User login
QI reduces daily labs and promotes sleep-friendly lab timing
Background: Daily labs are often unnecessary on clinically stable inpatients. Additionally, daily labs are frequently drawn very early in the morning, resulting in sleep disruptions. No prior studies have attempted an EHR-based intervention to simultaneously improve both frequency and timing of labs.
Study design: Quality improvement project.
Setting: Resident and hospitalist services at a single academic medical center.
Synopsis: After surveying providers about lab-ordering preferences, an EHR shortcut and visual reminder were built to facilitate labs being ordered every 48 hours at 6 a.m. (rather than daily at 4 a.m.). Results included 26.3% fewer routine lab draws per patient-day per week, and a significant increase in sleep-friendly lab order utilization per encounter per week on both resident services (intercept, 1.03; standard error, 0.29; P < .001) and hospitalist services (intercept, 1.17; SE, .50; P = .02).
Bottom line: An intervention consisting of physician education and an EHR tool reduced daily lab frequency and optimized morning lab timing to improve sleep.
Citation: Tapaskar N et al. Evaluation of the order SMARTT: An initiative to reduce phlebotomy and improve sleep-friendly labs on general medicine services. J Hosp Med. 2020;15:479-82.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Daily labs are often unnecessary on clinically stable inpatients. Additionally, daily labs are frequently drawn very early in the morning, resulting in sleep disruptions. No prior studies have attempted an EHR-based intervention to simultaneously improve both frequency and timing of labs.
Study design: Quality improvement project.
Setting: Resident and hospitalist services at a single academic medical center.
Synopsis: After surveying providers about lab-ordering preferences, an EHR shortcut and visual reminder were built to facilitate labs being ordered every 48 hours at 6 a.m. (rather than daily at 4 a.m.). Results included 26.3% fewer routine lab draws per patient-day per week, and a significant increase in sleep-friendly lab order utilization per encounter per week on both resident services (intercept, 1.03; standard error, 0.29; P < .001) and hospitalist services (intercept, 1.17; SE, .50; P = .02).
Bottom line: An intervention consisting of physician education and an EHR tool reduced daily lab frequency and optimized morning lab timing to improve sleep.
Citation: Tapaskar N et al. Evaluation of the order SMARTT: An initiative to reduce phlebotomy and improve sleep-friendly labs on general medicine services. J Hosp Med. 2020;15:479-82.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Daily labs are often unnecessary on clinically stable inpatients. Additionally, daily labs are frequently drawn very early in the morning, resulting in sleep disruptions. No prior studies have attempted an EHR-based intervention to simultaneously improve both frequency and timing of labs.
Study design: Quality improvement project.
Setting: Resident and hospitalist services at a single academic medical center.
Synopsis: After surveying providers about lab-ordering preferences, an EHR shortcut and visual reminder were built to facilitate labs being ordered every 48 hours at 6 a.m. (rather than daily at 4 a.m.). Results included 26.3% fewer routine lab draws per patient-day per week, and a significant increase in sleep-friendly lab order utilization per encounter per week on both resident services (intercept, 1.03; standard error, 0.29; P < .001) and hospitalist services (intercept, 1.17; SE, .50; P = .02).
Bottom line: An intervention consisting of physician education and an EHR tool reduced daily lab frequency and optimized morning lab timing to improve sleep.
Citation: Tapaskar N et al. Evaluation of the order SMARTT: An initiative to reduce phlebotomy and improve sleep-friendly labs on general medicine services. J Hosp Med. 2020;15:479-82.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Medical comanagement did not improve hip fracture outcomes
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
Background: Medical comanagement of hip fracture patients is common. Prior evidence comes from mostly single-center studies, with most improvements being in process indicators such as length of stay and staff satisfaction.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement Program database.
Synopsis: With the NSQIP database targeted user file for hip fracture of 19,896 patients from 2016 to 2017, unadjusted analysis showed patients in the medical comanagement cohort were older with higher burden of comorbidities, higher morbidity (19.5% vs. 9.6%, odds ratio, 2.28; 95% CI, 1.98-2.63; P < .0001), and higher mortality rate (6.9% vs. 4.0%; OR, 1.79; 95% CI, 1.44-2.22; P < .0001). Both cohorts had similar proportion of patients participating in a standardized hip fracture program. After propensity score matching, patients in the comanagement cohort continued to show inferior morbidity (OR, 1.82; 95% CI, 1.52-2.20; P < .0001) and mortality (OR, 1.36; 95% CI, 1.02-1.81; P = .033).
This study failed to show superior outcomes in comanagement patients. The retrospective nature and propensity matching will lead to the question of unmeasured confounding in this large multinational database.
Bottom line: Medical comanagement of hip fractures was not associated with improved outcomes in the NSQIP database.
Citation: Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: A propensity score–matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2020;15:468-74.
Dr. Lockwood is a hospitalist and chief of quality, performance, and patient safety at the Lexington (Ky.) VA Health Care System.
What is the best approach for managing CIED infections?
The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
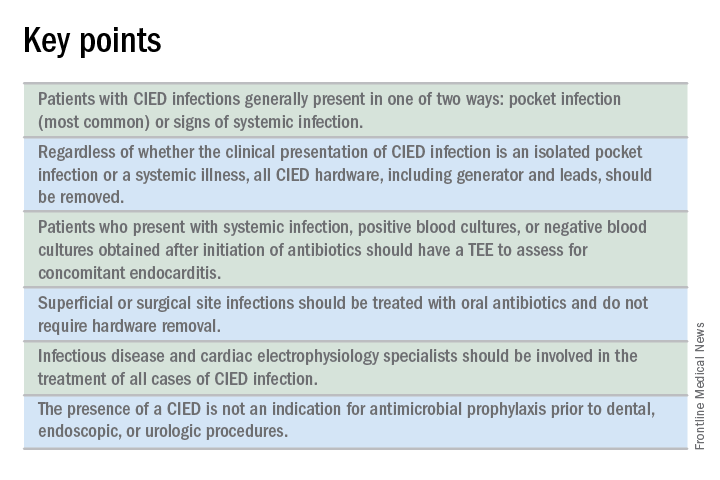
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
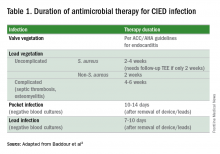
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.
References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.
The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.

References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.

The case
A 72-year-old man with ischemic cardiomyopathy and left-ventricular ejection fraction of 15% had an cardioverter-defibrillator implanted five years ago for primary prevention of sudden cardiac death. He was brought to the emergency department by his daughter who noticed erythema and swelling over the generator pocket site. His temperature is 100.1° F. Vital signs are otherwise normal and stable.
What are the best initial and definitive management strategies for this patient?
When should a cardiac implanted electronic device (CIED) infection be suspected?
CIED infections generally present in one of two ways: as a local generator pocket infection or as a systemic illness.1 Around 70% of CIED infections present with findings related to the generator pocket site. Findings in such cases include pain, swelling, erythema, induration, and ulceration. Systemic findings range from vague constitutional symptoms to overt sepsis. It is important to note that systemic signs of infection are only present in the minority of cases. Their absence does not rule out a CIED infection.1,2 As such, hospitalists must maintain a high index of suspicion in order to avoid missing the diagnosis.
Unfortunately, it can be difficult to distinguish between a CIED infection and less severe postimplantation complications such as surgical site infections, superficial pocket site infections, and noninfected hematoma.2
What are the risk factors for CIED infections?
The risk factors for CIED infection fit into three broad categories: procedure, patient, and pathogen.
Procedural factors that increase risk of infection include lack of periprocedural antimicrobial prophylaxis, inexperienced physician operator, multiple revision procedures, hematoma formation at the pocket site, and increased amount of hardware.1
Patient factors center on medical comorbidities, including congestive heart failure, diabetes mellitus, chronic kidney disease, immunosuppression, and ongoing oral anticoagulation.1
The specific pathogen also plays a role in infection risk. In one cohort study of 1,524 patients with CIEDs, patients with Staphylococcus aureus bacteremia ended up having confirmed or suspected CIED infections in 54.6% of cases, compared with just 12.0% of patients who had bacteremia with gram-negative bacilli.3
Which bacteria cause most CIED infections?
The vast majority of CIED infections are caused by gram-positive bacteria.1,4 An Italian study of 1,204 patients with CIED infection reported that pathogens isolated from extracted leads were gram-positive bacteria in 92.5% of infections.4 Staph species are the most common pathogens. Coagulase-negative staphylococcus and Staphylococcus aureus accounted for 69% and 13.8% of all isolates, respectively. Of note, 33% of CoNS isolates and 13% of S. aureus isolates were resistant to oxacillin in that study.4
Which initial diagnostic tests should be performed?
Patients should have two sets of blood cultures drawn from two separate sites, prior to administration of antibiotics.1 Current guidelines recommend against aspiration of a suspected infected pocket site because the sensitivity for identifying the causal pathogen is low while the risk of introducing infection to the generator pocket is substantial.1 In the event of CIED removal, pocket tissue and lead tips should be sent for gram stain and culture.1
Do all patients require a transesophageal echocardiogram?
Guidelines recommend a transesophageal echocardiogram (TEE) if suspicion for CIED infection is high based on positive blood cultures, antibiotic initiation prior to culture collection, ongoing systemic illness, or other suggestive signs.1,2 Positive transthoracic echocardiogram findings (for example, a valve vegetation) do not obviate the need for TEE because of the possibility of other relevant complications (including endocarditis and perivalvular extension) for which TEE has a greater sensitivity.1
What is the approach to antimicrobial therapy?
Since most infections involve staphylococcus species, initial empiric antimicrobial therapy should cover both oxacillin-sensitive and oxacillin-resistant strains. Thus, intravenous vancomycin is an appropriate initial choice.1 Culture and sensitivity results should then guide specific therapy decisions.1 Table 1 provides a summary of strategies for antimicrobial selection and duration.
Should all patients undergo complete device removal?
All patients with CIED infection require complete device removal, even if the infection is suspected to be confined to the generator pocket and blood cultures remain negative.2 Patients with superficial or surgical site infections without CIED infection do not require complete device removal. Rather, those cases can be managed with a 7- to 10-day course of oral antimicrobials.1
After device removal, what is the appropriate timing for installing a new device?
The decision to implant a replacement device is often made with input from infectious disease and cardiac electrophysiology experts. The decision must consider both infection and device-related concerns. Importantly, repeat blood cultures must be negative, and any infection in the pocket site should be controlled prior to installing a new device.2
Should all patients with a CIED receive antimicrobial prophylaxis prior to invasive dental, urologic, or endoscopic procedures?
No. Merely having a CIED is not considered an indication for antimicrobial prophylaxis.2
Back to the case
Two sets of blood cultures are drawn, and vancomycin is started as empiric therapy. The culture results show CoNS species, and antimicrobial resistance testing shows oxacillin-resistance.
TEE shows a vegetation on one of the leads but no vegetation on any of the heart valves. Cardiac electrophysiology is consulted and performs a percutaneous extraction of the entire device (generator and leads). Starting on the day of extraction, the patient undergoes two weeks of intravenous antimicrobial therapy with vancomycin. The patient, his family, and the electrophysiology team discuss the patient’s wishes, indications, and potential burdens related to device replacement.
He ultimately decides to have a replacement device installed. An infectious disease specialist is consulted to help define appropriate timing of the new device installation procedure.
Bottom line
The patient clearly had a CIED infection which required TEE, extraction of his CIED, and prolonged antimicrobial therapy.
Dr. Davisson is a primary care physician at Community Health Network in Indianapolis, Ind. Dr. Lockwood is a hospitalist at the Lexington VA Medical Center. Dr. Sweigart is a hospitalist at the University of Kentucky, Lexington, and the Lexington VA Medical Center.

References
1. Baddour LM, Epstein AE, Erickson CC, et al. Update on Cardiovascular Implantable Electronic Device Infections and Their Management: A Scientific Statement From the American Heart Association. Circulation. 2010;121(3):458-77.
2. Baddour LM, Cha YM, Wilson WR. Infections of Cardiovascular Implantable Electronic Devices. N Engl J Med. 2012;367(9):842-9.
3. Uslan DZ, Sohail MR, St. Sauver JL, et al. Permanent Pacemaker and Implantable Cardioverter Defibrillator Infection: A Population-Based Study. Arch Intern Med. 2007;167(7):669-75.
4. Bongiorni MG, Tascini C, Tagliaferri E, et al. Microbiology of Cardiac Implantable Electronic Device Infections. Europace. 2012;14(9):1334-9.