesium level and glycemic control.7–11 A recent study reported that plasma triglyceride and glucose levels are the main determinants of the plasma magnesium concentration in patients with type 2 diabetes.12
Our patient’s diabetes was uncontrolled, as evidenced by her hemoglobin A1c level of 9.7% and her random serum glucose level of 224 mg/dL. Therefore, it is more likely that “uncontrolled diabetes mellitus” (in addition to diuretic use) was the cause of her symptomatic severe hypomagnesemia rather than controlled diabetes mellitus.
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
- Mendes EM, Meireles-Brandão L, Meira C, Morais N, Ribeiro C, Guerra D. Primary hypoparathyroidism presenting as basal ganglia calcification secondary to extreme hypocalcemia. Clin Pract 2018; 8(1):1007. doi:10.4081/cp.2018.1007
- Vadiveloo T, Donnan PT, Leese GP. A population-based study of the epidemiology of chronic hypoparathyroidism. J Bone Miner Res 2018; 33(3):478-485. doi:10.1002/jbmr.3329
- Hendy GN, Cole DEC, Bastepe M. Hypoparathyroidism and pseudohypoparathyroidism. In: De Groot LJ, Chrousos G, Dungan K, et al, eds. Endotext [Internet], South Dartmouth (MA): MDText.com, Inc.; 2017. www.ncbi.nlm.nih.gov/books/NBK279165. Accessed August 20, 2018.
- Rosa RG, Barros AJ, de Lima AR, et al. Mood disorder as a manifestation of primary hypoparathyroidism: a case report. J Med Case Rep 2014; 8:326. doi:10.1186/1752-1947-8-326
- Almandoz JP, Gharib H. Hypothyroidism: etiology, diagnosis, and management. Med Clin North Am 2012; 96(2):203–221. doi:10.1016/j.mcna.2012.01.005
- Fouda UM, Fouda RM, Ammar HM, Salem M, Darouti ME. Impetigo herpetiformis during the puerperium triggered by secondary hypoparathyroidism: a case report. Cases J 2009; 2:9338. doi:10.1186/1757-1626-2-9338
- Tosiello L. Hypomagnesemia and diabetes mellitus. A review of clinical implications. Arch Intern Med 1996; 156(11):1143–1148. pmid: 8639008
- Pham PC, Pham PM, Pham PA, et al. Lower serum magnesium levels are associated with more rapid decline of renal function in patients with diabetes mellitus type 2. Clin Nephrol 2005; 63(6):429–436. pmid:15960144
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20(1):3–17. doi:10.1177/0885066604271539
- Resnick LM, Altura BT, Gupta RK, Laragh JH, Alderman MH, Altura BM. Intracellular and extracellular magnesium depletion in type 2 (non-insulin-independent) diabetes mellitus. Diabetologia 1993; 36(8):767–770. pmid:8405745
- Pun KK, Ho PW. Subclinical hyponatremia, hyperkalemia and hypomagnesemia in patients with poorly controlled diabetes mellitus. Diabetes Res Clin Pract 1989; 7(3)163–167. pmid: 2605984
- Kurstjens S, de Baaij JH, Bouras H, Bindels RJ, Tack CJ, Hoenderop JG. Determinants of hypomagnesemia in patients with type 2 diabetes mellitus. Eur J Endocrinol 2017; 176(1):11–19. doi:10.1530/EJE-16-0517
A 67-year-old woman with bilateral hand numbness
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
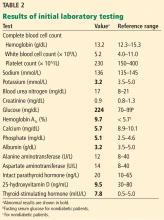
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
A 67-year-old woman presents to the emergency department after 8 weeks of progressive numbness and tingling in both hands, involving all fingers. The numbness has increased in severity in the last 3 days. She also has occasional numbness around her mouth. She reports no numbness in her feet.
She says she underwent thyroid surgery twice for thyroid cancer 10 years ago. Her medical history also includes type 2 diabetes mellitus (diagnosed 1 year ago), hypertension, dyslipidemia, and diastolic heart failure (diagnosed 5 years ago).
Her current medications are:
- Metformin 1 g twice a day
- Candesartan 16 mg once a day
- Atorvastatin 20 mg once a day
- Furosemide 40 mg twice a day
- Levothyroxine 100 μg per day
- Calcium carbonate 1,500 mg twice a day
- A vitamin D tablet twice a day, which she has not taken for the last 2 months.
She admits she has not been taking her medications regularly because she has been feeling depressed.
On physical examination, she is alert and oriented but appears anxious. She is not in respiratory distress. Her blood pressure is 150/90 mm Hg and her pulse is 92 beats per minute and regular. There is a thyroidectomy scar on the anterior neck. Her jugular venous pressure is not elevated. Her heart sounds are normal without extra sounds. She has no pulmonary rales and no lower-extremity edema.
The Phalen test and Tinel test for carpal tunnel syndrome are negative in both hands. Using a Katz hand diagram, the patient reports tingling and numbness in all fingers, both palms, and the dorsum of both hands. Tapping the area over the facial nerve does not elicit twitching of the facial muscles (ie, no Chvostek sign), but compression of the upper arm elicits carpal spasm (ie, positive Trousseau sign). There is no evidence of motor weakness in her hands. The rest of the physical examination is unremarkable.
POSSIBLE CAUSES OF NUMBNESS
1. Based on the initial evaluation, which of the following is the most likely cause of our patient’s bilateral hand numbness?
- Hypocalcemia due to primary hypoparathyroidism
- Carpal tunnel syndrome due to primary hypothyroidism
- Diabetic peripheral neuropathy
- Vitamin B12 deficiency due to metformin
- Hypocalcemia due to low serum calcitonin
All the conditions above except low serum calcitonin can cause bilateral hand paresthesia. Our patient most likely has hypocalcemia due to primary hypoparathyroidism.
Hypocalcemia
In our patient, bilateral hand numbness and perioral numbness after stopping vitamin D and a positive Trousseau sign strongly suggest hypocalcemia. The classic physical findings in patients with hypocalcemia are the Trousseau sign and the Chvostek sign. The Trousseau sign is elicited by inflating a blood pressure cuff above the systolic blood pressure for 3 minutes and observing for ischemia-induced carpopedal spasm, wrist and metacarpophalangeal joint flexion, thumb adduction, and interphalangeal joint extension. The Chvostek sign is elicited by tapping over the area of the facial nerve below the zygoma in front of the tragus, resulting in ipsilateral twitching of facial muscles.
Although the Trousseau sign is more sensitive and specific than the Chvostek sign, neither is pathognomonic for hypocalcemia.1 The Chvostek sign has been reported to be negative in 30% of patients with hypocalcemia and positive in 10% of normocalcemic individuals.1 The Trousseau sign, however, is present in 94% of hypocalcemic patients vs 1% of normocalcemic individuals.2
Primary hypoparathyroidism secondary to thyroidectomy. Postsurgical hypoparathyroidism is the most common cause of primary hypoparathyroidism. It results from ischemic injury or accidental removal of the parathyroid glands during anterior neck surgery.3,4 The consequent hypocalcemia can be transient, intermittent, or permanent. Permanent postsurgical hypoparathyroidism is defined as persistent hypocalcemia with insufficient parathyroid hormone (PTH) for more than 12 months after neck surgery; however, some consider 6 months to be enough to define the condition.5–7
The incidence of postsurgical hypoparathyroidism varies considerably with the extent of thyroid surgery and the experience of the surgeon.6,8 In the hands of experienced surgeons, permanent hypoparathyroidism occurs in fewer than 1% of patients after total thyroidectomy, whereas the rate may be higher than 6% with less-experienced surgeons.5,9 Other risk factors for postsurgical hypoparathyroidism include female sex, autoimmune thyroid disease, pregnancy, and lactation.5
Pseudohypoparathyroidism is a group of disorders characterized by renal resistance to PTH, leading to hypocalcemia, hyperphosphatemia, and elevated serum PTH. It is also associated with phenotypic features such as short stature and short fourth metacarpal bones.
Calcitonin deficiency. Calcitonin is a polypeptide hormone secreted from the parafollicular (C) cells of the thyroid gland. After total thyroidectomy, calcitonin levels are expected to be reduced. However, the role of calcitonin in humans is unclear. One study has shown that calcitonin is possibly a vestigial hormone, given that no calcitonin-related disorders (excess or deficiency) have been reported in humans.10
Carpal tunnel syndrome due to hypothyroidism
Our patient also could have primary hypothyroidism as a result of thyroidectomy. Hypothyroidism can cause bilateral hand numbness due to carpal tunnel syndrome, which is mediated by mucopolysaccharide deposition and synovial membrane swelling.11 One study reported that 29% of patients with hypothyroidism had carpal tunnel syndrome.12 Symptoms of carpal tunnel syndrome in hypothyroid patients may occur despite thyroid replacement therapy.13
Carpal tunnel syndrome is a clinical diagnosis. Patients usually experience hand paresthesia in the distribution of the median nerve. Provocative physical tests for carpal tunnel syndrome include the Tinel test, the Phalen test, and the Katz hand diagram, which is considered the best of the 3 tests.14,15 Based on how the patient marks the location and type of symptoms on the diagram, carpal tunnel syndrome is rated as classic, probable, possible, or unlikely (Table 1).14,16,17 The sensitivity of a classic or probable diagram ranges from 64% to 80%, while the specificity ranges from 73% to 90%.14,15
Carpal tunnel syndrome is less likely to be the cause of our patient’s symptoms, as her Katz hand diagram indicates only “possible” carpal tunnel syndrome. Her perioral numbness and positive Trousseau sign make hypocalcemia a more likely cause.
Diabetic peripheral neuropathy
Sensory peripheral neuropathy is a recognized complication of diabetes mellitus. However, neuropathy in diabetic patients most commonly manifests initially as distal symmetrical ascending neuropathy starting in the lower extremities.18 Therefore, diabetic peripheral neuropathy is less likely in this patient since her symptoms are limited to her hands.
Vitamin B12 deficiency
Metformin-induced vitamin B12 deficiency is another possible cause of peripheral neuropathy. It might be secondary to metformin-induced changes in intrinsic factor levels and small-intestine motility with resultant bacterial overgrowth, as well as inhibition of vitamin B12 absorption in the terminal ileum.19
However, metformin-induced vitamin B12 deficiency is not the most likely cause of our patient’s neuropathy, since she has been taking this drug for only 1 year. Vitamin B12 deficiency with consequent peripheral neuropathy is more likely in patients taking metformin in high doses for 10 or more years.20
Laboratory results and electrocardiography
Table 2 shows the patient’s initial laboratory results. Of note, her serum calcium level is 5.7 mg/dL (reference range 8.9–10.1). Electrocardiography in the emergency department shows:
- Prolonged PR interval (23 msec)
- Wide QRS complexes (13 msec)
- Flat T waves
- Prolonged corrected QT interval (475 msec)
- Occasional premature ventricular complexes.
CLINICAL MANIFESTATIONS OF HYPOCALCEMIA
2. Which of the following is not a manifestation of hypocalcemia?
- Tonic-clonic seizures
- Cyanosis
- Cardiac ventricular arrhythmias
- Acute pancreatitis
- Depression
Hypocalcemia can cause a wide range of clinical manifestations (Table 3), the extent and severity of which depend on the severity of hypocalcemia and how quickly it develops. The more acute the hypocalcemia, the more severe the manifestations.21
Tetany can cause seizures
Hypocalcemia is characterized by neuromuscular hyperexcitability, manifested clinically by tetany.22 Manifestations of tetany are numerous and include acral paresthesia, perioral numbness, muscle cramps, carpopedal spasm, and seizures. Tetany is the hallmark of hypocalcemia regardless of etiology. However, certain causes are associated with peculiar clinical manifestations. For example, chronic primary hypoparathyroidism may be associated with basal ganglia calcifications that can result in parkinsonism, other extrapyramidal disorders, and dementia (Table 4).6
Airway spasm can be fatal
A serious manifestation of acute severe hypocalcemia is spasm of the glottis muscles, which may cause cyanosis and, if untreated, death.21
Ventricular arrhythmias
Another potential fatal complication of acute severe hypocalcemia is polymorphic ventricular tachycardia due to prolongation of the QT interval, which is readily identified with electrocardiography.23
Hypocalcemia does not cause pancreatitis
Hypercalcemia, rather than hypocalcemia, may cause acute pancreatitis.24 Conversely, acute pancreatitis may cause hypocalcemia due to precipitation of calcium in the abdominal cavity.25
Psychiatric manifestations
In addition to depression, hypocalcemia is associated with psychiatric manifestations including anxiety, confusion, and emotional instability.
STEPS TO DIAGNOSIS OF HYPOCALCEMIA
First step: Confirm true hypocalcemia
Calcium circulates in the blood in 3 forms: bound to albumin (40% to 45%), bound to anions (10% to 15%), and free (ionized) (45%). Although ionized calcium is the active form, most laboratories report total serum calcium.
Since changes in serum albumin concentration affect the total serum calcium level, it is imperative to correct the measured serum calcium to the serum albumin concentration. Each 1-g/dL decrease in serum albumin lowers the total serum calcium by 0.8 mg/dL. Thus:
Corrected serum calcium (mg/dL) =
measured total serum calcium (mg/dL) +
0.8 (4 − serum albumin [g/dL]).
If the patient’s serum calcium level remains low when corrected for serum albumin, he or she has true hypocalcemia, which implies a low ionized serum calcium. Conversely, pseudohypocalcemia means that the measured calcium level is low but the corrected serum calcium is normal.
Using this formula, our patient’s corrected calcium level is calculated as 5.7 + 0.8 (4 – 3.2) = 6.3 mg/dL, indicating true hypocalcemia.
PHOSPHATE IS OFTEN HIGH WHEN CALCIUM IS LOW
In addition to hypocalcemia, our patient has an elevated phosphate level (Table 2).
3. Which of the following hypocalcemic disorders is not associated with hyperphosphatemia?
- End-stage renal disease
- Primary hypoparathyroidism
- Pseudohypoparathyroidism
- Vitamin D3 deficiency
- Rhabdomyolysis
Vitamin D deficiency is not associated with hyperphosphatemia.
Second step in evaluating hypocalcemia: Check phosphate, magnesium, creatinine
The major causes of hypocalcemia can be categorized according to the serum phosphate level: high vs normal or low (Table 5).
High-phosphate, low-calcium states. In the absence of concurrent end-stage renal disease and an excessive phosphate load, primary hypoparathyroidism is the most likely cause of hypocalcemia associated with hyperphosphatemia.
PTH increases serum ionized calcium by26,27:
- Increasing bone resorption
- Increasing reabsorption of calcium from the distal renal tubules
- Increasing the activity of 1-alpha-hydroxylase, responsible for conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 (the most biologically active vitamin D metabolite); 1,25-dihydroxyvitamin D increases the absorption of calcium and phosphate from the intestine.
Conversely, PTH decreases reabsorption of phosphate from proximal renal tubules, resulting in hypophosphatemia. Therefore, low serum PTH (primary hypoparathyroidism) or a PTH-resistant state (pseudohypoparathyroidism) results in hypocalcemia and hyperphosphatemia.26,27
Both end-stage renal disease and rhabdomyolysis are associated with high serum phosphate levels. The kidney normally excretes excess dietary phosphate to maintain phosphate homeostasis; however, this is impaired in end-stage renal disease, leading to hyperphosphatemia. In rhabdomyolysis, it is mainly the transcellular shift of phosphate into the extracellular space from myocyte injury that raises phosphate levels.
Normal- or low-phosphate, low calcium states. Hypocalcemia can also result from vitamin D deficiency, but this cause is associated with a low or normal serum phosphate level. In such cases, hypocalcemia causes secondary hyperparathyroidism with consequent renal phosphate loss and, thus, hypophosphatemia.27
Third step: Check serum intact PTH and 25-hydroxyvitamin D levels
Hypocalcemia stimulates secretion of PTH. Therefore, hypocalcemia with elevated serum PTH is caused by disorders that do not impair PTH secretion, including chronic renal failure and vitamin D deficiency (Table 5). Conversely, hypocalcemia with low or normal serum PTH levels suggests primary hypoparathyroidism.
Our patient’s serum PTH level is 20 ng/mL, which is within the reference range. This does not discount the diagnosis of primary hypoparathyroidism. Although most patients with primary hypoparathyroidism have low or undetectable serum PTH levels, some have normal PTH levels if some degree of PTH production is preserved.5,7,28–30 In these patients, the remaining functioning parathyroid tissue is not enough to maintain a normal serum calcium level, resulting in hypocalcemia. As a result, hypocalcemia stimulates the remaining parathyroid tissue to its maximum output, producing PTH levels usually within the lower or middle-normal range.30 In such patients, the terms parathyroid insufficiency and relative primary hypoparathyroidism are more precise than primary hypoparathyroidism.
Postsurgical hypoparathyroidism with an inappropriately normal PTH level is usually seen in patients with disorders that impair intestinal calcium absorption or bone resorption.31 In our patient’s case, the “normal” serum PTH level is likely due to maximal stimulation of remaining functioning parathyroid tissue by severe hypocalcemia, which is a result of her discontinuation of calcium and calcitriol therapy and her vitamin D deficiency.
CASE RESUMED: NO RESPONSE TO INTRAVENOUS CALCIUM GLUCONATE
The patient is given 2 10-mL ampules of 10% calcium gluconate diluted in 100 mL of 5% dextrose in water over 20 minutes intravenously. Electrocardiographic monitoring is continued. Two hours later, her measured serum calcium is only 5.8 mg/dL, with no improvement in her symptoms.
A continuous infusion of calcium gluconate is started: 12 ampules of calcium gluconate are added to 380 mL of 5% dextrose in water and infused at 40 mL/hour (infused rate of elemental calcium = 1.3 mg/kg/hour); 3 hours later, her measured serum calcium level is still only 5.8 mg//dL; at 6 hours it is 5.9 mg/dL, and her symptoms have not improved.
4. Which of the following is the most appropriate next step?
- Change the calcium gluconate to calcium chloride
- Increase the infusion rate to 1.5 mg of elemental calcium/kg/hour
- Give a bolus of 2 10-mL ampules of 10% calcium gluconate intravenously over 1 minute
- Give additional oral calcium tablets
- Check the serum magnesium level
Treatment of hypocalcemia can involve intravenous or oral calcium therapy.
Intravenous calcium is indicated for patients with any of the following6,32:
- Moderate to severe neuromuscular irritability (eg, acral paresthesia, carpopedal spasm, prolonged QT interval, seizures, laryngospasm, bronchospasm)
- Acute hypocalcemia with corrected serum calcium level less than 7.6 mg/dL, even if the patient is asymptomatic
- Cardiac failure.
One 10-mL ampule of 10% calcium gluconate contains 93 mg of elemental calcium; 1 or 2 ampules are typically diluted in 50 to 100 mL of 5% dextrose in water and infused slowly over 15 to 20 minutes. Rapid administration of intravenous calcium is contraindicated, as it may produce cardiac arrhythmias and possibly cardiac arrest. Therefore, intravenous calcium should be given slowly while continuing electrocardiographic monitoring.33
Since the effect of 1 ampule of calcium gluconate lasts only 2 to 3 hours, most patients with symptomatic hypocalcemia require continuous intravenous calcium infusion. The recommended dose of infused elemental calcium is 0.5 to 1.5 mg/kg/hour.34 Several ampules are added to 500 to 1,000 mL of 5% dextrose in water or 0.9% normal saline and infused at a rate appropriate for the patient’s corrected calcium and symptoms.
Oral calcium and vitamin D supplements can be given initially to patients with a corrected serum calcium level of 7.6 mg/dL or greater, with or without mild symptoms, if they can tolerate oral intake. However, this is not the treatment of choice for resistant acute hypocalcemia, as in this case.
Calcium chloride has no advantages over calcium gluconate. Further, it can be associated with local irritation and may result in tissue necrosis if extravasation occurs.35
Increasing the infusion rate of calcium gluconate to the maximum recommended dose may improve the patient’s ionized calcium level and symptoms somewhat. However, it is not the best option for this patient, given that she did not respond to 2 ampules of calcium gluconate followed by continuous infusion of 1.3 mg/kg/hour for 6 hours.
Calcium gluconate bolus. Similarly, giving the patient an additional 2 ampules of calcium gluconate over 1 minute would not be recommended, as rapid administration of intravenous calcium gluconate (eg, over 1 minute) is contraindicated.
Check magnesium
If hypocalcemia persists despite intravenous calcium therapy, as in our patient, further investigation or action is required. An important cause of persistent hypocalcemia is severe hypomagnesemia. Severe hypomagnesemia (serum magnesium < 0.8 mg/dL) causes resistant hypocalcemia by several mechanisms:
- Inducing PTH resistance32,36,37
- Decreasing PTH secretion32,36
- Decreasing calcitriol production.
The decrease in calcitriol production is a direct effect of hypomagnesemia, but it is also an indirect effect of low PTH secretion, which inhibits the enzyme 1-alpha-hydroxylase. Thus, conversion of 25-hydroxyvitamin D3 to calcitriol is impaired, leading to low calcitriol production.
Our patient could have hypomagnesemia due to furosemide use and uncontrolled diabetes mellitus. Hypocalcemia resistant to calcium therapy may occasionally respond to magnesium therapy even if the serum magnesium level is normal. This may be due to depleted intracellular magnesium salt levels.6,38 Rarely, severe hypermagnesemia can also be associated with hypocalcemia due to inhibition of PTH secretion.37,39
CASE RESUMED
Our patient’s serum magnesium level is 0.6 mg/dL (reference range 1.7–2.4 mg/dL). She is given 2 g of magnesium sulfate in 60 mL of 0.9% normal saline infused over 1 hour, followed by a continuous infusion of magnesium sulfate (12 g diluted in 250 mL of 0.9% normal saline, infused over 24 hours). On repeat testing 4 hours later, her serum magnesium level is 0.7 mg/dL, and at 8 hours later it is 0.9 mg/dL. She is subsequently started on oral magnesium oxide 600 mg per day. The magnesium sulfate infusion is continued for another 24 hours.
PREVENTING HYPERCALCIURIA
Patients with low PTH (primary hypoparathyroidism) may have hypercalciuria due to decreased renal tubular calcium reabsorption. Two important measures can minimize hypercalciuria in such patients:
- Keeping the serum calcium level in the low-normal range4,5,40
- Giving a thiazide diuretic (eg, hydrochlorothiazide 12.5–50 mg daily) with a low-salt diet.41,42
A thiazide diuretic is usually started once the 24-hour urine calcium reaches 250 mg.6 Thiazides are thought to enhance both proximal and distal renal tubular calcium reabsorption.43,44
PRIMARY HYPOPARATHYROIDISM: LONG-TERM MANAGEMENT
Long-term management of primary hypoparathyroidism requires calcium and vitamin D supplementation.
Calcium supplements. The most commonly prescribed calcium preparations are calcium carbonate and calcium citrate (containing 40% and 20% elemental calcium, respectively). Calcium carbonate, which is less expensive than calcium citrate, binds with phosphate intake and requires an acidic environment for absorption, and so it is better absorbed when taken with meals. Because calcium citrate does not require an acidic environment for absorption, it is the calcium preparation of choice for patients on proton pump inhibitors, or patients with achlorhydria or constipation.45 Calcium doses vary widely, with most hypoparathyroid patients requiring 1 to 2 g of elemental calcium daily.6
Vitamin D supplements. To promote intestinal absorption, calcium is combined with vitamin D in a fixed-dose preparation given in divided doses.46 Calcitriol (1,25-dihydroxyvitamin D3) is the most active metabolite of vitamin D, with rapid onset and offset of action, and it is the preferred form of vitamin D therapy for patients with hypoparathyroidism. If calcitriol is not available or is not affordable, alphacalcidol (1-alpha-hydroxyvitamin D3) is another option. This is a synthetic analogue of vitamin D that is already hyroxylated at the C1 position. After oral intake, it is hydroxylated in the liver to form calcitriol.
Since renal production of calcitriol is PTH-dependent, in hypoparathyroidism the conversion of 25-hydroxyvitamin D3 to calcitriol is limited. Therefore, vitamin D3 (cholecalciferol) and vitamin D2 (ergocalciferol) are not the preferred forms of vitamin D for such patients. However, either can be added to calcitriol, as they may have extraskeletal benefits.7
CASE CONCLUDED
Our patient presented with primary parathyroid insufficiency associated with vitamin D deficiency. Therefore, in addition to calcitriol and calcium combined with vitamin D in a fixed-dose preparation, her management included vitamin D3 for her vitamin D deficiency.
She was discharged on these medications. At a follow-up visit 3 weeks later, her measured serum calcium level was 8.6 mg/dL. She reported gradual resolution of her symptoms. She was also referred to a psychiatrist for her depression.
TAKE-HOME POINTS
- Hypocalcemia causes neuromuscular excitability, manifested clinically by tetany.
- Common causes of hypocalcemia include vitamin D deficiency, hypomagnesemia, renal failure, and primary hypoparathyroidism.
- The first step in evaluating hypocalcemia is to correct the measured serum calcium to the serum albumin concentration.
- Laboratory testing for hypocalcemia should include serum phosphorus, magnesium, creatinine, PTH, and 25-hydroxyvitamin D3.
- Primary hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and low serum PTH.
- Moderate to severe manifestations of hypo-
calcemia and acute hypocalcemia (< 7.6 mg/dL), even if asymptomatic, warrant intravenous calcium therapy. - Correction of hypomagnesemia is essential to treat hypocalcemia, especially if resistant to intravenous calcium therapy.
- The goal of chronic management of primary hypoparathyroidism includes correcting the serum calcium level to a low-normal range, the serum phosphorus level to an upper-normal range, and prevention of hypercalciuria.
Acknowledgments: The authors wish to thank Mr. Michael Edward Tierney of the School of Medicine, University of Sydney, Australia, for his linguistic editing of the manuscript.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.
- Jesus JE, Landry A. Images in clinical medicine. Chvostek’s and Trousseau’s signs. N Engl J Med 2012; 367:e15.
- Urbano FL. Signs of hypocalcemia: Chvostek’s and Trousseau’s. Hosp Physician 2000; 36:43–45.
- Chisthi MM, Nair RS, Kuttanchettiyar KG, Yadev I. Mechanisms behind post-thyroidectomy hypocalcemia: interplay of calcitonin, parathormone, and albumin—a prospective study. J Invest Surg 2017; 30:217–225.
- Shoback DM, Bilezikian JP, Costa AG, et al. Presentation of hypoparathyroidism: etiologies and clinical features. J Clin Endocrinol Metab 2016; 101:2300–2312.
- Stack BC Jr, Bimston DN, Bodenner DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: postoperative hypoparathyroidism—definitions and management. Endocr Pract 2015; 21:674–685.
- Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med 2008; 359:391–403.
- Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017; 7:172.
- Coimbra C, Monteiro F, Oliveira P, Ribeiro L, de Almeida MG, Condé A. Hypoparathyroidism following thyroidectomy: predictive factors. Acta Otorrinolaringol Esp 2017; 68:106–111.
- Thomusch O, Machens A, Sekulla C, Ukkat J, Brauckhoff M, Dralle H. The impact of surgical technique on postoperative hypoparathyroidism in bilateral thyroid surgery: a multivariate analysis of 5846 consecutive patients. Surgery 2003; 133:180–185.
- Hirsch PF, Lester GE, Talmage RV. Calcitonin, an enigmatic hormone: does it have a function? J Musculoskelet Neuronal Interact 2001; 1:299–305.
- Karne SS, Bhalerao NS. Carpal tunnel syndrome in hypothyroidism. J Clin Diagn Res 2016; 10:OC36–OC38.
- Duyff RF, Van den Bosch J, Laman DM, van Loon BJ, Linssen WH. Neuromuscular findings in thyroid dysfunction: a prospective clinical and electrodiagnostic study. J Neurol Neurosurg Psychiatry 2000; 68:750–755.
- Palumbo CF, Szabo RM, Olmsted SL. The effects of hypothyroidism and thyroid replacement on the development of carpal tunnel syndrome. J Hand Surg Am 2000; 25:734–739.
- Katz JN, Stirrat CR, Larson MG, Fossel AH, Eaton HM, Liang MH. A self-administered hand symptom diagram for the diagnosis and epidemiologic study of carpal tunnel syndrome. J Rheumatol 1990; 17:1495–1498.
- Katz JN, Stirrat CR. A self-administered hand diagram for the diagnosis of carpal tunnel syndrome. J Hand Surg Am 1990; 15:360–363.
- Calfee RP, Dale AM, Ryan D, Descatha A, Franzblau A, Evanoff B. Performance of simplified scoring systems for hand diagrams in carpal tunnel syndrome screening. J Hand Surg Am 2012; 37:10–17.
- D’Arcy CA, McGee S. The rational clinical examination. Does this patient have carpal tunnel syndrome? JAMA 2000; 283:3110–3117.
- Marchettini P, Lacerenza M, Mauri E, Marangoni C. Painful peripheral neuropathies. Curr Neuropharmacol 2006; 4:175–181.
- Kibirige D, Mwebaze R. Vitamin B12 deficiency among patients with diabetes mellitus: is routine screening and supplementation justified? J Diabetes Metab Disord 2013;12:17.
- Akinlade KS, Agbebaku SO, Rahamon SK, Balogun WO. Vitamin B12 levels in patients with type 2 diabetes mellitus on metformin. Ann Ib Postgrad Med 2015; 13:79–83.
- Tohme JF, Bilezikian JP. Hypocalcemic emergencies. Endocrinol Metab Clin North Am 1993; 22:363–375.
- Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain 1991; 114:527–540.
- Benoit SR, Mendelsohn AB, Nourjah P, Staffa JA, Graham DJ. Risk factors for prolonged QTc among US adults: Third National Health and Nutrition Examination Survey. Eur J Cardiovasc Prev Rehabil 2005; 12:363–368.
- Khoo TK, Vege SS, Abu-Lebdeh HS, Ryu E, Nadeem S, Wermers RA. Acute pancreatitis in primary hyperparathyroidism: a population-based study. J Clin Endocrinol Metab 2009; 94:2115–2118.
- McKay C, Beastall GH, Imrie CW, Baxter JN. Circulating intact parathyroid hormone levels in acute pancreatitis. Br J Surg 1994; 81:357–360.
- Talmage RV, Mobley HT. Calcium homeostasis: reassessment of the actions of parathyroid hormone. Gen Comp Endocrinol 2008; 156:1–8.
- Friedman PA, Gesek FA. Calcium transport in renal epithelial cells. Am J Physiol 1993; 264:F181–F198.
- Jensen PV, Jelstrup SM, Homøe P. Long-term outcomes after total thyroidectomy. Dan Med J 2015; 62:A5156.
- Ritter K, Elfenbein D, Schneider DF, Chen H, Sippel RS. Hypoparathyroidism after total thyroidectomy: incidence and resolution. J Surg Res 2015; 197:348–353.
- Promberger R, Ott J, Kober F, Karik M, Freissmuth M, Hermann M. Normal parathyroid hormone levels do not exclude permanent hypoparathyroidism after thyroidectomy. Thyroid 2011; 21:145–150.
- Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surgery 2015; 4:82–90.
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ 2008; 336:1298–1302.
- Tohme JF, Bilezikian JP. Diagnosis and treatment of hypocalcemic emergencies. Endocrinologist 1996; 6:10–18.
- Carroll R, Matfin G. Endocrine and metabolic emergencies: hypocalcaemia. Ther Adv Endocrinol Metab 2010; 1:29–33.
- Kim MP, Raho VJ, Mak J, Kaynar AM. Skin and soft tissue necrosis from calcium chloride in a deicer. J Emerg Med 2007; 32:41–44.
- Tong GM, Rude RK. Magnesium deficiency in critical illness. J Intensive Care Med 2005; 20:3–17.
- Cholst IN, Steinberg SF, Tropper PJ, Fox HE, Segre GV, Bilezikian JP. The influence of hypermagnesemia on serum calcium and parathyroid hormone levels in human subjects. N Engl J Med 1984; 310:1221–1225.
- Ryzen E, Nelson TA, Rude RK. Low blood mononuclear cell magnesium content and hypocalcemia in normomagnesemic patients. West J Med 1987; 147:549–553.
- Koontz SL, Friedman SA, Schwartz ML. Symptomatic hypocalcemia after tocolytic therapy with magnesium sulfate and nifedipine. Am J Obstet Gynecol 2004; 190:1773–1776.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Porter RH, Cox BG, Heaney D, Hostetter TH, Stinebaugh BJ, Suki WN. Treatment of hypoparathyroid patients with chlorthalidone. N Engl J Med 1978; 298:577–581.
- Clarke BL, Brown EM, Collins MT, et al. Epidemiology and diagnosis of hypoparathyroidism. J Clin Endocrinol Metab 2016; 101:2284–2299.
- Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 2005; 115:1651–1658.
- Costanzo LS. Localization of diuretic action in microperfused rat distal tubules: Ca and Na transport. Am J Physiol 1985; 248:F527–F535.
- Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab 2016; 101:2273–2283.
- Scotti A, Bianchini C, Abbiati G, Marzo A. Absorption of calcium administered alone or in fixed combination with vitamin D to healthy volunteers. Arzneimittelforschung 2001; 51:493–500.