User login
One lab finding, 2 vastly different causes
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
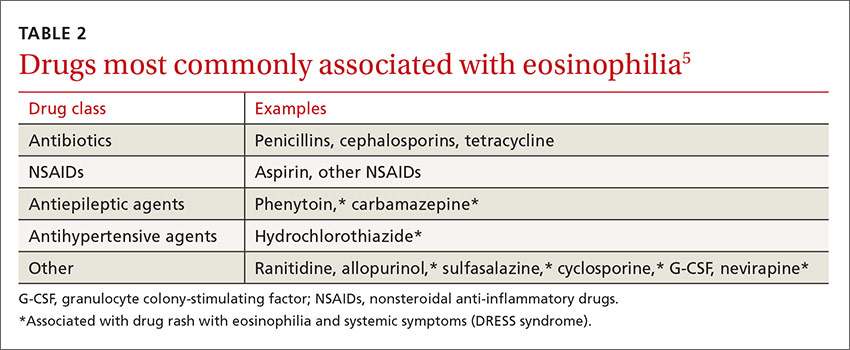
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3
Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3
Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
CASE 1
A 13-month-old boy who was recently adopted from Ethiopia presented to a primary care physician with a 3-week history of bloody diarrhea accompanied by flatulence and bloating. Stool cultures were positive for Campylobacter and Shigella. He was prescribed azithromycin but saw only moderate improvement. He was then referred to the Infectious Diseases Department. Neonatal, pregnancy, and immunization histories were unknown and a review of systems was unremarkable. On exam, the child looked well; he weighed 9.6 kg (15th percentile), was 69.5 cm long (<3rd percentile), and his head circumference was 45 cm (10th percentile). Head and neck, cardiorespiratory, and abdominal examinations were unremarkable.
A complete blood count (CBC) showed an elevated white blood cell (WBC) count of 26 x 109/L (normal: 4-10 x 109/L) with predominant eosinophilia (10.4 x 109/L or 40.1% of WBCs; normal: <0.45 x 109/L or 0%-8%). Hemoglobin and platelets were within normal limits. Stool testing for ova and parasites showed Strongyloides stercoralis larvae. Strongyloides serology was negative and Filaria serology was equivocal.
CASE 2
A 15-year-old boy was assessed for a 3-week history of fever and eosinophilia. He had enlarged cervical lymph nodes, a new rash, and had lost 4 pounds. He denied gastrointestinal symptoms, dyspnea, headaches, or chest pain. His past medical and family histories were unremarkable and he reported no drug use or allergies. He had traveled to Cuba with his family for 15 days 3 months prior to presentation. He recalled diarrhea while traveling, which resolved spontaneously. He and his family had traveled “off the beaten track,” eating foods prepared at local establishments and swimming in local rivers. He received pre-travel immunizations.
On examination, he appeared unwell, though his vital signs were normal. He had diffuse lymphadenopathy and a petechial rash on his chest, back, upper buttocks, legs, and feet. Cardiorespiratory and abdominal examinations were unremarkable. A CBC revealed an elevated WBC count of 76.9 x 109/L with predominant eosinophilia (71.5 x 109/L or 92% of WBCs). Hemoglobin, platelets, electrolytes, and liver function tests were normal. The patient was referred to a tertiary care center and was admitted to the hospital. Stool testing for ova and parasites, as well as serology for parasitic infections, was negative. A bone marrow aspiration and biopsy were performed and revealed the diagnosis of acute lymphoblastic leukemia (ALL).
DISCUSSION
These 2 cases highlight how the presentation of eosinophilia can vary and how important it is to maintain a broad differential diagnosis (TABLE 11-4). Causes of eosinophilia are numerous and can be divided into 3 categories: primary, secondary, and idiopathic.1,5 Hematologic malignancy, where eosinophilia is clonal, is an example of a primary etiology. Causes of secondary eosinophilia include infectious diseases, drugs (TABLE 25), autoimmune disorders, and allergic conditions. Prolonged eosinophilia that is >3 x 109/L is associated with end-organ damage. Dermatologic, pulmonary, gastrointestinal, and cardiac involvement is most common.2

Eosinophilia associated with parasitic infection

In returning travelers and international adoptees, multicellular helminthic parasites are the most common causes of eosinophilia, with eosinophilia occurring during tissue migration or penetration.1,3
Schistosomiasis is a chronic parasitic infection of the human vascular system. It is transmitted by contact with contaminated fresh water, where cercariae penetrate the skin. High prevalence areas include Africa and Southeast Asia. Acute infection can result in Katayama fever—a febrile illness with prominent eosinophilia that occurs 4 to 7 weeks after exposure.4 Diagnosis is primarily clinical with appropriate epidemiology, as serology may be negative early in infection. Praziquantel is the treatment of choice, though dosing varies by species, so expert consultation should be considered.
Soil-transmitted helminths, such as Ascaris (Ascaris lumbricoides), whipworm (Trichuris trichiura), and hookworm (Ancyclostoma duodenale and Necator americanus), can also cause eosinophilia during larval tissue migration. Following infection by ingestion or skin penetration, an acute respiratory illness, termed Löffler’s syndrome, can develop with associated eosinophilia.1 Once the helminths reach the adult stage, eosinophilia subsides. Patients are most commonly treated with albendazole 400 mg orally for 3 days.4
Fascioliasis is common in sheep-rearing areas. Humans are infected through ingestion of aquatic plants (eg, watercress). Parasitic migration through the duodenal wall and liver parenchyma can lead to fever, right upper quadrant pain, and eosinophilia. The incubation period is 6 to 12 weeks. Diagnosis during acute infection is by serology.4
Filarial infections, eg lymphatic filariasis, loiasis, and onchocerciasis, can also cause eosinophilia. The rise in eosinophils can be triggered by either the adult worms or circulating microfilariae.4 Treatment of fascioliasis and filarial infections varies and expert consultation is recommended.
Eosinophilia associated with primary hematologic malignancy
Eosinophilia is a rare presentation of hematologic malignancy. Acute myeloid leukemia, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia, and myeloproliferative disorders have all been associated with eosinophilia. Hepatosplenomegaly, generalized lymphadenopathy, and cytopenias in other cell lines are often noted. Also, the degree of eosinophilia is often more pronounced (>5 x 109/L). Patients with suspected hematologic malignancy should be urgently referred for expert consultation.5
A systematic approach to patients with eosinophilia
Consider the following approach in the assessment of patients with eosinophilia seen in the ambulatory care setting. Inpatients or patients being seen in developing areas may require a modified approach.
History. All patients with eosinophilia should have a thorough history taken, with particular attention paid to travel history. A travel history should make note of dates, duration and location of travel, and any relevant exposures, such as arthropod bites or swimming in freshwater. Dietary habits, such as ingestion of seafood, game, or undercooked meat can also be helpful in making a diagnosis.3,4
Physical exam. In addition to a general physical examination, the following features may be helpful in determining the etiology of eosinophilia. Wheeze is characteristic of parasites in a lung migration phase (eg, strongyloidiasis and ascariasis) or asthma. Hepatomegaly can be seen with liver flukes, visceral larva migrans, or schistosomiasis. Periorbital edema can be observed with Trichinella infection. Loa loa, a type of filarial infection, produces a transient, migratory angioedema, often localized to the wrists and large joints (termed Calabar swelling). Dermatitis of varying intensity may suggest filarial infection, schistosomiasis, or atopy. Perianal dermatitis is observed with strongyloidiasis. Cutaneous larva migrans is characterized by a linear, serpiginous rash.3,4
Laboratory investigations. Investigation will vary depending on the patient’s history, exposures, exam findings, and degree of eosinophilia. Any patient who is unwell or has significant eosinophilia (≥3 x 109/L) may warrant more urgent referral to infectious disease, travel medicine, or hematology. Basic laboratory investigations should include a CBC with differential, routine serum chemistries, and liver enzymes. In the setting of significant eosinophilia, an electrocardiogram, cardiac enzyme levels, and a chest x-ray should be obtained to screen for end-organ damage related to eosinophilia.3-5
In patients in whom you suspect hematologic malignancy, bone marrow aspiration and biopsy are often needed to make the diagnosis.5
Parasitic infections are most often diagnosed on stool examination for ova and parasites or by serology. Stool should be collected on 3 separate days to increase diagnostic yield. Certain species of Schistosoma can also be diagnosed on direct microscopy of urine specimens. Serologic assays are available for schistosomiasis, strongyloidiasis, Toxocara, fascioliasis, filariasis, and Trichinella. Further investigations for filiariasis, including blood films, eye exam, and skin snips will vary with filarial species, so expert consultation should be considered.3,4
Our patients. The first patient with strongyloidiasis was treated with ivermectin 200 µg/kg/day orally for 2 days and experienced symptomatic improvement and resolution of eosinophilia. The second patient with ALL was admitted and referred to hematology and received induction chemotherapy. Treatment was well tolerated and the patient was discharged one week later, with appropriate follow-up.
THE TAKEAWAY
Eosinophilia is commonly encountered in primary care. The approach to eosinophilia and the differential diagnosis can be challenging. The correct diagnosis was reached in both cases by maintaining a broad differential diagnosis. Obtaining a travel and exposure history is fundamental, although noninfectious causes, including allergy, malignancy, and drug reaction, must always be considered.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.
1. Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-521.
2. Tefferi A, Gotlib J, Pardanani A. Hypereosinophilic syndrome and clonal eosinophilia: point-of-care diagnostic algorithm and treatment update. Mayo Clin Proc. 2010;85:158-164.
3. Schulte C, Krebs B, Jelinek T, et al. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407-411.
4. Checkley AM, Chiodini PL, Dockrell DH, et al; British Infection Society and Hospital for Tropical Diseases. Eosinophilia in returning travellers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1-20.
5. Tefferi A, Patnaik MM, Pardanani A. Eosinophilia: secondary, clonal and idiopathic. Br J Haematol. 2006;133:468-492.